Abstract
We previously discovered that actinorhodin, a benzoisochromanequinone antibiotic produced by Streptomyces coelicolor A3(2), serves as a catalyst facilitating the oxidation of ascorbic acid and cysteine (PNAS 48:17,152, 2014). In the present study, we screened for similar ascorbic acid-oxidizing activity in the culture broth of various Streptomyces spp., and discovered marked activity in the culture broth of Streptomyces vietnamensis. The principle active compound was granaticin, a pigmented antibiotic that is structurally related to actinorhodin. The absence of any metals in the purified granaticin fraction indicated that granaticin was an organocatalyst. Granaticin catalyzed the oxidation of L-ascorbic acid, generating L-dehydroascorbic acid and hydrogen peroxide (H2O2) at a 1:1 stoichiometric ratio, with 15 times higher reactivity than that of actinorhodin at an optimum pH of 7.0. Granaticin also oxidizes sulfhydryl compounds, including L-cysteine and glutathione. Growth inhibitory assays demonstrated that knockout mutants of the catalase gene exhibit high sensitivity to granaticin. The results suggest that the bactericidal activity of granaticin is exerted by the oxidation of sulfhydryl groups of cellular components and the toxicity of H2O2 generated during the oxidation reaction.
Subject terms: Biochemistry, Microbiology, Chemistry
Introduction
Microbial products have a vast structural and functional diversity. Since the discovery of penicillin and streptomycin, many compounds of microbial origin have played a significant role as therapeutic agents for various diseases1. Some compounds have also contributed to the progress of basic science as tools for studying the mechanistic details of biological functions2,3. The activities of these microbial products are mostly exerted through their association with a specific enzyme or other macromolecules, causing inhibition or disorder of the corresponding bioprocesses.
Previously, we discovered that actinorhodin (Fig. 1), a pigment antibiotic produced by Streptomyces coelicolor A3(2), retained its catalytic activity4. The addition of purified actinorhodin facilitated the oxidation of L-ascorbic acid and cysteine according to the following equations:
Because actinorhodin did not change its structure after these reactions, it was concluded that the promotion of the reaction was due to the catalytic function of actinorhodin.
Figure 1.

Chemical structure of actinorhodin and granaticin.
Catalysts are grouped into types according to their physicochemical features and modes of action. Among these types, the major group is metal-containing catalysts, which are widely used in industrial chemical synthesis. However, the reactions carried out within living organisms are mostly mediated by biocatalysts. The major class of biocatalysts are enzymes, which are proteinaceous macromolecules, but some RNA molecules, termed ribozymes, also exhibit a catalytic function. Enzymes are utilized as an effective tool in industrial production because of their superior reactivity, including high stereoselectivity and low economic and environmental burdens.
The third group of biocatalysts is organocatalysts. Organocatalysts are catalytic organic molecules that do not contain metals5. The occurrence of catalytic activity in some organic compounds led to the hypothesis that the activity of some organic molecules that facilitates an asymmetrical chemical reaction is fundamental to the introduction and spread of homochirarity in the living creatures6,7. Although organic molecules were used as catalysts in the early chemical synthesis performed during the 1800s, their effective introduction to chemical synthesis began only after the year 20006,7. Since then, the number of studies referring to the usefulness of organocatalysts especially in the enantio-selective synthesis has increased remarkably, reflecting their growing potential7. Proline and imidazolidinones are well-characterized organocatalysts8–10. In 2021, the Nobel Prize in Chemistry was awarded to Drs MacMillan and List for the development of asymmetric organocatalysts11. Our previous discovery of actinorhodin was the first instance of an organocatalyst produced by microbial secondary metabolism.
In the present study, we screened for additional Streptomyces metabolites exhibiting ascorbic acid-oxidizing activity to further explore the occurrence of organocatalysts in microbial metabolites. We discovered that granaticin, a benzoisochromanequinone antibiotic produced by Streptomyces vietnamensis, is catalytically active. This discovery not only highlights the use of microbial products as organocatalysts, which may be important in industrial applications, but also deepens the insights into the diversity of action mechanism of antibiotics and their role in the natural environment.
Results
Identification of granaticin as a catalytic compound
To study the occurrence of organocatalytic activity in Streptomyces metabolites, we assessed the L-ascorbic acid-oxidizing activity in the culture supernatant of various Streptomyces strains (see “Methods”), and discovered that the culture broth of S. vietnamensis exhibited marked O2 consumption when it was mixed with 10 mM L-ascorbic acid (Fig. 2A). The same level of O2-consuming activity was observed even after heat treatment at 95 °C for 30 min (data not shown), indicating that the catalyst is a heat-stable molecule.
Figure 2.

O2 consumption due to catalytic activity of granaticin. The dissolved O2 concentration in 10 mM L-ascorbic acid solution added to the culture supernatant of S. vietnamensis (A) and purified granaticin (0.03 mM) (B) was monitored using an oxygen electrode. The time points when the S. vietnamensis culture supernatant (A) and purified granaticin (B) were added are indicated by solid arrows. The open arrow in (B) indicates the time point for the addition of 100 units catalase. The dotted lines are the data from the measurement without the addition of culture supernatant (A) and with the addition of catalase (B).
The culture broth of S. vietnamensis was purple in color, which was likely due to the production of a purple benzoisochromanequinone antibiotic, known as granaticin (Fig. 1)12. Previously, we proposed that the catalytic activity of actinorhodin is based on its quinone structure, since a weak but distinct L-ascorbic acid oxidizing activity was observed with several quinolic compounds of plant origin4. These previous observations led us to speculate granaticin-induced catalytic activity was present in the culture supernatant of S. vietnamensis. Therefore, the principle active compound was isolated using a previously published purification method for granaticin13. As expected, the L-ascorbic acid-oxidizing activity was fractionated into the same fraction as that of granaticin.
