Abstract
Non-small-cell lung cancer (NSCLC) is one of the most threatening malignant tumors to human health, with the overall 5-year survival rate being less than 30%. Regulatory T cells (Tregs), a functional subset of T cells, maintain immunologic immunological self-tolerance and homeostasis. Accumulating evidence has uncovered their implicated roles in various cancers in recent years. In NSCLC, they are associated with staging, therapeutic efficacy, and prognosis by infiltrating in tissues and thereby attenuating immunologic anticancer effects in patients. Tumor-associated Tregs display distinct immune signatures in NSCLC compared to thymus-derived Tregs, playing an important role in remodeling the tumor microenvironment (TME). Targeting Tregs has become a novel direction for NSCLC patients, such as disrupting their immune-suppressive functions, blocking their trafficking into tumors, and inhibiting their development and/or activation. This review is aimed at elucidating the molecular mechanisms of tumor-associated Tregs in NSCLC and providing therapeutic targets relevant to Tregs.
1. Epidemiology and Prevention of NSCLC
Lung cancer is one of the most commonly diagnosed cancers and the leading cause of cancer death worldwide in the year 2020, with an estimated 2.2 million new cases and 1.8 million deaths, which represents more than one in ten (11.4%) cancers diagnosed and approximately one in five (18.0%) deaths [1]. Traditionally, 85% of all cases are histopathologically classified as non-small-cell lung cancer (NSCLC) [2]. While treatment options comprise surgery, chemoradiotherapy, and targeted therapy, patients with NSCLC are often diagnosed with metastatic diseases or develop resistance to the drugs, resulting in a frustrating five-year overall survival of only less than 30% currently [3–5].
In recent years, tumor immunity has become a hot spot. The emergence of immunotherapy led by anti-immune checkpoint molecules has improved the overall survival of a subset of patients with advanced NSCLC. However, patients with positive tumor PD-L1 expression may still experience poor outcomes and severe adverse effects or even have a deterioration of their disease defined as hyperprogression [6, 7]. In recent years, accumulated evidence has uncovered the implicated roles of regulatory T cells (Tregs) in various cancers. In this review, we review the latest progress in the mechanisms of Tregs in promoting tumors and propose perspectives and therapeutic strategies targeting Tregs in NSCLC.
2. Summary of Suppressive Mechanisms of Tregs
Tregs, an immunosuppressive subset of CD4-positive (CD4+) T cells, are first found to serve to maintain immunological self-tolerance and homeostasis. Accumulated evidence has uncovered their implicated roles in autoimmune diseases and cancer [8–12]. They can be classified into two major groups based on their developmental origin, including thymus-derived Treg cells (tTregs) and peripheral Treg cells (pTregs) in vivo or induced Treg cells (iTregs) in vitro [12–14].
In the development of tTregs, several downstream signaling pathways are activated through the interaction between CD25 molecule (also known as interleukin-2 receptors, IL-2Rα), which are highly expressed on the membrane [14–16], and interleukin-2 (IL-2) [17], resulting in an increase of FOXP3 expression [18–20]. Elevation of FOXP3 can further promote the level of immunosuppressive receptors (i.e., CTLA-4, TIGIT, LAG-3, NRP1, CD39, and CD73) expressed on Tregs and enhance the secretion of multiple inhibitory cytokines such as IL-10, TGF-β, and IL -35, thus endowing Tregs with an immunosuppressive function [21–23]. In contrast, under costimulation of IL-2, TGF-β, or other cytokines, peripheral CD4+ T cells can be differentiated into a new cell subset with highly expressed FOXP3 that are designated pTregs or iTregs [24–27].
Effector Treg cells (eTregs), characterized by their high ability to suppress immunity, perform immunosuppressive functions mainly through cell contact-dependent and cytokine-mediated pathways. Cytotoxic T lymphocyte antigen 4 (CTLA-4) and IL-2R are two key molecules that mediate this process (Figure 1). eTregs can inhibit the costimulatory signals in effector T cells by expressing membrane and producing soluble CTLA-4, which can bind to CD80 or CD86 molecule that is expressed on antigen-presenting cells (APCs) [28–32]. Besides, eTregs can hamper the activation and tumor-killing capacity of CD8+ T cells and natural killer (NK) cells via depriving IL-2 owing to its high-affinity receptor CD25 on the cell surface [33–35]. They can also attenuate the function of APCs and effector T cells by secreted or intracellular inhibitory molecules, such as IL-10, TGF-β, and IL-35, or cell-killing factors such as granzyme and perforin (Figure 1) [36–39].
Figure 1.
