Abstract
Purpose
Apart from the global disease burden of acute COVID-19 disease, the health complications arising after recovery have been recognized as a long-COVID or post-COVID-19 syndrome. Evidences of long-COVID symptoms involving various organ systems are rapidly growing in literature. The objective was to perform a rapid review and evidence mapping of systemic complications and symptoms of long-COVID and underlying pathophysiological mechanisms.
Methods
Publications reporting clinical trials, observational cohort studies, case–control studies, case-series, meta-analysis, and systematic reviews, focusing on the squeal of the disease, consequences of COVID-19 treatment/hospitalization, long-COVID, chronic COVID syndrome, and post acute COVID-19 were reviewed in detail for the narrative synthesis of frequency, duration, risk factors, and pathophysiology.
Results
The review highlights that pulmonary, neuro-psychological, and cardiovascular complications are major findings in most epidemiological studies. However, dysfunctional gastrointestinal, endocrine, and metabolic health are recent findings for which underlying pathophysiological mechanisms are poorly understood. Analysis of the clinical trial landscape suggests that more than 50% of the industry-sponsored trials are focused on pulmonary symptoms. In contrast to the epidemiological trends and academic trials, cardiovascular complications are not a focus of industry-sponsored trials, suggestive of the gaps in the research efforts.
Conclusion
The gap in epidemiological trends and academic trials, particularly concerning cardiovascular complications not being a focus of industry-sponsored trials is suggestive of the gaps in research efforts and longer follow-up durations would help identify other long-COVID-related health issues such as reproductive health and fertility.
Supplementary Information
The online version contains supplementary material available at 10.1007/s15010-022-01835-6.
Keywords: Post-COVID syndrome, Post-acute COVID syndrome, Long-COVID, Pathophysiology, Clinical trials
Introduction
Coronavirus disease 2019 (COVID-19) is an upper and lower respiratory tract infection caused by SARS-CoV-2. Globally, 226 million confirmed cases of COVID-19, including 4.6 million deaths reported to the World Health Organization (WHO) as of 17 September 2021 (https://covid19.who.int/). Recently, there has been increasing evidence of long-term complications post-COVID recovery, and reports point to the broad tissue-tropism of the COVID-19 affecting multiple other organ systems. Evidences suggest that clinical symptoms in long-COVID are overlapping yet distinct from acute COVID-19 and could persist in recovered patients for weeks to months, adding to the overall disease burden (including clinical and economic burden) [1].
The National Institute for Health and Care Excellence (NICE; UK) guideline for long-term effects of COVID-19 defines the Post-COVID-19 syndrome as a set of persistent physical, cognitive, and/or psychological symptoms that continue for more than 12 weeks after illness and which are not explained by the alternative diagnosis (https://www.nice.org.uk/guidance/ng188). Long-COVID refers to the signs and symptoms that persist or develop after the acute COVID-19. Patients suffering from these conditions are known as COVID long-haulers. The definitions of the persistent post-COVID syndrome, chronic COVID syndrome, or post-acute COVID-19 syndrome overlap but are not consistent across studies (Table 1) [2, 3].
Table 1.
Integrative classification of COVID-19 and long-COVID
| Classification | Reference time point for relapse of COVID-19 symptoms |
|---|---|
| Classification by NICE (UK) (https://www.nice.org.uk/guidance/ng188) | |
| Acute COVID-19 | < 4 Weeks |
| Ongoing symptomatic COVID-19 | 4–12 Weeks |
| Post-COVID-19 syndrome | > 12 Weeks |
| Classification by Fernández-de-Las-Peñas et al. [4] | |
| Potentially infection related-symptoms | < 4–5 Weeks |
| Acute post-COVID symptoms | Week 5 to Week 12 |
| Long post-COVID symptoms | Week 12 to Week 24 |
| Persistent post-COVID symptoms | > 24 weeks |
A prospective cohort study of 4182 COVID-19 cases showed that 13.3%, 4.5%, and 2.5% of individuals had the symptoms of long-COVID for ≥ 28 days, ≥ 8 weeks, and ≥ 12 weeks, respectively [5]. Higher age and number of symptoms appearing in the first week of illness were identified as the strongest predictor of Post-COVID syndrome (OR 4.6 (95% CI 3.3–6.5)). The incidence of long-COVID was estimated to be 10–35% of post-COVID cases, which may reach up to 85% in patients with the severe form of COVID-19 and hospitalization [6–8]. Demographics of the patient, pre-existing comorbidities, the severity of the acute phase COVID-19, hospitalization and type of intensive care treatment, post-infection hyper-inflammatory status, and degree of fibro-proliferative changes in various organs, etc. are considered major risk factors for long-COVID [1, 7, 9].
This review aims to map current knowledge on common as well as emerging long-COVID symptoms, complications, and underlying pathophysiological mechanisms in various organ dysfunction (excluding secondary infections due to prolonged hospitalization, immune suppression, or steroid therapy). Emphasis is also provided to review the active industry efforts in ongoing clinical trials to gauge the current trends in this space. The correlation of these two findings would help identify the areas that require immediate attention as knowledge of long-COVID evolves.
Review methodology
We performed a narrative review of the literature using Boolean search string consisting of keywords like “SARS COV-2”, “COVID-19”, “post-COVID-19”, “Post-acute COVID-19”, “Post-COVID Syndrome”, “COVID-19 sequelae”, “persistence of symptoms”, “long-term health consequences”, from literature repositories such as CoronaCentral (https://coronacentral.ai), PubMed, Web of Science, and Google Scholar. The relevant articles were identified and manually reviewed for relevance with the context. The review aimed to cover the emerging trends and diversity of evidence (rather than the depth/granularity) in long-COVID.
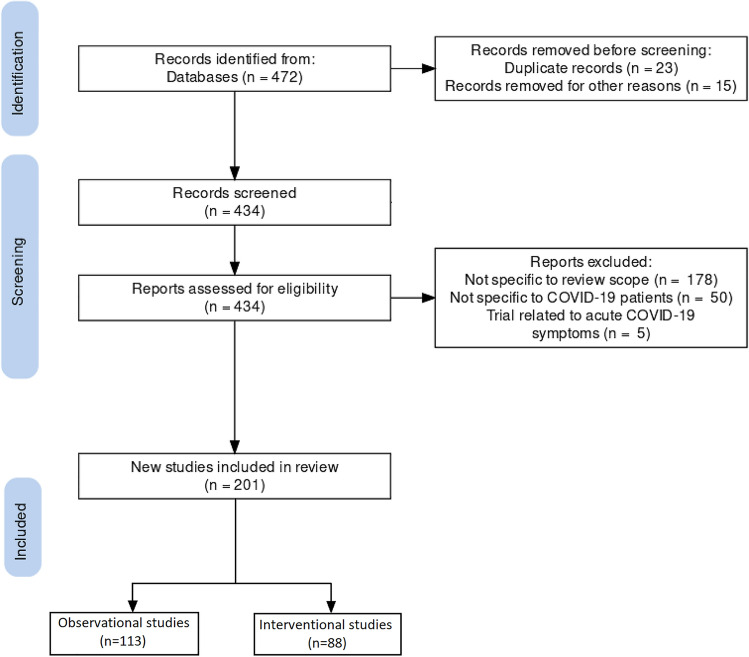
For the clinical trial landscape, we downloaded the clinical trial details from the portal of https://www.clinicaltrials.gov/ using keywords such as post-COVID syndrome, Long-COVID and were manually reviewed. A total of 472 trials were downloaded from the portal as of 1 September 2021 and were analyzed based on parameters of trial objectives, study type, and outcome measures. Clinical trials that were not focused on specific organ systems such as behavioral changes, rehabilitation programs, vaccine responses, serosurveillance were not analyzed (Fig. 1). Similarly, clinical trials focused on acute COVID-19 symptoms or health issues of caregivers, nurses, family or relatives were excluded from the analysis. In total, 201 clinical trials were selected for final analysis as per the PRISMA statement and review methodology (Fig. 1).
Fig. 1.
