Abstract
The effect of a water-soluble trimalonic acid derivative of fullerene, carboxyfullerene, against Streptococcus pyogenes infection was tested. Pretreatment with carboxyfullerene was able to protect mice from S. pyogenes infection in an air pouch model. S. pyogenes-induced death and skin injury were inhibited dose dependently by carboxyfullerene. Administration of carboxyfullerene via the peritoneum and air pouch at 3 h post-S. pyogenes infection was able to protect 33% of mice from death. Surveys of exudates of the air pouch of carboxyfullerene-treated mice revealed that survival of infiltrating neutrophils was prolonged and that the bacteria were eliminated as a result of enhanced bactericidal activity of the neutrophils. Furthermore, carboxyfullerene was able to directly inhibit in vitro growth of S. pyogenes. These data suggest that carboxyfullerene can be considered an antimicrobial agent for group A streptococcus infection.
The incidence of infection with the group A streptococcus (GAS) Streptococcus pyogenes has increased worldwide and is still associated with a high mortality rate, especially when GAS infection induces necrotizing fasciitis and streptococcal toxic shock syndrome (1, 3, 11, 14, 15, 21). Certain virulence factors involved in the pathogenesis of GAS infection have been reported. These include cell surface molecules such as M protein, opacity factor, the hyaluronic acid capsule, C5a peptidase, and the streptococcal inhibitor of complement, as well as secreted products such as pyogenic exotoxins, cysteine proteinase, streptolysins O and S, hyaluronidase, streptokinase, and other enzymes (3, 12, 15). Empirical therapy for GAS infection includes antibiotics, aggressive surgery, and intravenous administration of immunoglobulin (21, 22).
Buckminsterfullerenes (fullerene [C60]) have attracted much attention since their discovery and large-scale synthesis. Fullerene is characterized as a “radical sponge” because of its unique cage structure, which allows it to interact effectively with free radicals (7). However, native C60 is soluble only in organic solvents and so cannot be applied to medical therapy. A water-soluble trimalonic acid derivative of fullerene (carboxyfullerene [C63(COOH)6]) has been synthesized and has been found to be an effective neuroprotective antioxidant both in vitro and in vivo (2, 9). Carboxyfullerene is a powerful free radical scavenger and can protect cells from apoptosis in various systems (4, 5). In previous studies, we found that carboxyfullerene was able to inhibit the development of Escherichia coli-induced meningitis (18) by modulating the activity of neutrophils (16). In the present study, the effect of carboxyfullerene on gram-positive bacterial infections was evaluated. Using an air pouch GAS infection model, which mimics localized fasciitis (8), we found that carboxyfullerene was able to inhibit GAS infection by both modulating the bactericidal activity of infiltrating neutrophils and directly inhibiting the growth of S. pyogenes.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6) mice were purchased from The Jackson Laboratory, Bar Harbor, Maine, or from Charles River Japan, Inc. (Atsugi, Japan). They were maintained on standard laboratory chow and water ad libitum in our animal center. The animals were raised and cared for in accordance with guidelines established by the National Science Council of the Republic of China. Eight- to 12-week-old female mice were used in all experiments.
Carboxyfullerene.
Two regioisomers of water-soluble carboxylic acid C60 derivatives with C3 or D3 symmetry were synthesized as described previously (2). Both C63(COOH)6 (C3) and C63(COOH)6 (D3) are effective free radical scavengers. In this study, we used C63(COOH)6 (C3) dissolved in phosphate-buffered saline (PBS) (2 mg/ml).
Bacteria.
S. pyogenes A-20 (type M1, T1; opacity factor negative) was isolated from the blood of a patient with necrotizing fasciitis at the National Cheng Kung University Hospital. S. pyogenes NZ-131 (type M49, T14) was a gift from D. R. Martin, New Zealand Communicable Disease Center, Porirua. Genotyping of A-20 revealed the presence of speA, speB, and speC, whereas NZ-131 had just speB (8). S. pyogenes was cultured in tryptic soy broth containing 0.5% yeast extract (TSBY) (Difco Laboratories, Detroit, Mich.) for 12 h at 37°C and then subcultured in fresh broth (1:50, vol/vol) for another 3 h. The concentration of bacteria was determined with a spectrophotometer (Beckman Instruments, Somerset, N.J.), with an optical density at 600 nm of 1 being equal to 108 CFU/ml (20).