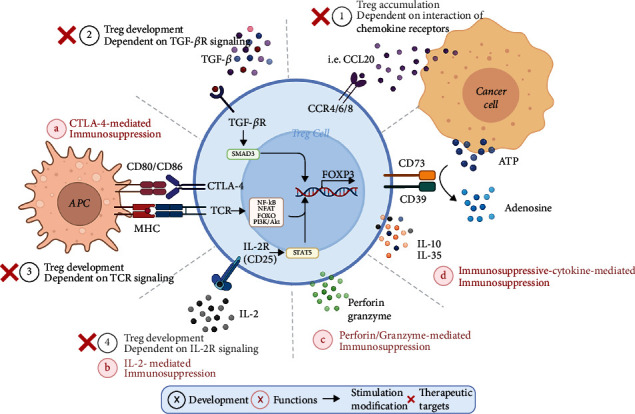
Prime mechanisms of Treg-mediated immunosuppression and associated therapeutic targets in NSCLC. Black: in the tumor microenvironment (TME), the development of regulatory T cells (Tregs) depends on several main factors that contribute to the FOXP3 expression: (1) the interaction of chemokine receptors, (2) TGF-βR signaling between Treg and cancer cells, (3) TCR, and (4) IL-2R signaling in Tregs. Red: Tregs express the high-affinity IL-2 receptor binding to and sequestering IL-2 to reduce its availability to effector T cells. They also express cytotoxic T lymphocyte antigen 4 (CTLA-4), which binds to CD80 and CD86, with a higher affinity than CD28, on antigen-presenting cells (APCs), thereby transmitting suppressive signals to these cells. In addition, Tregs can produce immunosuppressive cytokines, granzymes, and perforin to inhibit immunity. Red cross: different therapeutic approaches have been explored in downregulating Treg cell expansion mediated by chemokine or TGF-β in the TME. In addition, inhibitors targeting TCR and IL-2R signaling have been tested in reducing Treg cell activation and proliferation in patients.
3. Current Advances of Tumor-Associated Tregs in NSCLC
3.1. Infiltration of Tumor-Associated Tregs and Tumor Prognosis
Tregs are associated with oncogenesis, invasion, metastasis, reoccurrence, drug responses, and prognosis of patients in multiple cancers by remodeling the immune-suppressive microenvironment [40]. Tumor-associated Tregs account for 10–50% of CD4+ T cells in tumors, with only 2–5% of those in the peripheral blood of healthy individuals by contrast [12, 41]. However, they have displayed distinct immune signatures and activated immunophenotypes. Three groups of tumor-associated Tregs have been found in tumors, including tumor-resident, tissue-resident Tregs, and those from the circulation [42–44]. However, their origins and relationship are still unclear that whether tumor-infiltrated Tregs originate in the tumor-associate tissues or from the circulation. Treg infiltration has been a negative prognostic factor for patients with NSCLC. Increased number and enhanced activity have been found with Tregs in multiple tissues of patients, including tumors, metastatic lymph nodes, and the peripheral blood, which is highly associated with the staging and the occurrence of metastasis and recurrence NSCLC [45–47].
Besides, the frequency of Tregs in NSCLC contributes to resistance or even hyperprogressive diseases after chemotherapies and immunotherapies. Liu et al. [48] reported that the efficacy of platinum-based chemotherapy in NSCLC decreased with the increasing ratio of FOXP3+ Treg and CD8+ T, suggesting the abundance of eTregs in tumor sites was an independent factor for poor response to platinum-based chemotherapy. Consistent results were also found in a mouse model. Pircher et al. [49] demonstrated that the number of foxp3+ Tregs could increase when treated with platinum-based chemotherapy combined with cetuximab. eTregs potently attenuated the NK-mediated anticancer effects and the antibody-dependent cell-mediated cytotoxicity (ADCC) against CD8+ T cells.
3.2. Characteristics of Tumor-Associated Tregs and Tumor Prognosis
Compared to the natural tTregs, the key features of tumor-associated Tregs are highly activated and differentiated effector Tregs, with higher affinity to the T cell receptor (TCR). Transcriptomic data showed that several immune checkpoints (i.e., interleukin-1 receptor 2, PD-1 ligands, and CCR8 chemokine) were upregulated to maintain their suppressive role in tumors [50]. Higher amounts of immunosuppressive molecules can increase and expand in tumor tissues, inducing stronger immune suppression [41, 43, 44, 51].
It is worth noting that members of the TNF receptor superfamily (TNFRSF) also play a crucial role in the development and maturation of Tregs, which has not been detailed in our previous text. Molecules such as GITR (also known as TNFRSF18), OX40 (also known as TNFRSF4), and TNFR2 (also known as TNFRSF1B) can function as costimulatory agents in regulating the expression of the FOXP3 gene [52]. In NSCLC, TNFR2+ Tregs presented in the peripheral blood and pleural effusion of NSCLC patients were more proliferative and expressed a higher degree of CTLA-4 molecules to mediate immunosuppression than TNFR2- Tregs [53, 54]. Furthermore, TNFRSF9 was proposed to promote the immune-suppressive activity of Tregs in the TME in NSCLC [55]. We still need to identify new molecules of Tregs in order to better isolate them and explore their roles in the TME.
3.3. Tumor-Associated Tregs in the TME
Different cells and molecules in the TME also provide favorable conditions for tumor-associated Tregs to exert immunosuppressive functions (Figure 1). Recent studies in several kinds of tumors revealed that insufficient glucose supply and increased intracellular glycolysis in cancer cells could provide Tregs rich in lactic acid and fatty acids, promoting their proliferation [56, 57]. Besides, TGF-β, ATP, IDO, and some other molecules produced by tumor cells also enhanced the immunosuppressive function of Tregs in tumor tissue [58–60]. In NSCLC, tumor cells from patients with higher disease stage or lymph node metastasis generated more TGF-β than their counterparts [61], which could not only expand the infiltration of eTregs in the TME but potentiate the immunosuppressive function by elevating the expression of inhibitory molecules B7H1 and GITRL on the surface of APC cells [62].
