Abstract
Hypertension characterized by low circulating renin activity accounts for roughly 25%–30% of primary hypertension in humans and can be modeled experimentally via deoxycorticosterone acetate (DOCA)-salt treatment. In this model, phenotypes develop in progressive phases, although the timelines and relative contributions of various mechanisms to phenotype development can be distinct between laboratories. To explore interactions among environmental influences such as diet formulation and dietary sodium (Na) content on phenotype development in the DOCA-salt paradigm, we examined an array of cardiometabolic endpoints in young adult male C57BL/6J mice during sham or DOCA-salt treatments when mice were maintained on several common, commercially available laboratory rodent “chow” diets including PicoLab 5L0D (0.39% Na), Envigo 7913 (0.31% Na), Envigo 2920x (0.15% Na), or a customized version of Envigo 2920x (0.4% Na). Energy balance (weight gain, food intake, digestive efficiency, and energy efficiency), fluid and electrolyte homeostasis (fluid intake, Na intake, fecal Na content, hydration, and fluid compartmentalization), renal functions (urine production rate, glomerular filtration rate, urine Na excretion, renal expression of renin, vasopressin receptors, aquaporin-2 and relationships among markers of vasopressin release, aquaporin-2 shedding, and urine osmolality), and blood pressure, all exhibited changes that were subject to interactions between diet and DOCA-salt. Interestingly, some of these phenotypes, including blood pressure and hydration, were dependent on nonsodium dietary components, as Na-matched diets resulted in distinct phenotype development. These findings provide a broad and robust illustration of an environment × treatment interaction that impacts the use and interpretation of a common rodent model of low-renin hypertension.
Keywords: deoxycorticosterone, diet, fluid, hypertension, metabolism
INTRODUCTION
In 1943, Selye first published the hypertensive effects of a combined chronic administration of deoxycorticosterone acetate (DOCA) with a high dietary sodium (Na) load (1). Although the DOCA-salt model had been demonstrated to cause nephrosclerosis in chicks, dogs, rats, and monkeys, at the time of Selye’s publication, the blood pressure effects of DOCA-salt had not been previously evaluated. Selye showed that the hypertension and proteinuria phenotypes induced by DOCA-salt in rats were dependent on the synergy between DOCA and dietary Na, as treating animals with DOCA or dietary Na alone had no major effects across the 7 wk of study. This and many subsequent studies have established the DOCA-salt model as a convenient experimental model of hypertension characterized by low circulating renin-angiotensin system (RAS) activity (2, 3), as occurs in roughly 25%–30% of humans with primary hypertension (4). In addition to causing hypertension and renal function changes through mechanisms involving the brain RAS (5–7), DOCA-salt treatment stimulates resting metabolic rate (RMR) through activation of the brain RAS (8–11). Thus, DOCA-salt treatment has emerged as a simple experimental manipulation that activates the brain RAS and thereby multiple neurohormonal and autonomic mechanisms, to control an array of functions within cardiometabolic physiology.
Appreciation of the influence of various experimental conditions for animal studies is rapidly expanding, primarily reflecting an increased understanding of the influence of environmental variables (food sources, lighting types and timing, ambient temperature and humidity, seasonal variability, etc.) on cardiometabolic physiology. Since its first application for hypertension research, variations in the application of the DOCA-salt model have been adopted by different research teams (1–3, 10, 12). For example, some investigators utilize subcutaneous pellets or pumps to continuously deliver DOCA, whereas others utilize daily injections of the compound. Furthermore, some utilize DOCA, whereas others use distinct deoxycorticosterone salts instead of the acetate formulation (12). In addition, some investigators increase Na content of fluids offered to the animals, whereas others change the Na content of the food, with or without simultaneous manipulation of potassium supply. Finally, the DOCA-salt model has been used with and without uninephrectomy surgery, and across an array of species, including both rats and mice.
Here we have used one version of the DOCA-salt model in C57BL/6J male mice to investigate the importance of diet formulation, including dietary Na content, on an array of cardiometabolic phenotypes that develop in this model. In this model, mice were implanted with a subcutaneous DOCA pellet and provided both water and 0.15 M NaCl to drink, along with ad libitum access to a commercially available dietary formulation. It was determined that whereas some phenotypes are consistently developed, other phenotypes exhibited quantitative and even qualitative differences depending on the diet formulation. Surprisingly, some of these changes were independent of the dietary Na content. Results from these studies underscore the importance of environmental variables during the use of DOCA-salt treatment and illustrate the importance of clear methodological reporting. Ultimately, these results may explain contradictory results of studies using the DOCA-salt model at separate laboratories that supply distinct “chow” diets to experimental animals.
MATERIALS AND METHODS
Animals
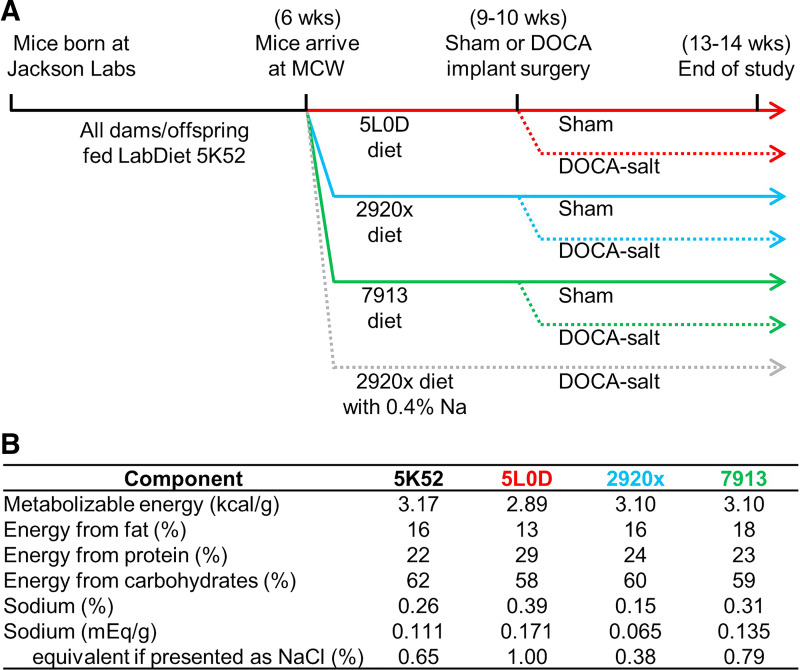
Male C57BL/6J mice were purchased from the Jackson Laboratories (stock no. 000664), where they were supplied LabDiet 5K52 diet, and arrived at the Medical College of Wisconsin at 6 wk of age (Fig. 1A). Mice were randomly assigned to one experimental diet on arrival and remained on that diet for the remainder of the study. All animal studies were performed in accordance with expectations laid out in the Guide for the Care and Use of Laboratory Animals, 8th edition (13), after approval by the MCW Institutional Animal Care and Use Committee.
Figure 1.
Experimental design and diet compositions. A: experimental timelines. B: dietary compositions.
Housing and Diets
Mice were housed in ventilated racks at 22°C–23°C with a 14:10 light:dark cycle, with ad libitum access to deionized (18.2 MΩ-cm) and autoclaved water, and ad libitum access to one experimental diet assigned at arrival. Experimental diets (Fig. 1B) included PicoLab 5L0D, Envigo 7913, Envigo 2920x, or a customized version of 2920x with Na added to supply 0.4% Na. Diets were provided in standard pellet form unless otherwise indicated.
DOCA-Salt Treatment
Between 9 and 10 wk of age, mice underwent sham surgery, where the subcutaneous space was exposed before suturing, or were implanted with a 50-mg pellet of DOCA (Sigma-Aldrich), pressed into a 0.25-in. diameter pellet via manual press (Parr), as we have previously used in studies of blood pressure, drinking, and metabolic responses to DOCA-salt treatment (8, 9, 14–16) in the subcutaneous space caudal to the scapulae while under isoflurane anesthesia, as previously described (10, 11). Mice were then administered one dose of meloxicam for postoperative analgesia, and topical bacitracin-neomycin-polymyxin antibiotic ointment was applied to the incision site. Notably, in this paradigm, animals did not undergo uninephrectomy, which is occasionally used to accelerate the genesis of phenotypes in the DOCA-salt paradigm. Mice that received DOCA implants were then additionally offered a second drink bottle for the remainder of the studies (in addition to deionized, autoclaved water), which contained 0.15 mol/L NaCl in deionized, autoclaved water. At euthanasia, the presence of some fraction of undissolved pellet was confirmed, to ensure that the pellet was not lost during the study (e.g., if the animal chewed or clawed the pellet out).
Tissue Collection and Blood Analyses
At the conclusion of studies, animals were euthanized by CO2 asphyxiation. Whole blood was collected into tubes coated with lithium heparin before analysis by an iSTAT blood chemistry analyzer. Alternatively, in a subset of mice, whole blood was collected into standard microcentrifuge tubes and allowed to coagulate on ice for at least 10 min before being centrifuged (10 min at 10,000 g) to isolate serum. Serum was then analyzed for sodium content using a flame atomic absorption spectrophotometer (Jenway).
Metabolic Caging
In a subset of cohorts, mice were placed into single mouse-sized metabolic cages (Nalgene/Tecniplast) for four consecutive days, as previously described (17, 18). Food intake, water and/or 0.15 mol/L NaCl, urine output, and fecal output were quantified daily. Because specific gravities of individual urine collections were not determined, urine production is reported as mass per day rather than volume per day. Urine and feces were quantitatively collected and stored at −80°C until further analyses. Food was provided in powdered form during metabolic cage studies.
Urinalyses
Urine collected in metabolic cage studies was analyzed for various solutes as previously (10, 11, 19, 20). Osmolality was determined by freezing-point depression osmometry (OsmoPro). Sodium content was assessed using a flame atomic absorption spectrophotometer (Jenway). Creatinine was assessed using a kit for Jaff’s reaction (Cat. No. DICT-500) from BioAssay Systems. Aldosterone was assessed using an ELISA kit from R&D Systems (Cat. No. KGE016). Copeptin was assessed using an ELISA kit from USCN/Cloud-Clone Corp (Cat. No. CEA365Mu). Aquaporin-2 was assessed using an ELISA assay kit (Cat. No. MBS456968) from MyBioSource. Protein content was assessed by Pierce bicinchoninic acid (BCA) assay from Thermo Fisher (Cat. No. 23227). UNOx was assessed using a kit from Abnova (Cat. No. KA4344).
Bomb Calorimetry
Caloric density of food and fecal samples were analyzed using a semimicro bomb calorimeter (Parr Instruments), as previously described (18). Calories absorbed were calculated as calories ingested per day minus calories lost to feces per day. Digestive efficiency was calculated as calories absorbed, divided by calories ingested. Energy efficiency was calculated as body mass gains, divided by calories absorbed, over the treatment timeframe (3 wk).
Fecal Na
Fecal Na was determined from feces collected in metabolic cages, by ashing fecal samples [using procedures previously described by Titze et al. (21)] and then reconstituting ash in 10% nitric acid. Samples were then analyzed for Na content using a flame atomic absorption spectrophotometer (Jenway).
Multiplexed Phenotyping
Multiplexed metabolic phenotyping (metabolic rates, food intake, fluid intake, and physical activity) was performed using a 16-cage Promethion (Sable Systems International) system, as previously described (18, 22). Briefly, animals underwent NMR on Monday morning, were housed continuously in the Promethion system until the following Friday afternoon, and then again underwent body composition analysis by NMR. Data from Monday and Tuesday were discarded as “acclimation,” and heat production, food and fluid intake behaviors, and physical activity were analyzed from data collected in the final 2 days of recording. To analyze meal and drink patterning, default parameters defining meals were used as suggested by the manufacturer (specifically, for food: minimum intake of 30 s and 0 g/maximum uptake per meal 1 g/maximum pause before ending meal 150 s; for water: minimum intake 10 s and 0 g/maximum uptake per bout 1 g/maximum pause before ending bout 30 s). We have added these details to the methods section.
Gene Expression
Renal expression of an array of genes was assessed using quantitative PCR. Primers and conditions were identical to our previous publications (20).
Body Composition and Sodium Pools
Body composition was analyzed using time-domain nuclear magnetic resonance spectroscopy (NMR), and fluid compartmentalization was calculated using bioimpedance spectroscopy, as previously described (19). Fat-free mass was calculated as total body mass minus fat mass. Total body water (TBW) was calculated as 73.2% of fat-free mass. Hydration is calculated as the ratio of total body water to total body mass. Osmotically active Na was calculated as the volume of extracellular fluid multiplied by the concentration of Na in plasma, plus the volume of intracellular fluid multiplied by 5 mmol/L. Total body Na was determined by ashing, as previously described by Titze et al. (21). Osmotically inactive Na was calculated as the difference between total Na and osmotically active Na.
Blood Pressure
Blood pressure was assessed using tail-cuff plethysmography (Visitech Systems), as previously described, and validated (23). Briefly, animals were restrained on a warmed pad and subjected to thirty 1-min inflation/deflation cycles whereas blood flow through the tail is assessed by photoelectric sensor. Animals were tested 5 days/wk, with one 30-min session per day. Data from the initial 3 wk were discarded as “acclimation/training,” and then data collected across 5 d each subsequent week were averaged within animal to generate a single weekly systolic blood pressure (BP) value per animal per week.
Transdermal Glomerular Filtration Rate
Transdermal glomerular filtration rate (tGFR) was assessed using instrumentation from MediBeacon, as described in detail by others (24). Briefly, mice were anesthetized via isoflurane inhalation, the back was shaved to expose the skin, and a miniaturized fluorescence detector was pressed against the skin and held in place by tape. A bolus of fluorescein-isothiocyanate (FITC)-conjugated sinistrin tracer was then injected into the retroorbital sinus (0.15 mg/g). Decay of the transdermal fluorescence signal over the following 90 min was then recorded and analyzed to calculate half-life and thereby tGFR.
