Abstract
Background:
Neck pain (NP) affects as much as 70% of individuals at some point in their lives. Systematic reviews indicate that manual treatments can be moderately effective in the management of chronic, nonspecific NP. However, there is a paucity of studies specifically evaluating the efficacy of osteopathic manipulative treatment (OMT).
Objective:
To evaluate the efficacy of OMT in reducing pain and disability in patients with chronic NP.
Design:
Single-blinded, cross-over, randomized controlled trial.
Setting:
University-based, osteopathic manipulative medicine outpatient clinic.
Participants:
97 participants, 21–65 years old, with chronic, nonspecific NP.
Interventions:
Participants were randomized to two trial arms: immediate OMT intervention or waiting period first. The intervention consisted of 3–4 OMT sessions over 4–6 weeks, after which the participants switched groups.
Main outcome measures:
Primary outcome measures were pain intensity (average and current) on the numerical rating scale and Neck Disability Index. Secondary outcomes included PROMIS-29 health domains and Fear Avoidance Beliefs Questionnaire. Outcomes obtained prior to the cross-over allocation were evaluated using general linear models and after adjusting for baseline values.
Results:
38 and 37 participants were available for the analysis in the OMT and waiting period groups, respectively. The results showed significantly better primary outcomes in the immediate OMT group for reductions in average pain (−1.02, 95%CI:(−1.72, −0.32), p=0.005), current pain (−1.02, 95%CI:(−1.75, −0.30), p=0.006), disability (−5.30%, 95%CI:(−9.2%, −1.3%), p=0.010) and improved secondary outcomes (PROMIS) related to sleep (−3.25, 95%CI: (−6.95, −1.54), p=0.003), fatigue (−3.26, 95%CI:(−6.04, −0.48), p=0.022), and depression (−2.59, 95%CI:(−4.73, −0.45), p=0.018). The effect sizes were in the clinically meaningful range between 0.5 and 1 standard deviation. No study-related serious adverse events were reported.
Conclusions:
OMT is relatively safe and effective in reducing pain and disability along with improving sleep, fatigue, and depression in patients with chronic NP immediately following treatment delivered over approximately 4–6 weeks.
Trial registration:
Keywords: Cervical spine, Neck pain, Osteopathic manipulative medicine, Disability
Introduction
Neck pain (NP) is one of the three most frequently reported musculoskeletal complaints 1,2. It affects as much as 70% of individuals at some point in their lives 3,4, is the fourth leading cause of years lived with disability 5, and this outlook has not changed significantly in recent decades 6. Most people with NP do not recover completely and may experience recurrence of the symptoms 1–5 years later 7–10. Forty-four percent of patients with chronic NP consult their general practitioners annually, one third of these patients are referred to paramedical or medical specialists, and a majority of them receive some form of conservative treatment, which may include medication, physical therapy, and other interventions (e.g., manual treatment, postural therapy, acupuncture, etc.) 11. Consequently, NP results in a significant socioeconomic burden, predominantly due to lost wages and work absenteeism, but also due to healthcare costs 12.
Most NP cases do not involve specific structural pathologies and are often referred to as “nonspecific,” “soft tissue,” or “mechanical” NP 13. Causes and prognostic factors are numerous, complex, and include psychosocial determinants 4,7,14. Thus, a syndromic diagnosis has been recommended to manage the majority of NP and searching for specific tissue pathology can become counterproductive 15. Informed by scientific evidence, various clinical guidelines endorse conservative, symptomatic management of nonspecific NP 16–19. Among these guidelines, physical exercise and some form of manual treatment are the most frequently recommended interventions 20.
Recent meta-analyses and systematic reviews of randomized clinical trials (RCTs) indicate that manual treatment, such as mobilization (involving non-thrust techniques), manipulation (involving thrust techniques), and massage are effective interventions for the management of NP 21–23. However, these reviews did not consider osteopathic manipulative treatment (OMT) as a distinctive modality. OMT employs a wide variety of manual techniques to diagnose and treat musculoskeletal dysfunction, including mobilization and manipulation 24–26, though these techniques can also be used by other health professionals.
To date, there are only three published RCTs on the efficacy of OMT in the management of chronic NP: two from Germany 27,28, and one from Brazil 29. Schwerla et al. showed the superiority of OMT over sham ultrasound on several measures of pain and function 27. Similarly, Rotter et al. demonstrated significant improvements in pain and disability among musicians with chronic NP who received OMT in comparison to those who received no intervention 28. Finally, Groisman et al. evaluated OMT in addition to exercise, which resulted in better outcomes than exercise alone 29. The purpose of the current RCT was to add to the sparse data on the efficacy of OMT for chronic NP. We hypothesized that OMT intervention will result in a significant reduction in pain and disability when compared with the waiting period control group.
Methods
Trial design.
The RCT reported here was primarily designed to validate several tests for head-neck motor control as objective measures of pain interference 30. To maximize the number of data points for the validation of these tests, while allowing all participants to receive real treatment, a cross-over design was adopted (Figure 1). In this paper, we present a part of the study that involves patient-reported outcomes of OMT intervention.
Figure 1.
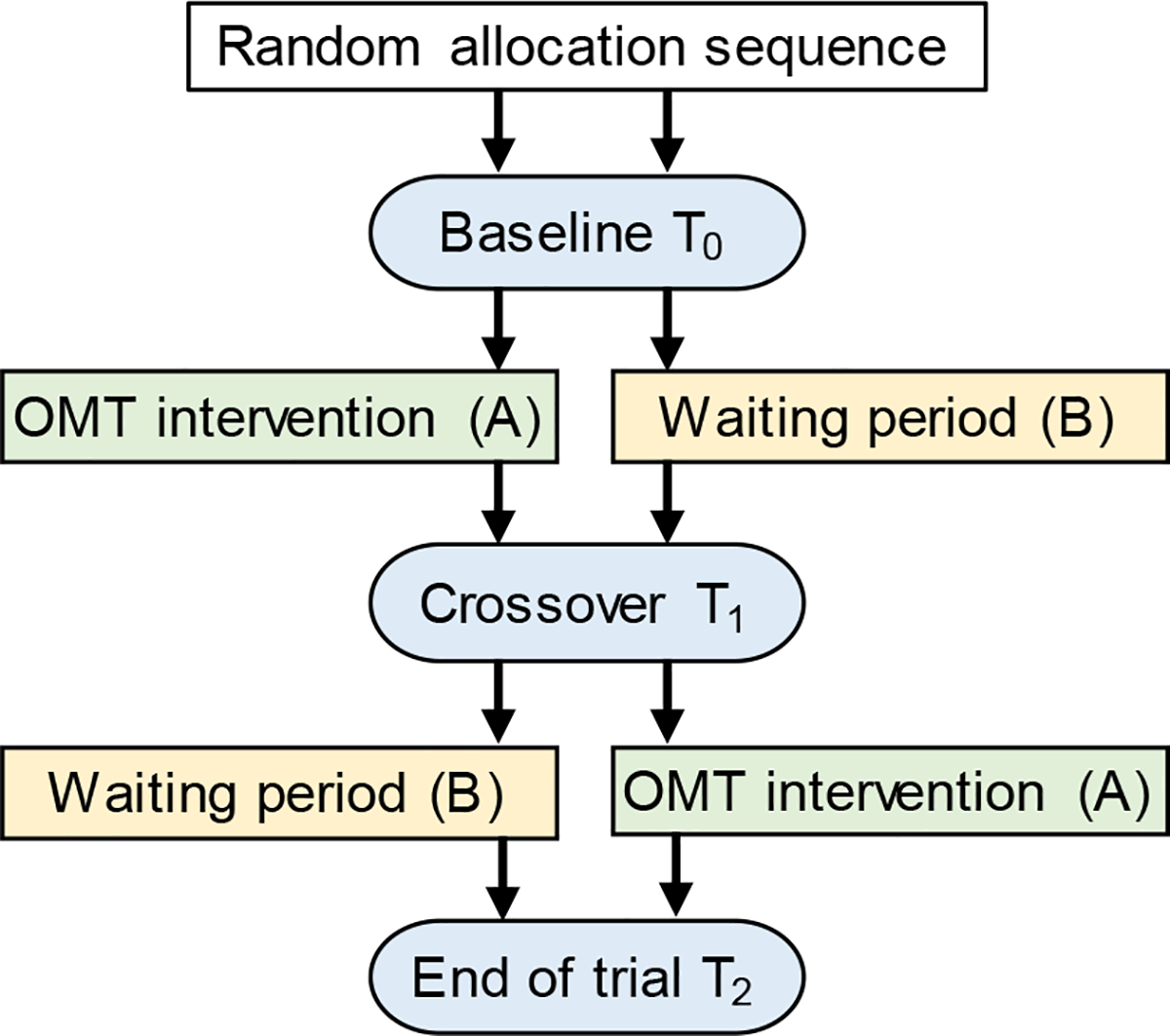
Schematic of the randomized, cross-over, controlled trial design. Subjects in the AB arm received treatment (A – OMT intervention) followed by no treatment (B - waiting period); whereas subjects in the BA arm received no treatment (B - waiting period) followed by treatment (A – OMT intervention). Patient-reported outcomes were collected at baseline (T0), cross-over point (T1), and end of the trial (T2). These three time points were spaced approximately 4–6 weeks.