Air pouch model of infection.
Mice were anesthetized by ether inhalation and then injected subcutaneously with 1 ml of air for three consecutive days to form an air pouch. Two days later, 0.1 ml of bacterial suspension containing 1 × 109 S. pyogenes A-20 cells or 2 × 109 S. pyogenes NZ-131 cells was inoculated into the air pouch (8). The 100% lethal doses (LD100) of S. pyogenes A-20 and NZ-131 by air pouch injection in B6 mice are 1 × 109 cells and 2 × 109 cells, respectively. The animals were observed every day for a total of 5 days. In carboxyfullerene inhibition experiments, the mice were given an air pouch injection of carboxyfullerene immediately post-S. pyogenes injection or as late as 3 h post-S. pyogenes injection. In some experiments, carboxyfullerene was given via both air pouch and intraperitoneal injections. Survival curves were determined. Tissues around the air pouch were excised 24 h after bacterial inoculation, fixed in 10% formaldehyde, and embedded in paraffin. The 5-μm-thick tissues were sliced and stained with hematoxylin and eosin. Infiltrating cells in the air pouch were collected by injecting 1 ml of PBS into the air pouch and aspirating the exudates by syringe with an 18-gauge needle (8). Numbers of cells were determined with a hemocytometer, and cell viability was determined by eosin Y exclusion.
Bacterial growth curves.
S. pyogenes A-20 was cultured in TSBY at 37°C overnight, and then the bacterial suspension was subcultured (1:50, vol/vol) in fresh TSBY for another 8 h. At the time of subculture, different concentrations of carboxyfullerene were added to the bacterial suspension, and the growth of bacteria at different times was determined with a spectrophotometer by measuring the absorbance at 600 nm. For exact quantification of bacteria, bacterial suspensions collected at different times were plated on blood agar and incubated for 24 h at 37°C. The results of one of three experiments are reported.
Bactericidal activity of neutrophils.
Neutrophils were purified from the blood of naïve B6 mice by Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) centrifugation (17). The neutrophils were resuspended (106 in 1 ml) in 24-well plates (Falcon; Becton-Dickinson Labware, Paramus, N.J.) and incubated for 4 h in RPMI 1640 medium containing 10% fetal calf serum with different concentrations of carboxyfullerene. Carboxyfullerene at 200 μg/ml is not toxic to neutrophils in 4-h culture. The neutrophils treated with carboxyfullerene were washed twice with PBS, and the cells were cocultured with S. pyogenes A-20 at ratio of 20:1 (bacteria to neutrophils) in RPMI 1640 medium for another hour. After 1 h of incubation, the supernatant was collected by centrifugation and plated on blood agar for quantification of bacteria.
RESULTS
