Abstract
We studied the in vitro emergence of resistance to daptomycin using three methods: spontaneous resistance incidence, serial passage in the presence of increasing drug concentrations, and chemical mutagenesis. No spontaneously resistant mutants were obtained for any organism tested (<10−10 for Staphylococcus aureus, <10−9 for Staphylococcus epidermidis, <10−9 for Enterococcus faecalis, <10−9 for Enterococcus faecium, <10−8 for Streptococcus pneumoniae). Population analysis demonstrated that bacterial susceptibility to daptomycin is heterogeneous. Assay results were sensitive to calcium concentration and culture density, both of which can affect apparent resistance rates. Stable S. aureus mutants were isolated by both serial passage in liquid media and chemical mutagenesis. The daptomycin MICs for these isolates were 8- to 32-fold higher than for the parental strain. Many mutants with high MICs (>12.5 μg/ml) had significant growth defects but did not display phenotypes typical of S. aureus small colony variants. The voltage component (Δψ) of the bacterial membrane potential was increased in three independent resistant isolates. In vivo data showed that some daptomycin-resistant mutants had lost significant virulence. For other mutants, the degree of in vitro resistance was greater than the change in in vivo susceptibility. These results suggest that infection with some daptomycin-resistant organisms may still be easily treatable.
Daptomycin is a novel lipopeptide antibiotic with potent bactericidal activity in vitro against most clinically important gram-positive pathogens, including vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, glycopeptide-intermediate S. aureus, coagulase-negative staphylococci, and penicillin-resistant Streptococcus pneumoniae (12, 19, 25, 26, 28). The mechanism of action, while not yet fully elucidated, appears to involve disruption of bacterial plasma membrane function (1–5). Daptomycin was efficacious in phase 2 clinical trials in skin and soft tissue infection and bacteremia (28). Additional phase 2 and 3 clinical studies are ongoing.
Results from in vitro studies and clinical trials indicate that resistance to daptomycin is rare (16, 28; P. Courvalin, personal communication; G. W. Kaatz, personal communication). However, one group has reported relatively high resistance rates in vitro (18). In an effort to resolve this discrepancy, we determined spontaneous resistance rates and analyzed technical factors influencing those rates. In addition, the possible utility of resistant mutants in pinpointing daptomycin's mechanism of action prompted us to isolate and analyze resistant organisms.
(Portions of this work were presented at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy [N. Oliver, T. Andrew, T. Li, and J. Silverman, poster F-117], San Diego, Calif., 24 to 27 September 1998.)
MATERIALS AND METHODS
Strains, media, and antibiotics.
Bacteria were propagated at 37°C. Staphylococci were grown in Mueller-Hinton broth (MHB; Becton, Dickinson, Cockeysville, Md.), enterococci in brain heart infusion (Becton, Dickinson), and S. pneumoniae in Todd-Hewitt broth plus 5% horse serum. MIC testing was performed according to NCCLS guidelines for broth microdilution (23) except that all cultures were grown at 37°C. In addition, daptomycin MICs were determined using MHB supplemented with 50 mg of Ca2+ per liter (MHBc). Modifications for determining nisin MICs were made as previously described (27). Vancomycin, ampicillin, gentamicin, and nisin were purchased from Sigma Chemical Company (St. Louis, Mo.). Complete defined media was obtained from JRH Biosciences (Lenexa, Kans.).
Heterogeneity assay.
Heterogeneous susceptibility was measured based on methods developed by de Lencastre et al. (8). Overnight cultures were plated at dilutions (made in MHBc) ranging from 100 to 10−7 on Mueller-Hinton agar (MHA) or MHA plus 50 mg of CaCl2 per liter containing twofold dilutions of daptomycin over the range 0.125 to 64 μg/ml.
Serial-passage mutagenesis.
On day 1, MHBc containing daptomycin at 0.25, 0.5, 1, or 2 times the MIC was inoculated with S. aureus (strain Sa42) from a single colony. Cultures were incubated overnight at 37°C with shaking. From the highest concentration that supported growth, cultures were diluted 1:10,000 into fresh media plus daptomycin at twofold dilutions. This process was continued for 21 days or until three successive cultures failed to show any decrease in susceptibility. Putative mutants were colony purified for three generations on MHA, prior to further characterization.
Chemical mutagenesis.