Flowchart for scouting long-COVID-related studies from the clinical trial registry
Impact of neurological and psychological health
Neuropsychological sequelae are frequent long-COVID symptoms, irrespective of the severity of the disease [10]. The most commonly observed neurological symptoms across the studies are fatigue (28.3–67.8%), sleep disturbance (30.8–56.5%), headache (44%), cognitive impairment and memory loss (25.4–34.2%), attention disorder (26.0%), post-traumatic stress disorder (PTSD) (22.2%), anxiety and depression (4.3–6.5%) [3, 11–15]. Retrospective data analysis from electronic health records (EHR) of 236,000 + COVID-19 survivors showed that the estimated incidence of neurological/psychiatric diseases in the following six months was 33.6%, out of which 12.8% were newly diagnosed. Anxiety disorder was the most common occurrence with 17.4%, and 19.2% in overall cohorts vs. ICU treated cohorts, respectively [16]. Neurological complications observed during the long-COVID course have been termed as “post-COVID-19 neurological syndrome (PCNS)” [17]. PCNS includes complications ranging from acute course diseases such as stroke to a rare and long course disease such as Guillain–Barre syndrome for up to six months post-COVID diagnosis [16, 18]. Not limited to the epidemiological trends of common symptoms, recent case studies have also reported the occurrence of new-onset focal or generalized seizures [19], refractory status epilepticus [20], hearing loss, and vestibule-cochlear neuritis [21, 22]. There are also reports of spinal cord disease, such as longitudinally extensive transverse myelitis (LETM) [23]. Long-COVID patients may also experience “brain fog” and show symptoms similar to those of myalgic encephalomyelitis or mast cell activation syndrome [24].
The emerging trends also indicate the involvement of Proust's madeleine brain network, referring to olfactory processes physiologically linked with memory and emotions through the limbic system and consequent symptoms [25]. COVID-19 infection has been shown to be associated with limbic encephalitis, as confirmed with MRI imaging [26]. The limbic system consists of brain structures between the cerebral cortex, the brain stem, primarily the Hippocampus, which acts as our brain's memory center, and the Amygdala, which regulates emotional responses such as pleasure, fear, anxiety, and anger. They also share an extensive neuronal network with the thalamus, that is the relay center for motor and sensory signals, hypothalamus that mediates essential hormonal regulation as well as responses like thirst, hunger, mood, etc., and basal ganglia involved in habit formation, movement, and learning, implying that dysfunction of the limbic system could result in a wide range of clinical symptoms [27–30]. The latter may imply that post-COVID-19 symptoms such as dysregulation of executive functions (Dysexecutive syndrome), Cognitive impairment, Myoclonus, Seizures, Dementia and Alzheimer' like symptoms, etc. may be attributed to multiple factors operative in tandem and involving multiple centers in the brain; further research on these topics is needed to elucidate the COVID-19 driven neuropathological mechanisms [31–34].
Impact on pulmonary functions
Several studies have reported persistent respiratory ailments in COVID-19 patients after being discharged, including radiological abnormalities persisting for > 28 days since symptom onset, irrespective of clinical severity [35]. Based on a cohort of Veteran Affair (VA) EHR database consisting of 73,435 COVID-19 survivors, the excess disease burden at six months was estimated for respiratory signs and symptoms, respiratory failure, insufficiency, and arrest with a hazard ratio of 28.5 (95% CI 26.4–30.5 and 3.4 (95% CI 2.7–3.9), respectively [36]. A systematic review based on the analysis of 5440 patients reported the signs and symptoms of chest pain, dyspnea, cough/sputum production at a frequency of 89%, 61%, and 59%, respectively, in the long-COVID-19 disease course [7]. Additionally, postmortem histopathological assessments demonstrated significant fibrotic remodeling with characteristic findings such as fibroblast proliferation, micro-honeycombing, and airspace obliteration. These were thought to explain acute respiratory distress syndrome (ARDS) as a long-term complication in COVID-19 [37]. Interestingly, the incidence of long-COVID was much lower in asthmatic patients, and altered immune profile with ongoing inhaled glucocorticoids medication is hypothesized to play a role in partial protection from long-COVID in asthmatics [38].
Impact on cardiovascular system
COVID-19 infection has an impact on the cardiovascular system that can be intermediate, long-lasting, or persistent. Patients with COVID-19 can experience hypoxia, hypotension, and shock resulting in myocardial injury that forms the basis for further complications, combined with COVID-19-associated coagulopathy, immunothrombosis, hyper-inflammation syndrome, myocarditis, acute coronary syndrome, cardiac arrest, cardiac arrhythmia, cardiomyopathy, and heart failure. These aspects have been extensively reviewed by others [39, 40]. The high disease burden of cardiovascular diseases was reported in the US Veteran Affair electronic medical records comprising > 73,000 survivors of acute-COVID-19, including hypertension (HR: 15.2 (95% CI 11.5–18.6)), cardiac dysrhythmias (HR: 8.4 (95% CI 7.2–9.5)), circulatory signs and symptoms (HR: 6.7 (95% CI 5.2–8.0)), coronary atherosclerosis (HR: 4.4 (95% CI 3.0–5.7)) and heart failure (HR: 3.9 (95% CI 3.0–4.8)) [36].
In a case series study, Postural Orthostatic Tachycardia Syndrome (POTS), characterized by chronic orthostatic intolerance and abnormal heart rate with upward postures, was demonstrated in middle-aged women with COVID-19 disease, which was thought to be caused by a viral or bacterial infection, and chronic inflammatory or autoimmune response [41]. Non-pulmonary etiologies of breathing difficulties (dyspnea) in COVID-19 disease were attributed to pathologies such as acute myocarditis, cardiomyopathy, exacerbation of heart failure, or atypical presentation of the cardio-renal syndrome [42]. In addition, acute kidney injury among patients hospitalized due to COVID-19 was a common finding (up to 46% of cases) and believed to be associated with long-term cardiovascular events, resulting in a complication known as a cardiorenal syndrome or reno-cardiac syndrome [43, 43].
Impact on gastro-intestinal and biliary system
During the first wave of the COVID-19 pandemic, up to 30.9% of patients presented with gastrointestinal (GI) symptoms upon follow-up of three months [45]. The EHR data from the US Department of Veteran Affair database of 73,435 patients, who survived more than 30 days after the first diagnosis, suggested the increased risk in terms of HR for oesophageal disorders (HR 6.9 (95% CI 4.6–9.1)), gastrointestinal disorders (HR 3.6 (95% CI 2.2–4.9)), dysphagia (HR 2.8 (95% CI 1.8–3.8)) and abdominal pain (HR 5.7 (95% CI 3.7–7.6) and found an increase in the use of laxatives, histamine blockers, and antacids among COVID-19 patients [36]. The most commonly reported symptoms were loss of appetite (24%), nausea (18%), acid reflux (18%) and diarrhea (15%), and others such as abdominal distension (14%), belching (10%), vomiting (9%), abdominal pain (7%), and bloody stools (2%), etc. However, an intriguing finding was the observation of an inverse relationship between gastrointestinal symptoms such as nausea, emesis, and diarrhea and the severity of COVID-19 [46]. In other case reports, patients initially presented to the emergency department with demonstrable thickening of the gallbladder wall, peri-vesicular liquid, and absence of gallstones later diagnosed as COVID-19 infection by RT-PCR [47]. In that line, other reports have shown that patients recovered from COVID-19 disease with critical cardiopulmonary involvement but showed characteristics of severe cholangitis and intrahepatic microangiopathy in histopathology and clinically [48]. In an interesting case report, the patient with Post COVID-19 cholangiopathy progressed to end-stage liver disease and required highly invasive interventions like liver transplantation [47]. More detailed studies are necessary to establish the epidemiological relationship delineating the progression of hepato-biliary manifestation and appropriate disease management strategy during the long-COVID period [47, 49].
Impact on endocrine and metabolic functions
Endocrine and metabolic conditions such as diabetes mellitus have been reported as long-COVID symptoms, including newly diagnosed diabetes and severe metabolic complications of pre-existing diabetes, e.g., diabetic ketoacidosis [50]. A retrospective analysis of 538 COVID-19 survivors in China suggested that 7.4% of patients reported diabetes at a median of 97 days (range: 91–116 days) post-discharge [15]. On the same line, the annualized incidence rate of new-onset diabetes was estimated as 2.9% over a mean follow-up of 4.6 months in a large cohort study involving 47,780 discharged COVID-19 patients in the UK [51]. History of reduced renal function, poor glycogenic control, diabetic ketoacidosis, or hypoglycemia-related hospitalization in the past five years was identified as additional risk factors associated with poor outcomes. The relationship between COVID-19 and diabetes seems bidirectional—pre-existing diabetes as a risk factor for COVID-19 severity and hospitalization versus COVID-19 infection resulting in new-onset diabetes during the long-COVID course [50, 52]. Also, for further assessment, the CoviDIAB Project (a global registry of patients with COVID-19–related diabetes) has been initiated jointly by King's College, London, and Monash University, Australia (covidiab.e-dendrite.com), providing an opportunity for detailed longitudinal follow-up of COVID-19 patients with diabetes-related morbidities.