Ventilation
Whole body plethysmography was measured using a custom-built, 200-mL cylindrical Plexiglas plethysmograph as previously described (25). Briefly, animals were placed into the temperature-controlled chamber and subjected to room air breathing (20–30 min) followed by either a 10-min hypoxia challenge (: 0.12; : 0.0; balanced with nitrogen) or hypercapnia challenge (: 0.21; : 0.07; balanced with nitrogen). Hypoxia and hypercapnia studies were repeated twice each on separate days. Data analyzed represent stable breathing void of behavioral or noneupneic breathing patterns. Tidal volume was determined using established methods (26).
RESULTS
Energy Homeostasis
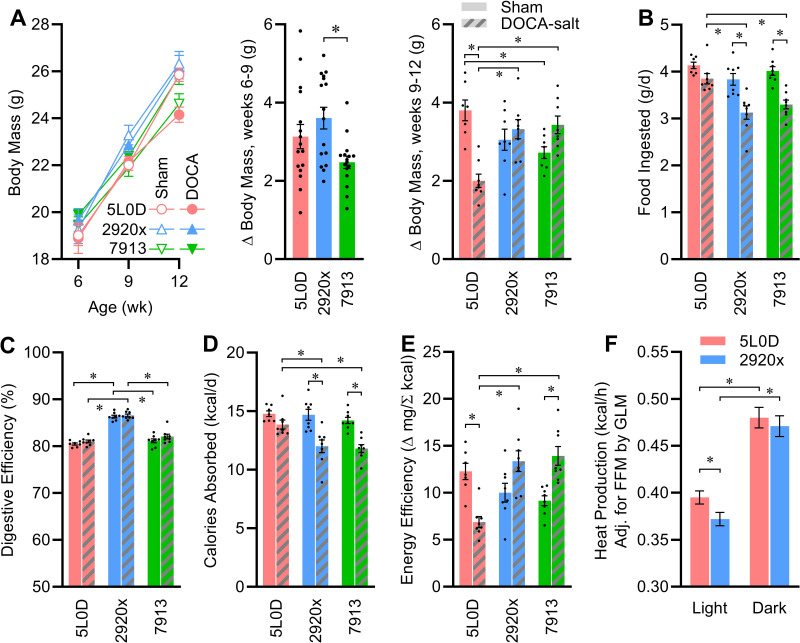
All mice were matched for body mass and composition on arrival. Although body mass gains were modestly increased in the mice fed 2920x diet in the 3-wk interval before DOCA-salt treatment was initiated, DOCA-salt induced diet-dependent alterations in weight gain (Fig. 2A). Strikingly, although DOCA-salt caused reduced weight gain in mice fed 5L0D, DOCA-salt had no significant effect on weight gain in mice fed 2920x or 7913. Diets and DOCA-salt had interactive effects to influence the masses of individual organs (Table 1). Notably among these, DOCA-salt caused increased liver, inguinal, interscapular, perigonadal, and perirenal fat pad masses and reduced spleen mass in some but not all diet groups. Renal mass was increased by DOCA-salt in all diet groups.
Figure 2.
Interactive effects of diet and DOCA-salt treatment on energy balance. A: body masses vs. time, change in body mass between weeks 6 and 9: one-way ANOVA P = 0.01; and change in body mass between weeks 9 and 12: two-way ANOVA diet P = 0.45, DOCA P = 0.14, diet × DOCA P < 0.01. B: food intake: two-way ANOVA diet P < 0.01, DOCA P < 0.01, diet × DOCA P = 0.09. C: digestive efficiency: two-way ANOVA diet P < 0.01, DOCA P = 0.06, diet × DOCA P = 0.66. D: calories absorbed per day: two-way ANOVA diet P < 0.01, DOCA P < 0.01, diet × DOCA P = 0.07. E: energy efficiency: two-way ANOVA diet P = 0.04, DOCA P = 0.21, diet × DOCA P < 0.01. F: aerobic heat production determined by O2/CO2 respirometry and averaged across the entire light phase or dark phase, and estimated marginal means are presented at covariate values of FFM = 19.31 g, fat mass = 5.93 g; light phase: FFM P < 0.01, fat P = 1.00, diet P = 0.04/dark phase: FFM P = 0.01, fat P = 0.85, diet P = 0.53. For A–F, n = 8 for all groups except n = 7 for week 12 5L0D + sham, and data are presented as means ± SE, and *P < 0.05 by Tukey’s multiple comparisons procedure. DOCA, deoxycorticosterone acetate; FFM, Fat-free mass.
Table 1.
Tissue masses after 3 wk of DOCA-salt or sham treatment
| End Point | 5L0D (n = 8, 8) |
2920x (n = 8, 8) |
7913 (n = 8, 8) |
P Value |
||
|---|---|---|---|---|---|---|
| Diet | DOCA | Diet × DOCA | ||||
| Body mass, g | ||||||
| Sham | 25.9 ± 0.3 | 26.3 ± 0.5 | 24.6 ± 0.4 | <0.01 | 0.52 | <0.01 |
| DOCA-salt | 24.2 ± 0.3 | 26.2 ± 0.5 | 25.8 ± 0.4 | |||
| Interscapular fat, mg | ||||||
| Sham | 63 ± 8 | 83 ± 19 | 61 ± 3 | 0.15 | 0.07 | 0.11 |
| DOCA-salt | 67 ± 6 | 85 ± 5 b | 99 ± 7a,b | |||
| Perigonadal fat, mg | ||||||
| Sham | 232 ± 27 | 376 ± 53 | 331 ± 19 | <0.01 | <0.01 | 0.45 |
| DOCA-salt | 330 ± 24 | 525 ± 50b | 523 ± 28 | |||
| Perirenal fat, mg | ||||||
| Sham | 50 ± 2 | 93 ± 14 | 76 ± 9 | <0.01 | <0.01 | 0.97 |
| DOCA-salt | 85 ± 9 | 129 ± 13 | 116 ± 6 | |||
| Inguinal fat, mg | ||||||
| Sham | 151 ± 30 | 184 ± 29 | 142 ± 9 | 0.12 | <0.01 | 0.08 |
| DOCA-salt | 167 ± 12 | 209 ± 14 | 237 ± 11a | |||
| Liver, g | ||||||
| Sham | 1.05 ± 0.12 | 1.10 ± 0.08 | 1.12 ± 0.03 | <0.01 | <0.01 | 0.03 |
| DOCA-salt | 1.27 ± 0.13 | 1.79 ± 0.08a,b | 1.70 ± 0.03a,b | |||
| Heart ventricles, mg | ||||||
| Sham | 106 ± 4 | 116 ± 3 | 94 ± 3c | <0.01 | 0.59 | <0.01 |
| DOCA-salt | 103 ± 2 | 106 ± 2 | 103 ± 4 | |||
| Adrenal glands, mg | ||||||
| Sham | 4.2 ± 0.3 | 3.3 ± 0.3 | 2.8 ± 0.5 | 0.04 | 0.11 | 0.41 |
| DOCA-salt | 2.9 ± 0.6 | 2.6 ± 0.4 | 2.6 ± 0.2 | |||
| Kidneys, mg | ||||||
| Sham | 335 ± 8 | 335 ± 7 | 300 ± 9 | 0.14 | <0.01 | 0.04 |
| DOCA-salt | 415 ± 11a | 436 ± 10a,b | 432 ± 11 a | |||
| Spleen, mg | ||||||
| Sham | 61 ± 3 | 69 ± 5 | 57 ± 3 | <0.01 | <0.01 | 0.09 |
| DOCA-salt | 45 ± 1a | 59 ± 2b | 55 ± 2 | |||
| Tibia, cm | ||||||
| Sham | 1.91 ± 0.06 | 1.88 ± 0.05 | 1.79 ± 0.04 | 0.85 | 0.12 | 0.18 |
| DOCA-salt | 1.80 ± 0.07 | 1.96 ± 0.04 | 1.84 ± 0.05 | |||
Data are presented as means ± SE. Data analyzed by two-way repeated-measures ANOVA. aP < 0.05 vs. diet-matched sham, bP < 0.05 vs. 5L0D + DOCA, and cP < 0.05 vs. 2920-sham by Tukey’s multiple comparison procedure. DOCA, deoxycorticosterone acetate.
To account for within- and between-group variation due to differences in body mass and body composition, tissue masses were additionally compared by generalized linear model (GLM), considering the covariates of body mass and tibia length; such corrections qualitatively changed interpretation of the main and interactive effects of diet and DOCA treatments on perirenal fat, liver, kidney, and adrenal masses (Table 2).
Table 2.
Tissue masses after 3 wk of DOCA-salt or sham treatment, corrected for body mass or tibia length by GLM
| End Point | 5L0D (n = 8, 8) |
2920x (n = 8, 8) |
7913 (n = 8, 8) |
P Value |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Corrected model | Body mass | Tibia length | Diet | DOCA | Diet × DOCA | ||||
| Interscapular fat, mg | |||||||||
| Sham | 83 ± 45 | 106 ± 36 | 112 ± 37 | 0.32 | 0.07 | 0.48 | 0.30 | 0.53 | 0.75 |
| DOCA-salt | 28 ± 38 | 111 ± 36 | 105 ± 35 | ||||||
| Perigonadal fat, mg | |||||||||
| Sham | 224 ± 35 | 333 ± 32 | 355 ± 32 | <0.01 | <0.01 | 0.56 | <0.01 | <0.01 | 0.93 |
| DOCA-salt | 400 ± 34 | 485 ± 32 | 509 ± 31 | ||||||
| Perirenal fat, mg | |||||||||
| Sham | 49 ± 76 | 83 ± 69 | 178 ± 69 | 0.79 | 0.53 | 0.39 | 0.90 | 0.40 | 0.34 |
| DOCA-salt | 208 ± 73 | 136 ± 69 | 106 ± 66 | ||||||
| Inguinal fat, mg | |||||||||
| Sham | 120 ± 24 | 175 ± 21 | 173 ± 22 | 0.02 | 0.14 | 0.45 | 0.05 | <0.01 | 0.71 |
| DOCA-salt | 180 ± 23 | 205 ± 22 | 232 ± 22 | ||||||
| Liver, g | |||||||||
| Sham | 1.01 ± 0.09 | 1.06 ± 0.08 | 1.14 ± 0.08 | <0.01 | 0.04 | 0.03 | <0.01 | <0.01 | 0.06 |
| DOCA-salt | 1.32 ± 0.09 | 1.80 ± 0.08 | 1.66 ± 0.08 | ||||||
| Heart ventricles, mg | |||||||||
| Sham | 107 ± 3 | 115 ± 3 | 94 ± 3 | <0.01 | 0.13 | 0.03 | <0.01 | 0.68 | 0.02 |
| DOCA-salt | 104 ± 3 | 107 ± 3 | 102 ± 3 | ||||||
| Adrenal glands, mg | |||||||||
| Sham | 3.9 ± 0.5 | 3.2 ± 0.4 | 2.9 ± 0.4 | 0.44 | 0.64 | 0.79 | 0.21 | 0.09 | 0.85 |
| DOCA-salt | 3.0 ± 0.4 | 2.6 ± 0.4 | 2.6 ± 0.4 | ||||||
| Kidneys, mg | |||||||||
| Sham | 338 ± 14 | 329 ± 13 | 305 ± 13 | <0.01 | 0.13 | 0.50 | 0.19 | <0.01 | 0.65 |
| DOCA-salt | 424 ± 14 | 434 ± 13 | 416 ± 12 | ||||||
| Spleen, mg | |||||||||
| Sham | 59 ± 4 | 68 ± 3 | 58 ± 3 | <0.01 | 0.52 | 0.98 | 0.02 | <0.01 | 0.32 |
| DOCA-salt | 46 ± 3 | 59 ± 3 | 55 ± 3 | ||||||
Data analyzed by GLM and presented as (EMM) ± SE after consideration of covariates at body mass = 25.44 g, tibia length = 1.86 cm. DOCA, deoxycorticosterone acetate. EMM, estimated marginal means; GLM, generalized linear model.
Diet-dependent changes in weight gain were accompanied by some interactive effects of diet and DOCA-salt on food intake behavior (Fig. 2B). Furthermore, digestive efficiency was modified by diet but not by DOCA-salt (Fig. 2C). As a result, caloric absorption rates were modified by diet and DOCA-salt (Fig. 2D). Energy efficiency (an inverse metric of total energy expenditure) was also therefore strongly influenced by an interaction between diet and DOCA-salt (Fig. 2E). In brief, DOCA-salt caused increased total energy expenditure in mice fed 5L0D but reductions in mice fed the other diets. Importantly, the relative changes in resting metabolism versus activity-dependent energy expenditure cannot be dissected by these approaches.
These results indicated that DOCA-salt caused distinct effects in 5L0D versus 2920x and 7913 diets. Thus, we next sought to further dissect the effect of DOCA-salt in mice fed 5L0D versus 2920x, as 2920x represents a purified soy-free diet that is commonly used by investigators that seek to avoid confounding effects of phytoestrogens. To dissect the energy expenditure effects of DOCA-salt in mice fed 5L0D versus 2920x diets, a separate cohort of mice was placed into a Promethion multiplexed metabolic phenotyping system during the third week of DOCA-salt treatment. Consistent with an increased energy expenditure in mice fed 5L0D, and with this increase mediated through exaggerated RMR, heat production rates were greater in mice fed 5L0D specifically during the light phase (Fig. 2F). No differences in locomotor activity were noted, though reductions in food, fluid, and Na intake in the 2920x group were confirmed (Tables 3 and 4). Interestingly, although total intakes differed, analysis of meal patterning failed to implicate meal count, size, duration, etc. as primary causes of total intake differences. Together, these data demonstrate that DOCA-salt has robust effects on various aspects of energy homeostasis, and that these effects are strongly modified by diet.
Table 3.