LC–MS analysis of the resultant purified active fraction showed a single peak at 443 m/z (ESI, negative) (Fig. 3A). The UV–visible absorption spectrum of this fraction containing peaks at 220, 285, 490, 525 and 570 nm (Fig. 3B) was identical to that of granaticin previously reported by James and Edwards14. These results confirm that the isolated fraction contained purified granaticin. The metallic ion content of the fraction was determined using an inductively coupled plasma optical emission spectrometer (ICP-OES), but none of the 71 metallic ion species that could be identified by the analytical method were detected.
Figure 3.
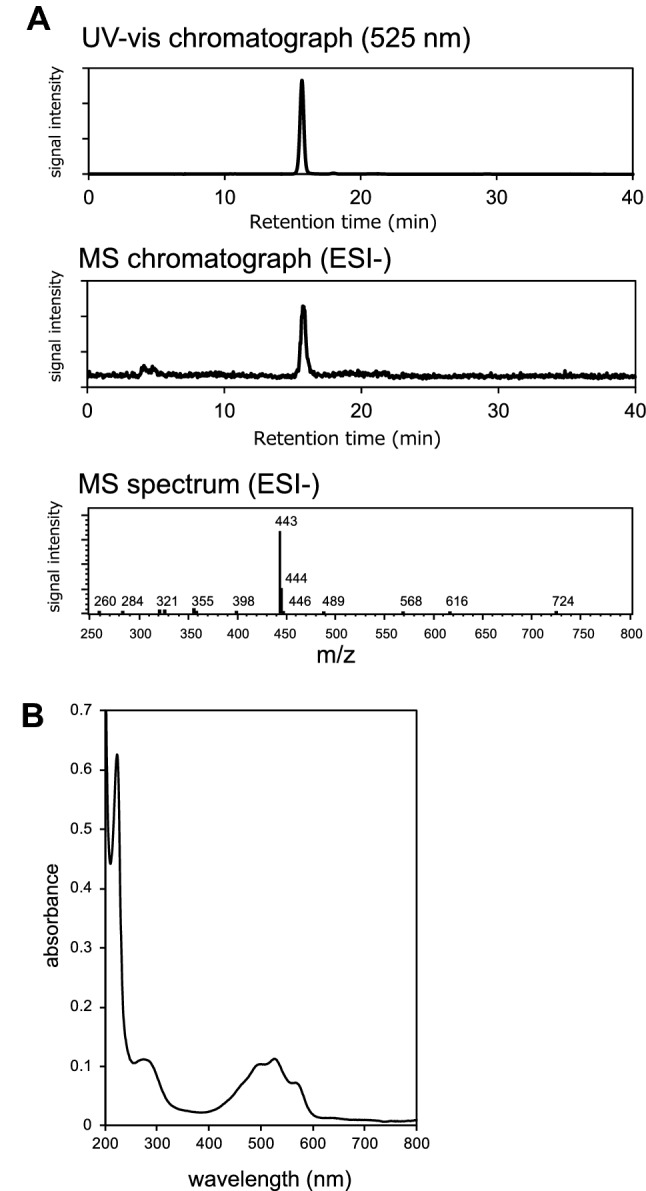
Spectra of the purified catalytic substance. LC–ESI–MS chromatogram (A) and UV–vis absorption spectrum (B) of the purified active compound. The single peak detected at 15.6 min (panel A, upper) was 443 m/z in the negative analytical mode according to MS analysis (panel A, lower), which coincided with the [M-H]- m/z of granaticin. The UV–vis absorption spectrum of the purified fraction (panel B) containing peaks at 220, 285, 490, 525 and 570 nm was identical to the profile of purified granaticin reported by James and Edwards14.
Catalytic activity of granaticin
-
(i)
Specific activity
The L-ascorbic acid oxidizing activity of purified granaticin was determined. As shown in Fig. 2B, the dissolved O2 concentration in the reaction solution started to decrease immediately after mixing with granaticin. The addition of catalase reduced the ratio of O2 consumption (Fig. 2B dashed line), indicating the occurrence of H2O2 in the reaction mixture. LC–MS analysis of the reaction mixture revealed the presence of granaticin at the starting concentration, and the absence of any derivatives (Supporting information figure S1), indicating that granaticin catalyzes the oxidation of L-ascorbic acid without changing its structure or being destroyed.
The specific activity of granaticin in L-ascorbic acid oxidation was 53.7 U/μmol, which is approximately 15-fold that of actinorhodin (3.5 U/μmol)4.
-
(ii)
Reaction products and stoichiometry
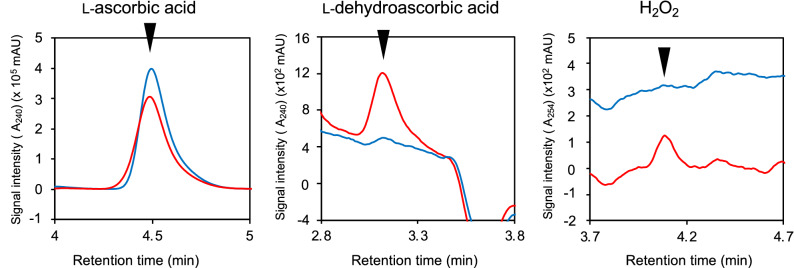
Our previous work demonstrated that actinorhodin catalyzes the oxidation of L-ascorbic acid, generating L-dehydroascorbic acid and H2O24. To determine whether this was the case with granaticin, the content of the reaction mixture was analyzed by HPLC. The two peaks generated by the reaction with granaticin corresponded to those of L-dehydroascorbic acid and H2O2 (Fig. 4). This indicates that granaticin catalyzes the same reaction as actinorhodin.
The stoichiometry of granaticin was determined (Fig. 5). The substrates (L-ascorbic acid and O2) and products (L-dehydroascorbic acid and H2O2) were present at a 1:1:1:1 stoichiometric ratio.
-
(iii)
Dependence on pH and temperature
The pH and temperature dependence of the catalytic activity of granaticin was assessed. The highest activity was observed at pH 7.0 (Fig. 6A). The oxidation of L-ascorbic acid occurred spontaneously at pH 10 and higher, as described previously4. The activity increased with increasing temperature from 25 to 40 °C (Fig. 6B). The activity at temperatures higher than 40 °C could not be measured because of the heat sensitivity of the electrode system. Although data were slightly fluctuated, preincubation at temperatures between 30 and 100 °C for 1 h did not significantly affect the catalytic activity (Fig. 6C).
