Abstract
The dynamin-like GTPases Mitofusin 1 and 2 (Mfn1 and Mfn2) are essential for mitochondrial function, which has been principally attributed to their regulation of fission/fusion dynamics. Here, we report that Mfn1 and 2 are critical for glucose-stimulated insulin secretion (GSIS) primarily through control of mitochondrial DNA (mtDNA) content. Whereas Mfn1 and Mfn2 individually were dispensable for glucose homeostasis, combined Mfn1/2 deletion in β-cells reduced mtDNA content, impaired mitochondrial morphology and networking, and decreased respiratory function, ultimately resulting in severe glucose intolerance. Importantly, gene dosage studies unexpectedly revealed that Mfn1/2 control of glucose homeostasis was dependent on maintenance of mtDNA content, rather than mitochondrial structure. Mfn1/2 maintain mtDNA content by regulating the expression of the crucial mitochondrial transcription factor Tfam, as Tfam overexpression ameliorated the reduction in mtDNA content and GSIS in Mfn1/2-deficient β-cells. Thus, the primary physiologic role of Mfn1 and 2 in β-cells is coupled to the preservation of mtDNA content rather than mitochondrial architecture, and Mfn1 and 2 may be promising targets to overcome mitochondrial dysfunction and restore glucose control in diabetes.
Subject terms: Diabetes, Mitochondria, Type 2 diabetes
Sidarala et al. examine the importance of the mitochondrial structural proteins, Mitofusins 1 and 2 (Mfn1/2), in diabetes. They find that Mfn1/2 control blood glucose by preserving mitochondrial DNA content, rather than mitochondrial structure.
Introduction
Mitochondrial dynamics, the balance of fusion and fission of mitochondrial networks, is essential to control mitochondrial structure. Mitochondrial fission and fusion are governed by several key proteins that maintain mitochondrial quality control1,2. Fission is primarily regulated by the GTPase dynamin-related protein 1 (Drp1), while fusion is controlled by both outer and inner mitochondrial membrane machinery. The dynamin-related large GTPase optic atrophy protein 1 (Opa1) controls inner membrane fusion, while outer membrane fusion is controlled by two GTPases known as Mitofusin 1 and 2 (Mfn1 and Mfn2). Mitofusin 1 and 2 share ~80% sequence similarity and contain homologous functional domains, suggesting they play both overlapping and unique roles in metabolic function largely ascribed to their control of mitochondrial structure3.
Imbalances in mitochondrial dynamics in metabolically active tissues have been implicated in many human diseases, including neurodegenerative conditions, cancer, cardiovascular disease, and diabetes2. Mitochondria provide the energy necessary for β-cell insulin release4,5, and abnormalities in mitochondrial structure and bioenergetics have been observed in the β-cells of humans with type 2 diabetes (T2D6,7). Further, mitochondrial defects have been recently found to precede the development of T2D in human β-cells8. Thus, strategies to understand and overcome mitochondrial dysfunction in β-cells are of great appeal for the treatment of diabetes. However, the role of the core fission/fusion machinery in β-cells is less clear. Reduced β-cell Mfn2 expression has been observed secondary to the deposition of toxic islet amyloid polypeptide oligomers observed in T2D as well as in mouse models of T2D9,10, yet the functions of Mfn1 and Mfn2 in β-cells in vivo are unexplored.
Here, we elucidate a key physiologic role for Mfn1 and 2 in the maintenance of glucose homeostasis that unexpectedly occurs through the preservation of β-cell mtDNA content, rather than mitochondrial structure. Utilizing several genetic mouse models, high resolution imaging, and transcriptomic profiling, we show that Mfn1 and 2 act in tandem to preserve β-cell mitochondrial health and, consequently, glucose-stimulated insulin secretion (GSIS). Mfn1 and 2 maintain mtDNA content by preventing loss of the mitochondrial transcription factor Tfam, which is a master regulator of mtDNA copy number control and mtRNA expression11,12. Indeed, adenoviral overexpression of Tfam ameliorates reductions in mtDNA content and GSIS in Mfn1/2-deficient β-cells, illustrating the critical importance of mtDNA copy number control by Mfn1/2 to promote glucose homeostasis.
Results
Combined deficiency of Mfn1 and Mfn2 in β-cells leads to severe glucose intolerance and reduced glucose-stimulated insulin secretion
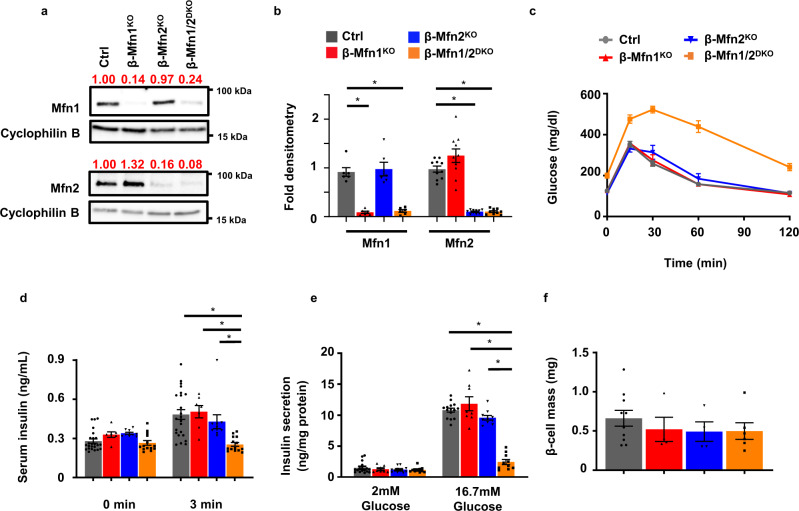
Mitochondrial health is vital to support β-cell insulin secretory responses to glucose or other nutrient stimuli. We hypothesized that Mfn1 and Mfn2 are required for GSIS in β-cells through their regulation of mitochondrial function. To test this hypothesis, we generated mice bearing β-cell specific deletion of Mfn1 alone (Mfn1loxP/loxP; Ins1-Cre), Mfn2 alone (Mfn2loxP/loxP; Ins1-Cre), and combined deletion of both Mfn1 and 2 (Mfn1loxP/loxPMfn2loxP/loxP; Ins1-Cre), hereafter known as β-Mfn1KO, β-Mfn2KO, and β-Mfn1/2DKO mice, respectively. Conditional alleles targeted the canonical G-1 GTPase motif of Mfn1 (exon 4) or Mfn2 (exon 6) (Fig. S1a13). Ins1-Cre and floxed alone controls were phenotypically indistinguishable from one another, exhibiting no differences in body weight or glycemic control (Fig. S1b, c). Therefore, they were pooled as controls for subsequent analyses. β-Mfn1KO, β-Mfn2KO, and β-Mfn1/2DKO mice each exhibited an efficient reduction in Mfn1 and/or Mfn2 islet protein expression when compared to littermate controls (Ctrl; Fig. 1a, b). We did not observe significant compensatory changes in Mfn2 expression in β-Mfn1KO islets, nor in Mfn1 expression in β-Mfn2KO islets (Fig. 1a, b). Further, we did not observe differences in expression of the other core fission/fusion proteins, Drp1 and Opa1, following deletion of Mfn1 and/or Mfn2 (Fig. S1d).
Fig. 1. Loss of both Mfn1 and Mfn2 in β-cells impairs glucose tolerance and glucose-stimulated insulin release.
a Expression of Mfn1 and Mfn2 by Western blot (WB) in islets isolated from 8-10-week-old Ctrl, β-Mfn1KO, β-Mfn2KO and β-Mfn1/2DKO mice. Cyclophilin B serves as a loading control. Representative of six independent mice/group for Mfn1 and 10 independent mice/group for Mfn2. Band intensity of each lane of displayed representative blot (shown as fold change compared to Ctrl, expression normalized to cyclophilin B) above each blot in red. b Densitometry (normalized to cyclophilin B) from studies in Fig. 1A. n = 6/group for Mfn1 and 10/group for Mfn2. Data are presented as Mean ± SEM. *p < 0.0005 by one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. c Blood glucose concentrations measured during IPGTT of 8-week-old Ctrl (n = 28), β-Mfn1KO (n = 7), β-Mfn2KO (n = 6) and β-Mfn1/2DKO (n = 15) littermates. Data are presented as Mean ± SEM. (p < 0.0005 by two-way ANOVA for β-Mfn1/2DKO mice vs Ctrl followed by Sidak’s post-hoc test for multiple comparisons). d Serum insulin concentrations (n = 7-24/group) measured during in vivo glucose-stimulated insulin release testing in 8-week-old Ctrl (n = 24), β-Mfn1KO (n = 7), β-Mfn2KO (n = 10) and β-Mfn1/2DKO (n = 14) littermates. Data are presented as mean ± SEM. *p < 0.0005 for β-Mfn1/2DKO mice vs Ctrl and β-Mfn1KO, p = 0.004 for β-Mfn1/2KO vs β-Mfn2KO by one-way ANOVA followed by Sidak’s post-hoc test for multiple comparisons. e Glucose-stimulated insulin secretion following static incubations in 2 mM and 16.7 mM glucose, performed in isolated islets of 8-week-old Ctrl (n = 16), β-Mfn1KO (n = 8), β-Mfn2KO (n = 11) and β-Mfn1/2DKO (n = 10) littermates. Data are presented as mean from independent mice ± SEM. *p < 0.0005 by one-way ANOVA followed by Sidak’s post-hoc test for multiple comparisons. f Pancreatic β-cell mass measured in 10-week-old Ctrl (n = 10), β-Mfn1KO (n = 4), β-Mfn2KO (n = 4) and β-Mfn1/2DKO (n = 6) littermates. Data are presented as mean ± SEM. Uncropped western blots and source data are provided as a Source Data file.
While loss of either Mfn1 or Mfn2 alone has been shown to impair glucose homeostasis in other metabolic tissues3,14, we were surprised to find that both β-Mfn1KO and β-Mfn2KO mice exhibited normal glucose homeostasis during an intraperitoneal glucose tolerance test (IPGTT; Fig. 1c). We also observed no changes in insulin secretion in single β-Mfn1KO or β-Mfn2KO mice after glucose administration in vivo (Fig. 1d) or in isolated islets (Fig. 1e). However, combined deletion of both Mfn1 and Mfn2 resulted in severe glucose intolerance (Fig. 1c; p < 0.005 by one-way ANOVA vs. Ctrl), due to markedly reduced GSIS (Figs. 1d, e and S1e).
To evaluate other etiologies of glucose intolerance in β-Mfn1/2DKO mice, we first measured β-cell mass. We observed no significant differences in β-cell mass between genotypes (Fig. 1f). There were also no differences in body weight or peripheral insulin sensitivity between the groups (Fig. S1f, g). Taken together, these studies suggest that Mfn1 and 2 are individually dispensable for glycemic control and GSIS, but together play vital complementary roles for the maintenance of glucose tolerance and β-cell insulin release.
Mfn1 and 2 regulate β-cell mitochondrial structure and respiratory function
Given the connections between mitochondrial function and dynamics, we asked whether mitochondrial respiration and architecture would be defective in the islets of β-Mfn1/2DKO mice, as has been reported in other models of Mfn1/2-deficiency15–19. Indeed, islets isolated from β-Mfn1/2DKO mice displayed a reduced glucose-stimulated oxygen consumption rate (OCR; Figs. 2a and S2a) when compared to Ctrl islets. We did not observe differences in glycolysis as measured by extracellular acidification rate (ECAR; Fig. S2a). Differences in glucose-stimulated OCR between Ctrl and β-Mfn1/2DKO islets were no longer observed following exposure to the Complex III inhibitor antimycin A, suggesting that Mfn1/2-deficient islets primarily possess a defect in β-cell mitochondrial respiration (Figs. 2a and S2a).
