Abstract
Cognitive deficits are central attendant symptoms of major depressive disorder (MDD) with a crucial impact in patients’ everyday life. Thus, it is of particular clinical importance to understand their pathophysiology. The aim of this study was to investigate a possible relationship between brain structure and cognitive performance in MDD patients in a well-characterized sample. N = 1007 participants (NMDD = 482, healthy controls (HC): NHC = 525) were selected from the FOR2107 cohort for this diffusion-tensor imaging study employing tract-based spatial statistics. We conducted a principal component analysis (PCA) to reduce neuropsychological test results, and to discover underlying factors of cognitive performance in MDD patients. We tested the association between fractional anisotropy (FA) and diagnosis (MDD vs. HC) and cognitive performance factors. The PCA yielded a single general cognitive performance factor that differed significantly between MDD patients and HC (P < 0.001). We found a significant main effect of the general cognitive performance factor in FA (Ptfce-FWE = 0.002) in a large bilateral cluster consisting of widespread frontotemporal-association fibers. In MDD patients this effect was independent of medication intake, the presence of comorbid diagnoses, the number of previous hospitalizations, and depressive symptomatology. This study provides robust evidence that white matter disturbances and cognitive performance seem to be associated. This association was independent of diagnosis, though MDD patients show more pronounced deficits and lower FA values in the global white matter fiber structure. This suggests a more general, rather than the depression-specific neurological basis for cognitive deficits.
Subject terms: Diagnostic markers, Neuroscience
Introduction
Cognitive deficits are attendant symptoms of major depressive disorder (MDD) as defined by the International Classification of Disease (ICD-11) [1] that occur in two-thirds of depressed patients [2]. Deficits were described in several domains of cognition including executive function, attention, concentration, learning, memory, and psychomotor processing speed [2–5], while automatic stages of processing seem to be less affected than controlled, effortful processing domains [6, 7].
Understanding the causes of cognitive dysfunction in depression is of high clinical relevance. Some cognitive deficits seem to persist after remission [8–10], and increase with every MDD episode [11, 12]. They are associated with reductions in psychosocial functioning in MDD [13, 14] with consequences for occupation, social interactions, and health. First, patients suffering from cognitive deficits are less likely to obtain and sustain a job [15]. Second, they have problems maintaining household, or social and family relationships [16]. Lastly, cognitive deficits appear to increase proneness to relapse [17] and suicidal ideations [18], by reducing social support [16], impairing treatment success [19], and compromising problem-solving capacities [20]. The treatment of cognitive deficits could therefore improve MDD patients’ functioning.
The neurobiological perspective might shed light on the underpinnings of cognitive deficits in MDD. Following lesion studies, the classic neurobiological view of cognitive deficits focused on the impairment of specialized brain regions responsible for unique cognitive operations. In doing so, complex models were established that focus on the prefrontal cortex, hippocampus, anterior cingulate cortex, and basal ganglia [21].
The brain is, however, characterized by a network of complex, reciprocal anatomical connections. Thus, the connectome perspective that higher cognitive functioning depends upon the integration of various inputs from specialized regions, seems more consistent with the brain’s architecture [22]. One possible measure of the microstructure of interconnecting fibers is diffusion-tensor imaging (DTI), a noninvasive, affordable, and efficient measurement to estimate fiber microstructure, reflecting myelination, axon density, axon diameter, and the number of fibers [23, 24]. White matter microstructure assessed by means of DTI has shown strong associations with cognitive performance in multiple studies in healthy controls (HC) and patient groups. In HC, cognitive performance measures were linked to fiber integrity in frontal association fibers [25–27], like the corpus callosum (CC), the cingulum bundle (CB), the superior longitudinal fasciculi (SLF), or the inferior fronto-occipital fasciculi (IFOF) [28, 29]. Likewise, similar associations of DTI-based measures of fiber integrity and cognitive deficits have already been described in different brain disorders, e.g., stroke [30], Parkinsons disease [31], small-vessel disease [32], multiple sclerosis [33], diabetes [34], substance abuse [22], schizophrenia [35, 36], or bipolar disorder [37].
However, the role of white matter integrity regarding cognitive deficits of MDD patients has attracted less attention, albeit MDD was associated with reductions in fiber microstructure in the IFOF, the uncinate fasciculi (UF), the thalamic radiation (TR), the corticospinal tract (CT), and the inferior longitudinal fasciculi (ILF) and the SLF, the CB, and the CC compared with HC [38–41] and changes in the white matter connectome [42, 43].
Unfortunately, studies investigating the association between these microstructural abnormalities and cognitive deficits in MDD are sparse: In geriatric depression, associations between cognitive deficits and brain microfiber structure were found in overall prefrontal white matter, the CC, the TR, and the UF [44–46]. However, we are not aware of a study investigating white matter disturbances using DTI over the entire age and severity range of MDD patients.
Another open question is the specificity of these potential alterations in MDD. The aim of this study was, thus, to the extent of previous results to the entire severity spectrum of MDD patients and to compare the association between fiber microstructure and cognitive deficits with HC. First, we expect MDD patients to perform worse on cognitive tests. These deficits should decline, but still be detectable in remitted patients (hypothesis 1). Second, we expect that MDD patients have lower fiber microstructure compared with HC in the IFOF, the UF, the TR, the CT, the SLF and the ILF, the CB, and the CC (hypothesis 2). Further, as associations between white matter microstructure and cognitive deficits were already shown for a wide range of disorders, we do not expect that the association of cognitive test measures and white matter integrity is restricted to MDD patients. Rather, we would assume that the magnitude of the association between white matter and cognitive functioning should be similar between MDD patients and HC (hypothesis 3).
Materials and methods
Participants
N = 1007 participants (MDD: N = 482, Mage = 37.12, 311♀, HC: N = 525, Mage = 31.68, 321♀, Table 1, Supplement 1) were selected from the FOR2107 cohort assessed at two scanning sites—Marburg and Münster (the general description of the study [47] and the magnetic resonance imaging (MRI) quality-assurance protocol [48] are provided elsewhere). Participants were recruited through newspaper advertisements or in psychiatric hospitals.
Table 1.
