Abstract
Immunocompromised patients are at risk of developing toxoplasma encephalitis (TE). Standard therapy regimens (including sulfadiazine plus pyrimethamine) are hampered by severe side effects. While atovaquone has potent in vitro activity against Toxoplasma gondii, it is poorly absorbed after oral administration and shows poor therapeutic efficacy against TE. To overcome the low absorption of atovaquone, we prepared atovaquone nanosuspensions (ANSs) for intravenous (i.v.) administration. At concentrations higher than 1.0 μg/ml, ANS did not exert cytotoxicity and was as effective as free atovaquone (i.e., atovaquone suspended in medium) against T. gondii in freshly isolated peritoneal macrophages. In a new murine model of TE that closely mimics reactivated toxoplasmosis in immunocompromised hosts, using mice with a targeted mutation in the gene encoding the interferon consensus sequence binding protein, i.v.-administered ANS doses of 10.0 mg/kg of body weight protected the animals against development of TE and death. Atovaquone was detectable in the sera, brains, livers, and lungs of mice by high-performance liquid chromatography. Development of TE and mortality in mice treated with 1.0- or 0.1-mg/kg i.v. doses of ANS did not differ from that in mice treated orally with 100 mg of atovaquone/kg. In conclusion, i.v. ANSs may prove to be an effective treatment alternative for patients with TE.
Toxoplasma gondii is an intracellular protozoan parasite of humans and animals with worldwide distribution. Up to 70% of adults are asymptomatically infected with this parasite (26, 32). The acute stage of infection passes by asymptomatically in the majority of cases, whereas the latent stage of infection is characterized by the presence of parasites in cysts in the central nervous system and muscle tissues (32). Immunocompromised hosts, such as patients with AIDS and organ transplant recipients, are at risk of reactivation of the infection by rupture of cysts (32). Toxoplasmic encephalitis (TE) is the most common clinical feature of reactivated disease in AIDS patients (34, 39) and is the most frequent infectious cause of focal intracerebral lesions in these patients (18, 33). If untreated, reactivation of disease leads to the death of the patient. Despite the fact that a variety of approaches have been developed in an effort to find an efficient and well-tolerated therapy regimen, the standard therapy regimen is still hampered by severe adverse effects (26). The standard therapy regimen includes pyrimethamine and sulfadiazine, which cause bone marrow suppression, hematologic toxicity, and/or life-threatening allergic reactions (11, 25, 28, 31, 42). Therefore, in up to 50% of cases, the standard regimen must be replaced by an alternative regimen of less-effective drugs (27).
A variety of new drugs with high in vitro activity against T. gondii and fewer side effects have been developed (2, 4–6, 8, 9). However, to date, insufficient passage through the blood-brain barrier (BBB) and/or insufficient bioavailability of these drugs has limited their in vivo use. The hydroxynaphthoquinone atovaquone is a potent inhibitor of the respiratory chain of parasites (17, 38) and is used for patients with Pneumocystis carinii pneumonia (46). It has potent in vitro activity against both the tachyzoite and cyst forms of T. gondii (2, 24). In a mouse model of acute toxoplasmosis, atovaquone showed excellent activity (2, 41). In addition, it reduced the number of cysts and prolonged the time to death in a model of chronic toxoplasmosis of CBA/Ca mice (3, 15). Atovaquone is a highly lipophilic substance which, when administered orally in tablet form, is absorbed slowly and irregularly. Absorption is improved by the simultaneous intake of food (23, 40). Intravenous (i.v.) injection of an atovaquone solution is not a feasible alternative to oral administration because of the poor solubility of this compound in the solvent mixtures acceptable for i.v. administration.
Improved bioavailability of low-solubility therapeutic agents can be achieved by administering them as nanosuspensions (36, 37). Using high-pressure homogenization, drug crystals of small, highly homogeneous sizes can be produced for i.v. injection. Furthermore, surface modifications allow targeting of such crystals to specific organs (1, 10, 37a). In this regard, the type of surfactant was shown to influence the passage of drugs through the BBB (1, 30).
Oral treatment of acutely infected mice with atovaquone-loaded nanocapsules resulted in prolonged survival compared to that associated with oral treatment of mice with atovaquone suspensions (45). Furthermore, in mice latently infected with T. gondii, oral treatment with atovaquone-loaded nanocapsules reduced cyst numbers (45). However, since mice did not develop TE, the influence of atovaquone nanocapsules on survival and/or time to death could not be investigated (45).
Studies of the efficacy of drugs with activity against T. gondii are commonly performed in murine models of both acute and chronic-progressive infections (3, 7). However, these models do not reflect the course of TE in humans after reactivation. We therefore established a new mouse model that more closely reflects the reactivation of infection in immunocompromised hosts. In analogy to studies by Suzuki et al. (47) using gamma-interferon (IFN-γ)-deficient mice, mice deficient in the interferon consensus sequence binding protein (ICSBP), which lack interleukin-12 (IL-12) p40 production (21, 43), were orally infected with T. gondii and subsequently treated with sulfadiazine. After discontinuation of sulfadiazine, reactivation of latent disease results in development of TE. This new model of reactivation was used to test the therapeutic efficacy of atovaquone nanosuspensions (ANSs) after i.v. injection.
MATERIALS AND METHODS
T. gondii
Tachyzoites of the BK strain, kindly provided by K. Janitschke (Robert-Koch-Institut, Berlin, Germany), were harvested from the peritoneal cavities of C57/BL6 mice infected intraperitoneally (i.p.) 2 to 3 days previously. Parasites were counted by using a hemocytometer and employed in in vitro experiments. Cysts of the ME49 strain of T. gondii were obtained from brains of NMRI mice (obtained from the animal facility of the Institute for Infection Medicine, Benjamin Franklin Medical Center, Berlin, Germany) that had been infected i.p. with 10 cysts 2 to 3 months previously. Mice were sacrificed by asphyxiation with CO2, and their brains were removed and triturated in phosphate-buffered saline (PBS). An aliquot of the brain suspension was used to determine the number of cysts in the preparation.
Mice.
Inbred male ICSBP−/− mice (C57/BL6 background), kindly provided by I. Horak (Free University of Berlin), were bred and maintained under specific-pathogen-free conditions in the animal facility of the Institute for Infection Medicine. Mice were 6 to 8 weeks old when used.
Cells.