Fig. 2. Combined Mfn1 and Mfn2 deficiency impairs mitochondrial respiration and structure in β-cells.
a OCR measured following exposure to 2 mM glucose, 20 mM glucose, and 2 µM antimycin A in islets isolated from 8-10-week-old Ctrl (n = 9) and β-Mfn1/2DKO (n = 8) mice by a Seahorse flux analyzer. Data are presented as mean from independent mice ± SEM. *p = 0.0176 by one-way ANOVA followed by Sidak’s post-hoc test for multiple comparisons. b Representative transmission EM images of β-cells from 12-week-old Ctrl, β-Mfn1KO, β-Mfn2KO and β-Mfn1/2DKO littermates. n = 3/group. Mitochondria are highlighted with red arrows. c Quantification of mitochondrial morphology in Ctrl, β-Mfn1KO, β-Mfn2KO, and β-Mfn1/2DKO β-cells from transmission EM images (∼125 independent mitochondria scored/animal). n = 3/group. Data are presented as mean ± SEM. *p = 0.0087 for Aspect Ratio, p = 0.004 for Form factor and Circularity, p = 0.037 for Perimeter by one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. d Imaris® generated three-dimensional reconstruction of deconvolution immunofluorescence Z-stack images at 100× magnification stained for SDHA (see inset image—red) from pancreatic sections of Ctrl and β-Mfn1/2DKO mice. β-cells were identified by insulin co-staining (inset: insulin—green). Each unique color represents a separate β-cell mitochondrial network cluster. Representative image of six independent mice/group. e β-cell mitochondrial morphology and network analysis of deconvolution immunofluorescence Z-stack images at 100× magnification stained for SDHA (and insulin) from pancreatic sections of Ctrl, β-Mfn1KO, β-Mfn2KO and β-Mfn1/2DKO mice by MitoAnalyzer. n = 6/group (∼150 β-cells/animal were quantified). Data are presented as mean ± SEM. *p = 0.0006 for Average mitochondrial volume, p = 0.0089 for Sphericity, p = 0.0032 for Branches per mitochondria, p = 0.0039 for Branch junctions by one-way ANOVA followed by Sidak’s post-hoc test for multiple comparisons. Source data are provided as a Source Data file.
Loss of key mediators of mitochondrial fusion disrupt mitochondrial network balance and favor increased mitochondrial fission, often leading to the appearance of increased punctate or spherical mitochondria20. We thus examined mitochondrial structure in Mfn1- and/or Mfn2-deficient β-cells by several independent and complementary methods. First, we assessed β-cell mitochondrial ultrastructure by transmission electron microscopy (TEM). While β-cells from all groups had the expected appearance of electron dense insulin granules, β-cells from β-Mfn1/2DKO mice had reductions in mitochondrial aspect ratio, form factor, and perimeter, and increased mitochondrial circularity (Fig. 2b, c), indicating smaller, punctate mitochondria. We observed no overt differences in ER morphology and proximity to mitochondria in β-cells from control and β-Mfn1/2DKO mice (Fig. S2b), however.
Secondly, we examined mitochondrial networking utilizing high-resolution deconvolution imaging of the mitochondrial marker succinate dehydrogenase A (SDHA). We initially used Imaris® imaging software to generate three-dimensional (3D) reconstructions of β-cell mitochondria to grossly evaluate mitochondrial networking21,22. Consistent with our findings using TEM, the number of 3D mitochondrial networks appeared similar between Ctrl and single β-Mfn1KO or β-Mfn2KO β-cells (Fig. S2c). However, Mfn1/2-double deficient mice showed a dramatic increase in the frequency of smaller β-cell mitochondrial networks (Fig. 2d).
Next, we analyzed 3D deconvolution imaging of β-cell SDHA using the Mitochondria Analyzer, a validated and unbiased quantification tool used to evaluate mitochondrial morphology and network/branch complexity22,23. This confirmed that measures of mitochondrial morphology (reduced mitochondrial volume and increased sphericity) and network/branch complexity (reduced branch number and branch junctions) were significantly impaired in β-Mfn1/2DKO mice, commensurate with an increase in punctate/spherical mitochondria due to decreased mitochondrial fusion (Fig. 2e). Together, these data demonstrate that together Mfn1 and 2 maintain β-cell mitochondrial structure and function.
Mfn1 and 2 maintain β-cell mtDNA content
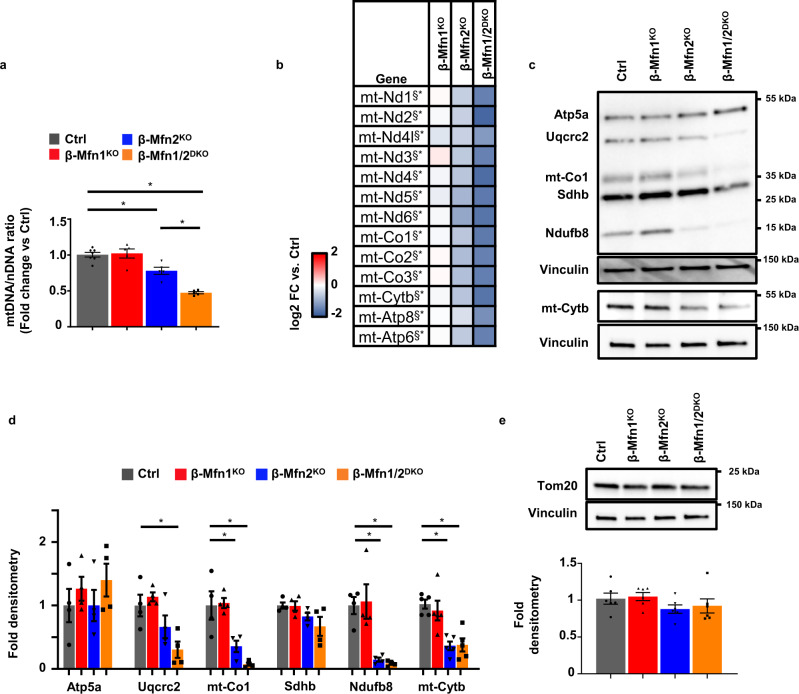
Mitochondrial DNA copy number is vital for β-cell function and glucose homeostasis24,25. Thus, we next examined whether mtDNA content was altered by loss of Mfn1 and/or Mfn2 in β-cells. We observed a small, yet significant, reduction in mtDNA content in islets of β-Mfn2KO mice that was not observed in islets of β-Mfn1KO mice (Fig. 3a). Interestingly, mtDNA depletion substantially worsened in the islets of β-Mfn1/2DKO mice (Fig. 3a), suggesting that loss of Mfn1 exacerbates the depletion of mtDNA caused by Mfn2 deficiency. We next assessed expression of the 13 mitochondrially encoded genes and observed a consistent and significant reduction in mtRNA expression in β-Mfn2KO islets that was exacerbated in β-Mfn1/2DKO mice (Fig. 3b). Concordantly, examination of protein expression of mitochondrial OXPHOS subunits by Western blot revealed reductions of the mitochondrially encoded subunits of Complex IV, mt-Co1, and Complex III, mt-Cytb, in β-Mfn2KO islets, and β-Mfn1/2DKO islets (Fig. 3c, d). We also observed significant reductions of nuclear encoded subunits of Complex I (Ndufb8) in both β-Mfn2KO and β-Mfn1/2DKO islets and Complex III (Uqcrc2) in β-Mfn1/2DKO islets, but no significant depletion of nuclear encoded Complex II (Sdhb) and Complex V (Atp5a) (Fig. 3c, d). Depletion of mtDNA content in β-Mfn1/2DKO islets did not appear to be secondary to changes in mitochondrial mass, as we did not observe reductions in Atp5a (Fig. 3c, d) or in the mitochondrial outer membrane protein Tom20 (Fig. 3e), a common marker of mitochondrial mass26. These studies reveal that both Mfn1 and 2 (and Mfn2 alone to a lesser degree) are necessary to maintain mtDNA copy number in β-cells.
Fig. 3. Mfn1/2-deficient β-cells exhibit reduced mtDNA content.
a Relative mtDNA content measured by qPCR (normalized to nuclear DNA expression) in isolated islets of 8-week-old Ctrl (n = 7), β-Mfn1KO (n = 5), β-Mfn2KO (n = 5) and β-Mfn1/2DKO (n = 5) littermates. Data are presented as mean ± SEM. *p = 0.0037 for β-Mfn2KO vs Ctrl, p < 0.0005 for β-Mfn1/2DKO vs Ctrl by one-way ANOVA followed by Sidak’s post-hoc test for multiple comparisons. b Heatmap representing relative expression (compared to littermate controls; Ctrl) of mitochondrial encoded transcripts in islets isolated from β-Mfn1KO (n = 4), β-Mfn2KO (n = 5) and β-Mfn1/2DKO (n = 5) mice. §* Benjamini-Hochberg FDR (Padj) < 0.05 comparing β-Mfn2KO (§) and β-Mfn1/2DKO (*) to Ctrl. c Expression of OXPHOS subunit proteins by WB in islets isolated from 8 to 10-week-old Ctrl, β-Mfn1KO, β-Mfn2KO, and β-Mfn1/2DKO mice. Representative of four independent mice/group. d OXPHOS subunit densitometry (normalized to Vinculin) from studies in Fig. 3D. n = 4/group; Data are presented as mean ± SEM. *p = 0.02 for Uqcrc2, p < 0.05 for mt-co1, p < 0.001 for Ndufb8, p < 0.005 for mt-Cytb by one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. e Expression of Tom20 by WB in islets isolated from 8 to 10-week-old Ctrl, β-Mfn1KO, β-Mfn2KO, and β-Mfn1/2DKO mice. Representative image (top) of six independent mice/group. Tom20 densitometry, normalized to vinculin (bottom). n = 6/group. Data are presented as mean ± SEM. Uncropped western blots and source data are provided as a Source Data file.
Defects in β-cell mitochondrial fusion induce glucose intolerance upon loss of mtDNA content
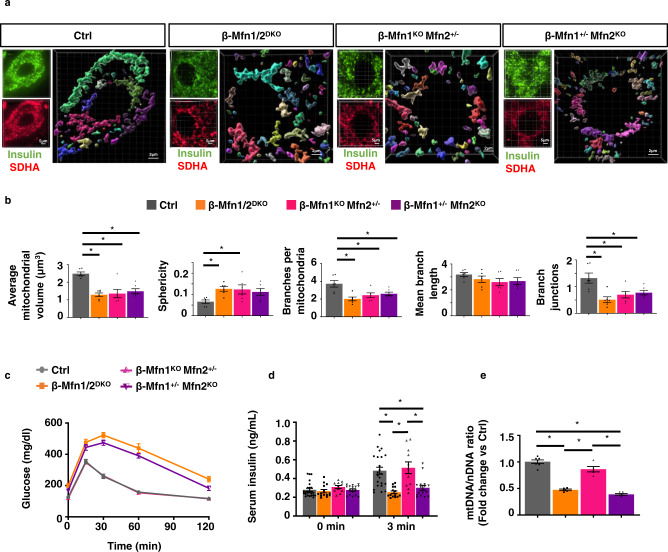
Our observations showing distinct and overlapping contributions of Mfn1 and 2 in the regulation of β-cell mitochondrial function led us to hypothesize that loss of mitochondrial fusion would lead to glucose intolerance due to mtDNA depletion. However, approaches to clarify the specific contribution of mtDNA depletion to metabolic dysfunction following imbalances in mitochondrial dynamics have been elusive. To this end, we generated animals harboring only a single functional allele of either Mfn1 (β-Mfn1+/−Mfn2KO) or Mfn2 (β-Mfn1KOMfn2+/−) to ascertain the relative importance of mtDNA content and structure in the maintenance of glycemic control. For robustness of interpretation, these experiments were performed alongside littermate controls and β-Mfn1/2DKO mice as previously presented (Figs. 1–3). As expected, neither β-Mfn1+/−Mfn2KO nor β-Mfn1KOMfn2+/− mice developed changes in β-cell mass, body weight, or insulin sensitivity (Fig. S3a–c). Interestingly, 3D quantification of mitochondrial morphology and network integrity revealed that, similar to β-Mfn1/2DKO mice, a single allele of either Mfn1 or Mfn2 was not sufficient to maintain β-cell mitochondrial structure, leading to increases in punctate mitochondria consistent with loss of mitochondrial fusion (Fig. 4a, b).