-
(iv)
Substrate specificity
The substrate specificity of granaticin was determined by reacting granaticin with various substrates. The substrate selectivity profile was similar to that of actinorhodin4, but the reactivity was different (Table 1). Compared to L-ascorbic acid (100%), the reaction of its stereoisomer D-isoascorbic acid was relatively weak (77.3%), indicating that the catalysis occurs asymmetrically. Much weaker but distinctive reactions were observed with L-cysteine methyl ester (1.5%), L-cysteine (0.6%), dithiothreitol (0.4%), D-cysteine (0.4%), and glutathione (0.1%). The narrow but distinctive difference in the reactivity with cysteine isomers also indicates the asymmetricity of the reaction. Oxidation of N-acetyl-L-cysteine, DL-penicillamine, DL-homocysteine, lipoamide, and α-lipoic acid was not observed when O2 consumption was monitored for 30 min.
Figure 4.
HPLC chromatogram of the reactants. The peaks corresponding to L-ascorbic acid, L-dehydroascorbic acid, and H2O2 before (blue line) and after (red line) the 5 min reaction with granaticin are shown.
Figure 5.

Stoichiometric analysis of granaticin catalysis. Time course of the concentration of L-ascorbic acid (L-ASC), O2, L-dehydroascorbic acid (L-DHA), and H2O2 in the oxidation reaction catalyzed by granaticin.
Figure 6.
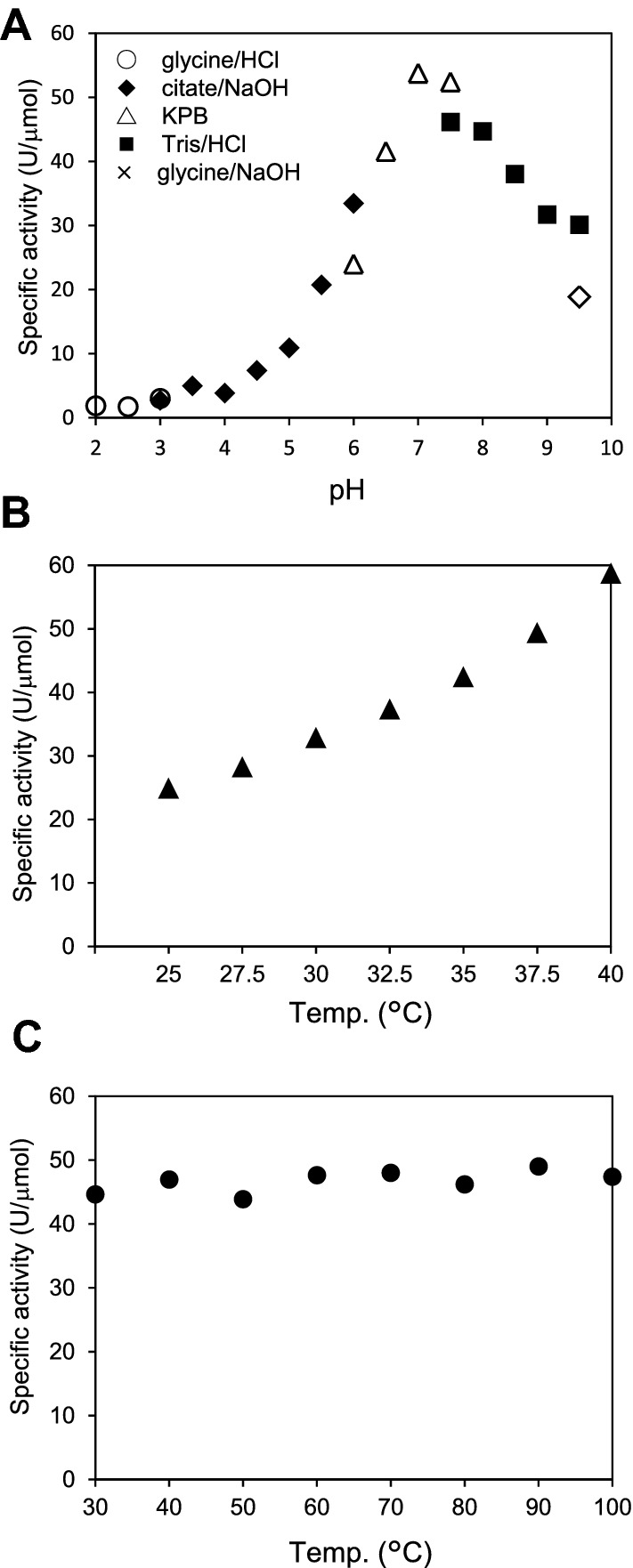
Effect of pH and temperature on granaticin activity. The catalytic activity of granaticin was measured at various pH values at 30 °C (A) and at various temperatures at pH 7.0 (B). The activity at temperatures higher than 40 °C could not be measured because of the operation limits of the oxygen electrode. The thermostability of the compound (C) was also studied by incubating granaticin solution at various temperatures for 1 h prior to the activity measurement. A representative result of repeated experiments is shown.
Table 1.
Substrate specificity.
| Substrates | Catalytic activity (U/μmol) | |
|---|---|---|
| Granaticin | Actinorhodin | |
| L-ascorbic acid | 52.63 | 3.50 |
| D-iso-ascorbic acid | 40.66 | 3.01 |
| L-cysteine methyl ester | 0.77 | 0.56 |
| L-cysteine | 0.31 | 0.02 |
| Dithiothreitol | 0.22 | 0.34 |
| D-cysteine | 0.19 | NT |
| Glutathione | 0.06 | 0.01 |
| N-acetyl-L-cysteine | ND | ND |
| DL-penicillamine | ND | ND |
| DL-homocysteine | ND | ND |
| Lipoamide | ND | NT |
| α-lipoic acid | ND | NT |
ND not detected, NT not tested.
