Abstract
Inhalation exposure to cigarette smoke and e-cigarette aerosol is known to alter the respiratory immune system, particularly cytokine signaling. In assessments of health impacts of tobacco product use, cytokines are often measured using a variety of sample types, from serum to airway mucosa. However, it is currently unclear whether and how well cytokine levels from different sample types and the airway locations they represent are correlated, making comparing studies that utilize differing sample types challenging. To address this challenge, we compared baseline cytokine signatures in upper and lower airways and systemic samples and evaluated how groups of coexpressed cytokines change with tobacco product use. Matched nasal lavage fluid (NLF), nasal epithelial lining fluid (NELF), sputum, and circulating serum samples were collected from 14 nonsmokers, 13 cigarette smokers, and 17 e-cigarette users and analyzed for levels of 22 cytokines. Individual cytokine signatures were first compared across each sample type, followed by identification of cytokine clusters within each sample type. Identified clusters were then evaluated for potential alterations following tobacco product use using eigenvector analyses. Individual cytokine signatures in the respiratory tract were significantly correlated (NLF, NELF, and sputum) compared with randomly permutated signatures, whereas serum was not significantly different from random permutations. Cytokine clusters that were similar across airway sample types were modified by tobacco product use, particularly e-cigarettes, indicating a degree of uniformity in terms of how cytokine host defense and immune cell recruitment responses cooperate in the upper and lower airways. Overall, cluster-based analyses were found to be especially useful in small cohort assessments, providing higher sensitivity than individual signatures to detect biologically meaningful differences between tobacco use groups. This novel cluster analysis approach revealed that eigencytokine patterns in noninvasive upper airway samples simulate cytokine patterns in lower airways.
Keywords: cytokine clusters, tobacco product use, unsupervised machine learning, upper and lower airways
INTRODUCTION
Because of their important roles in pulmonary responses and related disease outcomes, cytokines are commonly measured as biomarkers of exposure and/or effects, including our previous assessments of respiratory immune system responses to cigarette and e-cigarette exposure (1–3). The types and levels of cytokines expressed at baseline or in response to toxic insults depend on the tissue and cell type and are often used as biomarkers of respiratory health and disease. Samples that are used to evaluate respiratory health and associated biological responses include those collected from the upper and lower airway, such as nasal and sputum samples, and from circulation, blood/serum samples. Nasal samples can be collected as nasal lavage fluid (NLF) and nasal epithelium lining fluid (NELF) samples, both of which represent relatively noninvasive methods of collecting nasal mucosal samples (4). Sputum samples represent the lower airways and can either be produced spontaneously or induced by inhaling nebulized saline solutions (5, 6). However, there are substantial limitations to sputum sampling, including the invasiveness of the procedure and that not every individual is able to produce sputum. Cytokines can also be measured in serum, but the degree to which cytokine production and disease- or exposure-induced alterations differs between circulating blood and the airway has been questioned (7, 8). Hence, whether and how cytokine levels from upper airways, lower airways, and serum correlate with each other in health and/or disease are not clear.
This difficulty in interpreting the applicability of findings in one sample type to another can be seen in tobacco products health effects research. Inhalation exposure to cigarette smoke and e-cigarette aerosol are known to alter biological processes, including inflammation, host-defense status, and immune cell activation (9–11). Both in vitro and in vivo studies have attempted to assess the effects of e-cigarettes on respiratory immune cytokine production; however, translation of effects to the entire respiratory tract has been difficult (12–15). For example, studies have identified alterations of immune mediators within the nasal passage, but the applicability of these observed changes to the lower airway were unclear (2, 16). Therefore, what remains understudied is whether the impact of tobacco product use is consistent throughout the respiratory tract, and whether easy-to-sample respiratory compartments, such as NLF and NELF, are representative of lower airway cytokine changes in smokers and e-cigarette users.
This study set out to fill this critical research gap by leveraging a unique multicompartment cytokine profiling data set obtained from human volunteers, consisting of nonsmokers, cigarette smokers, and e-cigarette users. First, baseline cytokine profiles were characterized across matched samples collected from different compartments commonly analyzed in biomedical research applications (i.e., NLF, NELF, sputum, and serum) to evaluate the degree of similarity or differences between compartment-specific signatures. Then we evaluated how cytokines cluster together and demonstrated comodulated patterns that can be modified with tobacco product use, using eigenvector-based methods. Lastly, cluster-based findings were compared with more traditional analyses based on individual cytokine distributions, to gage the utility of the newer cluster-based approach. Findings from NELF samples were additionally replicated in a separate validation cohort. Collective results elucidate novel comodulated patterns of cytokines that are altered by tobacco product use and importantly identify that cytokine changes in nasal samples can be predictive of similar changes in the lower airways.
MATERIALS AND METHODS
Primary Study Cohort Overview
Healthy, young adults between 18 and 50 yr of age (subject number n = 44, Table 1) were recruited from 2014 to 2017 for study participation using inclusion and exclusion criteria described previously (2). Participants included current nonsmokers, not regularly exposed to tobacco products (n = 14). Participants also included self-identified current cigarette smokers, who reported smoking a mean of 12 cigarettes per day (n = 13), and self-identified current electronic cigarette (e-cigarette) users (n = 17), who reported a mean of 82 puffs/day with an average nicotine content of 12 mg/mL. E-cigarette users reported utilization of pen and tank style devices and the majority used fruity and sweet flavors. Pod-based/disposable e-cigarette devices were not utilized in this cohort as samples were collected from 2014 to 2017, before pod and disposable e-cigarettes becoming available/popular in the market. E-cigarette users reporting tobacco use exceeding five cigarettes per week were categorized as dual users and excluded from this study. Participants included both males and females and multiple races/ethnicities (Table 1). Use status of e-cigarette users and cigarette smokers was confirmed via serum cotinine analysis. Cotinine is a nicotine metabolite and indicates recent exposure to nicotine-containing products. Cotinine was measured in paired serum samples from each participant via ELISA per manufacturer instructions (CALBIOTECH, El Cajon, CA). This protocol was approved by the University of North Carolina at Chapel Hill Biomedical Institutional Review Board (No. 13–3454) and all methods were performed in accordance with relevant guidelines and regulations including obtaining written informed consent.
Table 1.
Overview of subject characteristics from the primary study cohort
| All Subjects | Nonsmokers | Cigarette Smokers | E-Cigarette Users | Significance Across Smoking Status* | |
|---|---|---|---|---|---|
| Total n | 44 | 14 | 13 | 17 | |
| Sex | |||||
| Male | 24 (57.1) | 7 (50.0) | 5 (38.5) | 12 (70.1) | 0.36 |
| Female | 20 (42.9) | 7 (50.0) | 8 (61.5) | 5 (29.9) | 0.36 |
| Race | |||||
| White | 28 (63.6) | 10 (71.4) | 7 (53.8) | 11 (64.7) | 0.51 |
| Black | 10 (22.7) | 2 (14.3) | 6 (46.2) | 2 (11.8) | 0.51 |
| Asian | 4 (9.1) | 1 (7.1) | 0 (0.0) | 3 (17.6) | 0.51 |
| Other | 2 (4.5) | 1 (7.1) | 0 (0.0) | 1 (5.9) | 0.51 |
| Race dichotomized | |||||
| White | 28 (63.6) | 10 (71.4) | 7 (53.8) | 11 (64.7) | 0.82 |
| Non-White | 16 (36.4) | 4 (28.6) | 6 (46.2) | 6 (35.3) | 0.82 |
| Ethnicity | |||||
| Non-Hispanic | 39 (88.6) | 11 (78.6) | 13 (100.0) | 15 (88.2) | 0.38 |
| Hispanic | 5 (11.4) | 3 (21.4) | 0 (0.0) | 2 (11.8) | 0.38 |
| Age, yr | 28 [18–47] | 29 [21–39] | 29.8 [22–47] | 26 [18–38] | 0.12 |
| BMI, kg/m2 | 29.1 [18.7–43.7] | 27.7 [20.0–37.7] | 29.2 [20.9–40.4] | 30.4 [21.7–43.7] | 0.55 |
| Cotinine, pg/mL | 103.5 [0–593.6] | 0 [0–0] | 181.4 [57.2–593.6] | 129.0 [0–288.8] | 8.35E-05 |
| Prior cigarette smoking | 25 (56.8) | 0 (100) | 13 (100) | 12 (70.5) | 1.71E-05 |
Values include the overall count (% of total) for all rows except age, BMI, and cotinine, which represent the overall average [min–max]. *Significance derived from Fisher’s exact tests across categorical data, and ANOVA across continuous data comparing against smoking groups. BMI, body mass index.
The distribution of demographic variables across subjects was evaluated to establish demographic statistics and to determine if there were demographic variables that differed across tobacco use groups. Fisher’s exact tests of independence were used to evaluate potential changes in categorical variable distributions (i.e., ethnicity, race, and sex), comparing cigarette smokers versus nonsmokers, as well as e-cigarette users versus nonsmokers. One-way ANOVA tests were used to evaluate potential changes in continuous variable distributions [i.e., age, body mass index (BMI), and cotinine levels], comparing cigarette smokers versus nonsmokers, as well as e-cigarette users versus nonsmokers. These calculations were carried out using baseline, default statistical software in R (v4.0.2) (17). No demographic variables demonstrated significant differences across smoking status groups (Table 1).