Murine peritoneal cells were collected from peritoneal cavities of locally bred female BALB/c mice 5 days after i.p. injection of 0.5 ml of sterile 3% Brewer's thioglycolate (Difco Laboratories, Detroit, Mich.) (44). Donor mice were killed in a CO2 atmosphere, and their cells were harvested by performing peritoneal cavity lavage, using a 20-gauge needle, twice with 5 ml of sterile Dulbecco's PBS (BioWhittaker, Walkersville, Md.) containing 2% heat-inactivated fetal calf serum (Biochrom, Berlin, Germany). Harvested cells were pooled and kept on ice. Cells were counted with a hemocytometer, and viability was determined by trypan blue exclusion (Biochrom). Differential counts were performed on fixed Pappenheim smears. Giemsa and May-Grünwald solutions were obtained from Merck (Darmstadt, Germany). Enumeration of harvested cells revealed means ± standard deviations (SDs) of 75.8% ± 8.2% mononuclear cells and 24.2% ± 8.2% lymphocytes but no neutrophils or other cells. Approximately 4 × 106 to 5 × 106 macrophages were harvested per mouse. Cells were suspended in RPMI 1640 containing 10% fetal calf serum, 2 mM l-glutamine, 100 μg of streptomycin/ml, and 100 U of penicillin G/ml at a density of 106 macrophages per ml and seeded in Chamber slides (Nunc, Roskilde, Denmark) or 96-well flat-bottom plates (Nunc) (200 μl/well). All cell culture reagents were purchased from Biochrom. After 3 h of incubation (37°C, 5% CO2), nonadherent cells were removed by washing the slides or plates three times with culture medium.
Atovaquone.
Atovaquone, 2-[trans-4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthoquinone, was provided by Glaxo-Wellcome (Hamburg, Germany). Atovaquone suspensions for in vitro experiments were prepared by adding atovaquone to culture medium in 1% (wt/vol) dimethyl sulfoxide (Fluka, Deisenhofen, Germany). Atovaquone suspensions for oral administration were prepared by sonicating atovaquone in an aqueous solution of 0.25% (wt/vol) sodium carboxymethyl cellulose (Sigma-Aldrich, Deisenhofen, Germany) and 0.05% (wt/vol) Tween 20 (Sigma-Aldrich).
ANSs.
ANSs were produced by high-pressure homogenization under aseptic conditions. Using an Ultra Turrax T 25 (Janke and Kunkel, Staufen, Germany), atovaquone powder (Glaxo-Wellcome) was dispersed in an aqueous surfactant solution consisting either of 0.3% Tween 80 (ICI Surfactants, Eversberg, Belgium), 0.3% Poloxamer 188 (CH Erbslöh, Düsseldorf, Germany), and 0.05% sodium cholate (Sigma-Aldrich) or of 1.0% Tween 80. The coarse predispersion obtained was homogenized in a Gaulin Micron LAB 40 high-pressure homogenizer (APV Deutschland, Lübeck, Germany) by applying pressures of 1.5 × 107 (2 cycles), 5 × 107 (2 cycles), and 1.5 × 108 (20 cycles) Pa (16). Particles were preserved in thimerosal (Sigma-Aldrich) at a concentration of 0.001% (wt/vol). Iso-osmolarity was achieved by adjustment with glycerol (Sigma-Aldrich) at a concentration of 2.25% (wt/vol).
Particle size and width of distribution (polydispersity index) were determined by photon correlation spectroscopy (Zetasizer IV; Malvern Instruments, Herrenberg, Germany) and laser diffraction (LS 230; Coulter Electronics, Miami, Fla.). The mean diameter ± SD measured by photon correlation spectroscopy was 279 ± 7 nm, and the mean polydispersity index was 0.18 ± 0.01; 99% (vol/vol) of the particles had a diameter of less than 1.741 μm.
In vitro experiments.
Confluent monolayers of macrophages were infected with the BK strain of T. gondii at parasite-to-cell ratios of 1:2 to 8:1. After 1 h of incubation at 37°C, free parasites were rinsed off by washing the monolayers two times with cell culture medium. After 20, 48, and 72 h of infection, cells were stained by the Pappenheim method. Infected cells and the number of parasites per cell were determined microscopically. A parasite-to-cell ratio of 1:2 or 2:1 was chosen for investigations of the activity of ANSs. One hour after infection, ANS or free drug (atovaquone suspension) was added at concentrations of 0.1 to 30.0 μg/ml. Both the number of infected cells and the number of parasites per cell were counted after 20 and 48 h.
The cytotoxicities of ANS and free drug to macrophages were assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (35). Fifty microliters of an MTT solution (Sigma-Aldrich; 2.5 mg of MTT/ml in PBS) was added to cells that had been incubated for 20 h at 37°C in a 5% CO2 incubator, and incubation was continued for 4 h. Absorbance was measured at 550 nm in an automated enzyme-linked immunosorbent assay plate reader (Tecan, Crailsheim, Germany). Percent viability was determined by comparison to untreated cells. All experiments were performed in triplicate and repeated at least twice.
In vivo experiments.
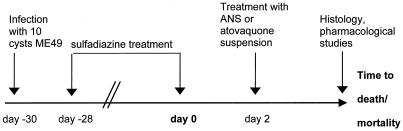
ICSBP−/− mice were orally infected with 10 cysts of the ME49 strain of T. gondii. Mice were treated with sulfadiazine (Sigma-Aldrich) in drinking water (400 mg/liter) for 3 weeks beginning 2 days after infection (47). Two days after discontinuation of sulfadiazine, mice were treated with different concentrations of ANS (0.1, 1.0, and 10.0 mg/kg of body weight) administered as a single i.v. dose every other day. Control mice were treated with diluent only (a solution of Tween 80 at a concentration equivalent to that in a 10.0-mg/kg ANS solution) in the same manner. As a separate treatment control, ICSBP−/− mice were treated with a 100-mg/kg atovaquone suspension orally every other day. At day 8 after discontinuation of sulfadiazine—the time point at which control mice showed symptoms of disease and/or began to succumb—their brains, livers, lungs, and sera were obtained and fixed for histological examination or stored at −40°C for high-performance liquid chromatography (HPLC) analysis.
Histology.