Growth inhibition activity of granaticin
We previously hypothesized that the antibiotic activity of actinorhodin is at least partly due to the toxicity of H2O2 generated along with the catalytic reaction, based on the observation that the addition of catalase rescued the growth of S. aureus exposed to actinorhodin on solid media4. The same assay using the filter disc diffusion method showed that the growth inhibition effect of granaticin on S. aureus was also ameliorated by the addition of catalase (data not shown).
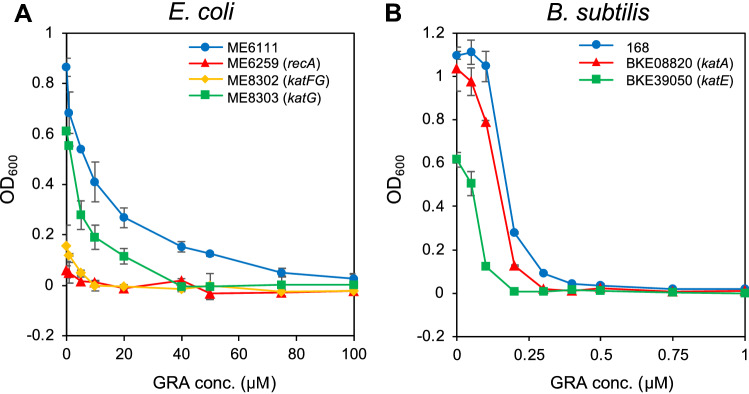
We next studied the sensitivity of catalase-deficient strains of E. coli and B. subtilis (Table 2). Figure 7 shows the dose effect of granaticin on the early growth (5 h cultivation) of these mutants in Luria–Bertani (LB) liquid medium. Granaticin is known to be an antibiotic that statically inhibits the growth of gram-positive bacteria15. As expected, the sensitivity of E. coli was far lower than that of B. subtilis (ca, 100-foled difference). However, in both bacteria, the catalase-deficient mutants exhibited higher sensitivity to granaticin than the control strain. In E. coli, ME8302 lacking the two catalase genes (katF and katG) was more sensitive than ME8303, lacking active katG but retaining katF (Fig. 6A). We also performed the assay with an E. coli mutant (ME6259) for recA, the master regulator of the SOS response system16, and found that it had a higher sensitivity than the control strain (ME6111) (Fig. 6A). A similar profile was obtained for JM109 (recA-) and JM105 (recA +) strains (data not shown). Similarly, the mutant for katA (BKE08820) and katE (BKE39050) of B. subtilis exhibited higher sensitivities than the control strain (Fig. 6B). These results support our hypothesis that the toxicity of granaticin is based on the effect of H2O2 that is generated by its catalytic reaction. The high sensitivity of recA mutants could be due to their disability to repair the damage caused in DNA structure by the oxidative agent.
Table 2.
Genotype of E. coli and B. subtilis mutants used in this study.
| Strain | Genotype |
|---|---|
| E. coli | |
| ME6111 | asn thi thyA str |
| ME6259 | asn thi thy str recA |
| ME8302 |
leuB6 proC83 purE42 trpE28 his-208 argG77 ilvA681 met-160 thi-1 ara-14 lacY1 galK2 xyl-5 mtl-1 azi-6 rpsL109 tonA23 tsx-67 supE44 malA38 xthA katF3 kat-3 cysI::Tn10 |
| ME8303 |
leuB6 proC83 purE42 trpE38 his-208 argG77 ilvA681 metA160 thi-1 ara-14lacY1 galK2 xyl-5 mtl-1 azi-6 rpsL109 tonA23 tsx-67 supE44 malA38 xthA katG17::Tn10 |
| JM105 | endA hsdR(rk-mk +) rpsL sbcBC thi-1 Δ(lac-proAB) F'[traD36 proAB lacIqZΔM15] |
| JM109 | recAl supE44 endA1 hsdR17 gryA96 thi Δ(lac-proAB) F'[traD36 proAB lacIqZΔM15] |
| B. subtilis | |
| BKE08820 | katA |
| BKE39050 | katE |
Figure 7.
Inhibition of bacterial growth by granaticin. Growth inhibition of E. coli (A) and B. subtilis (B). Strains were inoculated into LB liquid medium containing various concentrations of granaticin, and the optical density at 600 nm (OD600) was measured after 5 h of cultivation. The data are the means of three measurements. The error bars represent the standard deviation.
Discussion
In the present study, we determined that granaticin serves as a catalyst to facilitate the oxidation of L-ascorbic acid to L-dehydroascorbic acid. The lack of structural changes or depletion during this reaction, as well as the lack of metallic content, indicate that granaticin is an organocatalyst. The relatively narrow but distinctive difference in the reactivity with the stereoisomers of ascorbic acid and cysteine (Table 1) indicates that the catalytic reaction occurs asymmetrically. This finding reinforces the notion that these types of functional molecules are present in microbial products.
Granaticin and actinorhodin belong to the same class of compounds, termed benzoisochromanequinones, which share a naphthoquinone structure (Fig. 1). We previously showed that some plant-derived natural products, which commonly retain a quinone structure, also exhibit catalytic activity, facilitating the oxidation of ascorbic acid4. Thus, we speculate that the quinone structure is fundamental to the catalytic function of this class of organocatalysts. We hypothesize that the structural interconversion between quinone and quinol within the catalytic molecule mediates the oxidation of ascorbic acid to dehydroascorbic acid and the reduction of O2 to H2O2 (Fig. 8). Granaticin differs from actinorhodin in terms of its specific activity, optimum pH, and substrate specificity. It is likely that the differences in the structure surrounding the quinone backbone affect the catalytic properties of these molecules. A detailed understanding of the correlation of their chemical structure with the reactivity will broaden the field of organocatalysts.
Figure 8.