Descriptive statistics of the sample used in this study.
| MDD (N = 482) | HC (N = 525) | Test statistic | P value | Cohen’s d | |
|---|---|---|---|---|---|
| Age, M ± SD | 37.12 ± 13.47 | 31.68 ± 11.87 | t(962.3) = −6.77a | <0.001 | 0.436 |
| Sex, f/m | 311/171 | 321/204 | χ²(1) = 1.23b | 0.268 | – |
| IQMVT, M ± SD | 113.79 ± 13.71 | 114.98 ± 13.72 | t(1005) = 1.38c | 0.170 | 0.087 |
| Education years, M ± SD | 13.15 ± 2.76 | 13.98 ± 2.42 | t(1005) = 5.05c | <0.001 | 0.329 |
| BDI Sum, M ± SD | 17.99 ± 11.19 | 2.50 ± 2.15 | t(505.68) = −29.68a | <0.001 | 2.640 |
| TMT-A, M ± SD | 26.16 ± 10.36 | 22.52 ± 8.40 | t(927.0) = −6.10a | <0.001 | 0.401 |
| TMT-B, M ± SD | 57.12 ± 24.30 | 46.89 ± 17.30 | t(861.5) = −7.64a | <0.001 | 0.521 |
| DSST, M ± SD | 55.97 ± 12.17 | 65.45 ± 11.05 | t(973.2) = 12.9a | <0.001 | 0.827 |
| RAVLT-S, M ± SD | 55.73 ± 9.87 | 60.15 ± 8.27 | t(941.7) = 7.68a | <0.001 | 0.501 |
| RAVLT-R, M ± SD | 13.14 ± 2.99 | 13.90 ± 1.89 | t(799.2) = 4.75a | <0.001 | 0.336 |
| CBTT-f, M ± SD | 8.65 ± 1.84 | 9.44 ± 1.96 | t(1005) = 6.63c | <0.001 | 0.418 |
| CBTT-b, M ± SD | 8.04 ± 1.91 | 9.00 ± 1.71 | t(969.0) = 8.42a | <0.001 | 0.541 |
| d2, M ± SD | 168.05 ± 43.05 | 194.00 ± 42.86 | t(1005) = 9.58c | <0.001 | 0.604 |
| LNS, M ± SD | 15.83 ± 3.20 | 16.96 ± 3.09 | t(1005) = 5.70c | <0.001 | 0.360 |
| VF-C, M ± SD | 23.20 ± 6.01 | 25.45 ± 5.63 | t(1005) = 6.15c | <0.001 | 0.388 |
| VF-P, M ± SD | 11.32 ± 4.22 | 12.44 ± 4.41 | t(1005) = 4.11c | <0.001 | 0.259 |
| VF-A, M ± SD | 15.27 ± 3.48 | 16.90 ± 3.26 | t(1005) = 7.62c | <0.001 | 0.481 |
| General Cognitive Performance factor, M ± SD | −0.37 ± 1.03 | 0.34 ± 0.84 | t(1005) = −12.04 | <0.001 | 0.935 |
| Number of hospitalizations, M ± SD | 1.66 ± 2.16 | – | – | – | – |
| Medication Load Index, M ± SD | 1.32 ± 1.43 | – | – | – | – |
| Comorbid diagnosis (yes/no) | 203/279 | – | – | – | – |
BDI Sum beck depression inventory, CBTT-f/b Corsi block-tapping test, forwards/backwards, d2 d2 test of attention, DSST digit symbol substitution test, HC healthy control, IQMVT Intelligence quotient evaluated with the multiple-choice vocabulary test version B (dt. “Mehrfachwahl-Wortschatz-Test Version B”), LNS letter–number–sequences test, M mean, MDD major depressive disorder, RAVLT-S/B Rey Auditory Verbal Learning Test, sum of all correct words/recognition, SD standard deviation, TMT-A/B trail making test, Version A/B, VF-C/P/A verbal fluency test, category/phonemic/alternating.
aTwo-sample t test assuming unequal variance, bPearson χ² test, ctwo-sample t test assuming equal variance.
The FOR2107 cohort was approved by the Ethics Committees of the Medical Faculties, University of Marburg and University of Münster. All experiments were performed in accordance with the ethical guidelines and regulations. All participants gave written informed consent prior to examination. They received financial compensation for participation after the testing session.
Trained personnel confirmed psychiatric diagnoses or the lack thereof using the Structural Clinical Interview for DSM-IV-TR (SCID-IV) [49]. MDD patients were considered if they reported a current or lifetime diagnosis of MDD (severe, moderate, mild, (partially) remitted episode). Remission was defined as the absence of DSM-IV-TR diagnostic criteria for a MDD episode for at least two months at the time of the interview. Partial remission classifies patients with subclinical symptoms (i.e., symptoms are insufficient to fulfill the diagnostic criteria of an MDD episode but severe enough to interfere with daily functioning) or if the time of recovery was shorter than 2 months.
Questionnaires, tests, and other clinical characteristics
In the FOR2107 cohort, all participants underwent neurocognitive testing in five subdomains of cognition: (1) executive functioning and sustained attention, 2) long and short-term memory performance, (3) visuospatial working memory, (4) verbal working memory, and (5) semantic processing. For a detailed description of the neurocognitive test battery, see Supplement 2. The general intelligence quotient (IQMVT) was estimated with the German version of the multiple-choice vocabulary intelligence test (MVT). Participants provided their highest educational degree. Education years were then estimated according to the typical time it takes to acquire the said degree.
To correct for typical clinical characteristics associated with MDD the following questionnaires and scores were used: The Beck Depression Inventory (BDI) [50] to assess current symptomatology, the number of prior hospitalizations provided by the participants in an interview, the Medication Load Index [51], a composite measure of total medication load reflecting daily dose and number of prescriptions irrespective of active components, and the presence of any comorbidities provided by the SCID-I interview.
Analysis
Analysis 1: factor analysis
To address hypothesis 1, we conducted an explorative principal component analysis (PCA; KMO = 0.888; Bartlett (66) = 4308.3, P < 0.001) with varimax rotation to reduce the 12 neuropsychological test scores to fewer variables. The component scores for each extracted factor were computed using a regression approach. Demographic data and the component analysis were analyzed using IBM SPSS Statistics 26 (SPSS Inc., Chicago, IL, USA).
Analyses 2 and 3: diffusion-tensor imaging
The DTI data acquisition, quality-assurance protocol, and preprocessing steps have already been published [38]. Detailed information can be found in Supplements 3. Analysis was performed with FSL5.0.10 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/, FMRIB, Oxford Center for Functional MRI of the Brain, University of Oxford, Department of Clinical Neurology, John Radcliffe Hospital, Oxford, UK) [52–54]. Tensor-derived maps were generated and fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) for each voxel per participant were estimated [55]. FA is defined as the normalized variance of the three eigenvalues about their mean. FA quantifies directional diffusion, MD is the average of all three eigenvalues, AD is equivalent to the first eigenvalue reflecting the primary diffusion direction which representing tract orientation, and RD is the mean of the second and third eigenvalue, representing motion perpendicular to the tract. As the number of fibers, fiber crossings, and general fiber orientation can also influence diffusion metrics in healthy fiber structure [56], the values should be interpreted with caution. Nonetheless, increased MD and decreased FA are measures of neuronal injury, while increased RD measures demyelination and increased AD axonal damage [57].