Organs were excised, fixed in a solution containing 10% formalin, 70% ethanol, and 5% acetic acid, and embedded in paraffin. Sagittal sections of brains and cross-sections of livers and lungs were stained with hematoxylin and eosin (H&E) according to standard procedures or by the immunoperoxidase method with rabbit anti-T. gondii immunoglobulin G antibody (12). All reagents used for fixation and H&E staining were obtained from Merck. For staining by the immunoperoxidase method, deparaffinized sections were incubated with swine sera at 1:10 (DAKO, Carpinteria, Calif.) and with the primary antibody, rabbit anti-T. gondii. For production of rabbit anti-T. gondii antibodies, rabbits were orally infected with ME 49 cysts and then treated with sulfadiazine (300 mg/liter), and sera were harvested after a boost with T. gondii (RH strain) 15 days after infection. After being rinsed with modified PBS (12), sections were incubated with swine anti-rabbit immunoglobulin (1:100) (DAKO). As final steps, sections were incubated with rabbit antiperoxidase (1:100) (DAKO) and with diaminobenzidine (DAKO) development solution after being rinsed.
Sections stained with H&E were evaluated for inflammatory changes, and sections stained by the immunoperoxidase method were evaluated for numbers of T. gondii cysts and T. gondii tachyzoites or antigens. A total of six sections of each organ from three mice in each experimental group were evaluated for numbers of inflammatory foci and numbers of T. gondii cysts, tachyzoites, and antigens.
Atovaquone concentrations in tissues and serum.
Weighed tissue samples of mouse organs (50 to 300 mg) were homogenized in 5 ml of an extraction solution, consisting of 2% (vol/vol) isoamyl alcohol and 98% (vol/vol) hexane, in a glass-Teflon homogenizer (19). Serum samples (0.1-ml volumes) were each diluted in 5 ml of extraction solution. After addition of 1 ml of phosphate buffer, samples were rotated for 20 min in a rotating mixer (20). Suspensions were centrifuged for 10 min at 2,800 × g and 10°C. Supernatant (4 ml) was evaporated to dryness in a rotating vacuum centrifuge. The dry residue was redissolved in the mobile phase (a solution consisting of 50% [vol/vol] acetonitrile and 5% [vol/vol] methanol; pH 2.65) (13, 19, 20). The samples were chromatographed on a reversed-phase column (Spherisorb C1; Waters) with a C18 precolumn. The absorbance of the eluate at 253 nm was monitored in a UV detector (model LC 95; Perkin-Elmer, Überlingen, Germany). The linear calibration function was calculated by means of least-squares regression analysis using computer software (SQS 98; Perkin-Elmer). The detection limit of this method was 0.6 mg/liter of serum. The limit of quantitation for tissues was approximately 0.5 mg/kg of tissue. Interassay precision for serum (coefficient of variation) ranged from 7.4 to 15.1%. The level of recovery from spiked serum ranged from 98.1 to 108.1%. Replicate extractions yielded the following extraction rates for the first extraction: 100.0% (serum), 63.6% (brain), 78.1% (liver), and 78.1% (lung).
Statistical analysis.
Fisher's exact test was used to compare survival rates. Differences in numbers of inflammatory foci and parasite foci were analyzed using the Student t test.
RESULTS
Activity of ANS against T. gondii in vitro.
To test atovaquone activity in vitro, we established a cell culture system using freshly isolated murine peritoneal macrophages. Both numbers of infected cells (as a marker for infectivity) and numbers of parasites per cell (as a marker for intracellular replication) were determined. Increasing the parasite-to-cell ratio resulted in increasing numbers of infected cells with increasing incubation time (Table 1). The numbers of parasites per cell increased with increasing incubation time (Table 2).
TABLE 1.
Rates of infection of macrophages after incubation with T. gondiia
| Time postinfection (h) | Rate of infection (% ± SD) at parasite-to-cell ratio of:
|
||
|---|---|---|---|
| 1:2 | 2:1 | 8:1 | |
| 20 | 24 ± 11 | 63 ± 19 | 99 ± 2 |
| 48 | 57 ± 30 | 91 ± 16 | 100 ± 1 |
| 72 | 100 ± 1 | 100 ± 1 | 100 ± 1 |
Cells were infected by the BK strain of T. gondii at the indicated parasite-to-cell ratios for 1 h. After free parasites were rinsed off, macrophages were incubated for 20, 48, and 72 h.
TABLE 2.
Number of parasites/cell following infection of macrophages with T. gondiia
| Time postinfection (h) | No. of parasites/cell at parasite-to-cell ratio of:
|
||
|---|---|---|---|
| 1:2 | 2:1 | 8:1 | |
| 20 | <10 | 10–30 | >30 |
| 48 | >30 | >30 | >30 |
| 72 | >30 | >30 | >30 |
Cells were infected by the BK strain of T. gondii at the indicated parasite-to-cell ratios for 1 h. After free parasites were rinsed off, macrophages were incubated for 20, 48, and 72 h.
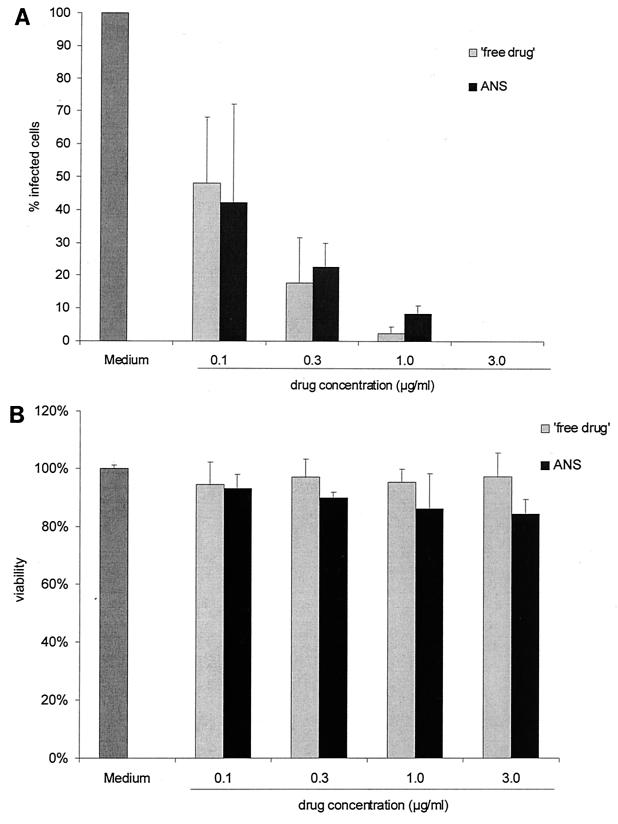
Based on these experiments, the in vitro efficacy of the ANS was tested using parasite-to-cell ratios of 1:2 and 2:1. ANS or free drug (atovaquone drug suspension) was added after 1 h of infection, and the cells were incubated for 20 or 48 h. Figure 1A shows the numbers of macrophages that were infected at a parasite-to-cell ratio of 2:1 and treated with drugs at different concentrations for 48 h. Both ANS and free drug at concentrations of 0.1 μg/ml led to a significant decrease in the number of infected cells (Fig. 1A) and the number of parasites per cell (data not shown). Parasite growth was completely inhibited at concentrations of >1.0 μg/ml of ANS as well as atovaquone free drug. The activities of ANS and free drug did not differ when cells were incubated for only 20 h (data not shown). In addition, concentrations of >0.3 μg of ANS or free drug/ml resulted in complete inhibition of parasite growth when a lower parasite-to-cell ratio (1:2) was used (data not shown). There were no detectable cytotoxic effects on macrophages of either ANS or free drug at effective concentrations of up to 3.0 μg/ml (Fig. 1B). At concentrations of 10 and 30 μg/ml, cell viability decreased to 62.2 and 36.5%, respectively. These results confirm the excellent in vitro activity and low cytotoxicity of atovaquone. The in vitro activities and cytotoxicities of ANS and free drug did not differ.
