Abstract
Purpose
To compare sleeve gastrectomy (SG) to SG associated with Rossetti fundoplication (SG + RF) in terms of de novo gastro-esophageal reflux disease (GERD) after surgery, weight loss, and postoperative complications.
Materials and methods
Patients affected by morbid obesity, without symptoms of GERD, who were never in therapy with proton pump inhibitors (PPIs), were randomized into two groups. One group underwent SG and the other SG + RF. The study was stopped on February 2020 due to the COVID pandemic.
Results
A total of 278 patients of the programmed number of 404 patients were enrolled (68.8%). De novo esophagitis was considered in those patients who had both pre- and postoperative gastroscopy (97/278, 34.9%). Two hundred fifty-one patients (90.3%) had completed clinical follow-up at 12 months. SG + RF resulted in an adequate weight loss, similar to classic SG at 12-month follow-up (%TWL = 35. 4 ± 7.2%) with a significantly better outcome in terms of GERD development. One year after surgery, PPIs were necessary in 4.3% SG + RF patients compared to 17.1% SG patients (p = 0.001). Esophagitis was present in 2.0% of SG + RF patients versus 23.4% SG patients (p = 0.002). The main complication after SG + RF was wrap perforation (4.3%), which improved with the surgeon’s learning curve.
Conclusion
SG + RF seemed to be an effective alternative to classic SG in preventing de novo GERD. More studies are needed to establish that an adequate learning curve decreases the higher percentage of short-term complications in the SG + RF group.
Graphical abstract
Keywords: Rossetti sleeve, Nissen sleeve, GERD, Obesity
Introduction
Sleeve gastrectomy (SG) is the most performed bariatric intervention in the world [1]. A recent systematic review and meta-analysis reported an increase of 19% of gastro-esophageal reflux disease (GERD) after SG and a 23% rate of new onset GERD, with erosive esophagitis (EE) in 30% of patients, and Barrett’s esophagus (BE) in 6% [2]. The use of proton pump inhibitors (PPIs) increased in 38% of patients. Other authors pointed out a development of EE after SG in 15.5 to 66.7% of patients [3–9].
The cause of an increase in GERD after SG may be related to alimentary behavior and surgery itself. Changes in the anatomy of the esophago-gastric (EG) junction area, dissection of the angle of His and potential damage to the sling fibers [10], increased intragastric pressure [7] reduced compliance [11], and eventual vagus nerve damage [12] are all factors that may alter the physiological anti-reflux systems [2].
On the other hand, some authors reported an improvement of GERD after SG, explained by weight loss, which reduces the gastro-esophageal pressure, and by decreased acid production due to the reduced volume of the stomach [13, 14].
We began performing a combined SG + Rossetti anti-reflux fundoplication (RF) to improve both obesity and GERD [15] in our center in 2015. Sleeve associated with fundoplication has been described by other authors with some variations, mostly regarding the type of fundoplication [16–21]. In previous manuscripts, we described the feasibility and the efficacy of SG + RF [22, 23].
The purpose of this study was to compare the outcomes of standard SG and SG + RF, in patients without preoperative GERD, evaluated in terms of de novo GERD after surgery, weight loss, and postoperative complications.
Materials and methods
Study design
The study was designed as a monocentric, two-arm (1:1), randomized, controlled clinical trial. All the surgical procedures were performed in a high-volume bariatric surgery center (Policlinico San Marco, GSD, Zingonia (BG), Italy), from May 2017 to February 2020. Patients were randomized, and data were collected in a prospectively held database and analyzed by another autonomous Research Center (Research Centre on Public Health (CESP) of the University of Milan-Bicocca, Monza (MB), Italy). The study was approved by the Ethics Committee and was conducted according to the Declaration of Helsinki.
Inclusion criteria were morbid obesity suitable for surgery according to the Italian Bariatric Society lines [24]; no preoperative typical or atypical reflux symptoms; no preoperative therapy with PPIs; and no esophagitis at the esophago-gastro-duodenoscopy (EGDS) when it was performed before surgery. The original protocol followed the 2016 Italian guidelines for bariatric surgery [24]; hence, the need for EGDS was assessed by clinical judgment. During the course of the study, we decided to perform EGDS preoperatively in all patients because it could add more objective information about GERD.
Exclusion criteria were clinical dysphagia; previous surgery for obesity or procedures on the EG junction; and any contraindication to laparoscopic surgery.
Eligible subjects were randomly assigned to one of the two arms according to the randomization code that was generated at CESP. In the experimental arm, patients underwent SG + RF. In the control arm, patients underwent standard SG.