Adults with chronic NP were randomized to two trial arms: Sequence (AB) - immediate OMT (A-active intervention) followed by a waiting period (B-inactive control), or Sequence (BA) - a waiting period (B-inactive control) followed by OMT (A-active intervention), with 1:1 allocation ratio. Subjects starting in the immediate treatment group received 3–4 OMT sessions (approximately once a week, allowing a minimum of 3 and a maximum of 14 days between the OMT sessions). Subjects starting in the waiting period group did not receive any treatment. After 4–6 weeks from baseline, patients switched group assignments (Figure 1). Questionnaires with patient-reported outcomes were administered to all participants at baseline (T0), at a cross-over time point (T1), and at the end of the study (T2). Participants remained in the study between 9 and 16 weeks, including the orientation session and the final follow-up on adverse events (AEs).
The study was registered with ClinicalTrials.gov (NCT# 02261259) and approved by the Michigan State University’s Biomedical and Health Institutional Review Board prior to enrollment. A Data and Safety Monitoring Board (DSMB) consisted of two individuals who were not associated with the study institution and one individual from a different college at the study institution. Both data and safety monitoring plan and DSMB were approved by the National Center for Complementary and Integrative Health (NCCIH). Additionally, a third-party monitor (Westat Corp., Rockville, MD, USA) conducted pre- enrollment and subsequent annual site visits.
Participants.
Eligible participants were adults with chronic, nonspecific NP who met the inclusion/exclusion criteria listed in Table 1. ”Chronic” was defined as pain lasting for a minimum of 3 months or longer 31. The “nonspecific” definition was taken from The Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders 13, which excludes any NP that is associated with serious local pathology or systemic disease (see exclusion criteria in Table 1). The eligibility of participants was verified with a two-step process. First, an online screening questionnaire addressed most of the inclusion/exclusion criteria (paper version was provided to those who did not have access to internet). Second, upon passing the online screening, participants were invited to the lab, signed an informed consent form, and were examined by a PM&R board-certified physician (LLP) who ruled out “red flags” and verified the eligibility criteria.
Table 1.
Inclusion and exclusion criteria.
| Inclusion Criteria |
| • Age 21–65 years |
| • Independently ambulatory |
| • Able to speak and read English |
| • Able to understand study procedures and to comply with them for the entire length of the study. |
| • Willing to be randomized to either immediate treatment-first or waiting period-first trial arms. |
| • Musculoskeletal pain - primarily in the cervical region lasting longer than 3 months |
| • Pain rating equal to or greater than 3/10 as indicated on the Numeric Rating Scale for Pain |
| • Neck Disability Index equal to or greater than 30% |
|
Exclusion Criteria |
| • Inability or unwillingness of individual to give written informed consent. |
| • Physical therapy or any other form of manual medicine (e.g., Osteopathic Manipulative Medicine, Chiropractic Manipulation, etc.), acupuncture or spinal injections within one month prior to study enrollment |
| • Workers’ compensation benefits in the past 3 months or ongoing medical legal issues |
| • Possibly pregnant |
| • Extreme obesity (BMI>36) |
| • Currently using electrical implants (e.g., cardiac pacemakers, drug delivery pumps, etc.) |
| History of: |
| • Spinal surgery |
| • Spinal fracture |
| • Spinal infection (e.g., osteomyelitis) |
| • Cancer |
| Unresolved symptoms from: |
| • Head trauma |
| • Inner ear infection with associated balance and coordination problems |
| • Orthostatic hypotension |
| • Uncontrolled hypertension |
| • Vestibular disorder (e.g. vertigo) |
| Current diagnosis of: |
| • Significant spinal deformity (e.g., scoliosis > 20 degrees, torticollis) |
| • Ankylosing spondylitis |
| • Spondylolisthesis grades III or IV |
| • Rheumatoid arthritis |
| • Osteoporosis |
| • Angina or congestive heart failure symptoms |
| • Active bleeding or infection in the neck |
| • Blindness |
| • Seizures |
| • Neurologic disease (e.g., Parkinson’s disease, multiple sclerosis, cerebral palsy, Alzheimer’s disease, amyotrophic lateral sclerosis, stroke or transient ischemic attack in the past year, cervical dystonia) |
| Conditions recognized by a physician any time during the study: |
| • Significant or worsening signs of neurologic deficits (e.g., diminished sensation, altered reflexes, and motor deficits) |
| • Symptoms are not consistent with mechanical findings |
| • Other conditions impeding protocol implementation |
Study participants were recruited from the general population in the Greater Lansing and surrounding areas between June 2014 and July 2018. Recruitment strategies included personal communication, direct mail (using numerous databases), emails and advertisements (e.g., newspaper, radio, handouts, flyers, websites, and social media - such as, but not limited to, Facebook or Twitter). The advertisement flyers were also placed in several area clinics specializing in pain management or musculoskeletal disorders. The participants were compensated with a $100 gift card upon the completion of the study.
Interventions.
All treatments were delivered by one of five osteopathic physicians (LAD, JJR, TJF, MAZ, and LLP) specializing in OMT. The physicians initially evaluated patients for somatic dysfunction, which could be related to NP, in the thoracic spine, rib cage, cervical spine and cranium as implemented by Licciardone et al. 26. Then, the physician addressed the diagnosed dysfunction with treatment, using a high-velocity, low-amplitude (HVLA) thrust technique to the cervical spine region and any (or none) combination of the following 4 techniques: (i) soft tissue, (ii) muscle energy, (iii) myofascial, (iv) articulatory. These techniques are defined as follows 32: (i) soft tissue – techniques that involve lateral stretching, deep pressure, and/or separation of muscle origin and insertion; (ii) muscle energy – a method in which the patient’s muscle are contracted from a specific position, in a specific direction, and against physician’s counterforce; (iii) myofascial – techniques utilizing continual palpatory feedback to alleviate restriction of the musculature and fascia; (iv) articulatory – a method in which a low velocity/moderate to high amplitude force is applied to a dysfunctional joint. All participants were positioned to attempt HVLA maneuver by the treating physician. Individuals that could not tolerate this maneuver did not receive HVLA in that session, resulting in an “HVLA attempted-not performed” entry in the treatment log. The clinician re-evaluated the degree of somatic dysfunction during the treatment session and repeated or changed the technique. Treatment sessions were up to approximately 30 minutes in duration.
The OMT techniques utilized by treating physicians may vary for many reasons, including the degree of restriction, acuteness of symptoms, provider preference, or patient tolerance/cooperation. However, no specific manual treatment technique demonstrates superior clinical outcomes (i.e., manipulation vs. mobilization) and, thus, there is no optimal technique that can be recommended for treating NP 33,34. Therefore, the selection of an appropriate treatment protocol for our study was made based on the most commonly used manual techniques used by osteopathic physicians in the United States 24–26. This protocol fit a more pragmatic research design, in which the efficacy of OMT was evaluated under “usual treatment” conditions 35. At the same time, implementing cervical HVLA as the primary treatment modality and limiting physician’s choices to four optional techniques made the treatment semi-standardized. The waiting period group served as an inactive control. Any non-study related manipulation (chiropractic or osteopathic), physical therapy, massage, acupuncture, and spinal injections were prohibited for both groups throughout the duration of the study. Medication usage was not limited.