FIG. 1.
(A) Number of macrophages infected after incubation with T. gondii at a parasite-to-cell ratio of 2:1, rinsing off of free parasites 1 h later, and a 48-h treatment with ANS or free drug at different concentrations. (B) Viability of macrophages after 20 h of incubation with ANS or free drug, determined by MTT assay.
Mortality of ANS-treated mice following reactivation of T. gondii infection.
In analogy to a murine model of reactivated disease in IFN-γ−/− mice, recently reported by Suzuki et al. (47), we established a murine model of reactivation using ICSBP−/− mice. These mice lack IL-12 p40 production, resulting in the impairment of IL-12-dependent IFN-γ production (21, 43).
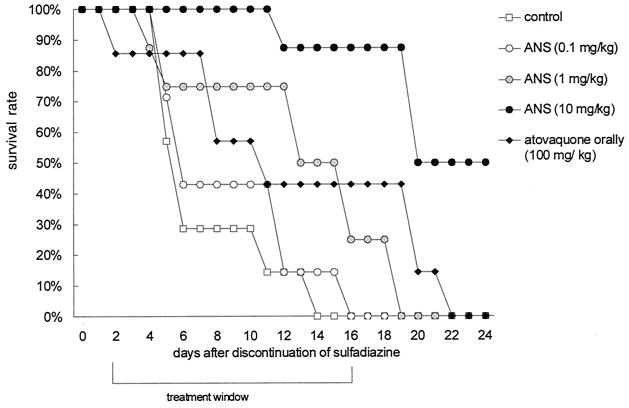
ICSBP−/− mice were orally infected with 10 cysts of T. gondii and treated with sulfadiazine starting at day −28 (Fig. 2). Within a few days of discontinuation of sulfadiazine 28 days later (day zero), mice developed piloerection, huddled, and lost mobility and weight. All mice died within 2 weeks after discontinuation of sulfadiazine (data not shown). Intravenous treatment of T. gondii-infected mice with the ASN at doses of between 0.1 and 10 mg/kg of body weight every other day starting from day 2 until day 16 after discontinuation of sulfadiazine resulted in dose-dependent increases in time to death and/or survival (Fig. 3). Fifty percent of mice treated with the ANS at concentrations of 10 mg/kg of body weight survived until day 24, the end of the study period. In contrast, all control mice treated with Tween 80, the stabilizing surfactant, at concentrations equivalent to those used in ASN dilutions died by day 14 (Fig. 3). Survival rates of mice orally treated with atovaquone suspension (100 mg/kg every other day) were significantly lower than those of mice treated i.v. with ASN at a dose of 10 mg/kg (P < 0.001); all mice treated orally died within 22 days after discontinuation of sulfadiazine, despite administration of a dose 10-fold higher than that administered i.v. (Fig. 3). Survival rates of mice orally treated with 100 mg of atovaquone/kg and mice treated i.v. with 1 mg/kg did not differ significantly. At the end of treatment, on day 16, survival rates in mice treated with 0.1, 1, or 10 mg of ANS/kg compared with controls were 0% (P > 0.999), 25% (P = 0.467), and 88% (P = 0.001), respectively (Fig. 3). By day 24, the rate of survival was 50% in mice treated with 10 mg of ANS/kg and 0% in all other groups.
FIG. 2.
Model of reactivated toxoplasmosis in ICSBP−/− mice.
FIG. 3.
Survival rates of ICSBP−/− mice i.v. treated with surfactant solution (control) (n = 7) or ANS (0.1, 1, or 10 mg/kg of body weight) (n = 8) every other day from day 2 until day 16 after discontinuation of sulfadiazine. One group of mice was treated orally with 100 mg of atovaquone suspension/kg at the same time points (n = 7). Survival rates presented are representative of three independent experiments.
Histological changes in mice with reactivated T. gondii infection.
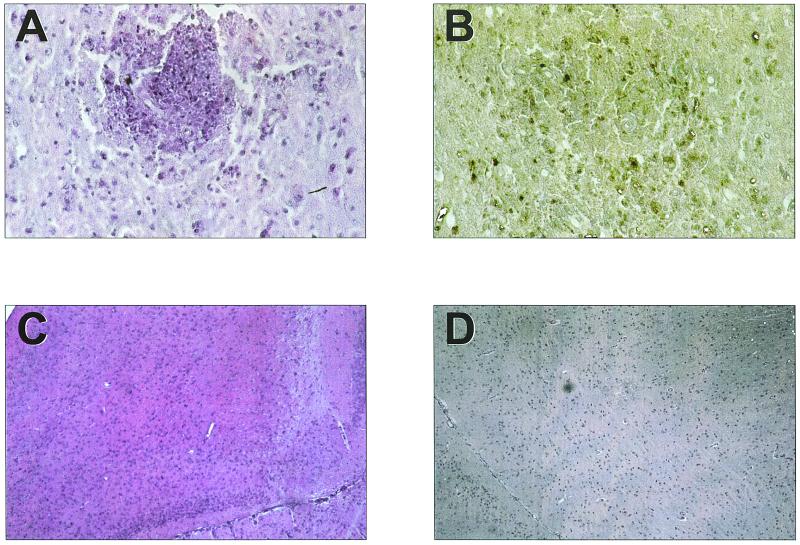
Treatment of infected ICSBP−/− mice with sulfadiazine resulted in latent infections involving the development of brain cysts (275 ± 26/brain). Following discontinuation of sulfadiazine, remarkable histological changes were observed in brains of ICSBP−/− mice (Fig. 4A). At day 8 after discontinuation of sulfadiazine, the brains of untreated mice and of mice treated with Tween 80 showed inflammatory changes consistent with TE, characterized by infiltration of mononuclear cells around vessels and, to a lesser extent, in meninges (Fig. 4A; Table 3). Immunohistochemical studies revealed that foci of parasitophorous vacuoles and/or free parasitic antigen were associated with inflammatory foci (Fig. 4B). These changes were similar to those found in patients with TE and most likely contributed to the death of mice (M. Deckert-Schlüter, University of Bonn, Bonn, Germany, personal communication).