Considering COVID-19 pandemic status, Italian Bariatric Society recommended performing only emergent and urgent surgery [25]. Therefore, in agreement with the statistician, the study was stopped in January 2020.
Surgical technique
Standard SG and SG + RF techniques were largely described in previous manuscripts [15, 22, 23]. In both interventions, four trocars were used: 10 mm left sub-costal (optic view), 5 mm epigastric (liver retraction), 5 mm right hypochondrium (left hand), and 15 mm mesogastrium (right hand). The dissection of the gastro-colic ligament began at 3–4 cm from the pylorus and reached the left diaphragmatic pillar. At this point, the two interventions differed. In the patients undergoing SG + RF, the phreno-esophageal membrane was dissected. A minimal opening of the posterior crura was made to allow the retro-esophageal passage of the fundus. A 1.5–2 cm long, floppy, 360° fundoplication was created over a 38Fr oro-gastric boogie, with two interrupted, non-absorbable, extracorporeal Roeder knots. No sutures on the esophagus were performed. After the formation of the fundoplication, the two procedures were continued and concluded in the same way. The stomach was sectioned over a 38Fr oro-gastric boogie with a linear articulable stapler (Tristaple Signia™ stapling system, Medtronic, Dublin, Ireland). The choice of the type of cartridge depended on the tissue thickness, as usual. The main difference between the two interventions was the fact that at the level of the fundoplication, a black cartridge was used. An indocyanine green (ICG) test was performed at the end of the SG + RF intervention, to check the correct vascularization of the wrap, using IMAGE1 S™ RUBINA™ system (KARL STORZ SE & CO. KG, Tuttlingen, Germany).
Postoperative management and follow-up
On postoperative day two, an upper gastro-intestinal (UGI) series with oral water-soluble contrast was performed. If negative, the patient started a liquid diet on the same day. If there were any signs of leakage after the UGI series, a computed tomography (CT) scan with oral water-soluble contrast was performed. If the postoperative course was regular, the patient was discharged on the third or fourth postoperative day. Patients had clinical follow-up at 1, 3, 6, and 12 months after surgery; some patients who had preoperative EGDS repeated the exam 12 months after surgery, but this was not specified in the initial protocol.
Statistical analysis
Descriptive statistics were used to summarize patient baseline characteristics and clinical data. Continuous variables were compared using non-parametric tests, Mann–Whitney-Wilcoxon test, or Wilcoxon signed-rank test for matched samples. The differences between groups were compared with Fisher’s exact test. A p value < 0.05 was considered statistically significant. The analyses were performed with the software SAS version 9.4 for Windows.
Results
Patient characteristics
A total of 278 patients of the programmed number of 404 patients were enrolled (68.8%). The study was early stopped for COVID pandemic. One hundred thirty-eight patients were randomized in the experimental group and underwent SG + RF; the remaining 140 patients were randomized in the control group and underwent normal SG. All the patients enrolled in the study respect the inclusion criteria shown in Table 1. All the interventions were performed in laparoscopy. The mean operative time was not significant different between SG + RF and SG (47.4 ± 17.4 vs 48.4 ± 15.1 min, p = 0.585). 62.8% of the SG + RF were performed by an expert surgeon, who had performed this procedure more than 100 times, and the remaining 37.2% of the SG + RF were performed by the rest of the surgical equipe. Considering only the expert surgeon, the mean operative time for normal SG was 32.1 ± 4.9 min and 38.5 ± 13.1 min for SG + RF (+ 6.4 min in SG + RF, p = 0.015). Considering the rest of the surgeons of the equipe, the mean operative time for normal SG was 49.3 ± 14.9 min and 62.3 ± 12.9 min for SG + RF (+ 12.9 min in SG + RF, p = 0.006−3). The relatively small difference in global mean operative time for SG + RF and SG was related to the fact that most of the SG + RF were performed by the expert surgeon and most of the normal SG were performed by the rest of the equipe.
Table 1.