Inhibition of GAS infection by carboxyfullerene.
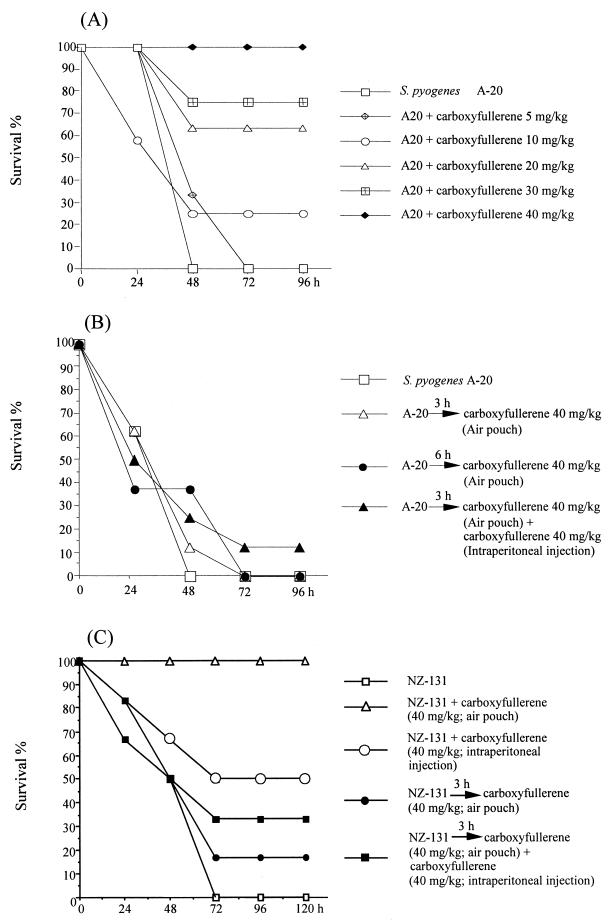
In the air pouch model of GAS infection, inoculation of 109 S. pyogenes A-20 cells (the LD100 for mice at 48 h) causes hair loss, suppuration, and bleeding on the skin around the air pouch at 24 h (8); the mice die at 48 h. The effect of carboxyfullerene on GAS-induced mortality was evaluated. As shown in Fig. 1A, carboxyfullerene pretreatment inhibited S. pyogenes A-20-induced death dose dependently. Injection of 10 mg of carboxyfullerene/kg of body weight resulted in 25% survival (2 of 8 mice), 20 mg/kg resulted in 63% survival (5 of 8), 30 mg/kg resulted in 75% survival (6 of 8), and 40 mg/kg completely protected mice from S. pyogenes A-20-induced death (Fig. 1A). In control mice, the structure of the epidermis, dermis, and subcutaneous fat on the skin around the air pouch was destroyed, but in carboxyfullerene-treated mice, the skin remained intact (Fig. 2). Thus, carboxyfullerene can inhibit S. pyogenes A-20-induced death as well as skin injury.
FIG. 1.
Inhibition of GAS infection by carboxyfullerene. (A) Carboxyfullerene inhibits S. pyogenes A-20-induced death in B6 mice dose dependently. Groups of four B6 mice were inoculated via the air pouch with 109 S. pyogenes A-20 cells per mouse. Various doses of carboxyfullerene were administrated via air pouch immediately after injection of S. pyogenes A-20. The mice were monitored for death every day for a total of 4 days. A total of eight mice were pooled in two experiments. Carboxyfullerene alone at 40 mg per kg has no effect on mice. (B) Therapeutic effect of carboxyfullerene against S. pyogenes A-20 infection in B6 mice. Groups of four B6 mice were inoculated via the air pouch with 109 S. pyogenes A-20 cells per mouse. Carboxyfullerene (40 mg per kg per mouse) was administrated via air pouch at 3 or 6 h postinfection. In one group, carboxyfullerene was administrated via both air pouch and intraperitoneal injection (40 mg per kg per route) at 3 h after infection. The mice were monitored for death every day for a total of 4 days. A total of eight mice were pooled in two experiments. (C) Therapeutic effect of carboxyfullerene against S. pyogenes NZ-131 infection in B6 mice. Groups of six B6 mice were inoculated via the air pouch with 2 × 109 S. pyogenes NZ-131 cells per mouse. In the preventive experiment, carboxyfullerene (40 mg per kg per mouse) was administrated immediately after inoculation of S. pyogenes NZ-131 via air pouch or intraperitoneal injection. In the therapeutic experiments, carboxyfullerene (40 mg per kg per mouse) was administrated at 3 h postinfection via air pouch or combined air pouch and intraperitoneal injection. The mice were monitored for death every day for 5 days (n = 6).
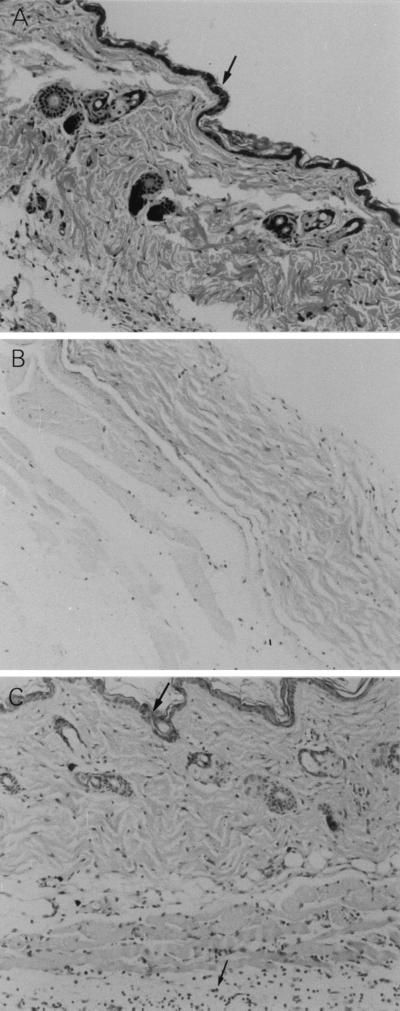
FIG. 2.