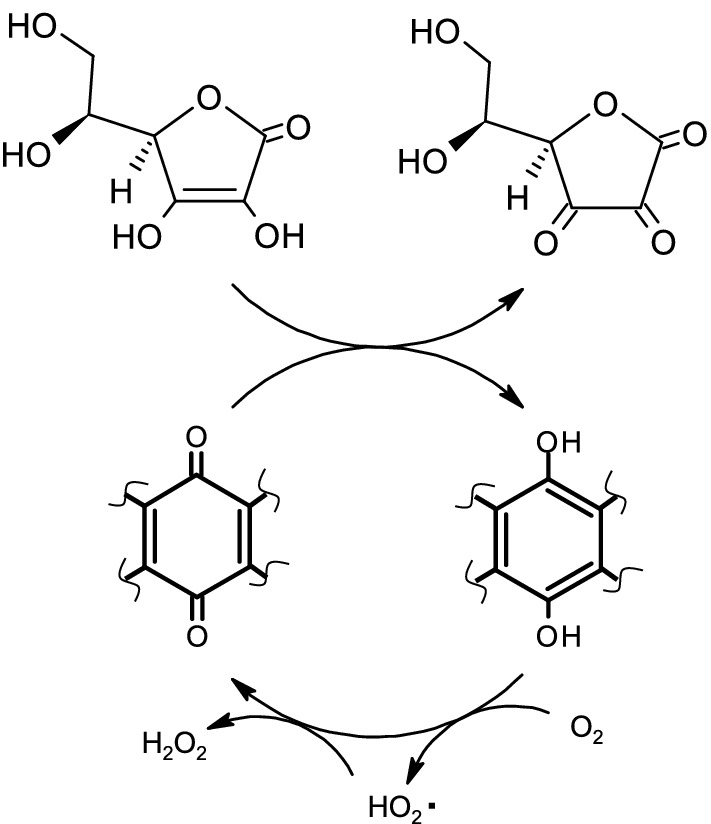
Hypothetical mechanism of catalysis. Possible mechanism of ascorbic acid oxidation catalyzed by granaticin and other quinolic substances. The catalytic reaction occurs because of the autonomous regeneration of the quinone form, via electron transfer from the quinol form to molecular oxygen.
Granaticin was first identified in the culture broth of Streptomyces olivaceus based on its antibiotic activity against gram-positive bacteria17. It was also cytotoxic to mammalian cells. Chang et al.18 discovered that an antibiotic termed litmomycin, which is produced by Streptomyces litmogenes, exhibited antitumor activity against leukemia in mice, and that the chemical structure of litmomycin was identical to that of granaticin. Early studies on the bactericidal mechanisms of granaticin suggest that its interaction with RNA or related molecules is fundamental to its antibiotic activity. Ogilvie et al.15 studied the effects of granaticin on Bacillus subtilis and concluded that it interferes with the aminoacylation of leucyl transfer RNA, thereby inhibiting translation. However, other studies have proposed different targets of inhibition, such as RNA synthesis by RNA polymerase15, DNA synthesis by reverse transcriptase19, and maturation of ribosomal RNA20.
Independently of its possible interaction with RNA or related molecules, Sturdik and Drobnica21 determined that granaticin is reactive to sulfhydryl groups by observing its effect on sulfhydryl compounds and sulfhydryl enzymes, including glyceraldehyde-3-phosphate dehydrogenase, and proposed that this kind of activity is fundamental to the cytotoxicity of granaticin. This is consistent with the report by Gibson-Clay et al.22, who observed the inhibitory effect of granaticin on the activity of pyruvate decarboxylase, the central metabolic enzyme, depending on its activity on the oxidation and reduction of the SH group of lipoamide, which is associated with the E2 domain.
The ambiguity of the action mechanism of granaticin can be explained by its organocatalytic activity. Previously, we hypothesized that the bactericidal activity of actinorhodin is, at least in part, due to the H2O2 produced during catalysis, based on the observation that the addition of catalase reduced the level of growth inhibition4. H2O2 generated via the catalytic reaction exerts toxic effects on cellular functions. This is also supported by the antibiotic activity measurements performed in the present study (Fig. 7). The high sensitivity of the catalase mutants to granaticin suggests that the generation of H2O2 within or on the surface of the target cell, along with the oxidation of cellular material, is fundamental to the antibiotic and cytotoxic activity of this compound. The similar feature of the recA mutants is interpreted as the oxidative effect of H2O2 damages the chromosomal DNA and therefore significantly affects the viability of the recA mutant strains, which lack the ability to express the DNA repair system16. The oxidative damage to nucleic acid structures may be correlated with the abovementioned hypothesis that granaticin exerts its cytotoxicity by introducing damage to RNA and related functions.
Recently, Nass et al.23 reported that γ-actinorhodin, an actinorhodin derivative, exhibits unique antibacterial activity. The bactericidal activity of γ-actinorhodin was supposed to be due to the generation of reactive oxygen species (ROS), as the S. aureus mutant for superoxide dismutase was highly sensitive to the substance. Similar observations have been reported for the bactericidal activity of a plant-derived bioactive quinone termed thymoquinone24, a chemically synthesized anticancer and antibacterial compound YM155 with a naphthoimidazole structure25, and 1,4-naphtoquinones exhibiting antibacterial activity and cytotoxicity26. These compounds may also exert their toxicity by generating ROS. The mode of action of these other bioactive quinolic substances reinforces the view that the toxicity of granaticin is also based on the generation of ROS.
In addition to ROS generation, inactivation of proteins due to the oxidation of sulfhydryl groups could be the basis of the observed toxicity of granaticin and related compounds. Although the specific activity was relatively low, in the present study we determined that granaticin promotes the oxidation of cysteine. This suggests that the sulfhydryl group, which is exposed to the protein surface, could be the target of oxidation by granaticin. This agrees with the abovementioned observation that granaticin inhibits sulfhydryl enzymes. Overall, the lines of evidence strongly suggest the existence of a novel mechanism for the action of antibiotics and bioactive substances based on their catalytic activity.
The reactivity of granaticin at neutral pH and moderate temperature indicates that its catalytic function can proceed under standard conditions. The granaticin biosynthetic gene cluster exists in the genome of various Streptomyces and other actinomycetes27,28. Deng et al.29 suggested that the ability to produce granaticin is distributed via horizontal gene transfer. Thus, the newly discovered function of this natural product is widespread in the natural environment, and it is readily available for various oxidative reactions. Taken together, these features with regard to granaticin support the occurrence of an unprecedent role, function, and utilization of microbial products.