Metabolic parameters assessed via Promethion multiplexed phenotyping system, body composition changes assessed by NMR, estimates of calories committed to growth, and total unaccounted calories (hypothesized to be a metric of anaerobic energy expenditure)
| End Point | 5L0D + DOCA (n = 9) |
2920x + DOCA (n = 9) |
P Value |
||
|---|---|---|---|---|---|
| FFM | Fat | Diet | |||
| Body mass, g | |||||
| (uncorrected) | 25.35 ± 0.56 | 26.39 ± 0.78 | 0.30 | ||
| Fat-free mass (FFM), g | |||||
| (uncorrected) | 19.22 ± 0.44 | 19.40 ± 0.47 | 0.79 | ||
| Fat mass, g | |||||
| (uncorrected) | 5.86 ± 0.11 | 6.01 ± 0.25 | 0.59 | ||
| Light phase heat, kcal/h | |||||
| (uncorrected) | 0.394 ± 0.009 | 0.374 ± 0.012 | 0.19 | ||
| (EMM) | 0.395 ± 0.007 | 0.372 ± 0.007 | <0.01 | 1.00 | 0.04 |
| Dark phase heat, kcal/h | |||||
| (uncorrected) | 0.479 ± 0.011 | 0.0472 ± 0.014 | 0.69 | ||
| (EMM) | 0.480 ± 0.011 | 0.471 ± 0.011 | 0.01 | 0.85 | 0.53 |
| Locomotor activity, m/d | |||||
| (uncorrected) | 374.25 ± 30.17 | 342.46 ± 33.94 | 0.49 | ||
| (EMM) | 371.21 ± 29.05 | 345.50 ± 29.05 | 0.10 | 0.07 | 0.54 |
| Food intake, g/d | |||||
| (uncorrected) | 4.94 ± 0.33 | 3.65 ± 0.23 | 0.01 | ||
| (EMM) | 4.96 ± 0.28 | 3.62 ± 0.28 | 0.14 | 0.96 | <0.01 |
| Meals per phase, n | |||||
| Light phase | 25.6 ± 3.0 | 23.6 ± 3.0 | |||
| Dark phase | 39.9 ± 1.5 | 34.3 ± 3.1 | |||
| Total fluid intake, mL/d | |||||
| (uncorrected) | 18.50 ± 1.21 | 9.80 ± 1.56 | <0.01 | ||
| (EMM) | 18.46 ± 1.26 | 9.85 ± 1.26 | 0.04 | 0.16 | <0.01 |
| Total Na intake, mEq/d | |||||
| (uncorrected) | 1.28 ± 0.14 | 0.67 ± 0.13 | 0.01 | ||
| (EMM) | 1.30 ± 0.13 | 0.65 ± 0.13 | 0.08 | 0.79 | <0.01 |
| Δ Fat mass, Mon–Fri | |||||
| (grams) | −0.57 ± 0.12 | −1.05 ± 0.13 | 0.01 | ||
| (kcal equivalent @ 9 kcal/g) | −5.15 ± 1.10 | −9.43 ± 1.13 | |||
| Δ Fat-free mass, Mon–Fri | |||||
| (grams) | 0.24 ± 0.10 | 0.08 ± 0.11 | 0.28 | ||
| (kcal equivalent @ 4 kcal/g) | 2.18 ± 0.87 | 0.75 ± 0.95 | |||
| Calories to growth, Mon–Fri | |||||
| (kcal) | −4.18 ± 1.21 | −9.10 ± 1.17 | 0.01 | ||
| Calories consumed, Mon–Fri | |||||
| (kcal) | 160.71 ± 9.84 | 69.85 ± 8.48 | <0.01 | ||
| Calories absorbed, Mon–Fri | |||||
| (digestive efficiency from Fig. 2D; %) | 81.0 ± 0.8 | 86.6 ± 0.8 | <0.01 | ||
| (kcal) | 130.18 ± 7.97 | 60.49 ± 7.35 | <0.01 | ||
| Total aerobic expenditure, Mon–Fri | |||||
| (kcal) | 40.38 ± 0.96 | 39.07 ± 1.08 | 0.38 | ||
| Unaccounted energy, Mon–Fri | |||||
| (kcal) | 93.97 ± 7.58 | 30.53 ± 7.03 | <0.01 | ||
| (% intake) | 72 ± 2 | 38 ± 14 | 0.03 | ||
Data presented as means ± SE. Data analyzed by t test when uncorrected for covariates, or by GLM when correcting for fat-free mass (FFM) and fat mass. Estimated marginal means (EMM) ± SE values are reported after consideration of covariates at FFM = 19.31 g, fat mass = 5.93 g. DOCA, deoxycorticosterone acetate. GLM, generalized linear model.
Table 4.
Ingestive behaviors as assessed via Promethion multiplexed phenotyping system
| End Point | 5L0D + DOCA (n = 9) |
2920x + DOCA (n = 9) |
P Value |
||
|---|---|---|---|---|---|
| Diet | Phase | Diet × Phase | |||
| Meals per phase, n | |||||
| Light phase | 25.6 ± 3.0 | 23.6 ± 3.0 | 0.17 | <0.01 | 0.54 |
| Dark phase | 39.9 ± 1.5 a | 34.3 ± 3.1 a | |||
| Mass consumed per meal, mg | |||||
| Light phase | 65 ± 7 | 64 ± 6 | 0.28 | 0.03 | 0.10 |
| Dark phase | 84 ± 5 | 66 ± 8 | |||
| Meal duration, min | |||||
| Light phase | 1.98 ± 0.18 | 2.43 ± 0.23 | 0.17 | <0.01 | 0.45 |
| Dark phase | 2.51 ± 0.17 | 2.78 ± 0.19 | |||
| Intermeal interval, min | |||||
| Light phase | 29.04 ± 3.51 | 33.11 ± 4.32 | 0.16 | <0.01 | 0.99 |
| Dark phase | 15.01 ± 0.66a | 19.19 ± 1.82a | |||
| Total food intake within phase, g | |||||
| Light phase | 1.61 ± 0.21 | 1.36 ± 0.10 | <0.01 | <0.01 | 0.07 |
| Dark phase | 3.30 ± 0.23a | 2.15 ± 0.23a,b | |||
| Total fluid intake within phase, mL | |||||
| Light phase | 5.83 ± 0.52 | 3.14 ± 0.60 | <0.01 | <0.01 | <0.01 |
| Dark phase | 12.96 ± 0.80a | 6.92 ± 1.06a,b | |||
| Total Na intake within phase, mEq | |||||
| Light phase | 0.46 ± 0.07 | 0.23 ± 0.04 | <0.01 | <0.01 | 0.08 |
| Dark phase | 0.85 ± 0.09a | 0.41 ± 0.08b | |||
| Fraction of total Na intake from drink, % | |||||
| Light phase | 33.5 ± 6.2 | 51.7 ± 8.5 | 0.03 | 0.96 | 0.16 |
| Dark phase | 28.6 ± 5.7 | 56.2 ± 8.0 | |||
| Total water intake within phase, mL | |||||
| Light phase | 4.65 ± 0.49 | 2.23 ± 0.42b | <0.01 | <0.01 | <0.01 |
| Dark phase | 11.15 ± 0.63a | 5.12 ± 0.78a,b | |||
| Total 0.15 M saline intake within phase, mL | |||||
| Light phase | 1.18 ± 0.32 | 0.91 ± 0.25 | 0.81 | <0.01 | 0.60 |
| Dark phase | 1.81 ± 0.52 | 1.81 ± 0.53 | |||
| Pref. for 0.15 M saline within phase, % | |||||
| Light phase | 20.1 ± 5.7 | 34.8 ± 8.0 | 0.07 | 0.08 | 0.69 |
| Dark phase | 13.2 ± 3.5 | 24.1 ± 4.7 | |||
Data presented as means ± SE. Data analyzed by two-way repeated-measures ANOVA. aP < 0.05 vs. light phase within treatment group, bP < 0.05 vs. 5L0D + DOCA in same phase by Tukey’s multiple comparison procedure. DOCA, deoxycorticosterone acetate.
Renal Function
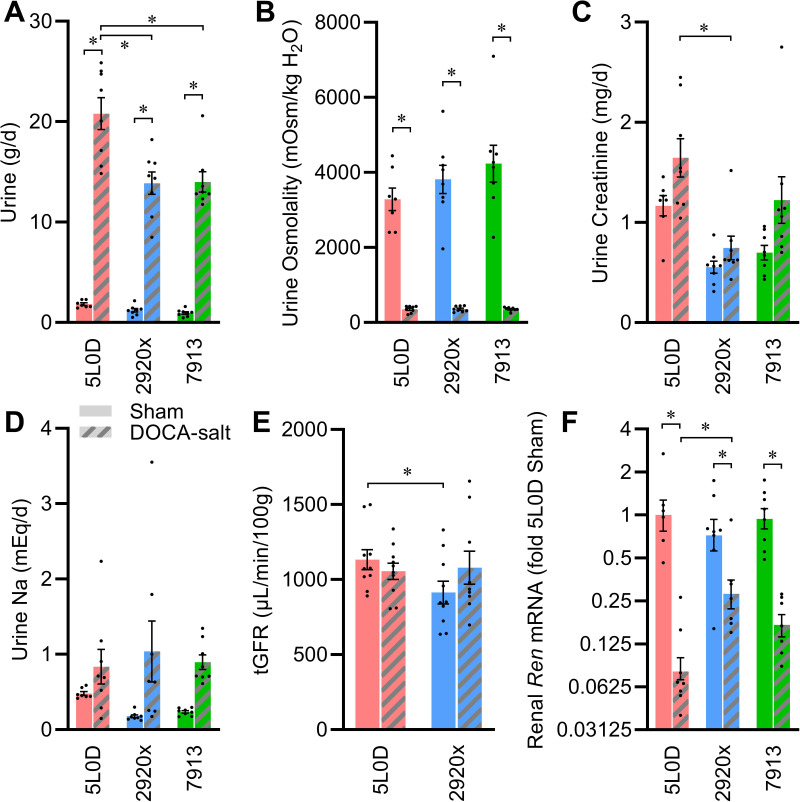
Urine production rates were similar across the three standard diet groups in sham-treated animals, and although DOCA-salt treatment caused expected increases in urine production rates, this effect was much greater in mice supplied 5L0D diet than the other diets (Fig. 3A). Urine osmolality was unaffected by diet, and DOCA-salt had a similar effect to cause dilution of urine in all three diet groups (Fig. 3B). Urine creatinine concentration was modified by diet, DOCA, and an interaction between these two factors (Table 5). Urine creatinine elimination per day differed by diet under both sham and DOCA-salt conditions, and the effect of DOCA-salt to increase urine creatinine elimination was dependent on the diet (Fig. 3C). Urine protein concentrations were dependent on diet, and similarly suppressed in all diet groups after DOCA-salt (Table 5). Total urine protein elimination per day was dependent on diet, but DOCA-salt had no effect on this end point. Urine Na concentration was dependent on diet in the sham-treated groups, and DOCA-salt caused reduced urine Na concentration in all diet groups. Total daily urine Na elimination was similar across diets, and similarly increased by DOCA-salt in all dietary conditions (Fig. 3D). Total urinary nitrate and nitrite (UNOx) excretion was modified by diet, and DOCA-salt caused diet-dependent reductions in this end point. Transdermal glomerular filtration rate (tGFR) was higher in sham-treated mice fed 5L0D versus 2920x, but DOCA-salt treatment normalized tGFR to an intermediate level in animals on both diets (Fig. 3E).
Figure 3.
Renal function effects of diet and DOCA-salt. A: urine production rate: two-way ANOVA diet P < 0.01, DOCA P < 0.01, diet × DOCA P < 0.01. B: urine osmolality: two-way ANOVA diet P = 0.25, DOCA P < 0.01, diet × DOCA P = 0.25. C: urine creatinine per day: two-way ANOVA diet P < 0.01, DOCA P < 0.01, diet × DOCA P = 0.47. D: urine Na per day: two-way ANOVA diet P = 0.90, DOCA P < 0.01, diet × DOCA P = 0.46. E: transdermal glomerular filtration rate: two-way ANOVA diet P = 0.22, DOCA P = 0.57, diet × DOCA P = 0.13. F: renal renin mRNA: two-way ANOVA diet P = 0.11, DOCA P < 0.01, diet × DOCA P < 0.01. For A–D and F, n = 8 for all groups except n = 7 for 5L0D + sham; for E, n = 10 for all groups. For A–F, data are presented as means ± SE, and *P < 0.05 by Tukey’s multiple comparisons procedure. DOCA, deoxycorticosterone acetate.
Table 5.
Urinalyses after 3 wk of DOCA-salt or sham treatment
| End Point | 5L0D (n = 8, 8) |
2920x (n = 8, 8) |
7913 (n = 8, 8) |
P Value |
||
|---|---|---|---|---|---|---|
| Diet | DOCA | Diet × DOCA | ||||
| Urine creatinine, mg/mL | ||||||
| Sham | 0.65 ± 0.07 | 0.50 ± 0.06a | 0.79 ± 0.09 | 0.01 | <0.01 | 0.04 |
| DOCA-salt | 0.08 ± 0.01b | 0.06 ± 0.01b | 0.08 ± 0.01b | |||
| Urine osmolytes, mOsmol/kgH2O/d | ||||||
| Sham | 5.8 ± 0.2a | 4.2 ± 0.3 | 3.6 ± 0.2 | <0.01 | <0.01 | 0.61 |
| DOCA-salt | 7.3 ± 0.8 | 4.8 ± 0.4c | 4.8 ± 0.1c | |||
| Potential renal solute load, mOsmol/kgH2O/d | ||||||
| Sham | 8.43 ± 1.70 | 6.40 ± 0.54d | 7.42 ± 0.45 | <0.01 | 0.43 | 0.08 |
| DOCA-salt | 9.60 ± 0.84 | 6.13 ± 1.23c | 7.21 ± 0.61c | |||
| Potential renal solute load, minus urine osmolytes, mOsmol/kgH2O/d | ||||||
| Sham | 2.65 ± 1.90 | 2.21 ± 0.44 | 3.78 ± 0.78 | 0.01 | 0.01 | 0.45 |
| DOCA-salt | 2.33 ± 1.87 | 1.30 ± 0.73 | 2.38 ± 0.51 | |||
| Urine protein, mg/mL | ||||||
| Sham | 38.7 ± 2.0a | 49.2 ± 4.4 | 54.3 ± 5.4 | 0.04 | <0.01 | 0.04 |
| DOCA-salt | 3.2 ± 0.3b | 3.8 ± 0.3b | 3.3 ± 0.2b | |||
| Urine protein, mg/d | ||||||
| Sham | 69.3 ± 1.9 | 54.7 ± 4.0 | 47.5 ± 2.9 | <0.01 | 0.30 | 0.96 |
| DOCA-salt | 65.5 ± 7.3 | 50.5 ± 2.5 | 45.4 ± 1.8c | |||
| Urine Na, mEq/L | ||||||
| Sham | 0.27 ± 0.01 | 0.16 ± 0.01a,d | 0.26 ± 0.02 | <0.01 | <0.01 | <0.01 |
| DOCA-salt | 0.04 ± 0.01b | 0.06 ± 0.01b | 0.07 ± 0.01b | |||
| UNOx, μmol/L | ||||||
| Sham | 211 ± 21a | 245 ± 58a | 443 ± 31 | <0.01 | <0.01 | <0.01 |
| DOCA-salt | 1 ± 0b | 1 ± 0b | 1 ± 0b | |||
| UNOx, mmol/d | ||||||
| Sham | 0.37 ± 0.02a,d | 0.25 ± 0.03a | 0.41 ± 0.04 | <0.01 | <0.01 | <0.01 |
| DOCA-salt | 0.03 ± 0.01b | 0.01 ± 0.01b | 0.01 ± 0.00b | |||
Data presented as means ± SE and analyzed by two-way ANOVA. aP < 0.05 vs. 7913-sham, bP < 0.05 vs. diet-matched sham, cP < 0.05 vs. 5L0D-DOCA, dP < 0.05 vs. 5L0D-sham by Tukey’s multiple comparison procedure. DOCA, deoxycorticosterone acetate.