Apart from the above metabolites, tumor cells can also escape from immune-mediated tumor surveillance by recruiting Tregs by expressing a variety of chemokines (Figure 1). Zhang et al. [63] reported that CCL20 secretion by tumor cells could recruit Tregs via cooperating with its receptor CCR6 in NSCLC. Moreover, the TME can posttranslationally regulate the expression of FOXP3 in Tregs. It has been shown that the AREG protein secreted by tumor cells of lung adenocarcinoma can maintain the Treg suppressive function via the EGFR/GSK-3β/FOXP3 axis in vitro and in vivo [64].
Several studies have shown that oncogene mutations can regulate the differentiation process of Tregs. One of the most studied has been KRAS, a fundamental driver of lung tumorigenesis, generally affecting 20-40% of NSCLC patients. The incidence is higher in smokers than in nonsmokers (30% vs. 10%) [65]. KRAS mutations in lung cancer cells were found to promote the differentiation of more CD4+ T cells into Tregs in the TME by increasing the secretion of TGF-β and IL-10, thereby increasing the number of cells in the TME [66, 67].
The progression and metastatic capacity of solid tumors are also influenced by some other immune cells in the TME. Macrophage receptor with collagenous structure (MARCO) expressed on the surface of tumor-associated macrophages (TAM) has been reported to promote the proliferation of Tregs and the inhibitory cytokine IL-10 secretion in NSCLC [68]. Besides, at the protumor inflammatory stage of lung cancer, TGF-α stimulation can upregulate MHC-II molecules on the surface of alveolar type II cells to trigger Treg expansion and promote the tumorigenesis of inflammation-driven lung adenocarcinoma [69].
4. Research Progress and Clinical Applications of Targeting Tumor-Associated Tregs
Because Tregs play roles in tumor immunity, measurements targeting them have emerged in tumor treatment in recent years, and three categories of approaches have been explored: disrupting their immune-suppressive functions, blocking their trafficking into tumors, and inhibiting their development and/or activation. Currently, therapeutic strategies targeting Tregs in oncology including NSCLC often involve two or more of the above to enhance antitumor immunity.
4.1. Disrupting Treg Cell Immune-Suppressive Functions in NSCLC
Monoclonal antibodies against cell membrane markers of Tregs have been currently the most commonly used method to inhibit the IL-2-mediated immunosuppressive function of Tregs (Figure 1). Given the constitutive and high expression of CD25 by most Tregs and its crucial role in eTreg cell maintenance, CD25 has attracted attention as a potential target in Treg depletion [70–73]. In clinical studies, CD25-blocking monoclonal antibody daclizumab administration has led to a marked and prolonged decrease in Tregs in patients with melanoma [74]. In NSCLC, preclinical results demonstrated that Treg depletion blocked by CD25 in combination with cytotoxic therapy might be beneficial as a treatment strategy. Ganesan et al. [75] found that mice bearing early NSCLC treated with daclizumab and chemotherapy exhibited significantly increased tumor cell death and extended survival associated with infiltration CD8+ T cells.
4.2. Blocking Treg Trafficking into Tumors in NSCLC
Previous studies have revealed mechanisms that lead to intratumoral Treg accumulation involve the interaction of chemokine receptor-expressing activated Tregs and the chemokines produced in the TME (Figure 1) [12, 76–78]. CCR4, a key chemokine receptor highly expressed on the surface of Tregs, has been a promising target in oncology herein [43, 79]. A monoclonal antibody, mogamulizumab, which depletes CCR4+ Tregs, could suppress tumor growth, having been approved for adult T cell leukemia or lymphoma [79].
However, the antitumor efficacy of this drug in solid tumors is unclear due to the less amount of surface CCR4 expression on Tregs in the TME. Indeed, clinical trial results did not show a significant synergistic antitumor effect for combined therapy using mogamulizumab and docetaxel or nivolumab, an anti-PD-1 agent, in advanced and perioperative NSCLC patients (https://www.clinicaltrials.gov/, NCT trial numbers: NCT02358473, NCT02946671), which may be attributed to the small number of patients enrolled. Recently, efficacy and safety of Treg depletion with mogamulizumab in combination with immune checkpoint inhibitors (anti-CTLA-4 or PD-1 or PD-L1 molecules) are being explored and determined in numerous kinds of advanced solid tumors, such as liver cancer, gastric cancer, and pancreatic cancer (NCT trial numbers: NCT02281409, NCT01929486, and NCT02476123), on which we could pin our hopes. In addition, CCR4 is not only expressed on Treg but similarly on the surface of conventional T cells, bringing about off-target immune side effects. These data highlight the need to identify molecules specific to tumor-accumulated Tregs as therapeutic targets.
Promisingly, there have still been several alternative molecules associated with Treg cell recruitment that may also effectively prevent tumor progression in solid tumors. The study conducted by Alvisi et al. [80] reported that transcription factor IRF4 could bind to BATF, reducing Treg cell recruitment by regulating the expression of chemokine receptors on Tregs. Besides, IRF4 can also downregulate the expression of inhibitory factors in Tregs, such as TNF receptor superfamily molecules and ICOS, thereby inhibiting the immunosuppression in the TME. Generally, chemokines are produced by cells in the TME, including tumor cells and TAMs, which can also be combated to reduce Tregs' accumulation. Researchers have found that docetaxel could rescue immunity functions against tumor cells by reducing their secretion of CCL20 interacted with CCR6+ Tregs [63].
