Abstract
Background
The decision of when to discontinue systemic treatment after achieving remission in psoriasis is an important question. In this systematic review, we sought to evaluate time to relapse after the discontinuation of systemic treatment in psoriasis patients.
Methods
Systematic searches of PubMed, Cochrane Library, and Embase databases were performed for randomized controlled studies reporting time to relapse after discontinuation of systemic drugs in psoriasis patients. In addition, pharmaceutical companies were contacted by the authors regarding missing data from the identified publications. In each publication, the time to psoriasis relapse and the timing of drug discontinuation were carefully assessed. The level of psoriasis control at the time of drug discontinuation and the definition used for psoriasis relapse were taken into account.
Results
Thirty articles published before April 2021 were included in the systematic review. Four articles focused on conventional systemic treatments with methotrexate and/or cyclosporine, nine focused on tumor necrosis factor (TNF) antagonists, eight focused on interleukin-17 (IL-17) antagonists, eight focused on IL-12/23 or IL-23 antagonists, and one focused on tofacitinib and apremilast. Different definitions were used to define psoriasis treatment success at the time of drug discontinuation. Similarly, heterogeneous criteria were used to define psoriasis relapse. Comparison between drugs was performed indirectly (i.e. across studies) for most drugs. Considering time of 50% loss of maximum Psoriasis Area Severity Index (PASI) improvement, a shorter median time to psoriasis relapse was observed with traditional systemic treatment (~ 4 weeks) compared to biological agents (from 12 to ~ 34 weeks). When using stringent relapse criteria, such as loss of PASI 90, a longer time to relapse after treatment cessation was observed with IL-23 antagonists (21–42 weeks) versus IL-17 antagonists (7–24 weeks).
Conclusion
Biological agents are associated with a longer time to relapse than oral systemic agents after drug discontinuation. Among biologicals, IL-23 antagonists are associated with the longest time to relapse. These findings may have clinical consequences for the selection of systemic agents when intermittent treatment is necessary.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40257-022-00679-y.
Key Points
| Different definitions are used to define psoriasis relapse after treatment discontinuation, which limits the comparison between studies. |
| Biological agents are associated with a longer time to relapse than oral systemic agents. |
| Among biologicals, interleukin-23 antagonists are associated with the longest time to relapse. |
Introduction
Biologics for psoriasis generally promote excellent disease control [1–3]. Recent practical data from experienced centers and international registries have shown both sustained treatment benefits and fairly good long-term safety with no major increase in serious infections, cancer, or mortality after more than 5 years of treatment [4–6].
Although discontinuation of biologics is associated with a risk of relapse, it may be advisable for several reasons, including a desire expressed by patients to reduce the treatment burden, treatment costs that cannot be covered, the occurrence of adverse events, and specific circumstances such as pregnancy. Time to relapse following treatment discontinuation appears to be very short with some therapeutic agents [7]. Most registration programs for new dermatology drugs include studies with a treatment withdrawal period to assess the potential for rebound effects and to determine the time to psoriasis relapse. The treatment withdrawal period can also be used to assess the effectiveness of treatment reintroduction.
The analysis of the dynamics of psoriasis relapse during the treatment withdrawal period provides relevant information regarding the potential usage of the drug in the clinic.
This systematic review aims to estimate the psoriasis relapse time after systemic drug discontinuation in psoriasis.
Material and Methods
The systematic review was prospectively registered at the PROSPERO register under number CRD42021252182.
Inclusion Criteria
We included randomized controlled trial studies that investigated the time to relapse following discontinuation of systemic treatment with fumarates, acitretin, methotrexate, cyclosporine, infliximab, etanercept, adalimumab, certolizumab pegol, ustekinumab, secukinumab, ixekizumab, brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, tofacitinib, or apremilast in patients with plaque psoriasis.
Literature Search
For the literature search, we have used the recent Cochrane skin group systematic review from November 2019 [1]. From the 140 studies included, we selected those that included a withdrawal period. A supplementary literature search from the earliest available date to April 2021 was performed in the PubMed, Cochrane Library, and Embase databases using the keywords ("Psoriasis"[Mesh] OR "Plaque psoriasis"[tiab]) AND ("Fumarates"[Mesh] OR "acid fumaric"[tiab] OR "Acitretin"[Mesh] OR "Acitretin"[tiab] OR "Methotrexate"[Mesh] OR "Methotrexate"[tiab] OR "Cyclosporine"[Mesh] OR "Cyclosporine"[tiab] OR "Ciclosporine"[tiab] OR "Infliximab"[Mesh] OR "Infliximab"[tiab] OR "Etanercept"[Mesh] OR "Etanercept"[tiab] OR "Adalimumab"[Mesh] OR "Adalimumab"[tiab] OR "Certolizumab Pegol"[Mesh] OR "Certolizumab Pegol"[tiab] OR "Ustekinumab"[Mesh] OR "Ustekinumab"[tiab] OR "Secukinumab"[Mesh] OR "Secukinumab"[tiab] OR "Ixekizumab"[Mesh] OR "Ixekizumab"[tiab] OR "Brodalumab"[Mesh] OR "Brodalumab"[tiab] OR “Bimekizumab”[Mesh] OR “Bimekizumab”[tiab] OR "Guselkumab"[Mesh] OR "Guselkumab"[tiab] OR "Tildrakizumab"[Mesh] OR "Tildrakizumab"[tiab] OR "Risankizumab"[Mesh] OR "Risankizumab"[tiab] OR "Tofacitinib"[Mesh] OR "Tofacitinib"[tiab] OR "Apremilast"[Mesh] OR "Apremilast"[tiab]) AND ("Drug Tapering"[Mesh] OR Recurrence[Mesh] OR Recurrence[tiab] OR "Withdrawal"[tiab] OR "Withdrawn"[tiab] OR "Discontinue"[tiab] OR "Discontinuation"[tiab] or "Stop"[tiab] OR "Stopped"[tiab] OR "Maintenance"[tiab] OR "Relapse"[tiab] OR "Interruption"[tiab]) AND ("Randomized Controlled Trial"[Publication Type] OR "Randomized"[tiab]) (see the “Supplementary Information” in the electronic supplementary material). The reference lists of included articles were also screened manually for additional studies. Moreover, we contacted individual pharmaceutical companies to provide data missing from the published studies on the time of relapse post-drug withdrawal.