N-Methyl-N′-nitro-N-nitrosoguanidine (MNNG; Sigma) mutagenesis was performed using methods developed by Miller (21) and Chatterjee (7). Mid-exponential cultures of S. aureus (ca. 3 × 108/ml) were washed twice with 0.03 M phosphate buffer (pH 7.0) and resuspended in 0.05 M Tris-acetate (pH 6.0)–10 mM MgCl2 (TAM). Then, 50 μg of MNNG per ml was added, and cells were incubated at 37°C to achieve 50% killing (30 min). Bacteria were washed three times with TAM, incubated overnight at 37°C in MHBc, and plated on selective media for 48 h. Putative mutants were purified on MHA prior to further characterization. To ensure that all mutants were independent, only one mutant was isolated from each selection plate.
Virulence titration and daptomycin protection studies.
For virulence titrations, five CD-1 female mice per group (Charles River Laboratories, Wilmington, Mass.) were inoculated intraperitoneally (i.p.) with 10-fold dilutions ranging from 105 to 108 CFU in phosphate-buffered saline (PBS) containing 6% hog gastric mucin (Sigma) or PBS-mucin only. The 50 and 100% lethal doses (LD50 and LD100) were calculated based on the number of mice surviving after 7 days. For daptomycin protection studies, mice were inoculated i.p. with two times the LD100. Daptomycin was dissolved in PBS and administered subcutaneously immediately and at 4 h postinfection. The 50% protective dose (PD50) was calculated via the method of probits (9) from the number of mice surviving after 7 days.
Membrane potential measurements.
Accumulation of the membrane-permeant anion tetraphenyl phosphonium bromide (TPP) was used to measure the voltage component of the bacterial membrane potential (Δψ) (15, 17, 20). Overnight cultures of S. aureus were diluted 1:1,000 into fresh MHBc, grown for 2 h at 37°C, and then shifted to room temperature and grown for an additional 2 h. Samples were collected for protein determination via the Coomassie protein assay (Pierce Chemical, Rockford, Ill.). Four 4-ml aliquots were withdrawn from the remaining culture. One was treated with 8% butanol for 10 min prior to labeling to produce a background value. Cells were labeled with 10 μM [3H]TPP (29 Ci/mmol; Moravek Biochemicals, Brea, Calif.) for 10 min, then filtered through GF/C filters (Whatman, Mardstone, United Kingdom). Filters were washed with 0.15 M NaCl and dried; the radioactivity was determined in a liquid scintillation counter. The Nernst equation, Δψ = [−(RT/F)ln([TPP+]in/[TPP+]out)], and a 4.2-μl intracellular volume per mg of protein (determined as previously described [24]) were used to calculate Δψ.
RESULTS AND DISCUSSION
Spontaneous resistance testing.
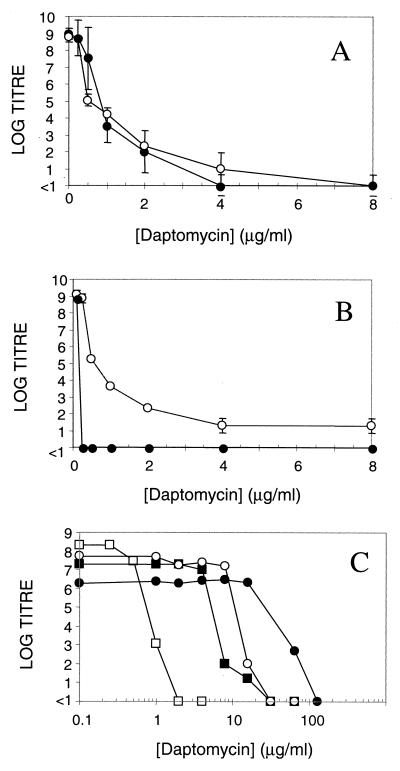
Previous studies of daptomycin resistance have yielded conflicting results, with rates varying by up to 3 orders of magnitude for some species (16, 18, G. W. Kaatz, personal communication; P. Courvalin, personal communication; protocol summary report no. B8B FP 2001 [Lilly Research Laboratories, Greenfield, Ind. 1986]) To resolve this discrepancy, we carried out additional resistance studies. Initially, we isolated colonies on MHA containing daptomycin at concentrations above the MIC. However, these isolates were not resistant. They failed to grow when restreaked to MHA containing daptomycin and, following purification on MHA, yielded MICs identical to those for the parent strain. The appearance of these colonies indicates that susceptibility to daptomycin is heterogeneous, as has been described for methicillin and vancomycin (6, 13, 14). This is demonstrated in Fig. 1A. Population heterogeneity may contribute to differences in apparent resistance rates.