4.3. Inhibiting Treg Development and/or Activation in NSCLC
Several directions have been considered to deactivate and convert Tregs into effective T cells enhancing immunity to various cancers. These include nonspecific cytotoxic agents, strategies selectively targeting molecules important for Treg differentiation and maturation and regulating gene expression in the nucleus of premature T cells.
4.3.1. Nonspecific Cytotoxic Agents
Traditional chemotherapeutics, such as cyclophosphamide, are effective in Treg depletion. Cyclophosphamide could significantly reduce the number of circulating Tregs in the peripheral blood of colorectal cancer patients [81, 82]. Low-dose cyclophosphamide combined with CD25 monoclonal antibody had better efficacy than the anti-CD25 mouse model with NSCLC receiving radiotherapy [83]. Furthermore, whether the addition of cytotoxic agent, cyclophosphamide or doxorubicin, can improve the efficacy of anti-PD-1 therapy by modulating tumor environment in NSCLC patients with PD-L1 expression less than 10% remains unclear to be figured out (NCT trial number: NCT03808480).
4.3.2. Treg Signaling Pathway Inhibitors
Two tyrosine kinase inhibitors, imatinib and dasatinib, have been found to inhibit LCK molecules on the surface of T cells as an off-target effect, impairing maintenance and immunosuppressive activity of Tregs by blocking TCR signaling (Figure 1) [84]. Redin and his colleagues reported that dasatinib could synergize with PD-1 inhibitor to impair tumor growth in NSCLC experimental mouse models. They uncovered that inactivated SFK targeted by dasatinib could inhibit the phosphorylation of STAT5 and SMAD3, which were downstream molecules of CD25 and TGF-βR, respectively, hereby inhibiting the TGF-β-induced differentiation process of Tregs. However, the combination of dasatinib and EGFR-TKI drug did not prolong the survival time of patients with EGFR mutations in NSCLC [85–87]. Its benefit remains to be determined when combined with PD-1 antibody in patients with advanced NSCLC (NCT trial number: NCT04284202, NCT02750514).
The phosphoinositide 3-kinase pathway (PI3K) is an important aspect of Treg cell development and function, which mediates signaling downstream of the TCR [88–90]. The inhibitor's deficiency of PI3Kδ by inhibitor could inhibit Treg cell activation and augment immunity to control cancer in mice via CD8+ T cells [91, 92]. Ahmad et al. reported a consistent result in a mouse lung cancer model that anticancer vaccine coadministered with the PI3Kδ inhibitor reduced the number of T cells whereas the number of effector T cells increased, leading to a decrease in tumor volume [91]. The efficacy and safety results of PI3Kδ inhibitor INCB050465 combined with pembrolizumab are currently being evaluated in a phase I trial in patients with advanced tumors, including NSCLC (NCT trial number: NCT02646748).
4.3.3. Molecules Mediating Treg Cell Development
The transformation between the immunosuppressive Tregs and immune cells has been a hotspot in tumor immunity fields. Notably, there is a close relation between Treg and Th17 cells, where the Th17 cell subset has been discovered with similar properties to Tregs, both originated from a common precursor. Recent studies uncovered the association between their balance and the progression in different kinds of tumors [93–95]. Th17/Treg ratio was lower in patients' tumors and peripheral blood tissues than in healthy individuals, as demonstrated in studies focused on various solid tumors, including NSCLC [96–98].
Interestingly, cytokines in the TME are important in the differentiation of CD4+ T cell subsets, which depend upon the balance of expression of certain transcriptional factors. For instance, TGF-β can inhibit the differentiation of Th17 cells while inducing more Treg precursor cells to differentiate into Tregs via elevating FOXP3 expression. On the contrary, the mediation of cytokines such as IL-1𝛽 and IL-6 contributes to Tregs secreting more increased amounts of IFN-γ and IL-17 and losing their original immunosuppressive function. This finally transforms Tregs into another two subsets of cells, Th1 or Th17, which will mediate immune clearance and inflammatory responses, respectively [99–101].
Mechanistically, Yu et al. [102] discovered that interferon regulatory factor 4 (IRF4) could induce more Th17 cells than Tregs in the malignant pleural effusion of patients with NSCLC via downregulating the expression of HELIOS, one of the dominant genes in Treg cell development. Moreover, curcumin has also been found to promote the conversion of Tregs to Th1 cells in NSCLC by inhibiting the transcription of FOXP3 and promoting the expression of IFN-γ, which is necessary for Th1 cells [103]. Hence, inducing the differentiation of Tregs into other T cell subsets harboring antitumor functions can be promising in addressing tumors within the TME to be explored in clinical practice.
5. Future Perspectives
Treg infiltration into tumors is a contributor to poor prognosis via hindering effective immune response against tumor cells in NSCLC. Recent research has uncovered molecular mechanisms underlying their antitumor immunity. Despite the monumental advance of checkpoint blockade immunotherapy, high morbidity and mortality and different responses in NSCLC patients necessitate the consideration of alternative targets, where Tregs are most notably modulating immunosuppressive cells in the TME. Therefore, we should explore therapeutic targets against Tregs to reinvigorate cancer immunity in cancer treatment.
Conflicts of Interest
The authors have no conflict of interest to declare.