FIG. 4.
Inflammatory foci (A) and corresponding parasite foci (parasitophorous vacuoles and parasitic antigen) (B) in brains of ICSBP−/− mice at day 8 after discontinuation of sulfadiazine (magnification, ×400); intact brain tissue, without inflammation, under treatment with sulfadiazine (C) and under treatment with ANS (10 mg/kg of body weight) (D) at day 8 after discontinuation of sulfadiazine (magnification, ×100).
TABLE 3.
Numbers of inflammatory foci, toxoplasma tachyzoites and antigens, and toxoplasma cysts in brains of T. gondii-infected ICSBP−/− mice 8 days after reactivation
| Treatment | No. of inflammatory foci (mean ± SD)a | No. of parasites (mean ± SD)b | No. of cysts (mean ± SD)b |
|---|---|---|---|
| Surfactantc | 5.3 ± 3.2 | 100.8 ± 66.0 | 10.3 ± 5.9 |
| ASN (10 mg/kg, i.v.) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| ASN (1 mg/kg, i.v.) | 3.3 ± 2.3 | 22.7 ± 21.2 | 4.3 ± 3.9 |
| ASN (0.1 mg/kg, i.v.) | 5.0 ± 1.9 | 104.8 ± 31.8 | 13.5 ± 10.6 |
| Atovaquone, oral suspension | 2.2 ± 1.2 | 19.5 ± 11.7 | 1.7 ± 1.2 |
Counted in two quadratic sections of 2 by 2 mm in brains of three mice per group, 100× magnification.
Counted in two quadratic sections of 2 by 2 mm in brains of three mice per group, 400× magnification.
As an additional control showed results identical to that of untreated mice.
In contrast, brains of mice treated with ASN at 10 mg/kg showed neither inflammatory foci nor foci of parasitophorous vacuoles and/or parasite antigen (Fig. 4D; Table 3), whereas numbers of inflammatory and parasitic foci increased with decreasing doses of ANS (Table 3). Numbers of inflammatory and parasitic foci in mice treated orally with atovaquone suspension did not differ significantly from those in mice treated with the ANS (1 mg/kg) (Table 3). Differences in treatment efficacy indicate that the organ distribution pattern of i.v. ANS differs from that of atovaquone absorbed from the gastrointestinal tract. The treatment efficacy of i.v. ANS was most likely caused by the transport of nanosuspension across the BBB.
Reactivation of latent infection in ICSBP−/− mice also resulted in inflammatory foci associated with the development of parasites in livers and lungs, consistent with mild hepatitis and pneumonitis (data not shown) (M. Deckert-Schlüter, personal communication).
Levels of atovaquone in serum and organs.
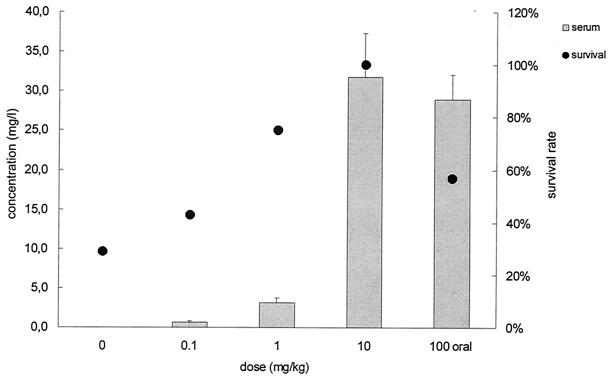
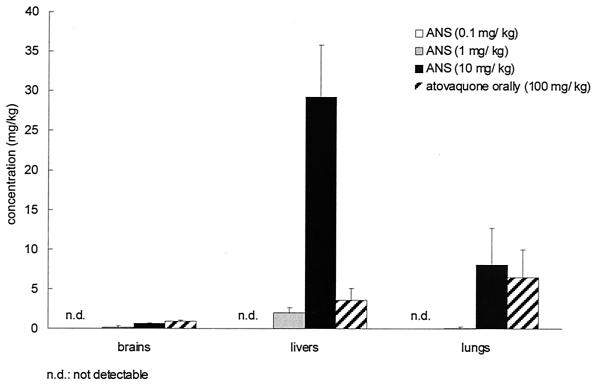
Sera, brains, lungs, and livers of ICSBP−/− mice were obtained on day 8, homogenized, and analyzed for atovaquone by HPLC. Increasing the dose of ANS resulted in increased atovaquone concentrations in serum and higher survival rates (Fig. 5). Oral treatment with atovaquone suspension at 100 mg/kg resulted in serum drug levels comparable to those measured after i.v. treatment with 10 mg of ANS/kg (P = 0.563) (Fig. 5). Atovaquone was detected in brain, liver, and lung tissues when mice were i.v. treated with ANS at doses of 1 and 10 but not 0.1 mg/kg; atovaquone was also detected in brain, liver, and lung tissues following oral treatment with 100 mg of drug suspension/kg (Fig. 6).
FIG. 5.
Concentration of atovaquone in sera of ICSBP−/− mice i.v. treated with surfactant solution (control) or ANS (0.1, 1, or 10 mg/kg) or treated orally with atovaquone suspension every other day. Serum samples were obtained 3 h after the last dosing. Values were derived from three mice per group that were sacrificed at day 8 after discontinuation of sulfadiazine (means ± SDs, left ordinate). Survival rates at day 8 refer to the right ordinate.
FIG. 6.
Concentrations of atovaquone in brain, liver, and lung tissues of ICSBP−/− mice i.v. treated with surfactant solution (control) or ANS (0.1, 1, or 10 mg/kg) every other day from day 2 until day 16 after discontinuation of sulfadiazine. One group of mice was treated orally with 100 mg of atovaquone suspension/kg at the same time points. Values were derived from three mice per group that were sacrificed at day 8 after discontinuation of sulfadiazine (means ± SDs) and are representative of three independently performed experiments.