FIG. 1.
Susceptibility to daptomycin is heterogeneous in S. aureus. (A) Susceptibility is heterogeneous in both laboratory (Sa42) and clinical (Sa675) isolates (●, Sa42; ○, Sa675). Data are mean values ± the standard deviation. (B) Heterogeneous susceptibility in Sa42 is affected by calcium (○, MHA [MIC = 1 μg/ml]; ●, MHA plus 50 mg of Ca2+ per liter [MIC = 0.3 μg/ml]). Data are mean values ± the standard deviation. (C) Heterogeneous susceptibility is observed in daptomycin-resistant mutants (□, Sa42 [parent]; ■, Sa278 [class 1]; ○, Sa295 [class 2]; ●, Sa291 [class 3]). Representative data are shown.
Experimental factors that increase population heterogeneity could increase apparent resistance rates. The activity of daptomycin is calcium dependent (10), and the levels of free calcium in MHA are variable (23). We determined the effect of calcium levels on population heterogeneity. As shown in Fig. 1B, the addition of 1 mM CaCl2 to the test agar results in homogeneous susceptibility and eliminates the appearance of falsely resistant colonies. Differences in calcium levels may have contributed to past differences in spontaneous resistance rates. Consistent with this idea, it has previously been demonstrated that resistance rates in liquid media increase as calcium levels decrease (29).
Based on the studies described above, we performed all resistance testing by plating overnight cultures on MHA supplemented with 1 mM CaCl2 and daptomycin at 8 times the MIC. Eight laboratory (American Type Culture Collection, Rockville, Md.) and eight clinical isolates (provided by D. Snydman, New England Medical Center, Boston, Mass.) were tested. No spontaneously resistant mutants were obtained, yielding resistance rates of <10−10 for S. aureus (n = 4 strains tested), <10−9 for Staphylococcus epidermidis (n = 4), <10−9 for Enterococcus faecalis (n = 4), <10−9 for E. faecium (n = 2), and <10−9 for S. pneumoniae (n = 2). These values support earlier reports of low resistance rates (16; G. W. Kaatz, personal communication; P. Courvalin, personal communication). As described above, earlier reports of higher rates of spontaneous resistance (18) may reflect variations in calcium levels in the test media and the effects of population heterogeneity.
Serial passage and chemical mutagenesis.
Two methodologies were employed to obtain daptomycin-resistant S. aureus. One mutant (Sa278) was obtained via 21-day serial passage in liquid culture. (Pulsed-field gel electrophoresis fingerprinting confirms that Sa278 is a derivative of the parent strain [data not shown]). Eleven additional independent mutants (Sa284 to Sa293 and Sa295) with varied colony morphology, growth phenotypes, and antibiotic resistance were isolated via chemical mutagenesis. The phenotypes of all mutants are summarized in Table 1. MIC increases were relatively modest (8- to 32-fold), consistent with the findings of previous serial passage experiments (18, 22). Daptomycin resistance is stable during repeated passage in the absence of continued drug selection (data not shown).
TABLE 1.
