Abstract
Objectives
In this study, we aimed to investigate vaccine effectiveness (VE) against SARS-CoV-2 infections among adolescents aged 12 to 17 years in Malaysia and examine potential VE differences after full vaccination.
Methods
We consolidated data on COVID-19 testing, vaccination, and outcomes for all public school-going adolescents in Malaysia from September 1, 2021, to December 31, 2021, and estimated the VE against SARS-CoV-2 infections during this period. Cases were defined as positive tests, either by reverse transcriptase- PCR (RT-PCR) or rapid antigen (RTK-Ag) testing, while controls were negative tests. Secondarily, we restricted the analysis to all tests performed in December 2021 and compared VE by month of full vaccination.
Results
A total of 175,880 eligible tests (53.4% or 93,995 RT-PCR tests) were included. After full vaccination with BNT162b2, VE against SARS-CoV-2 infections was 65.7% (95% confidence interval [CI] 64.4, 66.9) over the study period. When restricted to tests in December 2021, VEs for those fully vaccinated in September 2021, October 2021, and November 2021 were comparable (60.6% [95% CI 23.7, 81.5], 56.9% [95% CI 51.1, 62.0], and 65.7% [95% CI 59.8, 70.7] respectively).
Conclusions
Among adolescents, full vaccination with BNT162b2 offered considerable protection against SARS-CoV-2 infections over at least three months without substantial evidence of waning.
Keywords: COVID-19 Vaccines, SARS-CoV-2, BNT162b2, Effectiveness, Adolescent, Malaysia
Introduction
Vaccinating against the SARS-CoV-2 virus to achieve high population immunity is essential to realise health and socio-economic goals driving the global COVID-19 vaccination strategy (World Health Organization. Strategy to achieve global COVID-19 vaccination by mid-2022.). In Malaysia, the national vaccination programme for COVID-19 began in February 2021 using a diverse platform of vaccines (Suah et al., 2021). It was demonstrated that they offer considerable protection, particularly against severe outcomes (Suah et al., 2022, 2021). Beginning September 2021, the vaccination roll-out was extended nationwide to cover adolescents (≥12 years old) with primarily the BNT162b2 vaccine. By the end of 2021, 89.4% of Malaysia's adolescents were fully vaccinated. In a phase 3 clinical trial for BNT162b2, high efficacy was observed in adolescents aged 12 to 15 years (Frenck Jr et al., 2021). An initial real-world study from Israel (Reis et al., 2021) reported 90% vaccine effectiveness (VE) against documented SARS-CoV-2 infection on days 7 to 21 after the second dose, while another study from the United States (Olson et al., 2022) demonstrated >90% VE against hospitalisation and intensive care unit (ICU) admission. Subsequently, however, a study from Singapore estimated VE against confirmed SARS-CoV-2 infection at only 59% and observed a gradual waning of immunity over 4 months from completion of the second dose (Chiew et al., 2022). Notably, real-world evidence in low- and middle-income countries is lacking. We aimed to address this by evaluating VE against SARS-CoV-2 infections among adolescents in Malaysia (which is a low- and middle-income country) using a test-negative design.
Methods
The study population comprised all public school-going adolescents (aged 12 to 17 years) registered under the Ministry of Education Malaysia. From this school registry, we conducted a test-negative design by deterministically linking databases for: (i) supervised and approved reverse transcriptase-PCR (RT-PCR) and antigen rapid tests (RTK-Ag), (ii) COVID-19 vaccination recipients, and (iii) confirmed COVID-19 cases, including their eventual clinical outcomes. All RT-PCR and RTK-Ag tests conducted from September 1, 2021, until December 31, 2021, were included in the primary analysis, with cases defined as positive tests for SARS-CoV-2 infection (RT-PCR or RTK-Ag), whereas controls were negative tests. Further details on the test-negative design are provided in the Supplementary Material.
VE was calculated from 100 x (1- incidence rate ratio), where incidence rate ratio is the adjusted odds ratio of full vaccination (≥14 days after the second dose) in cases over controls, estimated in a logistic regression. Secondarily, we limited the analysis to all tests performed in December 2021 and compared VE by month of full vaccination to examine potential VE difference(s) after full vaccination. All analyses were executed in R Software Version 4.1.2. A significance level of 5% was used for inference.