Renal expression of renin (Ren) mRNA was similar across diet groups in the sham condition; however, the level of suppression by DOCA-salt treatment was strongly diet dependent with the strongest suppression in the 5L0D group (Fig. 3F). Renal angiotensinogen (Agt) expression was modified by an interaction between diet and DOCA-salt treatment (Table 6). No consistent changes were observed in renal expression of angiotensin-converting enzyme (Ace) or the ANG AT1A receptor (Agtr1a). Expression of various transporters, receptors, and channels were also analyzed, and although diet modified expression of some genes (such as Avpr2, Aqp2, and Scnn1g) and DOCA-salt treatment modified expression of some genes (such as Nkcc2a, Aqp4, Scnn1a, Scnn1b, Scnn1g, and Atp1a1), none exhibited consistent interactions between diet and DOCA-salt.
Table 6.
Renal gene expression after 3 wk of DOCA-salt or sham treatment
| Gene | 5L0D Sham (n = 8) |
5L0D DOCA (n = 8) |
2920x Sham (n = 8) |
2920x DOCA (n = 8) |
7913 Sham (n = 8) |
7913 DOCA (n = 8) |
P Value |
||
|---|---|---|---|---|---|---|---|---|---|
| Diet | DOCA | Diet × DOCA | |||||||
| Ren | 1.00 (0.77–1.27) | 0.08 (0.07–0.10)a | 0.72 (0.56–0.93) | 0.28 (0.22–0.35)a,b | 0.94 (0.80–1.11) | 0.17 (0.14–0.20)a | 0.11 | <0.01 | <0.01 |
| Agt | 1.00 (0.85–1.18) | 0.96 (0.88–1.05) | 0.75 (0.66–0.85) | 1.27 (1.18–1.36)a | 0.69 (0.60–0.79) | 1.04 (1.00–1.08) | 0.32 | <0.01 | 0.03 |
| Ace | 1.00 (0.79–1.27) | 0.63 (0.47–0.83) | 0.93 (0.63–1.38) | 0.75 (0.60–0.94) | 0.58 (0.29–1.17) | 0.55 (0.40–0.74) | 0.57 | 0.46 | 0.88 |
| Agtr1a | 1.00 (0.89–1.13) | 0.91 (0.74–1.12) | 1.00 (0.67–1.48) | 0.80 (0.68–0.95) | 0.76 (0.56–1.02) | 0.94 (0.86–1.03) | 0.88 | 0.88 | 0.63 |
| Ncc | 1.00 (0.79–1.27) | 2.60 (1.71–3.98) | 0.39 (0.17–0.89) | 1.23 (0.78–1.95) | 0.70 (0.37–1.35) | 0.82 (0.42–1.59) | 0.39 | 0.16 | 0.71 |
| Nhe3 | 1.00 (0.71–1.42) | 1.06 (0.66–1.70) | 0.40 (0.18–0.88) | 0.58 (0.35–0.95) | 0.46 (0.20–1.03) | 0.42 (0.24–0.75) | 0.35 | 0.83 | 0.93 |
| Nkcc2a | 1.00 (0.81–1.24) | 1.39 (1.07–1.82) | 0.50 (0.33–0.75) | 1.12 (0.92–1.37) | 0.39 (0.20–0.73) | 0.86 (0.65–1.13) | 0.19 | 0.04 | 0.78 |
| Avpr2 | 1.00 (0.81–1.23) | 0.81 (0.70–0.94) | 0.43 (0.35–0.53) | 0.55 (0.50–0.60) | 0.52 (0.44–0.61) | 0.56 (0.49–0.65) | <0.01 | 0.79 | 0.48 |
| Aqp1 | 1.00 (0.83–1.21) | 0.99 (0.74–1.33) | 0.61 (0.41–0.90) | 0.85 (0.70–1.03) | 0.42 (0.21–0.83) | 0.63 (0.47–0.85) | 0.27 | 0.46 | 0.86 |
| Aqp2 | 1.00 (0.81–1.23) | 1.03 (0.88–1.20) | 0.39 (0.30–0.52) | 0.66 (0.55–0.79) | 0.49 (0.37–0.65) | 0.66 (0.52–0.83) | 0.01 | 0.15 | 0.59 |
| Aqp3 | 1.00 (0.70–1.43) | 0.66 (0.46–0.97) | 0.55 (0.28–1.09) | 0.41 (0.28–0.61) | 0.22 (0.08–0.61) | 0.46 (0.27–0.79) | 0.35 | 0.97 | 0.61 |
| Aqp4 | 1.00 (0.83–1.20) | 2.65 (2.35–2.98)a | 1.01 (0.86–1.19) | 1.88 (1.66–2.12)a | 1.18 (1.02–1.37) | 2.42 (2.17–2.70)a | 0.30 | <0.01 | 0.45 |
|
Scnn1a
(ENaC-α) |
1.00 (0.71–1.41) | 2.52 (1.80–3.53) | 0.57 (0.32–1.00) | 1.95 (1.57–2.41) | 0.37 (0.19–0.74) | 1.45 (0.97–2.16) | 0.27 | <0.01 | 0.90 |
|
Scnn1b
(ENaC-β) |
1.00 (0.82–1.22) | 1.53 (1.33–1.75) | 0.68 (0.53–0.86) | 1.23 (1.08–1.39) | 0.53 (0.35–0.80) | 1.17 (1.03–1.34) | 0.15 | <0.01 | 0.72 |
|
Scnn1g
(ENaC-γ) |
1.00 (0.92–1.09) | 1.59 (1.42–1.77) | 0.64 (0.54–0.75) | 1.13 (1.00–1.27) | 0.71 (0.62–0.81) | 0.71 (0.59–0.85) | <0.01 | <0.01 | 0.55 |
|
Atp1a1
(Na-K-ATPase) |
1.00 (0.78–1.28) | 1.62 (1.22–2.17) | 0.62 (0.37–1.03) | 1.50 (1.21–1.86) | 0.37 (0.16–0.82) | 1.06 (0.73–1.54) | 0.33 | 0.04 | 0.83 |
Data presented as means (± SE) fold of 5L0D-sham group and analyzed by two-way ANOVA. aP < 0.05 vs. diet-matched sham, bP < 0.05 vs. 5L0D + DOCA by Tukey’s multiple-comparisons procedure. DOCA, deoxycorticosterone acetate.
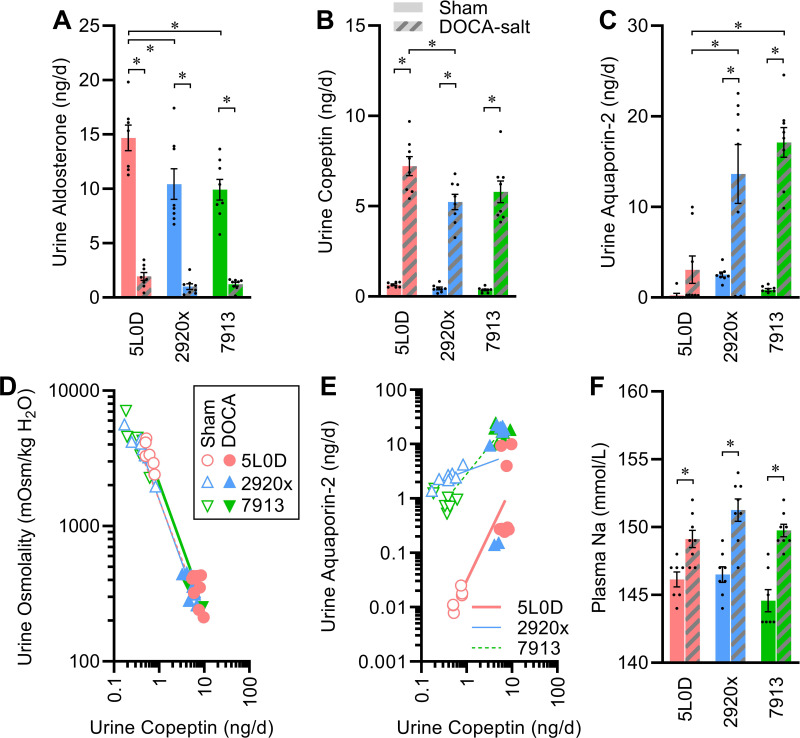
Elimination of aldosterone to urine (as a metric of aldosterone secretion rates) was dependent on the diet, with increased elimination in the 5L0D group, and DOCA-salt similarly caused suppression of aldosterone elimination in all mice (Fig. 4A). Similarly, elimination of copeptin to urine [as a metric of arginine vasopressin (AVP) secretion] was increased by DOCA-salt in all diet groups, though this induction was greatest in the 5L0D group (Fig. 4B). Surprisingly then, urine aquaporin-2 [AQP2, proposed as a metric of AQP2 mobilization in the collecting duct (27)] content differed by diet and was increased by DOCA-salt in a diet-dependent manner, with the lowest AQP2 shedding observed in mice fed 5L0D (Fig. 4C). Regression analyses were then utilized to examine relationships among endpoints. First, as expected, urine osmolality strongly correlated with copeptin regardless of diet or DOCA-salt treatment, and relationships (i.e., slopes of regression lines) were indistinguishable among diet groups (Fig. 4D). Second, comparison of urine AQP2 versus copeptin content illustrated the induction of each by DOCA-salt within each diet group (i.e., a positive slope for each line), but also a diet-dependent modulation of these relationships (i.e., slopes differed by diet, P < 0.0001; Fig. 4E). Consistent with these observations, renal mRNA expression of the AVP type 2 receptor (Avpr2) and AQP2 (Aqp2) genes were altered by diet, with highest expression in the 5L0D group (Table 6). These results support the concepts that diet strongly modifies the relationship between AVP secretion and AQP2 mobilization, and as a result, diet modifies the effect of DOCA-salt on these endpoints.
Figure 4.
Endocrine signals and renal function effects of diet and DOCA-salt. A: urine aldosterone elimination rate per day: two-way ANOVA diet P < 0.01, DOCA P < 0.01, diet × DOCA P = 0.05. B: urine copeptin elimination rate per day: two-way ANOVA diet P = 0.02, DOCA P < 0.01, diet × DOCA P = 0.07. C: urine aquaporin-2 shedding rate per day: two-way ANOVA diet P < 0.01, DOCA P < 0.01, diet × DOCA P < 0.01. D: regression modeling of relationship between urine osmolality vs. urine copeptin elimination rate: slopes P < 0.01 vs. slope of zero, and comparison of slopes P = 0.49. E: regression modeling of relationship between urine aquaporin-2 shedding rate vs. urine copeptin elimination rate: slopes are all P < 0.05 vs. slope of zero, and comparison of slopes P < 0.01. F: plasma Na concentration: two-way ANOVA diet P = 0.03, DOCA P < 0.01, diet × DOCA P = 0.22. For A–F, n = 8 for all groups except n = 7 for 5L0D + sham and 7913 + sham. For A–F, summary data are presented as means ± SE, and *P < 0.05 by Tukey’s multiple comparisons procedure. DOCA, deoxycorticosterone acetate.
Blood Chemistries
Plasma sodium concentrations were similar for mice in all sham-treated diet groups and increased by DOCA-salt in all diet groups (Fig. 4F). Plasma potassium was also similar across sham-treated diet groups and suppressed by DOCA-salt in all groups, but this suppression was also dependent on diet (Table 7). Similar interactive effects between DOCA-salt and diet were observed for plasma chloride, calcium, blood urea nitrogen (BUN), and anion gap.
Table 7.