Sample Collection
Samples were collected across compartments relevant to the respiratory tract, including nasal lavage fluid (NLF), nasal epithelial lining fluid (NELF), and sputum samples. Circulating serum samples were also collected as a more distal compartment comparison. NLF samples were specifically collected as described previously (2, 16, 18). Briefly, 8 mL of normal sterile saline was repeatedly sprayed into the nostrils (4 mL per nostril) and gently expelled into a sample collection cup by the participant. Samples were then filtered and centrifuged to remove cells and debris. The cell-free fraction was then frozen at −80°C until analysis. NELF samples were collected as described previously (3, 4). Briefly, each nostril was moistened with 100 μL of normal sterile saline (PCCA, Houston, TX). A strip of Leukosorb matrix (Pall, Port Washington, NY) cut to fit within the nasal passage was then inserted into each nostril and clamped with a padded nose clamp for 2 min. The strips were then removed and frozen at −20°C until elution. A strip was randomly selected from either nostril for each subject and used for analysis. The strips were eluted as described previously with 1% BSA with 0.05% Triton X-100 in Dulbecco’s phosphate-buffered saline (DPBS) and centrifugation. The NELF eluate was stored at −20°C for analysis. Sputum samples were collected as described previously (19, 20). Briefly, nebulized hypertonic saline was inhaled over three 7-min periods at 3%, 4%, and 5% saline, followed by participant expectoration of induced sputum in a collection cup. Samples were then separated into cellular and cell-free fractions by mucous removal and centrifugation. The cell-free fraction was frozen at −80°C until analysis. Serum samples were collected by venipuncture and collection of whole blood into serum separator tubes (Becton, Dickinson and Company, Franklin Lakes, NJ). Tubes were then centrifuged, and isolated serum was frozen until analysis.
Data Analysis
Protein extraction and measurement of cytokine levels.
Proteins were analyzed from the collected samples using singleplex and multiplex ELISA kits per manufacturer instructions. Specifically, a V-Plex Proinflammatory Panel 1 was used to measure interferon γ (IFNγ), interleukin 1β (IL1β), interleukin 6 (IL6), interleukin 8 (IL8, also known as CXCL8), interleukin 10 (IL10), interleukin 12 p70 (IL12p70), interleukin 13 (IL13), and tumor necrosis factor α (TNFα; Mesoscale Discovery, Rockville, MD). A V-Plex Chemokine Panel 1 was used to measure Eotaxin, Eotaxin3, interferon γ-induced protein (IP10, also known as CXCL10), macrophage inflammatory protein 1-α (MIP1α), macrophage inflammatory protein 1-β (MIP1β), monocyte chemoattractant protein-1 (MCP1), and thymus and activation regulated chemokine (TARC; Mesoscale Discovery, Rockville, MD). A custom V-Plex panel was used to measure Fractalkine (CX3CL1), interferon-inducible T cell α chemoattractant (ITAC), interleukin 4 (IL4), interleukin 17B (IL17B), interleukin 17 D (IL17D), small inducible cytokine A-1 (I309), and monokine induced by interferon-γ (MIG, also known as CXCL9; Mesoscale Discovery, Rockville, MD). An OptEIA ELISA kit was used to measure IL8 (Becton, Dickinson and Company). Due to the limited dynamic range for IL8 in this the V-plex Proinflammatory Panel 1, IL8 was quantified in NELF samples by a separate single-plex OptEIA ELISA (Becton, Dickinson and Company). This analysis yielded measures across 22 total cytokines, at cytokine concentration values, in pg/mL, for each sample collected. Concentration values were adjusted for differences in processing and dilution volumes. Overall descriptive statistics for these cytokine distributions were carried out per compartment, for the entire cohort and for smoking subgroups, using baseline statistical software in R.
Cross-compartment analysis of overall cytokine signatures.
A descriptive statistical analysis focused on nonsmokers was first carried out to estimate baseline cytokine signatures across each compartment. The average and standard deviation of each cytokine concentrations across all nonsmokers were calculated and visualized using the ggplot2 package in R (21), resulting in baseline cytokine signatures for each compartment (i.e., NLF, NELF, sputum, and serum). Cytokine signatures were tested for normality using the shapiro.test package in R (22) before and after pseudo log2-transformation (pslog2). This involved increasing cytokine concentrations by one and then log-transformed to avoid negative values and to center and scale the data. All signatures were nonnormally distributed, which is commonly seen in human biological samples. The degree of similarity between each compartment’s baseline cytokine signature was then evaluated using the Spearman’s rank correlation test, resulting in correlation R and P values. To further evaluate the likelihood of each compartment exhibiting cytokine signatures that may correlate by chance, we incorporated a random permutation analysis. Specifically, random cytokine signatures were generated using the Sample function in R, allowing for bootstrapping (i.e., random sampling with replacement) (23). Values were randomly selected from the measured cytokines to produce 500 random cytokine signatures (per compartment) that were then tested for correlation with each other, as well as the observed cytokine signatures. Resulting correlation R and P values were compared and visualized to provide further information surrounding how well each compartment’s signature correlated in comparison to random noise. To conduct a similar cross-compartment analysis within tobacco product users, the same set of statistics was additionally carried out in cigarette smokers as well as in e-cigarette users.
Deriving baseline cytokine clusters and eigencytokine values.
Cytokine clusters at baseline were identified as those exhibiting comodulation patterns initially across nonsmokers, separated per compartment. Groups of cytokines were identified as those that partitioned into clusters defined while minimizing within-cluster variance (postnormalization) to result in cytokines that were thus “coexpressed” in individuals that were not exposed to tobacco products. Toward this goal, cytokine concentrations (pslog2-transformed) were analyzed using K-means clustering, one of the most frequently used unsupervised machine learning algorithms for dividing a data set into clusters (24). In this method, k represents the number of clusters and the centroid corresponds to the mean of the points assigned to a cluster. For the current analysis, the Hartigan–Wong K-means algorithm was implemented through the baseline statistical packages in R to derive the clusters by minimizing total within-cluster variation and maximizing total between-cluster variation (25). Cluster numbers were determined through a combination approach, first employing the elbow method using the factoextra package in R, wherein a range of optimal cluster numbers were derived based on the percentage of variation explained by the first eigenvector of the cytokines in each cluster as a function of the cluster numbers (26). Then, visual inspection of two-dimensional (2-D) and three-dimensional (3-D) principal component analysis (PCA) plots was carried out using the factoextra and rgl packages, respectively (27). The final k value (number of clusters) of 3 was determined as the one producing the most distinct cytokine clusters throughout all compartments.
To obtain aggregate-level measures of cytokine profiles across each cluster, “eigencytokine” values were derived as eigenvectors of the first principal component of each cluster. These eigencytokines captured the majority of variance across cytokines and thus represented a collective measure of their profiles, parallel to a previous method that derived “eigenmetabolites” (28). Eigencytokine values were first derived for the clusters in nonsmokers, and then using the same cluster assignments, eigencytokines were derived within samples from cigarette smokers and e-cigarette users, separately. Prior to running principal component analysis (PCA), pseudo-transformed cytokine concentrations were scaled within each compartment. Weights of each cytokine on their respective eigenvectors were calculated and visualized alongside each eigenvector generated by using the ggplot2 package in R (21). These weights represent the magnitude and direction of the cytokine on the eigencytokine. It is notable that other clustering approaches exist and were examined as alternative approaches (e.g., hierarchical clustering), but these approaches yielded clusters with data distributions that were not as optimal in comparison to k-means. Specifically, some groupings contained just single cytokines and others the majority of cytokines, resulting in less biologically meaningful principal component analysis and final derivation of eigencytokines.
Identifying consensus clusters across respiratory regions and their alterations associated with tobacco product use.
Each cytokine cluster was compared between the respiratory regions, with cytokine assignments specifically visualized using Venn diagrams. Cytokines that were consistently grouped together as clusters across each of the respiratory samples were identified and termed “Consensus Clusters.” These represented cytokines that consistently demonstrated baseline comodulation patterns across the NLF, NELF, and sputum samples. Cytokine Consensus Clusters were next evaluated for potential changes in aggregate levels associated with smoking status, leveraging the derived eigencytokine values. Here, Wilcoxon rank-sum tests were used to compare eigencytokines in cigarette smokers versus nonsmokers, e-cigarette users versus nonsmokers, and e-cigarette users versus smokers, separately per compartment (R software). Resulting statistical P values were adjusted for multiple tests (referred to as “Padj” values) using false-discovery rate (FDR) q values (29).
Biological implications of identified consensus clusters.
To further elucidate the biological implications of the Consensus Clusters, and the potential implications of their alterations associated with tobacco use, a pathway enrichment analysis was carried out. Specifically, the list of cytokines in each Consensus Cluster was queried separately within the Ingenuity Pathway Analysis Knowledgebase and analyzed for enrichment within canonical pathways. A pathway was identified as enriched when it contained more cytokines than expected by random chance, defined using a Benjamini-Hochberg (BH)-corrected P < 0.05 from a modified right-tailed Fisher’s exact test (30, 31).
Identifying individual cytokine alterations associated with tobacco product use.
To evaluate how the cluster-derived results compared against results produced when evaluating cytokines individually, cytokines were next evaluated individually for potential changes associated with smoking status. Multiple approaches were implemented here with the goal of more fully capturing any potential changes across smoking status at the individual cytokine level. These approaches spanned crude, adjusted, and stratified statistical models.
Similar to the cluster-based statistics, Wilcoxon rank-sum tests were first used to compare cytokine signatures in cigarette smokers versus nonsmokers, and e-cigarette users versus nonsmokers, separately per compartment (R software). Although no demographic variables demonstrated significant differences across smoking status (Table 1), we performed additional analyses to include a potential confounder that demonstrated the largest (though not significant) difference across smoking subgroups: age. Here, analysis of variance (ANOVA) tests were first run as a crude model to compare individual cytokine profiles across smoking statuses (three-group comparison), which provided the foundation for analysis of covariance (ANCOVA) tests while controlling for age. Stratified analyses were also carried out, in which participants were evaluated in subgroups according to demographics (i.e., sex, race, ethnicity, and BMI) to further investigate potential changes associated with smoking status that may not be apparent when analyzing participants collectively. After stratifying, Wilcoxon rank-sum tests were carried out comparing nonsmokers versus cigarette smokers and nonsmokers versus e-cigarette users. Resulting statistical P values were adjusted (Padj) for multiple tests using false-discovery rate (FDR) q values (29).