Outcomes.
The primary clinical outcome measures were pain intensity and disability. Participants rated their current pain and the average pain over the last 7 days on a 11-point numeric rating scale (NRS) anchored with “no pain” at 0 and “worst pain imaginable” at 10. Disability was measured as a percentage using the Neck Disability Index (NDI) 36. The secondary clinical outcomes included PROMIS-29 v1.0 health domains (pain interference, satisfaction with participation in social roles, sleep disturbance, fatigue, depressive symptoms, anxiety, and physical function) 37 and the Fear Avoidance Beliefs Questionnaire (FABQ) physical activity and work subscales 38. The questionnaires, along with additional items aimed at rechecking exclusion criteria, were administered online at each time point using REDCap 39 and in-person interviews. All source data collected in paper format were transcribed into the REDCap database using a double data entry method.
Any unfavorable and unintended signs, symptoms, or disease occurring during the study were considered potential AEs, even if they were not related to the study 40. Study personnel queried participants about such signs and symptoms immediately after each visit, followed-up within 3 days and weekly via email, telephone, and in-person contacts until resolution. Participants rated the severity of their adverse signs or symptoms on a 11-point NRS. Because the variability of AE severity was not known a priori and such symptoms are not limited to the cervical region, we selected 2 NRS points as a threshold for classifying a symptom as an AE based on the minimal clinically important difference (MCID) recommended for general musculoskeletal pain 41,42. In other words, an increase in any adverse symptom severity during the study by more than 2 points from the previous assessment rendered the symptom an AE. The principal investigator (JC) graded their relatedness to this project (0 is not related and 4 is definitely related), expectancy (expected or unexpected) and severity (1 is mild and 4 is life-threatening) using the Common Terminology Criteria for Adverse Events (CTCAE v4.03) 40.
Sample size.
This study was powered for medium effect size of 0.7 (Cohen’s d) between patients with NP and healthy individuals in head/neck motor control tests. To detect these differences with power of 0.80 or greater in two-sided tests at 0.05 significance level, a sample of 34 subjects in each group was required. Therefore, adding a small margin for participant attrition, the targeted accrual of completed cases was set at 36 per group. This target was exceeded with 44 participants randomized to the OMT-first Sequence (AB) and 43 to the waiting period-first Sequence (BA) (Figure 1 and 2).
Figure 2.
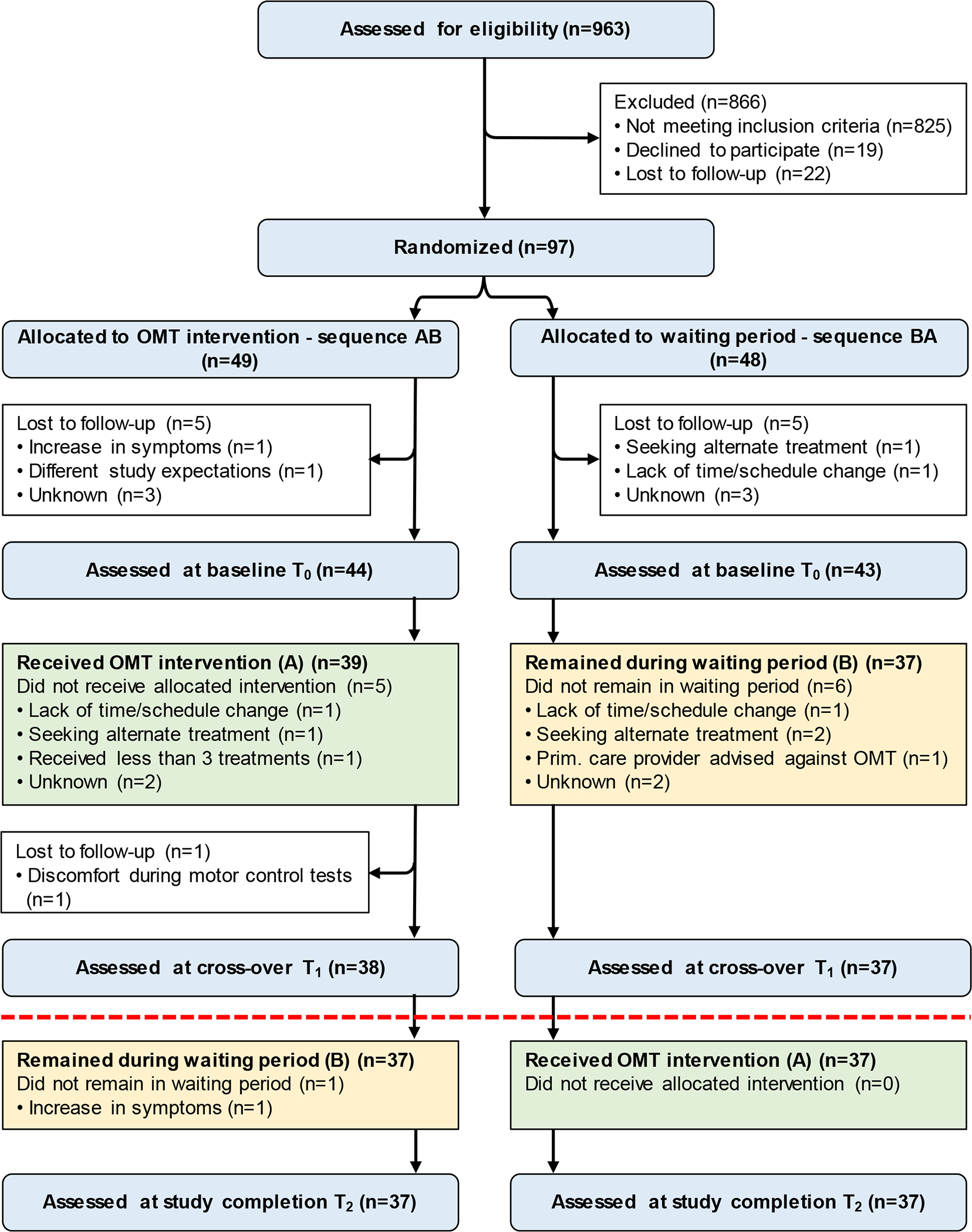
CONSORT flow diagram of participants. Because of the carryover effects in the primary outcomes, the comparison between study groups was carried out prior to the cross-over allocation at T1 (indicated with a red dashed line) with 38 participants in the immediate OMT intervention group and 37 participants in the waiting period group.
Because carryover effects were present in the primary outcome measures (see results), statistical analyses were performed using data from the first stage of the study (T1); timepoint prior to the cross-over allocation (i.e., comparison between immediate OMT vs. waiting period groups). Given the available sample size at T1 of 38 participants in the OMT group and 37 participants in the waiting period group, the effect size of Cohen’s d=0.66 was detectable in unadjusted analyses as statistically significant with power of 0.80 or greater in two-sided tests at 0.05 level of significance. In the analyses with adjustment for baseline, because of correlation of approximately 0.6 between T0 (baseline) and T1 measures, the error variance was reduced, and the detectable effect size was d=0.53.
Randomization and Blinding.
A randomization module in REDCap was used to assign subject’s group. The allocation table was generated by a computer and locked once the project had started. REDCap revealed group assignment for each subject at the time of enrollment. Therefore, there was no way to predict any participant’s allocation before enrollment or change it afterwards.
The PI, statistician, and treating team physicians were all blinded to group assignment (i.e., OMT or waiting period). An approximately 2-month lead-up period between the study commencement and enrollment of the first participant prevented the physicians from knowing whether a participant is receiving treatment immediately after enrollment or after the waiting period (Figure 1). Only the study coordinator and research assistants involved in coordinating clinical treatment had knowledge of group assignment. Study participants were instructed not to discuss their group assignment with the treating physicians and other study personnel.
Statistical methods.
A modified intent-to-treat analysis was used, which included all participants who were randomized but also completed at least one post-baseline assessment. Participants’ characteristics and outcome measures at baseline were summarized with descriptive statistics. Carryover effects were evaluated by comparing summed outcomes for two time periods (T0 to T1 and T1 to T2) between two allocation sequences (AB and BA) (Figure 1). Because tests of carryover effects are typically not powered 43, we estimated the effect sizes (Cohen’s d) and applied a commonly used cut-off of d=0.33 for clinical importance of the differences in patient-reported outcome data44. The characteristics of dropouts were compared between experimental groups using t- or chi-square tests as appropriate.
