Abstract
Polymorphonuclear neutrophils (PMN) are attracted to sites of infection. They have the potential to deliver antimicrobial agents to these sites if the agents enter the cells and do not alter migration. Penicillin G did not enter cells and was not transported by PMN. We found that azithromycin, ciprofloxacin, levofloxacin, moxifloxacin, and telithromycin were concentrated in PMN and transported toward a chemoattractant. These antimicrobial agents were released from the PMN and inhibited the growth of bacteria on test plates.
Neutrophils and antimicrobial agents have several potential interactions that may be synergistic for combating infection. Antimicrobial agents make bacteria more susceptible to killing by neutrophils even at subinhibitory concentrations (1, 8, 14). Neutrophils target sites of infection, concentrate at these sites, and thus may serve as an antimicrobial agent delivery mechanism (4). In order for neutrophils to function as an effective means of transporting antimicrobial agents to sites of infection, several criteria must be met: the agent should not interfere with neutrophil migration, the agent should be concentrated in the neutrophil, and the agent should be released in an active form at the site of infection. We examined several agents, including newer quinolones and a ketolide, in an in vitro system. Penicillin G was utilized because previous studies (13, 15) showed that this antibiotic is not concentrated in neutrophils.
MATERIALS AND METHODS
Determination of MICs.
The MICs of antimicrobial agents for the organisms used in the assays were determined by the broth dilution method (6).
Antimicrobial agents.
Azithromycin was provided by Pfizer Pharmaceuticals, New York, N.Y.; ciprofloxacin was provided by Miles Pharmaceuticals, West Haven, Conn.; levofloxacin was provided by R. W. Johnson Pharmaceutical Research Institute, Spring House, Pa.; telithromycin was supplied by Hoechst Marion Roussel, Romainville, France; and moxifloxacin was supplied by Bayer Corporation, West Haven, Conn. Penicillin G was obtained from Sigma Chemical Company, St. Louis, Mo. A stock solution of azithromycin was made by initially dissolving azithromycin in ethanol and then diluting this with Hanks balanced salt solution (HBSS; BioWhittaker Inc., Walkersville, Md.). Stock solutions of levofloxacin, ciprofloxacin, and moxifloxacin were made in sterile water. A stock solution of telithromycin was made by resuspending powder in 1% HCl and sterile water. The reported serum protein binding of the antibiotics used was as follows: azithromycin, 7 to 50% (depending on concentration); ciprofloxacin, 20 to 40%; levofloxacin, 24 to 38%; moxifloxacin, 30 to 45%; penicillin G, 60%; and telithromycin, 70%.
Bacterial strains.
Streptococcus pyogenes ATCC 12344 (American Type Culture Collection, Manassas, Va.) was kept on chocolate agar plates and subcultured every other day. For each transport experiment, a 6-h culture of the organism in tryptic soy broth (TSB; Difco Laboratories, Detroit, Mich.) grown at 37°C in 5% CO2 was made. Overnight cultures of Micrococcus luteus ATCC 9341, Staphylococcus aureus ATCC 27217, and Escherichia coli ATCC 011B4 in TSB were used in bioassay plates. Bacteria used for the assays were selected based on their susceptibility to the antibiotic being studied.
Isolation of PMN.
Purified polymorphonuclear neutrophils (PMN) were obtained from normal, heparinized (10 U of heparin [Lymphomed Fujisawa USA Inc., Deerfield, Ill.] per ml) human venous blood by a Ficoll-Hypaque separation procedure adapted from Ferrante and Thong (3). Nine milliliters of fresh, heparinized human blood was layered onto a gradient consisting of 1 ml of Ficoll-Hypaque (ICN Biomedicals, Aurora, Ohio), 2 ml of One Step Polymorphs (Accurate Chemicals and Scientific Corp., Westbury, N.Y.), and 1 ml of neutrophil isolation medium (Cardinal Associates, Santa Fe, N.M.). The blood with the separation medium was then centrifuged at 200 × g for 25 min to produce a layer of PMN. PMN were removed and washed three times with HBSS (Whittaker M. A. Bioproducts, Walkersville, Md.) with 10 U of heparin per ml. Red blood cells were lysed with 0.22% NaCl. Cells (95% PMN) were resuspended in HBSS and counted using a hemocytometer.