Separate validation study cohort analysis.
To bolster the external validity of this study’s findings, another data set, referred to as the “validation data set,” was included in this study which focused on the evaluation of NELF samples. Healthy, young adults between 19 and 43 yr of age (n = 48, Table 2) were recruited from 2015 to 2017 for study participation as described previously (3). Participants included current nonsmokers, characterized as never smokers (n = 20). Participants also included self-described current cigarette smokers (n = 14), who smoked a mean of 12 cigarettes per day, and self-described current electronic cigarette (e-cigarette) users (n = 14), who reported using a mean of 74 puffs/day. Inclusion and exclusion criteria were published previously (3). Participants included both males and females and multiple races/ethnicities (Table 2) and similar to the cohort described in Table 1, the status of e-cigarette users and cigarette smokers was confirmed via serum cotinine analysis. This protocol was approved by the University of North Carolina at Chapel Hill Biomedical Institutional Review Board (No. 13–2246) and all methods were performed in accordance with relevant guidelines and regulations. NELF samples were collected and analyzed for cytokine profiles across 28 cytokines: Eotaxin, Eotaxin3, granulocyte-macrophage-colony-stimulating factor (GMCSF), IFNγ, interleukin 10 (IL10), interleukin 12 p40 (IL12p40), IL12p70, interleukin 13 (IL13), interleukin 15 (IL15), interleukin 16 (IL16), interleukin 17 (IL17), interleukin 1 α (IL1α), interleukin 1β (IL1β), interleukin 2 (IL2), IL4, IL6, interleukin 7 (IL7), IL8, IP10, MCP1, monocyte chemoattractant protein-4 (MCP4), macrophage-derived chemokine (MDC, also known as CCL22), MIP1α, macrophage inflammatory protein 1-β (MIP1β), TARC, TNFα, tumor necrosis factor-β (TNFβ), and vascular endothelial growth factor (VEGF). These notably overlap with 15 of the 22 cytokines measured in the primary study cohort. Cytokine levels were measured using methods parallel to those described in the primary study cohort, and are previously published (2). In the current study, NELF cytokine data were organized and analyzed using the exact same methods as detailed above for the primary study cohort analyses. These steps included the identification of baseline cytokine clusters in nonsmokers, and the statistical analysis for cluster-based and individual cytokine-based changes associated with smoking status.
Table 2.
Overview of subject characteristics from the separate validation study cohort
| All Subjects | Nonsmokers | Cigarette Smokers | E-Cigarette Users | Significance Across Smoking Status* | |
|---|---|---|---|---|---|
| Total n | 48 | 20 | 14 | 14 | |
| Sex | |||||
| Male | 24 (50.0) | 6 (30.0) | 8 (57.1) | 10 (71.4) | 0.11 |
| Female | 24 (50.0) | 14 (70.0) | 6 (42.9) | 4 (28.6) | 0.11 |
| Race | |||||
| White | 33 (68.8) | 16 (80.0) | 7 (50.0) | 10 (71.4) | 0.18 |
| Black | 11 (22.9) | 2 (10.0) | 7 (50.0) | 2 (14.3) | 0.18 |
| Asian | 4 (8.3) | 2 (10.0) | 0(0.0) | 2 (14.3) | 0.18 |
| Race dichotomized | |||||
| White | 33 (68.8) | 16 (80.0) | 7 (50.0) | 10 (71.4) | 0.33 |
| Non-White | 16 (33.3) | 4 (20.0) | 7 (50.0) | 4 (28.6) | 0.33 |
| Ethnicity | |||||
| Non-Hispanic | 45 (93.7) | 17 (85.0) | 14 (100.0) | 15 (100.0) | 0.32 |
| Hispanic | 3 (6.3) | 3 (15.0) | 0 (0.0) | 1 (0.0) | 0.32 |
| Age, yr | 28 [19–43] | 28 [19–43] | 32.1 [21–40] | 24 [19–38] | 0.01 |
| BMI, kg/m2 | 27.1 [18.1–45.9] | 26.1 [18.1–41.2] | 27.8 [20.7–38.3] | 27.9 [20.1–45.9] | 0.66 |
| Cotinine, pg/mL | 164.8 [0–442.1] | 0.7 [0–14.7] | 209.5 [4.3–442.1] | 117.3 [3.52–413.4] | 5.76E-07 |
Values include the overall count (% of total) for all rows except the last three (age, BMI, and cotinine), which represent the overall average [min–max]. *Significance derived from Fisher’s exact tests across categorical data, and ANOVA across continuous data comparing against smoking groups. BMI, body mass index.
RESULTS
Primary Study Cohort Characteristics and Overall Cytokine Distributions
Overall demographic characteristics were summarized for the 44 participants in the primary cohort (Table 1). Of these subjects, about half were male (57%), most identified as White (64%), and a majority identified as non-Hispanic (89%). The cohort had an average age of 28 (range of 18–47) yr, along with an average BMI of 29.1 (18.7–43.7). Demographic characteristics remained largely consistent across each group of nonsmokers, cigarette smokers, and e-cigarette users. Serum cotinine levels had an average of 103.5 (range of 0–593.6) pg/mL across all subjects and 0 (0–0) pg/mL, 129.0 (0–288.8) pg/mL, 181.4 (57.2–593.6) pg/mL for nonsmokers, e-cigarette users, and cigarette smokers, respectively. Serum cotinine was the only variable that had statistically significant differences across smoking statuses and, based on previous work, was expected to be elevated in both e-cigarette users and cigarette smokers who use nicotine-containing products (2).
Overall cytokine distributions across all subjects and according to tobacco product use are summarized in Supplemental Table S1; all Supplemental material is available at https://doi.org/10.15139/S3/LJH0G4). Among the most highly expressed cytokines across all subjects was IP10 (overall highest in NLF; 3rd in NELF; 2nd in sputum and serum), Fractalkine (2nd highest in NLF and NELF; 1st in sputum and serum), and IL8 (3rd highest in NLF; 1st in NELF; 2nd in sputum; 11th in serum). Among the lowest expressed cytokines across all subjects was I309 (overall lowest in NLF and sputum; 2nd lowest in NELF; 15th in serum) and IL4 (2nd lowest in NLF, sputum, and serum; overall lowest in NELF).
Cross-Compartmental Comparison of Cytokine Signatures
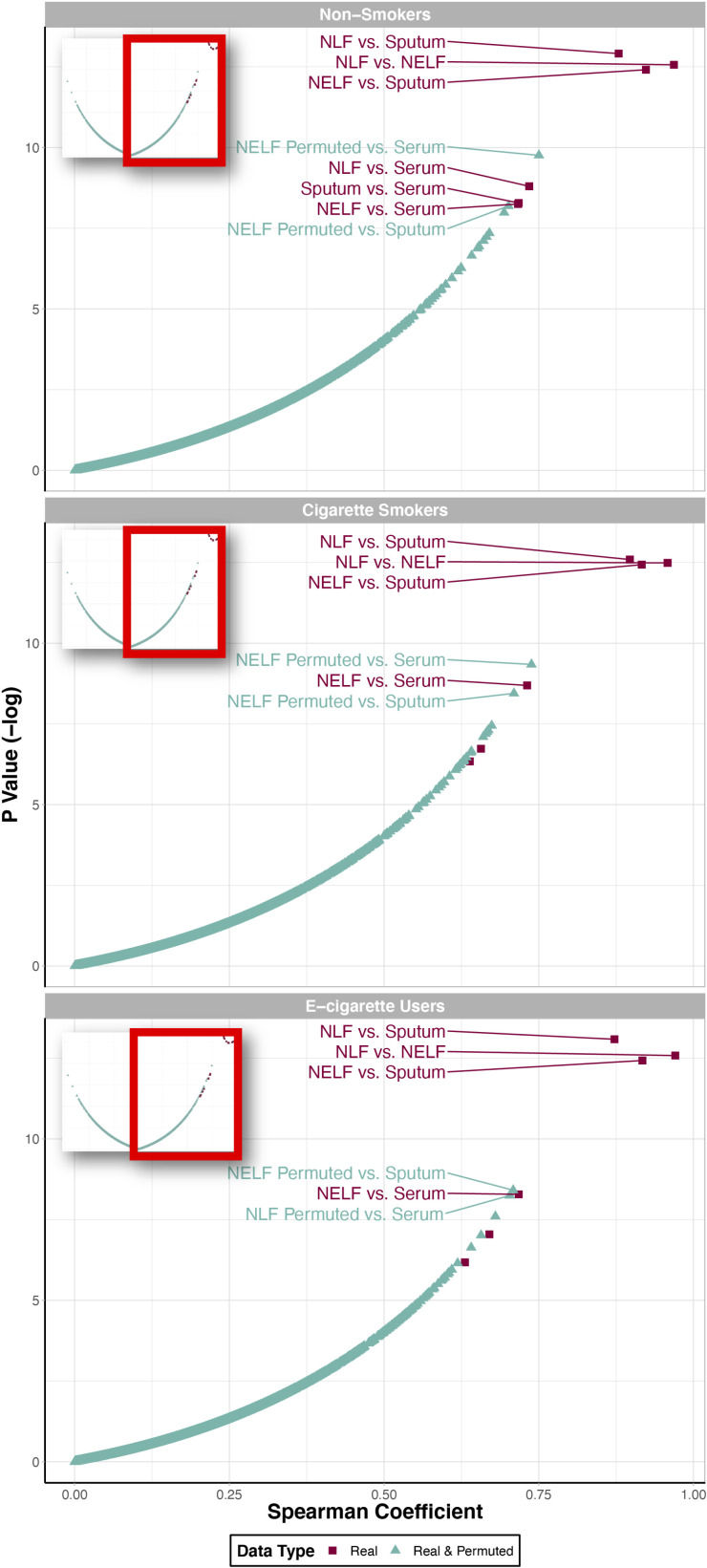
Average cytokine concentrations of nonsmokers were calculated (Supplemental Table S1) and visualized by compartment to first determine baseline cytokine signatures that exist in the absence of tobacco product use exposures (Fig. 1; see Supplemental Fig. S1 for median cytokine concentrations). Visual inspection of these signatures clearly demonstrates that NLF, NELF, and sputum samples are largely similar, whereas serum samples have differing profiles. To statistically evaluate these trends, correlation analyses were carried out comparing each compartment. These correlation tests identified statistically significant correlations between every compartment included in this analysis (R > 0.7, P < 0.05). As expected, cytokine signatures in NLF, NELF, and sputum samples were the most significantly correlated, and serum samples were the least correlated to the other respiratory-derived samples (Fig. 2). Comparisons between upper and lower airway sample types all had a greater degree of correlation indicated by higher Spearman’s correlation coefficients (all of which were significant) than correlations between sampled airway compartments and serum. These trends remained consistent when also comparing cytokine distribution profiles between sample types in cigarette smokers and e-cigarette users, with serum samples consistently demonstrating the lowest correlation to the respiratory-derived samples (Table 3). These findings confirm cytokine measurements acquired from serum samples may not always represent patterns occurring within the respiratory tract.