The unadjusted estimates of treatment effects on the outcomes were obtained from the t-tests comparing the two experimental groups at T1. The adjusted estimates of treatment effects were obtained from general linear models relating outcomes at T1 to experimental group and baseline value of the outcome. The least square (LS) means were output from these models, and differences between them by experimental group were tested. The adjusted effect sizes were estimated as differences between LS means divided by the square root of the mean squared error. Improvement in outcomes were considered to be clinically meaningful if the effect sizes for experimental group differences were between 0.5 and 1 standard deviation (SD) 44,45. All analyses were performed using SAS 9.4 statistical software (SAS 9.4 Copyright © 2013, SAS Institute Inc., Cary, NC, USA).
Results
The recruitment and enrollment for this study began in June 2014 and was completed in August 2017 when the targeted accrual of 36 participants per trial arm was exceeded. Out of 963 screened volunteers, 97 met the eligibility criteria, agreed to participate, and were randomized (Figure 2). On average, participants in this study were just over 40 years old, suffered from NP for more than 8 years, had average pain rating over 5 on a 0–10 scale, and the majority were females (Table 2).
Table 2.
Baseline characteristics of participants with chronic neck pain (NP) by trial arm.
| Characteristic | Arm 1 (Sequence AB: OMT then waiting period) N=44 Mean (SD) or N (%) |
Arm 2 (Sequence BA: waiting period then OMT) N=43 Mean (SD) or N (%) |
|---|---|---|
| Age (years) | 40.70 (13.12) | 43.40 (13.97) |
| Sex | ||
| Female | 33 (75%) | 33 (77%) |
| Male | 11 (25%) | 10 (23%) |
| Race | ||
| American Indian or Alaska Native | 1 (2.3%) | 1 (2.3%) |
| Asian | 4 (9.1%) | 1 (2.3%) |
| Black or African American | 1 (2.3%) | 3 (7%) |
| More than one race | 2 (4.5%) | 2 (4.7%) |
| White | 36 (81.8%) | 36 (83.7%) |
| Ethnicity | ||
| Hispanic or Latino | 7 (15.9%) | 2 (4.7%) |
| Not Hispanic or Latino | 37 (84.1%) | 39 (90.7%) |
| Unknown | 0 (0%) | 2 (4.7%) |
| Height (m) | 1.69 (0.07) | 1.69 (0.08) |
| Weight (kg) | 77.77 (14.93) | 80.01 (15.51) |
| BMI (kg/m2) | 27.31 (4.71) | 28.17 (5.47) |
| Duration of NP (years) | 8.29 (8.73) | 10.13 (8.92) |
| Average pain | 5.52 (1.36) | 5.28 (1.74) |
| Current pain | 4.86 (2.00) | 5.14 (2.02) |
| NDI (%) | 35.8 (9.9) | 37.4 (8.9) |
| FABQ work | 13.84 (8.32) | 14.67 (8.83) |
| FABQ physical activity | 12.02 (5.80) | 12.44 (4.17) |
| PROMIS Profile pain interference | 58.29 (7.52) | 59.73 (5.60) |
| PROMIS Profile satisfaction with participation in social roles | 45.34 (9.47) | 44.34 (5.93) |
| PROMIS Profile sleep disturbance | 56.46 (6.90) | 57.00 (7.05) |
| PROMIS Profile fatigue | 59.01 (7.31) | 56.48 (7.80) |
| PROMIS Profile depression | 50.13 (9.37) | 51.77 (9.16) |
| PROMIS Profile anxiety | 52.88 (9.54) | 54.46 (8.54) |
| PROMIS Profile physical function | 46.10 (6.61) | 45.01 (6.60) |
OMT=osteopathic manipulative treatment; SD=standard deviation; BMI=Body Mass Index; NDI=Neck Disability Index; FABQ=Fear Avoidance Beliefs Questionnaire; PROMIS= Patient-Reported Outcomes Measurement Information System.
Participants received a total of 298 OMT sessions. HVLA to the cervical region was performed, or at least attempted, in 96.6% of sessions (n=288/298), with cavitation occurring 60.4% (n=174/288) of time. There were 3 sessions where somatic dysfunction was not identified in the cervical region, thus HVLA was not attempted, and 7 sessions where HVLA was not attempted as per protocol or not documented as such (i.e., protocol deviations occurred). In addition to HVLA, patients also received muscle energy (88.3%), myofascial (55.4%), articulatory (45.3%), and soft tissue (5.4%) treatment techniques.
There were carryover effects present in the primary outcomes (persistence of improvements in outcomes following the OMT intervention) for average pain (t(73)=−1.41, p=.16, d=0.33), current pain (t(73)=−2.16, p=0.03, d=0.52), and NDI (t(73)=−1.84, p=0.07, d=0.48) (Figure 3), as well as in the secondary outcomes of PROMIS depression (t(73)=−1.63, p=0.11, d=0.37) and PROMIS sleep disturbance (t(73)=−2.84, p<0.01, d=0.66). Therefore, further analyses were limited to the T1 time point corresponding to the first stage of the study prior to the cross-over allocation. Due to the scheduling of laboratory and clinical visits, this time period (T0 (baseline) to T1) was significantly longer for the participants in the OMT group than in the waiting group (mean (SD) 5.2(0.8) vs. 4.4(0.8) weeks, respectively; (t(73)=−3.99, p<0.01). Six participants dropped out from each group during this time, however, their characteristics did not differ (Table 3). Consequently, 38 participants in the OMT group and 37 participants in the waiting period group were available for comparison (Figure 2).
Figure 3.
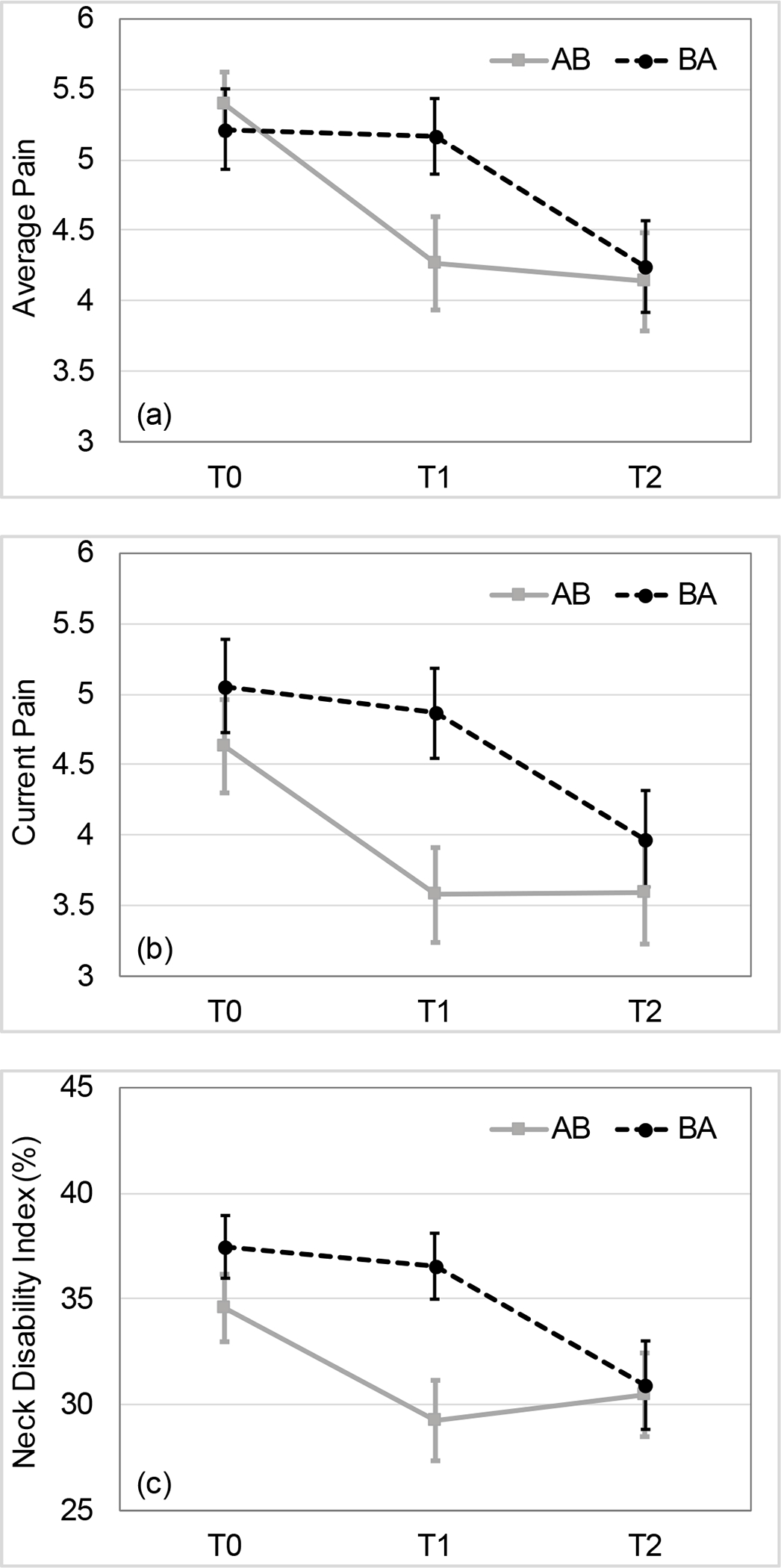
Carryover effects in the primary outcome measures by trial arm. Both unadjusted and adjusted analyses revealed the significant group differences (P<0.05) at T1 time point for average pain (a), current pain (b), and Neck Disability Index (NDI) (c). The allocation sequences indicate OMT intervention as A and waiting period as B. Error bars indicate standard errors. T0 - baseline, T1 - cross-over time point, T2 - study completion.