Intracellular antimicrobial agent determination by bioassay.
A total of 5 × 106 PMN/ml were tumbled at 37°C for 1 h with either 0.1 μg of azithromycin/ml, 4 μg of ciprofloxacin/ml, 6 μg of levofloxacin/ml, 4.5 μg of moxifloxacin/ml, 10 μg of penicillin G/ml, or 0.1 μg of telithromycin/ml. These concentrations are similar to published peak concentrations in serum for humans after usual doses. After the incubation period, the samples were centrifuged at 150 × g, the supernatants were decanted, and the pellets were blotted to remove excess liquid. The remaining pellets were then transferred to a 1.5-ml Eppendorf tube containing 150 μl of silicone oil (General Electric Company, Waterford, N.Y.) and microcentrifuged at 12,000 rpm for 3 min. This brought the PMN pellet to the base of the tube and kept the fluid supernatant on top separated by the oil. Supernatant and silicone oil were removed by pipette. A sterile cotton swab was used to wipe carefully around the PMN pellets to remove any remaining liquid. The PMN pellets were freeze-thawed three times (using dry ice-acetone slurry and a 37°C water bath) to lyse the cells. Then, 18 μl of sterile water was added to each pellet, and the pellets were triturated and placed into wells in seeded agar plates. Microscopic examination indicated that all PMN were lysed.
Tryptic soy agar (TSA) was seeded with either M. luteus, E. coli, or S. aureus. An overnight culture of the bacteria in TSB (1 ml per 50 ml of TSA for M. luteus or 1 ml per 100 ml of TSA for E. coli or S. aureus) plus 1 ml of 1 M HEPES per 100 ml of TSA was mixed, and 30 ml of this mixture was poured into each 150- by 15-mm petri dish (Becton Dickinson Labware, Lincoln Park, N.J.) and allowed to solidify. A standard curve relating the size of the zone of inhibition to the antimicrobial agent concentration was prepared by placing 20 μl of four concentrations of each antimicrobial agent in 4 mm-diameter wells and incubating these known standards on the same plates as the samples.
M. luteus plates were used for azithromycin, penicillin G, and telithromycin; S. aureus plates were used for moxifloxacin; and E. coli plates were used for ciprofloxacin and levofloxacin. The plates were incubated at 37°C in 5% CO2 overnight. The diameters of the cleared zones were then measured and plotted along a line created from the standards to determine the quantity of antimicrobial agent released from the PMN. The intracellular/extracellular (I/E) volume ratios were calculated utilizing a value for intracellular water volume as previously determined (10).
Preparation of plates for transport of antimicrobial agents by PMN.
Double-layer agar plates were made with a bottom layer of chemotaxis agar and a top layer of TSA as previously described (4). Wells of 3 mm in diameter were cut in the agar 4 mm apart in a triplet pattern. Each plate contained one control sample (PMN incubated without antimicrobial agent) in addition to samples of PMN incubated with antibiotic.
PMN (5 × 106/ml) were tumbled with concentrations of antimicrobial agents similar to peak levels in serum reported for patients after usual doses (2; F. Namour, D. H. Wessels, and M. Pascual, Abstr. 37th IDSA Meet., abstr. 976, 1999): 0.1 μg of azithromycin/ml, 4 μg of ciprofloxacin/ml, 6 μg of levofloxacin/ml, 4.5 μg of moxifloxacin/ml, 10 μg of penicillin G/ml, or no antimicrobial agent at 37°C for 1 h. The PMN were washed twice by centrifugation at 150 × g for 10 min, and the supernatant was discarded to remove extracellular antimicrobial agents. A lower concentration of telithromycin (0.1 μg/ml) was used rather than the usual serum telithromycin level of 2.3 μg/ml, because the zone of inhibition at this level was too large to measure in our system. Eight microliters of cell suspension (approximately 2 × 105 PMN) was placed in each of the middle wells of a triplet in the agar plates. A 10−7 M concentration of formyl-methionine-leucine-phenylalanine (fMLP) was used as a chemoattractant and placed in the outer wells. HBSS was placed in the inner well (Fig. 1). Plates were incubated for 3 h at 37°C in 5% CO2 to allow migration of neutrophils. After incubation, one set of plates was fixed with 100% methanol, followed by phosphate-buffered formalin (12). The agar was removed, and plates were stained with Giemsa stain. Neutrophil migration toward the chemoattractant and medium wells was measured under a microscope.