Figure 1.

Cytokine profiles across compartments in nonsmokers, representing baseline cytokine signatures that exist in the absence of tobacco product use exposures. Experimentally measured cytokine concentrations are plotted, with average levels as the lines and standard deviations as the shaded region, by compartment. Cytokines are ordered from those with the highest (left) to lowest (right) average concentration in NLF samples. NLF, nasal lavage fluid.
Figure 2.
Comparison of cytokine distribution similarity across compartments stratified by smoking status, identified through correlation and random permutation analyses. Two “data types” are included here: 1) “Real” data (in red boxes), which refer to the correlation results between two experimentally observed cytokine concentration datasets and 2) “real and simulated” data (in turquoise triangles), which refer to the correlation results between one experimentally observed cytokine concentration data set (i.e., the “real” data set) and one simulated dataset that was randomly generated based on the original data set. Points were labeled with the compartment comparison text identifiers if they were highly correlated and statistically significant (R > 0.7 and P < 0.05).
Table 3.
Correlations between cytokine signatures across each compartment stratified by smoking status
| Compartment Comparison | Spearman’s Coefficient | P Value |
|---|---|---|
| Nonsmokers | ||
| NLF and NELF | 0.968 | 3.50E-06 |
| NELF and sputum | 0.923 | 4.08E-06 |
| NLF and sputum | 0.879 | 2.47E-06 |
| NLF and serum | 0.735 | 1.51E-04 |
| Sputum and serum | 0.718 | 2.53E-04 |
| NELF and serum | 0.717 | 2.61E-04 |
| Cigarette smokers | ||
| NLF and NELF | 0.958 | 3.77E-06 |
| NELF and sputum | 0.916 | 3.99E-06 |
| NLF and sputum | 0.897 | 3.40E-06 |
| NLF and serum | 0.639 | 1.76E-03 |
| Sputum and serum | 0.657 | 1.19E-03 |
| NELF and serum | 0.731 | 1.68E-04 |
| E-cigarette users | ||
| NLF and NELF | 0.971 | 3.43E-06 |
| NELF and sputum | 0.918 | 4.01E-06 |
| NLF and sputum | 0.872 | 2.07E-06 |
| NLF and serum | 0.670 | 8.73E-04 |
| Sputum and serum | 0.631 | 2.08E-03 |
| NELF and serum | 0.718 | 2.53E-04 |
Correlation results are summarized as Spearman’s rank coefficients and P values for each compartment comparison. NELF, nasal epithelial lining fluid; NLF, nasal lavage fluid.
Additional analyses were included to evaluate how well cytokine signatures that were randomly permuted (i.e., “simulated” signatures) correlated with experimentally observed signatures (i.e., “real” signatures). This permutation analysis demonstrated that simulated data were not as correlated or statistically significant as the correlations between the real experimental data set, with the clear exception of serum measurements. To detail, there was a clear grouping of the top-correlated compartments, all representing experimentally measured “real” values between 1) NLF versus sputum, 2) NLF versus NELF, and 3) NELF versus sputum samples, in nonsmokers, cigarette smokers, and e-cigarette users (Fig. 2). These data suggest that resulting correlations between respiratory samples were very unlikely due to chance. In comparison, the “real” serum samples demonstrated correlations to the other “real” samples that overlapped in significance with the simulated datasets, suggesting that these relationships may be occurring due to random chance. Together, these analyses demonstrate a consistently high degree of similarity between immune mediator signatures in upper and lower airways samples, throughout each tobacco product use group.
Baseline Cytokine Clusters and Eigencytokine Values
A clustering approach was carried out to identify groups of cytokines that exhibited comodulation patterns across subjects at baseline (in nonsmokers). Employing a combination approach of the elbow method and visual inspection of 2-D and 3-D PCA plots, a final cluster number of three (i.e., k = 3 from k-means clustering) was determined as that producing the most distinct cytokine clusters throughout all compartments (Fig. 3). The first principal component of the eigenvectors of these clusters captured 84.1%, 85.1%, 88.4%, and 90.9% of the variance in cytokine levels in NLF, NELF, sputum, and serum, respectively. These variance values supported the use of the eigenvector of the first principal component to derive aggregate values for each of the clusters, which we termed “eigencytokines.” Clusters were largely abundance driven as seen in Fig. 4 visualizing NLF cytokine concentrations and eigenvector weights. The specific cluster assignments for each cytokine are also included in Supplemental Table S2, with many of the same cytokines clustering consistently throughout each respiratory region (as further discussed as respiratory Consensus Clusters in the results below). Cytokines demonstrated largely different cluster assignments in serum samples.
Figure 3.

Cytokine principal component plots showing clusters at baseline (in nonsmokers). The cytokine concentration distributions are visualized according to the evaluated compartments, NLF, NELF, sputum, and serum. Cytokine concentration values were transformed using data reduction approaches (i.e., PCA) to illustrate the overall trends in cytokine groupings in 2-D. The illustrated principal components show the resulting reduced values, which captured the majority of variance in data across samples. Cytokine clusters are shown as shaded shapes within these PCA plots. NELF, nasal epithelial lining fluid; NLF, nasal lavage fluid; PCA, principal component analysis; 2-D, two dimensional.
Figure 4.
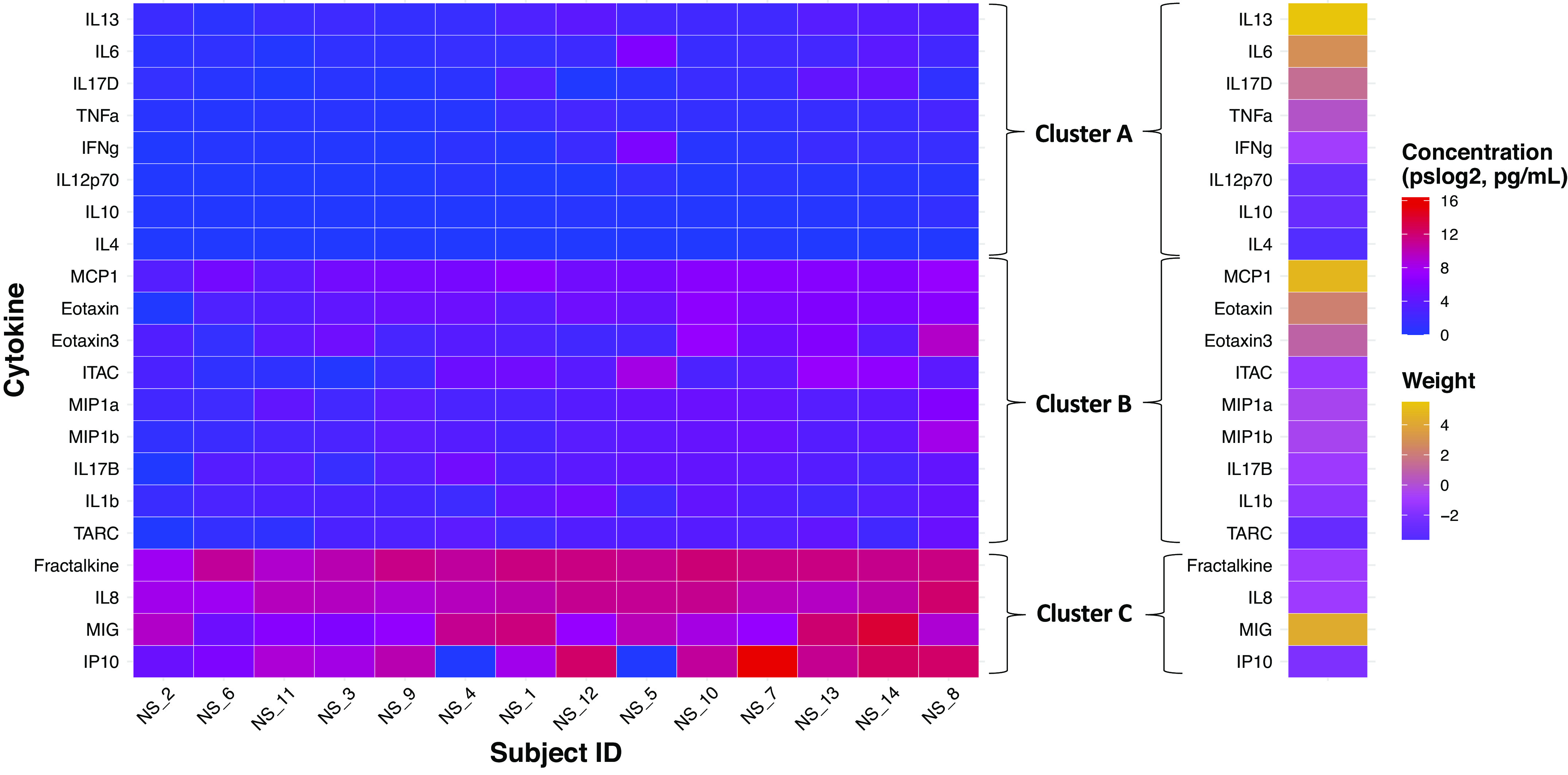
Individual cytokine levels and eigencytokine weights for those identified in NLF in the example derivation of cytokine clusters. Subjects are ordered from lowest to highest average cytokine concentration from left to right. Within each cluster, cytokines are ordered from lowest to highest average cytokine concentration from bottom to top. NLF, nasal lavage fluid; NS, nonsmoker.