The impact of COVID-19 infection on endocrine and metabolic functions can be seen beyond diabetes mellitus. From the literature, emerging trends related to thyroid diseases and corticosteroid dependent metabolism is observed—hypothyroidism (5%) in mild cases, thyrotoxicosis (20%) among hospitalized patients [53]. Recently, sporadic case reports of acute and sub-acute thyroiditis associated with COVID-19 have been published in different countries [54–56]. In previous SARS-CoV outbreaks, other endocrine or hormonal dysfunctions observed after three months from recovery were central hypocortisolism (39%) and hypothyroidism (5%) through its effects on the hypothalamic-pituitary axis [53]. Similarly, the impact of COVID-19 on the endocrine system, mainly the hypothalamic-pituitary axis and thyroid gland, have been considered to be mediated through direct or indirect cellular injury induced by COVID-19, resulting in deregulated hormonal levels such as TSH and free tri-iodothyronine (T3), ACTH and cortisol, etc. However, besides a few initial reports, more systematic epidemiology studies on the endocrine dysfunction after acute-COVID-19 recovery is still lacking.
Impact on reproductive system and fertility
Dysfunctions in the reproductive system and reduced fertility are often long-term findings beyond the time frame for which the longitudinal data are available after two pandemic waves. Recent early evidence from autopsies suggests the involvement of reproductive pathologies due to COVID-19 infection. A systematic study related to the impact of COVID-19 on reproduction and fertility is yet unavailable to validate the early findings at an epidemiological scale.
In autopsies of testicular samples from fatal cases of COVID-19 patients, orchitis characterized by histopathological changes such as damaged germ cells, thickened basement membrane, and leucocyte migration was observed [57]. In other studies, histopathological abnormalities such as inflammation of seminiferous tubules, erythrocytes exudation, and infiltration of inflammatory cells have been reported [58, 59]. Several studies have reported the inconsistent results of SARS-COV-2 presence in the testicular tissues, semen, and prostatic secretions, and these aspects have been previously reviewed in the literature [60]. Another study demonstrated SARS-COV-2 associated decrease in total motility and total motile sperm count without affecting semen volume and sperm concentration [61]. Findings such as reduction in semen volume, sperm production/count, increased DNA fragmentation, reduced sperm motility, scrotal discomfort, and enhanced production of inflammatory molecules such as C-reactive protein suggest testicular injury and viral orchitis [58, 62–64]. At least three possible speculations on the mechanism behind the SARS-CoV-2 virus-mediated impact on male reproductive functions: (1) direct pathogenic impact on testicular cells with higher ACE2 expression, (2) body fever contributes to an increase in testicular temperature, which may cause poor sperm quality, in terms of sperm density, motility, and morphology, (3) secondary immune response leading to autoimmune orchitis [65].
In addition, the impact of SARS-COV-2 on the hypothalamus-pituitary-gonad axis has been studied, which could also impact reproductive health. A cohort study of 45 patients (male) revealed that low testosterone and di-hydro-testosterone levels (68.6% and 48.6%, respectively) were associated with altered secretion of gonadotropins [66]. Other studies have pointed out a reciprocal correlation between testosterone levels, T/LH ratio, and inflammation-associated molecules [61, 67]. The blood-testis barrier (BTB) provides an isolated microenvironment and protection for sperm present in the testis [64]. However, SARS-CoV-2 were recovered from semen samples from acute and recovering male patients but not from vaginal and cervical fluids in females [58, 68, 69]. It is hypothesized that severe inflammation elicited due to an infection can impair the BTB leading to orchitis and/or hypogonadism, severely affecting male fertility and testicular function. To date, most studies point out that SARS-COV-2 infection may affect male fertility. Recent studies showing recovery of SARS-CoV-2 from semen samples only open the speculations about sexual transmission.
Epidemiology and case-series studies on the impact of COVID-19 infection in female reproductive health and fertility are scarce [70]. Apprehensions such as the ACE-II gene being located at the Xp22 locus on the X chromosome—an area known to escape the X-inactivation—may be responsible for higher expression of ACE-II enzyme on cell membranes in women [53]. Several observations from different countries have revealed that the frequency of worse outcomes was lower in women than men, which could be a function of other factors such as lower TMPRSS2 expression (required for virus activation and entry) and immune-competency [71]. Nevertheless, clinical observation and literature on long-COVID impact on female reproductive health and fertility are not yet available; comprehensive long-term follow-ups are required to unveil the longitudinal epidemiological trends.
Impact on immune system, multi-organ damage and other symptoms
Impairment or deregulation of multiple organ systems in long COVID is hypothesized to result from hyper-inflammatory changes and cytokine storm caused by SARS-CoV-2 [41, 72, 73]. In a prospective cohort study comprising of 201 long-COVID cases, functional impairment of pancreas, liver, heart, lung, kidney, and spleen was reported in 40%, 28%, 26%, 11%, 4%, and 4% of cases, respectively, with 29% patients showing multiorgan impairment after median 141 days following the COVID-19 infection. In severe long-COVID cases, evidences of myocarditis and pancreatitis were also seen along with fat accumulation. They increased liver volume as comorbidities independent of obesity, hypertension, diabetes mellitus, and heart disease status [74]. Similar to adults, multisystemic comorbidities were shown in the post-COVID-19 surveillance study in children and adolescents (n = 186), which showed the gastrointestinal system in 92% patients, cardiovascular in 80%, respiratory in 70%, with hematologic (76%), and mucocutaneous (74%) changes [75, 76].
Other long-COVID symptoms include anosmia (loss of smell), ageusia (loss of taste), dermatological manifestations, and myopathies. Anosmia persists after COVID, but the duration varies with the persistence of symptoms in 15.3% of patients after 60 days and 4.7% after six months with a predictive association with baseline severity of olfactory dysfunction (P 0.001) [77]. However, there are evidences that the choice of assessment methods in patient-reported measures such as taste or smell can also result in inconsistencies in reported persistence. A more standardized method could provide accurate observations and correlations between objective and patient-reported outcomes [78]. Interestingly, a study identified the geographical patterns in the prevalence of gustatory disturbances—Americas 66. 8%, (95% CI 54.8–78.8%), Europe 57.2% (95% CI 52.4–62.0%), Middle East 38.8% (95% CI 27.5–50.2%) and East Asia 13.1% (95% CI 0.1–26.1%) although the results are directional and must be interpreted with cautions [79].
In a case report, myopathic changes characterized by muscle membrane potential change, loss of myosin, and reduced myosin: actin ratio were reported in patients with a history of mild SARS-CoV-2 infection [80]. The American Academy of Dermatology (AAD) and the International League of Dermatological Societies (ILDS) also reported dermatological manifestations of COVID-19 infection (www.aad.org/covidregistry) with complication as morbilliform (22%), pernio-like (18%), urticarial (16%), macular erythema (13%), vesicular (11%), papulosquamous (9.9%) and retiform purpura (6.4%) [81]. While this provides a spectrum of skin affections in long-COVID, it is unsuitable for epidemiological interpretation due to the small sample size. In follow-up studies, manifestations like Papulo-squamous eruptions and Pernio were reported to last for a median of 20 days and 12 days, respectively, with the persistence of symptoms for over 60–70 days in some cases [73].
Cancer has also been anticipated as prospective long-term sequelae of COVID-19 infections due to overlap with mechanisms like elevated pro-inflammatory cytokines (e.g., IL-1, IL-6, IL-8, and TNF-α), T-cell depletion, and activation of oncogenic pathways (e.g., JAK‐STAT and NF‐κB pathways), and oxidative stress-driven by COVID-19 infection, which could collectively set the stage for malignant transformation and cancer development [82]. Cellular transformations such as the formation of multinucleated giant cells as well as changes in the phosphorylation landscape, protein–protein interaction of SARS‐CoV endoribonuclease Nsp15 with the retinoblastoma tumor suppressor protein (pRb), and S2 subunit of SARS‐CoV‐2 and the p53 and BRCA1/2 proteins has been demonstrated, which further supportive of the cancer risks in COVID-19 patients in the long run [82]. However, this remains theoretical speculation unless supported by real-world epidemiological trends.
Putative patho-physiological mechanisms involved in long-COVID
Owing to a relatively recent discovery, knowledge about the pathophysiology and biological factors involved in the long-COVID symptoms is incomplete and rapidly evolving. Some studies have suggested the long-COVID symptoms to be persistent or residual effects of acute-phase COVID-19 or the treatment regimen used for its treatment [83].