Data Extraction
The authors (Marie Masson Regnault, Jason Shourick, and Carle Paul) independently searched the databases for potentially relevant article titles and abstracts based on the inclusion criteria. Full text articles were then retrieved. We used the modified Cochrane Collaboration tool to assess risk of bias for randomized controlled trials. Bias was assessed as a judgment (high, low, or unclear) for individual elements from five domains (selection, performance, detection, attrition, and reporting bias) (see the “Supplementary Information” in the electronic supplementary material). The authors were independently involved in all stages of study selection and data extraction in terms of the number of patients enrolled in all of the studies, the number of patients in the withdrawal period, the criteria used for treatment withdrawal, the criteria defining relapse, post-withdrawal follow-up time, and the time to relapse.
Results
Article Selection
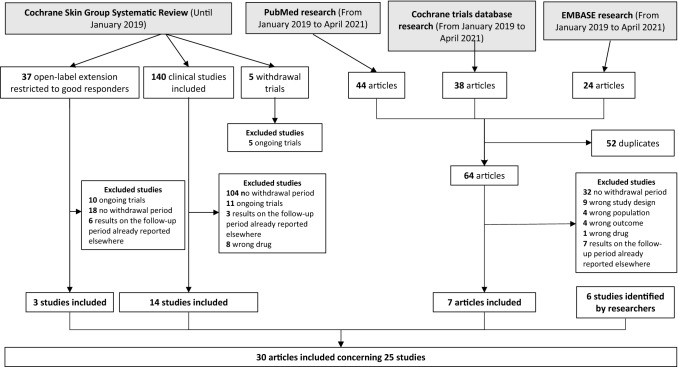
Overall, 30 original articles published before April 2021 were included in this review, but several papers report on the same study. Thus, we included 25 studies that report on 27 drug data points about time to relapse (Fig. 1). They are presented in Table 1. No randomized controlled trial was found investigating time to relapse with fumarates or acitretin. Four studies focused on conventional systemic treatments with methotrexate and/or cyclosporine [8–11], nine focused on tumor necrosis factor (TNF) antagonists [12–20], six focused on interleukin-17 (IL-17) antagonists [21–27, 37], six focused on IL-12/23 or IL-23 antagonists [15, 28–34], and one study each focused on tofacitinib [35] and apremilast [36]. The number of patients enrolled in these withdrawal-period studies varied from 14 to 917 (mean ± standard deviation, 217.75 ± 191.7; median [interquartile range (IQR)] 186 [84–356.5]).
Fig. 1.
Article selection
Table 1.
Characteristics of the treatment withdrawal period and relapse reported in the studies
| Drugs | References | No. of patients | Dose regimen | Efficacy criteria | No. of patients in withdrawal group | Relapse criteria (bold = criteria for retreatment) | Last treatment administration | Withdrawal period | Follow-up period (weeks) | Time to relapse after the last injection |
|---|---|---|---|---|---|---|---|---|---|---|
| Conventional systemic treatment | ||||||||||
| Methotrexate | Heydendael 2003 [9] | 44 | 15 mg/week | PASI 90 | 17 | 50% loss of maximum PASI improvement or treatment needed | W16 | – | – | Median: 4 weeks |
| Cyclosporine | Okubo 2011 [8] | 143 | 3 mg/kg/day | PASI 75 | 25 | Required treatment course | – | – | – | Median: 66.5 days (~ 9.5 weeks) mean: 182.0 ± 232.7 days (range 0–906) |
| Heydendael 2003 [9] | 44 | 3 mg/kg/day | PASI 90 | 14 | 50% loss of maximum PASI improvement or treatment needed | – | – | – | Median: 4 weeks | |
| Ellis 1995 [10] | 61 | 3 mg/kg/day | PGA 0–1 | 20 | Two-point worsening on a 7-point scale | W4 | W4 to W16 | 12 | Median: 6 weeks* | |
| Ho 1999 [11] | 400 | 5 mg/kg/day | PASI 75 | 192 | Required treatment course | W12 | Until relapse | 52 | Median: 112 days (~ 16 weeks) | |
| TNF antagonist | ||||||||||
| Etanercept | Gordon 2006 [12] | 652 | 3 arms: 50 mg twice weekly, 25 mg twice weekly, 25 mg once weekly | PASI 50 | 409 |
Loss of PASI 75 Loss of PASI 50 50% loss of maximum PASI improvement |
W24 | Until relapse | Until relapse |
Loss of PASI 75—median: ~ 8 weeks (57 days) Loss of PASI 50—median: ~ 13 weeks (91 days) 50% loss in maximum PASI improvement—median: 12 weeks (85 days) |
| Moore 2007 [13] | 2546 | 50 mg twice weekly | PGA ≤ 2 | 917 | Loss of PGA ≤ 2 | W12 | W12 to W20 | 8 | Median: 33 days (~ 4.7 weeks) mean: 39.6 days | |
| Ortonne 2009 [14] | 363 | 50 mg twice weekly | PGA ≤ 2 | 240 | Loss of PGA ≤ 2 | W12 | Until relapse | Until relapse | Median: 51 days (~ 7 weeks) mean: 72 (SD 46) days | |
| Griffiths 2010 [15] | 903 | 50 mg twice weekly vs. ustekinumab | PASI 75 | 245 | Loss of PGA ≤ 2 | W11 | Until relapse | Until relapse | Median: 7.3 weeks | |
| Adalimumab | Menter 2008 [16] | 1212 | 80 mg W0 and 40 mg every other week | PASI 75 | 240 | Loss of PASI 50 and gain of more than 6 points in PASI score | W32 | W33 to W52 | 20 | Median: > 23 weeks* |
| Papp 2011 [17] | 1468 | 80 mg W0 and 40 mg/week | PGA 0–1 | 525 | Loss of PGA ≤ 2 | W32 | W33 to W52 | 20 | Median: 141 days (IQR 93–202) (~ 20.1 weeks) | |
| Infliximab | Gottlieb 2004 [18] | 249 | 5 mg/kg (W0, W2, W6) | PASI 75 | 99 |
Loss of PASI 75 Loss of PGA ≤ 2 |
W6 | W6 to W26 | 20 |
Loss of PASI 75—median: ~ 15 weeks* Loss of PGA ≤ 2—median unpublished |
| Menter 2007 [19] | 835 | 5 mg/kg (W0, W2, W6) | – | 149 | Loss of PASI 75 | W6 | W14 to W50 | 36 | Median between 8 and 16 weeks | |
| Certolizumab | Reich 2012 [20] | 176 | 200 or 400 mg q2w | PASI 75 or PGA 0–1 | 148 | 50% loss of maximum PASI improvement | W10 | W12 to W36 | 24 | Median: 22 weeks (200 mg) or 20 weeks (400 mg) |
| IL-17 antagonist | ||||||||||
| Secukinumab | Rich 2013 [21] | 404 | 150 mg regimens at W0, 4, and 8 | PASI 75 | 67 | One-third loss of maximum PASI improvement | W8 | 45 weeks | 45 | Median: 24 weeks* |
| Mrowietz 2015 [22] | 966 | 300 or 150 mg W1, 2, 3, 4, and 8 | PASI 75 | 423 | 20% loss of maximum PASI improvement and loss of PASI 75 | W12 | W12 to W52 | 40 | 20% loss of maximum PASI improvement—median: 20 weeks (150 mg) and 24 weeks (300 mg) | |