Blood chemistries after 3 wk of DOCA-salt or sham treatment
| End Point | 5L0D (n = 8, 8) |
2920x (n = 8, 8) |
7913 (n = 8, 8) |
P Value |
||
|---|---|---|---|---|---|---|
| Diet | DOCA | Diet × DOCA | ||||
| Plasma Na, mmol/L | ||||||
| Sham | 146.1 ± 0.6 | 146.5 ± 0.6 | 144.6 ± 0.8 | 0.03 | <0.01 | 0.22 |
| DOCA-salt | 149.1 ± 0.6a | 151.3 ± 0.8a | 149.8 ± 0.5a | |||
| Plasma K, mmol/L | ||||||
| Sham | 6.4 ± 0.2 | 6.2 ± 0.2 | 6.1 ± 0.1 | <0.01 | <0.01 | 0.08 |
| DOCA-salt | 4.0 ± 0.1a | 3.1 ± 0.1a,b | 3.2 ± 0.0a,b | |||
| Plasma Cl, mmol/L | ||||||
| Sham | 115.9 ± 1.0 | 117.3 ± 1.1 | 117.9 ± 1.1 | 0.09 | <0.01 | <0.01 |
| DOCA-salt | 108.1 ± 1.2a | 103.9 ± 1.7a | 100.6 ± 0.8a,b | |||
| Plasma iCa, mmol/L | ||||||
| Sham | 0.99 ± 0.06 | 1.06 ± 0.05 | 0.87 ± 0.07 | 0.09 | 0.74 | 0.06 |
| DOCA-salt | 0.90 ± 0.05 | 1.03 ± 0.04 | 1.02 ± 0.04 | |||
| Blood glucose, mg/dL | ||||||
| Sham | 157 ± 5 | 182 ± 9 | 162 ± 8 | 0.08 | 0.06 | 0.48 |
| DOCA-salt | 145 ± 7 | 158 ± 13 | 158 ± 4 | |||
| BUN, mg/dL | ||||||
| Sham | 32.3 ± 1.1 | 21.4 ± 1.0c | 22.6 ± 0.9c | <0.01 | <0.01 | <0.01 |
| DOCA-salt | 14.0 ± 1.0a | 9.3 ± 0.6a,b | 8.5 ± 0.6a,b | |||
| Hematocrit, %PCV | ||||||
| Sham | 44.3 ± 0.9 | 46.6 ± 0.8 | 44.0 ± 0.5 | 0.01 | 0.03 | 0.56 |
| DOCA-salt | 43.8 ± 1.0 | 44.5 ± 0.7 | 42.5 ± 0.3 | |||
| Total CO2, mmol/L | ||||||
| Sham | 22.4 ± 0.5 | 19.6 ± 1.2 | 22.3 ± 1.4 | <0.01 | <0.01 | <0.01 |
| DOCA-salt | 29.0 ± 0.8a | 30.0 ± 1.3a,d | 36.5 ± 1.0a,b,d | |||
| Anion gap, mmol/L | ||||||
| Sham | 15.1 ± 0.9 | 17.0 ± 1.3 | 11.4 ± 1.5e | <0.01 | <0.01 | 0.32 |
| DOCA-salt | 17.0 ± 1.0 | 20.7 ± 1.6 | 17.0 ± 0.9a | |||
Data presented as means ± SE and analyzed by two-way repeated measures ANOVA. aP < 0.05 vs. diet-matched sham, bP < 0.05 vs. 5L0D + DOCA, cP < 0.05 vs. 5L0D + sham, dP < 0.05 vs. 2920 + DOCA, eP < 0.05 vs. 2920 + sham by Tukey’s multiple-comparisons procedure. DOCA, deoxycorticosterone acetate; PCV, packed cell volume.
Fluid and Electrolyte Turnver
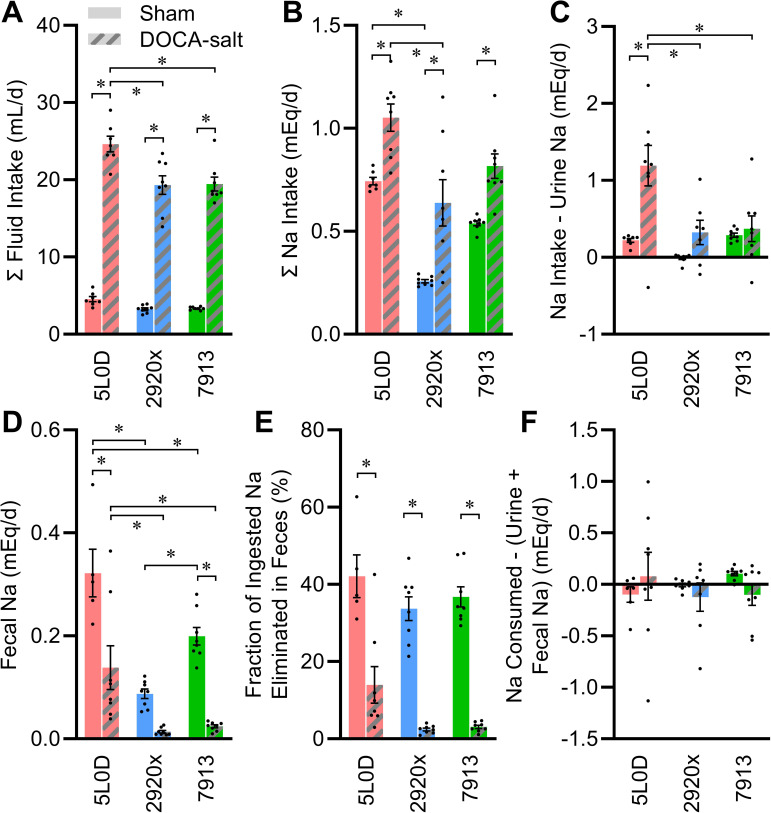
Fluid intake was similar between sham-treated diet groups (Fig. 5A). Treatment with DOCA-salt caused a robust increase in total fluid intake (water plus 0.15 M NaCl) in all diet groups; however, mice maintained on 5L0D exhibited an exaggerated intake relative to the other two groups. Mice in all diet groups exhibited a similar aversion to 0.15 M NaCl (5L0D: 9 ± 1, 2920x: 15 ± 3, 7913: 12 ± 2% total fluid, P = 0.27 by one-way ANOVA).
Figure 5.
Comprehensive analysis of Na balance. A: total fluid intake rate: two-way ANOVA diet P < 0.01, DOCA P < 0.01, diet × DOCA P < 0.01. B: total daily Na intake, including both fluid and food sources: two-way ANOVA diet P < 0.01, DOCA P < 0.01, diet × DOCA P = 0.68. C: apparent Na retention determined by subtracting urine Na from consumed Na: two-way ANOVA diet P < 0.01, DOCA P < 0.01, diet × DOCA P = 0.01. D: fecal Na elimination rate: two-way ANOVA diet P < 0.01, DOCA P < 0.01, diet × DOCA P = 0.04. E: fraction of daily Na intake that is eliminated to feces: two-way ANOVA diet P < 0.01, DOCA P < 0.01, diet × DOCA P = 0.69. F: calculated Na retention determined by subtracting urine and fecal Na from consumed Na: two-way ANOVA diet P = 0.85, DOCA P = 0.64, diet × DOCA P = 0.30. For A–F, n = 8 for all groups except n = 7 for 5L0D. For A–F, summary data are presented as means ± SE, and *P < 0.05 by Tukey’s multiple comparisons procedure. DOCA, deoxycorticosterone acetate.
Total daily electrolyte intake was then calculated from food intake and fluid intake data. Total daily intake of Na was dependent on diet, though diet did not significantly alter the increase in Na intake induced by DOCA-salt (Fig. 5B). Notably, this result paralleled the modulatory effect of diet on Na intake when animals were studied in the Promethion multiplexed system, as described above (Tables 3 and 4). Mice fed 5L0D diet exhibited greater Na intake under sham conditions, and mice on this diet exhibited a much stronger increase in Na intake with DOCA-salt treatment. Because paired collections of food and fluid intakes along with urine collections were available, it was possible to compare the flux of sodium through the animals. In the sham-treated animals, Na intake and urine Na output were closely balanced; however, DOCA-salt treatment caused a decoupling of this relationship in a diet-dependent manner (Fig. 5C). In particular, mice fed 5L0D exhibited a large apparent sodium retention (i.e., intake minus elimination to urine) after DOCA-salt treatment. This apparent retention was explained, however, by fecal water and electrolyte analyses.
Elimination of water to the feces was strongly dependent on diet, with sizeable losses in the 5L0D and 7913 groups, but less in the 2920 group (Table 8). These losses indicate that roughly 20%–40% of water elimination per day in sham-treated animals goes to the feces. DOCA-salt had no appreciable effect on water loss to feces, and therefore because urine production rates were increased so greatly by DOCA-salt, fecal water elimination accounted for only roughly 6% of total daily water turnover. Rate of elimination of Na to feces was strongly influenced by diet and DOCA-salt, and an interaction between these two variables (Fig. 5D).
Table 8.
Fecal analyses
| End Point | 5L0D (n = 10) |
2920x (0.4% Na) (n = 10) |
2920x (n = 10) |
P Value |
||
|---|---|---|---|---|---|---|
| Diet | DOCA | Diet × DOCA | ||||
| Fecal water content, g/d | ||||||
| Sham | 0.85 ± 0.06 | 0.28 ± 0.03a | 0.55 ± 0.07 | <0.01 | 0.94 | 0.03 |
| DOCA-salt | 1.17 ± 0.22 | 0.19 ± 0.01b | 0.34 ± 0.04b | |||
| Fecal water, % ingested water | ||||||
| Sham | 18 ± 1 | 8 ± 1 | 12 ± 3 | <0.01 | 0.90 | 0.23 |
| DOCA-salt | 23 ± 4 | 6 ± 1b | 10 ± 2b | |||
Data presented as means ± SE and analyzed via two-way ANOVA. aP < 0.05 vs. 5L0D + sham, bP < 0.05 vs. 5L0D + DOCA by Tukey’s multiple-comparisons procedure. DOCA, deoxycorticosterone acetate.
Fecal elimination of Na accounted for ∼37% of Na ingested by sham-treated mice, versus only ∼6.5% of ingested Na in DOCA-salt-treated mice (Fig. 5E). Fecal elimination of Na was greatest in the 5L0D group under sham conditions, and although fecal Na elimination was reduced by DOCA-salt in all groups, this loss was suppressed the least in mice maintained on 5L0D. Thus, consideration of all forms of intake versus the combination of urine and fecal Na elimination mechanisms illustrates that the animals were in a state of total Na balance regardless of diet or DOCA-salt treatment (Fig. 5F). Furthermore, these findings demonstrate that fecal elimination of water and Na account for a large fraction of total water and Na turnover, and that this elimination route is highly variable depending on both diet and experimental treatment.
Blood Pressure and Dietary Na
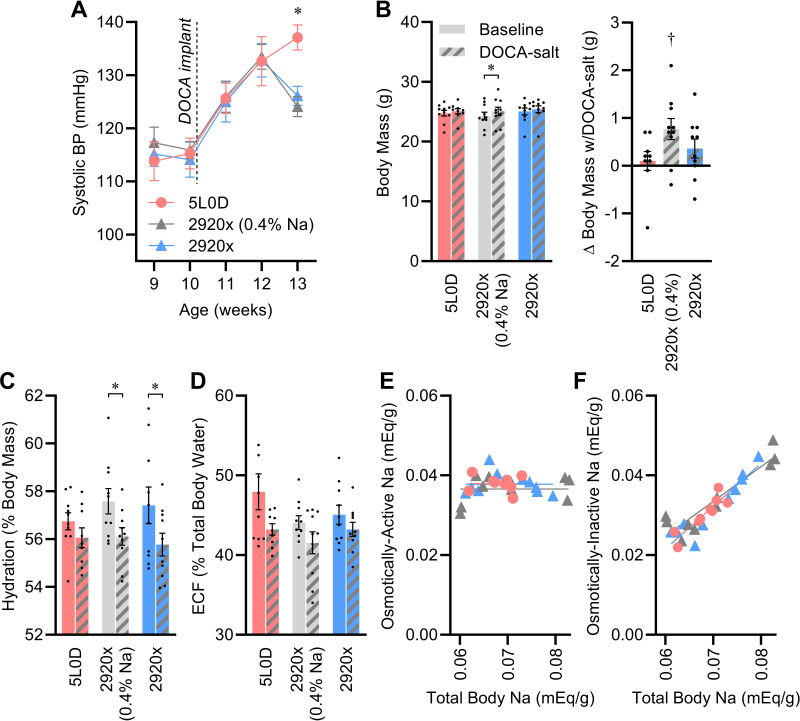
To further explore the interactions between dietary Na content and DOCA-salt, one cohort of animals was studied using only the 2920x and 5L0D diet groups, plus a new customized diet group in which Na was added by the manufacturer to the 2920x formulation to match the Na content of 5L0D. Using these three groups, we could therefore dissect the contribution of dietary Na, versus other components, in the differential effects of 2920x versus 5L0D.
Systolic BP, assessed by tail-cuff plethysmography, was similar among all animals in the presurgical phase, and increased as expected after DOCA-salt initiation at 10 wk of age (Fig. 6A). Interestingly, BP trajectories diverged after week 12 of age, as mice fed 2920x diet exhibited a drop in systolic BP whereas mice fed 5L0D continued an upward trajectory in week 13 of age. Profoundly, the drop in BP observed in the 2920x diet groups occurred regardless of Na content; BP dropped in both standard (0.15% Na) 2920x and customized (0.4% Na) 2920x groups.
Figure 6.
Sodium and nonsodium components of diets modify the effects of DOCA-salt on blood pressure, fluid compartmentalization, and Na pools. A: systolic BP determined by tail-cuff plethysmography: two-way repeated measures ANOVA diet P = 0.73, time/DOCA P < 0.01, diet × time/DOCA P = 0.03. B: body mass at end of study: two-way ANOVA diet P = 0.77, DOCA P < 0.01, diet × DOCA P = 0.09. C: hydration, determined by NMR and calculated as fraction of total body water per total body mass: two-way ANOVA diet P = 0.77, DOCA P < 0.01, diet × DOCA P = 0.39. D: fraction of total body water contained in extracellular space: two-way ANOVA diet P = 0.13, DOCA P < 0.01, diet × DOCA P = 0.54. E: regression analysis of osmotically active Na vs. total body Na stores: slopes do not differ from zero (P values range 0.33–0.74), and comparison of slopes P = 0.58. F: regression analysis of osmotically inactive Na vs. total body Na stores; slopes all significantly differ from zero (P values are all <0.01), and comparison of slopes P = 0.59. For A–F, n = 10 for all groups except n = 9 for 5L0D + DOCA. For A–F, summary data are presented as means ± SE, *P < 0.05 by Tukey’s multiple comparisons procedure, and †P < 0.05 vs. zero by one-sample t test. BP, blood pressure; DOCA, deoxycorticosterone acetate.
Body composition and fluid compartmentalization were assessed in the presurgical phase (week 10) and in week 14. Body mass modestly increased with DOCA-salt treatment, and this effect was greatest in the high-Na version of 2920x (Fig. 6B). Fat-free mass and total body water mass were largely unaffected by diet or DOCA-salt, and therefore because of expanded total body mass, hydration (i.e., total body water per total body mass) was reduced by DOCA-salt and this effect was most obvious in the two groups fed high- or standard-Na 2920x (Fig. 6C and Table 9). The relative distribution of fluid between extracellular (ECF) and intracellular (ICF) spaces was also affected by DOCA-salt treatment, with a modest shift of fluid from the ECF to ICF space that was not modified by diet (Fig. 6D).