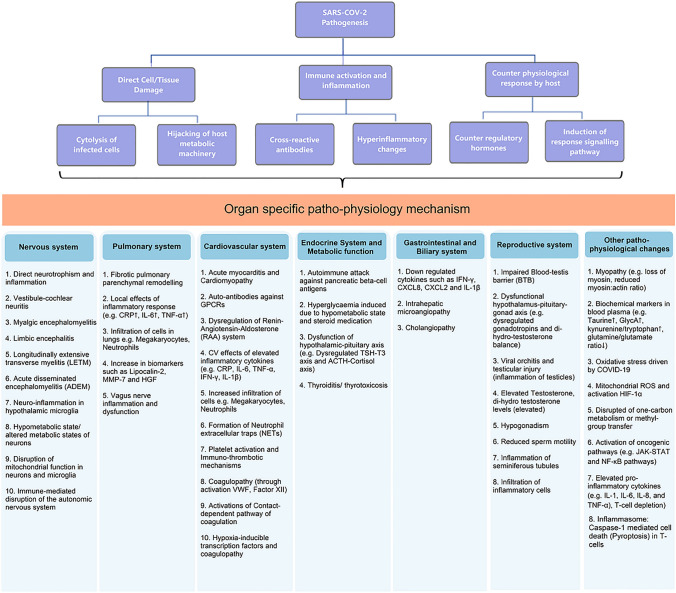
In more generalized terms, one or more of the following mechanisms would relate to subsequent organ-specific damages in long-COVID: (1) Virus entry through ACE-II receptors followed by direct cell damage, (2) Immune system activation including autoimmune response due to cross-reacting antibodies, and (3) Counter response by the host such as non-specific activation of the immune system, overproduction of counter-regulatory hormones and cytokines causing further damage to host cells. These effects are implemented through responsive signaling pathways such as increased phosphorylation of NF-kB and JAK-STAT pathway molecules [84]. Depending on the organ-specific microenvironment, these mechanisms drive the specific pathophysiological changes and clinical symptoms (Fig. 2).
Fig. 2.
Pathophysiological mechanisms in long-COVID or post-COVID syndrome. Based on the current knowledge, mechanisms that are involved in long COVID are complex and interrelated. Three major categories of the pathophysiological changes are: (1) Direct cellular/tissue injury caused due to cytotoxicity or by hijacking host metabolic machinery such as mitochondrial functioning or methyl group transfer; (2) Immune activation and inflammation, this can either target the host cells through antigen cross-reactivity or induce cell damage due to inflammatory changes including cytokines/chemokines and cellular infiltrations; (3) Counter physiological response corresponds to altered hormonal changes or responsive intracellular signaling pathways. The combination of the above mechanisms (upper panel, purple boxes) and depending on the viral tissue-tropism and microenvironment organ-specific pathophysiological changes are responsible for the respective clinical symptoms (lower panel, in blue shaded boxes). Abbreviations: C-Reactive Protein (CRP); Interferon gamma (IFN-γ); Tumor necrosis factor-α (TNF-α); Interleukin-1β (IL-1β); Interleukin-1 (IL-1); Interleukin-6 (IL-6); Interleukin-8 (IL-8); Matrix metalloproteinase-7 (MMP-7); Hepatocyte growth factor (HGF); GPCR—G-protein coupled receptors; von Willebrand factor (vWF);); Thyroid stimulating hormone (TSH); Triiodothyronine (T3); Adrenocorticotropic hormone (ACTH); Hypoxia-inducible factor 1α (HIF-1α); Reactive oxygen species (ROS); Chemokine (C-X-C motif) Ligand (CXLC-2, CXCL-8 etc.)
Concerning the neurological complications, virus neurotropism and subsequent neuroinflammation leading to pathophysiological impacts on the cortex, limbic system, and brain stem and hypothalamic–pituitary–adrenal axis would explain most of the observed symptoms [10, 19, 21–24, 85–87]. Although there are limited case reports of fully diagnosed dysfunctional Limbic system, it is hypothesized that several clinical symptoms such as emotional and cognitive disturbances, anxiety, depression, uncoordinated movements, partial seizures, and myoclonus can be partly explained in the post-COVID context [30, 30, 33]. The metabolic changes such as elevated taurine (p = 3.6 × 10–3), reduced glutamine/glutamate ratio (p = 6.95 × 10–8), elevated GlycA, and kynurenine/tryptophan ratio indicate possible liver damage, generalized tissue repair, or immune function [83]. SARS-CoV-2 virus-mediated disruption of mitochondrial function in neurons and microglia can also explain some neuropsychiatric symptoms, e.g., cognitive impairment and fatigue syndrome [88]. Furthermore, the hypometabolic state of the cells was thought to contribute to the pathogenesis resulting in symptoms like fatigue, insomnia, anosmia, ageusia, and cognitive impairment in long-COVID patients [89, 90]. Some neurological complications may also result from immune-mediated destruction of the peripheral nervous system (potentially due to cross-reacting antigens), as seen in Guillain–Barre syndrome, or coagulopathies and cerebrovascular accidents seen in stroke [17, 18, 91].
Immune suppression during COVID-19 treatment and/or the degree of fibrosis remodeling in lung, heart, and vascular tissue during the active phase of COVID-19 could result in respiratory symptoms [2]. In an interesting analysis, it was suggested that long-COVID symptoms have remarkable overlapping with those of pernicious anemia caused by deficiency of vitamin B12, disturbances of one-carbon metabolism, and increased methyl-group requirements in COVID patients [92]. Metabolic alterations seem to bridge the respiratory, immunological, and endocrine dysfunctions in long-COVID. Hyperglycemia and glycolysis promote SARS-CoV-2 replication via mitochondrial reactive oxygen species production and activation of hypoxia-inducible factor-1α (HIF-1 α). The diabetogenic effect of COVID-19 has been postulated to be a combination of direct cell damages of pancreatic beta-cells, an autoimmune attack against pancreatic beta-cell antigens, and indirect tissue destruction due to hyper-inflammatory response [93–97]. In addition, the combined effect of hyperglycemia caused by a hypometabolic state and post-effects of steroid medication during acute COVID-19 adds to the inadequate blood glucose regulation. Overall, the pathological relationship between COVID-19 infection and diabetes is bidirectional: pre-existing hyperglycemia directly increases SARS-CoV-2 replication, and SARS-CoV-2 infection causes altered endocrine and exocrine pancreatic functions either by direct cell injury or through responsive immune/inflammatory response [95]. Other mechanisms affecting endocrine and metabolic processes are mediated through inflammatory changes in the hypothalamic-pituitary axis, thyroid, and adrenal cortex resulting in impaired T3 production and corticosteroid-dependent metabolism [53, 98].
SARS-CoV-2 pathogenesis in immune-thrombosis is complex, involving virus-induced endothelial cell injury, activation of the coagulation cascade, and immune-thrombosis. After virus-induced inflammasome activation in monocytes and/or macrophages, pro-inflammatory cytokines (e.g., IL-1 and IL-18) are released, activating cellular immune system players like neutrophils and platelets. Neutrophils release NETs, activate factor XII and start contact-dependent coagulation pathways. NETs can also bind to VWF, recruit platelets, and contribute to coagulopathies. Activated platelets can release pro-inflammatory cytokines and hypoxia-inducible transcription factors, which aid in clot formation [39]. A combination of pro-inflammatory and immune-thrombotic pathological processes seems to mediate the cardiovascular symptoms in long-COVID disease. However, in contrast to the previous hypothesis of direct viral cytotoxicity, low expression of ACE-II receptors on endothelial cells suggest that the damage to endothelial cells could be primarily due to immune reaction or bystander effects from neighboring infected cells [99, 100]. Additionally, functionally active auto-antibodies against G-protein coupled receptors in long-COVID can act as agonists for β2-adrenoceptor, α1-adrenoceptor, angiotensin II AT1-receptor, nociceptin-like opioid receptor or antagonist to muscarinic M2-receptor, MAS-receptor, and ETA-receptor to exert positive or negative chronotropic effects on the cardiovascular system [101]. Furthermore, following inflammatory changes, a caspase‐1‐dependent cell death—known as Pyroptosis was also demonstrated in acute and post-acute COVID-19 infection and hypothesized to mediate apoptosis in immune cells as well as cardiomyocytes [102].
Concurrently, ACE-II receptor is expressed in lung, heart, and kidney cells, forming a link between the pulmonary, cardiovascular, and renal systems during COVID-19 infection [103]. The glomerular and myocardial injury, along with pro-inflammatory changes and cytokine storm, might partly explain the complications of cardio-renal syndrome during post-COVID syndrome [43]. Lipocalin-2, matrix metalloproteinase-7, and hepatocyte growth factor combined with increased neutrophil activation are believed to mediate fibrotic changes and lung remodeling in these patients. Further, elevated CRP, IL-6, TNF-α, and neutrophil infiltration were associated with ARDS severity in COVID-19 patients [104–108]. Viral invasion of the vagus nerve and/or resultant neuroinflammatory response, followed by peripheral and central hypersensitivity, has been hypothesized as the additional mechanism behind impaired pulmonary functions and chronic fatigue, chest pain, dyspnoea, persistent cough, and ventilation deficit during long-COVID disease [109].