As tract-based spatial statistics (TBSS) reduces partial volume effects and registration misalignments [58], it was used for all DTI analyses (Supplements 3). To test for statistical significance, the nonparametric permutation testing implemented in FSL’s “randomize” [59] was used with 5000 permutations. Using the default options optimized for TBSS, threshold-free cluster enhancement (TFCE) was used to correct for multiple comparisons. Significance was determined by correcting for the family-wise error (FWE; P < 0.05) using the 95th percentile of the null distribution of permutated input data of the maximum TFCE scores [60]. For scatterplots and additional analyses in SPSS, the average FA per participant of significant clusters was extracted using FSL’s “fslstats”. The total intracranial volume (TIV) was extracted from T1 images using the Computational Anatomy Toolbox (CAT-12, http://www.neuro.uni-jena.de/cat, v933, Supplement 3). More detailed information about the statistics and general methods can be found in Supplement 4.
Results focus on FA as it is the most widely reported DTI measure. However, as the combination of different DTI metrics can be beneficial for interpretation, results in MD, RD, and AD are described in Supplement 5. To correct for scanner differences, two dummy coded variables (Marburg pre-body-coil change, Marburg post-body-coil change) with Münster as reference category were calculated.
To replicate the effects of reduced fiber integrity (lower FA, higher MD/RD) in MDD patients compared with HC (hypothesis 2) an ANCOVA with FA as the dependent variable, diagnosis (HC vs. MDD) as an independent variable was conducted (Analysis 2).
Further, to investigate the association of neurocognitive functioning with fiber structure, an ANCOVA with FA as the dependent variable, diagnosis (HC vs. MDD), the extracted neurocognitive factors, and their interaction with diagnosis as independent variables was conducted (Analysis 3). We expected a significant main effect of neurocognitive functioning irrespective of diagnosis (Hypothesis 3). Additional control analyses were performed including a complementary tractography-based connectome analysis and analyses using T1 structural data (Supplement 3). If not otherwise specified, all analyses included the following nuisance variables: age, sex, TIV, Marburg pre-body-coil, Marburg post-body-coil, IQMVT, and the number of education years.
Results
Analysis 1. Factor analysis for neuropsychological tests
As expected, MDD patients performed significantly worse compared to HC on all neuropsychological tests (all P < 0.001, Table 1) with small (e.g., for verbal fluency) to large (processing speed) effect sizes (Cohen’s d range:[0.26–0.83]). While the five-factor structure ((1) executive functioning and sustained attention, (2) long and short-term memory performance, (3) visuospatial working memory, (4) verbal working memory, and (5) verbal fluency) could be replicated with clear allocations of each test to one of the five factors (Supplement 6), the scree plot (Supplementary Fig. 1) from the explorative PCA strongly suggested a one-factor solution (EVfactor = 4.953) accounting for 41.28% of the variance and factor-loadings ranging from 0.461 to 0.777 (Supplementary Table 1).
Differences between MDD and HC in cognitive performance
GCP differed significantly between MDD patients and HC (F(1,1001) = 74.46, P < .001, η² = 0.069) with a medium sized effect even after taking age, sex, IQMVT and education years into account. More precisely, acute and (partially) remitted MDD patients differed from HC in their GCP (F(1,1001) = 34.40, P < 0.001, η² = 0.033, Fig. 1). Post hoc Bonferroni corrected tests revealed that HC differed from acute (P < 0.001, 95% confidence interval (CI): [0.67, 1.06]), partially remitted (P < 0.001, CI: [0.42, 0.91]) and completely remitted MDD patients (P < 0.001, CI: [0.25, 0.73]), respectively. Acute MDD patients presented with lower GCP compared with completely remitted MDD patients (P = 0.002, CI: [−0.64, −0.10]), but not compared with partially remitted MDD patients (P = 0.300, CI: [−0.48, 0.07]. Lastly, the difference between partially and completely remitted MDD patients was not significant (P = 0.877, CI: [−0.48, 0.14]). Likewise, GCP was negatively associated with depression severity (BDI) in the MDD subsample after controlling for age, sex, IQMVT, and education years (F(1,469) = 4.82, P = 0.029, η² = 0.010; b = −0.007).
Fig. 1. The general cognitive performance factor in HC and MDD patients.
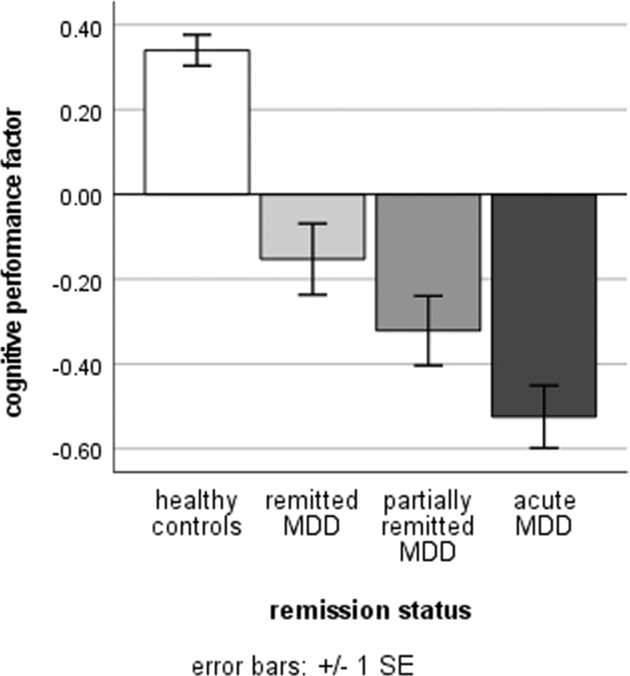
MDD patients were divided into remitted, partially remitted and acute MDD by the SCID-I diagnoses. HC healthy controls, MDD major depressive disorder, SE standard error.
Analysis 2. Group differences (MDD vs. HC)
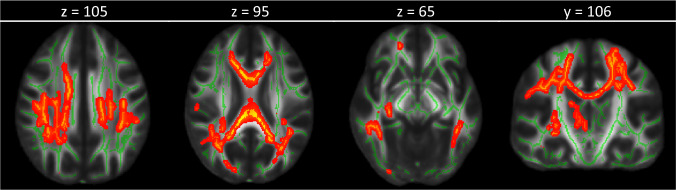
Prior to the inclusion of GCP in the model, the effect of diagnosis on FA was not significant (PFWE = 0.072). Significant effects were found for AD, but not in MD and RD (Supplement 2). However, when including only acute MDD patients and HC, a main effect of diagnosis was found in FA (Ptfce-FWE = 0.018, k = 15,111 voxels in three clusters, MNI-coordinates of the peak voxel from the largest cluster: x = 34, x = −18, 36, Fig. 2) in all eight anticipated fiber bundles (Supplementary Table 2).