| Blauvelt 2017 [23] (supplemental data from lab) | 1147 | 300 mg W1, 2, 3, 4, and then q8w | PASI 75 | 181 |
Loss of PASI 90 Loss of PASI 75 50% loss of maximum PASI improvement |
W52 | Until relapse | Until relapse |
Loss of PASI 90—median: 16 weeks* (300 mg) Loss of PASI 75—median: 20 weeks* (300 mg) 50% loss of maximum PASI improvement—median: 28.0 weeks (95% CI 24.14–32.00) |
|
| Ixekizumab | Gordon 2016, Blauvelt 2017 [24, 25] (supplemental data from lab) | 1296 | 160 mg at week 0, then 80 mg q2w or q4w | PGA 0–1 or PASI 90 | 191 |
Loss of PASI 100 Loss of PASI 90 Loss of PASI 75 Loss of PGA ≤ 2 |
W8 | W12 to W60 | 48 |
Loss of PASI 100—median: 12.1 weeks (9.0–13.0) Loss of PASI 90—median: 16.1 weeks (12.7–16.4) Loss of PASI 75—median: 20.1 weeks (17.1–20.6) Loss of PGA ≤ 2—median: 20 weeks |
| Brodalumab | Papp 2016 [26], Papp 2020 [27] | 661 | 140 or 210 mg q2w | PASI 75 or PGA 0–1 | 84 |
Loss of PASI 100 Loss of PASI 90 Loss of PGA 0–1 Loss of PGA ≤ 2 |
W10 | W12 to W120 | 108 |
Loss of PASI 100—median: 6 weeks (210 mg)* Loss of PASI 90—median: 7 weeks (210 mg)* Loss of PGA 0–1—median: 7 weeks (210 mg)* Loss of PGA ≤ 2—median: 56 days after start of placebo (~ 9 weeks after the last dose of treatment)/mean ± SD: 74.7 ± 50.5 days |
| Bimekizumab | Gordon 2021 [37] | 435 | 320 mg q4w | PASI 90 | 105 | Loss of PASI 75 | W16 | W20 to W56 | 36 |
Loss of PASI 100—median: 18 weeks* Loss of PASI 90—median: 24 weeks* Loss of PASI 75—median: 32 weeks |
| IL-12/23 or 1L-23 antagonist | ||||||||||
| Ustekinumab | Leonardi 2008 [28] (supplemental data from lab) | 766 | 45 or 90 mg q12w | PASI 75 | 160 |
Loss of PASI 90 Loss of PASI 75 50% loss of maximum PASI improvement |
W28 | W40 to W76 | 36 |
Loss of PASI 90—median: 20.6 weeks Loss of PASI 75—median: 15 weeks 50% loss of maximum PASI improvement—median unpublished |
| Griffiths 2010 [15] | 903 | 45 or 90 mg at W0 and W4 | PASI 75 | 399 | Loss of PGA ≤ 2 | W4 | Until relapse | Until relapse | Median: 14.4 weeks (45 mg) and 18.1 weeks (90 mg) | |
| Risankizumab | Blauvelt 2020 [29] | 507 | 150 mg at W0, W4, and then q12w | PGA 0–1 | 225 |
Loss of PASI 100 Loss of PGA 0 Loss of PASI 90 Loss of PGA 0–1 Loss of PGA ≤ 2 |
W16 | W28 to W52 | 24 |
Loss of PASI 100—median: 36 weeks* Loss of PGA 0—median: 36 weeks* Loss of PASI 90—median: 42 weeks Loss of PGA 0–1—median: 44 weeks* Loss of PGA ≤ 2—median: 295 days (IQR 211–428 days) ~ 54 weeks |
| Tildrakizumab | Kimball 2020, Warren 2020 [30, 31] | 1671 | 100/200 mg at W0, W4, q12w | PASI 75 | 233 |
Loss of PGA 0–1 Loss of PASI 90 Loss of PASI 75 50% loss of maximum PASI improvement |
W16 | W28 to W64 | 36 |
Loss of PGA 0–1—median 28 weeks (100 mg) and 28 weeks (200 mg) Loss of PASI 90—median 27.9 weeks (100 mg) and 32 weeks (200 mg) Loss of PASI 75—median 32.3 weeks (100 mg) and 36.6 weeks (200 mg) 50% loss of maximum PASI improvement—median: 24 weeks (100 or 200 mg) |
| Cantrell 2021 [32] (supplemental data from lab) | 772 | 100 mg at W0, W4, q12w | PASI 75 | 113 | 50% loss of maximum PASI improvement | W16 | W28 to W64 | 36 | 50% loss of maximum PASI improvement—median: 238 days (167–294) (~ 34 weeks) (100 mg) | |
| Guselkumab | Reich 2017, Gordon 2019 [33, 34] (supplemental data from lab) | 992 | 100 mg at W0, W4, and q8w | PASI 90 or IGA 0–1 | 329 |
Loss of PASI 100 Loss of PGA 0 Loss of PASI 90 Loss of PGA 0–1 Loss of PASI 75 50% loss of maximum PASI improvement |
W20 | W28 to W48 | 20 |
Loss of PASI 100—median: 20 weeks* Loss of PGA 0—median: 20.1 weeks Loss of PASI 90—median: 23 weeks Loss of PGA 0–1—median: 27.6 weeks Loss of PASI 75: median ~ 32 weeks* 50% loss of maximum PASI improvement—unpublished data |
| Small molecule inhibitors | ||||||||||
| Tofacitinib | Bissonnette 2015 [35] | 666 | 5 or 10 mg twice daily | PASI 75 and PGA 0–1 | 291 |
Loss of PASI 75 50% loss of maximum PASI improvement |
W24 | W24 to W40 | 16 |
Loss of a PASI 75—median: 8 weeks 50% loss of maximum PASI improvement—median: 16.1 weeks |
| Apremilast | Papp 2015 [36] | 844 | 30 mg twice daily | PASI 75 | 77 | Loss of PASI 75 | W32 | W32 to W52 | 20 | Median: 5.1 weeks (95% CI 4.1–8.1) |
CI confidence interval, IL interleukin, PASI Psoriasis Area Severity Index, IQR interquartile range, PGA/IGA Physician/Investigator’s Global Assessment scale, qXw every X weeks, TNF tumor necrosis factor, W week
*Data extrapolated from graphs provided in the study
Therapeutic Success Criteria Allowing Patients to Participate in the Withdrawal Period
The therapeutic success criteria determining inclusion of patients in the withdrawal period varied across studies. In three studies, patients had to attain Psoriasis Area Severity Index (PASI) 50 [12] or Physician’s Global Assessment (PGA) ≤ 2 [13, 14]. Patients had to achieve PASI 75 (± PGA 0–1), PASI 90 (± PGA 0–1), or only PGA 0–1 in 15 [8, 11, 15, 16, 18, 20–23, 26, 28, 30, 32, 35, 36], four [9, 24, 33, 37], and three studies [10, 17, 29], respectively. The therapeutic success criteria became more stringent in recent studies. Indeed, the achievement of PASI 90 or PGA 0–1 was used as a primary end point for the first time with the ixekizumab study published by Gordon et al. in 2016 and thereafter for the brodalumab, guselkumab, risankizumab, and bimekizumab studies [24]. The onset of the withdrawal period varied from 4 to 40 weeks after the beginning of treatment.