Growth phenotypes and MICs of mutants obtained via serial passage or chemical mutagenesisa
| Strain | Class | Growth phenotype on:
|
MIC (μg/ml)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Liquid media (OD600)
|
Solid media (colony size)
|
|||||||||
| MHB | CDM | MHA | SCV | Dapto | Vanc | Amp | Nisin | Gent | ||
| Sa42 | WT | 6.05 | 6.6 | +++ | +++ | 0.78 | 0.78 | 6.25 | 3.125 | 0.78 |
| Sa278b | 1 | 5.93 | 7.87 | +++ | +++ | 12.5 | 1.56 | 6.25 | 25 | 0.78 |
| Sa284 | 1 | 4.97 | 6.38 | +++ | +++ | 6.25 | 1.56 | 6.25 | 25 | 0.78 |
| Sa285 | 2 | 1.23 | NG | ++ | ++ | 6.25 | 0.78 | 3.13 | 25 | 0.39 |
| Sa286 | 3 | 0.63 | NG | + | + | 6.25 | 0.39 | ND | 6.25 | 0.78 |
| Sa287 | 1 | 3.89 | 6.59 | +++ | +++ | 6.25 | 1.56 | 6.25 | 25 | 1.56 |
| Sa288 | 1 | 5.34 | 8.17 | +++ | +++ | 6.25 | 0.78 | ND | 25 | 0.78 |
| Sa289 | 3 | 0.69 | 0.45 | + | + | 12.5 | 0.39 | ND | 3.125 | ND |
| Sa290 | 3 | 0.91 | NG | + | + | 12.5 | 0.39 | ND | ND | 1.56 |
| Sa291 | 3 | 0.71 | NG | + | + | 25 | 0.39 | 1.56 | 3.125 | 0.78 |
| Sa292 | 3 | 1.37 | NG | + | + | 12.5 | 0.78 | ND | ND | 0.78 |
| Sa293 | 3 | 0.43 | NG | + | + | 12.5 | 0.78 | ND | ND | 1.56 |
| Sa295 | 2 | 1.28 | NG | ++ | ++ | 12.5 | 1.56 | 6.25 | 6.25 | <0.1 |
Abbreviations: OD600, optical density at 600 nm; SCV, MHA plus hemin, thiamine, and menadione; Dapto, daptomycin; Vanc, vancomycin; Amp, ampicillin; Gent, gentamicin; WT, wild type; NG, no growth, 16 h; ND, not determined.
Obtained via serial passage techniques.
Mutants were grouped into three classes based on their growth phenotypes and antibiotic susceptibility (n ≥ 3 for all values). Class 1 mutants (including Sa278) exhibited normal growth rates and were cross-resistant to the peptide antibiotic nisin. Class 2 mutants had reduced growth rates on MHA, poor or no growth in complete defined media (CDM), and no antibiotic cross-resistance. Class 3 mutants showed severe growth defects in MHB and CDM, poor or no pigmentation on MHA, and no antibiotic cross-resistance. Poorly growing mutants (classes 2 and 3) were not rescued by the addition of menadione, hemin, and thiamine to MHA and were susceptible to gentamicin, suggesting that they were not classic S. aureus small colony variants. All classes of mutants retained the heterogeneous susceptibility of the parental strain (Fig. 1C) and exhibited homogeneous susceptibility in the presence of 1 mM Ca2+ (data not shown). Daptomycin remained bactericidal at 8 times the MIC against all classes of mutants (data not shown). None of the mutants demonstrated cross-resistance to vancomycin or ampicillin, a finding consistent with known differences in the mechanism of action of these drugs.
In vivo studies.
Selected mutants were tested for virulence in an acute murine IP infection model (Table 2). Sa278 and its daptomycin-sensitive parent had comparable LD50 values, consistent with similar growth rates in vitro. The PD50 for the resistant strain was <5-fold higher than that of the parent. This contrasts with a 16-fold increase in daptomycin MIC for Sa278 (verified by repeated testing [n = 40]) and suggests that some in vitro resistant strains will still be susceptible in vivo. The LD50 for a class 2 mutant was >20-fold higher (i.e., no animals died at the highest innoculum tested), meaning that a PD50 could not be determined. This loss of virulence may be a consequence of the growth defects observed in vitro; alternatively, this strain may be defective in the production of critical virulence factors. Whether these defects are a consequence of the acquisition of daptomycin resistance remains to be determined.
TABLE 2.
MIC, LD50, and PD50 values for selected strainsa
| Strain | Class | MIC (μg/ml) | LD50 (CFU) | PD50 (mg/kg) |
|---|---|---|---|---|
| Sa42 | WT | 0.78 | 4 × 106 | 0.22 |
| Sa278 | 1 | 12.5 | 1.3 × 106 | 1.03 |
| Sa295 | 2 | 12.5 | >108 | ND |
Abbreviations: WT, wild type; ND, not determined.
Membrane potential.