Fig. 4. A single allele of Mfn2, but not Mfn1, maintains glycemic control by preserving β-cell mtDNA content despite impaired mitochondrial structure.
a Imaris® generated three-dimensional reconstruction of deconvolution immunofluorescence Z-stack images at 100X magnification stained for SDHA (see inset image—red) from pancreatic sections of Ctrl, β-Mfn1/2DKO, β-Mfn1KOMfn2+/− and β-Mfn1+/−Mfn2KO mice. β-cells were identified by insulin co-staining (inset: insulin—green). Each unique color represents a separate β-cell mitochondrial network cluster. Representative image of six independent mice/group. b β-cell mitochondrial morphology and network analysis of deconvolution immunofluorescence Z-stack images from pancreatic sections stained for insulin and SDHA of Ctrl, β-Mfn1/2DKO, β-Mfn1KOMfn2+/−, and β-Mfn1+/−Mfn2KO mice by MitoAnalyzer. n = 6/group (50–200 β-cells/animal were quantified). *p = 0.005 for Average mitochondrial volume, p = 0.05 for Sphericity, p = 0.05 for Branches per mitochondria, p = 0.05 for Branch junctions by one-way ANOVA followed by Sidak’s post-hoc test for multiple comparisons. c Blood glucose concentrations measured during IPGTT of 8-week-old Ctrl (n = 28), β-Mfn1/2DKO (n = 15), β-Mfn1KOMfn2+/− (n = 13), and β-Mfn1+/−Mfn2KO (n = 17) littermates. (p < 0.0005 by two-way ANOVA for β-Mfn1+/−Mfn2KO and β-Mfn1/2DKO mice vs Ctrl followed by Sidak’s post-hoc test for multiple comparisons). d Serum insulin concentrations (n = 11–24/group) measured during in vivo glucose-stimulated insulin release testing in 8-week-old Ctrl (n = 24), β-Mfn1/2DKO (n = 14), β-Mfn1KOMfn2+/− (n = 11) and β-Mfn1+/−Mfn2KO (n = 16) littermates. *p < 0.0005 by one-way ANOVA followed by Sidak’s post-hoc test for multiple comparisons. e Relative mtDNA content measured by qPCR (normalized to nuclear DNA expression) in isolated islets of 8-week-old Ctrl (n = 7), β-Mfn1/2DKO (n = 5), β-Mfn1KOMfn2+/− (n = 5) and β-Mfn1+/−Mfn2KO (n = 5) littermates. Data are presented as mean ± SEM. *p < 0.0005 by one-way ANOVA followed by Sidak’s post-hoc test for multiple comparisons. Of note, studies in Ctrl and β-Mfn1/2DKO mice were performed together alongside all β-Mfn1KO, β-Mfn2KO, β-Mfn1KOMfn2+/− (n = 11) and β-Mfn1+/−Mfn2KO (n = 16) littermates and thus may appear twice for purposes of relevant comparisons (in Figs. 1C, D, 2D, E, 3A, and again in Fig. 4A–E). Source data are provided as a Source Data file.
Despite mitochondrial morphologic imbalances in both β-Mfn1+/−Mfn2KO and β-Mfn1KOMfn2+/− mice, only β-Mfn1+/−Mfn2KO mice developed glucose intolerance and impaired GSIS similar to that of β-Mfn1/2DKO mice (Fig. 4c, d). We also found significant reductions in mtDNA content associated with the loss of GSIS observed in β-Mfn1+/−Mfn2KO and β-Mfn1/2DKO mice (Fig. 4e). Thus, maintenance of a single functional allele of Mfn2, but not Mfn1, is able to preserve glucose homeostasis, mtDNA content, and insulin secretion, despite being insufficient to maintain β-cell mitochondrial network integrity. Importantly, these results disjoin β-cell function from changes in mitochondrial morphology, and implicate mtDNA copy number as the vital purveyor of glycemic control following loss of mitochondrial fusion.
Mfn1 and 2 deficiency induces a transcriptional signature consistent with defects in mitochondrial metabolism and alterations in mtDNA replication
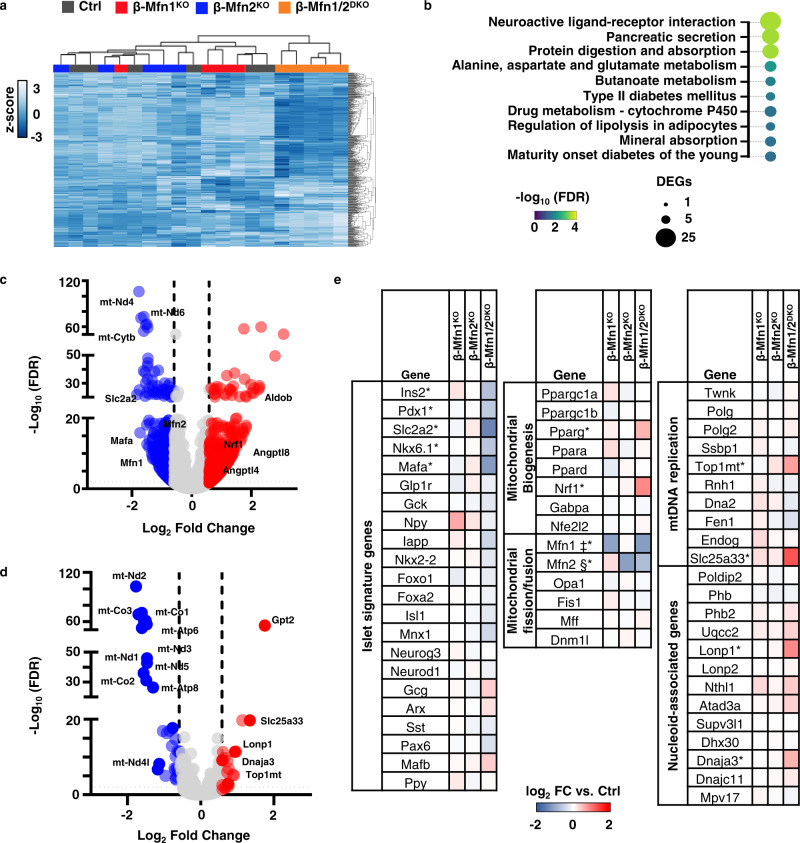
To begin to understand the mechanisms underlying control of mtDNA content by Mfn1 and 2, we analyzed bulk RNA sequencing (RNAseq) data generated from islets of β-Mfn1KO, β-Mfn2KO, and β-Mfn1/2DKO mice as well as littermate controls. Initial hierarchical clustering analyses based on the top 500 most highly expressed genes revealed that only β-Mfn1/2DKO islets clustered by genotype, while we did not observe discrete clustering across Ctrl or single β-Mfn1KO and β-Mfn2KO islets due to very few differences in expression between these groups (Figs. 5a and S4a, b). Gene ontology and pathway analyses of differentially expressed genes from β-Mfn1/2DKO islets revealed significant changes related to endocrine hormone/insulin secretion, β-cell signature genes, as well as several metabolic pathways (Figs. 5b and S4c, d), consistent with the defects in β-cell function and mitochondrial metabolism observed in β-Mfn1/2DKO mice.
Fig. 5. Mfn1/2-deficiency induces expression of genes associated with mtDNA replication.
a Hierarchical clustering of RNAseq samples displaying the 500 genes with the highest mean expression in islets of 12-week-old Ctrl (n = 6), β-Mfn1KO (n = 4), β-Mfn2KO (n = 5) and β-Mfn1/2DKO (n = 5) mice. b Pathway analysis of top differentially regulated pathways in β-Mfn1/2DKO islets (n = 5) compared to littermate Ctrl islets (n = 6), demarcated by both false discovery rate (FDR) and the number of significantly differentially expressed genes (DEGs) per pathway. c Volcano plot depicting differential RNA expression in islets of β-Mfn1/2DKO (n = 5) mice compared to littermate Ctrl mice (n = 6). Significantly differentially expressed genes demarcated by −log10 FDR > or < 2 and log2 fold change (FC) > or < 0.6. d Volcano plot depicting differential RNA expression of MitoCarta 2.0 targets in islets of β-Mfn1/2DKO (n = 5) mice compared to littermate Ctrl mice (n = 6). Significantly differentially expressed genes demarcated by −log10 FDR > or < 2 and log2 fold change (FC) > or < 0.6. e Differential RNA expression heatmap of selected genes from islets of β-Mfn1KO (n = 4), β-Mfn2KO (n = 5) and β-Mfn1/2DKO (n = 5) mice compared to littermate Ctrl mice (n = 6). up. ‡§* Benjamini-Hochberg FDR (Padj) < 0.05 comparing β-Mfn1KO(‡), β-Mfn2KO(§), and β-Mfn1/2DKO(*) to Ctrl.
The nearly 1000 significantly dysregulated genes in β-Mfn1/2DKO islets included downregulation of mitochondrial encoded transcripts, as well as key β-cell signature genes such as Slc2a2 (Glut2) and MafA (Fig. 5c and e). We also observed increases in expression of regulators of triglyceride metabolism, including Angptl4 and Angptl827, the glycolytic/gluconeogenic enzyme AldoB, which is highly upregulated in models of β-cell mitochondrial dysfunction and in T2D islets28,29, as well as Nrf1, a key regulator of mitochondrial biogenesis (Figs. 5c, e, and S4d30). To resolve additional mitochondrial specific effectors that were differentially regulated following the loss of Mfn1 and 2, we next overlaid our RNAseq data on MitoCarta 2.031, which contains a compendium of targets with strong evidence for localization to the mitochondria (Fig. 5d). This revealed increased expression of several genes related to mtDNA replication machinery or associated with the mitochondrial nucleoid (Fig. 5d, e), including the critical mtDNA topoisomerase Top1mt32, the mitochondrial co-chaperone Dnaja3/Tid1, which maintains mtDNA integrity33, and the mitochondrial pyrimidine transporter Slc25a33, which is essential for mtDNA replication34. We also observed upregulation in the mitochondrial AAA + protease LonP1, which interacts with and regulates the stability of numerous proteins at the mitochondrial nucleoid (Fig. 5d, e35–37). Importantly, we did not observe changes in expression of other fission/fusion genes or regulators of mtRNA transcription, mtDNA repair, sirtuins, or mitophagy (Fig. S4d). Taken together, these data indicate that Mfn1/2-deficiency induced a transcriptional signature consistent with an activation of mtDNA replication to potentially compensate for β-cell mtDNA depletion.
Mfn1 and 2 act through Tfam to regulate β-cell mtDNA copy number
The mechanisms underlying regulation of mtDNA by mitochondrial fusion have been controversial, and previous studies implicate several pathways that could reduce mtDNA content, including genome instability/increased mtDNA mutations, enhanced mitophagy, defects in mitochondria-associated ER membrane (MAM) function, or impaired mtDNA replication17,19,38–41. As our studies were completed in young mice, which bear infrequent mtDNA mutations42, and we did not observe differences in expression of mtDNA repair enzymes (Fig. S4d), we focused on assessments of mitophagy and mtDNA replication to better resolve the significant reduction of mtDNA observed in β-Mfn1/2DKO islets. We first assessed rates of mitophagy following incubation of control and β-Mfn1/2DKO islets with the cell permeable Mtphagy dye, which did not reveal differences in mitophagy between genotypes (Fig. S4e). We also did not observe a difference in frequency of autophagosomes bearing mitochondria by TEM, or changes in essential genes in the mitophagy pathway between control and β-Mfn1/2DKO islets (Figs. 2b, S2b and S4d). As an initial screen for MAM function, we measured MAM-dependent lipids, including cholesteryl esters and phospholipids43,44. However, we did not detect differences in total cholesterol, cholesteryl esters, or phosphatidylserine concentrations in isolated islets of control and β-Mfn1/2DKO mice (Fig. S4f, g).
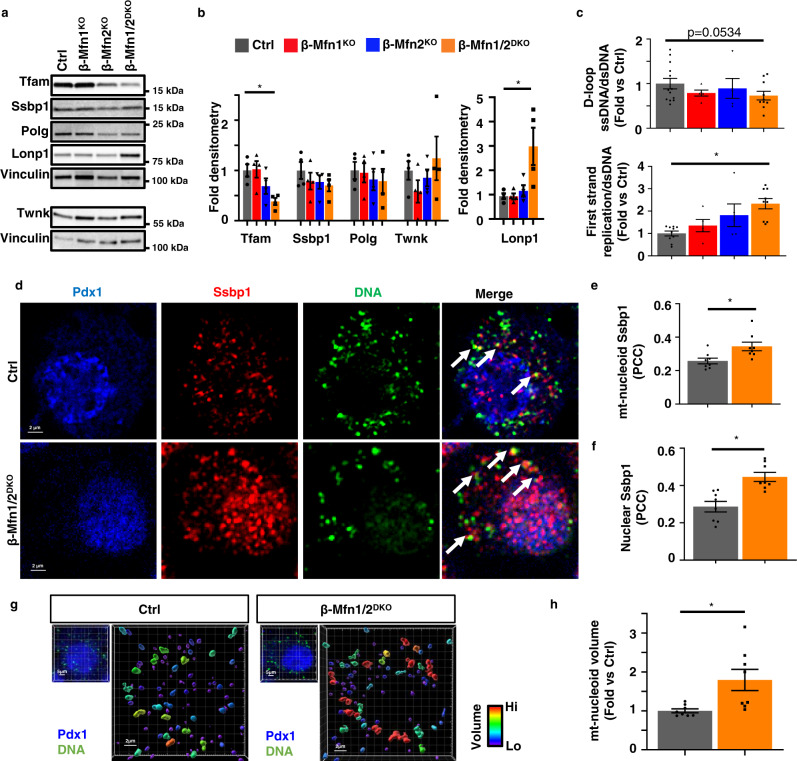
To assess regulators of β-cell mtDNA replication, we examined expression of the replisome proteins Ssbp1, Twinkle, and Polg. We also measured expression of Tfam, a master regulator of mtDNA copy number that promotes mitochondrial genome abundance via effects on mtDNA packaging, stability, and replication, and whose levels are proportional to mtDNA copy number11,45–48. While replisome proteins were largely unchanged, we observed a significant reduction in Tfam protein in β-Mfn1/2DKO islets (Fig. 6a, b). Tfam mRNA expression was unchanged in β-Mfn1/2DKO mice (Fig. S4d), suggesting the reduction in Tfam protein occurred post-transcriptionally. We further observed a significant increase in the level of LonP1 protein, which has been shown to lead to Tfam protein turnover49,50. We additionally confirmed reductions in Tfam and increases in LonP1 protein in β-Mfn1+/−Mfn2KO islets (Fig. S5), correlating with the mtDNA depletion, glucose intolerance, and impaired GSIS observed in these mice (Fig. 4c–e). In contrast, β-Mfn1KOMfn2+/− mice, which develop mitochondrial structural alterations without glucose intolerance or mtDNA depletion, did not exhibit changes in Tfam and LonP1 (Figs. S5 and 4c–e). These results suggest that changes in Tfam and LonP1 expression following Mfn1/2 deficiency were not due to increases in punctate mitochondria.