Relapse-Defining Criteria
Similarly, heterogenous criteria were used across different studies to define psoriasis relapse. Relapse was sometimes defined by multiple criteria in the same study (Table 1). The definition of relapse became more stringent in the most recent studies evaluating biological agents.
The duration of follow-up after treatment discontinuation varied from 8 to 108 weeks [mean 33.1 ± 21.58—median 30 (10–20)].
Time to Relapse
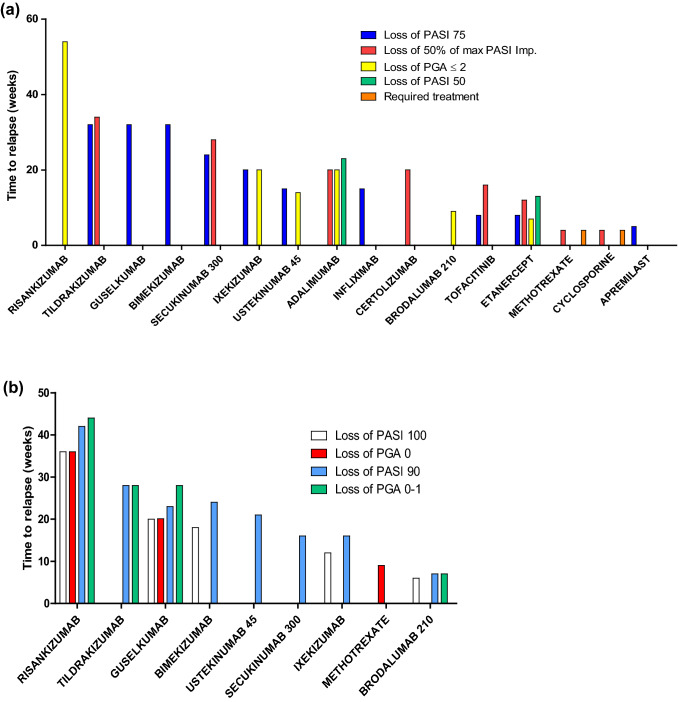
Given the variability in the relapse evaluation criteria, we have computed the time to relapse for each molecule under consideration, ranging from the longest to the shortest time to relapse (Fig. 2a, b). In most cases, the comparison between drugs was indirect. The time to loss of PASI 75 was compared between etanercept and ustekinumab in one study [15].
Fig. 2.
a Median time to relapse (in weeks) depending on the treatment, with less stringent criteria defining relapse, e.g., loss of PASI 75, loss of PASI 50, 50% loss of PASI maximum improvement, loss of PGA ≤ 2, or when patients felt they needed to resume systemic treatment. b Median time to relapse (in weeks) depending on the treatment, with stringent criteria defining relapse, e.g., loss of PASI 100, loss of PASI 90, or loss of PGA 0 or 1. Imp improvement, PASI Psoriasis Area Severity Index, PGA Physician’s Global Assessment
Oral Systemic Treatment
For patients treated with oral cyclosporine, four studies evaluated time to relapse in 25, 14, 20, and 192 patients, respectively [8–11]. Each study used a different relapse definition. Relapse was defined as the moment when patients felt a new course of cyclosporine was required, as a 2-point reduction in a 7-point severity scale, or as a 50% loss of maximum PASI improvement. The median length of time to the perceived need for a new course of cyclosporine treatment varied from 9.5 to 16 weeks. The median time to loss of 50% of maximum PASI improvement was about 4 weeks. For methotrexate patients in a small study (n = 17), relapse was defined as a 50% loss of maximum PASI improvement or when the patients felt treatment was needed [9]. Similarly to cyclosporine, the median time to loss of 50% of maximum PASI improvement was 4 weeks (Fig. 2a).
TNF Antagonists
Four studies focused on etanercept [12–15], two studies each focused on adalimumab [16, 17] and infliximab [18, 19], and one study focused on certolizumab pegol [20]. A higher number of patients were included in the withdrawal period in the TNF antagonist studies compared to conventional systemic treatment, ranging from 99 to 917 patients [mean 330.2 ± 257.8; median 240 (149–409)]. The duration of follow-up ranged from 8 to 36 weeks. Time to relapse was defined as loss of PGA ≤ 2 in five studies [13–15, 17, 18], an increase of more than 6 points in the PASI score in one study [16], a loss of PASI 50 in two studies [12, 16], a 50% reduction in maximum PASI response in two studies [12, 20], and a loss of PASI 75 in three studies [12, 18, 19]. The median time to loss of PGA ≤ 2 varied from 4.7 to 7.3 weeks for etanercept and was estimated to be about 20.1 weeks for adalimumab. The median time to loss of PASI 50 for etanercept and adalimumab was estimated to be about 13 and 23 weeks, respectively. The median time to loss of PASI 75 was about 8.1 weeks for etanercept and approximately 15 weeks for infliximab. The median time to loss of 50% loss of maximum PASI improvement was about 12 weeks for etanercept and 22 weeks and 20 weeks for certolizumab pegol 200 mg and 400 mg, respectively (Fig. 2a).