Baseline and demographic characteristics of patients enrolled in the study
| Baseline characteristics | Summary statistics | SG (n 140) | SG + RF (n 138) | TOTAL (n 278) | p-value |
|---|---|---|---|---|---|
| Age (years) | Mean ± SD | 41.3 ± 9.8 | 40.8 ± 11.1 | 41.1 ± 10.5 | 0.741 |
|
Sex F M |
%, n/Pts %, n/Pts |
71.4%, 100/140 28.6%, 40/140 |
78.3%, 108/138 21.7%, 30/138 |
74.8%, 208/278 25.1%, 70/278 |
0.189 |
| BMI (kg/m2) | Mean ± SD | 45.2 ± 7.0 | 43.4 ± 5.9 | 44.3 ± 6.6 | 0.017* |
| PPI | %, n/Pts | 0%, 0/140 | 0%, 0/138 | 0%, 0/278 | n.s |
| Reflux symptoms | %, n/Pts | 0%, 0/140 | 0%, 0/138 | 0%, 0/278 | n.s |
|
EGDS EE Hiatal Hernia |
N %, n/Pts %, n/Pts |
116 (82.8%) 0%, 0/116 10.3%, 12 /116 |
108 (78.3%) 0%, 0/108 16.7%, 18 /108 |
224 (80.6%) 0%, 0/224 13.4%, 30 /224 |
n.s 0.161 |
| CPAP | %, n/Pts | 46.4%, 65/140 | 37.7%, 52/138 | 42.1%, 117/278 | 0.143 |
| T2D | %, n/Pts | 8.6%, 12/140 | 5.0%, 7/138 | 6.8%, 19/278 | 0.235 |
| HTN | %, n/Pts | 32.1%, 45/140 | 26.1%, 36/138 | 29.1%, 81/278 | 0.272 |
SG sleeve gastrectomy; RF Rossetti fundoplication; sd standard deviation; F female; M male; BMI body mass index; PPIs proton pump inhibitor; EGDS esophago-gastro-duodenoscopy; EE erosive esophagitis; CPAP continuous positive airways pressure; T2D type 2 diabetes; HTN hypertension; n.s. not significant; N number of patients analyzed when different from the total; n/Pts number of patients out of the total; *statistically significant
New onset of GERD after surgery
None of the patients had preoperative symptoms of GERD and none was on PPIs. None of the patients who had preoperative EGDS (224/278, 80.6%) presented with EE. 30/224 (13.4%) had a small sliding hiatal hernia (< 2 cm). 97/278 (34.9%) patients had EGDS both pre- and postsurgery at a mean follow-up of 14.7 ± 5.4 months in SG group and of 16.9 ± 7.3 in SG + RF group. De novo esophagitis was considered in those patients who had both pre- and postoperative gastroscopy (33.6% in SG group and 36.2% in SG + RF group).
New onset GERD was higher in the SG group than in SG + RF group (Table 2), with a significantly higher rate of patients using PPIs 1 year after surgery (17.1% vs 4.3%, p = 0.001). The incidence of EE after SG was higher than after SG + RF (23.4% vs 2.0%, p = 0.002). In the SG group, the patients had grade A and grade B EE (54.4% and 45.5%). In the SG + RF group, only 1 patient, who did not respect the dietary indications, had grade A EE. GERD symptoms did not necessarily follow the finding of EE. In the SG group, only 5 out of the 11 patients with esophagitis referred GERD symptoms (45.5%). The only patient with EE in the SG + RF group did not report symptoms. On the contrary, 13 of the total of 84 patients without esophagitis reported reflux symptoms 1 year after surgery, 8 in the SG group and 5 in the SG + RF group.
Table 2.
PPIs consumption and de novo esophagitis after surgery
| Summary statistics | SG (n 140) | SG + RF (n 138) | p-value | |
|---|---|---|---|---|
| Using PPIs | %, n/Pts | 17.1%, 23/140 | 4.3%, 6/138 | 0.001* |
|
EGDS Total esophagitis A esophagitis B esophagitis |
N %, n/Pts %, n/Pts %, n/Pts |
47 (33.6%) 23.4%, 11/47 54.5%, 6/11 45.5%, 5/11 |
50 (36.2%) 2.0%, 1/50 100%, 2/2 0%, 0/2 |
0.002* |
SG sleeve gastrectomy; RF Rossetti fundoplication; PPIs proton pump inhibitor; EE erosive esophagitis; EGSD esophago-gastro-duodenoscopy; N number of patients analyzed when different from the total; n/Pts number of patients out of the total; *statistically significant
The finding of postoperative EE was not related to the preoperative endoscopic finding of small sliding HH (< 2 cm). Of the 12 patients with HH in the SG group, only one had postoperative grade B esophagitis, and among the 18 patients with HH in the SG + RF group, none had postoperative esophagitis. HH was not surgically repaired because it was < 2 cm and asymptomatic.