Inhibition of GAS-induced skin damage by carboxyfullerene. Groups of four B6 mice were inoculated via the air pouch with 109 S. pyogenes A-20 cells per mouse. Carboxyfullerene (40 mg/kg) was administrated immediately after injection of S. pyogenes A-20, and the mice were sacrificed at 24 h postinfection. The skin tissue around the air pouch was excised and fixed in 10% formaldehyde, as described in Materials and Methods. Five-micrometer-thick sections were stained with hematoxylin and eosin. (A) PBS-inoculated control. The arrow indicates intact epidermis. (B) S. pyogenes A-20 treatment. (C) S. pyogenes A-20 and carboxyfullerene treatment. The top arrow indicates intact epidermis; the bottom arrow indicates infiltrating neutrophils. Magnification, ×100.
The therapeutic effect of carboxyfullerene after GAS infection was examined next. As shown in Fig. 1B, the mice died within 48 h of injection of 109 S. pyogenes A-20 cells. Administration of 40 mg of carboxyfullerene/kg in the air pouch at 3 or 6 h postinjection only delayed the death of the mice; the mice still died by 72 h. When a combination of air pouch and intraperitoneal administration of 40 mg of carboxyfullerene/kg at 3 h postinfection was used, there was a marginal effect, as 12.5% of the mice survived. To repeat this experiment, we used another isolate, S. pyogenes NZ-131, which is less virulent than S. pyogenes A-20. The LD100 of NZ-131 is 2 × 109 cells at 72 h via air pouch inoculation. Pretreatment with carboxyfullerene protected the mice from S. pyogenes NZ-131-induced death in a dose-dependent manner (data not shown); 40 mg of carboxyfullerene/kg completely protected mice from S. pyogenes NZ-131-induced death (Fig. 1C). Intraperitoneal injection of 40 mg of carboxyfullerene/kg protected only 50% of mice from death; thus, intraperitoneal injection of carboxyfullerene is less effective than local injection in the air pouch at inhibiting S. pyogenes-induced death. Administration of 40 mg/kg in the air pouch at 3 h postinfection protected 17% of the mice from death. When combined air pouch and peritoneal injection at a total dose of 80 mg/kg was used, the protective effect increased to 33%. These results suggest that carboxyfullerene has a partial therapeutic effect on GAS infection. Its inhibitory effect is influenced by the dose of carboxyfullerene and the virulence of the S. pyogenes isolate used.
Carboxyfullerene not only prolongs the survival of infiltrating neutrophils but also accelerates the clearance of bacteria.