IL-17 Antagonists
Three studies were identified for secukinumab [21–23], two for ixekizumab [24, 25], two for brodalumab [26, 27], and one for bimekizumab [37]. The number of patients included varied from 67 to 423 patients [mean 171.6 ± 134.1; median 132 (84–188.5)]. The time to relapse was reported as a loss of PGA ≤ 2 in four studies [24–27], a one-third loss of maximum PASI improvement in one study [21], a loss of 50% of maximum PASI improvement in one study [23], a loss of PASI 75 for three studies [22, 24, 25, 37], a loss of PASI 90 in four studies [23, 24, 26, 37], a loss of PASI 100 in three studies [24, 26, 37], and a loss of PGA 0–1 in one study [26]. The median time to loss of PGA ≤ 2 was about 20 weeks for ixekizumab and 9 weeks for brodalumab. The median time to loss of PASI 75 varied from 20 to 24 weeks for secukinumab 300 mg. It was about 20 weeks for ixekizumab and estimated at 32 weeks for bimekizumab. The median time to loss of PGA 0–1 for brodalumab was estimated to be between 6 and 7 weeks. For brodalumab, ixekizumab, secukinumab 300 mg, and bimekizumab, the median time to loss of PASI 90 was estimated to be 7 weeks, 16 weeks, 16 weeks, and 24 weeks, respectively. For brodalumab, ixekizumab, and bimekizumab, the median time to loss of PASI 100 was estimated to be 6 weeks, 12 weeks, and 18 weeks, respectively (Fig. 2a, b).
IL-12/23 and IL-23 Antagonists
We included two studies for ustekinumab [15, 28], two for guselkumab [33, 34], one for risankizumab [29], and three for tildrakizumab [30–32]. The number of patients included in the withdrawal period ranged from 113 to 399 [mean 243.2 ± 105.8; median 229 (176.3–305)]. Time to relapse was reported as a loss of 50% maximum PASI improvement in nine studies [9, 12, 20, 23, 28, 30–35], a loss of PASI 75 in five studies [28, 30–33], a loss of PASI 90 in six studies [28, 29, 31–34], a loss of PGA 0–1 in three studies [26, 27, 29–31], and a loss of PASI 100 in two studies [29, 33]. The median time to loss of PASI 75 was about 15 weeks for ustekinumab compared to over 28 weeks for guselkumab and about 32.3 for tildrakizumab (100 mg). The median time to loss of PASI 90 was estimated at 20 weeks for ustekinumab, between 23 and 24 weeks for guselkumab, 27.9 weeks for tildrakizumab (100 mg), and 42 weeks for risankizumab. The median time to loss of PGA 0–1 was estimated to be 28 weeks for guselkumab, 28 weeks for tildrakizumab (100 mg), and 44 weeks for risankizumab (Fig. 2a, b).
Small Molecule Inhibitors
We found one tofacitinib study including 291 patients in the withdrawal period [35]. Patients who obtained PASI 75 and PGA 0–1 responses were selected to enter the withdrawal period. The relapse was defined as a 50% loss in maximum PASI improvement during initial treatment or a loss of a PASI 75 response. Median time to a 50% loss of maximum PASI response was 16.1 weeks. Median time to a loss of PASI 75 response was 8 weeks (Fig. 2a). We found one study for apremilast [36]. The relapse was defined as a loss of PASI 75. The median time to initial loss of PASI 75 after re-randomization at week 32 was 5.1 weeks (95% confidence interval 4.1–8.1) (Fig. 2a).
Drug Half-Lives
The mean terminal elimination half-life of different drugs was collected from product information or public assessment reports available on the European Medicines Agency’s (EMA) website. These are presented from the longest to the shortest maintenance response we determined in the previous subsection (Fig. 3).
Fig. 3.
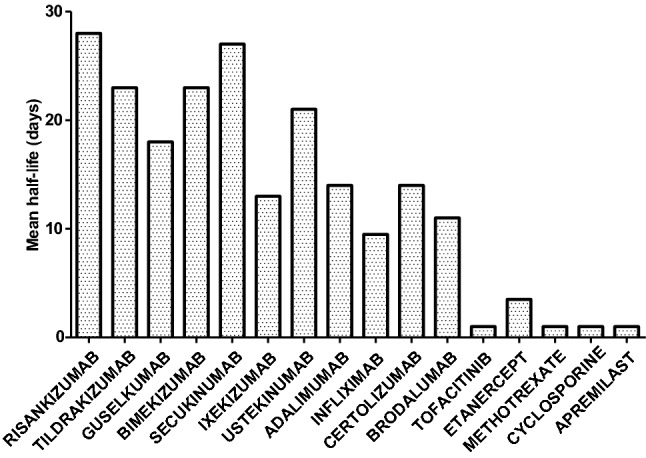
Mean terminal elimination half-life (in days) of evaluated drugs. Data were extracted from the product information or public assessment report for each drug (available at https://www.ema.europa.eu/en). Drugs are ranked from left to right from the longest to the shortest time to relapse.
Discussion
Systemic treatment is used as induction and maintenance therapy in patients with moderate to severe psoriasis. In a real-life setting, discontinuation of treatment may be triggered by life events such as pregnancy or concomitant disease. It may also be motivated by a patient’s desire to have drug-free intervals or to reduce the treatment burden.
Based on indirect comparison, overall, we noted a longer period of maintained response after discontinuation of biological agents as compared with oral systemic treatment. Moreover, treatment with IL-12/23 and IL-23 antagonists showed a tendency for a longer time to relapse compared to IL-17 and TNF antagonists. It should be noted that due to clinical trial constraints, the drug discontinuation was performed relatively early. It remains to be determined whether discontinuation after a long period in remission would produce the same results. Future studies are needed to investigate whether drug discontinuation after sustained complete remission of psoriasis can be associated with long-term psoriasis clearance in some patients. The time to relapse after treatment discontinuation is particularly interesting in clinical settings where the use of systemic agents may be detrimental. A recent example is the worldwide coronavirus disease 2019 (COVID-19) pandemic, during which practitioners were initially concerned about maintaining treatment for patients until reassuring data were published [38]. In 2010, Kamaria et al. produced a non-systematic review comparing biologics and their respective times to relapse [39]. They showed that alefacept offered the longest clinical benefit, followed by ustekinumab, infliximab, adalimumab, etanercept, and lastly, efalizumab. More recently, Wang et al. reported a short non-systematic review on time to relapse after biologic treatment withdrawal including observational studies. They suggested that IL-23 inhibitors are associated with a longer time to relapse after treatment withdrawal than TNF inhibitors [40].