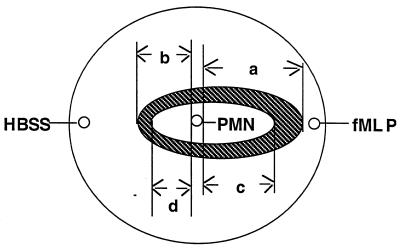
FIG. 1.
Cartoon of plate used for antimicrobial agent transport assay. The three small circles indicate 3-mm wells punched in agar plates. PMNs were placed in the center well and allowed to migrate toward the chemoattractant well (containing fMLP) or the medium well (containing HBSS). The striped oval indicates the pattern of PMN migration after 3 h. The inner oval indicates the area of inhibition of bacterial growth. a, directed PMN migration; b, nondirected PMN migration; c, inhibition of bacterial growth toward chemoattractant well; d, inhibition of bacterial growth toward medium well.
To determine the role of PMN migration in the transport of antimicrobial agents, cytochalasin B (1.0 μg/106 PMN) was used in some experiments to inhibit PMN movement (7). Cytochalasin B was dissolved in dimethyl sulfoxide (DMSO), and some experiments were performed with 1 μl of DMSO.
Since a component of the inhibition of bacterial growth could be related to antimicrobial agents diffusing out of the well independently of PMN movement, some experiments were performed with two HBSS washes of the PMN-containing well after the 3-h chemotaxis period.
Assay of release of antimicrobial agents from PMN.
After a 3-h incubation to allow the PMN to migrate, some plates were streaked with a 6-h broth culture of S. pyogenes using a wire loop and were incubated at 37°C in 5% CO2 overnight. The next morning, the zones of inhibition of bacterial growth toward the chemoattractant wells and the medium wells were measured under a microscope (Fig. 1).
RESULTS
MICs.
The MICs of the antimicrobial agents used for S. pyogenes were as follows: azithromycin, 0.25 μg/ml; ciprofloxacin, 0.312 μg/ml; levofloxacin, 0.625 μg/ml; moxifloxacin, 0.16 μg/ml; penicillin G, 0.156 μg/ml; and telithromycin, 0.0078 μg/ml. The MICs of the agents used for M. luteus were as follows: azithromycin, 0.125 μg/ml; ciprofloxacin, 1.25 μg/ml; levofloxacin, 1.25 μg/ml; moxifloxacin, 0.312 μg/ml; penicillin G, 0.156 μg/ml; and telithromycin, 0.312 μg/ml. The MICs of the agents used for S. aureus were as follows: azithromycin, 3.125 μg/ml; ciprofloxacin, 0.312 μg/ml; levofloxacin, 0.312 μg/ml; moxifloxacin, 0.078 μg/ml; penicillin G, 0.625 μg/ml; and telithromycin, 0.125 μg/ml. The MICs of the agents used for E. coli were as follows: ciprofloxacin, 0.0156 μg/ml; and levofloxacin, 0.0625 μg/ml.
PMN migration.