Fig. 2. Healthy controls had higher fractional anisotropy (FA) values compared with acute depressive patients.
FA values are displayed at Ptfce-FWE < 0.05 onto the FMRIB58 template. Slice position is noted above the brain images.
Analysis 3. ANCOVA including the general cognitive performance (GCP) factor
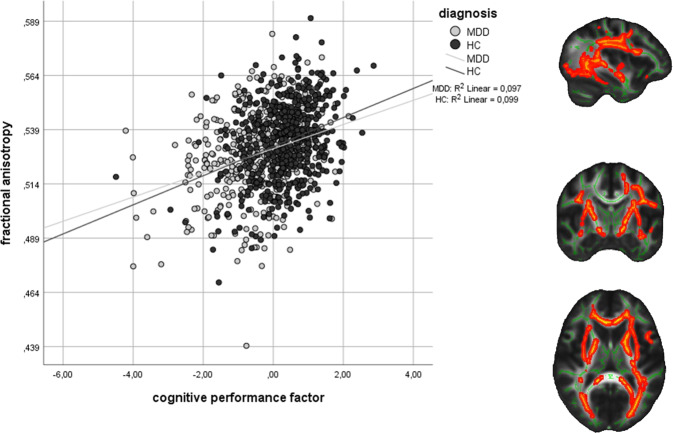
After including GCP into the model, neither the main effect of diagnosis (Ptfce-FWE = 0.264) nor a diagnosis × GCP interaction (Ptfce-FWE = 0.365) could be found for FA, while a significant main effect of diagnosis was still present for AD (Supplement 5). However, we found a significant main effect of GCP (Ptfce-FWE = 0.002, k = 43,700 voxels in one cluster, MNI-coordinates of the peak voxel: x = 31, y = −66, z = 11, Supplementary Table 3, Fig. 3) in a large bilateral cluster consisting of the CC, the IFOF, the anterior TR, and the SLF and ILF among other regions (Supplementary Table 2). Even after excluding (partially) remitted MDD patients to reduce variance and enhance differences with HC, this association between FA and GCP remained (Supplement 7). To verify that results were independent of the MRI scanner, two ANCOVAs with the mean FA values per participant from the significant cluster as the dependent variable were calculated in SPSS. This analysis confirmed that the GCP effect was present at both scanning sites, respectively (Marburg: F(1,628) = 31.67, P < 0.001, η² = .048, Münster: F(1,361) = 12.93, P < 0.001, η² = 0.035). As GCP and diagnosis are depending on each other, a linear ANCOVA might not be adequate to disentangle their effects. Thus, the analysis was repeated in SPSS using a nonparametric Generalized estimating equation (GEE) analysis. The results were confirmed using this method (main effect diagnosis: Wald-χ²(1) = 0.29, P = 0.589; main effect GCP: Wald-χ²(1) = 25.95, P < 0.001; interaction: Wald-χ²(1) = 0.40, P = 0.528). The tractography-based connectome analysis confirmed that the associations with GCP were widespread, including fibers connecting nearly all anatomical brain regions irrespective of the method of analysis (Supplement 8 and Supplementary Fig. 2). The effect in the MDD subgroup remained significant, even after taking medication intake, the presence of comorbid diagnoses, number of previous hospitalizations, and BDI into account in an additional ANCOVA in SPSS (Table 2). Except for verbal working memory performance, all subdomains of GCP were associated with the mean extracted FA from the significant cluster. The strongest effects were found for processing speed and sustained attention (Supplementary Table 4). A significant association with GCP was also present for MD and RD (Supplement 3). There was no positive correlation between GCP and gray matter volume at Ptfce-FWE = 0.217.
Fig. 3. Association of fractional anisotropy (FA) and the general cognitive performance factor in major depressive disorder (MDD) patients and healthy controls (HC).
FA values were extracted with a threshold of Ptfce-FWE < 0.01 and displayed onto the FMRIB58 template in the x = −36, y = −9, z = 11 planes in MNI space. The scatterplot depicts mean FA values of the significant cluster with a threshold of Ptfce-FWE < 0.05.
Table 2.
Using only the participants with MDD diagnosis, an ANCOVA was calculated with mean extracted FA values as the independent variable and medication intake, presence of a comorbid diagnosis, number of hospitalizations, and acute symptomatology on top of age, sex, TIV, scanner/side variables, IQMVT and number of education years in SPSS.
| Factor/covariate | F-statistic, df(1,459) | P value | η² |
|---|---|---|---|
| General cognitive performance | 12.611 | <0.001 | 0.027 |
| Age | 24.773 | <0.001 | 0.051 |
| Sex | 1.476 | 0.225 | 0.003 |
| TIV | 38.753 | <0.001 | 0.078 |
| Marburg pre-body-coil | 71.228 | <0.001 | 0.134 |
| Marburg post-body-coil | 43.299 | <0.001 | 0.086 |
| IQMVT | 1.081 | 0.299 | 0.002 |
| Number of education years | 3.821 | 0.051 | 0.008 |
| Medication Load Index | 0.835 | 0.361 | 0.002 |
| Comorbid disorder | 0.275 | 0.600 | 0.001 |
| Number of hospitalizations | 0.486 | 0.486 | 0.001 |
| BDI | 0.280 | 0.597 | 0.001 |
ANCOVA analysis of covariance, BDI Beck’s Depression Inventory Score, FA fractional anisotropy, IQMVT intelligence quotient evaluated with the multiple-choice vocabulary test version B (dt. “Mehrfachwahl-Wortschatz-Test Version B”), MDD major depressive disorder, TIV total intracranial volume.
Discussion
The aim of this study was to investigate the association between white matter fiber microstructure and cognitive deficits in MDD patients over the entire spectrum of the disorder. As expected, general cognitive performance was associated with FA in a large bilateral cluster consisting of the CC, the IFOF, the anterior TR, and the SLF and ILF among other regions. This effect seems to be driven by deficits in processing speed and sustained attention, semantic processing, and memory, while small (visuospatial working memory) or no associations (verbal working memory) could be found for working memory. The associations between fiber microstructure and general cognitive performance were very robust even after correction for general intelligence, educational achievement, medication intake, and presence of comorbid diagnoses, number of previous hospitalizations, and BDI in the MDD subsample. They were confirmed using a different method of analysis (tractography-based connectome analysis), and were found at both MRI scanners, respectively. Lastly, similar associations were found for other DTI measures of fiber integrity (MD and RD). While the interpretation of single DTI measures is limited [56], the combination of reduced FA and increased MD and RD values might suggest neuropathological processes as the basis for cognitive deficits. This effect seems to be confined to white matter microstructure and not gray matter volume, as no positive associations were found between cognitive performance and gray matter structural MRI data.