Methods
Bacterial strains and media
The Streptomyces strains used for catalytic activity screening were obtained from the National Institute of Technology and Evaluation (NITE), Chiba, Japan (Table 3). Staphylococcus aureus NBRC100910 was used for the growth inhibition assay, and was also obtained from the NITE. Escherichia coli strains used for the growth inhibition assay (Table 2) were obtained from the National Bioresource Project (NBRP) (https://nbrp.jp/en/). JM105 and JM109 were purchased from Takara Bio Inc. (Kyoto, Japan). Bacillus subtilis strains 168, BKE08820 (katA-deficient), and BKE39050 (katE-deficient) were also obtained from the NBRP.
Table 3.
Streptomyces strains used for screening.
| Species | NBRC no | Species | NBRC no |
|---|---|---|---|
| S. abikoensis | 13907 | S. ahygroscopicus | 13664 |
| S. albofaciens | 12833 | S. albulus | 13410 |
| S. amakusaensis | 12835 | S. argenteolus | 12841 |
| S. argenteolus | 13847 | S. cellulosae | 13027 |
| S. chattanoogensis | 12754 | S. fasiculatus | 12765 |
| S. griseolus | 12777 | S. hygroscopicus | 3977 |
| S. hygroscopicus | 13815 | S. inusitatus | 13601 |
| S. libani | 13452 | S. melanogenes | 12890 |
| S. noboritoensis | 13065 | S. olivaceiscleroticus | 13484 |
| S. olivoverticillatus | 13068 | S. sanglieri | 100784 |
| S. setonii | 13085 | S. sioyaensis | 12820 |
| S. somaliensis | 12916 | S. sporoclivatus | 100767 |
| S. verne | 13097 | S. vietnamensis | 104153 |
| S. violens | 13486 |
Streptomyces strains were cultured at 28 °C in Bennett’s/maltose (BM) medium containing 1 g/L yeast extract (Difco Laboratories, Detroit, Michigan), 1 g/L meat extract (Kyokuto, Tokyo, Japan), 2 g/L NZ amine (Wako Pure Chemical Industries Ltd., Osaka, Japan), and 10 g/L maltose (pH 7.2). For the antibiotic assay, E. coli and B. subtilis strains were cultured in Luria–Bertani (LB) medium containing 10 g/L tryptone (Difco), 5 g/L yeast extract (Difco), and 5 g/L NaCl. Agar was added to LB broth to a final concentration of 1.5% to prepare solid media. Chemicals were purchased from Kokusan (Tokyo, Japan), unless otherwise indicated.
Screening for catalytic activity
Screening for the organocatalytic activity in Streptomyces culture supernatants was performed by monitoring the decrease in dissolved O2 concentration using an oxygen electrode (Hansatech Instruments Ltd., Norfolk, UK). Each Streptomyces strain was cultured in a test tube (Φ18 × 180 mm) containing 10 ml BM liquid medium at 28 °C for 5 days with reciprocal shaking at 300 rpm. After growth, the supernatant was obtained by centrifugation at 10,400×g at 4 °C for 15 min. The substrate solution contained 10 mM L-ascorbic acid and 100 mM potassium phosphate buffer (pH 7.2). The reaction was initiated by adding 20 µl of culture supernatant to 1 ml substrate solution in an electrode cuvette and incubating at 30 °C for 5 min. The consumption of O2 during the 5 min reaction was monitored using an oxygen electrode according to the manufacturer’s instructions. To evaluate the O2 decrease due to spontaneous oxidation, the decrease in O2 concentration was measured in parallel in a negative control mixture with fresh culture medium in place of the culture supernatant.
Isolation of granaticin
S. vietnamensis NBRC 104153 was first inoculated into a test tube (Φ18 × 180 mm) containing 10 ml BM liquid medium and cultured at 28 °C for 5 days with reciprocal shaking at 300 rpm. Then, 1 ml of this preculture was inoculated into a baffled 500 ml Erlenmeyer flask containing 100 ml of BM medium. The culture was incubated at 28 °C with rotary shaking at 160 rpm for 1 day. The culture supernatant was obtained by centrifugation at 10,400×g at 4 °C for 15 min. The supernatant was added to an equivalent volume of ethyl acetate/methanol/acetic acid (80/20/5) to extract the active compounds. The organic phase was collected, evaporated to dryness, and dissolved in acetonitrile, and then fractionated using a Shimadzu LC-10Avp high-performance liquid chromatography (HPLC) system equipped with a COSMOSIL 5C18-MS-II column (4.6 mm i.d. × 150 mm; Nacalai Tesque, Inc., Kyoto, Japan) under the following conditions: column temperature, 40 °C; flow rate, 1 ml/min; photodiode array detector, 190–800 nm; mobile-phase solvent, 55% methanol; and isocratic elution for 15 min. The eluate corresponding to the isolated peak at 10.6 min was obtained and evaporated to dryness using a rotary evaporator. The dried material was then dissolved in methanol: water at a ratio of 55:45 due to its high solubility and stability, and was henceforth termed ‘granaticin solution’. The solution was maintained at 4 °C to avoid evaporation of methanol.
The molecular mass was determined by liquid chromatography electrospray ionization mass spectrometry (LC–ESI–MS; negative mode) with a Shimadzu LCMS-2010 system equipped with a COSMOSIL 5C18-PAQ column (4.6 mm i.d. × 150 mm; Nacalai Tesque) under the following conditions: column temperature, 40 °C; flow rate, 1 ml/min; and photodiode array detector, 190–800 nm; mobile phase, 0.5% (v/v) acetic acid in 50% methanol; isocratic elution for 40 min.