Observational studies provide additional information about real-life psoriasis relapse following the discontinuation of biologic agents in psoriasis. Bellinato et al. reported, in 53 patients treated with etanercept and achieving PASI 0/1, that 33 (62%) relapsed within 6 months after drug discontinuation. The median time to relapse (defined as PASI score variation ≥ 5) was 184 days (~ 26 weeks) [41]. Giunta et al. reported a median time to loss of PASI 50 of 182 days (range 84–308 days) (~ 26 weeks in patients experiencing a PASI 75 response to infliximab) [42]. For IL-17 antagonists, we reported a 46-day median time to psoriasis relapse for patients stopping brodalumab [7]. Umezawa et al. reported, in PASI 75 responders to ixekizumab, that approximately half of the patients relapsed (loss of PASI 50) within 5 months after drug discontinuation. [43]. Rivera et al. reported a median time from the last dose of biologics to the initiation of new systemic treatment (relapse) of 192.5 days (IQR 107–308 days) (~ 27 weeks) for patients treated with secukinumab [44]. In an 8-year multicenter retrospective study, Chiu et al. reported in 202 patients responding to ustekinumab a median time to relapse (loss of 50% of the PASI maximum improvement) of 6.7 ± 4.1 months. Possible clinical predictors for longer relapse times were being biologic-naive, having a shorter psoriasis duration, absence of arthritis or chronic kidney disease, absence of family history of psoriasis, and higher and more rapid response to treatment [45]. Rivera et al. reported a median time to relapse of 282 days in patients treated with guselkumab [44]. The conclusions about these findings are limited by the very large and variable definitions used for defining relapse. Down-titration and discontinuation strategies of a biological treatment are also a topic of research in other inflammatory diseases, such as rheumatoid arthritis. It has been shown that fixed-dose reduction of TNF inhibitors is associated with maintenance of efficacy, whereas drug withdrawal leads to disease relapse [46].
One important factor to consider when evaluating continued drug effects after treatment discontinuation is the persistence of the drug in the body. Based on pharmacokinetic data, maintained response following drug discontinuation appears to partially correlate with the mean drug half-lives. Overall, the longest time to relapse was recorded with drugs with the longest half-lives. The difference in efficacy maintenance between drugs cannot be solely explained by drug half-lives. Other factors may play a role, such as molecular target and site of action in the psoriasis inflammation cascade.
The main study limitation is the scarcity of published data currently available on clinical study withdrawal periods and the considerable variability in the criteria used to define optimal clinical response before treatment discontinuation and relapse during the withdrawal period. Relapse after the discontinuation of oral systemic agents and TNF antagonists have mostly been evaluated using less-stringent measures. Only limited data were available for traditional systemic treatments such as methotrexate and cyclosporine, and no data exists for acitretin or fumarates. A consensus approach to the definition of psoriasis relapse in the treatment withdrawal period of clinical studies is needed, taking both patient and physician perspectives into account. The absence of access to individual patient data does not allow assessment of the role of demographic factors [e.g., body mass index (BMI), age, sex] or clinical variables such as baseline disease severity or age of onset of psoriasis. The effects of discontinuation of systemic therapy in psoriasis patients are unclear. In the case of molecules with long-term efficacy after discontinuation, evidence suggests that it may be possible to increase the interval between doses in some patients for IL-23 antagonists. Further high-quality research is needed in this area, with particular emphasis on double-blind comparative randomized controlled trials in which biologic therapy or immunosuppressants are withdrawn, as well as registry data on the real-life efficacy and safety of biologic discontinuation in patients.
Conclusion
The data from this systematic review suggest that biological agents are associated with improved maintenance of the effect and longer time to relapse compared to oral systemic agents. Among biologicals and when considering the most stringent criteria, it seems that IL-23 antagonists are associated with a longer time to relapse post-withdrawal compared to other psoriasis treatments. These findings may have clinical consequences on the selection of systemic agents in certain situations. Indeed, when the need for systemic treatment discontinuation can be anticipated, such as for scheduled surgery, planned pregnancy, or long-term travel to countries with limited access to healthcare resources, IL-23 antagonists may be preferred based on their persistent effect.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank Maria Jazra (Janssen), Coraline Bellagarde (Novartis), Volker Koscielny (Almirall), Gaia Gallo (Lilly), and Philippe Kauffmann and Anouk Vaslet de Fontaubert (AbbVie) for providing missing data from some publications.
Declarations
Ethics declarations
Not applicable.
Funding
Not applicable.
Conflict of interest
MMR has received grants or personal fees from AbbVie, Lilly, Janssen, UCB, and Leo Pharma. MT is a speaker for Janssen. CP has received grants and personal fees from Amgen, AbbVie, Celgene, Eli Lilly, Novartis, Janssen, Pfizer, and Leo Pharma. JS and FJ have no conflicts of interest to declare.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Yes.
Code availability
On demand.
Author contributions
MMR, JS, and CP performed the literature research and the analysis of the data. All the authors participated in the interpretation of the results and in the writing of the manuscript.
Contributor Information
Marie Masson Regnault, Email: m.massonregnault@gmail.com.
Carle Paul, Email: paul.c@chu-toulouse.fr.