These results highlight that intact fiber microstructure is associated with fast and accurate communication between brain regions required for optimal cognitive functioning [22]. Previous studies have already postulated that fine-tuned prefrontal signaling—with too much or too little signaling reducing cognitive performance—could be fundamental for sustained attention [61]. If the communicating fibers between frontal areas and other brain regions are structurally impaired, as hinted at by reduced FA in those fibers, this could result in cognitive impairment. It must be noted, however, that the results in this study seem to be regionally unspecific, as multiple tracts in a large bilateral cluster were affected.
Second, we found that MDD patients performed consistently worse on all cognitive tasks in concordance with previous analyses [2]. While these effects were found most strongly in tests assessing processing speed, a wide range of cognitive processes were affected in MDD patients, reflected by the single general cognitive performance factor extracted in the PCA. The differences between HC and acute or (partially) remitted MDD patients, respectively, support and extent the well-known report that cognitive deficits in MDD—while being alleviated—seem to persist in remission [14]. Deficits in cognitive performance could influence the inhibition of inappropriate behavioral or emotional processes, planning of future behavior, and flexible problem solving [14]. Cognitive deficits in remitted MDD patients may play a crucial role in sustaining psychosocial functioning—socially, mentally as well as in more general societal functions like workplace productivity. The neurobiological underpinnings of FA reductions associated with cognitive performance in MDD patients are most likely complex. It is possible, that interactive effects of genetic influences and environmental stressors might result in more pronounced fiber integrity reductions and, hence, elevated cognitive deficits in MDD [8, 62]. The variation in time of white matter maturation differs regionally. Especially association and commissural white matter fibers responsible for higher cognitive functioning, continue to develop throughout adolescence to early and middle adulthood [36, 63]. This prolonged development is the underlying basis for white matter plasticity, which in turn would be necessary for environmental and (epi-)genetic factors to in turn influence fiber structure [63–65].
Third, in contrast to our hypothesis, MDD patients’ FA differed only marginally from HC. Previous studies [38, 66] have already drawn into question that MDD patients’ microstructure differs from HC on a general basis. Choi et al. argued that small sample sizes, tracts prone to artifacts, or other aspects of MDD pathology (e.g., course of illness, childhood maltreatment experiences, antidepressant treatment, or specific symptoms) could have produced the significant differences between MDD patients and HC in earlier studies [66]. Likewise, the significant reduction in FA values in acute MDD patients (i.e., those who take more medication and experience more severe symptoms) compared with HC in our well-powered analysis suggests that the lifetime MDD diagnosis by itself might not be the sole driving force of white matter alterations in patients.
Acute MDD patients take more psychiatric medications and have a worse course of illness than (partially) remitted MDD patients in our sample, this could explain this difference.
Future studies might use the results of this study to use white matter morphological abnormalities associated with cognitive deficits in MDD to guide the inquiry of new therapeutic options. It should be investigated whether current treatment options for cognitive deficits in MDD [3, 8, 67, 68] like biobehavioral interventions (e.g., exercise, sleep hygiene, healthy diet), pharmacological treatments (e.g., vortioxetine), neurostimulation techniques (e.g., transcranial magnetic stimulation), and psychotherapeutic interventions (e.g., cognitive behavioral therapy, cognitive remediation therapy) can be linked to changes in MDD’s fiber structure.
Limitations
Some limitations should be acknowledged. First, while DTI is a feasible and noninvasive technique, FA can also be influenced by other, non-pathological factors, e.g., the number and orientation of axons irrespective of fiber damage [56]. Second, while we tried to account for influences attributed to current psychiatric medication intake, the influence of therapy (including psychotherapy, electroconvulsive therapy, etc.), cannot be ruled out completely. Therefore, future studies should try to replicate the findings of this study in untreated MDD patients or systematically investigate the influences of treatment. Third, while MDD patients and HC did not differ in their IQMVT scores, we found a significant difference in their educational achievements. We have to note that, while the MVT is a robust and efficient estimator for general IQ, it is also prone to slight measuring inaccuracies [69] and captures crystallized intelligence rather than fluid intelligence. Hence, even after correcting for educational years and IQMVT confounding effects of general intelligence cannot be ruled out entirely. Lastly, this study is correlational, and cannot evaluate causal effects. To this end, longitudinal studies are needed that investigate fiber microstructural changes over the course of MDD or pre-post treatment. Regardless, the major strength of this study is the use of a large, well-characterized sample reflecting the entire spectrum of MDD patients.
Conclusion
Our findings highlight the importance of neurobiological wiring in cognitive performance in healthy controls and MDD patients. They provide robust evidence that global structural connectivity is associated with cognitive performance in MDD patients and HC. This association was independent of diagnosis, suggesting a general association between DTI measures of fiber integrity and cognitive performance. Efforts to treat cognitive deficits in MDD should, thus, consider the white matter as one of the underlying neural mechanisms.