Olfactory dysfunction (anosmia/parasomnia/hyposmia) have been reported in acute as well as long-COVID course and can be explained by one or more of the following mechanisms: (1) Preventing odorants access to olfactory cleft by mechanical obstruction due to inflammatory exudates, (2) direct damage to supporting cell of the olfactory epithelium (e.g., sustentacular cell) and (3) direct invasion and damage of olfactory neurons [110]. In recent reports involving gastrointestinal complications in long-COVID, down-regulation of key inflammatory genes such as IFN-γ, CXLC-2, CXCL-8, and IL-1β, as well as reduced pro-inflammatory dendritic cell subsets were demonstrated [46]. Variations in the prevalence of post-COVID gastrointestinal symptoms, shedding of SARS-CoV-2 virus replication-competent feces samples, and correlation with gastrointestinal symptoms at diagnosis/hospitalization have left a knowledge gap that requires more systematic research [111]. Epithelial lining structure with ACE-II expression in the bile duct and gall bladder is hypothesized to drive the clinical presentation of acalculous cholecystitis and viral RNA detection in the gallbladder [47, 112]. Also, inflammation leading to severe cholangitis and intrahepatic microangiopathy with progression to end-stage liver disease have been demonstrated in post-COVID patients [48, 113]. Hyper-inflammation, impaired blood-testes barrier, and resultant injuries such as viral orchitis, testicular injury, and hypogonadism were the major findings in relation to the reproductive system complications in long-COVID [58, 62–64]. SARS-COV-2 was also identified from several semen samples from infected patients, but sexual transmission of the disease has not yet been established.
Complications such as dermatological manifestations and cancer have been anticipated based on a sporadic case report and relevance with intracellular biochemical changes (e.g., oxidative stress, activation of oncogenic pathways). However, more extensive follow-up studies are required through international registries in the long-COVID context. Systematic epidemiological studies and in-depth assessment of underlying pathophysiological changes of the unexplored post-COVID complications can be expected in the near future.
Clinical trial activities in long-COVID syndrome
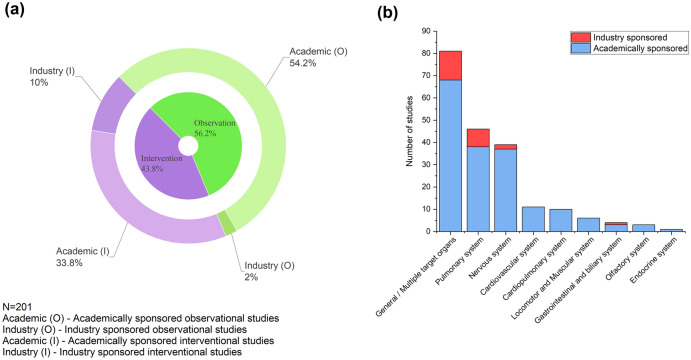
Initiation of clinical trials is generally seen as an indicator of active efforts to understand a disease or develop interventions/therapy. In this line, we reviewed the registered clinical trials at the portal of ClinicalTrials.gov (https://clinicaltrials.gov, data assessed as of 1 September 2021). We identified 201 clinical trials focused on any specific organ system, either through observational studies to understand the disease patterns or interventional studies focusing on post-COVID complications or organ systems. Among all the trials that were analyzed (N = 201), landscape showed a nearly equal split of observation and interventional trials (56.2% and 43.8%, respectively) (Fig. 3a). Among the long COVID studies, 88% of the trials were academically funded, and only 12% were sponsored by industry. The focus of most of the industry-sponsored trials was non-specific to any specific organ system effects. These trials measured outcomes like fatigue, breathing difficulty, etc., or aimed to assess the prevalence of long-COVID symptoms without focusing solely on one organ system (see supplementary material for details). However, among system-focused industry-funded clinical trials, the respiratory system is the most frequent target, followed by the nervous and gastrointestinal systems (Fig. 3b). Among academic clinical trials, specific organ system-focused studies were most commonly represented by pulmonary, nervous, and cardiovascular complications. From the landscape, it is evident that the cardiovascular and endocrine systems have failed to gain industry attention as long-COVID complications.
Fig. 3.
Overview of clinical trial activities related to the post-COVID syndrome. a Landscape of long-COVID clinical trials stratified by observational and interventional, followed by academic vs. industry sponsorship of the trials. b Organ system focus on clinical trials stacked by academic or industry sponsorship
Several epidemiological or COVID follow-up studies have widely presorted diseases like diabetes mellitus in patients recovered from acute COVID-19 infection and other emerging and long-term complications, such as metabolic, endocrine, reproductive health, and fertility, are poorly covered in clinical trials (e.g., thyroiditis, testosterone level, etc.). We expect the landscape to change as more epidemiological data becomes available. The clinical trial landscape also shows a low representation of pediatric trials (18/167, 10.8%)—only four trials specifically for children and 14 trials where children are recruited alongside adults (in the combined dataset). Clinical trials with more inclusive strategies for pediatric patients are required to generate additional trends in children diagnosed with COVID-19. Thus, we highlight the need for industry attention on emerging long-COVID symptoms (including children) and identify the gaps between recent epidemiological trends and the global clinical trial landscape in long-COVID disease.
Discussion and perspectives
The literature on sequelae of acute-COVID-19 infection, as long-COVID disease and the relevant pathophysiological mechanisms, are limited due to the relatively recent onset of this disease in humans. The epidemiological data from cohort and cross-sectional studies are rapidly increasing. They suggest that multiorgan dysfunction, including impairment of neuronal system, reduced lung function, and cardiovascular diseases are common in COVID-19 survivors. A combination of SARS-CoV-2 virus-mediated direct cytotoxicity, hyper-inflammation, cytokine storms, and responsive physiological changes in respective organ systems are responsible for specific post-COVID symptoms (Fig. 2). Additional speculations are derived from the clinical success of symptomatic treatments in post-COVID, such as using DPP4 antagonists in diabetes, anticoagulants like anti-WVF for thrombo-inflammations, steroids to control systemic inflammation, anti-GABAergic for neurological symptoms such as fatigue, and cognitive impairments for controlling the long-term impacts of the disease. The landscape is rapidly evolving. Recent reports and longitudinal follow-up have identified emerging and intriguing findings such as non-respiratory etiologies causing breathlessness, acute myocarditis, heart failure exacerbation, or atypical cardio-renal syndrome presentation in long-COVID. Despite early epidemiological reporting of gastrointestinal and metabolic symptoms, data are inconsistent, and pathophysiological mechanisms are unclear. In addition, changes in molecular signaling and biochemical pathways leading to oncogenic transformation of cells have been hypothesized, predicting an increase in cancerous conditions in the future.
Globally two large waves of the COVID-19 pandemic have been observed, of which the aging patients (> 60 yrs) and mid-age patients were the most affected population in the first and second wave, respectively [114]. The long-term follow-up for the second wave patients (young to the mid-age group)—being the primary subject of interest for reproductive health and fertility, could provide additional insights in this context. Hence, epidemiological data for fertility-related issues and cancers might take a few to several years before they can be regarded as statistically relevant complications of the post-COVID syndrome. Outcomes of this review emphasize that an ideal post-COVID rehabilitation program should comprise an integrative approach involving multidisciplinary support and individualized rehabilitation strategies [3, 115–117]. This review could provide a trigger to look at multisystemic aspects of the disease in this direction. In addition, current sporadic case reports of involvement of chronic cardiovascular complications, endocrine, metabolic and reproductive health, and infertility could get recognition as established post-COVID complications, as long-term epidemiological data become available from respective registries and longitudinal follow-ups. Also, we highlight the need for industry attention for health-related issues such as cardiovascular and reproductive health, which have been increasingly identified from post-COVID follow-up studies. Further investigations on long-COVID will have far-reaching effects on improving the post-COVID quality of life, healthcare system, society, and policymaking.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflict of interest
The authors declare no conflict of interest. The views, arguments, and opinions expressed in the article belong solely to the author. Affiliating organizations have no influence or liability concerning the same.
Ethics approval
This is a review article and ethical approval is not applicable.
Contributor Information
Ramesh Chandra Pandey, Email: rcp@alumni.iitm.ac.in.
Mukesh Kumar Gupta, Email: guptam@nitrkl.ac.in.