Authors' Contributions
Jiaqi Liang and Guoshu Bi contributed equally to this work.
References
- 1.Sung H., Ferlay J., Siegel R. L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians . 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Travis W. D., Brambilla E., Nicholson A. G., et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. Journal of Thoracic Oncology . 2015;10(9):1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 3.Allemani C., Matsuda T., Di Carlo V., et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet . 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbst R. S., Morgensztern D., Boshoff C. The biology and management of non-small cell lung cancer. Nature . 2018;553(7689):446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R. L., Miller K. D., Fuchs H. E., Jemal A. Cancer statistics, 2021. CA: a Cancer Journal for Clinicians . 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 6.Champiat S., Ferrara R., Massard C., et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nature Reviews. Clinical Oncology . 2018;15(12):748–762. doi: 10.1038/s41571-018-0111-2. [DOI] [PubMed] [Google Scholar]
- 7.Kamada T., Togashi Y., Tay C., et al. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proceedings of the National Academy of Sciences of the United States of America . 2019;116(20):9999–10008. doi: 10.1073/pnas.1822001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontenot J. D., Gavin M. A., Rudensky A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunology . 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 9.Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription FactorFoxp3. Science . 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 10.Khattri R., Cox T., Yasayko S. A., Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature Immunology . 2003;4(4):337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 11.Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. Journal of Immunology . 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 12.Togashi Y., Nishikawa H. Regulatory T cells: molecular and cellular basis for immunoregulation. Current Topics in Microbiology and Immunology . 2017;410:3–27. doi: 10.1007/82_2017_58. [DOI] [PubMed] [Google Scholar]
- 13.Abbas A. K., Benoist C., Bluestone J. A., et al. Regulatory T cells: recommendations to simplify the nomenclature. Nature Immunology . 2013;14(4):307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi S., Miyara M., Costantino C. M., Hafler D. A. FOXP3+ regulatory T cells in the human immune system. Nature Reviews. Immunology . 2010;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 15.Jordan M. S., Boesteanu A., Reed A. J., et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nature Immunology . 2001;2(4):301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 16.Weissler K. A., Caton A. J. The role of T-cell receptor recognition of peptide:MHC complexes in the formation and activity of Foxp3+ regulatory T cells. Immunological Reviews . 2014;259(1):11–22. doi: 10.1111/imr.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lio C. W., Hsieh C. S. A two-step process for thymic regulatory T cell development. Immunity . 2008;28(1):100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burchill M. A., Yang J., Vang K. B., et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity . 2008;28(1):112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long M., Park S. G., Strickland I., Hayden M. S., Ghosh S. Nuclear factor-κB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity . 2009;31(6):921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Luo C. T., Li M. O. Transcriptional control of regulatory T cell development and function. Trends in Immunology . 2013;34(11):531–539. doi: 10.1016/j.it.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borsellino G., Kleinewietfeld M., Di Mitri D., et al. Expression of ectonucleotidase CD39 by Foxp 3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood . 2007;110(4):1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 22.Kobie J. J., Shah P. R., Yang L., Rebhahn J. A., Fowell D. J., Mosmann T. R. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. Journal of Immunology . 2006;177(10):6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 23.Sarris M., Andersen K. G., Randow F., Mayr L., Betz A. G. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity . 2008;28(3):402–413. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coombes J. L., Siddiqui K. R., Arancibia-Cárcamo C. V., et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp 3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. The Journal of Experimental Medicine . 2007;204(8):1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis G. I., Reneer M. C., Vélez-Ortega A. C., McCool A., Martí F. Generation of induced regulatory T cells from primary human Naïve and memory T cells. Journal of Visualized Experiments . 2012;62(62) doi: 10.3791/3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu P., Santner-Nanan B., Hu M., et al. IL-10 potentiates differentiation of human induced regulatory T cells via STAT3 and Foxo 1. Journal of Immunology . 2015;195(8):3665–3674. doi: 10.4049/jimmunol.1402898. [DOI] [PubMed] [Google Scholar]