BMI variations and comorbidity improvements
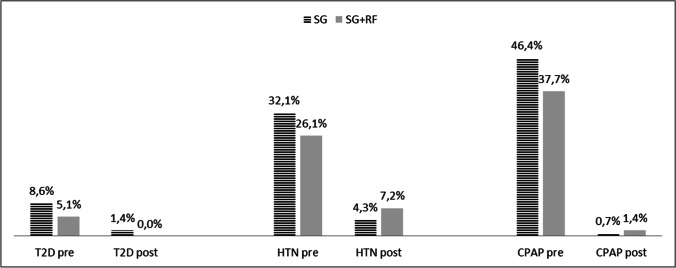
Two hundred fifty-one patients out of 278 (90.3%) had completed clinical follow-up at 12 months. One year after surgery, both groups reached a mean BMI < 30 kg/m2 (Fig. 1), with a %TWL > 20%, which is considered adequate [26]. The SG group had a %TWL of 35.4 ± 7.2%, with a mean BMI at 12 months of 29.1 ± 5.8 kg/m2. The SG + RF group had a %TWL of 32.2 ± 7.6%, with a mean BMI = 29.4 ± 5.0 kg/m2. Obstructive sleep apnea syndrome (OSAS), type 2 Diabetes (T2D), and hypertension (HTN) improved significantly 1 year after surgery (Fig. 2).
Fig. 1.
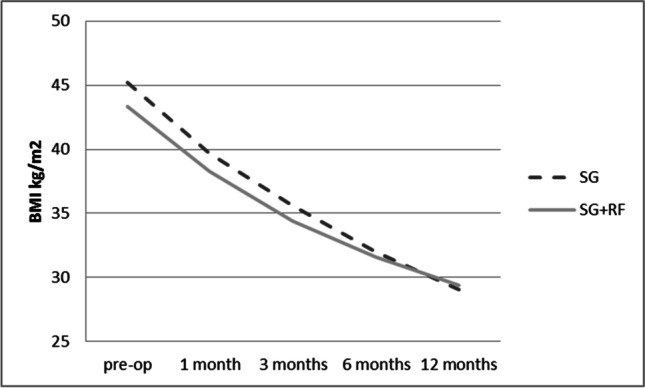
Variations of BMI before and after surgery are compared in the SG population and the SG + RF population. 126 patients out of 140 (90%) in the SG group and 125 out of 138 in the SG + RF group (90.6%) completed follow up. BMI = body mass index, SG = sleeve gastrectomy, SG + RF = sleeve gastrectomy + Rossetti fundoplication
Fig. 2.
Variations in co-morbidities measured as the necessity to assume at least 1 drug for pathology. The follow up was completed for 126/140 (90%) in the SG group and 125/138 in the SG + RF group (90.6%). T2D = type 2 diabetes, HTN = hypertension, CPAP = continuous positive airways pressure
Complications
Patients in the SG + RF group had a longer hospital stay than those in the SG group (Table 3). The percentage of reintervention for early and late complications was not different in the two groups. Both groups had a 0.7% of bleeding that needed laparoscopy and hemostasis on day 2 after surgery. Leakage, a well-known complication after sleeve, appeared in 1 out of 140 SG patients (0.7%), and never appeared in those with SG + RF. However, wrap perforation complicated 6 out of 138 patients who underwent SG + RF (4.3%). It did not occur in the patients who had had SG (p = 0.013).
Table 3.
Early and late surgical complications after SG and SG + RF
| Summary statistics | SG (n 140) | SG + RF (n 138) | p-value | |
|---|---|---|---|---|
| Hospital stay (days) | Mean ± SD | 3.1 ± 0.5 | 3.9 ± 4.0 | 0.0181* |
|
Early reintervention (< 1 month) Bleeding Leakage Wrap perforation |
%, n/Pts %, n/Pts %, n/Pts %, n/Pts |
1.4%, 2/140 0.7%, 1/140 0.7%, 1/140 0.0%, 0/140 |
5.2%, 7/138 0.7%, 1/138 0.0%, 0/138 4.3%, 6/138 |
0.0768 1.0000 0.3310 0.0133* |
|
Late reintervention (> 1 month) Weight regain GERD |
%, n/Pts %, n/Pts %, n/Pts |
0.7%, 1/140 0.0%, 0/140 0.7%, 1/140 |
1.4%, 2/138 1.4%, 2/138 0.0%, 0/138 |
0.5687 0.1608 0.3310 |
SG sleeve gastrectomy; RF Rossetti fundoplication; GERD gastro-esophageal reflux disease; n/Pts number of patients out of the total; *statistically significant
Leakage after SG was treated with an endogastric self-expandable metal stent (SEMS) placed endoscopically on day 5. On day 12, the SEMS was replaced, and a laparoscopic abdominal toilette and drainage was performed. The patient was discharged on day 32 after primary surgery. The SEMS was removed on day 39 with leakage resolution.