Table 3.
Characteristics of dropouts from baseline (T0) to crossover point (T1) by study group.
| Characteristic | OMT Group Dropouts N=6 Mean (SD) or N (%) |
Waiting Period Group Dropouts N=6 Mean (SD) or N (%) |
P-value |
|---|---|---|---|
| Age (years) | 38.33 (14.09) | 44.67 (15.36) | 0.47* |
| Sex | 0.99† | ||
| Female | 5 (83%) | 6 (100%) | |
| Male | 1 (17%) | 0 (0%) | |
| Duration of NP (years) | 6.22 (5.84) | 10.25 (6.87) | 0.30* |
| Average pain | 6.33 (1.03) | 5.67 (1.97) | 0.48* |
| Current pain | 6.33 (1.03) | 5.67 (2.16) | 0.51* |
| NDI (%) | 43.3 (8.1) | 36.8 (8.6) | 0.27* |
OMT=osteopathic manipulative treatment; SD=standard deviation; NP=neck pain; NDI=Neck Disability Index.
t-tests were used.
Chi-square test was used.
The results of comparisons between groups were similar for the adjusted and unadjusted analyses, with both analyses indicating significantly better outcomes in the OMT group for average pain, current pain, NDI, PROMIS sleep disturbance and depression (Table 4). The better outcome in the OMT group for PROMIS fatigue was significant in the adjusted but not in unadjusted analysis. The adjusted, between-group differences were as follows: average pain −1.02 (95% CI:(−1.72, −0.32), p=0.005), current pain −1.02 (95% CI:(−1.75, −0.30), p=0.006), disability −5.30% (95% CI:(−9.2%, −1.3%), p=0.010), sleep disturbance −3.25 (95% CI: (−6.95, −1.54), p=0.003), fatigue −3.26 (95% CI:(−6.04, −0.48), p=0.022), and depression −2.59 (95% CI:(−4.73, −0.45), p=0.018). The effect sizes for experimental group differences were in the clinically significant range between 0.5 and 1 SD 44,45 (Table 4).
Table 4.
Post-intervention outcomes by study group.
| Unadjusted Analyses * | Adjusted Analyses † | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | OMT Group Mean (SE) | Waiting Period Group Mean (SE) | Between-Group Difference (95% CI) ‡ | P-value, Effect Size (d) | OMT Groupy LS Mean (SE) | Waiting Period Group LS Mean (SE) | Between-Group Difference (95% CI) ‡ | P-value, Effect Size (d) |
| Average pain | 4.26 (0.33) | 5.16 (0.27) | −0.90 (−1.75, −0.05) | 0.039, d=0.49 | 4.20 (0.25) | 5.22 (0.25) | −1.02 (−1.72, −0.32) | 0.005, d=0.67 |
| Current pain | 3.58 (0.34) | 4.86 (0.32) | −1.28 (−2.21, −0.36) | 0.007, d=0.64 | 3.71 (0.25) | 4.73 (0.26) | −1.02 (−1.75, −0.30) | 0.006, d=0.65 |
| NDI (%) | 29.30 (1.9) | 36.50 (1.6) | −7.20 (−12.2, −2.3) | 0.005, d=0.68 | 30.2 (1.4) | 35.5 (1.4) | −5.30 (−9.2, −1.3) | 0.010, d=0.62 |
| FABQ work | 12.92 (1.53) | 13.92 (1.30) | −1.00 (−5.01, 3.01) | 0.621, d=0.11 | 13.31 (1.17) | 13.52 (0.18) | −0.21 (−3.53, 3.11) | 0.896, d=0.03 |
| FABQ physical activity | 11.39 (0.88) | 12.30 (0.82) | −0.91 (−3/30, 1.50) | 0.456, d=0.17 | 11.50 (0.52) | 11.74 (0.52) | −0.24 (−2.59, 1.49) | 0.593, d=0.13 |
| PROMIS Profile pain interference | 56.28 (1.34) | 57.75 (0.76) | −1.47 (−4.56, 1.63) | 0.349, d=0.22 | 57.04 (0.84) | 56.97 (0.85) | 0.07 (−2.32, 2.47) | 0.950, d=0.02 |
| PROMIS Profile satisfaction with participation in social roles | 45.97 (1.36) | 46.36 (1.02) | −0.39 (−3.80, 3.02) | 0.820, d=0.05 | 45.58 (1.00) | 46.76 (1.01) | −1.18 (−4.02, 1.65) | 0.408, d=0.19 |
| PROMIS Profile sleep disturbance | 51.97 (0.89) | 56.51 (1.34) | −4.54 (−7.73, −1.35) | 0.006, d=0.66 | 53.11 (0.95) | 56.36 (0.96) | −3.25 (−6.95, −1.54) | 0.003, d=0.72 |
| PROMIS Profile fatigue | 54.77 (1.02) | 56.65 (1.41) | −1.88 (−5.34, 1.58) | 0.283, d=0.25 | 54.09 (0.97) | 57.35 (0.99) | −3.26 (−6.04, −0.48) | 0.022, d=0.55 |
| PROMIS Profile depression | 46.60 (1.18) | 50.66 (1.46) | −4.06 (−7.78, −0.34) | 0.033, d=0.50 | 47.33 (0.75) | 49.92 (0.76) | −2.59 (−4.73, −0.45) | 0.018, d=0.56 |
| PROMIS Profile anxiety | 48.52 (1.23) | 51.28 (1.48) | −2.76 (−6.64, 1.11) | 0.159, d=0.33 | 49.23 (0.99) | 50.54 (1.00) | −1.31 (−4.13, 1.51) | 0.358, d=0.22 |
| PROMIS Profile physical function | 46.13 (1.19) | 44.00 (1.05) | 2.13 (−1.04, 5.30) | 0.184, d=0.31 | 45.75 (0.78) | 44.40 (0.79) | 1.35 (−0.87, 3.57) | 0.228, d=0.28 |
OMT=osteopathic manipulative treatment; SE=standard error; CI=confidence interval; LS=least square; NDI=Neck Disability Index; FABQ=Fear Avoidance Beliefs Questionnaire; PROMIS= Patient-Reported Outcomes Measurement Information System.
The unadjusted estimates of treatment effects on the outcomes were obtained from the t-tests comparing the two experimental groups.
The adjusted estimates of treatment effects were obtained from general linear models relating outcomes at T1 to experimental group and baseline value of the outcome.
Significant (p<0.05) differences are bolded.