Fig. 6. Mfn1 and 2 maintain expression of Tfam in β-cells.
a Expression of Tfam, key replisome proteins, and LonP1 by Western blot (WB) in islets isolated from 11-week-old Ctrl, β-Mfn1KO, β-Mfn2KO and β-Mfn1/2DKO mice. Representative of four independent mice per group. b Densitometry (normalized to Vinculin) from studies in Fig. 6A. n = 4/group; Data are presented as mean ± SEM. *p = 0.03 for Tfam, p = 0.01 for Lonp1 by one-way ANOVA followed by Sidak’s post-hoc test for multiple comparisons. c Quantification of single strand mtDNA products measured by qPCR in isolated islets from 8-week-old Ctrl (n = 13), β-Mfn1KO (n = 5), β-Mfn2KO (n = 5), and β-Mfn1/2DKO (n = 9) mice. Data are presented as mean ± SEM. *p = 0.0007 by one-way ANOVA followed by Sidak’s post-hoc test for multiple comparisons. d Deconvolution immunofluorescence image at 100× magnification of islets from Ctrl and β-Mfn1/2DKO mice stained for Ssbp1 (red), mtDNA (green), and Pdx1 (blue). Representative of eight independent experiments. White arrows demarcate co-localized Ssbp1 + mtDNA + structures. e Quantification of mitochondrial nucleoid Ssbp1 localization (Ssbp1 + mtDNA + co-localization) in Ctrl and β-Mfn1/2DKO β-cells from studies depicted in Fig. 6D by Pearson’s correlation coefficient (PCC). n = 8/group. Data are presented as mean ± SEM. *p = 0.0142 by two-tailed t test. (∼100 β-cells from each animal per group were analyzed). f Quantification of nuclear Ssbp1 localization in Ctrl and β-Mfn1/2DKO β-cells from studies depicted in Fig. 6D by Pearson’s correlation coefficient (PCC). n = 8/group. Data are presented as mean ± SEM. *p = 0.0008 by two-tailed t-test. (~100 β-cells from each animal per group were analyzed). g Imaris® generated 3D reconstruction of deconvolution immunofluorescence Z-stack images at 100× magnification stained for DNA of β-cells (see inset image – Pdx1, blue, DNA, green) from Ctrl and β-Mfn1/2DKO mice. Colors represent relative mitochondrial volume. Representative image of eight independent mice/group. h Quantification of relative nucleoid volume of Ctrl and β-Mfn1/2DKO β-cells from studies depicted in Fig. 6g. n = 8/group. Data are presented as mean ± SEM. *p = 0.0127 by two-tailed t-test. (~100 β-cells from each animal per group were analyzed). Uncropped western blots and source data are provided as a Source Data file.
To test for defects in mtDNA replication, we next assessed initiation of first strand replication. First strand synthesis in mitochondrial replication begins in the D-loop. However, a single stranded (ss) molecule that forms the classic D-loop structure called 7S, which is a non-replicative structure, shares this sequence. It is the elongation of the 7S sequence into the nearby region (Cytb) that demonstrates committed initiation of replication. Here we assessed the levels of ss and dsDNA at specific sequences by quantitative PCR to overcome expected sample limitations from studies with mouse pancreatic islets. Notably, import of nascent Tfam has been shown to increase the generation of 7S DNA due to its transcription initiation activity51. We found a trend toward reduced total ss 7S sequences normalized to ds mtDNA in β-Mfn1/2DKO islets (Fig. 6c). However, the rate of 7S sequence extension into committed first strand replication was increased in β-Mfn1/2DKO islets (Fig. 6c), which could represent a compensatory response to mtDNA depletion.
Increased first strand replication intermediates heighten the levels of displaced second strand template, which is bound by the single strand binding protein Ssbp152. Consistent with ss qPCR studies, in situ levels of Ssbp1 at the mtDNA nucleoid (detected by perinuclear anti-DNA antibody staining), were significantly elevated in Mfn1/2-deficient β-cells (Fig. 6d, e). The increase in Ssbp1 localization to mitochondrial nucleoids could represent binding to a higher quantity of single-stranded mtDNA replication intermediates and could also be consistent with observations of impaired completion of mtDNA replication previously reported following Mfn1/2-deficiency19,53. We also observed Ssbp1 nuclear localization (Fig. 6d and f), which has been previously demonstrated following mitochondrial stress54. Further, we detected an increase in the volume of mtDNA nucleoid structures in Mfn1/2-deficient β-cells by high-resolution 3D imaging (Fig. 6g, h), which are similar to previous reports of Tfam-deficiency but could also represent impaired nucleoid distribution19,55,56. Taken together, our observations of reduced mtDNA content, mtRNA levels, as well as changes in single-stranded mtDNA products, Ssbp1 localization, and nucleoid size following Mfn1/2-deficiency suggest a role for Tfam in mediating the effects of Mfn1/2 in β-cell function.
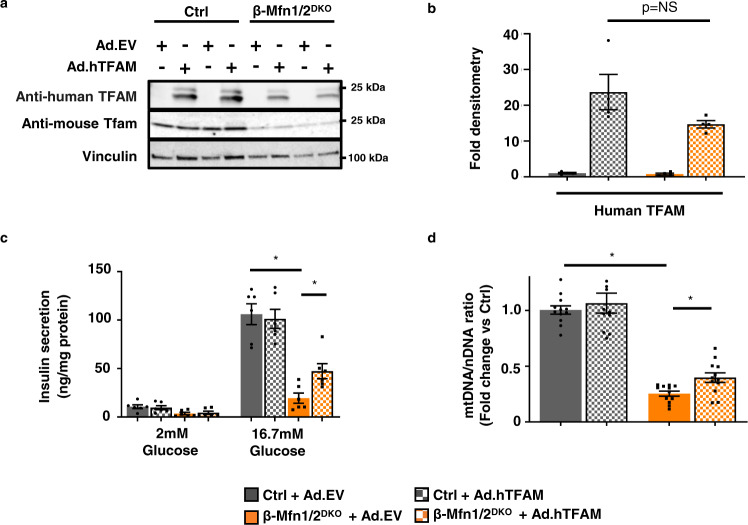
To test whether increasing mtDNA copy number was sufficient to improve β-cell function in fusion-deficient islets, we overexpressed Tfam in β-Mfn1/2DKO and littermate control islets. We transduced intact islets using adenoviral vectors encoding the human form of TFAM, thus allowing us to track overexpression of TFAM using human-specific TFAM anti-sera (Figs. 7a, b and S6a). Transduction efficiency in β-cells was estimated at ~40–50% (Fig. S6a). Despite limitations in transduction efficiency and expression, TFAM overexpression significantly ameliorated the reductions in both GSIS and mtDNA copy number in β-Mfn1/2DKO islets (Figs. 7c, d and S6b). Therefore, Mfn1 and 2 direct β-cell function, at least in part, through the regulation of Tfam-mediated mtDNA copy number control.
Fig. 7. Mfn1 and 2 maintain β-cell function through Tfam-mediated mtDNA copy number control.
a Expression of mouse-specific Tfam and human-specific TFAM by WB in islets isolated from 10 to 15-week-old Ctrl and β-Mfn1/2DKO mice, transduced with empty vector control (Ad.EV) or human TFAM-overexpressing (Ad.hTFAM) adenoviral particles. Representative of four independent mice/group. b Densitometry (normalized to cyclophilin B) from studies in Fig. 7A. n = 4/group; Data are presented as mean ± SEM. p = NS (non-significant) by two-tailed t test. c Glucose-stimulated insulin secretion following static incubations in 2 mM and 16.7 mM glucose, performed in isolated Ctrl and β-Mfn1/2DKO islets following transduction with Ad.EV or Ad.hTFAM adenoviral particles. n = 6/group. Data are presented as mean ± SEM. *p < 0.0005 for Ctrl + Ad.EV vs β-Mfn1/2DKO + Ad.EV, p = 0.0148 for β-Mfn1/2DKO + Ad.EV vs β-Mfn1/2DKO + Ad.hTFAM by one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. d Relative mtDNA content measured by qPCR (normalized to nuclear DNA expression) from isolated Ctrl and β-Mfn1/2DKO islets following transduction with Ad.EV or Ad.hTFAM adenoviral particles. n = 12/group. Data are presented as mean ± SEM. *p < 0.0005 for Ctrl + Ad.EV vs β-Mfn1/2DKO + Ad.EV, p = 0.007 for β-Mfn1/2DKO + Ad.EV vs β-Mfn1/2DKO + Ad.hTFAM by two-tailed t test. Uncropped western blots and source data are provided as a Source Data file.
Mitofusin agonists restore mtDNA content independent of changes in mitochondrial structure
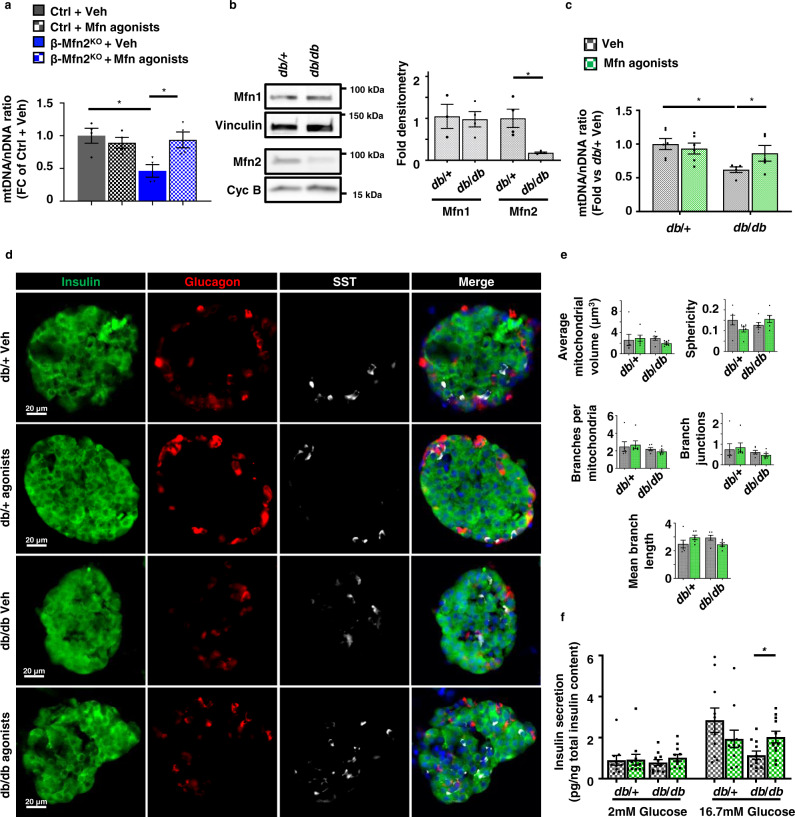
We next asked if pharmacologically targeting mitofusins could restore mtDNA levels in models of mitofusin deficiency independent of changes in mitochondrial structure. To this end, we employed recently described tandem pharmacological agonists, (2-{2-[(5-cyclopropyl-4-phenyl-4H-1,2,4-triazol-3-yl)sulfanyl]propanamido}−4H,5H,6H-cyclopenta[b]thiophene-3-carboxamide and 1-[2-(benzylsulf-anyl)ethyl]-3-(2-methylcyclohexyl)urea (hereafter called Mfn agonists), which together have been previously shown to improve mitochondrial morphology in either Mfn1- or Mfn2-deficient fibroblasts57. These agonists require some residual mitofusin levels to be effective57. Thus, we initially treated control and β-Mfn2KO islets, which exhibit reduced mtDNA content without changes in mitochondrial structure (Figs. 3a, 2b, c, and e). To avoid systemic effects of modifying mitochondrial fission/fusion balance in other metabolic tissues1, we utilized Mfn agonists on isolated islets. Interestingly, Mfn agonists restored mtDNA content to baseline in β-Mfn2KO islets without affecting mitochondrial morphology or inducing transcriptional regulators of mitochondrial biogenesis (Figs. 8a and S7a, b). As anticipated, Mfn agonists were ineffective in the setting of combined absence of Mfn1 and 2 and were unable to rescue reductions in mtDNA content or Tfam protein in β-Mfn1/2DKO islets (Fig. S7c, d).