Consensus Clusters across Respiratory Regions and Their Alterations Associated with Tobacco Product Use
Many of the cytokines were assigned to similar clusters based on comodulation patterns throughout the respiratory tract. To visualize how consistent these assignments were, Venn diagrams were produced comparing the lists of cytokines in each cluster, per compartment (Fig. 5). The cytokines that overlapped between the respiratory compartments were identified as “Consensus Clusters,” representing cytokines that consistently demonstrated comodulation patterns across regions of the respiratory tract. These specifically included six cytokines in Consensus Cluster A, seven cytokines in Consensus Cluster B, and four cytokines in Consensus Cluster C. To detail, Cluster A consisted of IFNγ, IL10, IL12p70, IL17D, IL4, TNFα. Cluster B consisted of Eotaxin, Eotaxin3, IL17b, IL1β, ITAC, MIP1α, and TARC. Cluster C consisted of Fractalkine, IL8, IP10, and MIG.
Figure 5.

Deriving “Consensus Clusters” of cytokines that consistently demonstrate comodulation patterns across regions of the respiratory tract commonly evaluated in clinical and toxicological research. Venn diagrams are used here to compare which cytokines clustered together across each respiratory compartment, yielding Consensus Cluster A, including cytokines involved in host defense (A); Consensus Cluster B, including cytokines involved in chemotactic immune cell recruitment (B); and Consensus Cluster C, including cytokines involved in immune cell adhesion and inflammation (C). NELF, nasal epithelial lining fluid; NLF, nasal lavage fluid.
The identified Consensus Clusters were then evaluated for potential comodulation alterations associated with smoking status. Eigencytokine values were used to statistically compare aggregate signatures for each cluster in cigarette smokers versus nonsmokers, as well as e-cigarette users versus nonsmokers. This analysis identified that Consensus Cluster A was significantly modified (Padj < 0.05) in e-cigarette users and nonsmokers in NLF samples. Consensus Cluster A was more highly expressed in e-cigarette users than nonsmokers. In addition, Consensus Cluster B was also modified in e-cigarette users and nonsmokers in NELF samples with Cluster B being more expressed in e-cigarette users than nonsmokers (Table 4). The general elevation in select cytokine cluster expression within e-cigarette users was also demonstrated through a comparison between tobacco use groups, where e-cigarette users showed significantly increased expression of cytokines within Consensus Cluster A in NLF samples and Consensus Cluster B in sputum samples in comparison to smokers (Table 4). Visualizations of the individual cytokine expression levels versus cluster-level eigencytokine values further demonstrated that minimal changes occurred at the individual cytokine level (Fig. 6A), whereas significant changes occurred at the cytokine cluster level associated with tobacco use, particularly in e-cigarette users compared with nonsmokers (Fig. 6B).
Table 4.
Baseline cytokine Consensus Cluster assignments and associated modulations in cigarette smokers and e-cigarette users
| Compartment | Cytokines Within Each Cluster | Nonsmokers, n = 14 vs. Cigarette Smokers, n = 13 Cluster Comparison |
Nonsmokers, n = 14 vs. E-Cigarette User, n = 17 Cluster Comparison |
Cigarette Smokers, n = 13 vs. E-Cigarette Users, n = 17 Cluster Comparison |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P Value | P adj | Eigencytokine Change in Smokers | P Value | P adj | Eigencytokine Change in E-Cigarette Users | P Value | P adj | Eigencytokine Change in E-Cigarette Users | ||
| Cluster A | ||||||||||
| NLF | IFNg, IL10, IL12p70, IL17D, IL4, TNFa | 7.93E-01 | 7.93E-01 | NA | 7.54E-09 | 2.26E-08 | Increased | 1.67E-08 | 5.01E-08 | Increased |
| NELF | 5.83E-01 | 9.43E-01 | NA | 1.61E-01 | 3.77E-01 | NA | 1.13E-01 | 2.63E-01 | NA | |
| Sputum | 9.43E-01 | 9.43E-01 | NA | 7.99E-01 | 7.99E-01 | NA | 5.63E-01 | 5.63E-01 | NA | |
| Cluster B | ||||||||||
| NLF | Eotaxin, Eotaxin3, IL17B, IL1b, ITAC, MIP1a, TARC | 2.39E-01 | 3.58E-01 | NA | 3.56E-01 | 5.34E-01 | NA | 8.37E-01 | 8.37E-01 | NA |
| NELF | 9.43E-01 | 9.43E-01 | NA | 3.77E-01 | 3.77E-01 | Increased | 2.63E-01 | 2.63E-01 | NA | |
| Sputum | 3.50E-01 | 5.25E-01 | NA | 1.47E-02 | 4.42E-02 | NA | 1.04E-03 | 3.12E-03 | Increased | |
| Cluster C | ||||||||||
| NLF | Fractalkine, IL8, IP10, MIG | 1.85E-01 | 3.58E-01 | NA | 5.97E-01 | 5.97E-01 | NA | 4.83E-01 | 7.24E-01 | NA |
| NELF | 7.56E-01 | 9.43E-01 | NA | 2.62E-01 | 3.77E-01 | NA | 2.13E-01 | 2.63E-01 | NA | |
| Sputum | 2.80E-01 | 5.25E-01 | NA | 1.73E-01 | 2.60E-01 | NA | 3.89E-02 | 5.84E-02 | NA | |
Cytokines corresponding to each consensus cluster are detailed, as well as statistical results indicating instances of significant modulation associated with tobacco use at the cluster-level. NA, not applicable; NELF, nasal epithelial lining fluid; NLF, nasal lavage fluid.
Figure 6.

Individual cytokine expression levels (A) and eigencytokine values (B) describing cluster-level trends for the derived Consensus Clusters across all subjects. Subjects were ordered first according to tobacco use status, starting with nonsmokers (NS) then cigarette smokers (CS) and e-cigarette users (Ecig). Within tobacco use groups, subjects are ordered from lowest to highest average cytokine concentration (or eigenvector value) from left to right. Within each cluster, cytokines are ordered from lowest to highest average cytokine concentration from bottom to top. This figure depicts minimal changes occurring at the individual cytokine level (A) and much more robust changes occurring at the cytokine cluster level associated with tobacco use (B), particularly in e-cigarette users compared with nonsmokers.
Biological Implications of Identified Consensus Clusters
Pathway enrichment analysis of these clusters showed that cytokines in Consensus Cluster A were enriched for processes relevant to host defense, including Role of Pattern Recognition Receptors in Recognition of Bacteria and Viruses (P = 7.9 × 10−14; Table 5). Cytokines in Consensus Cluster B were enriched for processes relevant to chemotactic immune cell recruitment, including IL17 signaling (P = 2.5 × 10−11). Cytokines in Consensus Cluster C were enriched for processes relevant to immune cell adhesion and inflammation, including granulocyte adhesion and diapedesis (P = 3.2 × 10−9), agranulocyte adhesion and diapedesis (P = 5.0 × 10−9), and neuroinflammation signaling pathway (P = 8.9 × 10−6).
Table 5.
Biological pathways that are associated with the Consensus Clusters identified throughout the respiratory samples (Consensus Clusters A–C)
| Pathway | P Value |
|---|---|
| (A) Consensus Cluster A | |
| Role of pattern recognition receptors in recognition of bacteria and viruses | 7.9E-14 |
| Role of cytokines in mediating communication between immune cells | 4.0E-13 |
| T helper cell differentiation | 1.6E-12 |
| Systemic lupus erythematosus in B-cell signaling pathway | 3.2E-12 |
| Altered T-cell and B-cell signaling in rheumatoid arthritis | 5.0E-12 |
| (B) Consensus Cluster B | |
| Granulocyte adhesion and diapedesis | 1.6E-11 |
| IL17 signaling | 2.5E-11 |
| Agranulocyte adhesion and diapedesis | 2.5E-11 |
| Differential regulation of cytokine production in macrophages and T helper cells by IL17A and IL17F | 3.2E-11 |
| Role of hypercytokinemia/hyperchemokinemia in the pathogenesis of influenza | 2.3E-08 |
| (C) Consensus Cluster C | |
| Granulocyte adhesion and diapedesis | 3.2E-09 |
| Agranulocyte adhesion and diapedesis | 5.0E-09 |
| Pathogenesis of multiple sclerosis | 8.3E-07 |
| IL17A signaling in gastric cells | 7.4E-06 |
| Neuroinflammation signaling pathway | 8.9E-06 |
The top five most significantly associated canonical pathways associated with each cluster are listed.
Individual Cytokine Alterations Associated with Tobacco Product Use
As a point of comparison for the cluster-derived statistical analyses, we also carried out cytokine profile comparisons between tobacco product user groups at the individual cytokine level. A Wilcoxon rank-sum test approach was first employed comparing each individual cytokine between nonsmokers versus cigarette smokers and nonsmokers versus e-cigarette users, paralleling the two-group comparison implemented in the cluster analysis. IL6 levels in sputum were the only changes to show significance, identified when comparing nonsmokers versus cigarette smokers (Supplemental Table S3). An additionally statistical approach was also carried out to adjust for covariates, focusing on the potential confounder that demonstrated the greatest change (though not significant) across smoking groups, namely, age. Although data were not normally distributed, ANOVA models were included here as additional approaches to the aforementioned nonparametric approaches, that could also incorporate covariates. Here, crude and adjusted ANOVA tests were carried out, which found that no cytokines displayed significant differences in levels associated with smoking status, in any compartment (Supplemental Table S4).