References
- 1.Mahmud R, Rahman MM, Rassel MA, Monayem FB, Sayeed SKJB, Islam MS, et al. Post-COVID-19 syndrome among symptomatic COVID-19 patients: a prospective cohort study in a tertiary care center of Bangladesh. PLoS ONE. 2021;16:e0249644. doi: 10.1371/journal.pone.0249644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oronsky B, Larson C, Hammond TC, Oronsky A, Kesari S, Lybeck M, et al. A review of persistent post-COVID syndrome (PPCS) Clin Rev Allergy Immunol. 2021 doi: 10.1007/s12016-021-08848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 Long-term effects of COVID-19: a systematic review and meta-analysis. medRxiv Prepr Serv Heal Sci. 2021 doi: 10.1101/2021.01.27.21250617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernández-de-Las-Peñas C, Torres-Macho J, Velasco-Arribas M, Arias-Navalón JA, Guijarro C, Hernández-Barrera V, et al. Similar prevalence of long-term post-COVID symptoms in patients with asthma: A case-control study. J Infect. 2021;83:237–279. doi: 10.1016/j.jinf.2021.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021 doi: 10.1016/j.arcmed.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabrera Martimbianco AL, Pacheco RL, Bagattini ÂM, Riera R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: a systematic review. Int J Clin Pract. 2021;75:e14357. doi: 10.1111/ijcp.14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreno-Pérez O, Merino E, Leon-Ramirez J-M, Andres M, Ramos JM, Arenas-Jiménez J, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a mediterranean cohort study. J Infect. 2021;82:378–383. doi: 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 10.Benzakour L, Assal F, Péron JA. Neuropsychological long-COVID: neurologic or psychiatric origin? Rev Med Suisse. 2021;17:822–826. [PubMed] [Google Scholar]
- 11.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Lorenzo R, Conte C, Lanzani C, Benedetti F, Roveri L, Mazza MG, et al. Residual clinical damage after COVID-19: a retrospective and prospective observational cohort study. PLoS ONE. 2020;15:e0239570. doi: 10.1371/journal.pone.0239570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Cruz RF, Waller MD, Perrin F, Periselneris J, Norton S, Smith L-J, et al. Chest radiography is a poor predictor of respiratory symptoms and functional impairment in survivors of severe COVID-19 pneumonia. ERJ Open Res. 2021;7:00655-2020. doi: 10.1183/23120541.00655-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrigues E, Janvier P, Kherabi Y, Le Bot A, Hamon A, Gouze H, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camargo-Martínez W, Lozada-Martínez I, Escobar-Collazos A, Navarro-Coronado A, Moscote-Salazar L, Pacheco-Hernández A, et al. Post-COVID 19 neurological syndrome: implications for sequelae’s treatment. J Clin Neurosci. 2021;88:219–225. doi: 10.1016/j.jocn.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen CM, Ramsamy S, Tarr AW, Tighe PJ, Irving WL, Tanasescu R, et al. Guillain-Barré syndrome variant occurring after SARS-CoV-2 vaccination. Ann Neurol. 2021;90:315–318. doi: 10.1002/ana.26144. [DOI] [PubMed] [Google Scholar]
- 19.Park S, Majoka H, Sheikh A, Ali I. A presumed case of new-onset focal seizures as a delayed complication of COVID-19 infection. Epilepsy Behav Reports. 2021;16:100447. doi: 10.1016/j.ebr.2021.100447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll E, Neumann H, Aguero-Rosenfeld ME, Lighter J, Czeisler BM, Melmed K, et al. Post-COVID-19 inflammatory syndrome manifesting as refractory status epilepticus. Epilepsia. 2020;61:e135–e139. doi: 10.1111/epi.16683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aasfara J, Hajjij A, Bensouda H, Ouhabi H, Benariba F. A unique association of bifacial weakness, paresthesia and vestibulocochlear neuritis as post-COVID-19 manifestation in pregnant women: a case report. Pan Afr Med J. 2021;38:30. doi: 10.11604/pamj.2021.38.30.27646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koumpa FS, Forde CT, Manjaly JG. Sudden irreversible hearing loss post COVID-19. BMJ Case Rep. 2020;13:e238419. doi: 10.1136/bcr-2020-238419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno-Escobar MC, Kataria S, Khan E, Subedi R, Tandon M, Peshwe K, et al. Acute transverse myelitis with Dysautonomia following SARS-CoV-2 infection: a case report and review of literature. J Neuroimmunol. 2021;353:577523. doi: 10.1016/j.jneuroim.2021.577523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Décary S, Gaboury I, Poirier S, Garcia C, Simpson S, Bull M, et al. Humility and acceptance: working within our limits with long COVID and myalgic encephalomyelitis/chronic fatigue syndrome. J Orthop Sport Phys Ther. 2021;51:197–200. doi: 10.2519/jospt.2021.0106. [DOI] [PubMed] [Google Scholar]
- 25.Guedj E, Lazarini F, Morbelli S, Ceccaldi M, Hautefort C, Kas A, et al. Long COVID and the brain network of Proust’s madeleine: targeting the olfactory pathway. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiveri L, Verrengia E, Muscia F, Nuzzaco G, Raimondi E, Vecchio E, et al. Limbic encephalitis in a COVID-19 patient? J Neurovirol. 2021;27:498–500. doi: 10.1007/s13365-021-00971-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catani M, Dell’acqua F, Thiebaut de Schotten M. A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev. 2013;37:1724–1737. doi: 10.1016/j.neubiorev.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Roxo MR, Franceschini PR, Zubaran C, Kleber FD, Sander JW. The limbic system conception and its historical evolution. Sci World J. 2011;11:2428–2441. doi: 10.1100/2011/157150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jo HJ, Kenney-Jung DL, Balzekas I, Welker KM, Jones DT, Croarkin PE, et al. Relationship between seizure frequency and functional abnormalities in limbic network of medial temporal lobe epilepsy. Front Neurol. 2019;10:488. doi: 10.3389/fneur.2019.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ardila A, Lahiri D. Executive dysfunction in COVID-19 patients. Diabetes Metab Syndr. 2020;14:1377–1378. doi: 10.1016/j.dsx.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark JR, Liotta EM, Reish NJ, Shlobin NA, Hoffman SC, Orban ZS, et al. Abnormal movements in hospitalized COVID-19 patients: a case series. J Neurol Sci. 2021;423:117377. doi: 10.1016/j.jns.2021.117377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Erausquin GA, Snyder H, Carrillo M, Hosseini AA, Brugha TS, Seshadri S. The chronic neuropsychiatric sequelae of COVID-19: the need for a prospective study of viral impact on brain functioning. Alzheimers Dement. 2021;17:1056–1065. doi: 10.1002/alz.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, Xu J, Hou Y, Leverenz JB, Kallianpur A, Mehra R, et al. Network medicine links SARS-CoV-2/COVID-19 infection to brain microvascular injury and neuroinflammation in dementia-like cognitive impairment. bioRxiv Prepr Serv Biol. 2021 doi: 10.1101/2021.03.15.435423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, Solis-Navarro L, Burgos F, Puppo H, et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology. 2021;27:328–337. doi: 10.1016/j.pulmoe.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 37.Grillo F, Barisione E, Ball L, Mastracci L, Fiocca R. Lung fibrosis: an undervalued finding in COVID-19 pathological series. Lancet Infect Dis. 2021;21:e72. doi: 10.1016/S1473-3099(20)30582-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Pachon E, Grau-Delgado J, Soler-Sempere MJ, Zamora-Molina L, Baeza-Martinez C, Ruiz-Alcaraz S, et al. Low prevalence of post-COVID-19 syndrome in patients with asthma. J Infect. 2021;82:276–316. doi: 10.1016/j.jinf.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adu-Amankwaah J, Mprah R, Adekunle AO, Ndzie Noah ML, Adzika GK, Machuki JO, et al. The cardiovascular aspect of COVID-19. Ann Med. 2021;53:227–236. doi: 10.1080/07853890.2020.1861644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansson M, Ståhlberg M, Runold M, Nygren-Bonnier M, Nilsson J, Olshansky B, et al. Long-haul post-COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the Swedish experience. JACC Case Reports. 2021;3:573–580. doi: 10.1016/j.jaccas.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali UA, Sadiq MS, Yunus MJ. Cardiorenal syndrome in COVID-19. BMJ Case Rep. 2021;14:e241914. http://casereports.bmj.com/content/14/4/e241914.abstract. 10.1136/bcr-2021-241914. [DOI] [PMC free article] [PubMed]
- 43.Farouk SS, Fiaccadori E, Cravedi P, Campbell KN. COVID-19 and the kidney: what we think we know so far and what we don’t. J Nephrol. 2020;33:1213–1218. doi: 10.1007/s40620-020-00789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32:151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Y-M, Shang Y-M, Song W-B, Li Q-Q, Xie H, Xu Q-F, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Livanos AE, Jha D, Cossarini F, Gonzalez-Reiche AS, Tokuyama M, Aydillo T, et al. Gastrointestinal involvement attenuates COVID-19 severity and mortality. medRxiv 2020;2020.09.07.20187666. http://medrxiv.org/content/early/2020/11/11/2020.09.07.20187666.abstract. 10.1101/2020.09.07.20187666.