Fig. 8. Mitofusin agonists restore mtDNA depletion following Mfn2 deficiency independent of changes in mitochondrial structure.
a Relative mtDNA content measured by qPCR (normalized to nuclear DNA expression) from isolated islets of Ctrl and β-Mfn2KO mice following treatment with vehicle (Veh) or 0.5 µM Mfn agonists for 24 h. n = 4/group. Data are presented as mean ± SEM. *p = 0.0138 for Ctrl + Veh vs β-Mfn2KO + Veh, p = 0.0302 for β-Mfn2KO + Veh vs β-Mfn2KO + Mfn agonists by one-way ANOVA followed by Sidak’s post-hoc test for multiple comparisons. b Expression of Mfn1 and Mfn2 by WB in islets isolated from 10 to 12-week-old db/ + and db/db mice. Vinculin and cyclophilin B serve as loading controls. Representative image (left) of three independent mice/group for Mfn1 and four independent mice/group for Mfn2. Mfn1 (n = 3) and Mfn2 (n = 4) densitometry, normalized to vinculin or cyclophilin B (right). Data are presented as mean ± SEM. *p = 0.0095 by two-tailed t test. c Relative mtDNA content measured by qPCR (normalized to nuclear DNA expression) from isolated islets of 10–12-week-old db/ + (n = 6) and db/db (n = 5) mice following treatment with vehicle (Veh) or 0.5 µM mitofusin agonists for 24 h. Data are presented as mean ± SEM. *p = 0.0038 for db/ + + Veh vs db/db + Veh, p = 0.0428 for db/db + Veh vs db/db + Mfn agonists by two-tailed t test. d Immunofluorescence images at 20× magnification of islets from 10 to 12-week-old db/ + and db/db mice stained for Insulin (green), Glucagon (red), Somatostatin (SST; white), and DAPI (blue) following treatment with vehicle (Veh) or 0.5 µM mitofusin agonists for 24 h. n = 3/group. e β-cell mitochondrial morphology and network analysis by MitoAnalyzer of deconvolution immunofluorescence Z-stack images, stained for SDHA (and insulin), from pancreatic islets of 10–12-week-old db/ + and db/db mice following treatment with vehicle (Veh) or 0.5 µM mitofusin agonists for 24 h. n = 6/group (∼150–200 β-cells/animal were quantified). Data are presented as mean ± SEM. f Glucose-stimulated insulin secretion following static incubations in 2 mM and 16.7 mM glucose, performed in isolated islets of 10–12-week-old db/ + and db/db mice following treatment with vehicle (Veh) or 0.5 µM mitofusin agonists for 24 h. n = 11/group. Data are presented as mean ± SEM. *p = 0.0092 by two-tailed t test. Uncropped western blots and source data are provided as a Source Data file.
We next evaluated the effects of Mfn agonists in other models of mitofusin deficiency, as Mfn2 expression is reduced in several models of β-cell dysfunction9,10. We assessed the expression of Mfn1 and 2 in islets isolated from the leptin receptor deficient db/db BKS mouse model, which develops obesity, progressive β-cell mitochondrial and insulin secretory dysfunction, and eventual β-cell failure58–62. We observed that Mfn2, but not Mfn1, protein levels were reduced in islets of 10–12-week-old db/db mice when compared to db/ + controls (Fig. 8b), measured at an age prior to β-cell failure58,59. Similar to observations in β-Mfn2KO islets, db/db islets exhibited reduced mtDNA content without alterations in mitochondrial structure (Fig. 8c and e). Concordantly, treatment with Mfn agonists rescued mtDNA levels in db/db islets, while leaving mitochondrial structure and islet architecture unchanged (Fig. 8c–e). We also observed that Mfn agonists improved GSIS in the islets of db/db mice (Fig. 8f). Taken together, these results indicate that Mfn agonists restore mtDNA content and improve GSIS independent of changes in mitochondrial structure. Moreover, these results extend the association between mitofusin deficiency, mtDNA depletion, and impaired GSIS in β-cells.
Discussion
Here, we identify that Mfn1 and 2 promote mitochondrial fitness to fuel glucose-stimulated insulin release in pancreatic β-cells and maintain glucose homeostasis. Mfn1 or Mfn2 are individually dispensable for β-cell function, but together coordinately control mitochondrial respiration, structure, and mtDNA content. Mfn1 and 2 were thought to regulate cellular metabolism principally through the control of mitochondrial architecture. Our single allele mouse models directly demonstrate that Mfn1/2-deficiency is coupled to glucose intolerance through mtDNA loss rather than mitochondrial structure. Further, we observe that increased mtDNA copy number following Tfam overexpression is capable of ameliorating impaired GSIS in fusion-deficient β-cells. Thus, our studies position the maintenance of mtDNA content as the principal task necessary for Mfn1 and 2 to regulate β-cell function.
Our description of a central role for Mfn1 and 2 in β-cells through mtDNA content rather than mitochondrial structure elucidates a previously unappreciated role for mitochondrial fusion on cellular metabolic function. Mfn1 and 2 act in a complementary manner to support mitochondrial metabolism from development to adulthood. Both germline and cardiomyocyte-specific deletion of Mfn1 and 2 are associated with developmental or early postnatal demise16,18, presumed to be primarily caused by defects in mitochondrial network dynamics. Later descriptions of reduced mtDNA levels in constitutive or inducible loss of Mfn1/2 in skeletal muscle did not discern the physiologic importance of mtDNA depletion versus structure following fusion-deficiency15,17. Our results clarify a primary physiologic impact of mtDNA depletion rather than mitochondrial structure following Mfn1/2 deficiency in β-cells that may also underlie the physiologic importance of Mfn1 and 2 in other metabolic tissues. To our knowledge, this is the first time that these functions of Mfn1/2 have been separated in a physiological context.
We observe that β-Mfn1/2DKO islets attempt unsuccessfully to compensate for mtDNA depletion. Our analyses of first strand replication suggest that Mfn1/2-deficient β-cells increase the activation of early steps in mtDNA replication, which is supported by increased Ssbp1 localization to nucleoids to potentially bind newly formed ss-mtDNA. Despite these changes, mtDNA content remains low in Mfn1/2-deficient β-cells, suggesting that the increased commitment to replication is insufficient. Indeed, increased nucleoid Ssbp1 may be engaged in binding abnormal single-stranded replication products due to fusion and replication defects19,53. The increased expression of Top1mt found in Mfn1/2-deficient β-cells (Fig. 5e) could also represent a response to increased mitochondrial replication stress63.
Loss of Mfn1/2 in β-cells leads to severe hyperglycemia, reduced GSIS, and mtDNA depletion, which bears similarity to a previous report of selective Tfam deficiency in β-cells25. Notably, previously described β-cell specific Tfam knockout mice developed progressive loss of β-cell mass with age25. We found that β-cell mass was unchanged in young β-Mfn1/2DKO mice, but it is possible that they would similarly develop glucotoxicity and an age-related loss of β-cell mass over time. Alternatively, complete depletion of mtDNA, which occurs in the setting of Tfam-deficiency but does not occur in β-Mfn1/2DKO mice, might be required for loss of β-cell mass. Furthermore, the transgenic RIP2-Cre used in the β-cell Tfam knockout model (in contrast to the highly specific Ins1-Cre knockin strain we used in β-Mfn1/2DKO mice) is known to result in off-target recombination25,64, which may in part underlie phenotypic differences between the models. In future experiments, it will be intriguing to examine Tfam and Mfn1/2-deficient mice generated using the same Cre-recombinase strains to determine the contribution of Tfam-dependent and independent functions on Mfn1/2 in β-cell health and function.
A related concern in the Mfn1/2-deficiency models is the use of a constitutive Cre strain, which may yield phenotypic defects due to recombination during β-cell development. However, an independent group found that mice with inducible Mfn1/2-deficiency only in adult β-cells developed a highly similar phenotype to constitutive β-Mfn1/2DKO mice65, suggesting that developmental defects are unlikely to be a major contributor to the phenotype we observe.
While our data suggest that Tfam mediates the effects of Mfn1/2 on mtDNA content and GSIS in β-cells, the mechanisms for post-transcriptional loss of Tfam in β-Mfn1/2DKO islets are unclear. Loss of Tfam may be due to increased LonP1 expression, which is known to degrade Tfam49,50. Further support for a role for LonP1 as a contributor to cellular dysfunction include studies demonstrating reduction of nuclear-encoded subunits of Complex I and III following increases in LonP1 expression66–68, an effect we also observe following Mfn1/2-deficiency (Fig. 3d). Mfn1/2-deficiency has also been linked to reductions in nuclear-encoded Complex I and III subunits via instability in respiratory supercomplexes, which have been observed following LonP1 overexpression15,66–68. However, the function and transcriptional regulation of LonP1 in β-cells are unknown and require future study.
Mfn2 is frequently observed at MAMs69,70. MAMs are essential for communication and Ca2+ transfer between the ER and mitochondria, are important for β-cell function and GSIS, and have been suggested to regulate mtDNA replication39–41,71–73. Thus, alterations in MAMs in β-Mfn1/2DKO islets could lead to impaired mtDNA replication. Our initial functional assessments of MAMs by measurement of MAM-dependent lipids did not reveal differences between control and β-Mfn1/2DKO islets (Fig. S4f, g). We also did not observe ER dilation or overt changes in mitochondrial-ER proximity upon analysis of transmission EM in β-Mfn1/2DKO islets (Figs. 2b and S2b). Additionally, a recent report found that inducible loss of Mfn1/2 in adult β-cells did not alter MAM-dependent ER calcium release65. Despite these findings, our results do not exclude the potential that impaired MAM function at β-cell mtDNA nucleoids contributes to the impairments in mtDNA replication and content we observe. Indeed, our results demonstrating the importance for a single allele of Mfn2 over Mfn1 in the maintenance of glycemic control and mtDNA content could suggest a role for Mfn2-dependent MAM function in β-cell health and function, as Mfn1 is not found at MAMs. Alternatively, the regulation of MAMs by Mfn2, as well as the MAM-dependent role of Mfn2 in mtDNA replication, remains controversial74,75. Recent work also implicates mitochondrial fusion as necessary to maintain the stoichiometry of the protein components within the mtDNA replisome19. Future studies will be valuable to dissect the molecular details underlying the connections between mitochondrial fusion, MAMs, and control of β-cell mtDNA replication.
Mitochondrial structural defects and age-related mtDNA depletion combine to impair β-cell function to increase T2D risk6,7,76–78, thus, improving mitochondrial function would have significant translational benefits to restore β-cell health in diabetes. Our studies demonstrate the feasibility of targeting mtDNA copy number to improve GSIS in the setting of mitofusin deficiency. While our results in db/db islets are promising, loss of the leptin receptor could lead to mitochondrial defects that are particularly amenable to improvement by Mfn agonists. Thus, it will be intriguing to determine if Mfn agonists can also reverse β-cell dysfunction in diet-induced models of obesity in vivo. Further, recently identified compounds targeting pyrimidine metabolism to induce Mfn1/2 transcription79 will be of interest to determine if they can improve β-cell function and mtDNA content. Our observation of the upregulation of mitochondrial pyrimidine transporter Slc25a33 in β-Mfn1/2DKO islets is consistent with links between pyrimidine metabolism and mitochondrial fusion, which suggest these compounds could hold promise to enhance β-cell mitochondrial function. As expression of MFN1 and MFN2 is reduced in cardiomyocytes and skeletal muscle of patients with diabetes, respectively80–82, activators of Mfn1/2 may have additional benefits beyond improving β-cell function. Future studies will be required to fully characterize the efficacy of overcoming mitochondrial defects in vivo to treat diabetes.