To further test whether individual cytokines showed significant changes associated with tobacco product use, additional analyses were carried out by stratifying the participants according to important demographics. Thus, we were able to evaluate whether changes may be apparent in select subgroups, specifically, in groups separated according to sex, race, age (<30 and >30 yr), and BMI (<25 and ≥25). Across all of these stratified analyses, only four significant findings resulted from these comparisons. These included IL6 changes in sputum samples of cigarette smokers that were female (vs. nonsmokers that were female); IL6 changes in sputum of cigarette smokers that were younger (< 30 yr old; vs. nonsmokers that were younger); and MIP1α and IP10 changes in NLF samples of e-cigarette users that were male (vs. nonsmokers that were male; Supplemental Table S5). Notably, this is similar to a prior study where Fractalkine and its receptor, CX3CR1, were found to have more muted responses in e-cigarette users along with MIG and IP10 in the nasal mucosa (3). Overall, there were still more changes that reached statistical significance through the eigencytokine-based approach in comparison to all of these individual cytokine-based analyses. These results collectively demonstrate that analyzing cytokines at the cluster level (as opposed to individual level) may lead to increased sensitivity and ability to detect pattern-level biological changes.
Separate Validation Study Cohort Results
Overall demographic characteristics were summarized for the 48 participants in the separate validation cohort (Table 2). Of these subjects, about half were male (50%), most identified as White (69%), and a majority identified as non-Hispanic (94%). The cohort had an average age of 28 (range of 19–43) yr, along with an average BMI of 27.1 (18.1–45.9). Demographic characteristics remained largely consistent across each group of nonsmokers, cigarette smokers, and e-cigarette users with the exception of age. Serum cotinine levels had an average of 164.8 (range of 0–442.1) pg/mL across all subjects and 0.7 (0–14.7) pg/mL, 209.5 (4.3–442.1) pg/mL, 117.3 (3.52–413.4) pg/mL for nonsmokers, cigarette smokers, and e-cigarette users, respectively. Serum cotinine was also significantly different across smoking statuses, as expected.
Analyzing NELF cytokine patterns in this additional data set resulted in the identification of three clusters that greatly paralleled the primary study findings (Fig. 7). The NELF eigenvector in the validation data set captured 85.3% of the variance in cytokine levels. Eigenvector weights for both the original and validation datasets were calculated (Supplemental Table S6). Despite the slightly reduced number of cytokines within the validation cohort, the NELF compartment clustered similarly and demonstrated similar statistical relationships with tobacco product use when comparing between the primary and validation datasets (Fig. 7 and Supplemental Table S7). These results provide evidence that the cluster-based findings in noninvasive samples, such as NELF, were reproducible, supporting future efforts to further validate their ability to predict cytokine changes in other compartments.
Figure 7.

Cytokine principal component plots showing clusters at baseline (in nonsmokers) in NELF samples from the primary cohort (left) and separate validation cohort (right). Cytokine concentration distributions were transformed using data reduction approaches (i.e., PCA) to illustrate the overall trends in cytokine groupings in 2-D. The illustrated principal components show the resulting reduced values, which captured the majority of variance in data across NELF samples. Cytokine clusters are shown as shaded shapes within these PCA plots. NELF, nasal epithelial lining fluid; PCA, principal component analysis; 2-D, two dimensional.
DISCUSSION
This study set out to evaluate cytokines signatures in multiple compartments throughout the body across varying tobacco use groups. We hypothesized that cytokine signatures derived from upper and lower regions of the airway (i.e., NLF, NELF, and sputum samples) would demonstrate greater similarity in comparison to serum samples and that groups of cytokines would be modulated in association with tobacco product use. There were three main findings from this analysis. First, analyses demonstrated high levels of correlation between cytokine signatures in each sampled region of the airway, with serum cytokine profiles demonstrating a less robust level of correlation that may have occurred by random chance. Second, groups of coexpressed cytokines identified through k-means clustering contained largely consistent lists of cytokines across the respiratory compartments, resulting in three respiratory Consensus Clusters involved in host defense, chemotactic immune cell recruitment, and immune cell adhesion and inflammation. These clusters were present in nonsmokers and also showed modifications in association with tobacco product use through eigenvector analyses, with the greatest modulations in e-cigarette users in comparison to cigarette smokers and nonsmokers. Third, these immune mediator responses were not apparent when analyzing data on an individual cytokine basis, suggesting that clustering-based approaches highlight important system-level findings of biological interactions and immune system responses associated with tobacco product use in smaller cohorts. Together, these findings suggest that a clustering approach increases statistical power to uncover cytokine differences associated with tobacco product use and that cytokine profiles identified in noninvasive nasal samples are representative of lower airway cytokine milieus.
This study analyzed cytokine signatures across compartments within nonsmokers, cigarette smokers, and e-cigarette users and found that in all groups, there were highly significant correlations between respiratory-derived signatures (i.e., in NLF, NELF, and sputum). These findings are of high importance, as they support the utility of noninvasive sample collection from the upper respiratory tract as accurate surrogates for evaluating responses within the lower respiratory tract, particularly when evaluating immune mediators. A similar approach is already being used by others, such as evaluating the potential for respiratory cancers using nasal samples (4, 32); therefore, this work is also a logical extension of utilizing upper respiratory biomarkers to predict outcomes in the lower airway. Although a sampling approach for lower airway disease using bronchoalveolar lavage (BAL) would be more direct (33), our work does support the utility and feasibility of the use of nasal biomarkers, as upper respiratory tract samples require less invasive methods in comparison to lower respiratory tract samples (4, 32). Analysis of serum samples collected in these same volunteers showed that serum cytokine profiles had a less robust level of correlation that, after including randomly permuted signatures, was found to overlap with correlation metrics sometimes occurring by chance. This finding is of high interest given the common use of serum samples in clinical and toxicological studies as potentially informing respiratory-relevant biology (34–37). Therefore, future clinical and toxicological studies that aim to evaluate respiratory-relevant outcomes should consider these potential differences that serum cytokine levels may be less representative of changes occurring in the respiratory tract than actual respiratory samples.
A cluster-based approach was used here to identify cytokines that exhibit comodulation patterns collectively across study participants. This method of data-driven clustering of cytokine profiles using unsupervised approaches to identify functional groupings of cosignaling cytokines has been used before to uncover cytokine modules associated with different respiratory diseases (38, 39). Clusters derived at baseline (in nonsmokers) were grouped similarly across regions of the respiratory tract and were modified in association with tobacco product use via eigenvector analyses, with the greatest changes occurring in e-cigarette users. The cytokines that were assigned similarly across respiratory tract samples were designated as Consensus Clusters, and these were found to be enriched for processes relevant to host defense (Consensus Cluster A), chemotactic immune cell recruitment (Consensus Cluster B), and immune cell adhesion and inflammation (Consensus Cluster C). In particular, e-cigarette users showed significantly increased expression of cytokines within Consensus Cluster A in NLF samples and Consensus Cluster B in sputum samples in comparison to smokers and nonsmokers, indicating potentially increased effects of e-cigarette use on cluster-specific cytokines compared with the other groups. These findings parallel those that have been previously reported by our research team. For instance, we have shown that nasal samples from e-cigarette users have suppressed expression of immune and inflammatory-response genes, including IL4, IL10, IL17B, and Fractalkine. Although these cytokines fall into different consensus clusters in our study, they were all implicated in mediating cytokine-cytokine receptor interactions (2). This suppression of host defense was also demonstrated at the functional-level, in which the nasal mucosa of e-cigarette users was found to have decreased immune response to live-attenuated influenza virus (3). Other studies have additionally found that humans exhibit increased risk of viral infection in association with tobacco product use (40, 41). Hence, cytokine consensus clustering can be used to uncover similar molecular phenotypes associated with toxicant exposures across different respiratory samples.
Cytokine alterations potentially associated with tobacco product use were also evaluated using more traditional approaches based on individual cytokine statistical comparisons. Despite the employment of several models (crude, adjusted, and stratified), there was an overall lack of significant findings, suggesting that clustering-based methods may extract more biologically relevant information from studies with smaller numbers of study participants and high-dimensional data. Findings were bolstered by the inclusion of the validation data set, which provided evidence of similar clustering patterns within the NELF compartment. There are many examples of previous studies that have evaluated changes in cytokine levels within clinical, epidemiological, and toxicological research (1–3, 42–48). However, these studies have primarily identified transcription-level changes between e-cigarette users and nonsmokers in airway samples and only shown correlation with protein production, not individual immune mediator differences (49). Thus, our lack of significance in individual cytokine differences between e-cigarette user and nonsmoker groups in airway samples is similar to prior studies (2, 3). In contrast, animal studies of e-cigarette exposures and ex vivo studies of human airway or systemic immune cells exposed to e-cigarettes have identified alterations of immune mediator production with exposure (49). Our findings do differ from past research comparing cigarette smokers and nonsmokers that have found significant effects on immune mediator protein production in the airway (50). This may be attributable to a variety of factors, including our relatively small n, moderate use rate of cigarettes compared with other studies, and the age of our cohort.
Another difference between our study and prior work is that in prior studies, there is an overall lack of integration of machine learning (e.g., k-means clustering and other modeling approaches) and aggregate cluster-level techniques when evaluating cytokine signatures in the clinical/toxicological setting. Similarly, previous studies have concluded that consensus clustering approaches for cytokine profiles not only increase statistical power to detect differences associated with clinical phenotypes but also provide the basis for additional mechanistic studies identifying the causality of exposure-related changes in cytokine clusters (38). For example, e-cigarette-induced increases in Consensus Clusters A and B warrant additional studies into the causality for these exposure-related groupings of cytokines. This study supports using methods to determine differences in collective responses and/or across biological pathways as opposed to using individual cytokines as proxies for immune system response, especially when dealing with small sample size where sensitivity may be gained by more system-level approaches.