References
- 1.Sbidian E, Chaimani A, Afach S, Doney L, Dressler C, Hua C, Mazaud C, Phan C, Hughes C, Riddle D, Naldi L, Garcia-Doval I, Le Cleach L. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2020;1:011535. doi: 10.1002/14651858.CD011535.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140:645–653. doi: 10.1016/j.jaci.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehncke W-H, Schön MP. Psoriasis. Lancet. 2015;386:983–994. doi: 10.1016/S0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- 4.Reich K, Mrowietz U, Radtke MA, Thaci D, Rustenbach SJ, Spehr C, Augustin M. Drug safety of systemic treatments for psoriasis: results from The German Psoriasis Registry PsoBest. Arch Dermatol Res. 2015;307:875–883. doi: 10.1007/s00403-015-1593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egeberg A, Ottosen MB, Gniadecki R, Broesby-Olsen S, Dam TN, Bryld LE, Rasmussen MK, Skov L. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178:509–519. doi: 10.1111/bjd.16102. [DOI] [PubMed] [Google Scholar]
- 6.Papp K, Gottlieb AB, Naldi L, Pariser D, Ho V, Goyal K, Fakharzadeh S, Chevrier M, Calabro S, Langholff W, Krueger G. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) J Drugs Dermatol. 2015;14:706–714. [PubMed] [Google Scholar]
- 7.Masson Regnault M, Konstantinou M-P, Khemis A, Poulin Y, Bourcier M, Amelot F, Bulaï Livideanu C, Paul C. Early relapse of psoriasis after stopping brodalumab: a retrospective cohort study in 77 patients. J Eur Acad Dermatol Venereol. 2017;31:1491–1496. doi: 10.1111/jdv.14387. [DOI] [PubMed] [Google Scholar]
- 8.Okubo Y, Natsume S, Usui K, Amaya M, Tsuboi R. Low-dose, short-term ciclosporin (Neoral®) therapy is effective in improving patients’ quality of life as assessed by Skindex-16 and GHQ-28 in mild to severe psoriasis patients. J Dermatol. 2011;38:465–472. doi: 10.1111/j.1346-8138.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 9.Heydendael VMR, Spuls PI, Opmeer BC, de Borgie CAJM, Reitsma JB, Goldschmidt WFM, Bossuyt PMM, Bos JD, de Rie MA. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658–665. doi: 10.1056/NEJMoa021359. [DOI] [PubMed] [Google Scholar]
- 10.Ellis CN, Fradin MS, Hamilton TA, Voorhees JJ. Duration of remission during maintenance cyclosporine therapy for psoriasis. Relationship to maintenance dose and degree of improvement during initial therapy. Arch Dermatol. 1995;131:791–795. doi: 10.1001/archderm.1995.01690190043008. [DOI] [PubMed] [Google Scholar]
- 11.Ho VC, Griffiths CE, Albrecht G, Vanaclocha F, León-Dorantes G, Atakan N, Reitamo S, Ohannesson A, Mørk NJ, Clarke P, Pfister P, Paul C. Intermittent short courses of cyclosporin (Neoral(R)) for psoriasis unresponsive to topical therapy: a 1-year multicentre, randomized study The PISCES Study Group. Br J Dermatol. 1999;141:283–291. doi: 10.1046/j.1365-2133.1999.02977.x. [DOI] [PubMed] [Google Scholar]
- 12.Gordon KB, Gottlieb AB, Leonardi CL, Elewski BE, Wang A, Jahreis A, Zitnik R. Clinical response in psoriasis patients discontinued from and then reinitiated on etanercept therapy. J Dermatolog Treat. 2006;17:9–17. doi: 10.1080/09546630500472838. [DOI] [PubMed] [Google Scholar]
- 13.Moore A, Gordon KB, Kang S, Gottlieb A, Freundlich B, Xia HA, Stevens SR. A randomized, open-label trial of continuous versus interrupted etanercept therapy in the treatment of psoriasis. J Am Acad Dermatol. 2007;56:598–603. doi: 10.1016/j.jaad.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Ortonne J-P, Taïeb A, Ormerod AD, Robertson D, Foehl J, Pedersen R, Molta C, Freundlich B. Patients with moderate-to-severe psoriasis recapture clinical response during re-treatment with etanercept. Br J Dermatol. 2009;161:1190–1195. doi: 10.1111/j.1365-2133.2009.09238.x. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths CEM, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, Guzzo C, Xia Y, Zhou B, Li S, Dooley LT, Goldstein NH, Menter A, ACCEPT Study Group Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118–128. doi: 10.1056/NEJMoa0810652. [DOI] [PubMed] [Google Scholar]
- 16.Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG, Strober BE, Kaul M, Gu Y, Okun M, Papp K. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58:106–115. doi: 10.1016/j.jaad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Papp K, Crowley J, Ortonne J-P, Leu J, Okun M, Gupta SR, Gu Y, Langley RG. Adalimumab for moderate to severe chronic plaque psoriasis: efficacy and safety of retreatment and disease recurrence following withdrawal from therapy. Br J Dermatol. 2011;164:434–441. doi: 10.1111/j.1365-2133.2010.10139.x. [DOI] [PubMed] [Google Scholar]
- 18.Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, Bala M, Marano CW, Menter A. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51:534–542. doi: 10.1016/j.jaad.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, Li S, Dooley LT, Arnold C, Gottlieb AB. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56:31.e1–15. doi: 10.1016/j.jaad.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Reich K, Ortonne J-P, Gottlieb AB, Terpstra IJ, Coteur G, Tasset C, Mease P. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab’ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180–190. doi: 10.1111/j.1365-2133.2012.10941.x. [DOI] [PubMed] [Google Scholar]
- 21.Rich P, Sigurgeirsson B, Thaci D, Ortonne J-P, Paul C, Schopf RE, Morita A, Roseau K, Harfst E, Guettner A, Machacek M, Papavassilis C. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402–411. doi: 10.1111/bjd.12112. [DOI] [PubMed] [Google Scholar]
- 22.Mrowietz U, Leonardi CL, Girolomoni G, Toth D, Morita A, Balki SA, Szepietowski JC, Regnault P, Thurston H, Papavassilis C, SCULPTURE Study Group Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double-blind, noninferiority trial (SCULPTURE) J Am Acad Dermatol. 2015;73:27–36.e1. doi: 10.1016/j.jaad.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Blauvelt A, Reich K, Warren RB, Szepietowski JC, Sigurgeirsson B, Tyring SK, Messina I, Bhosekar V, Oliver J, Papavassilis C, Frueh J, Langley RGB. Secukinumab re-initiation achieves regain of high response levels in patients who interrupt treatment for moderate to severe plaque psoriasis. Br J Dermatol. 2017;177:879–881. doi: 10.1111/bjd.15656. [DOI] [PubMed] [Google Scholar]