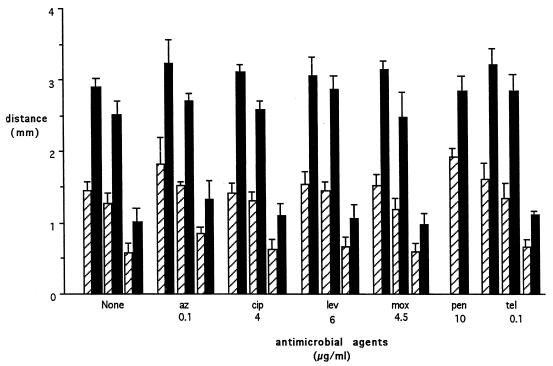
Results are expressed as mean ± standard error of the mean (SEM) and were analyzed with the paired Student t test. PMN migrated 2.92 ± 0.098 mm (n = 10) toward the chemoattractant well (directed migration) and 1.46 ± 0.098 mm (n = 10) toward the medium well (nondirected migration) (P < 0.05 [for the difference between directed and nondirected migration]). None of the antimicrobial agents studied affected migration distance. DMSO alone reduced directed migration by 16.7% ± 2.3% (P < 0.001) and nondirected migration by 15.3% ± 3.2% (P < 0.001). DMSO plus cytochalasin B reduced directed migration by 65.1% ± 1.8% (P < 0.001) and nondirected migration by 57.6% ± 3.1% (P < 0.001) (Fig. 1 and 2).
FIG. 2.
PMN movement toward fMLP (directed migration) or medium (nondirected migration). Hatched bars depict migration toward the HBSS well; and solid bars depict migration toward the fMLP well. Pairs for each condition were treated as follows (left to right): no additions, 1.0 μl of DMSO per well, and 1.12 μg of cytochalasin B plus 1.0 μl of DMSO per well. The antibiotics tested did not alter directed or nondirected migration. Bars are means ± SEM. At least three experiments were performed for each condition. Abbreviations: az, azithromycin; cip, ciprofloxacin; lev, levofloxacin; mox, moxifloxacin; pen, penicillin G; tel, telithromycin.
Uptake of antimicrobial agents.
Table 1 presents the intracellular and the extracellular antimicrobial agent concentrations and the I/E ratios. All antimicrobial agents studied except penicillin G were concentrated in the neutrophils. Telithromycin and azithromycin achieved the highest I/E ratios.
TABLE 1.
Antimicrobial agent uptake by PMNa
| Agent | Concn (μg/ml)
|
I/E ratio | |
|---|---|---|---|
| Extracellular | Intracellular | ||
| Azithromycin | 0.1 | 51.7 ± 4.3 | 517 |
| Ciprofloxacin | 4.0 | 24.8 ± 4.8 | 6.2 |
| Levofloxacin | 6.0 | 28.8 ± 4.8 | 4.8 |
| Moxifloxacin | 4.5 | 54 ± 10.5 | 12.0 |
| Penicillin G | 10 | 1.6 ± 0.2 | 0.16 |
| Telithromycin | 0.1 | 19.7 ± 2.7 | 197 |
Extracellular and intracellular antimicrobial agent concentrations for PMN incubated with the indicated concentrations of antimicrobial agents for 1 h were determined by bioassay. Results are means of at least three determinations.
Transport of antimicrobial agents by PMN.
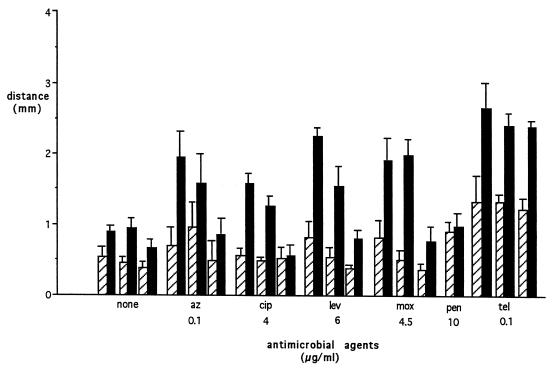
With the exception of penicillin G, the antimicrobial agents tested showed a marked increase in the zone of inhibition of bacterial growth in the direction coinciding with the greatest PMN migration (Fig. 3). DMSO alone reduced inhibition of bacterial growth toward fMLP by 16.6% ± 4.6% (P < 0.0001) and inhibition of growth toward HBSS by 12.5% ± 14.7% (P < 0.001). DMSO plus cytochalasin B reduced inhibition of growth toward fMLP by 44.5% ± 7.2% (P < 0.001) and toward HBSS by 14.4% ± 14.4% (P < 0.0001). Cytochalasin B, which inhibited directed PMN migration, caused a marked reduction in inhibition of bacterial growth, indicating that PMN movement and antimicrobial agent transport accounted for the increased zone of inhibition of bacterial growth. When PMN were studied without antimicrobial agents, there was a small zone of inhibition of bacterial growth that was greatest in the direction of greatest migration. This was probably due to the antibacterial substances present in the neutrophils (5). Telithromycin, a ketolide antimicrobial agent that achieves high concentrations inside PMN (11), showed the most potent inhibition. Although peak levels of telithromycin in serum after usual oral doses are 2.3 μg/ml, we used lower concentrations in our studies because at concentrations above 0.1 μg/ml, inhibition of bacterial growth extended into the medium and chemoattractant wells and could not be measured.