Table 9.
Body composition, fluid compartmentalization, and sodium pools
| End Point | 5L0D (n = 10) |
2920x (0.4% Na) (n = 10) |
2920x (n = 10) |
P Value |
||
|---|---|---|---|---|---|---|
| Diet | DOCA | Diet × DOCA | ||||
| Fat mass, g | ||||||
| Week 10 | 5.56 ± 0.15 | 5.18 ± 0.20 | 5.40 ± 0.29 | 0.68 | <0.01 | 0.26 |
| Week 14 | 5.86 ± 0.11 | 5.85 ± 0.21 | 6.06 ± 0.23 | |||
| Fat-free mass, g | ||||||
| Week 10 | 19.20 ± 0.42 | 19.12 ± 0.54 | 19.66 ± 0.54 | 0.82 | 0.32 | 0.36 |
| Week 14 | 19.22 ± 0.44 | 19.22 ± 0.53 | 19.36 ± 0.43 | |||
| Total body water, mL | ||||||
| Week 10 | 14.05 ± 0.31 | 14.00 ± 0.40 | 14.39 ± 0.40 | 0.82 | 0.32 | 0.36 |
| Week 14 | 14.07 ± 0.32 | 14.07 ± 0.39 | 14.17 ± 0.31 | |||
| Extracellular fluid, mL | ||||||
| Week 10 | 6.77 ± 0.44 | 6.16 ± 0.19 | 6.47 ± 0.20 | 0.40 | <0.01 | 0.49 |
| Week 14 | 6.07 ± 0.14 | 5.84 ± 0.24 | 6.12 ± 0.16 | |||
| Extracellular fluid, % TBW | ||||||
| Week 10 | 47.90 ± 2.25 | 44.06 ± 0.87 | 45.07 ± 1.21 | 0.13 | <0.01 | 0.54 |
| Week 14 | 43.23 ± 0.72 | 41.54 ± 1.38 | 43.22 ± 0.88 | |||
| Intracellular fluid, mL | ||||||
| Week 10 | 7.28 ± 0.27 | 7.84 ± 0.28 | 7.90 ± 0.33 | 0.44 | 0.03 | 0.46 |
| Week 14 | 7.99 ± 0.24 | 8.23 ± 0.31 | 8.06 ± 0.24 | |||
| Osmotically active Na, mEq | ||||||
| Week 10 | 1.02 ± 0.06 | 0.93 ± 0.03 | 0.98 ± 0.03 | 0.43 | 0.11 | 0.48 |
| Week 14 | 0.95 ± 0.02 | 0.92 ± 0.04 | 0.96 ± 0.02 | |||
| Osmotically active Na, mEq/g | ||||||
| Week 10 | 0.041 ± 0.002 | 0.038 ± 0.001 | 0.039 ± 0.001 | 0.28 | 0.03 | 0.73 |
| Week 14 | 0.038 ± 0.001 | 0.037 ± 0.001 | 0.038 ± 0.001 | |||
| Total body Na, mEq | ||||||
| Week 14 | 1.71 ± 0.04 | 1.77 ± 0.10 | 1.78 ± 0.06 | 0.74 | ||
| Total body Na, mEq/g | ||||||
| Week 14 | 0.068 ± 0.001 | 0.070 ± 0.003 | 0.070 ± 0.002 | 0.74 | ||
| Osmotically inactive Na, mEq | ||||||
| Week 14 | 0.76 ± 0.04 | 0.85 ± 0.08 | 0.82 ± 0.06 | 0.59 | ||
| Osmotically inactive Na, mEq/g | ||||||
| Week 14 | 0.030 ± 0.001 | 0.034 ± 0.003 | 0.032 ± 0.002 | 0.55 | ||
Data presented as means ± SE and analyzed via two-way ANOVA. DOCA, deoxycorticosterone acetate.
Using ECF and ICF volumes plus plasma Na measures, osmotically active Na stores were estimated at both timepoints using methods we recently reported (28). Diet had no effect on estimated osmotically active Na stores, but DOCA-salt caused a small reduction that was not modified by diet (Table 9). At the conclusion of the study after DOCA-salt treatment, total body Na content was also assessed by ashing. Total body Na content was similar across all three diet groups, and the difference between total and osmotically active Na, thereby reflecting the osmotically inactive Na pool, was similar across all three diet groups. Interestingly, regression analyses of active or inactive Na pools versus total body Na reveal that although osmotically active Na was not correlated with total body Na stores and no significant relationship was noted (Fig. 6E), osmotically inactive Na was positively and tightly correlated with total body Na (Fig. 6F).
Ventilatory Function
Previously we and others have demonstrated increased RMR in DOCA-salt-treated animals, and data presented above demonstrate differences in aerobic metabolism during the light phase between DOCA-salt-treated mice fed 5L0D versus 2920x (Fig. 2F, Tables 3 and 4). Thus, we sought to evaluate whether diets and DOCA-salt had modulatory effects on pulmonary function. Breathing characteristics were evaluated by plethysmography in sham and DOCA-salt-treated mice fed either 2920x or 5L0D diets (Table 10). When breathing room air (≈20.5% O2, ≈0.03% CO2), all four groups of mice exhibited similar tidal volumes and minute ventilation rates, though a small but statistically significant interaction between diet and DOCA-salt treatments was observed in breathing frequency. When breathing a hypoxic air mixture (12% O2), all four groups of mice exhibited similar increases in breathing frequency, tidal volumes, and minute ventilation rates. Finally, when breathing a hypercapnic air mixture (21% O2, 7% CO2), all four groups of mice again exhibited similar increases in breathing frequency, tidal volumes, and minute ventilation rates. Thus, it appears that this DOCA-salt treatment paradigm, regardless of diet, has minimal impact on ventilatory functions.
Table 10.
Ventilatory function as assessed by plethysmography
| End Point | Sham 2920x (n = 10) |
DOCA 2920x (n = 9) |
Sham 5L0D (n = 10) |
DOCA 5L0D (n = 10) |
P Value |
||
|---|---|---|---|---|---|---|---|
| Diet | DOCA | Diet × DOCA | |||||
| Room air | |||||||
| V̇e, mL/100 g/min | 87.1 ± 5.6 | 91.6 ± 4.9 | 90.5 ± 4.1 | 89.3 ± 4.6 | 0.91 | 0.74 | 0.56 |
| VT, mL/breath/100 g | 0.48 ± 0.03 | 0.47 ± 0.02 | 0.43 ± 0.02 | 0.46 ± 0.02 | 0.24 | 0.57 | 0.34 |
| Frequency, breath/min | 185 ± 4 | 196 ± 8 | 210 ± 6 | 192 ± 8 | 0.14 | 0.65 | 0.03 |
| Hypoxia | |||||||
| V̇e, mL/100 g/min | 128.4 ± 11.5 | 135.5 ± 7.6 | 133.0 ± 7.8 | 116.0 ± 8.0 | 0.41 | 0.58 | 0.19 |
| (% of room air) | 142 ± 8 | 140 ± 10 | 138 ± 9 | 127 ± 13 | 0.41 | 0.58 | 0.68 |
| VT, mL/breath/100 g | 0.51 ± 0.04 | 0.55 ± 0.02 | 0.53 ± 0.02 | 0.51 ± 0.02 | 0.71 | 0.79 | 0.31 |
| (% of room air) | 114 ± 6 | 115 ± 5 | 118 ± 5 | 111 ± 7 | 0.99 | 0.59 | 0.49 |
| Frequency, breath/min | 250 ± 10 | 247 ± 9 | 248 ± 7 | 229 ± 11 | 0.29 | 0.23 | 0.40 |
| (% of room air) | 125 ± 5 | 120 ± 4 | 116 ± 5 | 115 ± 6 | 0.22 | 0.50 | 0.64 |
| Hypercapnia | |||||||
| V̇e, mL/100 g/min | 283.6 ± 27.7 | 268.7 ± 13.7 | 256.1 ± 21.2 | 244.6 ± 14.1 | 0.21 | 0.52 | 0.93 |
| (% of room air) | 341 ± 15 | 312 ± 13 | 317 ± 18 | 311 ± 12 | 0.42 | 0.24 | 0.46 |
| VT, mL/breath/100 g | 0.83 ± 0.08 | 0.80 ± 0.04 | 0.74 ± 0.06 | 0.75 ± 0.03 | 0.22 | 0.91 | 0.71 |
| (% of room air) | 170 ± 7 | 178 ± 9 | 184 ± 6 | 171 ± 9 | 0.65 | 0.79 | 0.17 |
| Frequency, breath/min | 342 ± 7 | 336 ± 9 | 345 ± 3 | 323 ± 8 | 0.47 | 0.06 | 0.29 |
| (% of room air) | 200 ± 3 | 185 ± 10 | 172 ± 8 | 182 ± 6 | 0.03 | 0.76 | 0.08 |
Data presented as means ± SE and analyzed via two-way ANOVA. DOCA, deoxycorticosterone acetate; V̇e, minute ventilation; VT, tidal volume.
DISCUSSION
Increasing evidence supports interactions among environmental factors, such as diet, with other factors, such as genetics, in the pathogenesis of hypertension. In one notable example, Li et al. (29) previously used genome-wide analyses to map genomic loci that interact with sodium to increase blood pressure in participants of the Genetic Epidemiology Network of Salt-Sensitivity (GenSalt) and Multi-Ethnic Study of Atherosclerosis (META) studies and identified several loci that appear to contribute to salt sensitivity of BP. Similar associations between individual genes, epigenetic factors, and propensity for salt-sensitive hypertension have been explored in an array of rodent models (30, 31). Thus, the findings of the current study underscore the importance of considering both Na and other dietary components in studies attempting to identify interactive effects that influence salt-sensitive blood pressure phenotypes.
The current study identified an extensive array of cardiometabolic phenotypes, induced by a modest version of DOCA-salt treatment, that are modulated by a complex relationship with diet formulation. For example, somatic growth, feeding behaviors, and energy efficiency were quantitatively and qualitatively sensitive to diet. Whereas DOCA-salt-treated mice fed 5L0D diet exhibited increased total energy expenditure and thereby reduced weight gain, mice fed 7913 exhibited reduced food intake and reduced total energy expenditure, and ultimately a trend toward exaggerated weight gain in this timeframe. Thus, the effects of DOCA-salt treatment on energetic flux appeared to be opposite, depending on diet.
It is important to note that energy expenditure phenotypes assessed here were interpreted based on changes in energy efficiency. This ratio of weight gain to calories absorbed is an inverse metric of total energy expenditure, but does not distinguish contributions of resting metabolic rate, the thermic effect of food, adaptive thermogenesis, or physical activity. Thus, further dissection of these contributors to total energy expenditure may provide additional insights into the specific effects of dietary formulation on phenotypes induced by DOCA-salt. Indeed, we have previously demonstrated that DOCA-salt strongly stimulates resting metabolic rate in mice fed 7913, through a mechanism dependent on angiotensin AT1 receptors within the brain (8, 10, 11), but we have not assessed changes in other mechanisms contributing to total energy expenditure.
It is noteworthy that the current, modest version of the DOCA-salt paradigm (i.e., animals are provided ad libitum choice to consume excess Na, and mice are not uninephrectomized) did not result in gross cardiac or renal remodeling or damage despite causing increased blood pressure. Similarly, using radiotelemetric blood pressure transducers, we previously documented that this modest version of the DOCA-salt paradigm in C57BL/6J male mice fed the 7913 diet results in robust systolic and diastolic hypertension and bradycardia within a 3-wk treatment timeframe, and these effects were not associated with cardiac hypertrophy (10). It appears that more severe hypertension or a longer treatment period are required to induce gross tissue damage in this animal model, and it remains unclear whether interactions between diet and DOCA-salt treatments would persist or become masked under such conditions.
Analyses of renal function, including the elimination of Na to urine, are frequently used as metrics of total body Na turnover or even total Na consumption. In addition to illustrating interactions between diet and DOCA-salt treatment to modify various aspects of renal function, the current data set provides a robust illustration of the potential error of overinterpreting urine Na content, especially in the context of hypertension. Indeed, here we discovered that fecal elimination of both water and Na were dependent on the interactions between diet and DOCA-salt and that the failure to account for the large and variable elimination of Na to feces would erroneously lead investigators to conclude differences in Na accumulation and Na between diet groups. Modulation of fecal elimination of Na has previously been suggested as a novel therapeutic approach to hypertension (32, 33), and a role for mineralocorticoid receptors within the intestine in the control of Na absorption kinetics has been demonstrated (34). Thus, the current data set prompts questions about the fundamental validity of studies of Na balance that fail to consider changes in fecal elimination of Na.
Interestingly, diet modified the daily elimination of aldosterone to urine, which serves as a metric of the rate of aldosterone production or release. Mice fed 5L0D exhibited increased aldosterone elimination per day compared with mice fed 2920x or 7913. This pattern was not paralleled by, and therefore unlikely to be secondary to, changes in urine production rate or plasma Na. Urine aldosterone was then suppressed to similar levels by DOCA-salt regardless of diet. This likely reflects a reflexive suppression of endogenous aldosterone production in response to a combination of the administration of exogenous DOCA, increased blood pressure, increased plasma Na, and reduced circulating RAS activity as evidenced by reduced renal renin expression. Interestingly, aldosterone elimination under both sham and DOCA-salt conditions varied by diet in patterns that paralleled the fecal elimination of Na. These results are complementary to results from animals with genetic deletion of the mineralocorticoid receptor from intestinal epithelial cells, as those animals exhibited increased fecal Na content, attenuated reductions in plasma aldosterone concentrations with 3 wk of DOCA-salt treatment, and an attenuated blood pressure increase with 3 wk of DOCA-salt (34). Thus, there appears to be an interplay among dietary composition, mineralocorticoid production, and mineralocorticoid action on the kinetics of intestinal Na transport, which importantly contributes to electrolyte homeostasis and integrated hemodynamic control.