Characterization of the catalytic activity of granaticin
The catalytic activity of granaticin was studied using an oxygen electrode under the same conditions as those used for activity screening. The dissolved O2 concentration in a 1 ml reaction mixture containing 20 µl of 1 µM granaticin solution was monitored for 5 min using an oxygen electrode. One hundred units of catalase (Nacalai Tesque, Inc., Kyoto, Japan) were added if needed. For the negative control measurement, 20 µl of 55% methanol was added in place of the granaticin solution to confirm that the presence of methanol does not affect the accurate measurement. The O2 consumption was also measured using a non-contact O2 sensor chip system (OXY-1 SMA; PreSensPrecision Sensing GmbH, Regensburg, Germany) for reactions where oxidation occurred slowly. The reaction mixture was prepared under the same conditions and enclosed in a glass vial containing a sensor chip attached to its inner surface. The dissolved O2 concentration was recorded every 1 min for up to 30 min by scanning the chip with the chip reader from the outside of the vial. One unit of catalytic activity was defined as the amount of the compound that catalyzed the consumption of 1 µmol of O2 per min.
To identify the reaction products generated by the catalytic oxidation of L-ascorbic acid, the reaction mixture was incubated at 30 °C for 5 min, and formic acid was added to a final concentration of 1% to stop the reaction. The reactant was then analyzed by HPLC (Shimadzu LC-10Avp system) on a ZIC-HILIC column (4.6 mm i.d. × 150 mm; Merck KGaA, Darmstadt, Germany) operated under the following conditions: column temperature, 40 °C; isocratic elution of 100 mM ammonium acetate:CH3CN = 3:7 mobile phase); flow rate, 1 ml/min; and photo-diode array detector, 240 nm. H2O2 was detected by HPLC on a COSMOSIL Sugar-D column (4.6 mm i.d. × 250 mm; Nacalai Tesque, Inc.) under the following conditions: column temperature, 40 °C; isocratic elution of 80% acetonitrile mobile phase; flow rate, 1 ml/min; and photodiode array detector, 254 nm.
The temperature dependence of the catalytic activity of granaticin was determined by measuring the reduction in O2 in the reaction mixture incubated at various temperatures between 25 and 40 °C. The composition of the reaction mixture was the same as that above, except for the addition of 2 mM L-ascorbic acid. pH dependence was analyzed by adding 2 mM L-ascorbic acid to 100 mM of the following buffers: glycine/HCl buffer (pH 2.0–3.0), citate/NaOH buffer (pH 3.0–6.0), potassium phosphate buffer (pH 6.0–7.5), Tris–HCl buffer (pH 7.5–9.5), and glycine/NaOH buffer (pH 9.5). Heat tolerance was investigated by incubating the granaticin solution at various temperatures for 1 h prior to activity measurement using the oxygen electrode as before. The reaction mixture conditions were the same as those used for the temperature dependence analysis.
Metal analysis
All glassware was soaked in 0.3 M HNO3 overnight, and exhaustively rinsed with distilled water before use. Granaticin solution (20 μM) and 55% methanol (reference) were analyzed using an inductivity coupled plasma optical emission spectrometer (model ICPE-9000, Shimadzu, Kyoto, Japan). The analysis enabled the detection of the following 71 metals at concentrations > 5 ppb: lithium, beryllium, boron, sodium, magnesium, aluminum, silicon, phosphorus, sulfur, potassium, calcium, scandium, titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, zinc, gallium, germanium, arsenic, selenium, rubidium, strontium, yttrium, zirconium, niobium, molybdenum, ruthenium, rhodium, palladium, silver, cadmium, indium, tin, antimony, tellurium, iodine, cesium, barium, hafnium, tantalum, tungsten, rhenium, osmium, iridium, platinum, gold, mercury, thallium, lead, bismuth, lanthanum, cerium, praseodymium, neodymium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, thorium and uranium.
Growth inhibition analysis
E. coli and B. subtilis strains were precultured for 16 h with shaking at 135 rpm in glass tubes containing 5 ml LB liquid medium at 37 °C and 30 °C, respectively, and then inoculated to a final concentration of 0.5% into the culture medium under the same conditions as the preculture, to which granaticin was added at 0–100 μM (E. coli) and 0–1.0 μM (B. subtilis). After 5 h of cultivation, growth was quantified by measuring the optical density at 600 nm.
Supplementary Information
Acknowledgements
We thank Professor Tadashi Ema for helpful discussions and Professor Hiroaki Sumida for supporting the metal analysis.
Author contributions
T.N.: Conceptualization, Methodology, Investigation, Visualization, and Funding acquisition. N.E.: Investigation. R.N.: Investigation. K.U.: Conceptualization, Visualization, Writing-original draft, Supervision, Project administration.
Funding
T.N. obtained Grant-in-Aid for Young Scientists (B) of the Japan Society for the Promotion of Science (17K18159 and 20K15439) and a research grant from Nagase Foundation.
Data availability
All data are contained within the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-10877-7.