FIG. 3.
Inhibition of bacterial growth by migrating PMN loaded with antimicrobial agents. Hatched bars depict distance of inhibition of bacterial growth toward the HBSS well; solid bars depict distance of inhibition toward the fMLP well. Pairs for each condition were treated as described in the legend to Fig. 2. The penicillin G pair had no additions. Bars are means ± SEM. At least three experiments were performed for each condition. Abbreviations: az, azithromycin; cip, ciprofloxacin; lev, levofloxacin; mox, moxifloxacin; pen, penicillin G; tel, telithromycin.
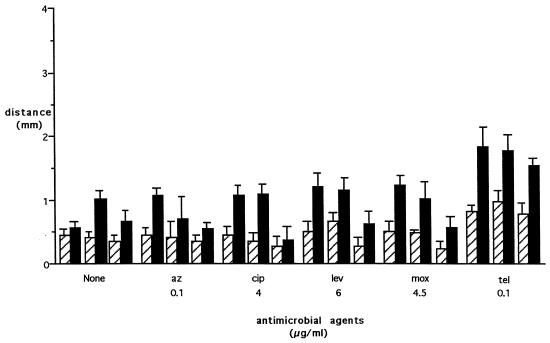
Washing of wells containing antibiotic-loaded PMN after 3 h (to allow for chemotaxis) resulted in smaller zones of bacterial inhibition (Fig. 4). For example, washing of the PMN loaded with 0.1 μg of telithromycin caused a 24% reduction (P = 0.02) of the zone of inhibition in the direction of fMLP. Inhibition of bacterial growth seen with directed migration was 1.99 times that of nondirected migration without washing of the wells, compared to a ratio of 2.21 after washing of the PMN. This indicates that the PMN were actually transporting and releasing the antimicrobial agents. Experiments performed with no PMN to determine the efficacy of washing the wells showed the following: for 1.25 μg of telithromycin per ml, washing of the well immediately resulted in a zone of bacterial inhibition that was 27% of that of the unwashed well and washing after 3 h resulted in a zone of inhibition that was 46% of that of the unwashed well.
FIG. 4.
Effect of washing of wells after chemotaxis on inhibition of bacterial growth by migrating PMN loaded with antimicrobial agents. Results are similar to those shown in Fig. 3, but the zones of inhibition are smaller. Hatched bars depict distance of inhibition of bacterial growth toward HBSS; solid bars depict distance of inhibition toward fMLP. Triplet pairs for each condition were treated as described in the legend to Fig. 2. DMSO alone reduced inhibition of bacterial growth toward fMLP by 8.5% ± 6.9% and did not affect inhibition of growth toward HBSS. DMSO plus cytochalasin B reduced inhibition of growth toward fMLP by 34.5% ± 11.0% and inhibition of growth toward HBSS by 29.8% ± 8.2%. Bars are means ± SEM. At least three experiments were performed for each condition. Abbreviations: az, azithromycin; cip, ciprofloxacin; lev, levofloxacin; mox, moxifloxacin; tel, telithromycin.
DISCUSSION
Antimicrobial agents and neutrophils have the potential to interact in ways that enhance the therapy for infectious diseases. Bacteria that are exposed to antimicrobial agents are often more susceptible to ingestion and killing by phagocytes (1, 14). Microbes that can survive inside phagocytes are killed by agents that penetrate the cell but not by agents that do not enter the phagocyte. Staphylococci inside neutrophils survive incubation with beta-lactam antibiotics in concentrations that kill extracellular organisms, since these agents do not enter neutrophils. Rifampin enters neutrophils and kills staphylococci residing in the phagocyte (9).