This study provides novel insight on the comparison of individual and clustered cytokine profiles across tobacco product use groups and body compartments, although it is not without limitations. Studies carried out in human volunteers are inherently subject to the inclusion of potential covariates that could influence results. For example, the tobacco products used, flavorings or additives included in each product, individual subject pack-years or use history, how the tobacco product was used (puff topography, frequency, alteration of devices, etc.), and socioeconomic status are known factors to influence smoking-related outcomes and were not variables that were collected in our study (14, 51, 52); however, the demographic variables that were collected did not exhibit significant differences in distributions across subgroups. In addition, bronchoalveolar lavage fluid (BALF) is another common sample used to derive cytokine concentrations and inform immune responses in the lower respiratory tract but was not included in this study (53). Inclusion of BALF, a much more invasive sampling technique, in a cross-compartmental analysis could further assess the consistency immune system responses across the entire respiratory tract. This study also included 44 individuals in the primary cohort and 48 in the separate validation cohort for cytokine data collected in NELF samples. Although both of these are relatively small cohorts, inherently influencing the statistical power, this study lends itself to future analyses validating the use of NELF samples as a surrogate for lower airway cytokine assessment. Using noninvasive NELF sampling could provide a method to elucidate understudied lower airway responses to tobacco product use in humans and better account for potential confounders known to have impacts on respiratory inflammation.
Conclusions
This study was one of the first to leverage an unsupervised machine learning approach to characterize potential cytokine patterns across tobacco product use and clinical samples. Our results provide evidence of a high degree of correlation among the upper and lower airway and less robust correlation between sampled respiratory compartments and serum samples. This is a novel and important prospective identification of correlations between immune mediators in paired upper and lower airways samples, indicating utility of nasal mediators as biomarkers of lower airway effects. A k-means clustering analysis revealed largely consistent cytokine groups, or “Consensus Clusters,” among sampled respiratory compartments. These clusters were associated with host defense, chemotactic immune cell recruitment, and immune cell adhesion and inflammation, suggesting that cytokine clusters identified in noninvasive nasal samples are representative of lower airway cytokine milieus. Lastly, the cluster analysis displayed a more robust differential profile between tobacco product users and nonsmokers than individual cytokines. Like previous studies (38, 39), our findings suggest that utilization of a clustering approach could potentially be more representative of biological interactions and immune system response than comparisons of individual values. Thus, together with the minimal risk associated with the collection of nasal samples, a clustering approach provides a broadly deployable system-level assessment of toxicological changes resulting from cigarette smoke or e-cigarette exposures.
DATA AVAILABILITY
The data Analytical Code in R is available in GitHub at https://github.com/Ragerlab.
SUPPLEMENTAL DATA
Raw data, metadata, Supplemental Fig. S1, and Supplemental Tables S1–S7: https://doi.org/10.15139/S3/LJH0G4.
GRANTS
This work was supported by the National Institutes of Health (NIH) Grants T32 ES007126, P50 HL120100, and P30ES010126. This work was funded by NIH Grant P50 HL120100. Research reported in this publication was in part supported by NIH and US Food and Drug Administration Center for Tobacco Products (CTP). Support was also provided through the University of North Carolina (UNC) Institute for Environmental Health Solutions (IEHS).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Food and Drug Administration.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.J. and M.E.R. conceived and designed research; A.N.P., J.R.H., H.W., C.R., N.E.A., and M.E.R. performed experiments; A.D.P., A.N.P., J.R.H., V.A., and J.E.R. analyzed data; A.D.P., M.E.R., A.N.P., I.J., and J.E.R. interpreted results of experiments; A.D.P., A.N.P., V.A., J.E.R., and M.E.R. prepared figures; A.D.P., A.N.P., I.J., J.E.R., and M.E.R. drafted manuscript; A.D.P., A.N.P., J.R.H., V.A., H.W., C.R., N.E.A., I.J., J.E.R., and M.E.R. edited and revised manuscript; A.D.P., A.N.P., J.R.H., V.A., H.W., C.R., N.E.A., I.J., J.E.R., and M.E.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the participants involved in this study for time and effort.
REFERENCES
- 1.Clapp PW, Jaspers I. Electronic cigarettes: their constituents and potential links to asthma. Curr Allergy Asthma Rep 17: 79, 2017. doi: 10.1007/s11882-017-0747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin EM, Clapp PW, Rebuli ME, Pawlak EA, Glista-Baker E, Benowitz NL, Fry RC, Jaspers I. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol 311: L135–L144, 2016. doi: 10.1152/ajplung.00170.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rebuli ME, Glista-Baker E, Hoffman JR, Duffney PF, Robinette C, Speen AM, Pawlak EA, Dhingra R, Noah TL, Jaspers I. Electronic-cigarette use alters nasal mucosal immune response to live-attenuated influenza virus. A clinical trial. Am J Respir Cell Mol Biol 64: 126–137, 2021. doi: 10.1165/rcmb.2020-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rebuli ME, Speen AM, Clapp PW, Jaspers I. Novel applications for a noninvasive sampling method of the nasal mucosa. Am J Physiol Lung Cell Mol Physiol 312: L288–L296, 2017. doi: 10.1152/ajplung.00476.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexis NE, Hu SC, Zeman K, Alter T, Bennett WD. Induced sputum derives from the central airways: confirmation using a radiolabeled aerosol bolus delivery technique. Am J Respir Crit Care Med 164: 1964–1970, 2001. doi: 10.1164/ajrccm.164.10.2104051. [DOI] [PubMed] [Google Scholar]
- 6.Bhowmik A, Seemungal TAR, Sapsford RJ, Devalia JL, Wedzicha JA. Comparison of spontaneous and induced sputum for investigation of airway inflammation in chronic obstructive pulmonary disease. Thorax 53: 953–956, 1998. doi: 10.1136/thx.53.11.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paats MS, Bergen IM, Bakker M, Hoek RAS, Nietzman-Lammering KJ, Hoogsteden HC, Hendriks RW, van der Eerden MM. Cytokines in nasal lavages and plasma and their correlation with clinical parameters in cystic fibrosis. J Cyst Fibros 12: 623–629, 2013. doi: 10.1016/j.jcf.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Saris A, Reijnders TDY, Nossent EJ, Schuurman AR, Verhoeff J, Asten S. V, Bontkes H, Blok S, Duitman J, Bogaard H-J, Heunks L, Lutter R, van der Poll T, Garcia Vallejo JJ; ArtDECO consortium and the Amsterdam UMC COVID study group. Distinct cellular immune profiles in the airways and blood of critically ill patients with COVID-19. Thorax 76: 1010–1019, 2021. doi: 10.1136/thoraxjnl-2020-216256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wills TA, Soneji SS, Choi K, Jaspers I, Tam EK. E-cigarette use and respiratory disorders: an integrative review of converging evidence from epidemiological and laboratory studies. Eur Respir J 57: 1901815, 2021. doi: 10.1183/13993003.01815-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer CMT, Dewitte-Orr SJ, Hornby KR, Zavitz CCJ, Lichty BD, Stämpfli MR, Mossman KL. Cigarette smoke suppresses type I interferon-mediated antiviral immunity in lung fibroblast and epithelial cells. J Interferon Cytokine Res 28: 167–179, 2008. doi: 10.1089/jir.2007.0054. [DOI] [PubMed] [Google Scholar]
- 11.Gualano RC, Hansen MJ, Vlahos R, Jones JE, Park-Jones RA, Deliyannis G, Turner SJ, Duca KA, Anderson GP. Cigarette smoke worsens lung inflammation and impairs resolution of influenza infection in mice. Respir Res 9: 53, 2008. doi: 10.1186/1465-9921-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ween MP, Whittall JJ, Hamon R, Reynolds PN, Hodge SJ. Phagocytosis and Inflammation: Exploring the effects of the components of E-cigarette vapor on macrophages. Physiol Rep 5: e13370, 2017. doi: 10.14814/phy2.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott A, Lugg ST, Aldridge K, Lewis KE, Bowden A, Mahida RY, Grudzinska FS, Dosanjh D, Parekh D, Foronjy R, Sapey E, Naidu B, Thickett DR. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax 73: 1161–1169, 2018. doi: 10.1136/thoraxjnl-2018-211663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Zyl-Smit RN, Binder A, Meldau R, Semple PL, Evans A, Smith P, Bateman ED, Dheda K. Cigarette smoke impairs cytokine responses and BCG containment in alveolar macrophages. Thorax 69: 363–370, 2014. doi: 10.1136/thoraxjnl-2013-204229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stabile AM, Marinucci L, Balloni S, Giuliani A, Pistilli A, Bodo M, Rende M. Long term effects of cigarette smoke extract or nicotine on nerve growth factor and its receptors in a bronchial epithelial cell line. Toxicol In Vitro 53: 29–36, 2018. doi: 10.1016/j.tiv.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Noah TL, Zhou H, Monaco J, Horvath K, Herbst M, Jaspers I. Tobacco smoke exposure and altered nasal responses to live attenuated influenza virus. Environ Health Perspect 119: 78–83, 2011. doi: 10.1289/ehp.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CRAN. What is R? Introduction to R (Online). https://www.r-project.org/about.html [2020 Nov 1].
- 18.Rebuli ME, Speen AM, Martin EM, Addo KA, Pawlak EA, Glista-Baker E, Robinette C, Zhou H, Noah TL, Jaspers I. Wood smoke exposure alters human inflammatory responses to viral infection in a sex-specific manner. A randomized, placebo-controlled study. Am J Respir Crit Care Med 199: 996–1007, 2019. doi: 10.1164/rccm.201807-1287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexis N, Lay J, Zeman K, Bennett W, Peden D, Soukup J, Devlin R, Becker S. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Allergy Clin Immunol 117: 1396–1403, 2006. doi: 10.1016/j.jaci.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Alexis NE, Eldridge MW, Peden DB. Effect of inhaled endotoxin on airway and circulating inflammatory cell phagocytosis and CD11b expression in atopic asthmatic subjects. J Allergy Clin Immunol 112: 353–361, 2003. doi: 10.1067/mai.2003.1651. [DOI] [PubMed] [Google Scholar]
- 21.Wickham H. ggplot2: Elegant Graphics for Data Analysis (Online). https://ggplot2.tidyverse.org [2020 Oct 8].