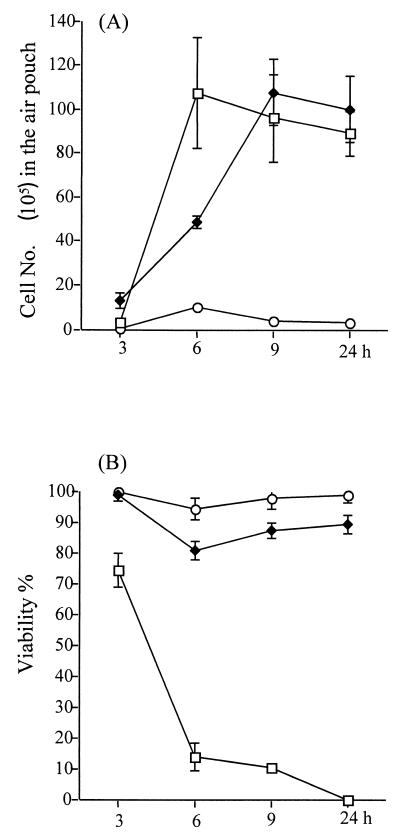
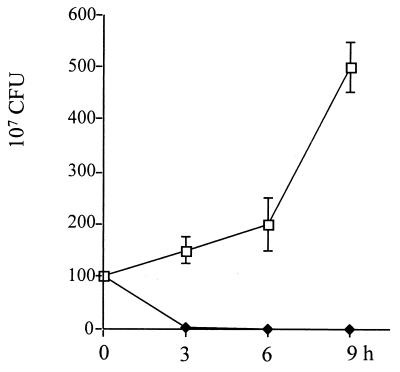
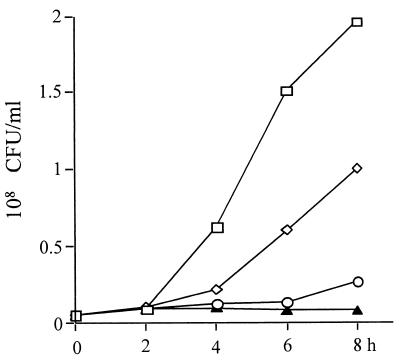
The working mechanism of carboxyfullerene-mediated inhibition was investigated next. Histological examination showed that neutrophils had infiltrated the subcutaneous area after carboxyfullerene treatment (Fig. 2C). Therefore, the infiltrating neutrophils in the exudates were collected from the air pouches, and their total numbers and viability were determined. It was found that neutrophil infiltration of the air pouches began at 3 h, reached a maximum at 6 h, and then declined from 9 h to 24 h after inoculation of S. pyogenes A-20. In carboxyfullerene-treated mice, the kinetics of neutrophil infiltration were slightly slower than in untreated controls but the total numbers of infiltrating cells did not differ between the two groups (Fig. 3A). However, the viability of the infiltrating neutrophils dropped significantly in untreated mice: at 6 h post-S. pyogenes A-20 injection, most of the infiltrating cells (>90%) were dead. In contrast, the viability of neutrophils in carboxyfullerene-treated mice remained at over 85% (Fig. 3B). Further examination of remnant bacteria in the exudates revealed that the bacteria were cleared quickly: no bacteria (<100 CFU/ml) were found in the exudates by 6 h after carboxyfullerene treatment. In contrast, bacteria continued to grow in the air pouches of untreated mice (Fig. 4). To avoid the carryover effects of remnant carboxyfullerene in the exudates, the exudates collected at different times were diluted at least 100× with PBS, and the lower limit of detection was 100 CFU/ml. The bactericidal activity of neutrophils enhanced by carboxyfullerene was further demonstrated. Peripheral neutrophils from naïve mice were isolated and incubated for 4 h with different concentrations (5 to 200 μg/ml) of carboxyfullerene and then washed with PBS to remove the carboxyfullerene, after which the bactericidal activity of neutrophils was determined. With no carboxyfullerene treatment, S. pyogenes A-20 was present at (3.2 ± 1.1) × 108 CFU/ml; with carboxyfullerene at 5, 50, and 200 μg/ml, numbers of bacteria were (3.8 ± 2.1) × 108, (1.1 ± 1.0) × 108, and (8.5 ± 2.1) × 106 CFU/ml, respectively. These results suggest that carboxyfullerene not only prolongs neutrophil survival but also enhances their bactericidal activity.
FIG. 3.
Numbers (A) and viability (B) of infiltrating neutrophils in air pouches after carboxyfullerene treatment of GAS infection. Groups of four B6 mice were inoculated via the air pouch with 109 S. pyogenes A-20 cells per mouse. Carboxyfullerene was administrated via air pouch immediately after injection of S. pyogenes A-20, and infiltrating cells were collected at different times postinfection, as described in Materials and Methods. Treatments: □, S. pyogenes A-20 only; ♦, S. pyogenes A-20 plus carboxyfullerene (40 mg/kg); ○, carboxyfullerene (40 mg/kg) only.
FIG. 4.
Bacteria in air pouches after carboxyfullerene treatment of GAS infection. Groups of four B6 mice were inoculated via the air pouch with 109 S. pyogenes A-20 cells per mouse. Carboxyfullerene was administered via air pouch immediately after injection of S. pyogenes A-20. Exudates containing bacteria were collected, and bacteria were counted at different times postinfection, as described in Materials and Methods. Treatments: □, S. pyogenes A-20 only; ♦, S. pyogenes A-20 plus carboxyfullerene (40 mg/kg).