Intracellular antimicrobial agents may be in the cytoplasm or concentrated in organelles (16). The rates of entry and egress vary according to the agents and are not well studied for most antimicrobial agents.
In order for an antimicrobial agent to be effectively transported, it must not interfere with the ability of the neutrophil to sense chemoattractants and to move normally. We modeled antimicrobial agent transport in an in vitro system and found that agents that are concentrated by neutrophils were effectively carried along a chemotactic gradient and inhibited the growth of a lawn of sentinel bacteria. Larger zones of inhibition were seen in the direction of the chemoattractant and thus in the direction of the greatest migration distance, indicating that neutrophils were actually transporting the antimicrobial agents. Studies with cytochalasin B, a potent inhibitor of neutrophil motility, showed that migrating neutrophils were necessary for optimal effect.
Transport of antimicrobial agents by PMN is only part of a complex series of events that includes entrance of the antibiotic into the PMN, interaction of the agent with intracellular organelles, and efflux of the active agent. The pharmacodynamics in vivo are also complex, since in most instances, levels of drug in the blood vary widely and levels in tissue may be very different from levels in blood. Thus, it is possible that antimicrobial agents that appear to be transported poorly diffuse out of the neutrophils rapidly during washing with antimicrobial agent-free media. If this is the case, then levels of antimicrobial agents in PMN should be expected to decrease markedly as levels in serum fall and transport should be ineffective.
The in vitro system that we used showed those agents that were highly concentrated and slowly released by PMN to be most effectively transported. This appears to be the case for azithromycin (18) and telithromycin (17).
The antimicrobial agents studied included penicillin G, which does not enter neutrophils and thus was not transported. The fluoroquinolones ciprofloxacin, levofloxacin, and moxifloxacin were all concentrated in the neutrophils and were effectively transported. Azithromycin and telithromycin were highly concentrated in the neutrophils and were transported very efficiently. We cannot explain why telithromycin transport was not inhibited effectively by cytochalasin B plus DMSO. A factor in the potency of the antimicrobial agents in inhibiting the growth of the sentinel lawn of S. pyogenes is the in vitro activity of the antimicrobial agent. The most active agent was telithromycin, but penicillin G, despite its potency, was not effectively transported.
Our results with ciprofloxacin differed from those reported in 1992 (4). At that time, it was noted that inhibition of bacterial growth was not significantly different in the direction of the chemoattractant (P = 0.11). However, there was a difference in the mean size of the zones of inhibition (1.40 versus 1.18 mm). Our present techniques have improved, resulting in increased chemotactic migration from 2.51 ± 0.16 mm in 1992 to 2.92 ± 0.098 mm at present. Nondirected migration was unchanged at 1.48 ± 0.12 mm in 1992 and 1.46 ± 0.098 mm at present. This change was most likely due to a different method of neutrophil isolation and lysis of red blood cells. The present system is also more sensitive in detecting zones of bacterial inhibition. For example, in the present study, 0.1 μg of azithromycin resulted in a 1.95 ± 0.337-mm zone of inhibition toward chemoattractant while in the 1992 study, 0.3 μg of azithromycin resulted in only a 1.0-mm zone of inhibition. Thus, transport of ciprofloxacin by PMN does occur.
The concept of antimicrobial agent transport by phagocytes is an attractive one. Neutrophils home toward products of microbes and molecules generated by the host cell's reactions to microbes and concentrate at the site of infection. If neutrophils carry intracellular antimicrobial agents to these infection sites and release the agents at these sites, this would be an effective way to enhance the treatment of infections. Further study will be needed to determine if this is clinically important.
ACKNOWLEDGMENTS
This work was supported by grants from Aventis Pharmaceuticals, Romainville, France; Ortho-McNeil Pharmaceutical, Raritan, N.J.; and Bayer Corporation, West Haven, Conn.