Daptomycin's mechanism of action is not clearly defined. Earlier research has suggested two likely targets: dissipation of the bacterial membrane potential (1) and inhibition of lipoteichoic acid synthesis (4, 5). We measured the membrane potential in the wild type and in selected class 1 mutants via the uptake of the permeant anion tetraphenyl phosphonium. As shown in Table 3, the voltage difference across the cytoplasmic membrane of daptomycin-resistant mutants is increased compared to the parent strain. This may be consistent with the membrane potential as the primary target. Higher levels of daptomycin may be necessary to fully dissipate the membrane potential in these mutants, accounting for their higher MICs. This may also account for their cross-resistance to nisin, which is known to dissipate membrane potential.
TABLE 3.
Δψ in class 1 mutantsa
| Strain | Class | Trial 1
|
Trial 2
|
||
|---|---|---|---|---|---|
| Δψ (mV) | % Change | Δψ (mV) | % Change | ||
| Sa42 | WT | −112 ± 3 | −127 ± 18 | ||
| Sa278 | 1 | −162 ± 7 | 45 | −174 ± 20 | 37 |
| Sa284 | 1 | −147 ± 8 | 31 | −173 ± 13 | 36 |
| Sa287 | 1 | ND | −161 ± 6 | 27 | |
Membrane potential was determined as described in the text. Data are the average of duplicate samples.
Abbreviations: WT, wild type; ND, not determined.
Conclusion.
Antibiotic resistance is a growing worldwide problem, requiring the development of novel agents to combat resistant organisms. The low spontaneous resistance rates, limited increases in MICs during serial passage, and ease of treatment of resistant isolates are encouraging for the clinical development of daptomycin. The low resistance potential in vitro may provide daptomycin with a low clinical resistance rate, prolonging the drug's utility. The higher resistance rates in one earlier report may be attributable to variances in experimental protocols. Preliminary analysis of resistant mutants supports alteration of membrane potential as a mechanism of action for daptomycin.
ACKNOWLEDGMENTS
We thank Jeff Alder, Frank Tally, and Grace Thorne for their helpful discussions. We are grateful to Glenn Kaatz for the gift of Sa675 and for making the initial observation of heterogeneous susceptibility to daptomycin.
REFERENCES
- 1.Alborn W E, Jr, Allen N E, Preston D A. Daptomycin disrupts membrane potential in growing Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35:2282–2287. doi: 10.1128/aac.35.11.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen N E, Alborn W E, Jr, Hobbs J N., Jr Inhibition of membrane potential-dependent amino acid transport by daptomycin. Antimicrob Agents Chemother. 1991;35:2639–2642. doi: 10.1128/aac.35.12.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boaretti M, Canepari P. Identification of daptomycin-binding proteins in the membrane of Enterococcus hirae. Antimicrob Agents Chemother. 1995;39:2068–2072. doi: 10.1128/aac.39.9.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boaretti M, Canepari P, del Mar Lleò M, Satta G. The activity of daptomycin on Enterococcus faecium protoplasts: indirect evidence supporting a novel mode of action on lipoteichoic acid synthesis. J Antimicrob Chemother. 1993;31:227–235. doi: 10.1093/jac/31.2.227. [DOI] [PubMed] [Google Scholar]
- 5.Canepari P, Boaretti M, del Mar Lleó M, Satta G. Lipoteichoic acid as a new target for activity of antibiotics: mode of action of daptomycin ( LY146032) Antimicrob Agents Chemother. 1990;34:1220–1226. doi: 10.1128/aac.34.6.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers H F. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin Microbiol Rev. 1997;10:781–791. doi: 10.1128/cmr.10.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatterjee A N. Use of bacteriophage-resistant mutants to study the nature of the bacteriophage receptor site of Staphylococcus aureus. J Bacteriol. 1969;98:519–527. doi: 10.1128/jb.98.2.519-527.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lencastre H, Figueiredo A M S, Urban C, Rahal J, Tomasz A. Multiple mechanisms of methicillin resistance and improved methods for detection in clinical isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35:632–639. doi: 10.1128/aac.35.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eliopoulous G M, Thauvin C, Gerson B, Moellering R C. In vitro activity and mechanism of action of A21978C1, a novel cyclic lipopeptide antibiotic. Antimicrob Agents Chemother. 1985;27:357–362. doi: 10.1128/aac.27.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finney D J. Probit analysis. 3rd ed. Cambridge, United Kingdom: Cambridge University Press; 1971. [Google Scholar]