Methods
Animals
Mfn1loxP/loxP and Mfn2loxP/loxP mice possessing loxP sites flanking exon 4 of the Mfn1 gene and exon 6 of the Mfn2 gene, respectively, were purchased from Jackson Laboratories13. Mfn1loxP and Mfn2loxP mice were mated to Ins1-Cre83 to generate experimental groups. Ins1-Cre–alone, Mfn1loxP/loxP and Mfn2loxP/loxP mice were phenotypically indistinguishable from each other and combined as controls (Ctrl; see Fig. S1b, c). Ins1-Cre–alone were also phenotypically indistinguishable from wild-type C57BL/6N controls, consistent with previous reports from our group and others21,83. All animals were maintained on the C57BL/6 N background. db/ + and db/db mice, maintained on the BKS background, were purchased from Jackson Laboratories. Animals were housed on a standard 12-h light/12-h dark cycle at room temperature and 30–70% humidity, with ad libitum access to food and water. All mice were fed PicoLab® Laboratory Rodent Diet 5L0D (LabDiet) containing 28% protein, 13% fat, and 57% carbohydrates. All studies and endpoints in all figures were completed using both male and female mice, and results from both sexes were combined in all experimental groups. Animal studies were approved by the University of Michigan Institutional Animal Care and Use Committee.
Mitochondrial respirometry
Islet respirometry was measured using an XF96 extracellular flux analyzer (Seahorse Bioscience) according to manufacturer’s instructions. Briefly, 20 similarly sized islets were plated per well of a Seahorse spheroid plate pre-treated with CellTak (Corning)84. Prior to respirometry profiling, islets were incubated for 1 h in an atmospheric CO2 incubator at 37 °C and supplemented with mouse islet culture media85,86 comprised of pH 7.4 unbuffered RPMI-1640 media (Seahorse Bioscience) and 2 mM glucose prior to flux analyzer assays. Analysis was performed at baseline, upon exposure to 20 mM glucose, and again after treatment with 2 µM antimycin A. OCR measurements were normalized to islet number confirmed by light microscopy (Zeiss Stemi 2000) after the completion of respirometry assays.
Transmission electron microscopy
Mouse islets were pelleted, fixed in 3% glutaraldehyde and 3% paraformaldehyde in 0.1 M Cacodylate buffer (CB; pH 7.2) overnight at 4 °C. Islets were then washed with PBS and centrifuged at 2000 rpm for 2 mins. Pre-warmed 2% agarose solution was then carefully added to islet pellets, centrifuged and allowed to cool at 4 °C for 30 min87. Samples were then subjected to osmification in 1.5% K4Fe(CN)6 + 2% OsO4 in 0.1 CB for 1 h, dehydrated by serial washes in EtOH (30%, 50%, 70%, 80%, 90%, 95% and 100%) and embedded in Spurr’s resin by polymerization at 60 °C for 24 h. Polymerized resins were then sectioned at 90 nm thickness using a Leica EM UC7 ultramicrotome and imaged at 70 kV using a JEOL 1400 TEM equipped with an AMT CMOS imaging system. Mitochondrial structures (Aspect Ratio, Perimeter, Circularity) were analyzed and quantified by ImageJ. Form factor assessments were analyzed by ImageJ and quantified as described22.
Imaging of islet and organelle morphology and subcellular localization analysis
Mitochondrial morphology and subcellular localization analyses were performed by immuno-staining using mouse pancreas tissue sections and dissociated islet cells21. Pancreatic tissue sections were fixed in 4% PFA overnight, embedded in paraffin, and sectioned. Following dewaxing and rehydration, antigen retrieval was performed with 10 mM citric acid (pH 6.0). Dissociated islet cells were fixed in 4% PFA for 15 min and centrifuged onto glass slides. Pancreatic sections and dissociated islets were then blocked using 5% donkey serum for 1 h at room temperature followed by immunostaining. Islet architecture was analyzed using isolated mouse islets fixed in 4% PFA overnight followed by a 20 min incubation with methylene-blue solution (Sigma) and OCT embedding. Cryo-sectioned islets were rinsed in 75% ethanol for 5 min to remove methylene blue, permeabilized with 0.2% Triton-X 100 for 15 min, blocked using 5% donkey serum for 1 h, and immunostained.
Images were captured with an IX81 microscope (Olympus) using an ORCA Flash4 CMOS digital camera (Hamamatsu). Immunostained pancreatic sections, cryo-sectioned and dispersed mouse islets were captured with Z-stack images and subjected to deconvolution (CellSens; Olympus). Mitochondrial morphology and nucleoids were visualized using 3D-renderings generated with Imaris® imaging software (Bitplane). Quantitative 3D assessments of mitochondrial morphology and network were performed on ImageJ using Mitochondria Analyzer plugin22. Co-localization analyses were performed on Z-stack images of immunostained dissociated islet cells using the Coloc2 plugin on ImageJ. Changes in relative nucleoid size were quantified from 3D deconvolution images of immuno-stained dissociated islet cells, detected by perinuclear anti-DNA antibody staining, using ImageJ.
mtDNA content and replication assays
Relative mtDNA content by qPCR with SYBR-based detection (Universal SYBR Green Supermix; Biorad) was conducted following DNA isolation with the Blood/Tissue DNeasy kit (Qiagen) according to manufacturer’s protocols. Primer sequences: mt9/mt11 (5′- GAGCATCTTATCCACGCTTCC-3′ and 5′- GGTGGTACTCCCGCTGTAAA-3′) for mtDNA and Ndufv1 (5′- CTTCCCCACTGGCCTCAAG-3′ and 5′- CCAAAACCCAGTGATCCAGC-3′) for nuclear DNA88. First strand mtDNA replication levels were measured by qPCR following digestion by Mnl I, which site specifically digest double stranded DNA89. Briefly, primers specific to 7 S mtDNA (5′-ACTCTTCTCTTCCATATGACTATCCC-3′ and 5′-GGCCCTGAAGTAAGAACCAGATGT-3′), whose amplicon contains a Mnl I site, were used to quantify relative single-stranded mtDNA forming the D-loop. Single-stranded DNA generated from the extension of 7S region was measured using primers specific to CYTB (5′-TGCATACGCCATTCTACGCTCA-3′ and 5′-GTGATTGGGCGGAATATTAGGCTTC-3′), which also contains an Mnl I site. Primers specific to COXI (5′-GCAGGAGCATCAGTAGACCTAACA-3′ and 5′-GCGGCTAGCACTGGTAGTGATAAT-3′), whose amplicon does not contain an Mnl I site, were used to quantify non-replicating double-stranded mtDNA. The ratio of 7S or CYTB amplicons to the COXI amplicon were then calculated after qPCR of Mnl I digested DNA to determine the relative amount of single stranded mtDNA at the D-loop strand or initiation of first strand replication, respectively.
RNA sequencing, differential expression, and functional analysis
Sequencing was performed by the Advanced Genomics Core at University of Michigan Medical School. Total RNA was isolated, and DNase-treated using commercially available kits (Omega Biotek and Ambion, respectively). Libraries were constructed and subsequently subjected to 151 bp paired-end cycles on the NovaSeq-6000 platform (Illumina). FastQC (v0.11.8) was used to ensure the quality of data. Reads were mapped to the reference genome GRCm38 (ENSEMBL), using STAR (v2.6.1b) and assigned count estimates to genes with RSEM (v1.3.1). Alignment options followed ENCODE standards for RNA-seq. FastQC was used in an additional post-alignment step to ensure that only high-quality data were used for expression quantitation and differential expression. Data were pre-filtered to remove genes with 0 counts in all samples. Differential gene expression analysis was performed using DESeq2, using a negative binomial generalized linear model (thresholds: linear fold change >1.5 or <−1.5, Benjamini-Hochberg FDR (Padj) < 0.05). Plots were generated using variations of DESeq2 plotting functions and other packages with R version 3.3.3. Genes were annotated with NCBI Entrez GeneIDs and text descriptions. Functional analysis, including candidate pathways activated or inhibited in comparison(s) and GO-term enrichments, was performed using iPathway Guide (Advaita). The RNA sequencing data generated in this study have been deposited in the Gene Expression Omnibus (GEO) database under accession code GSE194204.
Adenoviral transfection
Isolated mouse islets were distended with pre-warmed Accutase for 1 min at 37 °C followed by rapid inactivation with RPMI-1640 media (Thermo Fisher Scientific) supplemented with 10% Heat-inactivated FBS (Gemini Foundation), 1% Penicillin/Streptomycin (Thermo Fisher Scientific), 1% Antibiotic-Antimycotic (Thermo Fisher Scientific), 1 mM Sodium Pyruvate (Thermo Fisher Scientific) and 10 mM HEPES (Thermo Fisher Scientific)90. After two PBS washes, islets were resuspended in fresh RPMI media and transduced with 0.15 MOI of Ad.EV (expressing an empty vector) or Ad.hTFAM (expressing human-specific TFAM) for 48 h (viral particles purchased from Vector Biolabs). β-cell transduction was evaluated on dissociated islet cells by immunostaining with Pdx1 and human-specific TFAM anti-sera.
Flow cytometry
Following isolation and culture, live mouse islets were exposed to 100 nM Mtphagy dye (Dojindo Molecular Technologies) for 3 h to assess time-dependent accumulation of mitochondria to acidic organelles by the relative fluorescence intensity of the dye per cell91,92. Islets were then dissociated into single cells by incubating with pre-warmed 0.25% trypsin for 3 min at room temperature, stained with DAPI (Thermo Fisher Scientific) and Fluozin-3 (Thermo Fisher Scientific), and resuspended in phenol red–free RPMI-1640 culture medium (Thermo Fisher Scientific) containing 11.1 mM glucose supplemented with 1% Penicillin/Streptomycin (Thermo Fisher Scientific), 1% Antibiotic-Antimycotic (Thermo Fisher Scientific), 1 mM Sodium Pyruvate (Thermo Fisher Scientific) and 10 mM HEPES (Thermo Fisher Scientific)21. Samples were analyzed on an LSR Fortessa flow cytometer (BD Biosciences). Single cells were gated using forward scatter and side scatter (FSC and SSC, respectively) plots, DAPI staining was used to exclude dead cells, and Fluozin-3 was used to identify β-cells21,93. Mtphagy measurements in β-cells were made using 488 nm excitation laser with a 710 nm emission filter and analyzed using FlowJo (Tree Star Inc.). A total of 5000 β-cells was quantified from each independent islet preparation. Gating strategies with representative plots are provided in Supplementary Fig. S8.
Glucose and insulin measurements
All mice were fasted for 6 h prior to glucose and insulin measurements. For IPGTT or ITT, mice were injected intraperitoneally with 2 g/kg glucose or 0.75 U/kg regular human insulin (Novolin R; Novo Nordisk), respectively. Tail blood was collected for measurements of blood glucose. For in vivo insulin release measurements, tail blood was collected following glucose injection, centrifuged at 2000 rpm for 10 min at 4 °C and stored at −20 °C. Static insulin secretion assays on isolated islets were performed following 1 h incubations with 2 mM and 16.7 mM glucose in HEPES-supplemented KRB buffer containing 135 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 5 mM NaHCO3, 1.2 mM MgSO4.7H2O, 1 mM CaCl2, 10 mM HEPES and 0.1% BSA (pH 7.4). Samples were analyzed for insulin release using Mouse Ultrasensitive Insulin ELISA (ALPCO) as per the manufacturer’s protocol.
Islet isolation and cell culture
Mouse islets were isolated by collagenase (1 mg/ml) injection into the common bile duct followed by digestion at 37 °C, gradient centrifugation with histopaque (Sigma) and hand-picked. Islets were then cultured in RPMI-1640 media (Thermo Fisher Scientific) containing 11.1 mM glucose supplemented with 10% Heat-inactivated FBS (Gemini Foundation), 1% Penicillin/Streptomycin (Thermo Fisher Scientific), 1% Antibiotic-Antimycotic (Thermo Fisher Scientific), 1 mM Sodium Pyruvate (Thermo Fisher Scientific) and 10 mM HEPES (Thermo Fisher Scientific)85,86. Cell treatments included DMSO (Fisher) and antimycin A (Sigma). Mitofusin agonist treatment included an equimolar mixture of two previously described compounds (0.5 µM each57), which were administered for 24 h (2-{2-[(5-cyclopropyl-4-phenyl-4H-1,2,4-triazol-3-yl)sulfanyl]propanamido}-4H,5H,6H-cyclopenta[b]thiophene-3-carboxamide, CAS# 920868-45-7 and 1-[2-(benzylsulfanyl)ethyl]-3-(2-methylcyclohexyl)urea, CAS# 1007818-44-1; Enamine Ltd.).