DISCUSSION
Results of the present study reveal that ANSs possess distinct in vitro and in vivo activities against T. gondii. In vitro, both the ANS and atovaquone free drug were effective at concentrations of >1 μg/ml. No significant differences in the efficacies of the two atovaquone formulations were found. The excellent in vitro efficacy of both atovaquone formulations is most likely due to formation of drug precipitates in culture medium because of this drug's high lipophilicity and subsequent phagocytic uptake by cells. The efficacy of free atovaquone observed by us is in line with results reported in the literature (2, 41, 45). Araujo et al. (2) reported inhibition of parasite replication in human foreskin fibroblasts at an atovaquone concentration of 1.8 μg/ml. In MRC5 fibroblasts, the 50% inhibitory concentration of atovaquone against the parasite was estimated to be 0.024 μg/ml (41). At effective concentrations, we did not find cytotoxic activity for ANS or free drug. At concentrations of >5 μg/ml, cytotoxic effects were observed, as has been previously reported (41). Since ANS showed excellent activity against T. gondii at concentrations that were lower than serum concentrations observed in AIDS patients after treatment with 750 mg of atovaquone three times a day (22), we initiated in vivo studies.
To mimic the clinical situation in patients with reactivated disease as closely as possible, a new mouse model of reactivated TE was established. Intravenous treatment with ANS (10 mg/kg of body weight every other day) resulted in therapeutic effects. A survival rate of 88% was observed during the treatment period, whereas all control mice succumbed to TE. The therapeutic efficacy was further documented by a complete lack of histological signs of TE. In contrast, i.v. treatment with lower doses of ANS resulted in increased mortality correlating with an increase in the number of inflammatory foci in brains that were associated with parasites. When atovaquone suspension was administered orally at a dose of 100 mg/kg every other day, the survival rate was only 43% and histological signs of TE were observed. Interestingly, mortality and histological changes in these mice were similar to those observed in mice treated i.v. with ANS at 1 mg/kg. In murine models of latent (Swiss Webster mice) and chronic-progressive (CBA/Ca mice) infection with T. gondii, a reduction in the numbers of cysts and a 100% survival rate were reported following treatment with atovaquone suspension at a dose of 100 mg/kg/day (2, 3, 15). Furthermore, oral treatment with atovaquone suspension and atovaquone-loaded nanocapsules (15 mg/kg/day each) reduced the parasitic burden in Swiss Webster mice with latent T. gondii infections (45).
The vast majority of studies examining the therapeutic effect of antiparasitic drugs have been performed in murine models of either chronic-progressive or latent disease (2, 3, 29, 45). Despite the wide distribution of these models, they do not reflect the clinical situation, i.e., the reactivation of latent disease in immunocompromised patients (39). Therefore, we established a new murine model of reactivated disease based on a recent report by Suzuki et al. (47). Mice lacking the gene encoding ICSBP (and hence exhibiting impaired IL-12-dependent IFN-γ production) were infected orally with T. gondii and treated with sulfadiazine to establish latent infection. Shortly after discontinuation of sulfadiazine, the brains of mice developed remarkable inflammatory changes consistent with TE, and all untreated mice died within several days. In addition to the striking similarities in histological changes and mortality to those in patients with TE, the new model presented above offers several other advantages compared to conventional models. First, in contrast to the induction of reactivation by administration of immunosuppressive drugs or injection of antibodies against T-cell subsets and cytokines (reviewed by Denkers and Gazzinelli [14]), reactivation can be easily induced by discontinuation of sulfadiazine. Second, this approach results in a relatively synchronized development of TE within days, whereas in conventional models of chronic-progressive disease, TE takes weeks to develop; thus, the treatment period was shortened. Third, since all mice ultimately die after discontinuation of drugs, as do patients treated for TE, this model may also prove suitable for the study of drug regimens for maintenance therapy.
Atovaquone was detected by HPLC in the sera, brains, livers, and lungs of mice treated with ANS (1 and 10 mg/kg) and atovaquone suspension (100 mg/kg). The demonstration of atovaquone in brain tissue at low but measurable concentrations is particularly important. Increasing the dose of ANS resulted in increasing concentrations of the drug in serum, which correlated with survival. Intravenous administration of ANS at a dose of 10 mg/kg led to serum drug concentrations similar to those attained by oral administration of a 10-fold-higher dose of atovaquone suspension. Similar atovaquone concentrations were reported by Araujo et al. (2) for CBA/CA mice after oral administration of atovaquone on a similar treatment schedule.
In this first study focusing on treatment efficacy of an ANS, the drug levels in blood and concentrations in organs were analyzed at one time point. To assess precisely the improvement in bioavailability, the full plasma concentration/time profiles need to be measured for calculation of area under the curve, the maximum concentration of the drug in serum, and the time to maximum concentration of the drug in serum. We speculate that the absolute bioavailability of oral atovaquone is approximately 10%, since a 10-fold-higher oral dose of atovaquone resulted in serum drug levels comparable to those observed in mice treated i.v. with ANS. Since serum and organ levels were analyzed 3 h after drug administration and orally administered drugs often have a lag in absorption time, the absolute bioavailability may also be distinctly less than 10%.
The new model of TE described above, in combination with analytical studies of drug concentrations in tissues including brain, will allow detailed studies of the efficacy of new antiparasitic drugs. Furthermore, the influence of the stabilizing surfactant on the efficacy of drug carrier systems including nanosuspensions can be analyzed. In this regard, the type of surfactant was shown to influence the passage of drugs through the BBB (1, 30).
In conclusion, the present study has revealed that an ANS has a high in vitro activity against T. gondii and shows an excellent therapeutic effect in a new murine model of TE. Circumventing the low bioavailability of atovaquone by i.v. administration in the form of nanosuspensions may lead to improved treatment of immunocompromised patients with TE.
ACKNOWLEDGMENTS
We thank Imke Dillmann, Andrea Maletz, Solvy Wolke, Uschi Rüschendorf, Hildegard Hartwig, and the staff of the Animal Facilities, Department of Medical Microbiology and Immunology of Infection, Freie Universität Berlin, for excellent technical assistance.
This work was supported by grants from the Deutsche Forschungsgesellschaft (DFG Mu708/9, E and Li638/5-1).