- 27.Tran D. Q., Ramsey H., Shevach E. M. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood . 2007;110(8):2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onishi Y., Fehervari Z., Yamaguchi T., Sakaguchi S. Foxp 3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proceedings of the National Academy of Sciences of the United States of America . 2008;105(29):10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sage P. T., Paterson A. M., Lovitch S. B., Sharpe A. H. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity . 2014;41(6):1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wing J. B., Ise W., Kurosaki T., Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity . 2014;41(6):1013–1025. doi: 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Wing K., Onishi Y., Prieto-Martin P., et al. CTLA-4 control over Foxp 3+ regulatory T cell function. Science . 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 32.Qureshi O. S., Zheng Y., Nakamura K., et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science . 2011;332(6029):600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chinen T., Kannan A. K., Levine A. G., et al. An essential role for the IL-2 receptor in Treg cell function. Nature Immunology . 2016;17(11):1322–1333. doi: 10.1038/ni.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malek T. R., Yu A., Zhu L., Matsutani T., Adeegbe D., Bayer A. L. IL-2 family of cytokines in T regulatory cell development and homeostasis. Journal of Clinical Immunology . 2008;28(6):635–639. doi: 10.1007/s10875-008-9235-y. [DOI] [PubMed] [Google Scholar]
- 35.Pandiyan P., Zheng L., Ishihara S., Reed J., Lenardo M. J. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nature Immunology . 2007;8(12):1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 36.Collison L. W., Workman C. J., Kuo T. T., et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature . 2007;450(7169):566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 37.Jarnicki A. G., Lysaght J., Todryk S., Mills K. H. G. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. Journal of Immunology . 2006;177(2):896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 38.Steinbrink K., Wölfl M., Jonuleit H., Knop J., Enk A. H. Induction of tolerance by IL-10-treated dendritic cells. Journal of Immunology . 1997;159(10):4772–4780. [PubMed] [Google Scholar]
- 39.Grossman W. J., Verbsky J. W., Barchet W., Colonna M., Atkinson J. P., Ley T. J. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity . 2004;21(4):589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka A., Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Research . 2017;27(1):109–118. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saito T., Nishikawa H., Wada H., et al. Two FOXP3+CD4+ T cell subpopulations distinctly control the prognosis of colorectal cancers. Nature Medicine . 2016;22(6):679–684. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 42.Hoadley K. A., Yau C., Hinoue T., et al. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell . 2018;173(2):291–304.e6. doi: 10.1016/j.cell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azizi E., Carr A. J., Plitas G., et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell . 2018;174(5):1293–308.e36. doi: 10.1016/j.cell.2018.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plitas G., Konopacki C., Wu K., et al. Regulatory T cells exhibit distinct features in human breast cancer. Immunity . 2016;45(5):1122–1134. doi: 10.1016/j.immuni.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erfani N., Mehrabadi S. M., Ghayumi M. A., et al. Increase of regulatory T cells in metastatic stage and CTLA-4 over expression in lymphocytes of patients with non-small cell lung cancer (NSCLC) Lung Cancer . 2012;77(2):306–311. doi: 10.1016/j.lungcan.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Petersen R. P., Campa M. J., Sperlazza J., et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer . 2006;107(12):2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 47.Schneider T., Kimpfler S., Warth A., et al. Foxp3+ regulatory T cells and natural killer cells distinctly infiltrate primary tumors and draining lymph nodes in pulmonary adenocarcinoma. Journal of Thoracic Oncology . 2011;6(3):432–438. doi: 10.1097/JTO.0b013e31820b80ca. [DOI] [PubMed] [Google Scholar]
- 48.Liu H., Zhang T., Ye J., et al. Tumor-infiltrating lymphocytes predict response to chemotherapy in patients with advance non-small cell lung cancer. Cancer Immunology, Immunotherapy . 2012;61(10):1849–1856. doi: 10.1007/s00262-012-1231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pircher A., Gamerith G., Amann A., et al. Neoadjuvant chemo-immunotherapy modifies CD4+CD25+ regulatory T cells (Treg) in non-small cell lung cancer (NSCLC) patients. Lung Cancer . 2014;85(1):81–87. doi: 10.1016/j.lungcan.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Kim H. R., Park H. J., Son J., et al. Tumor microenvironment dictates regulatory T cell phenotype: upregulated immune checkpoints reinforce suppressive function. Journal for Immunotherapy of Cancer . 2019;7(1):p. 339. doi: 10.1186/s40425-019-0785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Simone M., Arrigoni A., Rossetti G., et al. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory cells. Immunity . 2016;45(5):1135–1147. doi: 10.1016/j.immuni.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahmud S. A., Manlove L. S., Schmitz H. M., et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nature Immunology . 2014;15(5):473–481. doi: 10.1038/ni.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan F., Du R., Wei F., et al. Expression of TNFR2 by regulatory T cells in peripheral blood is correlated with clinical pathology of lung cancer patients. Cancer Immunology, Immunotherapy . 2015;64(11):1475–1485. doi: 10.1007/s00262-015-1751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye L. L., Peng W. B., Niu Y. R., et al. Accumulation of TNFR2-expressing regulatory T cells in malignant pleural effusion of lung cancer patients is associated with poor prognosis. Annals of Translational Medicine . 2020;8(24):p. 1647. doi: 10.21037/atm-20-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cho J. W., Son J., Ha S. J., Lee I. Systems biology analysis identifies _TNFRSF9_ as a functional marker of tumor- infiltrating regulatory T-cell enabling clinical outcome prediction in lung cancer. Computational and Structural Biotechnology Journal . 2021;19:860–868. doi: 10.1016/j.csbj.2021.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Angelin A., Gil-de-Gómez L., Dahiya S., et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metabolism . 2017;25(6):1282–1293. doi: 10.1016/j.cmet.