- 22.RDocumentation. shapiro.test: Shapiro-Wilk Normality Test (Online). https://www.rdocumentation.org/packages/stats/versions/3.6.2/topics/shapiro.test [2020 Nov 27].
- 23.RDocumentation. sample: Random Samples and Permutations (Online). https://www.rdocumentation.org/packages/base/versions/3.6.2/topics/sample [2020 Nov 10].
- 24.Kanungo T, Mount DM, Netanyahu NS, Piatko CD, Silverman R, Wu AY. An efficient k-means clustering algorithm: analysis and implementation. IEEE Trans Pattern Anal Machine Intell 24: 881–892, 2002. doi: 10.1109/TPAMI.2002.1017616. [DOI] [Google Scholar]
- 25.Hartigan JA, Wong MA. Algorithm AS 136: a K-means clustering algorithm. J R Stat Soc Ser C (Appl Stat) 28: 100–108, 1979. doi: 10.2307/2346830. [DOI] [Google Scholar]
- 26.RDocumentation. factoextra: Extract and Visualize the Results of Multivariate Data Analyses (Online). https://www.rdocumentation.org/packages/factoextra/versions/1.0.3 [2020 Jul 1].
- 27.RDocumentation. rgl: 3D Visualization Using OpenGL (Online). https://www.rdocumentation.org/packages/rgl/versions/0.100.54. [2020 Jul 1]
- 28.Scott Chialvo CH, Che R, Reif D, Motsinger-Reif A, Reed LK. Eigenvector metabolite analysis reveals dietary effects on the association among metabolite correlation patterns, gene expression, and phenotypes. Metabolomics 12: 167, 2016. doi: 10.1007/s11306-016-1117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Storey JBA, Dabney A, Robinson D. qvalue: Q-Value Estimation for False Discovery Rate Control. R package version 2.22.0 (Online). https://www.bioconductor.org/packages/release/bioc/html/qvalue.html [2020 Jan 1].
- 30.QIAGEN. Understand Complex ‘Omics Data To Accelerate Your Research (Online). https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis/ [2019 Dec 1].
- 31.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57: 289–300, 1995. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 32.Hentschel J, Müller U, Doht F, Fischer N, Böer K, Sonnemann J, Hipler C, Hünniger K, Kurzai O, Markert UR, Mainz JG. Influences of nasal lavage collection-, processing- and storage methods on inflammatory markers–evaluation of a method for non-invasive sampling of epithelial lining fluid in cystic fibrosis and other respiratory diseases. J Immunol Methods 404: 41–51, 2014. doi: 10.1016/j.jim.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Davidson KR, Ha DM, Schwarz MI, Chan ED. Bronchoalveolar lavage as a diagnostic procedure: a review of known cellular and molecular findings in various lung diseases. J Thorac Dis 12: 4991–5019, 2020. doi: 10.21037/jtd-20-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain KK (Editor). Biomarkers of pulmonary diseases. In: The Handbook of Biomarkers. New York, NY: Humana Press, 2017, p. 673–688. [Google Scholar]
- 35.Bhargava M, Wendt CH. Biomarkers in acute lung injury. Transl Res 159: 205–217, 2012. doi: 10.1016/j.trsl.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blanco-Pérez JJ, Blanco-Dorado S, Rodríguez-García J, Gonzalez-Bello ME, Salgado-Barreira Á, Caldera-Díaz AC, Pallarés-Sanmartín A, Fernandez-Villar A, González-Barcala FJ. Serum levels of inflammatory mediators as prognostic biomarker in silica exposed workers. Sci Rep 11: 13348, 2021. doi: 10.1038/s41598-021-92587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z, Han S, Zhang J, Zheng P, Liu X, Zhang Y, Jia G. Metabolomics screening of serum biomarkers for occupational exposure of titanium dioxide nanoparticles. Nanotoxicology 15: 832–849, 2021. doi: 10.1080/17435390.2021.1921872. [DOI] [PubMed] [Google Scholar]
- 38.Cohen L, Fiore-Gartland A, Randolph AG, Panoskaltsis-Mortari A, Wong S-S, Ralston J, Wood T, Seeds R, Huang QS, Webby RJ, Thomas PG, Hertz T. A modular cytokine analysis method reveals novel associations with clinical phenotypes and identifies sets of co-signaling cytokines across influenza natural infection cohorts and healthy controls. Front Immunol 10: 1338, 2019. doi: 10.3389/fimmu.2019.01338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seys SF, Scheers H, Van den Brande P, Marijsse G, Dilissen E, Van Den Bergh A, Goeminne PC, Hellings PW, Ceuppens JL, Dupont LJ, Bullens DMA. Cluster analysis of sputum cytokine-high profiles reveals diversity in T(h)2-high asthma patients. Respir Res 18: 39, 2017. doi: 10.1186/s12931-017-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corriden R, Moshensky A, Bojanowski CM, Meier A, Chien J, Nelson RK, Crotty Alexander LE. E-cigarette use increases susceptibility to bacterial infection by impairment of human neutrophil chemotaxis, phagocytosis, and NET formation. Am J Physiol Cell Physiol 318: C205–C214, 2020. doi: 10.1152/ajpcell.00045.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaiha SM, Cheng J, Halpern-Felsher B. Association between youth smoking, electronic cigarette use, and COVID-19. J Adolesc Health 67: 519–523, 2020. doi: 10.1016/j.jadohealth.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fry RC, Rager JE, Zhou H, Zou B, Brickey JW, Ting J, Lay JC, Peden DB, Alexis NE. Individuals with increased inflammatory response to ozone demonstrate muted signaling of immune cell trafficking pathways. Respir Res 13: 89, 2012. doi: 10.1186/1465-9921-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bauer RN, Brighton LE, Mueller L, Xiang Z, Rager JE, Fry RC, Peden DB, Jaspers I. Influenza enhances caspase-1 in bronchial epithelial cells from asthmatic volunteers and is associated with pathogenesis. J Allergy Clin Immunol 130: 958–967.e14, 2012. doi: 10.1016/j.jaci.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rager JE, Bailey KA, Smeester L, Miller SK, Parker JS, Laine JE, Drobná Z, Currier J, Douillet C, Olshan AF, Rubio-Andrade M, Stýblo M, García-Vargas G, Fry RC. Prenatal arsenic exposure and the epigenome: altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood. Environ Mol Mutagen 55: 196–208, 2014. doi: 10.1002/em.21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bailey KA, Laine J, Rager JE, Sebastian E, Olshan A, Smeester L, Drobná Z, Stýblo M, Rubio-Andrade M, García-Vargas G, Fry RC. Prenatal arsenic exposure and shifts in the newborn proteome: interindividual differences in tumor necrosis factor (TNF)-responsive signaling. Toxicol Sci 139: 328–337, 2014. doi: 10.1093/toxsci/kfu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cook KD, Shpargel KB, Starmer J, Whitfield-Larry F, Conley B, Allard DE, Rager JE, Fry RC, Davenport ML, Magnuson T, Whitmire JK, Su MA. T follicular helper cell-dependent clearance of a persistent virus infection requires T cell expression of the histone demethylase UTX. Immunity 43: 703–714, 2015. doi: 10.1016/j.immuni.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rager JE, Yosim A, Fry RC. Prenatal exposure to arsenic and cadmium impacts infectious disease-related genes within the glucocorticoid receptor signal transduction pathway. Int J Mol Sci 15: 22374–22391, 2014. doi: 10.3390/ijms151222374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rager JE, Smeester L, Jaspers I, Sexton KG, Fry RC. Epigenetic changes induced by air toxics: formaldehyde exposure alters miRNA expression profiles in human lung cells. Environ Health Perspect 119: 494–500, 2011. doi: 10.1289/ehp.1002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalininskiy A, Kittel J, Nacca NE, Misra RS, Croft DP, McGraw MD. E-cigarette exposures, respiratory tract infections, and impaired innate immunity: a narrative review. Pediatric Medicine 4: 5–5, 2021. doi: 10.21037/pm-20-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shields PG, Berman M, Brasky TM, Freudenheim JL, Mathe E, McElroy JP, Song M-A, Wewers MD. A review of pulmonary toxicity of electronic cigarettes in the context of smoking: a focus on inflammation. Cancer Epidemiol Biomarkers Prev 26: 1175–1191, 2017. doi: 10.1158/1055-9965.EPI-17-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amigo H, Ale D, Varela N, Peruga A, Bustos P. Pattern of smoking and socioeconomic status in two cohorts of young adults. Rev Med Chil 146: 168–174, 2018. doi: 10.4067/s0034-98872018000200168. [DOI] [PubMed] [Google Scholar]
- 52.Soulet S, Duquesne M, Toutain J, Pairaud C, Mercury M. Impact of vaping regimens on electronic cigarette efficiency. Int J Environ Res Public Health 16 :4753, 2019. doi: 10.3390/ijerph16234753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noël-Georis I, Bernard A, Falmagne P, Wattiez R. Database of bronchoalveolar lavage fluid proteins. J Chromatogr B Analyt Technol Biomed Life Sci 771: 221–236, 2002. doi: 10.1016/s1570-0232(02)00114-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data, metadata, Supplemental Fig. S1, and Supplemental Tables S1–S7: https://doi.org/10.15139/S3/LJH0G4.
Data Availability Statement
The data Analytical Code in R is available in GitHub at https://github.com/Ragerlab.