Western blotting, immunostaining, and quantitative PCR
For Western blotting, mouse pancreatic islets were lysed with radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors (Calbiochem), and insoluble material was removed by centrifugation. Equal amounts of proteins were resolved on 4%–20% gradient Tris-glycine gels (Bio-Rad) and transferred to nitrocellulose membranes (Bio-Rad). Membranes were then blocked in 5% milk for 1 h and immunoblotting was performed using Cyclophilin B (1:5000; ThermoFisher, Catalog# PA1-027A), Drp1 (1:1000; CST #8570), Lonp1 (1:1000, ProteinTech, Catalog# 15440-1-AP), Mfn1 (1:1000; Abcam, Catalog# ab126575), Mfn2 (1:1000; Abcam, Catalog# ab56889), mt-Cytb (1:500; ProteinTech, Catalog# 55090-1-AP), Opa1 (1:1000, BD Transduction laboratories, Catalog# 612606), Total Oxphos antibody cocktail (1:1000; Abcam, ab110413), Polg (1:1000, Abcam, Catalog# ab128899), Ssbp1 (1:250; Atlas antibodies; Catalog# HPA002866), Anti-human TFAM (1:1000, PhosphoSolutions, Catalog# 1999-hTFAM), Anti-mouse TFAM (1:1000; PhosphoSolutions 2001-TFAM), Tom20 (1:1000; Cell Signaling Technology, Catalog# 42406), Twnk (1:1000, ProteinTech, Catalog# 13435-1-AP), Vinculin (1:5000; Millipore, Catalog# CP74) and species-specific HRP-conjugated secondary antibodies (Vector Laboratories). Immunostaining on pancreatic sections, cryo-sectioned and dispersed mouse islets was performed using Anti-DNA (1:50; American Research Products, Catalog# PA1-027A), Insulin (1:250; Dako, Catalog# A0564), Insulin (1:100, Abcam, Catalog# ab7842), Glucagon (1:2000; SantaCruz, Catalog# sc-13091), Pdx1 (1:250; Abcam, Catalog# ab47383), SDHA (1:100, Abcam, Catalog# ab14715), Ssbp1 (1:250; Atlas antibodies, Catalog# HPA002866), Somatostatin (1:500, Abcam, Catalog# ab30788) and species-specific Cy2, Cy3 and Cy5-conjugated secondary antibodies (Jackson Immunoresearch). β-cell mass was quantified as total insulin-positive area/total pancreatic area multiplied by pancreatic wet weight using stitched images of complete pancreatic sections86. All antibodies used for Western blotting and immunostaining are listed in Supplementary Table 1.
RNA samples were reverse transcribed using High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Quantitative reverse transcription PCR (qRT-PCR) was performed with SYBR-based detection (Universal SYBR Green Supermix; Biorad) using primers for Ppargc1a (5′-ACTATGAATCAAGCCACTACAGAC-3′ and 5′- TTCATCCCTCTTGAGCCTTTCG-3′), Nrf1 (5′-ACAGATAGTCCTGTCTGGGGAAA-3′ and 5′- TGGTACATGCTCACAGGGATCT-3′) and Tfam (5′-AGCTTCCAGGAGGCAAAGGATGAT-3′ and 5′-ACTTCAGCCATCTGCTCTTCCCAA-3′).
Cholesterol esterification and phosphatidylserine assay
Total amounts of free cholesterol and cholesterol esters were determined in mouse islet lysates as per the manufacturer’s instructions (Promega). Phosphatidylserine levels were measured in mouse islet lysates as per the manufacturer’s instructions (LSBio).
Statistics
In all figures, data are presented as means ± SEM, and error bars denote SEM, unless otherwise noted in the legends. Statistical comparisons were performed using unpaired two-tailed Student’s t tests, one-way or two-way ANOVA, followed by Tukey’s or Sidak’s post-hoc test for multiple comparisons, as appropriate (GraphPad Prism). A P value < 0.05 was considered significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
S.A.S was supported by the JDRF (CDA-2016-189, SRA-2018-539, COE-2019-861), the NIH (R01 DK108921, U01 DK127747), the Department of Veterans Affairs (I01 BX004444), the Brehm family, and the Anthony family. E.L.-D. was supported by the NIH (T32 AI007413 and T32-AG000114). G.L.P. was supported by the American Diabetes Association (19-PDF-063). B.A.K. was supported by the Department of Veterans Affairs (I01 BX004444). The JDRF Career Development Award to S.A.S. is partly supported by the Danish Diabetes Academy and the Novo Nordisk Foundation. We acknowledge the Microscopy, Imaging, and Cellular Physiology Core of the University of Michigan DRC (P30 DK020572) for assistance with imaging studies. We thank the University of Michigan Flow Cytometry Core for assistance with flow cytometry studies. Next-generation sequencing was carried out in the Advanced Genomics Core at the University of Michigan. We acknowledge support from the Bioinformatics Core of the University of Michigan’s Biomedical Research Core Facilities. We thank Drs. K. Claiborn, C. Rutledge, N. Desai, G. Rutter, A. Garner, A. Menon, M. Torres, and members of the Soleimanpour laboratory for helpful advice.
Source data
Author contributions
V.S. conceived, designed and performed experiments, interpreted results, drafted and reviewed the manuscript. J.Z. and E.L-D. designed and performed experiments, interpreted results, and reviewed the manuscript. G.L.P. and E.C.R. designed and performed experiments and interpreted results. E.M.W. designed and performed experiments, interpreted results, edited, and reviewed the manuscript. B.A.K. designed studies, interpreted results and reviewed the manuscript. S.A.S. conceived and designed the studies, interpreted results, drafted, edited, and reviewed the manuscript.
Peer review
Peer review information
Nature Communications thanks Raghu Mirmira and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
The authors declare that data supporting the findings of this study are available within the article and its Supplementary information files or from the corresponding author (S.A.S.) upon request. RNA sequencing data used for differential gene expression-, pathway- and GO- enrichment analyses have been deposited in the GEO database under the accession number GSE194204. The reference genome used for RNA sequencing was obtained from ENSEMBL (Mus_musculus - Ensembl genome browser 105). All uncropped western blots and data values for all figures are provided in the source data file. Source data are provided with this paper.
Code availability
High-throughput RNA sequencing data was analyzed using DESeq2 (v1.26.0) to determine differential gene expression. iPathway Guide (Advaita version 1910) was used to perform pathway and GO-term analysis.
Competing interests
The authors have declared that no conflict of interest exists.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Vaibhav Sidarala, Jie Zhu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-29945-7.
References
- 1.Sebastian D, Palacin M, Zorzano A. Mitochondrial dynamics: coupling mitochondrial fitness with healthy aging. Trends Mol. Med. 2017;23:201–215. doi: 10.1016/j.molmed.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 3.Schrepfer E, Scorrano L. Mitofusins, from mitochondria to metabolism. Mol. Cell. 2016;61:683–694. doi: 10.1016/j.molcel.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman BA, Li C, Soleimanpour SA. Mitochondrial regulation of beta-cell function: maintaining the momentum for insulin release. Mol. Asp. Med. 2015;42:91–104. doi: 10.1016/j.mam.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maechler P. Mitochondrial function and insulin secretion. Mol. Cell Endocrinol. 2013;379:12–18. doi: 10.1016/j.mce.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Anello M, et al. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia. 2005;48:282–289. doi: 10.1007/s00125-004-1627-9. [DOI] [PubMed] [Google Scholar]
- 7.Masini, M. et al. Ultrastructural alterations of pancreatic beta cells in human diabetes mellitus. Diabetes Metab. Res. Rev.33, e2894 (2017). [DOI] [PubMed]
- 8.Wigger L, et al. Multi-omics profiling of living human pancreatic islet donors reveals heterogeneous beta cell trajectories towards type 2 diabetes. Nat. Metab. 2021;3:1017–1031. doi: 10.1038/s42255-021-00420-9. [DOI] [PubMed] [Google Scholar]
- 9.Safia Costes T. G., Alexandra E. B., Sam S., Orian S. & Peter C. B. Disruption of the endoplasmic reticulum-mitochondria interface by toxic IAPP oligomers may compromise beta-cell mitochondrial network integrity and function in type 2 diabetes. In: Proceedings of the Endocrine Society Annual Meeting (ed Society E) (2015).
- 10.Stoehr JP, et al. Genetic obesity unmasks nonlinear interactions between murine type 2 diabetes susceptibility loci. Diabetes. 2000;49:1946–1954. doi: 10.2337/diabetes.49.11.1946. [DOI] [PubMed] [Google Scholar]
- 11.Campbell CT, Kolesar JE, Kaufman BA. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim. Biophys. Acta. 2012;1819:921–929. doi: 10.1016/j.bbagrm.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Filograna, R., Mennuni M., Alsina, D. & Larsson N. G. Mitochondrial DNA copy number in human disease: the more the better? FEBS Lett. 595, 976–1002 (2021). [DOI] [PMC free article] [PubMed]
- 13.Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17:491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell MB, Bush Z, McGinnis GR, Rowe GC. Adult skeletal muscle deletion of Mitofusin 1 and 2 impedes exercise performance and training capacity. J. Appl. Physiol. (1985) 2019;126:341–353. doi: 10.1152/japplphysiol.00719.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H, et al. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papanicolaou KN, et al. Mitofusins 1 and 2 are essential for postnatal metabolic remodeling in heart. Circ. Res. 2012;111:1012–1026. doi: 10.1161/CIRCRESAHA.112.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva Ramos E, et al. Mitochondrial fusion is required for regulation of mitochondrial DNA replication. PLoS Genet. 2019;15:e1008085. doi: 10.1371/journal.pgen.1008085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twig G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidarala, V. et al. Mitophagy protects beta cells from inflammatory damage in diabetes. JCI Insight5, e141138 (2020). [DOI] [PMC free article] [PubMed]
- 22.Chaudhry A, Shi R, Luciani DS. A pipeline for multidimensional confocal analysis of mitochondrial morphology, function, and dynamics in pancreatic beta-cells. Am. J. Physiol. Endocrinol. Metab. 2020;318:E87–E101. doi: 10.1152/ajpendo.00457.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemel I, Engelen BPH, Luber N, Gerards M. A hitchhiker’s guide to mitochondrial quantification. Mitochondrion. 2021;59:216–224. doi: 10.1016/j.mito.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Mulder H. Transcribing beta-cell mitochondria in health and disease. Mol. Metab. 2017;6:1040–1051. doi: 10.1016/j.molmet.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva JP, et al. Impaired insulin secretion and beta-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nat. Genet. 2000;26:336–340. doi: 10.1038/81649. [DOI] [PubMed] [Google Scholar]
- 26.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kersten S. Physiological regulation of lipoprotein lipase. Biochim. Biophys. Acta. 2014;1841:919–933. doi: 10.1016/j.bbalip.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Haythorne E, et al. Diabetes causes marked inhibition of mitochondrial metabolism in pancreatic beta-cells. Nat. Commun. 2019;10:2474. doi: 10.1038/s41467-019-10189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerst F, et al. The expression of aldolase B in islets is negatively associated with insulin secretion in humans. J. Clin. Endocrinol. Metab. 2018;103:4373–4383. doi: 10.1210/jc.2018-00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 31.Calvo SE, Clauser KR, Mootha VK. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016;44:D1251–D1257. doi: 10.1093/nar/gkv1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, et al. Human mitochondrial topoisomerase I. Proc. Natl Acad. Sci. USA. 2001;98:10608–10613. doi: 10.1073/pnas.191321998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng AC, Baird SD, Screaton RA. Essential role of TID1 in maintaining mitochondrial membrane potential homogeneity and mitochondrial DNA integrity. Mol. Cell Biol. 2014;34:1427–1437. doi: 10.1128/MCB.01021-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Noia MA, et al. The human SLC25A33 and SLC25A36 genes of solute carrier family 25 encode two mitochondrial pyrimidine nucleotide transporters. J. Biol. Chem. 2014;289:33137–33148. doi: 10.1074/jbc.M114.610808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zurita Rendon, O. & Shoubridge, E. A. LONP1 is required for maturation of a subset of mitochondrial proteins, and its loss elicits an integrated stress response. Mol. Cell Biol.38, e00412–17 (2018). [DOI] [PMC free article] [PubMed]
- 36.Venkatesh S, Lee J, Singh K, Lee I, Suzuki CK. Multitasking in the mitochondrion by the ATP-dependent Lon protease. Biochim. Biophys. Acta. 2012;1823:56–66. doi: 10.1016/j.bbamcr.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu B, et al. Roles for the human ATP-dependent Lon protease in mitochondrial DNA maintenance. J. Biol. Chem. 2007;282:17363–17374. doi: 10.1074/jbc.M611540200. [DOI] [PubMed] [Google Scholar]