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H., Franco F., Ho P. C. Metabolic regulation of Tregs in cancer: opportunities for immunotherapy. Trends Cancer. . 2017;3(8):583–592. doi: 10.1016/j.trecan.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Smith C., Chang M. Y., Parker K. H., et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discovery . 2012;2(8):722–735. doi: 10.1158/2159-8290.CD-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Speeckaert R., Vermaelen K., van Geel N., et al. Indoleamine 2,3-dioxygenase, a new prognostic marker in sentinel lymph nodes of melanoma patients. European Journal of Cancer . 2012;48(13):2004–2011. doi: 10.1016/j.ejca.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 60.Principe D. R., Doll J. A., Bauer J., et al. TGF-β: duality of function between tumor prevention and carcinogenesis. Journal of the National Cancer Institute . 2014;106(2):p. djt369. doi: 10.1093/jnci/djt369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Domagała-Kulawik J., Hoser G., Safianowska A., Grubek-Jaworska H., Chazan R. Elevated TGF-β1 concentration in bronchoalveolar lavage fluid from patients with primary lung cancer. Archivum Immunologiae et Therapiae Experimentalis (Warsz) . 2006;54(2):143–147. doi: 10.1007/s00005-006-0016-0. [DOI] [PubMed] [Google Scholar]
- 62.Ni X. Y., Sui H. X., Liu Y., Ke S. Z., Wang Y. N., Gao F. G. TGF-β of lung cancer microenvironment upregulates B7H1 and GITRL expression in dendritic cells and is associated with regulatory T cell generation. Oncology Reports . 2012;28(2):615–621. doi: 10.3892/or.2012.1822. [DOI] [PubMed] [Google Scholar]
- 63.Zhang C. Y., Qi Y., Li X. N., et al. The role of CCL20/CCR6 axis in recruiting Treg cells to tumor sites of NSCLC patients. Biomedicine & Pharmacotherapy . 2015;69:242–248. doi: 10.1016/j.biopha.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 64.Wang S., Zhang Y., Wang Y., et al. Amphiregulin confers regulatory T cell suppressive function and tumor invasion via the EGFR/GSK-3β/Foxp3 axis∗. The Journal of Biological Chemistry . 2016;291(40):21085–21095. doi: 10.1074/jbc.M116.717892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ricciuti B., Leonardi G. C., Metro G., et al. Targeting the KRAS variant for treatment of non-small cell lung cancer: potential therapeutic applications. Expert Review of Respiratory Medicine . 2016;10(1):53–68. doi: 10.1586/17476348.2016.1115349. [DOI] [PubMed] [Google Scholar]
- 66.Zdanov S., Mandapathil M., Abu Eid R., et al. Mutant KRAS conversion of conventional T cells into regulatory T cells. Cancer Immunology Research . 2016;4(4):354–365. doi: 10.1158/2326-6066.CIR-15-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Granville C. A., Memmott R. M., Balogh A., et al. A central role for Foxp 3+ regulatory T cells in K-Ras-driven lung tumorigenesis. PLoS One . 2009;4(3):p. e5061. doi: 10.1371/journal.pone.0005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.La Fleur L., Botling J., He F., et al. Targeting MARCO and IL37R on immunosuppressive macrophages in lung cancer blocks regulatory T cells and supports cytotoxic lymphocyte function. Cancer Research . 2021;81(4):956–967. doi: 10.1158/0008-5472.CAN-20-1885. [DOI] [PubMed] [Google Scholar]
- 69.Guo N., Wen Y., Wang C., et al. Lung adenocarcinoma-related TNF-α-dependent inflammation upregulates MHC-II on alveolar type II cells through CXCR-2 to contribute to Treg expansion. The FASEB Journal . 2020;34(9):12197–12213. doi: 10.1096/fj.202000166RR. [DOI] [PubMed] [Google Scholar]
- 70.Cheng G., Yuan X., Tsai M. S., Podack E. R., Yu A., Malek T. R. IL-2 receptor signaling is essential for the development of Klrg 1+ terminally differentiated T regulatory cells. Journal of Immunology . 2012;189(4):1780–1791. doi: 10.4049/jimmunol.1103768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pierson W., Cauwe B., Policheni A., et al. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3+ regulatory T cells. Nature Immunology . 2013;14(9):959–965. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smigiel K. S., Richards E., Srivastava S., et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. The Journal of Experimental Medicine . 2014;211(1):121–136. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smigiel K. S., Srivastava S., Stolley J. M., Campbell D. J. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunological Reviews . 2014;259(1):40–59. doi: 10.1111/imr.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rech A. J., Mick R., Martin S., et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Science Translational Medicine . 2012;4(134, article 134ra62) doi: 10.1126/scitranslmed.3003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ganesan A. P., Johansson M., Ruffell B., et al. Tumor-infiltrating regulatory T cells inhibit endogenous cytotoxic T cell responses to lung adenocarcinoma. Journal of Immunology . 2013;191(4):2009–2017. doi: 10.4049/jimmunol.1301317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Curiel T. J., Coukos G., Zou L., et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Medicine . 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 77.Hoelzinger D. B., Smith S. E., Mirza N., Dominguez A. L., Manrique S. Z., Lustgarten J. Blockade of CCL1 inhibits T regulatory cell suppressive function enhancing tumor immunity without affecting T effector responses. Journal of Immunology . 2010;184(12):6833–6842. doi: 10.4049/jimmunol.0904084. [DOI] [PubMed] [Google Scholar]