Supplementary information
Acknowledgements
This work is part of the German multicenter consortium “Neurobiology of Affective Disorders. A translational perspective on brain structure and function“, funded by the German Research Foundation (Deutsche Forschungsgemeinschaft DFG; Forschungsgruppe/Research Unit FOR2107). Principal investigators (PIs) with respective areas of responsibility in the FOR2107 consortium are: Work Package WP1, FOR2107/MACS cohort and brainimaging: Tilo Kircher (speaker FOR2107; DFG grant numbers KI 588/14-1, KI 588/14-2), Udo Dannlowski (co-speaker FOR2107; DA 1151/5-1, DA 1151/5-2), Axel Krug (KR 3822/5-1, KR 3822/7-2), Igor Nenadić (NE 2254/1-2, NE 2254/3-1, NE 2254/4-1), Carsten Konrad (KO 4291/3-1). WP2, animal phenotyping: Markus Wöhr (WO 1732/4-1, WO 1732/4-2), Rainer Schwarting (SCHW 559/14-1, SCHW 559/14-2). WP3, miRNA: Gerhard Schratt (SCHR 1136/3-1, 1136/3-2). WP4, immunology, mitochondriae: Judith Alferink (AL 1145/5-2), Carsten Culmsee (CU 43/9-1, CU 43/9-2), Holger Garn (GA 545/5-1, GA 545/7-2). WP5, genetics: Marcella Rietschel (RI 908/11-1, RI 908/11-2), Markus Nöthen (NO 246/10-1, NO 246/10-2), Stephanie Witt (WI 3439/3-1, WI 3439/3-2). WP6, multi-method data analytics: Andreas Jansen (JA 1890/7-1, JA 1890/7-2), Tim Hahn (HA7070/2-2, HA7070/3, HA7070/4), Bertram Müller-Myhsok (MU1315/8-2), Astrid Dempfle (DE 1614/3-1, DE 1614/3-2). CP1, biobank: Petra Pfefferle (PF 784/1-1, PF 784/1-2), Harald Renz (RE 737/20-1, 737/20-2). CP2, administration. Tilo Kircher (KI 588/15-1, KI 588/17-1), Udo Dannlowski (DA 1151/6-1), Carsten Konrad (KO 4291/4-1). Data access and responsibility: All PIs take responsibility for the integrity of the respective study data and their components. All authors and coauthors had full access to all study data. Acknowledgements and members by Work Package 1: Henrike Bröhl, Katharina Brosch, Bruno Dietsche, Rozbeh Elahi, Jennifer Engelen, Sabine Fischer, Jessica Heinen, Svenja Klingel, Felicitas Meier, Tina Meller, Julia-Katharina Pfarr, Kai Ringwald, Torsten Sauder, Simon Schmitt, Frederike Stein, Annette Tittmar, Dilara Yüksel (Dept. of Psychiatry, Marburg University). Mechthild Wallnig, Rita Werner (Core-Facility Brainimaging, Marburg University). Carmen Schade-Brittinger, Maik Hahmann (Coordinating Centre for Clinical Trials, Marburg). Michael Putzke (Psychiatric Hospital, Friedberg). Rolf Speier, Lutz Lenhard (Psychiatric Hospital, Haina). Birgit Köhnlein (Psychiatric Practice, Marburg). Peter Wulf, Jürgen Kleebach, Achim Becker (Psychiatric Hospital Hephata, Schwalmstadt-Treysa). Ruth Bär (Care facility Bischoff, Neukirchen). Matthias Müller, Michael Franz, Siegfried Scharmann, Anja Haag, Kristina Spenner, Ulrich Ohlenschläger (Psychiatric Hospital Vitos, Marburg). Matthias Müller, Michael Franz, Bernd Kundermann (Psychiatric Hospital Vitos, Gießen). Christian Bürger, Katharina Dohm, Fanni Dzvonyar, Verena Enneking, Stella Fingas, Katharina Förster, Janik Goltermann, Dominik Grotegerd, Hannah Lemke, Susanne Meinert, Nils Opel, Ronny Redlich, Jonathan Repple, Kordula Vorspohl, Bettina Walden, Dario Zaremba (Department of Psychiatry, University of Münster). Harald Kugel, Jochen Bauer, Walter Heindel, Birgit Vahrenkamp (Department of Clinical Radiology, University of Münster). Gereon Heuft, Gudrun Schneider (Department of Psychosomatics and Psychotherapy, University of Münster). Thomas Reker (LWL-Hospital Münster). Gisela Bartling (IPP Münster). Ulrike Buhlmann (Department of Clinical Psychology, University of Münster). We are deeply indebted to all participants of this study, the recruitment sites, and their staff. This work was further supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) (UD, grant numbers SFB-TRR58, Projects C09 and Z02), the Interdisciplinary Center for Clinical Research (IZKF) of the medical faculty of Münster (UD, grant number Dan3/012/17; NO, grant number SEED 11/18; TH grant MzH 3/020/20), “Innovative Medizinische Forschung” (IMF) of the medical faculty of Münster (JR, grant number RE221707), (EJL, grant number LE121703), and the Deanery of the Medical Faculty of the University of Münster.
Author contributions
Substantial contribution to conception and design: SM, NN, IA, and UD. Substantial contribution to the acquisition of the data: SM, NN, DG, IA, VE, HL, LW, FS, KB, SS, TM, JKP, KR, OS, MG, IN, AK, EJL, KT, KD, AW, TK, and UD. Analysis and interpretation of data: SM, NN, DG, JR, NRW, IA, VE, MG, TH, NO, RIS, TK, and UD. Drafting the article: SM. Revising it critically for important intellectual content: NN, DG, JR, NRW, IA, VE, HL, LW, FS, KB, SS, TM, JKP, KR, OS, MG, IN, AK, EJL, TH, KT, KD, AW, NO, RIS, TK, and UD. Final approval of the version to be published: SM, NN, DG, JR, NRW, IA, VE, HL, LW, FS, KB, SS, TM, JKP, KR, OS, MG, IN, AK, EJL, TH, KT, KD, AW, NO, RIS, TK, and UD.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Competing interests
TK received unrestricted educational grants from Servier, Janssen, Recordati, Aristo, Otsuka, neuraxpharm. This funding is not associated with the current work. On behalf of all other authors, the corresponding author states no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-021-01330-8.
References
- 1.World Health Organization. International classification of diseases for mortality and morbidity statistics (11th Revision). 2018. Retrieved from https://icd.who.int/.
- 2.Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychological Med. 2014;44:2029–40. doi: 10.1017/S0033291713002535. [DOI] [PubMed] [Google Scholar]
- 3.Bortolato B, Carvalho A, McIntyre R. Cognitive dysfunction in major depressive disorder: a state-of-the-art clinical review. CNSNDDT. 2015;13:1804–18. doi: 10.2174/1871527313666141130203823. [DOI] [PubMed] [Google Scholar]
- 4.Albert KM, Potter GG, McQuoid DR, Taylor WD. Cognitive performance in antidepressant-free recurrent major depressive disorder. Depression Anxiety. 2018;35:694–9. doi: 10.1002/da.22747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychological Bull. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartlage S, Alloy LB, Vázquez C, Dykman B. Automatic and effortful processing in depression. Psychological Bull. 1993;113:247–78. doi: 10.1037/0033-2909.113.2.247. [DOI] [PubMed] [Google Scholar]
- 7.Dannlowski U, Kersting A, Arolt V, Lalee-Mentzel J, Donges U-S, Suslow T. Unimpaired automatic processing of verbal information in the course of clinical depression. Depression Anxiety. 2006;23:325–30. doi: 10.1002/da.20173. [DOI] [PubMed] [Google Scholar]
- 8.Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Prim. 2016;2:16065. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 9.Baune BT, Malhi GS, Morris G, Outhred T, Hamilton A, Das P, et al. Cognition in depression: can we THINC-it better? J Affect Disord. 2018;225:559–62. doi: 10.1016/j.jad.2017.08.080. [DOI] [PubMed] [Google Scholar]