Inhibition of in vitro GAS growth by carboxyfullerene.
The direct effect of carboxyfullerene on in vitro growth of GAS was examined. As shown in Fig. 5, carboxyfullerene was able to inhibit the growth of S. pyogenes A-20 directly in an in vitro culture, and its inhibition was dose dependent. Based on the above data, we conclude that carboxyfullerene inhibits GAS infection both by enhancing the bactericidal activity of neutrophils and by directly inhibiting growth of S. pyogenes.
FIG. 5.
Inhibition of in vitro growth of S. pyogenes A-20 by carboxyfullerene. S. pyogenes A-20 (4 × 106cells/ml) was cultured with various concentrations of carboxyfullerene in TSBY, and bacterial growth was determined by counting colonies on blood agar plates, as described in Materials and Methods. One of three experiments is represented. Amount of carboxyfullerene: □, none; ⋄, 5 μg/ml; ○, 50 μg/ml; ▴, 200 μg/ml.
DISCUSSION
GAS not only causes serious diseases in humans but also is associated with a high mortality rate, especially in streptococcal toxic shock syndrome, which kills 30 to 60% of patients in 72 to 96 h (15). Penicillin, which has been the recommended drug for GAS infection (13), is less effective against severe infections because of its short postantibiotic effect and reduction of activity against stationary-phase organisms. Nowadays, therapy for invasive GAS infection consists mainly of clindamycin, aggressive surgery, and intravenous administration of immunoglobulins (15). However, inappropriate use of antibiotics can lead to the emergence of resistant bacteria. In this study, we demonstrate that carboxyfullerene inhibits GAS infection both through direct inhibition of bacterial growth and by enhancing the bactericidal activity of neutrophils. These findings suggest a new way to treat GAS infection.
Fullerenes have a unique cage structure that allows them to interact effectively with biomolecules and free radicals. These properties of fullerenes have generated great interest in their use in biomedical research (6, 7). It is necessary to convert hydrophobic C60 into one of its water-soluble derivatives before using it as a free radical scavenger or an antioxidant in medical or therapeutic applications. A water-soluble trimalonic acid derivative of C60, carboxyfullerene [C63(COOH)6], has recently been synthesized and has been reported to have protective effects in various systems. These effects include protection of cultured cortical neurons from excitotoxic injury in vitro, delaying of neuronal deterioration in a transgenic model of familial amylotrophic lateral sclerosis (2), blocking of apoptosis signaling induced by transforming growth factor-β in human hepatoma cells (5), and prevention of iron-induced oxidative injury in rat brain (9). In a previous study, we found that carboxyfullerene was able to inhibit E. coli-induced meningitis and that it did so through enhancing the host immune response rather than by direct antimicrobial activity (18). Furthermore, we found that carboxyfullerene was able to decrease the damage of infiltrating neutrophils at the blood-brain barrier during E. coli-induced meningitis (16). The bactericidal activity of neutrophils against E. coli in vitro was also shown to be enhanced by carboxyfullerene (unpublished data). Although the bactericidal activity of neutrophils against E. coli and S. pyogenes has been shown to be enhanced by carboxyfullerene, the phagocytic activity of neutrophils has not (unpublished data). Infiltrating neutrophils from carboxyfullerene-treated air pouches have greater viability than those from untreated controls, suggesting that carboxyfullerene might modulate neutrophil activity by enhancing the survival of activated neutrophils. Nevertheless, our data suggest that carboxyfullerene has dual roles: modulation of neutrophils to enhance its bactericidal activity and direct inhibition of S. pyogenes growth. In addition to its effects on neutrophils, other in vivo effects of carboxyfullerene administration have not been excluded; we are currently investigating this. Since we do not have appropriate methods of quantitating carboxyfullerene, its pharmacokinetics in vivo are not understood.