Wrap perforation was treated by laparoscopic valve resection. The mean time of presentation of wrap perforation was 2.4 ± 5.5 days. The diagnosis was performed by an abdominal CT scan with oral water-soluble contrast. The mean hospitalization time after revision surgery was 11.2 ± 2.6 days for 5 out of 6 patients (83.3%). One of the six patients had a longer hospital stay because he developed leakage after valve resection and needed SEMS placement on day 5. The SEMS was replaced on day 20 and was definitively removed on day 38 with leakage resolution.
Regarding late complications, one patient in the SG group needed a laparoscopic conversion to Roux-en-Y gastric bypass (RYGB) 28 months after primary surgery because of weight regain and GERD. In the SG + RF group, one patient needed a surgical revision 24 months after primary surgery because of valve disruption and weight regain, and underwent conversion to RYGB. Another patient had weight regain and underwent laparoscopic valve resection 28 months after primary surgery.
Conclusions
After a year of follow-up, SG + RF was effective both in terms of weight loss (Fig. 1) and prevention of de novo GERD (Table 2). A previous manuscript reported an improvement of preoperative GERD and esophagitis in patients who underwent SG + RF [22].
SG + RF had a longer operative time than normal SG: a mean of + 6.4 min when SG + RF was performed by an expert surgeon in both bariatric and GERD surgery (with already more than 100 SG + RF procedures), and a mean of + 12.9 min when SG + RF was performed by the rest of the surgical equipe.
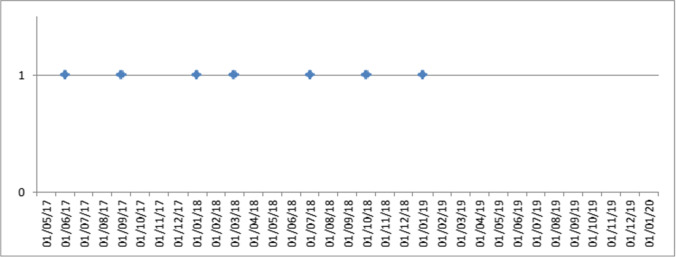
The group of patients who underwent SG + RF had a longer hospitalization than those who underwent SG (Table 3): respectively 3.9 ± 4.0 days vs 3.1 ± 0.5 days (p = 0.018). This was related to a higher frequency of wrap perforation after fundoplication (4.3%) than the classic leakage following normal SG (0.7%). Wrap perforation differed from leakage after SG and never occurred along the staple line. It seemed to be related to traumatism of the gastric serosa due to excessive manipulation of the fundus during fundoplication, and usually appeared within the first 2–3 days after surgery. The increased pressure in the wrap during feeding might overwhelm the serosa resistance. Perforation could also be associated to ischemic issues. For this reason, at the end of every fundoplication, the wrap was checked with ICG test. Presentation was usually pain irradiated to left shoulder, fever, and leukocytosis. CT scan with oral water-soluble contrast was diagnostic for perforation and peri-gastric collection. The therapeutic approach was laparoscopic resection of the valve associated with a toilette of the abdominal cavity. The rate of wrap perforation (4.3%), 6 times higher than classic leakage (0.7%), could be alarming, but it decreased with the learning curve of the surgeon (Fig. 3). In fact, during the last year of the study, from January 2019, no valve perforations were reported. In this period, 38 SG + RF were performed (27.5% of the total). Moreover, the hospital stay after wrap perforation and fundus resection was shorter (11.2 ± 2.6 days) than the postoperative course after leakage in SG (39 days). No classic leakages were seen after SG + RF. This could be explained by the valve protecting the higher part of the stomach. After revision surgery for wrap perforation, one leakage was reported.
Fig. 3.
Fundoplication perforation considering the trial’s time frame. Each point in the graph corresponds to an intervention. On the x-axis: date of intervention. On the y-axis: 0 = no perforation; 1 = perforation
While conducting this study, other than the efficacy of the surgical procedure itself, we noted the importance of the preoperative EGDS, even if it is not considered mandatory in the principal guidelines for bariatric surgery [24, 28, 29]. We found it essential for establishing the presence and the gravity of esophagitis, according to the LA classification. Moreover, from the literature, we know that more than 25% of patients without GERD symptoms may have unknown esophagitis [8]. The preoperative data about the condition of the EG junction mucosa may lead to the surgical choice of adding a fundoplication to SG, improving the postoperative condition of the patient.