- 24.Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, Reich K, Amato D, Ball SG, Braun DK, Cameron GS, Erickson J, Konrad RJ, Muram TM, Nickoloff BJ, Osuntokun OO, Secrest RJ, Zhao F, Mallbris L, Leonardi CL, UNCOVER-1 Study Group, UNCOVER-2 Study Group, UNCOVER-3 Study Group Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345–356. doi: 10.1056/NEJMoa1512711. [DOI] [PubMed] [Google Scholar]
- 25.Blauvelt A, Papp KA, Sofen H, Augustin M, Yosipovitch G, Katoh N, Mrowietz U, Ohtsuki M, Poulin Y, Shrom D, Burge R, See K, Mallbris L, Gordon KB. Continuous dosing versus interrupted therapy with ixekizumab: an integrated analysis of two phase 3 trials in psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1004–1013. doi: 10.1111/jdv.14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, Toth D, Langley RG, Cather J, Gottlieb AB, Thaçi D, Krueger JG, Russell CB, Milmont CE, Li J, Klekotka PA, Kricorian G, Nirula A. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273–286. doi: 10.1111/bjd.14493. [DOI] [PubMed] [Google Scholar]
- 27.Papp K, Menter A, Leonardi C, Soung J, Weiss S, Pillai R, Jacobson A. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1) Br J Dermatol. 2020;183:1037–1048. doi: 10.1111/bjd.19132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, Li S, Dooley LT, Gordon KB, PHOENIX 1 Study Investigators Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 29.Blauvelt A, Leonardi CL, Gooderham M, Papp KA, Philipp S, Wu JJ, Igarashi A, Flack M, Geng Z, Wu T, Camez A, Williams D, Langley RG. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis. JAMA Dermatol. 2020;156:1–11. doi: 10.1001/jamadermatol.2020.0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimball AB, Papp KA, Reich K, Gooderham M, Li Q, Cichanowitz N, La Rosa C, Blauvelt A. Efficacy and safety of tildrakizumab for plaque psoriasis with continuous dosing, treatment interruption, dose adjustments and switching from etanercept: results from phase III studies. Br J Dermatol. 2020;182:1359–1368. doi: 10.1111/bjd.18484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warren RB, Carrascosa JM, Fumero E, Schoenenberger A, Lebwohl MG, Szepietowski JC, Reich K. Time to relapse after tildrakizumab withdrawal in patients with moderate-to-severe psoriasis who were responders at week 28: post hoc analysis through 64 weeks from reSURFACE 1 trial. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16964. [DOI] [PubMed] [Google Scholar]
- 32.Cantrell W, Lee P, Mendelsohn AM, Rozzo SJ, Liao W. Efficacy and safety of tildrakizumab 100 mg for plaque psoriasis in patients randomized to treatment continuation vs treatment withdrawal with retreatment upon relapse in reSURFACE 1. J Eur Acad Dermatol Venereol. 2021 doi: 10.1111/jdv.17124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reich K, Armstrong AW, Foley P, Song M, Wasfi Y, Randazzo B, Li S, Shen Y-K, Gordon KB. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418–431. doi: 10.1016/j.jaad.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 34.Gordon KB, Armstrong AW, Foley P, Song M, Shen Y-K, Li S, Muñoz-Elías EJ, Branigan P, Liu X, Reich K. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Investig Dermatol. 2019;139:2437–2446.e1. doi: 10.1016/j.jid.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Bissonnette R, Iversen L, Sofen H, Griffiths CEM, Foley P, Romiti R, Bachinsky M, Rottinghaus ST, Tan H, Proulx J, Valdez H, Gupta P, Mallbris L, Wolk R. Tofacitinib withdrawal and retreatment in moderate-to-severe chronic plaque psoriasis: a randomized controlled trial. Br J Dermatol. 2015;172:1395–1406. doi: 10.1111/bjd.13551. [DOI] [PubMed] [Google Scholar]
- 36.Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RGB, Hu C, Stevens RM, Day RM, Gordon KB, Korman NJ, Griffiths CEM. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1) J Am Acad Dermatol. 2015;73:37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 37.Gordon KB, Foley P, Krueger JG, Pinter A, Reich K, Vender R, Vanvoorden V, Madden C, White K, Cioffi C, Blauvelt A. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475–486. doi: 10.1016/S0140-6736(21)00126-4. [DOI] [PubMed] [Google Scholar]
- 38.Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217–1218. doi: 10.1016/j.jaad.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamaria M, Liao W, Koo JY. How long does the benefit of biologics last? An update on time to relapse and potential for rebound of biologic agents for psoriasis. Psoriasis Forum. 2010;16:36–42. doi: 10.1177/247553031016a00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X-Y, Zhang C-L, Wang W-H. Time to relapse after treatment withdrawal for different biologics used to treat plaque psoriasis. Chin Med J (Engl) 2020;133:2998–3000. doi: 10.1097/CM9.0000000000001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellinato F, Girolomoni G, Gisondi P. Relapse of psoriasis in patients who asked to discontinue etanercept after achieving a stable clinical remission. Br J Dermatol. 2019;181:1319–1320. doi: 10.1111/bjd.18225. [DOI] [PubMed] [Google Scholar]
- 42.Giunta A, Papoutsaki M, Bianchi L, Chimenti S. Efficacy and safety of infliximab in long-term treatment of plaque-type psoriasis: a comparative retrospective study on two different (continuous vs. relapse related) regimens P2765. J Am Acad Dermatol. 2007;56(2):AB190. [Google Scholar]
- 43.Umezawa Y, Nobeyama Y, Hayashi M, Fukuchi O, Ito T, Saeki H, Nakagawa H. Drug survival rates in patients with psoriasis after treatment with biologics. J Dermatol. 2013;40:1008–1013. doi: 10.1111/1346-8138.12353. [DOI] [PubMed] [Google Scholar]
- 44.Rivera R, Martorell A, López A, Salgado L, Sahuquillo A, de la Cueva P, Herranz P, Ratón JA, Ferrán M, Izu R, Ruiz-Genao D, García-Donoso C, Carrascosa JM. Maintenance of response following discontinuation of guselkumab and secukinumab in Spanish patients who participated in the ECLIPSE study. J Eur Acad Dermatol Venereol. 2021;35:e65–e67. doi: 10.1111/jdv.16809. [DOI] [PubMed] [Google Scholar]
- 45.Chiu H-Y, Hui RC-Y, Tsai T-F, Chen Y-C, Chang Liao N-F, Chen P-H, Lai P-J, Wang T-S, Huang Y-H. Predictors of time to relapse following ustekinumab withdrawal in patients with psoriasis who had responded to therapy: an eight-year multicenter study. J Am Acad Dermatol. 2019 doi: 10.1016/j.jaad.2019.01.035. [DOI] [PubMed] [Google Scholar]
- 46.Down-titration and discontinuation strategies of tumour necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity—PubMed, (n.d.). https://pubmed.ncbi.nlm.nih.gov/31125448/ (Accessed Feb 4, 2022). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Yes.