The therapeutic effect of carboxyfullerene is greater against E. coli-induced meningitis than against S. pyogenes-induced septic shock. The LD100 of E. coli in the meningitis model was 5 × 105 cells, and E. coli in the brain was limited until 12 h after infection, so administration of carboxyfullerene as late as 9 h after infection still had a protective effect (18). In contrast, in S. pyogenes-induced septic shock, the number of bacteria (1 × 109 to 2 × 109 cells) inoculated into the air pouch is much higher than the number of E. coli cells used in the meningitis model. S. pyogenes replicates quickly and invades the bloodstream at 6 h postinjection, and it secretes cysteine protease. Once S. pyogenes spreads systemically, it is difficult to inhibit even by combined treatment with both air pouch and intraperitoneal injection. When the inoculation dose of S. pyogenes is lowered from 109 to 108 cells, the survival rate ranges from 20% in untreated mice to 80% in carboxyfullerene-treated mice (data not shown). The highest dosage used in this study (a total of 80 mg/kg by air pouch and intraperitoneal injection) was far below the toxic dose. The LD50 of fullerenol in mice by intraperitoneal injection is 1,000 mg/kg (19). For therapeutic purposes, the dosage can be increased further in future studies.
In a previous study, carboxyfullerene was unable to inhibit E. coli growth directly (18), but in this study it was able to inhibit S. pyogenes A-20 growth. Carboxyfullerene inhibits the growth of gram-positive, but not gram-negative, bacteria, as confirmed with several different strains of bacteria (unpublished observations). This difference is interesting and worth further investigation. We have also reported that carboxyfullerene is able to inactivate enveloped viruses such as dengue virus and Japanese encephalitis virus by a light-independent mechanism (10). It blocks virus replication at the attachment and penetration stages, suggesting a direct interaction between carboxyfullerene and virions. The inhibition of gram-positive bacteria such as S. pyogenes is an example of direct contact between carboxyfullerene and the gram-positive cell wall. Carboxyfullerene has not only free radical scavenging activity that might modulate neutrophil activity but also an antimicrobial effect on gram-positive bacteria such as S. pyogenes. This dual activity of carboxyfullerene permits this nonantibiotic compound to affect both phagocytes and the bacterium itself during infection.
ACKNOWLEDGMENTS
This work was supported by grant NHRI-GT-EX89B717L from the National Health Research Institute, Department of Health, Republic of China.
REFERENCES
- 1.Badgett J T, Hesterberg L K. Management of group A streptococcus pharyngitis with a second-generation rapid strep screen: Strep A OIA. Microb Drug Resist. 1996;2:371–376. doi: 10.1089/mdr.1996.2.371. [DOI] [PubMed] [Google Scholar]
- 2.Dugan L L, Turetsky D M, Du C, Lobner D, Wheeler M, Almli C R, Shen C K F, Luh T Y, Choi D W, Lin T S. Carboxyfullerenes as neuroprotective agents. Proc Natl Acad Sci USA. 1997;94:9434–9439. doi: 10.1073/pnas.94.17.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Efstratious A. Group A streptococci in the 1990s. J Antimicrob Chemother. 2000;45(Suppl.):3–12. doi: 10.1093/jac/45.suppl_1.3. [DOI] [PubMed] [Google Scholar]
- 4.Hsu S C, Wu C C, Luh T Y, Chou C K, Han S H, Lai M Z. Apoptotic signal of Fas is not mediated by ceramide. Blood. 1998;91:2658–2663. [PubMed] [Google Scholar]
- 5.Huang Y L, Shen C K F, Luh T Y, Yang H C, Hwang K C, Chou C K. Blockage of apoptotic signaling of transforming growth factor-β in human hepatoma cells by carboxyfullerene. Eur J Biochem. 1998;254:38–43. doi: 10.1046/j.1432-1327.1998.2540038.x. [DOI] [PubMed] [Google Scholar]