Esophageal manometry associated with EGDS may be the best way to select the best treatment for each patient, since 76.6% of the patients who underwent SG did not have GERD after 1 year. For this reason, it is important to select that 23.4% of patients affected by obesity without preoperative GERD who may benefit from the association between SG and fundoplication, to prevent de novo GERD. SG + RF may be the correct intervention for patients with preoperative EE (> B grade), and, to avoid de novo GERD, for those without GERD who have a hypotonic lesser esophageal sphincter (LES) at the manometry. Patients without GERD and with a normal LES may be the best candidate for normal SG.
The limits of this study are the lack of manometric and 24 h-PH metry studies in describing GERD. The role of 24 h-PH metry in bariatric patients is still debated [30]. In fact, it is more complicated to perform and require a higher level of patient compliance.
Further studies to associate preoperative EGDS with manometric analysis are ongoing. These could be an important diagnostic aid for correctly deciding the best intervention for each patient, in order to provide a tailored approach. Further studies with longer follow-up are needed to evaluate long-term maintenance of weight loss and GERD improvement in patients undergoing SG + RF.
Declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
All the authors declare no competing interests.
Footnotes
Key points
• Adding Rossetti fundoplication to sleeve reduces development of de novo GERD.
• Patients undergoing sleeve + Rossetti fundoplication had an adequate weight loss.
•Sleeve + Rossetti fundoplication is safe after an adequate learning curve.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/24/2022
A Correction to this paper has been published: 10.1007/s11695-022-06013-z
Contributor Information
Stefano Olmi, Email: stefano.olmi@gmail.com.
Giovanni Cesana, Email: giovanni.cesana@gmail.com.
Angela Gambioli, Email: a.gambioli@campus.unimib.it.
Marta Bonaldi, Email: met.89@hotmail.it.
Davide Ferrari, Email: erraridav93@gmail.com.
Matteo Uccelli, Email: matteo.uccelli@gmail.com.
Francesca Ciccarese, Email: fra.ciccarese@gmail.com.
De Carli Stefano, Email: stefanomariadecarli@gmail.com.
Giorgi Riccardo, Email: riccardogio@yahoo.it.
Mantovani Lorenzo, Email: lorenzo.mantovani@unimib.it.
References
- 1.Angrisani L, Santonicola A, Iovino P, Vitiello A, Higa K, Himpens J, Buchwald H, Scopinaro N. IFSO Worldwide Survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;28(12):3783–3794. doi: 10.1007/s11695-018-3450-2. [DOI] [PubMed] [Google Scholar]
- 2.Yeung KTD, Penney N, Ashrafian L, Darzi A, Ashrafian H. Does sleeve gastrectomy expose the distal esophagus to severe reflux?: a systematic review and meta-analysis. Ann Surg. 2020;271(2):257–265. doi: 10.1097/SLA.0000000000003275. [DOI] [PubMed] [Google Scholar]
- 3.Braghetto I, Csendes A. Prevalence of Barrett’s esophagus in bariatric patients undergoing sleeve gastrectomy. Obes Surg. 2016;26(4):710–714. doi: 10.1007/s11695-015-1574-1. [DOI] [PubMed] [Google Scholar]
- 4.Felsenreich DM, Kefurt R, Schermann M, Beckerhinn P, Kristo I, Krebs M, Prager G, Langer FB. Reflux, sleeve dilation, and Barrett’s esophagus after laparoscopic sleeve gastrectomy: long-term follow-up. Obes Surg. 2017;27(12):3092–3101. doi: 10.1007/s11695-017-2748-9. [DOI] [PubMed] [Google Scholar]
- 5.Sharma A, Aggarwal S, Ahuja V, Bal C. Evaluation of gastroesophageal reflux before and after sleeve gastrectomy using symptom scoring, scintigraphy, and endoscopy. Surg Obes Relat Dis. 2014 Jul-Aug;10(4):600–5. 10.1016/j.soard.2014.01.017. Epub 2014 Jan 28. PMID: 24837563. [DOI] [PubMed]