An unexpected finding of the current study was the determination that dietary formulation grossly alters the relationship between arginine vasopressin release (assessed via urine copeptin elimination) and aquaporin-2 shedding (considered a metric of AQP2 mobilization to the apical membrane (27)). Relative to the other diet groups, mice fed 5L0D diet exhibited increased renal expression of the vasopressin type 2 receptor (Avpr2) and AQP2 (Aqp2) in kidney, yet lower urine AQP2 shedding under sham treatment conditions, and a greater gain in AQP2 shedding rates after similar increases in vasopressin release were observed. These findings point to a diet-induced alteration in the vasopressin/aquaporin-2 signaling axis that may ultimately contribute to diet-induced changes in blood pressure and hydration control.
When interpreting the effects of DOCA-salt treatment on ingestive behaviors, especially with regard to the intake of sodium, it is important to appreciate that species-specific differences in the effect of DOCA, DOCA-salt, and other manipulations that impact circulating levels of adrenal steroids and the RAS have been reported. When considering the effect of DOCA-salt on sodium intake behaviors, here we have again [similar to previous reports (14)] demonstrated that DOCA-salt does not induce a “preference” for 0.15 M NaCl. Our previous work examining intake behaviors and preferences of mice for an array of aversive concentrations of NaCl demonstrate that mice exhibit a preference for dilute 0.03 M NaCl compared with deionized water, no preference for isotonic 0.15 M NaCl, and significant aversions to hypertonic 0.30 and 0.45 M NaCl solutions (11). After DOCA-salt treatment, mice exhibit “reduced aversion” or “increased acceptance” toward NaCl (meaning an alleviation of an aversion, toward 50% preference ratio) but not a traditional “preference” (meaning a preference ratio above 50%). Here, we extend those observations as we found that diet formulations do not strongly modify that effect of DOCA-salt treatment in C57BL/6J mice. These results complement and extend the observations of many other investigators [reviewed by Rowland and Fregly (35)] demonstrating that in contrast to rats (36–39), various species such as mice, Mongolian gerbils, Syrian hamsters, and others do not exhibit a sodium preference (i.e., increased preference ratio vs. water) or appetite (e.g., increased consumption when NaCl is presented at aversive concentrations) in response to an array of stimuli that modify circulating adrenal steroids or the circulating RAS, including DOCA, DOCA-salt, or adrenalectomy. As others have previously stated, fluid and electrolyte handling and seeking behaviors are different across species, and results from studies of mice versus rats (and other species) must be interpreted in context.
Another potentially unexpected finding of the current study was the determination that the blood pressure trajectory and hydration status during DOCA-salt treatment were sensitive to components of the diet beyond simply the Na content. Adding more Na to 2920x did not alter the trajectory of blood pressure, nor did it impact hydration changes. Further studies to understand the pro- or antihypertensive components of the 5L0D versus 2920x diets are obviously required. The observation that blood pressure diverged between these groups only in week 13, after following similar trajectories in earlier weeks of treatment (i.e., the “early” phase), supports changes in specific mechanisms that mediate the “developed” phase of DOCA-salt effects. As previously reviewed (2, 3), a major difference between these two phases is the recruitment of sympathetic nervous system activity, plasma catecholamines, and endothelin actions. Importantly, although these experiments were only performed using tail-cuff plethysmography, these animals were all analyzed concurrently as one cohort, and BP data represent only one of multiple complementary endpoints, which together increase confidence in the data set and conclusions regarding interactions between diet and DOCA at this timepoint. Furthermore, here we documented that arginine vasopressin release kinetics differ between 5L0D- and 2920x-fed animals during DOCA-salt treatment, which may also mechanistically contribute. Understanding how dietary components can alter these known contributor mechanisms may therefore explain the summative effects on blood pressure trajectories observed here. One likely contributor is the composition and activity of the gut microbiota, as salt-sensitive hypertension in the Dahl rat model is sensitive to dietary composition and gut microbial composition (40). Our previous work has demonstrated a major contribution of gut microbiota to anaerobic energy flux (41), and the potential to use unaccounted caloric flux (i.e., nonaerobic energy accumulation that is not used for growth) as a metric of anerobic metabolism in vivo (22). Although our previous work demonstrates that C57BL/6J mice typically expend ≈7%–10% of energy via anaerobic metabolism and a suppressive effect of antibiotics and high-fat diets on this form of energy expenditure (41–44), the current data set supports the notion that DOCA-salt treatment may increase this form of energy expenditure (Table 2). Future work is needed to understand the modulatory effect of DOCA-salt (and more generally sodium-sensitive hypertension) on the gut microbiota and anaerobic energy flux.
Analysis of osmotically active versus inactive Na pools also highlighted that although DOCA-salt had consistent effects to reduce osmotically active Na, diet formulations had no effect on total body Na retention in any pool. Interestingly, we found that there was no relationship between osmotically active Na and total body Na. In contrast, a tight, positive relationship was noted between osmotically inactive Na and total body Na. Titze and others have documented osmotically inactive Na pools in humans and rodents and proposed a potential role for this pool in the pathogenesis of hypertension (21, 45–49). Consistent with that body of literature, the current results indicate that in male C57BL/6J mice, the osmotically inactive Na pool apparently accounts for roughly 45% (here, ranging from 33% to 60%) of total body Na. Furthermore, these findings support the more generalized concept that osmotically inactive Na pools act to buffer osmotically active Na. Thus, future studies to clarify the role of osmotically inactive Na pools in the pathogenesis and maintenance of subtypes of hypertension (e.g., salt-sensitive or not) are warranted.
Concentrations of total CO2 in the blood and the anion gap values prompt the consideration that DOCA-salt treatment may have induced a metabolic acidosis in the mice, and that this effect was modified by the supplied diet. This interpretation is supported by some previous studies suggesting that DOCA-salt may induce metabolic acidosis (50, 51); however, this interpretation is challenged by other previous studies that indicate that DOCA-salt actually causes metabolic alkalosis in rats (52). The effects of DOCA-salt to cause changes in acid/base status are of interest, as such changes would be expected to impact respiratory function. For example, if the animals were in metabolic alkalosis, the respiratory system would be expected to respond by a compensatory decrease in alveolar ventilation. Given that we measured total ventilation (VE; alveolar + dead space) with an indirect approach (plethysmography), there may be subtle effects that we did not detect in these mouse models. In addition, the ventilatory control system adapts over time, meaning that any shift in acid/base status may initially alter basal breathing but over time the system finds the optimal balance between dead space to alveolar ventilation at a given pH. Finally, DOCA-salt treatment may lead to direct and indirect effects on ventilatory patterns that oppose expected changes in minute ventilation, as glucocorticoid signaling may offset effects of metabolic acid/base changes (53, 54). Determining whether a failure to detect changes in ventilation in response to diet or DOCA-salt in the current study stems from the timing of measurements relative to initiation of DOCA-salt treatment, inadequacy of plethysmographic methods to detect relevant changes in the respiratory system and its function or nuanced and opposing stimuli to modify breathing patterns will require additional future study.
Several notable limitations with the current study design must be noted. First, only male mice were studied to reduce the number of variables under consideration. Future work to study these effects in females, in both the nonpregnant and pregnant states, may uncover additional interactions of interest. Such studies during pregnancy, for example, may prove especially informative given that some hypertensive disorders of pregnancy such as preeclampsia are, relative to physiological pregnancy, characterized by low circulating renin activity (55). Second, systolic blood pressure was evaluated using tail-cuff plethysmography, which allowed for serial measurements of body composition by nuclear magnetic resonance (NMR) and bioimpedance spectroscopy (BIS). These methods cannot be safely applied to animals that are instrumented with metallic implants such as radiotelemetric transducers. Future studies to further characterize diet-specific effects of DOCA-salt on blood pressure trajectories should utilize more sophisticated methodologies. Third, only relatively young adult mice were studied, focusing on a timeframe between week 6 (immediately after puberty) through week 14 of life. Future studies to understand additional interactions with aging may also be of interest. Fourth, only the C57BL/6J strain of mice was studied. Increasing evidence supports interactions between genetics and diet in blood pressure control, and thus it follows that examination of the effect of diet formulation on cardiometabolic physiology in other strains of mice and in other species will likely uncover additional important interactive effects. Fifth, the use of the 5L0D, 7913, and 2920x diets were purely of convenience, as these diets are commonly used in the field, and we have specifically used these diets in our previous studies. Inclusion of all of these diets (e.g., including 7913 and a customized version of 2920x with Na content to match 7913 in blood pressure studies) or inclusion of other modified diets (e.g., diets with altered Na, fat, carbohydrate, protein, or micronutrient contents to all studies) may help to further isolate the “nonsodium” dietary components that contribute to the diet × DOCA-salt interactions documented herein.
Perspectives and Significance
In summary, it is increasingly clear that interactions among environmental and genetic factors influence cardiometabolic physiology. Here we present an extensive catalog of phenotypes induced by DOCA-salt treatment, often used to model hypertension with low circulating renin activity, and the modulatory effect of diet formulation on these phenotypes in a commonly utilized strain of mice. Many phenotypes were dependent on or sensitive to diet formulation, but unexpectedly some phenotypes were completely reversed depending on diet, and some phenotypes were sensitive to components of the diet beyond simply Na content. These findings underscore the importance of environmental factors on data interpretation and thorough methodological reporting in studies evaluating cardiometabolic phenotypes.
GRANTS
This work was supported by the MCW Biomedical Resource Center and the MCW Comprehensive Rodent Metabolic Phenotyping Core. The authors were supported by the National Institutes of Health Grants HL134850, HL084207, HL007852, HL144807, HL153101, HL122358, and ES005605), the American Heart Association (18EIA33890055, 20CDA3510121, 903246, 898067), the Children’s Research Institute (CRI22700), the American Physiological Society Postdoctoral Fellowship program, the MCW Clinical and Translational Science Institute (UL1TR001436), and the Advancing a Healthier Wisconsin Endowment to MCW.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.N.P., J.J.R., G.C.M. Jr., A.E.K., M.R.H., J.L.S., C.D.S., and J.L.G. conceived and designed research; C.N.P., M.L.R., K.K.W., V.O., K.B., C.C.G., D.T.B., J.J.R., G.C.M. Jr., A.J.K., J.L.S., and J.L.G. performed experiments; C.N.P., M.L.R., V.O., K.B., C.C.G., D.T.B., J.J.R., P.N., G.C.M. Jr., A.J.K., M.R.H., J.L.S., and J.L.G.analyzed data; C.N.P., M.L.R., V.O., K.B., C.C.G., J.J.R., P.N., G.C.M. Jr., A.J.K., A.E.K., M.R.H., J.L.S., C.D.S., and J.L.G.interpreted results of experiments; C.N.P. and J.L.G. prepared figures; C.N.P. and J.L.G. drafted manuscript; C.N.P., J.J.R., P.N., G.C.M. Jr., A.J.K., A.E.K., J.L.S., C.D.S., and J.L.G. edited and revised manuscript; C.N.P., M.L.R., K.K.W., V.O., K.B., C.C.G., D.T.B., J.J.R., P.N., G.C.M. Jr., A.J.K., A.E.K., M.R.H., J.L.S., C.D.S., and J.L.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Ko-Ting Lu, MS, Neil Rohde, and Olivia Eckes for technical assistance, and Dr. Allen J. Cowley, Jr., for critical review of the work.