- 11.Flandrois J P, Carret G, Pichat C, Peyret M. Mise en évidence d'un effet inoculum sur l'estimation de la concentration minimale inhibitrice du LY 146032. Pathol Biol. 1988;36:377–380. . (Summary in English.) [PubMed] [Google Scholar]
- 12.Hanberger H, Nilsson L E, Maller R, Isaksson B. Pharmacodynamics of daptomycin and vancomycin on Enterococcus faecalis and Staphylococcus aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob Agents Chemother. 1991;35:1710–1716. doi: 10.1128/aac.35.9.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartman B J, Tomasz A. Expression of methicillin resistance in heterogeneous strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1986;29:85–92. doi: 10.1128/aac.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howe R A, Wootton M, Walsh T R, Bennett P M, MacGowan A P. Expression and detection of hetero-vancomycin resistance in Staphylococcus aureus. J Antimicrob Chemother. 1999;44:675–678. doi: 10.1093/jac/44.5.675. [DOI] [PubMed] [Google Scholar]
- 15.Jolliffe L K, Doyle R J, Streips U N. The energized membrane and cellular autolysis in Bacillus subtilis. Cell. 1981;25:753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- 16.Kaatz G W, Seo S M, Reddy V N, Bailey E M, Rybak M J. Daptomycin compared with teicoplanin and vancomycin for therapy of experimental Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1990;34:2081–2085. doi: 10.1128/aac.34.11.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashket E R, Blanchard A G, Metzger W C. Proton motive force during growth of Streptococcus lactis cells. J Bacteriol. 1980;143:128–134. doi: 10.1128/jb.143.1.128-134.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liebowitz L D, Saunders J, Chalkley L J, Koornhof H J. In vitro selection of bacteria resistant to LY146032, a new cyclic lipopeptide. Antimicrob Agents Chemother. 1988;32:24–26. doi: 10.1128/aac.32.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louie A, Baltch A L, Ritz W J, Smith R P, Asperilla M. Comparison of in vitro inhibitory and bactericidal activities of daptomycin (LY 146032) and four reference antibiotics, singly and in combination, against gentamicin-susceptible and high-level-gentamicin-resistant enterococci. Chemotherapy. 1993;39:302–310. doi: 10.1159/000239141. [DOI] [PubMed] [Google Scholar]
- 20.Mates S M, Patel L, Kaback H R, Miller M H. Membrane potential in anaerobically growing Staphylococcus aureus and its relationship to gentamicin uptake. Antimicrob Agents Chemother. 1983;23:526–530. doi: 10.1128/aac.23.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 22.Mouton R P, Mulders S L T M. LY146032: activity and resistance development in vitro. J Antimicrob Chemother. 1987;20:513–517. doi: 10.1093/jac/20.4.513. [DOI] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Document M7–A5. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 24.Rottenberg H. The measurement of membrane potential and ΔpH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- 25.Rybak M J, Hershberger E, Moldovan T, Grucz R G. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against staphylococci and enterococci, including vancomycin-intermediate and -resistant strains. Antimicrob Agents Chemother. 2000;44:1062–1066. doi: 10.1128/aac.44.4.1062-1066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snydman D R, Jacobus N V, McDermott L A, Lonks J R, Boyce J M. Comparative in vitro activities of daptomycin and vancomycin against resistant gram-positive pathogens. Antimicrob Agents Chemother. 2000;44:3447–3450. doi: 10.1128/aac.44.12.3447-3450.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinberg D A, Hurst M A, Fujii C A, Kung A H C, Ho J F, Cheng F-C, Loury D J, Fiddes J C. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob Agents Chemother. 1997;41:1738–1742. doi: 10.1128/aac.41.8.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tally F P, Zeckel M, Wasilewski M M, Carini C, Berman C L, Drusano G L, Oleson F B., Jr Daptomycin: a novel agent for Gram-positive infections. Exp Opin Investig Drugs. 1999;8:1223–1238. doi: 10.1517/13543784.8.8.1223. [DOI] [PubMed] [Google Scholar]
- 29.Wale M C J, Wale L J, Greenwood D. Turbidimetric response of Staphylococcus aureus and Enterococcus faecalis to daptomycin. Eur J Clin Microbiol Infect Dis. 1988;7:809–812. doi: 10.1007/BF01975057. [DOI] [PubMed] [Google Scholar]