- 6.Tai CM, Huang CK, Lee YC, Chang CY, Lee CT, Lin JT. Increase in gastroesophageal reflux disease symptoms and erosive esophagitis 1 year after laparoscopic sleeve gastrectomy among obese adults. Surg Endosc. 2013;27(4):1260–1266. doi: 10.1007/s00464-012-2593-9. [DOI] [PubMed] [Google Scholar]
- 7.Mion F, Tolone S, Garros A, Savarino E, Pelascini E, Robert M, Poncet G, Valette PJ, Marjoux S, Docimo L, Roman S. High-resolution impedance manometry after sleeve gastrectomy: increased intragastric pressure and reflux are frequent events. Obes Surg. 2016;26(10):2449–2456. doi: 10.1007/s11695-016-2127-y. [DOI] [PubMed] [Google Scholar]
- 8.Genco A, Soricelli E, Casella G, Maselli R, Castagneto-Gissey L, Di Lorenzo N, Basso N. Gastroesophageal reflux disease and Barrett’s esophagus after laparoscopic sleeve gastrectomy: a possible, underestimated long-term complication. Surg Obes Relat Dis. 2017;13(4):568–574. doi: 10.1016/j.soard.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Soricelli E, Casella G, Baglio G, Maselli R, Ernesti I, Genco A. Lack of correlation between gastroesophageal reflux disease symptoms and esophageal lesions after sleeve gastrectomy. Surg Obes Relat Dis. 2018;14(6):751–756. doi: 10.1016/j.soard.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds JL, Zehetner J, Shiraga S, Lipham JC, Katkhouda N. Intraoperative assessment of the effects of laparoscopic sleeve gastrectomy on the distensibility of the lower esophageal sphincter using impedance planimetry. Surg Endosc. 2016;30(11):4904–4909. doi: 10.1007/s00464-016-4829-6. [DOI] [PubMed] [Google Scholar]
- 11.Coupaye M, Gorbatchef C, Calabrese D, Sami O, Msika S, Coffin B, Ledoux S. Gastroesophageal reflux after sleeve gastrectomy: a prospective mechanistic study. Obes Surg. 2018;28(3):838–845. doi: 10.1007/s11695-017-2942-9. [DOI] [PubMed] [Google Scholar]
- 12.van Rijn S, Rinsma NF, van Herwaarden-Lindeboom MY, Ringers J, Gooszen HG, van Rijn PJ, Veenendaal RA, Conchillo JM, Bouvy ND, Masclee AA. Effect of vagus nerve integrity on short and long-term efficacy of antireflux surgery. Am J Gastroenterol. 2016;111(4):508–515. doi: 10.1038/ajg.2016.42. [DOI] [PubMed] [Google Scholar]
- 13.Pallati PK, Shaligram A, Shostrom VK, Oleynikov D, McBride CL, Goede MR. Improvement in gastroesophageal reflux disease symptoms after various bariatric procedures: review of the bariatric outcomes longitudinal database. Surg Obes Relat Dis. 2014 May-Jun;10(3):502–7. 10.1016/j.soard.2013.07.018. Epub 2013 Aug 29. PMID: 24238733. [DOI] [PubMed]
- 14.Garay M, Balagué C, Rodríguez-Otero C, Gonzalo B, Domenech A, Pernas JC, Gich IJ, Miñambres I, Fernández-Ananín S, Targarona EM. Influence of antrum size on gastric emptying and weight-loss outcomes after laparoscopic sleeve gastrectomy (preliminary analysis of a randomized trial) Surg Endosc. 2018;32(6):2739–2745. doi: 10.1007/s00464-017-5972-4. [DOI] [PubMed] [Google Scholar]
- 15.Olmi S, Caruso F, Uccelli M, Cioffi S, Ciccarese F, Cesana G. Laparoscopic sleeve gastrectomy combined with Rossetti fundoplication (R-Sleeve) for treatment of morbid obesity and gastroesophageal reflux. Surg Obes Relat Dis. 2017;13(12):1945–1950. doi: 10.1016/j.soard.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Nocca D, Nedelcu M, Loureiro M, Palermo M, Silvestri M, Jong A, Ramos A. The Nissen sleeve gastrectomy: technical considerations. J Laparoendosc Adv Surg Tech A. 2020;30(11):1231–1236. doi: 10.1089/lap.2020.0651. [DOI] [PubMed] [Google Scholar]
- 17.Şen O, Türkçapar AG. Combined partial posterior fundoplication with laparoscopic sleeve gastrectomy for morbid obese patients with symptomatic GERD. Video case report. Int J Surg Case Rep. 2020;71:34–36. 10.1016/j.ijscr.2020.04.016. Epub 2020 May 8. PMID: 32428830; PMCID: PMC7235945. [DOI] [PMC free article] [PubMed]