REFERENCES
- 1.Alyautdin R, Gothier D, Petrov V, Kharkevich D, Kreuter J. Analgesic activity of the hexapeptide dalargin adsorbed on the surface of polysorbate 80-coated poly(butylcyanoacrylate) nanoparticles. Eur J Pharm Biopharm. 1995;41:44–48. [Google Scholar]
- 2.Araujo F G, Huskinson J, Remington J S. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob Agents Chemother. 1991;35:293–299. doi: 10.1128/aac.35.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo F G, Huskinson-Mark J, Gutteridge W E, Remington J S. In vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against the cyst form of Toxoplasma gondii. Antimicrob Agents Chemother. 1992;36:326–330. doi: 10.1128/aac.36.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araujo F G, Khan A A, Remington J S. Rifapentine is active in vitro and in vivo against Toxoplasma gondii. Antimicrob Agents Chemother. 1996;40:1335–1337. doi: 10.1128/aac.40.6.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araujo F G, Khan A A, Slifer T L, Bryskier A, Remington J S. The ketolide antibiotics HMR 3647 and HMR 3004 are active against Toxoplasma gondii in vitro and in murine models of infection. Antimicrob Agents Chemother. 1997;41:2137–2140. doi: 10.1128/aac.41.10.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araujo F G, Lin T, Remington J S. Synergistic combination of azithromycin and sulfadiazine for treatment of toxoplasmosis in mice. Eur J Clin Microbiol Infect Dis. 1992;11:71–73. doi: 10.1007/BF01971278. [DOI] [PubMed] [Google Scholar]
- 7.Araujo F G, Prokocimer P, Lin T, Remington J S. Activity of clarithromycin alone or in combination with other drugs for treatment of murine toxoplasmosis. Antimicrob Agents Chemother. 1992;36:2454–2457. doi: 10.1128/aac.36.11.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araujo F G, Shepard R M, Remington J S. In vivo activity of the macrolide antibiotics azithromycin, roxithromycin and spiramycin against Toxoplasma gondii. Eur J Clin Microbiol Infect Dis. 1991;10:519–524. doi: 10.1007/BF01963942. [DOI] [PubMed] [Google Scholar]
- 9.Araujo F G, Slifer T, Remington J S. Rifabutin is active in murine models of toxoplasmosis. Antimicrob Agents Chemother. 1994;38:570–575. doi: 10.1128/aac.38.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blunk T, Hochstrasser D F, Sanchez J C, Müller B W, Müller R H. Colloidal carriers for intravenous drug targeting: plasma protein adsorption patterns on surface-modified latex particles evaluated by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis. 1993;14:1382–1387. doi: 10.1002/elps.11501401214. [DOI] [PubMed] [Google Scholar]
- 11.Cohn J, McMeeking A, Cohen W. Evaluation of the policy of empiric treatment of suspected Toxoplasma encephalitis in patients with the acquired immunodeficiency syndrome. Am J Med. 1989;86:521–527. doi: 10.1016/0002-9343(89)90378-1. [DOI] [PubMed] [Google Scholar]
- 12.Conley F K, Jenkins K A, Remington J. Toxoplasma gondii infection of the central nervous system. Use of the peroxidase-antiperoxidase method to demonstrate toxoplasma in formalin fixed, paraffin embedded tissue sections. Hum Pathol. 1981;12:690–698. doi: 10.1016/s0046-8177(81)80170-0. [DOI] [PubMed] [Google Scholar]
- 13.De Angelis D V, Long J D, Kanics L L, Woolley J L. High-performance liquid chromatographic assay for the measurement of atovaquone in plasma. J Chromatogr. 1994;652:211–219. doi: 10.1016/0378-4347(93)e0387-6. [DOI] [PubMed] [Google Scholar]
- 14.Denkers E Y, Gazzinelli R T. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson D J, Huskinson-Mark J, Araujo F G, Remington J S. An ultrastructural study of the effect of treatment with atovaquone in brains of mice chronically infected with the ME49 strain of Toxoplasma gondii. Int J Exp Pathol. 1994;75:111–116. [PMC free article] [PubMed] [Google Scholar]
- 16.Grau J M, Kayser O, Müller R H. Nanosuspensions of poorly soluble drugs—reproducibility of small scale production. Int J Pharm. 2000;196:155–159. doi: 10.1016/s0378-5173(99)00411-1. [DOI] [PubMed] [Google Scholar]
- 17.Gutteridge W E. 566C80, an antimalarial hydroxynaphthoquinone with broad spectrum experimental activity against opportunistic parasitic infections of AIDS patients. J Protozool. 1991;38:141–143. [PubMed] [Google Scholar]
- 18.Handler M, Ho V, Whelan M. Intracerebral toxoplasmosis in patients with acquired immune deficiency syndrome. J Neurosurg. 1983;59:994–1001. doi: 10.3171/jns.1983.59.6.0994. [DOI] [PubMed] [Google Scholar]
- 19.Hannan S L, Ridout G A, Jones A E. Determination of the potent antiprotozoal compound atovaquone in plasma using liquid-liquid extraction followed by reversed-phase high-performance liquid chromatography with ultraviolet detection. J Chromatogr B. 1996;678:297–302. doi: 10.1016/0378-4347(95)00497-1. [DOI] [PubMed] [Google Scholar]
- 20.Hansson A G, Mitchell S, Jatlow P, Rainey P M. Rapid high-performance liquid chromatographic assay for atovaquone. J Chromatogr B. 1996;675:180–182. doi: 10.1016/0378-4347(95)00329-0. [DOI] [PubMed] [Google Scholar]
- 21.Holschke T, Löhler J, Kanno Y, Fehr T, Giese N, Rosenbauer F, Lou J, Knobeloch K P, Gabriele L, Waring J F, Bachmann M F, Zinkernagel R M, Morse H C, Ozato K, Horak I. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell. 1996;87:307–317. doi: 10.1016/s0092-8674(00)81348-3. [DOI] [PubMed] [Google Scholar]
- 22.Hughes W, Leoung G, Kramer F, Bozzette S A, Safrin S, Frame P. Comparison of atovaquone (566C80) with trimethoprim-sulfamethoxazole to treat Pneumocystis carinii pneumonia in patients with AIDS. N Engl J Med. 1993;328:1521–1527. doi: 10.1056/NEJM199305273282103. [DOI] [PubMed] [Google Scholar]