REFERENCES
- 1.Adinolfi L, Bonventre P F. Enhanced phagocytosis, killing, and serum sensitivity of Escherichia coli and Staphylococcus aureus treated with sub-MICs of imipenem. Antimicrob Agents Chemother. 1988;32:1012–1018. doi: 10.1128/aac.32.7.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amsden G. Tables of antimicrobial agent pharmacology. In: Mandell G, Bennett J, Dolin R, editors. Tables of antimicrobial agent pharmacology. 5th ed. Philadelphia, Pa: Churchill Livingstone; 2000. pp. 551–601. [Google Scholar]
- 3.Ferrante A, Thong Y H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol. 1980;36:109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- 4.Frank M O, Sullivan G W, Carper H T, Mandell G L. In vitro demonstration of transport and delivery of antibiotics by polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1992;36:2584–2588. doi: 10.1128/aac.36.12.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganz T. Antimicrobial peptides of leukocytes. Curr Opin Hematol. 1997;4:53–58. doi: 10.1097/00062752-199704010-00009. [DOI] [PubMed] [Google Scholar]
- 6.Jones R N, Barry A L, Gavan T L, Washington J A., II . Susceptibility tests: microdilution and macrodilution broth Procedures. In: Lennette E H, Balows A, Hausler W J Jr, Shadomy H J, editors. Manual of clinical microbiology. 4th ed. Washington, D.C.: American Society for Microbiology; 1985. pp. 972–977. [Google Scholar]
- 7.Klebanoff S, Clark R. The neutrophil: function and clinical disorders; New York, N.Y: Elsevier/North-Holland Biomedical Press; 1978. pp. 129–130. [Google Scholar]
- 8.Lam C, Georgopoulos A, Laber G, Schütze E. Therapeutic relevance of penicillin-induced hypersensitivity of staphylococcus aureus to killing by polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1984;26:149–154. doi: 10.1128/aac.26.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandell G, Vest T. Killing of intraleukocytic Staphylococcus aureus by rifampin: in-vitro and in-vivo studies. J Infect Dis. 1982;125:486–490. doi: 10.1093/infdis/125.5.486. [DOI] [PubMed] [Google Scholar]
- 10.Mandell G L. Interaction of intraleukocytic bacteria and antibiotics. J Clin Investig. 1973;52:1673–1679. doi: 10.1172/JCI107348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miossec-Bartoli C, Pilatre L, Peyron P, N'Diaye E-N, Collart-Dutilleul V, Maridonneau-Parini I, Diu-Hercend A. The new ketolide HMR3647 accumulates in the azurophil granules of human polymorphonuclear cells. Antimicrob Agents Chemother. 1999;43:2457–2462. doi: 10.1128/aac.43.10.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson R D, McCormack R T, Fiegel V D. Chemotaxis of human leukocytes under agarose; In: Gallin J I, Quie P G, editors. Leukocyte chemotaxis: methods, physiology, and clinical implications. New York, N.Y: Raven Press; 1978. pp. 25–42. [Google Scholar]
- 13.Prokesch R C, Hand W L. Antibiotic entry into human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1982;21:373–380. doi: 10.1128/aac.21.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Root R, Isturiz R, Molavi A, Metcalf J, Malech H. Interactions between antibiotics and human neutrophils in the killing of staphylococci. J Clin Investig. 1981;67:247–259. doi: 10.1172/JCI110020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwab J C, Mandell G L. The importance of penetration of antimicrobial agents into cells. Infect Dis Clin N Am. 1989;3:461–467. [PubMed] [Google Scholar]
- 16.Tulkens P. Intracellular distribution and activity of antibiotics. Eur J Clin Microbiol Infect Dis. 1991;10:100–106. doi: 10.1007/BF01964420. [DOI] [PubMed] [Google Scholar]
- 17.Vazifeh D, Preira A, Bryskier A, Labro M. Interactions between HMR 3647, a new ketolide, and human polymorphonuclear neutrophils. Antimicrob Agents Chemother. 1998;42:1944–1951. doi: 10.1128/aac.42.8.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wildfeuer A, Laufen H, Zimmermann T. Uptake of azithromycin by various cells and its intracellular activity under in Vivo conditions. Antimicrob Agents Chemother. 1996;40:75–79. doi: 10.1128/aac.40.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]