- 18.Del Genio G, Tolone S, Gambardella C, Brusciano L, Volpe ML, Gualtieri G, Del Genio F, Docimo L. Sleeve gastrectomy and anterior fundoplication (D-SLEEVE) prevents gastroesophageal reflux in symptomatic GERD. Obes Surg. 2020;30(5):1642–1652. doi: 10.1007/s11695-020-04427-1. [DOI] [PubMed] [Google Scholar]
- 19.Palermo M, Serra E, Duza G. N-sleeve gastrectomy: an option for obesity and GERD. Arq Bras Cir Dig. 2019;32(4):e1482. doi: 10.1590/0102-672020190001e1482.PMID:31859934;PMCID:PMC6918749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lasnibat JP, Braghetto I, Gutierrez L, Sanchez F. Sleeve gastrectomy and fundoplication as a single procedure in patients with obesity and gastroesophageal reflux. Arq Bras Cir Dig. 2017 Jul-Sep;30(3):216–221. 10.1590/0102-6720201700030012. PMID: 29019565; PMCID: PMC5630217. [DOI] [PMC free article] [PubMed]
- 21.Geagea T. Laparoscopic Nissen-Rossetti fundoplication. Surg Endosc. 1994;8(9):1080–1084. doi: 10.1007/BF00705724. [DOI] [PubMed] [Google Scholar]
- 22.Olmi S, Cesana G, D'Angiolella L, Bonaldi M, Uccelli M, Mantovani L. Sleeve gastrectomy with tailored 360° fundoplication according to Rossetti in patients affected by obesity and gastroesophageal reflux: a prospective observational study. Surg Obes Relat Dis. 2021 Jan 21:S1550–7289(21)00033–2. 10.1016/j.soard.2021.01.007. Epub ahead of print. PMID: 33622604. [DOI] [PubMed]
- 23.Olmi S, David G, Cesana G, Ciccarese F, Giorgi R, De Carli S, Uccelli M. Modified sleeve gastrectomy combined with laparoscopic Rossetti fundoplication and vascularization assessment with indocyanine green. Obes Surg. 2019;29(9):3086–3088. doi: 10.1007/s11695-019-03970-w. [DOI] [PubMed] [Google Scholar]
- 24.Foschi D, De Luca M, Sarro G, et al. Linee guida di Chirurgia dell’Obesità. Edizione 2016. SICOB.
- 25.Navarra, G., Komaei, I., Currò, G. et al. Bariatric surgery and the COVID-19 pandemic: SICOB recommendations on how to perform surgery during the outbreak and when to resume the activities in phase 2 of lockdown. Updates Surg 72, 259–268 (2020). https://doi-org.pros.lib.unimi.it/10.1007/s13304-020-00821-7 [DOI] [PMC free article] [PubMed]
- 26.Corcelles R, Boules M, Froylich D, Hag A, Daigle CR, Aminian A, Brethauer SA, Burguera B, Schauer PR. Total weight loss as the outcome measure of choice after Roux-en-Y gastric bypass. Obes Surg. 2016;26(8):1794–1798. doi: 10.1007/s11695-015-2022-y. [DOI] [PubMed] [Google Scholar]
- 27.Małczak P, Pisarska-Adamczyk M, Zarzycki P, Wysocki M, Major P. Hiatal hernia repair during laparoscopic sleeve gastrectomy: systematic review and meta-analysis on gastroesophageal reflux disease symptoms changes. Pol Przegl Chir. 2021;93(5):1–5. doi: 10.5604/01.3001.0014.9356. [DOI] [PubMed] [Google Scholar]
- 28.Campos GM, Mazzini GS, Altieri MS, Docimo S Jr, DeMaria EJ, Rogers AM; Clinical Issues Committee of the American Society for Metabolic and Bariatric Surgery. ASMBS position statement on the rationale for performance of upper gastrointestinal endoscopy before and after metabolic and bariatric surgery. Surg Obes Relat Dis. 2021 May;17(5):837–847. 10.1016/j.soard.2021.03.007. Epub 2021 Mar 19. PMID: 33875361. [DOI] [PubMed]
- 29.Fisher OM, Chan DL, Talbot ML, Ramos A, Bashir A, Herrera MF, Himpens J, Shikora S, Higa KD, Kow L, Brown WA. Barrett’s oesophagus and bariatric/metabolic surgery-IFSO 2020 position statement. Obes Surg. 2021;31(3):915–934. doi: 10.1007/s11695-020-05143-6. [DOI] [PubMed] [Google Scholar]
- 30.Assalia A, Gagner M, Nedelcu M, Ramos AC, Nocca D. Gastroesophageal reflux and laparoscopic sleeve gastrectomy: results of the First International Consensus Conference. Obes Surg. 2020;30(10):3695–3705. doi: 10.1007/s11695-020-04749-0. [DOI] [PubMed] [Google Scholar]