- 23.Hughes W T, Kennedy W, Shenep J L, Flynn P M, Hetherton S V, Fullen G. Safety and pharmacokinetics of 566C80, a hydroxynaphthoquinone with anti-Pneumocystis carinii activity: a phase I study in human immunodeficiency virus (HIV)-infected men. J Infect Dis. 1991;163:843–848. doi: 10.1093/infdis/163.4.843. [DOI] [PubMed] [Google Scholar]
- 24.Huskinson-Mark J, Araujo F G, Remington J S. Evaluation of the effect of drugs on the cyst form of Toxoplasma gondii. J Infect Dis. 1991;164:170–177. doi: 10.1093/infdis/164.1.170. [DOI] [PubMed] [Google Scholar]
- 25.Israelski D M, Remington J S. AIDS-associated toxoplasmosis. In: Sande M A, Volberding P A, editors. The medical management of AIDS. 3rd ed. Philadelphia, Pa: W. B. Saunders; 1992. pp. 319–345. [Google Scholar]
- 26.Israelski D M, Remington J S. Toxoplasmosis in the non-AIDS immunocompromised host. Curr Clin Top Infect Dis. 1993;13:322–356. [PubMed] [Google Scholar]
- 27.Katlama C, De Wit S, O'Doherty E, Van Glabeke M, Clumeck N. Pyrimethamine-clindamycin versus pyrimethamine-sulfadiazine as acute and long-term therapy for toxoplasmic encephalitis in patients with AIDS. Clin Infect Dis. 1996;22:268–275. doi: 10.1093/clinids/22.2.268. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman H E, Geisler P H. The hematologic toxicity of pyrimethamine (Daraprim) in man. Arch Ophthalmol. 1960;64:140–146. doi: 10.1001/archopht.1960.01840010142016. [DOI] [PubMed] [Google Scholar]
- 29.Khan A A, Araujo F G, Craft J C, Remington J S. Ketolide ABT-773 is active against Toxoplasma gondii. J Antimicrob Chemother. 2000;46:489–492. doi: 10.1093/jac/46.3.489. [DOI] [PubMed] [Google Scholar]
- 30.Kreuter J, Petrov V E, Kharkevich D A, Alyautdin R N. Influence of the type of surfactant on the analgesic effects induced by the peptide dalargin after its delivery across the blood-brain barrier using surfactant-coated nanoparticles. J Control Release. 1997;49:81–87. [Google Scholar]
- 31.Leport C, Raffi F, Matheron S. Treatment of central nervous system toxoplasmosis with pyrimethamine-sulfadiazine combination in 35 patients with the acquired immunodeficiency syndrome: efficacy of long-term continuous therapy. Am J Med. 1988;84:94–100. doi: 10.1016/0002-9343(88)90014-9. [DOI] [PubMed] [Google Scholar]
- 32.Liesenfeld O, Wong S Y, Remington J S. Toxoplasma in setting of AIDS. In: Merigan T C Jr, Bartlett J G, Bolognesi D, editors. Textbook of AIDS medicine. 2nd ed. Baltimore, Md: Williams & Wilkins; 1998. pp. 225–259. [Google Scholar]
- 33.Luft B J, Remington J S. AIDS commentary: toxoplasmic encephalitis. J Infect Dis. 1988;157:1–6. doi: 10.1093/infdis/157.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Luft B J, Remington J S. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 35.Mosmann T R. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 36.Müller R H, Benita S, Böhm B H L. Emulsions and nanosuspensions for the formulation of poorly soluble drugs. Stuttgart, Germany: medpharm Verlag GmbH; 1998. [Google Scholar]
- 37.Müller R H, Peters K. Nanosuspensions for the formulation of poorly soluble drugs. I. Preparation by size reducing technique. Int J Pharm. 1998;160:229–237. [Google Scholar]
- 37a.Müller, R. H., M. Lück, and J. Kreuter. 1997. German patent 197 45 950.1.
- 38.Pfefferkorn E R, Borotz S E, Nothnagel R F. Mutants of Toxoplasma gondii resistant to atovaquone (566C80) or decoquinate. J Parasitol. 1993;79:559–564. [PubMed] [Google Scholar]
- 39.Porter S B, Sande M A. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327:1643–1648. doi: 10.1056/NEJM199212033272306. [DOI] [PubMed] [Google Scholar]
- 40.Rolan P E, Mercer A J, Weatherly B C, Holdich T, Meire H, Peck R W. Examination of some factors responsible for a food-induced increase in absorption of atovaquone. Br J Clin Pharmacol. 1994;37:13–20. doi: 10.1111/j.1365-2125.1994.tb04232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romand S, Pudney M, Derouin F. In vitro and in vivo activities of the hydroxynaphthoquinone atovaquone alone or combined with pyrimethamine, sulfadiazine, clarithromycin, or minocycline against Toxoplasma gondii. Antimicrob Agents Chemother. 1993;37:2371–2378. doi: 10.1128/aac.37.11.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rousseau F, Pueyo S, Morlat P. Increased risk of toxoplasmic encephalitis in human immunodeficiency virus-infected patients with pyrimethamine-related rash. Clin Infect Dis. 1997;24:396–402. doi: 10.1093/clinids/24.3.396. [DOI] [PubMed] [Google Scholar]
- 43.Scharton-Kersten T, Contursi C, Masumi A, Sher A, Ozato K. Interferon consensus sequence binding protein-deficient mice display impaired resistance to intracellular infection due to a primary defect in interleukin 12 p40 induction. J Exp Med. 1997;186:1523–1534. doi: 10.1084/jem.186.9.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schöler N, Zimmermann E, Katzfey U, Hahn H, Müller R H, Liesenfeld O. Effect of solid lipid nanoparticles (SLN) on cytokine production and the viability of murine peritoneal macrophages. J Microencapsul. 2000;17:639–650. doi: 10.1080/026520400417685. [DOI] [PubMed] [Google Scholar]
- 45.Sordet F, Aumjaud Y, Fessi H, Derouin F. Assessment of the activity of atovaquone-loaded nanocapsules in the treatment of acute and chronic murine toxoplasmosis. Parasite. 1998;5:223–229. doi: 10.1051/parasite/1998053223. [DOI] [PubMed] [Google Scholar]
- 46.Spencer C M, Goa K L. Atovaquone: a review of its pharmacological properties and therapeutic efficacy in opportunistic infections. Drugs. 1995;50:176–196. doi: 10.2165/00003495-199550010-00011. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki Y, Kang H, Parmley S, Lim S, Park D. Induction of tumor necrosis factor-α and inducible nitric oxide synthase fails to prevent toxoplasmic encephalitis in the absence of interferon-γ in genetically resistant BALB/c mice. Microbes Infect. 2000;2:455–462. doi: 10.1016/s1286-4579(00)00318-x. [DOI] [PubMed] [Google Scholar]