There was a total of 187 AEs reported during this study and none of them were rated as serious (Severity Grade 4 or greater). Of these, only 37 AEs, reported by 27 participants (some participants reported more than one AE after a single OMT session), could be attributed to any of the 298 delivered OMT sessions, as these AEs were classified as being at least “possibly related” (relatedness Grade 2 or greater) and these AEs were expected. The other AEs were classified as either not related to the study (relatedness Grade 0 or 1) or were associated with the motor control testing sessions. One AE, involving an increase in rib pain following an OMT session, was rated as Severe (Grade 3). The remaining AEs were mild or moderate (Grade 1 or 2) increase in NP (n=16), muscle soreness (n=15), headache (n=2), and other (n=3). Almost all OMT-related AEs resolved completely; one with minor sequela and one unknown (lost to follow-up).
Discussion
The results from the current study indicate that OMT intervention is effective in reducing pain and disability in patients with chronic NP, as compared to no intervention. The participants in the OMT group also showed significant improvements in sleep, fatigue, and depression scores. It is possible that improvements in sleep disturbance and fatigue came from the reduction in average pain. Improvements in depression profiles were not hypothesized because the theorized mechanisms of manual treatment do not typically include modifications of psychosocial factors 46–48. However, there is a well-established link between chronic pain and depressive disorders, which share similar neurophysiological pathways 49,50. In fact, in a large cross-sectional study, Juan et al. demonstrated a positive correlation between NP intensity and depression, which was mediated by sleep quality 51. Sleep disturbance, pain, anxiety, depression, and fatigue (lack of energy), known as the SPADE cluster, often co-occur in the general population and are difficult to manage in a primary clinical practice 52. Concomitant improvements in pain, disability, depression, and sleep disturbance in the group receiving OMT intervention demonstrate its efficacy in addressing SPADE symptoms and point to the consistency in the results from the current study.
The significant group differences in the primary outcomes in our study ranged from 0.9 to 1.3 points for NRS pain scores and from 5.3% to 7.2% for NDI, depending on whether the adjusted or unadjusted means were considered. It is debated how much improvement in pain and disability can be considered clinically significant or important. Typically, the MCID is calculated as the smallest difference that patients perceive to be beneficial. It is frequently quoted as 1.3 points on NRS for mechanical NP, based on one study 53. However, due to methodological differences 54, the reported MCID values for NDI are inconsistent across studies and range widely between 6 and 38% 55–57. For that reason, Norman et al. proposed, and others supported, the 0.5 SD as a conservative estimate of an effect size that is likely to be clinically meaningful 44,45. By this measure, all statistically significant improvements in the outcomes observed in this study are clinically meaningful, as they ranged between 0.5 and 0.7 SD compared with controls.
There are only three published RCTs on the efficacy of OMT for chronic NP, by which our results can be directly compared. Schwerla et al.27 showed slightly larger differences in average and current pain (1.8 and 1.2, respectively) between the OMT and sham ultrasound groups. Similarly, slightly larger differences than in our study were reported for NDI between musicians who did and did not receive OMT (8.4%) 28. However, the participants in these two studies received 5 OMT sessions in comparison to 3–4 sessions in the current study. A dose-response effect could account for the differences in outcomes. The results from the study by Groisman et al. 29, in which the individuals with chronic NP received OMT once a week for 4 weeks in addition to general exercise, were closer to our results (OMT+ exercise versus exercise alone group differences were 1.4 NRS for average pain and 7.6% for NDI). Unfortunately, the dose-response to manual treatment for NP is unknown, but such data on chronic low back pain suggests an optimum of 12 sessions of spinal manipulation 58 or 6 sessions of OMT to achieve similar improvement 59. Thus, it is possible that an increase in the dose of OMT beyond 3–4 sessions would have been more effective in the current study.
There are many RCTs of the effects of other manual treatment techniques, broadly termed spinal manipulation and mobilization, on pain and disability in NP patients. Collectively, as described in a Cochrane systematic review of spinal manipulation and mobilization for NP, these techniques result in similar positive outcomes with small to medium effect sizes 23, which are on a par with the results of the current study demonstrating medium effect sizes (between 0.5 and 0.7). Given the complex biopsychosocial character of chronic pain 60, it should be reasonable to expect that a unimodal intervention may not address all the factors contributing to its persistence, thus producing modest outcomes 61. Indeed, the multimodal programs that combine manual treatment with exercise and physical therapy appear to be superior to unimodal interventions for chronic NP 22,31.
Increase in pain, soreness, and headache are common AEs experienced by patients after manual treatment for NP, including OMT 62–64. However, AEs are generally underreported in RCTs 65 and there are no standard selection criteria for reporting AEs 66. For example, none of the previous three RCTs on OMT for chronic NP addressed AEs in a systematic manner. Groisman et al. stated only that “no adverse events were reported” without describing how they were monitored 29. Similarly, Schwerla et al. stated that “no serious adverse events were recorded” and mentioned that in a few cases patients reported tiredness and symptoms in the area other than cervical spine 27. Finally, among 28 patients who received 5 treatments, Rotter et al. identified two AEs (tiredness and dizziness) through interviews and participant reports to the study center 28. It is, therefore, difficult to compare these numbers with the current study, in which we logged all unfavorable signs and symptoms after each visit and through weekly contacts, and then classified them systematically using the definitions and terminology endorsed by the National Institutes of Health 40. Despite this diligence, the incidence of 37 AEs related to the 298 OMT sessions (12%) in the current study appears to be much lower in comparison with the 22% incidence after other manual treatment sessions in RCTs that reported AEs with the appropriate detail 67. Had we counted all symptoms as AEs without the 2-point symptom intensity threshold, the incidence of AEs would have been higher. This discrepancy underscores the need for the standardized selection criteria in reporting AEs. Nevertheless, considering that all 37 AEs in this study were expected and no serious AEs occurred, it could be concluded that OMT is relatively safe as are other manual treatment modalities.
The lack of long-term follow-up is a major limitation of the current study, although the persistence of improvements in the primary clinical outcomes can be judged from the significant carryover effects that were sustained for at least 4 weeks in the second part of this cross-over experimental design. Participants were recruited based on self-reported NP and were not necessarily seeking care for their symptoms, which may not generalize to patients actively seeking care who might have shown bigger treatment effects. Due to the schedule of clinical visits, participants were randomized, and their group allocation was disclosed to them prior to the baseline assessment. This sequence makes the attrition rate larger (23%) by counting 10 participants who dropped out between the randomization and baseline assessment. Ideally, randomization should have been done after the baseline assessment, which would have given us a 14% attrition rate corresponding to the time period between T0 and T1. These attrition rates are in line with the 5 to 25% range typically seen in cluster randomized cross-over trials 68. In addition, the number and characteristics of dropouts did not differ between the study groups.
Also due to the scheduling logistics, outcomes in the OMT group at T1 (cross-over point) were assessed approximately 5 days later than in the waiting period group. However, this difference is unlikely to influence the outcomes, as the participants in this study suffered chronic NP, on average, for longer than 8 years. Furthermore, because this was a validation study for the head-neck motor control tests, it did not include an active treatment control group and was based on a single clinical center. Finally, due to carry-over effects, the analyses were conducted only up to the cross-over time point. Notwithstanding the above limitations, to date, this is only the fourth RCT on the efficacy of OMT for chronic NP. As such, it provides new data on this topic and will help in the design of future longitudinal or multi-center studies. Because physicians in the current study were relatively free to choose techniques and areas of the body to treat based on their clinical examination, the results should have broader generalizability to other osteopathic practices.
Conclusions
Our study demonstrated that OMT intervention is effective in reducing pain and disability along with improving sleep, fatigue, and depression in patients with chronic NP immediately following treatment delivered over approximately 4–6 weeks. OMT applied to the cervical region is relatively safe. Given that OMT is cost-effective 69, it could be recommended as an effective option in the management of chronic NP.
Acknowledgments:
The authors would like to thank Dr. Cody Priess for his significant role in the development of head-neck motor control tests. We would also like to pay our gratitude and respects to our late colleague, Timothy Francisco, DO, whose dedication and contribution to the osteopathic profession also made this work possible.
Funding support:
This publication was made possible by grant number U19AT006057 from the National Center for Complementary and Integrative Health (NCCIH) at the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCCIH.
Disclosures
Dr. Reeves is the Founder and President of Sumaq Life LLC. Sumaq Life LLC was formed to increase commercialization of government supported research discoveries to improve health outcomes, including technology to assess and treat back pain.