- 6.Kratshmer W, Lamb L D, Fostiropoulos K, Huffman D R. Solid C60: a new form of carbon. Nature. 1990;347:354–358. [Google Scholar]
- 7.Krusic P J, Wasserman E, Keizer P N, Morton J R, Preston K F. Radical reaction of C60. Science. 1991;254:1183–1185. doi: 10.1126/science.254.5035.1183. [DOI] [PubMed] [Google Scholar]
- 8.Kuo C F, Wu J J, Lin K Y, Tsai P J, Lee S C, Lin Y T, Lei H Y, Lin Y S. Role of streptococcal pyrogenic exotoxin B in the mouse model of group A streptococcal infection. Infect Immun. 1998;66:3931–3935. doi: 10.1128/iai.66.8.3931-3935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin A Y M, Chyi B Y, Wang S D, Yu H H, Kanakamma P P, Luh T Y, Chou C K, Ho L T. Carboxyfullerenes prevent iron-induced oxidative injury in the nigrostriatal dopaminergic system in rat brain. J Neurochem. 1999;72:1634–1640. doi: 10.1046/j.1471-4159.1999.721634.x. [DOI] [PubMed] [Google Scholar]
- 10.Lin Y L, Lei H Y, Wen Y Y, Luh T Y, Chou C K, Liu H S. Light-independent inactivation of dengue-2 virus by carboxyfullerene C3 isomer. Virology. 2000;275:258–262. doi: 10.1006/viro.2000.0490. [DOI] [PubMed] [Google Scholar]
- 11.Murray D L, Ohlendorf D H, Schlievert P M. Staphylococcal and streptococcal superantigens: their role in human diseases. ASM News. 1995;61:229–235. [Google Scholar]
- 12.Oliver C. Rheumatic fever—is it a problem? J Antimicrob Chemother. 2000;45(Suppl.):13–21. doi: 10.1093/jac/45.suppl_1.13. [DOI] [PubMed] [Google Scholar]
- 13.Peter G. Streptococcal pharyngitis: current therapy and criteria for evaluation of new agents. Clin Infect Dis. 1992;14(Suppl. 2):S218–S223. doi: 10.1093/clinids/14.supplement_2.s218. [DOI] [PubMed] [Google Scholar]
- 14.Spencer R C. Invasive streptococci. Eur J Clin Microbiol Infect Dis. 1995;14(Suppl.):S26–S32. [PubMed] [Google Scholar]
- 15.Steven D L. Streptococcal toxic shock syndrome associated with necrotizing fasciitis. Annu Rev Med. 2000;51:271–288. doi: 10.1146/annurev.med.51.1.271. [DOI] [PubMed] [Google Scholar]
- 16.Tsao, N., C. M. Wu, H. P. Hsu, C. C. Liu, T. Y. Luh, C. K. Chou, and H. Y. Lei. Inhibition of the increased permeability of blood-brain barrier in Escherichia coli-induced meningitis by carboxyfullerene. Fullerene Sci. Technol., in press.
- 17.Tsao N, Hsu H P, Lei H Y. TNF-α-induced cyclooxygenase 2 not only increases the vasopermeability of blood-brain barrier but also enhances the neutrophil survival in Escherichia coli-induced brain inflammation. Prostaglandins Other Lipid Mediat. 1999;57:371–382. doi: 10.1016/s0090-6980(99)00013-1. [DOI] [PubMed] [Google Scholar]
- 18.Tsao N, Kanakamma P P, Luh T Y, Chou C K, Lei H Y. Inhibition of Escherichia coli-induced meningitis by carboxyfullerence. Antimicrob Agents Chemother. 1999;43:2273–2277. doi: 10.1128/aac.43.9.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Useng T H, Kang J J, Wang H W, Cheng Y W, Chiang L Y. Suppression of microsomal cytochrome P450-dependent monooxygenases and mitochondrial oxidative phosphorylation by fullerenol, a polyhydroxylated fullerene C60. Toxicol Lett. 1997;93:29–37. doi: 10.1016/s0378-4274(97)00071-4. [DOI] [PubMed] [Google Scholar]
- 20.Wang S D, Huang K J, Lin Y S, Lei H Y. Sepsis-induced apoptosis of the thymocytes in mice. J Immunol. 1994;152:5014–5021. [PubMed] [Google Scholar]
- 21.Weiss K A, Laverdiere M. Group A streptococcus invasive infection: a review. Can J Surg. 1997;40:18–25. [PMC free article] [PubMed] [Google Scholar]
- 22.Wheeler C M, Roe M H, Kaplan E L, Schlievert P M, Todd J K. Outbreak of group A streptococcus septicemia in children: clinical, epidemiologic, and microbiological correlates. JAMA. 1991;266:533–537. [PubMed] [Google Scholar]