- 47.Balaphas A, Gkoufa K, Meyer J, Peloso A, Bornand A, McKee TA, et al. COVID-19 can mimic acute cholecystitis and is associated with the presence of viral RNA in the gallbladder wall. J Hepatol. 2020;73:1566–1568. doi: 10.1016/j.jhep.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roth NC, Kim A, Vitkovski T, Xia J, Ramirez G, Bernstein D, et al. Post-COVID-19 cholangiopathy: a novel entity. Am J Gastroenterol. 2021;116:1077–1082. doi: 10.14309/ajg.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 49.Alhassan SM, Iqbal P, Fikrey L, Mohamed Ibrahim MI, Qamar MS, Chaponda M, et al. Post COVID 19 acute acalculous cholecystitis raising the possibility of underlying dysregulated immune response, a case report. Ann Med Surg. 2020;60:434–7. https://www.sciencedirect.com/science/article/pii/S204908012030458110.1016/j.amsu.2020.11.031 [DOI] [PMC free article] [PubMed]
- 50.Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, et al. New-onset diabetes in COVID-19. N Engl J Med. 2020;383:789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sathish T, Anton MC, Sivakumar T. New-onset diabetes in “long COVID”. J Diabetes. 2021;13:693–694. doi: 10.1111/1753-0407.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Landstra CP, de Koning EJP. COVID-19 and diabetes: understanding the interrelationship and risks for a severe course. Front Endocrinol (Lausanne) 2021;12:649525. doi: 10.3389/fendo.2021.649525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lisco G, De Tullio A, Stragapede A, Solimando AG, Albanese F, Capobianco M, et al. COVID-19 and the endocrine system: a comprehensive review on the theme. J Clin Med. 2021;10:2920. doi: 10.3390/jcm10132920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mehmood MA, Bapna M, Arshad M. A case of post-COVID-19 subacute thyroiditis. Cureus. 2020;12:e12301. doi: 10.7759/cureus.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davoodi L, Oladi Z, Jafarpour H, Zakariaei Z, Soleymani E, Razavi A. A 33-year-old man with COVID-19 presented with subacute thyroiditis: a rare case report and literature review. New Microbes New Infect. 2021;41:100871. doi: 10.1016/j.nmni.2021.100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campos-Barrera E, Alvarez-Cisneros T, Davalos-Fuentes M. Subacute thyroiditis associated with COVID-19. Case Rep Endocrinol. 2020;2020:8891539. doi: 10.1155/2020/8891539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duarte-Neto AN, Monteiro RAA, da Silva LFF, Malheiros DMAC, de Oliveira EP, Theodoro-Filho J, et al. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77:186–197. doi: 10.1111/his.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open. 2020;3:e208292. doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Wan X, Xiao Q, Zhang Y, Sun W, Xie Y, et al. Value of 3D versus 2D speckle-tracking echocardiography for rv strain measurement: validation with cardiac magnetic resonance. JACC Cardiovasc Imaging. 2020;13:2056–2058. doi: 10.1016/j.jcmg.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 60.He Y, Wang J, Ren J, Zhao Y, Chen J, Chen X. Effect of COVID-19 on Male reproductive system—a systematic review. Front Endocrinol (Lausanne) 2021;12:677701. doi: 10.3389/fendo.2021.677701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pazir Y, Eroglu T, Kose A, Bulut TB, Genc C, Kadihasanoglu M. Impaired semen parameters in patients with confirmed SARS-CoV-2 infection: a prospective cohort study. Andrologia. 2021;53:1–6. doi: 10.1111/and.14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, Adams O, et al. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril. 2020;114:233–238. doi: 10.1016/j.fertnstert.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma L, Xie W, Li D, Shi L, Ye G, Mao Y, et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol. 2021;93:456–462. doi: 10.1002/jmv.26259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tian Y, Zhou L-Q. Evaluating the impact of COVID-19 on male reproduction. Reproduction. 2021;161:R37–44. doi: 10.1530/REP-20-0523. [DOI] [PubMed] [Google Scholar]
- 65.Abdelhamid MHM, Fellah AA, Elmarghani A, Al msellati IA. An assessment of men semen alterations in SARS-CoV-2: is fever the principal concern? Reprod Sci. 2022 doi: 10.1007/s43032-022-00889-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schroeder M, Schaumburg B, Müller Z, Parplys A, Jarczak D, Nierhaus A, et al. Sex hormone and metabolic dysregulations are associated with critical illness in male Covid-19 patients. medRxiv 2020;2020.05.07.20073817. http://medrxiv.org/content/early/2020/12/20/2020.05.07.20073817.abstract. 10.1101/2020.05.07.20073817.
- 67.Rastrelli G, Di Stasi V, Inglese F, Beccaria M, Garuti M, Di Costanzo D, et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021;9:88–98. doi: 10.1111/andr.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agarwal M, Basumatary S, Bhusan D, Pati BK. Detection of severe acute respiratory syndrome corona virus 2 in cervico-vaginal secretion of COVID-19-affected female: a prospective observational study from India. SAGE Open Med. 2021;9:20503121211022990. doi: 10.1177/20503121211022993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khoiwal K, Kalita D, Shankar R, Kumari R, Dhundi D, Bahadur A, et al. Identification of SARS-CoV-2 in the vaginal fluid and cervical exfoliated cells of women with active COVID-19 infection: a pilot study. Int J Gynaecol Obstet. 2021;153:551–553. doi: 10.1002/ijgo.13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li F, Lu H, Zhang Q, Li X, wang T, Liu Q, et al. Impact of COVID-19 on female fertility: a systematic review and meta-analysis protocol. BMJ Open 2021;11:e045524. http://bmjopen.bmj.com/content/11/2/e045524.abstract. 10.1136/bmjopen-2020-045524 [DOI] [PMC free article] [PubMed]
- 71.Strope JD, PharmD CHC, Figg WD. TMPRSS2: potential biomarker for COVID-19 outcomes. J Clin Pharmacol. 2020;60:801–807. doi: 10.1002/jcph.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Funke-Chambour M, Bridevaux P-O, Clarenbach CF, Soccal PM, Nicod LP, von Garnier C. Swiss recommendations for the follow-up and treatment of pulmonary long COVID. Respiration 2021. https://www.karger.com/. 10.1159/000517255. [DOI] [PMC free article] [PubMed]
- 73.McMahon DE, Gallman AE, Hruza GJ, Rosenbach M, Lipoff JB, Desai SR, et al. Long COVID in the skin: a registry analysis of COVID-19 dermatological duration. Lancet Infect Dis. 2021;21:313–314. doi: 10.1016/S1473-3099(20)30986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dennis A, Wamil M, Alberts J, Oben J, Cuthbertson DJ, Wootton D, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open 2021;11:e048391. http://bmjopen.bmj.com/content/11/3/e048391.abstract. 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed]
- 75.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in US. Children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parpas A, Yudd M, Dreisbach AW, Michaud J. Post COVID 19 multisystem inflammatory syndrome in an older adult. Ren Fail. 2021;43:530–532. doi: 10.1080/0886022X.2021.1895839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lechien JR, Chiesa-Estomba CM, Beckers E, Mustin V, Ducarme M, Journe F, et al. Prevalence and 6-month recovery of olfactory dysfunction: a multicentre study of 1363 COVID-19 patients. J Intern Med. 2021;290:451–461. doi: 10.1111/joim.13209. [DOI] [PubMed] [Google Scholar]
- 78.Paolo G. Does COVID-19 cause permanent damage to olfactory and gustatory function? Med Hypotheses. 2020;143:110086. doi: 10.1016/j.mehy.2020.110086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cirillo N. Taste alteration in COVID-19: Significant geographical differences exist in the prevalence of the symptom. J. Infect. Public Health 2021;14:1099–105. https://www.sciencedirect.com/science/article/pii/S1876034121001891. 10.1016/j.jiph.2021.07.002 [DOI] [PMC free article] [PubMed]
- 80.Rodriguez B, Nansoz S, Cameron DR, Z’Graggen WJ. Is myopathy part of long-Covid? Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2021;132:1241–1242. doi: 10.1016/j.clinph.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Freeman EE, McMahon DE, Lipoff JB, Rosenbach M, Kovarik C, Desai SR, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118–1129. doi: 10.1016/j.jaad.2020.06.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saini G, Aneja R. Cancer as a prospective sequela of long COVID-19. BioEssays. 2021;43:e2000331. doi: 10.1002/bies.202000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holmes E, Wist J, Masuda R, Lodge S, Nitschke P, Kimhofer T, et al. Incomplete systemic recovery and metabolic phenoreversion in post-acute-phase nonhospitalized COVID-19 patients: implications for assessment of post-acute COVID-19 syndrome. J Proteome Res. 2021;20:3315–3329. doi: 10.1021/acs.jproteome.1c00224. [DOI] [PubMed] [Google Scholar]