References
- 1.Hoy D, March L, Woolf A, et al. The global burden of neck pain: estimates from the global burden of disease 2010 study. Ann Rheum Dis. Jul 2014;73(7):1309–1315. [DOI] [PubMed] [Google Scholar]
- 2.Hurwitz EL, Randhawa K, Yu H, Cote P, Haldeman S. The Global Spine Care Initiative: a summary of the global burden of low back and neck pain studies. Eur Spine J Sep 2018;27(Suppl 6):796–801. [DOI] [PubMed] [Google Scholar]
- 3.Cote P, Cassidy JD, Carroll L. The Saskatchewan Health and Back Pain Survey. The prevalence of neck pain and related disability in Saskatchewan adults. Spine. Aug 1 1998;23(15):1689–1698. [DOI] [PubMed] [Google Scholar]
- 4.Hogg-Johnson S, van der Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976). Feb 15 2008;33(4 Suppl):S39–51. [DOI] [PubMed] [Google Scholar]
- 5.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. Dec 15 2012;380(9859):2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safiri S, Kolahi AA, Hoy D, et al. Global, regional, and national burden of neck pain in the general population, 1990–2017: systematic analysis of the Global Burden of Disease Study 2017. BMJ. Mar 26 2020;368:m791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll LJ, Hogg-Johnson S, van der Velde G, et al. Course and prognostic factors for neck pain in the general population: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976). Feb 15 2008;33(4 Suppl):S75–82. [DOI] [PubMed] [Google Scholar]
- 8.Cote P, Cassidy JD, Carroll LJ, Kristman V. The annual incidence and course of neck pain in the general population: a population-based cohort study. Pain. Dec 2004;112(3):267–273. [DOI] [PubMed] [Google Scholar]
- 9.Haldeman S, Carroll L, Cassidy JD, Schubert J, Nygren A. The Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders: executive summary. Spine. Feb 15 2008;33(4 Suppl):S5–7. [DOI] [PubMed] [Google Scholar]
- 10.Hush JM, Lin CC, Michaleff ZA, Verhagen A, Refshauge KM. Prognosis of acute idiopathic neck pain is poor: a systematic review and meta-analysis. Arch Phys Med Rehabil. May 2011;92(5):824–829. [DOI] [PubMed] [Google Scholar]
- 11.Borghouts J, Janssen H, Koes B, Muris J, Metsemakers J, Bouter L. The management of chronic neck pain in general practice. A retrospective study. Scand J Prim Health Care. Dec 1999;17(4):215–220. [DOI] [PubMed] [Google Scholar]
- 12.van Dongen JM, Ketheswaran J, Tordrup D, Ostelo R, Bertollini R, van Tulder MW. Health economic evidence gaps and methodological constraints in low back pain and neck pain: Results of the Research Agenda for Health Economic Evaluation (RAHEE) project. Best Pract Res Clin Rheumatol. Dec 2016;30(6):981–993. [DOI] [PubMed] [Google Scholar]
- 13.Guzman J, Hurwitz EL, Carroll LJ, et al. A new conceptual model of neck pain: linking onset, course, and care: the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976). Feb 15 2008;33(4 Suppl):S14–23. [DOI] [PubMed] [Google Scholar]
- 14.McLean SM, May S, Klaber-Moffett J, Sharp DM, Gardiner E. Risk factors for the onset of non-specific neck pain: a systematic review. J Epidemiol Community Health. Jul 2010;64(7):565–572. [DOI] [PubMed] [Google Scholar]
- 15.Guzman J, Haldeman S, Carroll LJ, et al. Clinical practice implications of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders: from concepts and findings to recommendations. Spine (Phila Pa 1976). Feb 15 2008;33(4 Suppl):S199–213. [DOI] [PubMed] [Google Scholar]
- 16.Bier JD, Scholten-Peeters WGM, Staal JB, et al. Clinical Practice Guideline for Physical Therapy Assessment and Treatment in Patients With Nonspecific Neck Pain. Phys Ther. Mar 1 2018;98(3):162–171. [DOI] [PubMed] [Google Scholar]
- 17.Blanpied PR, Gross AR, Elliott JM, et al. Neck Pain: Revision 2017. Clinical practice guidelines linked to the International Classification of Functioning, Disability and Health from the Orthopaedic Section of the American Physical Therapy Association. The Journal of orthopaedic and sports physical therapy. Jul 2017;47(7):A1–A83. [DOI] [PubMed] [Google Scholar]
- 18.Hawk C, Whalen W, Farabaugh RJ, et al. Best Practices for Chiropractic Management of Patients with Chronic Musculoskeletal Pain: A Clinical Practice Guideline. J Altern Complement Med Oct 2020;26(10):884–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whalen W, Farabaugh RJ, Hawk C, et al. Best-Practice Recommendations for Chiropractic Management of Patients With Neck Pain. J Manipulative Physiol Ther Nov 2019;42(9):635–650. [DOI] [PubMed] [Google Scholar]
- 20.Parikh P, Santaguida P, Macdermid J, Gross A, Eshtiaghi A. Comparison of CPG’s for the diagnosis, prognosis and management of non-specific neck pain: a systematic review. BMC Musculoskelet Disord. Feb 14 2019;20(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong JJ, Shearer HM, Mior S, et al. Are manual therapies, passive physical modalities, or acupuncture effective for the management of patients with whiplash-associated disorders or neck pain and associated disorders? An update of the Bone and Joint Decade Task Force on Neck Pain and Its Associated Disorders by the OPTIMa collaboration. Spine J. Dec 2016;16(12):1598–1630. [DOI] [PubMed] [Google Scholar]
- 22.Coulter ID, Crawford C, Vernon H, et al. Manipulation and Mobilization for Treating Chronic Nonspecific Neck Pain: A Systematic Review and Meta-Analysis for an Appropriateness Panel. Pain Physician. Mar 2019;22(2):E55–E70. [PMC free article] [PubMed] [Google Scholar]
- 23.Gross A, Langevin P, Burnie SJ, et al. Manipulation and mobilisation for neck pain contrasted against an inactive control or another active treatment. Cochrane Database Syst Rev. Sep 23 2015(9):CD004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fryer G, Morse CM, Johnson JC. Spinal and sacroiliac assessment and treatment techniques used by osteopathic physicians in the United States. Osteopathic medicine and primary care. 2009;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson SM, Kurtz ME. Osteopathic manipulative treatment techniques preferred by contemporary osteopathic physicians. J Am Osteopath Assoc. May 2003;103(5):219–224. [PubMed] [Google Scholar]
- 26.Licciardone JC, Kearns CM, King HH, et al. Somatic dysfunction and use of osteopathic manual treatment techniques during ambulatory medical care visits: a CONCORD-PBRN study. J Am Osteopath Assoc. May 2014;114(5):344–354. [DOI] [PubMed] [Google Scholar]
- 27.Schwerla F, Bischoff A, Nurnberger A, Genter P, Guillaume JP, Resch KL. Osteopathic treatment of patients with chronic non-specific neck pain: a randomised controlled trial of efficacy. Forsch Komplementmed. Jun 2008;15(3):138–145. [DOI] [PubMed] [Google Scholar]
- 28.Rotter G, Fernholz I, Binting S, et al. The effect of osteopathic medicine on pain in musicians with nonspecific chronic neck pain: a randomized controlled trial. Ther Adv Musculoskelet Dis. 2020;12:1759720X20979853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groisman S, Malysz T, de Souza da Silva L, et al. Osteopathic manipulative treatment combined with exercise improves pain and disability in individuals with non-specific chronic neck pain: A pragmatic randomized controlled trial. J Bodyw Mov Ther. Apr 2020;24(2):189–195. [DOI] [PubMed] [Google Scholar]
- 30.Popovich JM Jr., Reeves NP, Priess MC, Cholewicki J, Choi J, Radcliffe CJ. Quantitative measures of sagittal plane head-neck control: a test-retest reliability study. J Biomech. Feb 5 2015;48(3):549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Domingues L, Pimentel-Santos FM, Cruz EB, et al. Is a combined programme of manual therapy and exercise more effective than usual care in patients with non-specific chronic neck pain? A randomized controlled trial. Clin Rehabil. Dec 2019;33(12):1908–1918. [DOI] [PubMed] [Google Scholar]