- 10.Semkovska M, Quinlivan L, O’Grady T, Johnson R, Collins A, O’Connor J, et al. Cognitive function following a major depressive episode: a systematic review and meta-analysis. The Lancet Psychiatry. 2019;6:851–61. doi: 10.1016/S2215-0366(19)30291-3. [DOI] [PubMed] [Google Scholar]
- 11.Kessing LV. Cognitive impairment in the euthymic phase of affective disorder. Psychol Med. 1998;28:1027–38. doi: 10.1017/S0033291798006862. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry. 2000;48:674–84. doi: 10.1016/S0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- 13.Evans VC, Iverson GL, Yatham LN, Lam RW. The relationship between neurocognitive and psychosocial functioning in major depressive disorder: a systematic review. J Clin Psychiatry. 2014;75:1359–70. doi: 10.4088/JCP.13r08939. [DOI] [PubMed] [Google Scholar]
- 14.Baune BT, Miller R, McAfoose J, Johnson M, Quirk F, Mitchell D. The role of cognitive impairment in general functioning in major depression. Psychiatry Res. 2010;176:183–9. doi: 10.1016/j.psychres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 15.McIntyre RS, Xiao HX, Syeda K, Vinberg M, Carvalho AF, Mansur RB, et al. The prevalence, measurement, and treatment of the cognitive dimension/domain in major depressive disorder. CNS Drugs. 2015;29:577–89. doi: 10.1007/s40263-015-0263-x. [DOI] [PubMed] [Google Scholar]
- 16.Gonda X, Pompili M, Serafini G, Carvalho AF, Rihmer Z, Dome P. The role of cognitive dysfunction in the symptoms and remission from depression. Ann Gen Psychiatry. 2015;14:27. doi: 10.1186/s12991-015-0068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vicent-Gil M, Keymer-Gausset A, Serra-Blasco M, Carceller-Sindreu M, Diego-Adelino J, de, Trujols J, et al. Cognitive predictors of illness course at 12 months after first-episode of depression. Eur Neuropsychopharmacol. 2018;28:529–37. doi: 10.1016/j.euroneuro.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Keilp JG, Sackeim HA, Brodsky BS, Oquendo MA, Malone KM, Mann JJ. Neuropsychological dysfunction in depressed suicide attempters. Am J Psychiatry. 2001;158:735–41. doi: 10.1176/appi.ajp.158.5.735. [DOI] [PubMed] [Google Scholar]
- 19.Dawson EL, Caveney AF, Meyers KK, Weisenbach SL, Giordani B, Avery ET, et al. Executive functioning at baseline prospectively predicts depression treatment response. The Primary Care Companion for CNS Disorders. 2017;19. [DOI] [PMC free article] [PubMed]
- 20.Jaeger J, Berns S, Uzelac S, Davis-Conway S. Neurocognitive deficits and disability in major depressive disorder. Psychiatry Res. 2006;145:39–48. [DOI] [PubMed]
- 21.Malhi GS, Byrow Y, Fritz K, Das P, Baune BT, Porter RJ, et al. Mood disorders: neurocognitive models. Bipolar Disord. 2015;17:3–20. doi: 10.1111/bdi.12353. [DOI] [PubMed] [Google Scholar]
- 22.Lim KO, Helpern JA. Neuropsychiatric applications of DTI - a review. NMR Biomed. 2002;15:587–93. doi: 10.1002/nbm.789. [DOI] [PubMed] [Google Scholar]
- 23.Winston GP. The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant Imaging Med Surg. 2012;2:254–65. doi: 10.3978/j.issn.2223-4292.2012.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–80. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- 25.Grumbach P, Opel N, Martin S, Meinert S, Leehr EJ, Redlich R, et al. Sleep duration is associated with white matter microstructure and cognitive performance in healthy adults. Human Brain Mapping. 2020;41:4397–405. [DOI] [PMC free article] [PubMed]
- 26.Opel N, Martin S, Meinert S, Redlich R, Enneking V, Richter M, et al. White matter microstructure mediates the association between physical fitness and cognition in healthy, young adults. Sci Rep. 2019;9:12885. doi: 10.1038/s41598-019-49301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Repple J, Karliczek G, Meinert S, Förster K, Grotegerd D, Goltermann, J et al. Variation of HbA1c affects cognition and white matter microstructure in healthy, young adults. Mol Psychiatry. 2019;26:1399–408. [DOI] [PubMed]
- 28.Koch K, Wagner G, Schachtzabel C, Schultz CC, Güllmar D, Reichenbach JR, et al. Age-dependent visuomotor performance and white matter structure: a DTI study. Brain Struct Funct. 2013;218:1075–84. doi: 10.1007/s00429-012-0447-9. [DOI] [PubMed] [Google Scholar]
- 29.Turken A, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JDE. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. NeuroImage. 2008;42:1032–44. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du J, Wang Y, Zhi N, Geng J, Cao W, Yu L, et al. Structural brain network measures are superior to vascular burden scores in predicting early cognitive impairment in post stroke patients with small vessel disease. NeuroImage Clin. 2019;22:101712. doi: 10.1016/j.nicl.2019.101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haghshomar M, Dolatshahi M, Ghazi Sherbaf F, Sanjari Moghaddam H, Shirin Shandiz M, Aarabi MH. Disruption of inferior longitudinal fasciculus microstructure in Parkinson’s disease: a systematic review of diffusion tensor imaging studies. Front Neurol. 2018;9:598. doi: 10.3389/fneur.2018.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moonen JEF, Foster-Dingley JC, van den Berg-Huijsmans AA, Ruijter W, de, de Craen AJM, van der Grond J, et al. Influence of small vessel disease and microstructural integrity on neurocognitive functioning in older individuals: The DANTE Study Leiden. Ajnr Am J Neuroradiol. 2017;38:25–30. doi: 10.3174/ajnr.A4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Schependom J, Gielen J, Laton J, Sotiropoulos G, Vanbinst A-M, Mey Jde, et al. The effect of morphological and microstructural integrity of the corpus callosum on cognition, fatigue and depression in mildly disabled MS patients. Magn Reson Imaging. 2017;40:109–14. doi: 10.1016/j.mri.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 34.van Duinkerken E, Ryan CM, Schoonheim MM, Barkhof F, Klein M, Moll AC, et al. Subgenual cingulate cortex functional connectivity in relation to depressive symptoms and cognitive functioning in type 1 diabetes mellitus patients. Psychosom Med. 2016;78:740–9. doi: 10.1097/PSY.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 35.Holleran L, Kelly S, Alloza C, Agartz I, Andreassen OA, Arango C, et al. The relationship between white matter microstructure and general cognitive ability in patients with schizophrenia and healthy participants in the ENIGMA Consortium. Am J Psychiatry. 2020; 177:537–47. [DOI] [PMC free article] [PubMed]