References
- 1.Demain AL. Antibiotics: Natural products essential to human health. Med. Res. Rev. 2009;29:821–842. doi: 10.1002/med.20154. [DOI] [PubMed] [Google Scholar]
- 2.Kakeya H. Natural products-prompted chemical biology: Phenotypic screening and a new platform for target identification. Nat. Prod. Rep. 2016;33:648–654. doi: 10.1039/c5np00120j. [DOI] [PubMed] [Google Scholar]
- 3.Kato N, Takahashi S, Nogawa T, Saito T, Osada H. Construction of a microbial natural product library for chemical biology studies. Curr. Opin. Chem. Biol. 2012;16:101–108. doi: 10.1016/j.cbpa.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Nishiyama T, Hashimoto Y, Kusakabe H, Kumano T, Kobayashi M. Natural low-molecular mass organic compounds with oxidase activity as organocatalysts. Proc. Natl. Acad. Sci. U.S.A. 2014;111:17152–17157. doi: 10.1073/pnas.1417941111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalko PI, Moisan L. In the golden age of organocatalysis. Angew. Chem. Int. Ed. 2004;43:5138–5175. doi: 10.1002/anie.200400650. [DOI] [PubMed] [Google Scholar]
- 6.Enders D, Grondal C, Huttl MR. Asymmetric organocatalytic domino reactions. Angew. Chem. Int. Ed. Engl. 2007;46:1570–1581. doi: 10.1002/anie.200603129. [DOI] [PubMed] [Google Scholar]
- 7.List B. Introduction: Organocatalysis. Chem. Rev. 2007;107:5413–5415. doi: 10.1021/cr078412e. [DOI] [Google Scholar]
- 8.Ahrendt KA, Borths CJ, MacMillan DWC. New strategies for organic catalysis: The first highly enantioselective organocatalytic Diels-Alder reaction. J. Am. Chem. Soc. 2000;122:4243–4244. doi: 10.1021/ja000092s. [DOI] [Google Scholar]
- 9.Gaunt MJ, Johansson CC, McNally A, Vo NT. Enantioselective organocatalysis. Drug Discov. Today. 2007;12:8–27. doi: 10.1016/j.drudis.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Kucherenko AS, Siyutkin DE, Maltsev OV, Kochetkov SV, Zlotin SG. Asymmetric organocatalysis: From proline to highly efficient immobilized organocatalysts. Russ. Chem. B. 2012;61:1313–1320. doi: 10.1007/s11172-012-0177-4. [DOI] [Google Scholar]
- 11.Davide Castelvecchi ES. 'Elegant' catalysts that tell left from right scoop chemistry Nobel. Nature. 2021;598:247–248. doi: 10.1038/d41586-021-02704-2. [DOI] [PubMed] [Google Scholar]
- 12.Deng MR, Guo J, Zhu HH. Streptomyces vietnamensis GIMV4.0001: A granaticin-producing strain that can be readily genetically manipulated. J. Antibiot. 2011;64:345–347. doi: 10.1038/ja.2011.3. [DOI] [PubMed] [Google Scholar]
- 13.Ichinose K, et al. The granaticin biosynthetic gene cluster of Streptomyces violaceoruber Tu22: Sequence analysis and expression in a heterologous host. Chem. Biol. 1998;5:647–659. doi: 10.1016/S1074-5521(98)90292-7. [DOI] [PubMed] [Google Scholar]
- 14.James PDA, Edwards C. The effects of temperature on growth and production of the antibiotic granaticin by a thermotolerant Streptomycete. J. Gen. Microbiol. 1989;135:1997–2003. doi: 10.1099/00221287-135-7-1997. [DOI] [PubMed] [Google Scholar]
- 15.Ogilvie A, Wiebauer K, Kersten W. Inhibition of Leu-transfer ribonucleic acid synthetase in Bacillus subtilis by granaticin. Biochem. J. 1975;152:511–515. doi: 10.1042/bj1520511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maslowska KH, Makiela-Dzbenska K, Fijalkowska IJ. The SOS system: A complex and tightly regulated response to DNA damage. Environ. Mol. Mutagen. 2019;60:368–384. doi: 10.1002/em.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbaz R, Ettlinger L, Gäumann E, Kalvoda J, Keller-Schieriein W, Kradolfer F, Manukian BK, Neipp L, Prelog V, Reusser P, Zähner H. Stoffwechselproduke von Actinomyceten Granaticin. Helv. Chim. Acta. 1957;40:1262–1269. doi: 10.1002/hlca.19570400518. [DOI] [Google Scholar]
- 18.Chang CJ, Floss HG, Soong P, Chang CT. Identity of the antitumor antibiotic litmomycin with granaticin A. J. Antibiot. 1975;28:156. doi: 10.7164/antibiotics.28.156. [DOI] [PubMed] [Google Scholar]
- 19.Sethi ML. In vitro inhibition of viral DNA polymerase activity by litmomycin. J. Pharm. Sci. 1977;66:130–132. doi: 10.1002/jps.2600660138. [DOI] [PubMed] [Google Scholar]
- 20.Heinstein P. Mechanism of action of granaticin: Inhibition of ribosomal RNA maturation and cell cycle specificity. J. Pharm. Sci. 1982;71:197–200. doi: 10.1002/jps.2600710215. [DOI] [PubMed] [Google Scholar]
- 21.Sturdik E, Drobnica L. The cytotoxic action of granaticin, a sulfhydryl-reactive antibiotic, on Ehrlich ascites carcinoma cells. Neoplasma. 1983;30:3–6. [PubMed] [Google Scholar]
- 22.Gibson-Clay G, Byrn SR, Heinstein P. The interaction of granaticin with nucleic acids and pyruvate decarboxylase. J. Pharm. Sci. 1982;71:467–468. doi: 10.1002/jps.2600710427. [DOI] [PubMed] [Google Scholar]
- 23.Nass NM, et al. Revisiting unexploited antibiotics in search of new antibacterial drug candidates: The case of gamma-actinorhodin. Sci. Rep. 2017;7:17419. doi: 10.1038/s41598-017-17232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goel S, Mishra P. Thymoquinone inhibits biofilm formation and has selective antibacterial activity due to ROS generation. Appl. Microbiol. Biotechnol. 2018;102:1955–1967. doi: 10.1007/s00253-018-8736-8. [DOI] [PubMed] [Google Scholar]
- 25.Jang HJ, et al. Redirecting an anticancer to an antibacterial hit against methicillin-resistant Staphylococcus aureus. Front. Microbiol. 2019;10:350. doi: 10.3389/fmicb.2019.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravichandiran P, Sheet S, Premnath D, Kim AR, Yoo DJ. 1,4-naphthoquinone analogues: Potent antibacterial agents and mode of action evaluation. Molecules. 2019 doi: 10.3390/molecules24071437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khatri I, Subramanian S, Mayilraj S. Genome sequencing and annotation of Amycolatopsis azurea DSM 43854(T) Genom. Data. 2014;2:44–45. doi: 10.1016/j.gdata.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sung AA, Gromek SM, Balunas MJ. Upregulation and identification of antibiotic activity of a marine-derived Streptomyces sp. via co-cultures with human pathogens. Mar. Drugs. 2017 doi: 10.3390/md15080250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng MR, Guo J, Li X, Zhu CH, Zhu HH. Granaticins and their biosynthetic gene cluster from Streptomyces vietnamensis: Evidence of horizontal gene transfer. Anton. Leeuw. Int. J. G. 2011;100:607–617. doi: 10.1007/s10482-011-9615-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the manuscript.