- 84.Yang L, Xie X, Tu Z, Fu J, Xu D, Zhou Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct Target Ther. 2021;6:255. doi: 10.1038/s41392-021-00679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Assunção FB, Fragoso DC, Donoso Scoppetta TLP, Martins Maia AC. COVID-19-associated acute disseminated encephalomyelitis-like disease. AJNR Am J Neuroradiol. 2021;42:E21–E23. doi: 10.3174/ajnr.A6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baghbanian SM, Namazi F. Post COVID-19 longitudinally extensive transverse myelitis (LETM)-a case report. Acta Neurol Belg. 2020 doi: 10.1007/s13760-020-01497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim J-W, Abdullayev N, Neuneier J, Fink GR, Lehmann HC. Post-COVID-19 encephalomyelitis. Neurol Res Pract. 2021;3:18. doi: 10.1186/s42466-021-00113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stefano GB. Historical insight into infections and disorders associated with neurological and psychiatric sequelae similar to long COVID. Med Sci Monit Int Med J Exp Clin Res. 2021;27:e931447. doi: 10.12659/MSM.931447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guedj E, Campion JY, Dudouet P, Kaphan E, Bregeon F, Tissot-Dupont H, et al. (18)F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021;48:2823–2833. doi: 10.1007/s00259-021-05215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sollini M, Morbelli S, Ciccarelli M, Cecconi M, Aghemo A, Morelli P, et al. Long COVID hallmarks on [18F]FDG-PET/CT: a case-control study. Eur J Nucl Med Mol Imaging. 2021 doi: 10.1007/s00259-021-05294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.LaRovere KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, Maamari M, et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78:536–547. doi: 10.1001/jamaneurol.2021.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCaddon A, Regland B. COVID-19: a methyl-group assault? Med Hypotheses 2021;149:110543. https://www.sciencedirect.com/science/article/pii/S030698772100061X. 10.1016/j.mehy.2021.110543 [DOI] [PMC free article] [PubMed]
- 93.Unnikrishnan R, Misra A. Diabetes and COVID19: a bidirectional relationship. Nutr Diabetes. 2021;11:21. doi: 10.1038/s41387-021-00163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang J-K, Lin S-S, Ji X-J, Guo L-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lim S, Bae JH, Kwon H-S, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17:11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Khunti K, Davies MJ, Kosiborod MN, Nauck MA. Long COVID— metabolic risk factors and novel therapeutic management. Nat Rev Endocrinol. 2021;17:379–380. doi: 10.1038/s41574-021-00495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shi J, Fan J, Su Q, Yang Z. Cytokines and abnormal glucose and lipid metabolism. Front Endocrinol (Lausanne) 2019;10:703. doi: 10.3389/fendo.2019.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mao Y, Xu B, Guan W, Xu D, Li F, Ren R, et al. The adrenal cortex, an underestimated site of SARS-CoV-2 infection. Front Endocrinol (Lausanne) 2020;11:593179. doi: 10.3389/fendo.2020.593179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McCracken IR, Saginc G, He L, Huseynov A, Daniels A, Fletcher S, et al. Lack of evidence of angiotensin-converting enzyme 2 expression and replicative infection by SARS-CoV-2 in human endothelial cells. Circulation. 2021;143:865–868. doi: 10.1161/CIRCULATIONAHA.120.052824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Siddiqi HK, Libby P, Ridker PM. COVID-19—a vascular disease. Trends Cardiovasc Med. 2021;31:1–5. doi: 10.1016/j.tcm.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wallukat G, Hohberger B, Wenzel K, Fürst J, Schulze-Rothe S, Wallukat A, et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J Transl Autoimmun. 2021;4:100100. https://www.sciencedirect.com/science/article/pii/S2589909021000204. 10.1016/j.jtauto.2021.100100. [DOI] [PMC free article] [PubMed]
- 102.Plassmeyer M, Alpan O, Corley MJ, Premeaux TA, Lillard K, Coatney P, et al. Caspases and therapeutic potential of caspase inhibitors in moderate-severe SARS CoV2 infection and long COVID. Allergy. 2021 doi: 10.1111/all.14907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Apetrii M, Enache S, Siriopol D, Burlacu A, Kanbay A, Kanbay M, et al. A brand-new cardiorenal syndrome in the COVID-19 setting. Clin Kidney J. 2020;13:291–296. doi: 10.1093/ckj/sfaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chun HJ, Coutavas E, Pine AB, Lee AI, Yu VL, Shallow MK, et al. Immunofibrotic drivers of impaired lung function in postacute sequelae of SARS-CoV-2 infection. JCI Insight. 2021 doi: 10.1172/jci.insight.148476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Crestani B, Marchand-Adam S, Quesnel C, Plantier L, Borensztajn K, Marchal J, et al. Hepatocyte growth factor and lung fibrosis. Proc Am Thorac Soc. 2012;9:158–163. doi: 10.1513/pats.201202-018AW. [DOI] [PubMed] [Google Scholar]
- 106.Pardo A, Cabrera S, Maldonado M, Selman M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir Res. 2016;17:23. doi: 10.1186/s12931-016-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chan JF-W, Yip CC-Y, To KK-W, Tang TH-C, Wong SC-Y, Leung K-H, et al. Improved Molecular Diagnosis of COVID-19 by the Novel, Highly Sensitive and Specific COVID-19-RdRp/Hel Real-Time Reverse Transcription-PCR Assay Validated In Vitro and with Clinical Specimens. J. Clin. Microbiol. [Internet] 2020 22;58:e00310–20, /jcm/58/5/JCM.00310–20.atom. https://jcm.asm.org/content/58/5/e00310-20. 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed]
- 108.Almutairi AS, Abunurah H, Hadi Alanazi A, Alenazi F, Nagy H, Saad Almutairi N, et al. The immunological response among COVID-19 patients with acute respiratory distress syndrome. J Infect Public Health. 2021;14:954–959. doi: 10.1016/j.jiph.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Song W-J, Hui CKM, Hull JH, Birring SS, McGarvey L, Mazzone SB, et al. Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir Med. 2021;9:533–544. doi: 10.1016/S2213-2600(21)00125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saniasiaya J, Narayanan P. Parosmia post COVID-19: an unpleasant manifestation of long COVID syndrome. Postgrad Med J. 2021 doi: 10.1136/postgradmedj-2021-139855. [DOI] [PubMed] [Google Scholar]
- 111.Brooks EF, Bhatt AS. The gut microbiome: a missing link in understanding the gastrointestinal manifestations of COVID-19? Cold Spring Harb Mol Case Stud. 2021;7:a0060310. doi: 10.1101/mcs.a006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guo Y, Korteweg C, McNutt MA, Gu J. Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res. 2008;133:4–12. doi: 10.1016/j.virusres.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Durazo FA, Nicholas AA, Mahaffey JJ, Sova S, Evans JJ, Trivella JP, et al. Post-COVID-19 cholangiopathy-a new indication for liver transplantation: a case report. Transplant Proc. 2021;53:1132–1137. doi: 10.1016/j.transproceed.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Iftimie S, López-Azcona AF, Vallverdú I, Hernández-Flix S, de Febrer G, Parra S, et al. First and second waves of coronavirus disease-19: a comparative study in hospitalized patients in Reus. Spain PLoS One. 2021;16:e0248029. doi: 10.1371/journal.pone.0248029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Agostini F, Mangone M, Ruiu P, Paolucci T, Santilli V, Bernetti A. Rehabilitation setting during and after Covid-19: an overview on recommendations. J Rehabil Med. 2021;53:jrm00141. doi: 10.2340/16501977-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lutchmansingh DD, Knauert MP, Antin-Ozerkis DE, Chupp G, Cohn L, Dela Cruz CS, et al. A clinic blueprint for post-coronavirus disease 2019 RECOVERY: learning from the past, looking to the future. Chest. 2021;159:949–958. doi: 10.1016/j.chest.2020.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Parkin A, Davison J, Tarrant R, Ross D, Halpin S, Simms A, et al. A Multidisciplinary NHS COVID-19 service to manage post-COVID-19 syndrome in the community. J Prim Care Community Health. 2021;12:21501327211010990. doi: 10.1177/21501327211010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.