- 78.Tan M. C., Goedegebuure P. S., Belt B. A., et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. Journal of Immunology . 2009;182(3):1746–1755. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sugiyama D., Nishikawa H., Maeda Y., et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+regulatory T cells, evoking antitumor immune responses in humans. Proceedings of the National Academy of Sciences of the United States of America . 2013;110(44):17945–17950. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alvisi G., Brummelman J., Puccio S., et al. IRF4 instructs effector Treg differentiation and immune suppression in human cancer. The Journal of Clinical Investigation . 2020;130(6):3137–3150. doi: 10.1172/JCI130426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scurr M., Pembroke T., Bloom A., et al. Low-dose cyclophosphamide induces antitumor T-cell responses, which associate with survival in metastatic colorectal cancer. Clinical Cancer Research . 2017;23(22):6771–6780. doi: 10.1158/1078-0432.CCR-17-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scurr M., Pembroke T., Bloom A., et al. Effect of modified vaccinia Ankara-5T4 and low-dose cyclophosphamide on antitumor immunity in metastatic colorectal cancer: a randomized clinical trial. JAMA Oncology . 2017;3(10, article e172579) doi: 10.1001/jamaoncol.2017.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Son C. H., Bae J. H., Shin D. Y., et al. Combination effect of regulatory T-cell depletion and ionizing radiation in mouse models of lung and colon cancer. International Journal of Radiation Oncology • Biology • Physics . 2015;92(2):390–398. doi: 10.1016/j.ijrobp.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 84.Tanaka A., Nishikawa H., Noguchi S., et al. Tyrosine kinase inhibitor imatinib augments tumor immunity by depleting effector regulatory T cells. The Journal of Experimental Medicine . 2020;217(2) doi: 10.1084/jem.20191009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Creelan B. C., Gray J. E., Tanvetyanon T., et al. Phase 1 trial of dasatinib combined with afatinib for epidermal growth factor receptor- (EGFR-) mutated lung cancer with acquired tyrosine kinase inhibitor (TKI) resistance. British Journal of Cancer . 2019;120(8):791–796. doi: 10.1038/s41416-019-0428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haura E. B., Tanvetyanon T., Chiappori A., et al. Phase I/II study of the Src inhibitor dasatinib in combination with erlotinib in advanced non-small-cell lung cancer. Journal of Clinical Oncology . 2010;28(8):1387–1394. doi: 10.1200/JCO.2009.25.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson M. L., Riely G. J., Rizvi N. A., et al. Phase II trial of dasatinib for patients with acquired resistance to treatment with the epidermal growth factor receptor tyrosine kinase inhibitors erlotinib or gefitinib. Journal of Thoracic Oncology . 2011;6(6):1128–1131. doi: 10.1097/JTO.0b013e3182161508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huynh A., DuPage M., Priyadharshini B., et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nature Immunology . 2015;16(2):188–196. doi: 10.1038/ni.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vahl J. C., Drees C., Heger K., et al. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity . 2014;41(5):722–736. doi: 10.1016/j.immuni.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 90.Levine A. G., Arvey A., Jin W., Rudensky A. Y. Continuous requirement for the TCR in regulatory T cell function. Nature Immunology . 2014;15(11):1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahmad S., Abu-Eid R., Shrimali R., et al. Differential PI3Kδ signaling in CD4(+) T-cell subsets enables selective targeting of T regulatory cells to enhance cancer immunotherapy. Cancer Research . 2017;77(8):1892–1904. doi: 10.1158/0008-5472.CAN-16-1839. [DOI] [PubMed] [Google Scholar]
- 92.Ali K., Soond D. R., Pineiro R., et al. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature . 2014;510(7505):407–411. doi: 10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Acosta-Rodriguez E. V., Rivino L., Geginat J., et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nature Immunology . 2007;8(6):639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 94.Crome S. Q., Wang A. Y., Levings M. K. Translational mini-review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clinical and Experimental Immunology . 2010;159(2):109–119. doi: 10.1111/j.1365-2249.2009.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peck A., Mellins E. D. Plasticity of T-cell phenotype and function: the T helper type 17 example. Immunology . 2010;129(2):147–153. doi: 10.1111/j.1365-2567.2009.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duan M. C., Han W., Jin P. W., et al. Disturbed Th17/Treg balance in patients with non-small cell lung cancer. Inflammation . 2015;38(6):2156–2165. doi: 10.1007/s10753-015-0198-x. [DOI] [PubMed] [Google Scholar]
- 97.Li S., Li Y., Qu X., Liu X., Liang J. Detection and significance of TregFoxP3(+) and Th17 cells in peripheral blood of non-small cell lung cancer patients. Archives of Medical Science . 2014;10(2):232–239. doi: 10.5114/aoms.2014.42573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao L., Yang J., Wang H. P., Liu R. Y. Imbalance in the Th17/Treg and cytokine environment in peripheral blood of patients with adenocarcinoma and squamous cell carcinoma. Medical Oncology . 2013;30(1):p. 461. doi: 10.1007/s12032-013-0461-7. [DOI] [PubMed] [Google Scholar]
- 99.Koenen H. J., Smeets R. L., Vink P. M., van Rijssen E., Boots A. M. H., Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood . 2008;112(6):2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 100.Yang X. O., Nurieva R., Martinez G. J., et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity . 2008;29(1):44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou L., Lopes J. E., Chong M. M., et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature . 2008;453(7192):236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu W., Ji N., Gu C., et al. IRF4 is correlated with the conversion to a Th17-like phenotype in regulatory T cells from the malignant pleural effusion. International Journal of General Medicine . 2021;Volume 14:6009–6019. doi: 10.2147/IJGM.S330389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zou J. Y., Su C. H., Luo H. H., et al. Curcumin converts Foxp 3+ regulatory T cells to T helper 1 cells in patients with lung cancer. Journal of Cellular Biochemistry . 2018;119(2):1420–1428. doi: 10.1002/jcb.26302. [DOI] [PubMed] [Google Scholar]