- 38.Sebastian D, et al. Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J. 2016;35:1677–1693. doi: 10.15252/embj.201593084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman JR, et al. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis, S. C., Uchiyama, L. F. & Nunnari J. ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science353, aaf5549 (2016). [DOI] [PMC free article] [PubMed]
- 41.Murley A, et al. ER-associated mitochondrial division links the distribution of mitochondria and mitochondrial DNA in yeast. Elife. 2013;2:e00422. doi: 10.7554/eLife.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma H, et al. Germline and somatic mtDNA mutations in mouse aging. PLoS One. 2018;13:e0201304. doi: 10.1371/journal.pone.0201304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Area-Gomez E. Assessing the function of mitochondria-associated ER membranes. Methods Enzymol. 2014;547:181–197. doi: 10.1016/B978-0-12-801415-8.00011-4. [DOI] [PubMed] [Google Scholar]
- 44.Vance JE. Molecular and cell biology of phosphatidylserine and phosphatidylethanolamine metabolism. Prog. Nucleic Acid Res. Mol. Biol. 2003;75:69–111. doi: 10.1016/S0079-6603(03)75003-X. [DOI] [PubMed] [Google Scholar]
- 45.Ekstrand MI, et al. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet. 2004;13:935–944. doi: 10.1093/hmg/ddh109. [DOI] [PubMed] [Google Scholar]
- 46.Kang D, Kim SH, Hamasaki N. Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion. 2007;7:39–44. doi: 10.1016/j.mito.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 47.Kang I, Chu CT, Kaufman BA. The mitochondrial transcription factor TFAM in neurodegeneration: emerging evidence and mechanisms. FEBS Lett. 2018;592:793–811. doi: 10.1002/1873-3468.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsson NG, et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet. 1998;18:231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- 49.Matsushima Y, Goto Y, Kaguni LS. Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM) Proc. Natl Acad. Sci. USA. 2010;107:18410–18415. doi: 10.1073/pnas.1008924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu B, et al. Phosphorylation of human TFAM in mitochondria impairs DNA binding and promotes degradation by the AAA+ Lon protease. Mol. Cell. 2013;49:121–132. doi: 10.1016/j.molcel.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gensler S, et al. Mechanism of mammalian mitochondrial DNA replication: import of mitochondrial transcription factor A into isolated mitochondria stimulates 7S DNA synthesis. Nucleic Acids Res. 2001;29:3657–3663. doi: 10.1093/nar/29.17.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pohjoismaki JL, Goffart S. Of circles, forks and humanity: topological organisation and replication of mammalian mitochondrial DNA. Bioessays. 2011;33:290–299. doi: 10.1002/bies.201000137. [DOI] [PubMed] [Google Scholar]
- 53.Gustafson MA, Sullivan ED, Copeland WC. Consequences of compromised mitochondrial genome integrity. DNA Repair. 2020;93:102916. doi: 10.1016/j.dnarep.2020.102916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan K, et al. Mitochondrial SSBP1 protects cells from proteotoxic stresses by potentiating stress-induced HSF1 transcriptional activity. Nat. Commun. 2015;6:6580. doi: 10.1038/ncomms7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kasashima K, Sumitani M, Endo H. Human mitochondrial transcription factor A is required for the segregation of mitochondrial DNA in cultured cells. Exp. Cell Res. 2011;317:210–220. doi: 10.1016/j.yexcr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 56.West AP, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rocha AG, et al. MFN2 agonists reverse mitochondrial defects in preclinical models of Charcot-Marie-Tooth disease type 2A. Science. 2018;360:336–341. doi: 10.1126/science.aao1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo S, et al. Inactivation of specific beta cell transcription factors in type 2 diabetes. J. Clin. Investig. 2013;123:3305–3316. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishida E, Kim-Muller JY, Accili D. Pair feeding, but not insulin, phloridzin, or rosiglitazone treatment, curtails markers of beta-cell dedifferentiation in db/db mice. Diabetes. 2017;66:2092–2101. doi: 10.2337/db16-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li J, et al. Amorphous solid dispersion of Berberine mitigates apoptosis via iPLA2beta/Cardiolipin/Opa1 pathway in db/db mice and in Palmitate-treated MIN6 beta-cells. Int. J. Biol. Sci. 2019;15:1533–1545. doi: 10.7150/ijbs.32020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu J, et al. Rhein protects pancreatic beta-cells from dynamin-related protein-1-mediated mitochondrial fission and cell apoptosis under hyperglycemia. Diabetes. 2013;62:3927–3935. doi: 10.2337/db13-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao Z, et al. Protection of pancreatic beta-cells by group VIA phospholipase A(2)-mediated repair of mitochondrial membrane peroxidation. Endocrinology. 2010;151:3038–3048. doi: 10.1210/en.2010-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pommier Y, Sun Y, Huang SN, Nitiss JL. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol. 2016;17:703–721. doi: 10.1038/nrm.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wicksteed B, et al. Conditional gene targeting in mouse pancreatic ss-Cells: analysis of ectopic Cre transgene expression in the brain. Diabetes. 2010;59:3090–3098. doi: 10.2337/db10-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Georgiadou E. et al. Mitofusins <em>Mfn1</em> and <em>Mfn2</em> are required to preserve glucose-but not incretin- stimulated beta cell connectivity and insulin secretion. bioRxiv, 2020.2004.2022.055384 (2021). [DOI] [PMC free article] [PubMed]
- 66.Besse A, Brezavar D, Hanson J, Larson A, Bonnen PE. LONP1 de novo dominant mutation causes mitochondrial encephalopathy with loss of LONP1 chaperone activity and excessive LONP1 proteolytic activity. Mitochondrion. 2020;51:68–78. doi: 10.1016/j.mito.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peter B, et al. Defective mitochondrial protease LonP1 can cause classical mitochondrial disease. Hum. Mol. Genet. 2018;27:1743–1753. doi: 10.1093/hmg/ddy080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quiros PM, et al. ATP-dependent Lon protease controls tumor bioenergetics by reprogramming mitochondrial activity. Cell Rep. 2014;8:542–556. doi: 10.1016/j.celrep.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 69.Sebastian D, et al. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc. Natl Acad. Sci. USA. 2012;109:5523–5528. doi: 10.1073/pnas.1108220109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 71.Dingreville F, et al. Differential effect of glucose on ER-mitochondria Ca(2+) exchange participates in insulin secretion and glucotoxicity-mediated dysfunction of beta-cells. Diabetes. 2019;68:1778–1794. doi: 10.2337/db18-1112. [DOI] [PubMed] [Google Scholar]
- 72.Madec AM, Perrier J, Panthu B, Dingreville F. Role of mitochondria-associated endoplasmic reticulum membrane (MAMs) interactions and calcium exchange in the development of type 2 diabetes. Int. Rev. Cell Mol. Biol. 2021;363:169–202. doi: 10.1016/bs.ircmb.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 73.Rieusset J. Role of endoplasmic reticulum-mitochondria communication in type 2 diabetes. Adv. Exp. Med. Biol. 2017;997:171–186. doi: 10.1007/978-981-10-4567-7_13. [DOI] [PubMed] [Google Scholar]
- 74.Cosson P, Marchetti A, Ravazzola M, Orci L. Mitofusin-2 independent juxtaposition of endoplasmic reticulum and mitochondria: an ultrastructural study. PLoS One. 2012;7:e46293. doi: 10.1371/journal.pone.0046293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Filadi R, et al. Mitofusin 2 ablation increases endoplasmic reticulum-mitochondria coupling. Proc. Natl Acad. Sci. USA. 2015;112:E2174–E2181. doi: 10.1073/pnas.1504880112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nile DL, et al. Age-related mitochondrial DNA depletion and the impact on pancreatic Beta cell function. PLoS One. 2014;9:e115433. doi: 10.1371/journal.pone.0115433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cree LM, et al. Age-related decline in mitochondrial DNA copy number in isolated human pancreatic islets. Diabetologia. 2008;51:1440–1443. doi: 10.1007/s00125-008-1054-4. [DOI] [PubMed] [Google Scholar]
- 78.Chinnery PF, et al. Mitochondrial DNA haplogroups and type 2 diabetes: a study of 897 cases and 1010 controls. J. Med. Genet. 2007;44:e80. doi: 10.1136/jmg.2007.048876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miret-Casals L, et al. Identification of new activators of mitochondrial fusion reveals a link between mitochondrial morphology and pyrimidine metabolism. Cell Chem. Biol. 2018;25:268–278.e264. doi: 10.1016/j.chembiol.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 80.Bach D, et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54:2685–2693. doi: 10.2337/diabetes.54.9.2685. [DOI] [PubMed] [Google Scholar]
- 81.Montaigne D, et al. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation. 2014;130:554–564. doi: 10.1161/CIRCULATIONAHA.113.008476. [DOI] [PubMed] [Google Scholar]
- 82.Zorzano A, Liesa M, Palacin M. Mitochondrial dynamics as a bridge between mitochondrial dysfunction and insulin resistance. Arch. Physiol. Biochem. 2009;115:1–12. doi: 10.1080/13813450802676335. [DOI] [PubMed] [Google Scholar]
- 83.Thorens B, et al. Ins1(Cre) knock-in mice for beta cell-specific gene recombination. Diabetologia. 2015;58:558–565. doi: 10.1007/s00125-014-3468-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taddeo EP, et al. Individual islet respirometry reveals functional diversity within the islet population of mice and human donors. Mol. Metab. 2018;16:150–159. doi: 10.1016/j.molmet.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pearson G, et al. Clec16a, Nrdp1, and USP8 form a ubiquitin-dependent tripartite complex that regulates beta-cell mitophagy. Diabetes. 2018;67:265–277. doi: 10.2337/db17-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Soleimanpour SA, et al. The diabetes susceptibility gene Clec16a regulates mitophagy. Cell. 2014;157:1577–1590. doi: 10.1016/j.cell.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soleimanpour SA, et al. Calcineurin signaling regulates human islet {beta}-cell survival. J. Biol. Chem. 2010;285:40050–40059. doi: 10.1074/jbc.M110.154955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kolesar JE, Wang CY, Taguchi YV, Chou SH, Kaufman BA. Two-dimensional intact mitochondrial DNA agarose electrophoresis reveals the structural complexity of the mammalian mitochondrial genome. Nucleic Acids Res. 2013;41:e58. doi: 10.1093/nar/gks1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karamanlidis G, et al. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ. Res. 2010;106:1541–1548. doi: 10.1161/CIRCRESAHA.109.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reissaus CA, et al. A versatile, portable intravital microscopy platform for studying beta-cell biology in vivo. Sci. Rep. 2019;9:8449. doi: 10.1038/s41598-019-44777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iwashita H, et al. Live cell imaging of mitochondrial autophagy with a novel fluorescent small molecule. ACS Chem. Biol. 2017;12:2546–2551. doi: 10.1021/acschembio.7b00647. [DOI] [PubMed] [Google Scholar]
- 92.Konig J, et al. Mitochondrial contribution to lipofuscin formation. Redox Biol. 2017;11:673–681. doi: 10.1016/j.redox.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jayaraman S. A novel method for the detection of viable human pancreatic beta cells by flow cytometry using fluorophores that selectively detect labile zinc, mitochondrial membrane potential and protein thiols. Cytometry A. 2008;73:615–625. doi: 10.1002/cyto.a.20560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article and its Supplementary information files or from the corresponding author (S.A.S.) upon request. RNA sequencing data used for differential gene expression-, pathway- and GO- enrichment analyses have been deposited in the GEO database under the accession number GSE194204. The reference genome used for RNA sequencing was obtained from ENSEMBL (Mus_musculus - Ensembl genome browser 105). All uncropped western blots and data values for all figures are provided in the source data file. Source data are provided with this paper.
High-throughput RNA sequencing data was analyzed using DESeq2 (v1.26.0) to determine differential gene expression. iPathway Guide (Advaita version 1910) was used to perform pathway and GO-term analysis.