REFERENCES
- 1.Selye H, Hall CE, Rowley EM. Malignant hypertension produced by treatment with desoxycorticosterone acetate and sodium chloride. Can Med Assoc J 49: 88–92, 1943. doi: 10.1016/s0002-8703(43)90306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yemane H, Busauskas M, Burris SK, Knuepfer MM. Neurohumoral mechanisms in deoxycorticosterone acetate (DOCA)-salt hypertension in rats. Exp Physiol 95: 51–55, 2010. doi: 10.1113/expphysiol.2008.046334. [DOI] [PubMed] [Google Scholar]
- 3.Schenk J, McNeill JH. The pathogenesis of DOCA-salt hypertension. J Pharmacol Toxicol Methods 27: 161–170, 1992. doi: 10.1016/1056-8719(92)90036-Z. [DOI] [PubMed] [Google Scholar]
- 4.Laragh JH. Biochemical profiling and the natural history of hypertensive diseases: low-renin essential hypertension, a benign condition. Circulation 44: 971–974, 1971. doi: 10.1161/01.cir.44.6.971. [DOI] [PubMed] [Google Scholar]
- 5.Itaya Y, Suzuki H, Matsukawa S, Kondo K, Saruta T. Central renin-angiotensin system and the pathogenesis of DOCA-salt hypertension in rats. Am J Physiol Heart Circ Physiol 251: H261–H268, 1986. doi: 10.1152/ajpheart.1986.251.2.H261. [DOI] [PubMed] [Google Scholar]
- 6.Kubo T, Yamaguchi H, Tsujimura M, Hagiwara Y, Fukumori R. Blockade of angiotensin receptors in the anterior hypothalamic preoptic area lowers blood pressure in DOCA-salt hypertensive rats. Hypertens Res 23: 109–118, 2000. doi: 10.1291/hypres.23.109. [DOI] [PubMed] [Google Scholar]
- 7.Park CG, Leenen FH. Effects of centrally administered losartan on deoxycorticosterone-salt hypertension rats. J Korean Med Sci 16: 553–557, 2001. doi: 10.3346/jkms.2001.16.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claflin KE, Sandgren JA, Lambertz AM, Weidemann BJ, Littlejohn NK, Burnett CM, Pearson NA, Morgan DA, Gibson-Corley KN, Rahmouni K, Grobe JL. Angiotensin AT1A receptors on leptin receptor-expressing cells control resting metabolism. J Clin Invest 127: 1414–1424, 2017. doi: 10.1172/JCI88641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilzendeger AM, Cassell MD, Davis DR, Stauss HM, Mark AL, Grobe JL, Sigmund CD. Angiotensin type 1a receptors in the subfornical organ are required for deoxycorticosterone acetate-salt hypertension. Hypertension 61: 716–722, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, Sigmund CD. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (DOCA)-salt in C57 mice. Hypertension 57: 600–607, 2011. doi: 10.1161/HYPERTENSIONAHA.110.165829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedens DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab 12: 431–442, 2010. doi: 10.1016/j.cmet.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douma LG, Solocinski K, Holzworth MR, Crislip GR, Masten SH, Miller AH, Cheng KY, Lynch IJ, Cain BD, Wingo CS, Gumz ML. Female C57BL/6J mice lacking the circadian clock protein PER1 are protected from nondipping hypertension. Am J Physiol Regul, Integr Comp Physiol 316: R50–R58, 2019. doi: 10.1152/ajpregu.00381.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, D.C.: National Acadamies Press, 2011. [Google Scholar]
- 14.Zanaty M, Seara FAC, Nakagawa P, Deng G, Mathieu NM, Balapattabi K, Karnik SS, Grobe JL, Sigmund CD. β-Arrestin-biased agonist targeting the brain AT(1)R (angiotensin II type 1 receptor) increases aversion to saline and lowers blood pressure in deoxycorticosterone acetate-salt hypertension. Hypertension 77: 420–431, 2021. doi: 10.1161/HYPERTENSIONAHA.120.15793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandgren JA, Linggonegoro DW, Zhang SY, Sapouckey SA, Claflin KE, Pearson NA, Leidinger MR, Pierce GL, Santillan MK, Gibson-Corley KN, Sigmund CD, Grobe JL. Angiotensin AT1A receptors expressed in vasopressin-producing cells of the supraoptic nucleus contribute to the osmotic control of vasopressin. Am J Physiol Regul, Integr Comp Physiol 314: R770–R780, 2018. doi: 10.1152/ajpregu.00435.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jo F, Jo H, Hilzendeger AM, Thompson AP, Cassell MD, Rutkowski DT, Davisson RL, Grobe JL, Sigmund CD. Brain endoplasmic reticulum stress mechanistically distinguishes the saline-intake and hypertensive response to deoxycorticosterone acetate-salt. Hypertension 65: 1341–1348, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziegler AA, Grobe CC, Reho JJ, Jensen ES, Thulin JD, Segar JL, Grobe JL. Short-term housing in metabolic caging on measures of energy and fluid balance in male C57BL/6J mice (Mus musculus). J Am Assoc Lab Anim Sci 61: 132–139, 2022. doi: 10.30802/AALAS-JAALAS-21-000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grobe JL. Comprehensive assessments of energy balance in mice. Methods Mol Biol 1614: 123–146, 2017. doi: 10.1007/978-1-4939-7030-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segar JL, Balapattabi K, Reho JJ, Grobe CC, Burnett CML, Grobe JL. Quantification of body fluid compartmentalization by combined time-domain nuclear magnetic resonance and bioimpedance spectroscopy. Am J Physiol Regul Integr Comp Physiol 320: R44–R54, 2021. doi: 10.1152/ajpregu.00227.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Littlejohn NK, Siel RB Jr, Ketsawatsomkron P, Pelham CJ, Pearson NA, Hilzendeger AM, Buehrer BA, Weidemann BJ, Li H, Davis DR, Thompson AP, Liu X, Cassell MD, Sigmund CD, Grobe JL. Hypertension in mice with transgenic activation of the brain renin-angiotensin system is vasopressin dependent. Am J Physiol Regul Integr Comp Physiol 304: R818–R828, 2013. doi: 10.1152/ajpregu.00082.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Titze J, Bauer K, Schafflhuber M, Dietsch P, Lang R, Schwind KH, Luft FC, Eckardt KU, Hilgers KF. Internal sodium balance in DOCA-salt rats: a body composition study. Am J Physiol Renal Physiol 289: F793–F802, 2005. [Erratum in Am J Physiol Renal Physiol 290: F561–F562, 2006]. doi: 10.1152/ajprenal.00096.2005. [DOI] [PubMed] [Google Scholar]
- 22.Soto JE, Burnett CML, Ten Eyck P, Abel ED, Grobe JL. Comparison of the effects of high-fat diet on energy flux in mice using two multiplexed metabolic phenotyping systems. Obesity (Silver Spring) 27: 793–802, 2019. doi: 10.1002/oby.22441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandgren JA, Deng G, Linggonegoro DW, Scroggins SM, Perschbacher KJ, Nair AR, Nishimura TE, Zhang SY, Agbor LN, Wu J, Keen HL, Naber MC, Pearson NA, Zimmerman KA, Weiss RM, Bowdler NC, Usachev YM, Santillan DA, Potthoff MJ, Pierce GL, Gibson-Corley KN, Sigmund CD, Santillan MK, Grobe JL. Arginine vasopressin infusion is sufficient to model clinical features of preeclampsia in mice. JCI insight 3: e99403, 2018. doi: 10.1172/jci.insight.99403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scarfe L, Schock-Kusch D, Ressel L, Friedemann J, Shulhevich Y, Murray P, Wilm B, de Caestecker M. Transdermal measurement of glomerular filtration rate in mice. J Vis Exp 140: 58520, 2018. doi: 10.3791/58520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan K, Echert AE, Massat B, Puissant MM, Palygin O, Geurts AM, Hodges MR. Chronic central serotonin depletion attenuates ventilation and body temperature in young but not adult Tph2 knockout rats. J Appl Physiol (1985) 120: 1070–1081, 2016. doi: 10.1152/japplphysiol.01015.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics 16: 81–87, 1955. doi: 10.1542/peds.16.1.81. [DOI] [PubMed] [Google Scholar]
- 27.Wen H, Frokiaer J, Kwon TH, Nielsen S. Urinary excretion of aquaporin-2 in rat is mediated by a vasopressin-dependent apical pathway. J Am Soc Nephrol 10: 1416–1429, 1999. doi: 10.1681/ASN.V1071416. [DOI] [PubMed] [Google Scholar]
- 28.Reho JJ, Nakagawa P, Mouradian GC Jr, Grobe CC, Saravia FL, Burnett CM, Kwitek AE, Kirby JR, Segar JL, Hodges MR, Sigmund CD, Grobe JL. Methods for the comprehensive in vivo analysis of energy flux, fluid homeostasis, blood pressure, and ventilatory function in rodents. Front Physiol 13: 855054, 2022. doi: 10.3389/fphys.2022.855054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li C, He J, Chen J, Zhao J, Gu D, Hixson JE, Rao DC, Jaquish CE, Gu CC, Chen J, Huang J, Chen S, Kelly TN. Genome-wide gene-sodium interaction analyses on blood pressure: the genetic epidemiology network of salt-sensitivity study. Hypertension 68: 348–355, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armando I, Villar VA, Jose PA. Genomics and pharmacogenomics of salt-sensitive hypertension. Curr Hypertens Rev 11: 49–56, 2015. doi: 10.2174/1573402111999150521102331. [DOI] [PubMed] [Google Scholar]
- 31.Hirohama D, Fujita T. Evaluation of the pathophysiological mechanisms of salt-sensitive hypertension. Hypertens Res 42: 1848–1857, 2019. doi: 10.1038/s41440-019-0332-5. [DOI] [PubMed] [Google Scholar]
- 32.Linz D, Wirth K, Linz W, Heuer HO, Frick W, Hofmeister A, Heinelt U, Arndt P, Schwahn U, Böhm M, Ruetten H. Antihypertensive and laxative effects by pharmacological inhibition of sodium-proton-exchanger subtype 3-mediated sodium absorption in the gut. Hypertension 60: 1560–1567, 2012. doi: 10.1161/HYPERTENSIONAHA.112.201590. [DOI] [PubMed] [Google Scholar]
- 33.Spencer AG, Labonte ED, Rosenbaum DP, Plato CF, Carreras CW, Leadbetter MR, Kozuka K, Kohler J, Koo-McCoy S, He L, Bell N, Tabora J, Joly KM, Navre M, Jacobs JW, Charmot D. Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med 6: 227ra36, 2014. doi: 10.1126/scitranslmed.3007790. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura T, Kurihara I, Kobayashi S, Yokota K, Murai-Takeda A, Mitsuishi Y, Morisaki M, Kohata N, Oshima Y, Minami Y, Shibata H, Itoh H. Intestinal mineralocorticoid receptor contributes to epithelial sodium channel-mediated intestinal sodium absorption and blood pressure regulation. J Am Heart Assoc 7: e008259, 2018. doi: 10.1161/JAHA.117.008259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowland NE, Fregly MJ. Characteristics of thirst and sodium appetite in mice (Mus musculus). Behav Neurosci 102: 969–974, 1988. doi: 10.1037//0735-7044.102.6.969. [DOI] [PubMed] [Google Scholar]
- 36.Stellar E, Epstein AN. Neuroendocrine factors in salt appetite. J Physiol Pharmacol 42: 345–355, 1991. [PubMed] [Google Scholar]
- 37.Epstein AN. The dependence of the salt appetite of the rat on the hormonal consequences of sodium deficiency. J Physiol (Paris) 79: 496–498, 1984. [PubMed] [Google Scholar]
- 38.Krause EG, Sakai RR. Richter and sodium appetite: from adrenalectomy to molecular biology. Appetite 49: 353–367, 2007. doi: 10.1016/j.appet.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richter CP. Increased salt appetite in adrenalectomized rats. Am J Physiol 115: 155–161, 1936. doi: 10.1152/ajplegacy.1936.115.1.155. [DOI] [Google Scholar]
- 40.Abais-Battad JM, Saravia FL, Lund H, Dasinger JH, Fehrenbach DJ, Alsheikh AJ, Zemaj J, Kirby JR, Mattson DL. Dietary influences on the Dahl SS rat gut microbiota and its effects on salt-sensitive hypertension and renal damage. Acta Physiol (Oxf) 232: e13662, 2021. doi: 10.1111/apha.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riedl RA, Burnett CML, Pearson NA, Reho JJ, Mokadem M, Edwards RA, Kindel TL, Kirby JR, Grobe JL. Gut microbiota represent a major thermogenic biomass. Function (Oxf) 2: zqab019, 2021. doi: 10.1093/function/zqab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bahr SM, Weidemann BJ, Castro AN, Walsh JW, deLeon O, Burnett CML, Pearson NA, Murry DJ, Grobe JL, Kirby JR. Risperidone-induced weight gain is mediated through shifts in the gut microbiome and suppression of energy expenditure. EBioMedicine 2: 1725–1734, 2015. doi: 10.1016/j.ebiom.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burnett CM, Grobe JL. Dietary effects on resting metabolic rate in C57BL/6 mice are differentially detected by indirect (O2/CO2 respirometry) and direct calorimetry. Mol Metab 3: 460–464, 2014. doi: 10.1016/j.molmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burnett CM, Grobe JL. Direct calorimetry identifies deficiencies in respirometry for the determination of resting metabolic rate in C57Bl/6 and FVB mice. Am J Physiol Endocrinol Metab 305: E916–E924, 2013. doi: 10.1152/ajpendo.00387.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiig H, Luft FC, Titze JM. The interstitium conducts extrarenal storage of sodium and represents a third compartment essential for extracellular volume and blood pressure homeostasis. Acta Physiol 222: e13006, 2018. doi: 10.1111/apha.13006. [DOI] [PubMed] [Google Scholar]
- 46..Titze J, Lang R, Ilies C, Schwind KH, Kirsch KA, Dietsch P, Luft FC, Hilgers KF. Osmotically inactive skin Na+ storage in rats. Am J Physiol Renal Physiol 285: F1108–F1117, 2003. doi: 10.1152/ajprenal.00200.2003. [DOI] [PubMed] [Google Scholar]
- 47.Heer M, Frings-Meuthen P, Titze J, Boschmann M, Frisch S, Baecker N, Beck L. Increasing sodium intake from a previous low or high intake affects water, electrolyte and acid-base balance differently. Br J Nutr 101: 1286–1294, 2009. doi: 10.1017/S0007114508088041. [DOI] [PubMed] [Google Scholar]
- 48.Titze J. Water-free sodium accumulation. Semin Dial 22: 253–255, 2009. doi: 10.1111/j.1525-139X.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 49.Schafflhuber M, Volpi N, Dahlmann A, Hilgers KF, Maccari F, Dietsch P, Wagner H, Luft FC, Eckardt KU, Titze J. Mobilization of osmotically inactive Na+ by growth and by dietary salt restriction in rats. Am J Physiol Renal Physiol 292: F1490–F1500, 2007. doi: 10.1152/ajprenal.00300.2006. [DOI] [PubMed] [Google Scholar]
- 50.Madias NE, Bossert WH, Adrogué HJ. Ventilatory response to chronic metabolic acidosis and alkalosis in the dog. J Appl Physiol Respir Environ Exerc Physiol 56: 1640–1646, 1984. doi: 10.1152/jappl.1984.56.6.1640. [DOI] [PubMed] [Google Scholar]
- 51.Adler S, Zett B, Anderson B, Fraley DS. Effect of volume expansion on renal citrate and ammonia metabolism in KCl-deficient rats. J Clin Invest 56: 391–400, 1975. doi: 10.1172/JCI108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ziomber A, Machnik A, Dahlmann A, Dietsch P, Beck FX, Wagner H, Hilgers KF, Luft FC, Eckardt KU, Titze J. Sodium-, potassium-, chloride-, and bicarbonate-related effects on blood pressure and electrolyte homeostasis in deoxycorticosterone acetate-treated rats. Am J Physiol Renal Physiol 295: F1752–F1763, 2008. doi: 10.1152/ajprenal.00531.2007. [DOI] [PubMed] [Google Scholar]
- 53.Fournier S, Allard M, Gulemetova R, Joseph V, Kinkead R. Chronic corticosterone elevation and sex-specific augmentation of the hypoxic ventilatory response in awake rats. J Physiol 584: 951–962, 2007. doi: 10.1113/jphysiol.2007.141655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weissman C, Askanazi J, Forse RA, Hyman AI, Milic-Emili J, Kinney JM. The metabolic and ventilatory response to the infusion of stress hormones. Ann Surg 203: 408–412, 1986. doi: 10.1097/00000658-198604000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shah DM. The role of RAS in the pathogenesis of preeclampsia. Curr Hypertens Rep 8: 144–152, 2006. doi: 10.1007/s11906-006-0011-1. [DOI] [PubMed] [Google Scholar]