- 36.Kochunov P, Coyle TR, Rowland LM, Jahanshad N, Thompson PM, Kelly S, et al. Association of white matter with core cognitive deficits in patients with schizophrenia. JAMA Psychiatry. 2017;74:958–66. doi: 10.1001/jamapsychiatry.2017.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poletti S, Bollettini I, Mazza E, Locatelli C, Radaelli D, Vai B, et al. Cognitive performances associate with measures of white matter integrity in bipolar disorder. J Affect Disord. 2015;174:342–52. doi: 10.1016/j.jad.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 38.Meinert S, Repple J, Nenadic I, Krug A, Jansen A, Grotegerd D, et al. Reduced fractional anisotropy in depressed patients due to childhood maltreatment rather than diagnosis. Neuropsychopharmacology. 2019;44:2065–72. doi: 10.1038/s41386-019-0472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Repple J, Meinert S, Grotegerd D, Kugel H, Redlich R, Dohm K, et al. A voxel-based diffusion tensor imaging study in unipolar and bipolar depression. Bipolar Disord. 2017;19:23–31. doi: 10.1111/bdi.12465. [DOI] [PubMed] [Google Scholar]
- 40.Wise T, Radua J, Nortje G, Cleare AJ, Young AH, Arnone D. Voxel-based meta-analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biol Psychiatry. 2016;79:293–302. doi: 10.1016/j.biopsych.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 41.van Velzen LS, Kelly S, Isaev D, Aleman A, Aftanas LI, Bauer J, et al. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol Psychiatry 2019;25:1511–25. [DOI] [PMC free article] [PubMed]
- 42.Lange SC, de, Scholtens LH, van den Berg LH, Boks MP, Bozzali M, Cahn W, et al. Shared vulnerability for connectome alterations across psychiatric and neurological brain disorders. Nat Hum Behav. 2019;3:988–98. doi: 10.1038/s41562-019-0659-6. [DOI] [PubMed] [Google Scholar]
- 43.Repple J, Mauritz M, Meinert S, Lange SC de, Grotegerd D, Opel N, et al. Severity of current depression and remission status are associated with structural connectome alterations in major depressive disorder. Mol Psychiatry. 2019;25:1550–8. [DOI] [PubMed]
- 44.Sexton CE, McDermott L, Kalu UG, Herrmann LL, Bradley KM, Allan CL, et al. Exploring the pattern and neural correlates of neuropsychological impairment in late-life depression. Psychological Med. 2012;42:1195–202. doi: 10.1017/S0033291711002352. [DOI] [PubMed] [Google Scholar]
- 45.Shimony JS, Sheline YI, D’Angelo G, Epstein AA, Benzinger TLS, Mintun MA, et al. Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry. 2009;66:245–52. doi: 10.1016/j.biopsych.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mettenburg JM, Benzinger TL, Shimony JS, Snyder AZ, Sheline YI. Diminished performance on neuropsychological testing in late life depression is correlated with microstructural white matter abnormalities. NeuroImage. 2012;60:2182–90. doi: 10.1016/j.neuroimage.2012.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kircher T, Wöhr M, Nenadic I, Schwarting R, Schratt G, Alferink J, et al. Neurobiology of the major psychoses: a translational perspective on brain structure and function-the FOR2107 consortium. Eur Arch Psychiatry Clin Neurosci. 2019;269:949–62. doi: 10.1007/s00406-018-0943-x. [DOI] [PubMed] [Google Scholar]
- 48.Vogelbacher C, Möbius TWD, Sommer J, Schuster V, Dannlowski U, Kircher T, et al. The Marburg-Münster affective disorders cohort study (MACS): a quality assurance protocol for MR neuroimaging data. NeuroImage. 2018;172:450–60. doi: 10.1016/j.neuroimage.2018.01.079. [DOI] [PubMed] [Google Scholar]
- 49.Wittchen H-U, Wunderlich U, Gruschwitz S, Zaudig M. SKID I. Strukturiertes Klinisches Interview für DSM-IV. Achse I: Psychische Störungen. Interviewheft und Beurteilungsheft. Eine deutschsprachige, erweiterte Bearbeitung der amerikanischen Originalversion des SKID I. Hogrefe: Göttingen, 1997.
- 50.Beck AT, Steer RA, Brown GK. Manual for beck depression inventory II (BDI-II). San Antonio, TX: Psychology Corporation; 1996.
- 51.Redlich R, Almeida JJR, Grotegerd D, Opel N, Kugel H, Heindel W, et al. Brain morphometric biomarkers distinguishing unipolar and bipolar depression. A voxel-based morphometry-pattern classification approach. JAMA Psychiatry. 2014;71:1222–30. doi: 10.1001/jamapsychiatry.2014.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. Fsl. NeuroImage. 2012;62:782–90. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 53.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 54.Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens TEJ, et al. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45:S173–86. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 55.Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–88. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 56.Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. NeuroImage. 2013;73:239–54. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- 57.Feldman HM, Yeatman JD, Lee ES, Barde LHF, Gaman-Bean S. Diffusion tensor imaging: a review for pediatric researchers and clinicians. J Dev Behav Pediatrics JDBP. 2010;31:346–56. doi: 10.1097/DBP.0b013e3181dcaa8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 59.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. NeuroImage. 2014;92:381–97. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 61.Bast T, Pezze M, McGarrity S. Cognitive deficits caused by prefrontal cortical and hippocampal neural disinhibition. Br J Pharmacol. 2017;174:3211–25. doi: 10.1111/bph.13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- 63.Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40:1044–55. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 64.Keedwell PA, Chapman R, Christiansen K, Richardson H, Evans J, Jones DK. Cingulum white matter in young women at risk of depression: the effect of family history and anhedonia. Biol Psychiatry. 2012;72:296–302. doi: 10.1016/j.biopsych.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 65.Meinert S, Leehr EJ, Grotegerd D, Repple J, Förster K, Winter NR, et al. White matter fiber microstructure is associated with prior hospitalizations rather than acute symptomatology in major depressive disorder. Psychol Med. 2021:1–9. 10.1017/S0033291720002950. Epub ahead of print. [DOI] [PubMed]
- 66.Choi KS, Holtzheimer PE, Franco AR, Kelley ME, Dunlop BW, Hu XP, et al. Reconciling variable findings of white matter integrity in major depressive disorder. Neuropsychopharmacology. 2014;39:1332–9. doi: 10.1038/npp.2013.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frampton JE. Vortioxetine: a review in cognitive dysfunction in depression. Drugs. 2016;76:1675–82. doi: 10.1007/s40265-016-0655-3. [DOI] [PubMed] [Google Scholar]
- 68.Knight MJ, Baune BT. Cognitive dysfunction in major depressive disorder. Curr Opin Psychiatry. 2018;31:26–31. doi: 10.1097/YCO.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 69.Satzger W, Fessmann H, Engel RR. Liefern HAWIE-R, WST und MWT-B vergleichbare IQ-Werte? Z für Differentielle und Diagnostische Psychologie. 2002;23:159–70. doi: 10.1024//0170-1789.23.2.159. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.