- 32.Education Council on Osteopathic Principles. Glossary of Osteopathic Terminology. In: Giusti R, ed. Chevy Chase, MD: American Association of Colleges of Osteopathic Medicine; 2017. [Google Scholar]
- 33.Gross A, Miller J, D’Sylva J, et al. Manipulation or mobilisation for neck pain: a Cochrane Review. Man Ther Aug 2010;15(4):315–333. [DOI] [PubMed] [Google Scholar]
- 34.Paanalahti K, Holm LW, Nordin M, et al. Three combinations of manual therapy techniques within naprapathy in the treatment of neck and/or back pain: a randomized controlled trial. BMC Musculoskelet Disord. Apr 23 2016;17:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. May 8 2015;350:h2147. [DOI] [PubMed] [Google Scholar]
- 36.Vernon H The Neck Disability Index: state-of-the-art, 1991–2008. J Manipulative Physiol Ther. Sep 2008;31(7):491–502. [DOI] [PubMed] [Google Scholar]
- 37.Hays RD, Spritzer KL, Schalet BD, Cella D. PROMIS((R))-29 v2.0 profile physical and mental health summary scores. Qual Life Res. Jul 2018;27(7):1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landers MR, Creger RV, Baker CV, Stutelberg KS. The use of fear-avoidance beliefs and nonorganic signs in predicting prolonged disability in patients with neck pain. Man Ther. Jun 2008;13(3):239–248. [DOI] [PubMed] [Google Scholar]
- 39.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. Jul 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Common Terminology Criteria for Adverse Events (CTCAE). In: U.S. Department of Health and Human Services, ed: National Institutes of Health; 2010. [Google Scholar]
- 41.Farrar JT, Young JP Jr., LaMoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. Nov 2001;94(2):149–158. [DOI] [PubMed] [Google Scholar]
- 42.Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain Aug 2004;8(4):283–291. [DOI] [PubMed] [Google Scholar]
- 43.Senn S Statistical issues in drug development. 2nd ed: John Wiley & Sons; 2009. [Google Scholar]
- 44.Sloan JA, Cella D, Hays RD. Clinical significance of patient-reported questionnaire data: another step toward consensus. J Clin Epidemiol. Dec 2005;58(12):1217–1219. [DOI] [PubMed] [Google Scholar]
- 45.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. May 2003;41(5):582–592. [DOI] [PubMed] [Google Scholar]
- 46.Bialosky JE, Beneciuk JM, Bishop MD, et al. Unraveling the Mechanisms of Manual Therapy: Modeling an Approach. The Journal of orthopaedic and sports physical therapy. Jan 2018;48(1):8–18. [DOI] [PubMed] [Google Scholar]
- 47.Gyer G, Michael J, Inklebarger J, Tedla JS. Spinal manipulation therapy: Is it all about the brain? A current review of the neurophysiological effects of manipulation. J Integr Med. Sep 2019;17(5):328–337. [DOI] [PubMed] [Google Scholar]
- 48.Hennenhoefer K, Schmidt D. Toward a Theory of the Mechanism of High-Velocity, Low-Amplitude Technique: A Literature Review. J Am Osteopath Assoc. Oct 1 2019;119(10):688–695. [DOI] [PubMed] [Google Scholar]
- 49.Humo M, Lu H, Yalcin I. The molecular neurobiology of chronic pain-induced depression. Cell Tissue Res. Jul 2019;377(1):21–43. [DOI] [PubMed] [Google Scholar]
- 50.Sheng J, Liu S, Wang Y, Cui R, Zhang X. The Link between Depression and Chronic Pain: Neural Mechanisms in the Brain. Neural Plast. 2017;2017:9724371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Juan W, Rui L, Wei-Wen Z. Chronic neck pain and depression: the mediating role of sleep quality and exercise. Psychol Health Med Feb 4 2020:1–7. [DOI] [PubMed] [Google Scholar]
- 52.Kroenke K, Talib TL, Stump TE, et al. Incorporating PROMIS Symptom Measures into Primary Care Practice-a Randomized Clinical Trial. J Gen Intern Med. Aug 2018;33(8):1245–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cleland JA, Childs JD, Whitman JM. Psychometric properties of the Neck Disability Index and Numeric Pain Rating Scale in patients with mechanical neck pain. Arch Phys Med Rehabil. Jan 2008;89(1):69–74. [DOI] [PubMed] [Google Scholar]
- 54.Beaton DE, Boers M, Wells GA. Many faces of the minimal clinically important difference (MCID): a literature review and directions for future research. Curr Opin Rheumatol. Mar 2002;14(2):109–114. [DOI] [PubMed] [Google Scholar]
- 55.Lauche R, Langhorst J, Dobos GJ, Cramer H. Clinically meaningful differences in pain, disability and quality of life for chronic nonspecific neck pain - a reanalysis of 4 randomized controlled trials of cupping therapy. Complement Ther Med. Aug 2013;21(4):342–347. [DOI] [PubMed] [Google Scholar]
- 56.MacDermid JC, Walton DM, Avery S, et al. Measurement properties of the neck disability index: a systematic review. The Journal of orthopaedic and sports physical therapy. May 2009;39(5):400–417. [DOI] [PubMed] [Google Scholar]
- 57.Schellingerhout JM, Verhagen AP, Heymans MW, Koes BW, de Vet HC, Terwee CB. Measurement properties of disease-specific questionnaires in patients with neck pain: a systematic review. Qual Life Res. May 2012;21(4):659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haas M, Vavrek D, Peterson D, Polissar N, Neradilek MB. Dose-response and efficacy of spinal manipulation for care of chronic low back pain: a randomized controlled trial. Spine J. Jul 1 2014;14(7):1106–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Licciardone JC. Short-term dosing of manual therapies for chronic low back pain. Spine J. Jun 1 2014;14(6):1085–1086. [DOI] [PubMed] [Google Scholar]
- 60.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. Jul 2007;133(4):581–624. [DOI] [PubMed] [Google Scholar]
- 61.Cholewicki J, Pathak PK, Reeves NP, Popovich JM Jr. Model Simulations Challenge Reductionist Research Approaches to Studying Chronic Low Back Pain. The Journal of orthopaedic and sports physical therapy. Jun 2019;49(6):477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Degenhardt BF, Johnson JC, Brooks WJ, Norman L. Characterizing Adverse Events Reported Immediately After Osteopathic Manipulative Treatment. J Am Osteopath Assoc. Mar 1 2018;118(3):141–149. [DOI] [PubMed] [Google Scholar]
- 63.Kranenburg HA, Schmitt MA, Puentedura EJ, Luijckx GJ, van der Schans CP. Adverse events associated with the use of cervical spine manipulation or mobilization and patient characteristics: A systematic review. Musculoskelet Sci Pract. Apr 2017;28:32–38. [DOI] [PubMed] [Google Scholar]
- 64.Paanalahti K, Holm LW, Nordin M, Asker M, Lyander J, Skillgate E. Adverse events after manual therapy among patients seeking care for neck and/or back pain: a randomized controlled trial. BMC Musculoskelet Disord. Mar 12 2014;15:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gorrell LM, Engel RM, Brown B, Lystad RP. The reporting of adverse events following spinal manipulation in randomized clinical trials-a systematic review. Spine J. Sep 2016;16(9):1143–1151. [DOI] [PubMed] [Google Scholar]
- 66.Mayo-Wilson E, Fusco N, Hong H, Li T, Canner JK, Dickersin K. Opportunities for selective reporting of harms in randomized clinical trials: Selection criteria for non-systematic adverse events. Trials. Sep 5 2019;20(1):553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carnes D, Mars TS, Mullinger B, Froud R, Underwood M. Adverse events and manual therapy: a systematic review. Man Ther. Aug 2010;15(4):355–363. [DOI] [PubMed] [Google Scholar]
- 68.Moerbeek M The cluster randomized crossover trial: The effects of attrition in the AB/BA design and how to account for it in sample size calculations. Clin Trials. Aug 2020;17(4):420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verhaeghe N, Schepers J, van Dun P, Annemans L. Osteopathic care for low back pain and neck pain: A cost-utility analysis. Complement Ther Med. Oct 2018;40:207–213. [DOI] [PubMed] [Google Scholar]