Results
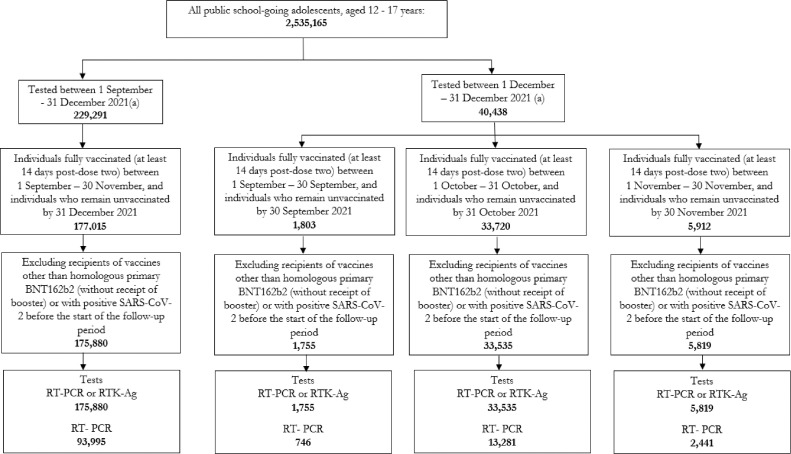
From September 1, 2021 until December 31, 2021, there were 175,880 eligible tests (53.4% or 93,995 RT-PCR tests, 46.6% or 81,885 RTK-Ag tests) by adolescents aged between 12 and 17 years (Figure 1 ), of which 79,484 (45.2%) were fully vaccinated.
Figure 1.
Study participants for the overall vaccine effectiveness and the vaccine effectiveness by time since vaccination against SARS-CoV-2 infection
Individuals who were tested by reverse transcriptase-PCR(RT-PCR) or rapid antigen (RTK-Ag) or RT-PCR only, between September 1, 2021, to December 31, 2021, and fully vaccinated with homologous BNT162b2 or unvaccinated between September 1, 2021, and November 30, 2021, aged 12 to 17 years and had no confirmed SARS-CoV-2 infections before the outcomes observed.
Table 1 lists the VE estimates. Overall, VE against SARS-CoV-2 infections was 65.7% (95% CI 64.4, 66.9). When restricted to only RT-PCR tests, VE was 63.4% (95% CI 64.4, 66.9). Among eligible study participants, there were 48 ICU admissions (49.8/100,000 persons-tested) and 11 COVID-19–related deaths (11.4/100,000 persons-tested) among unvaccinated adolescents, compared to 8 ICU admissions (10.1/100,000 persons-tested) and no COVID-19 related deaths among those fully vaccinated.
Table 1.
Overall BNT162b2 Vaccine effectiveness against SARS-CoV-2 infection among adolescents and by time since vaccination
| Tested by RT-PCR or RTK-Ag (September 1, 2021 –December 31, 2021) |
Tested by RT-PCR (September 1, 2021 –December 31, 2021) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total | Test-positive | VE | CI (95%) | Total | Test-positive | VE | CI (95%) | |
| Overall vaccine effectiveness | ||||||||
| Unvaccinated | 96,396 | 41,928 | ref | ref | 65,292 | 38,893 | ref | ref |
| Fully Vaccinated | 79,484 | 8,312 | 65.7 | 64.4, 66.9 | 28,703 | 7,056 | 63.4 | 64.4, 66.9 |
| Tested by RT-PCR or RTK-Ag (December 1, 2021 – December 31, 2021) |
Tested by RT-PCR (December 1, 2021 – December 31, 2021) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Month fully vaccinated | ||||||||
| Unvaccinated | 1,563 | 363 | ref | ref | 700 | 337 | ref | ref |
| September | 192 | 10 | 60.6 | 23.7, 81.5 | 46 | 5 | 76.2 | 41.5, 92.1 |
| October | 31,972 | 3,606 | 56.9 | 51.1, 62.0 | 12,581 | 3,292 | 62.8 | 56.4, 68.3 |
| November | 4,256 | 390 | 65.7 | 59.8, 70.7 | 1,745 | 372 | 70.2 | 64.0, 75.3 |
The estimates were adjusted for (i) age, (ii) sex, (iii) states of residence, (iv) strata (urban/rural), (v) school types (daily schools, boarding schools, special needs schools), and (vi) number of baseline (before September 1, 2021) tests. The analysis for the overall vaccine effectiveness was compared between the individuals tested between September 1, 2021, to December 31, 2021, and fully vaccinated between September 1, 2021, to November 30, 2021, and the unvaccinated group. The analysis for the waning effectiveness compared between individuals tested in December 2021 and fully vaccinated in September 2021 (3 to 4 months post-vaccination during the outcomes observation period in December), October 2021 (2 to 3 months post-vaccination during the outcomes observation period in December), November 2021 (1 to 2 months post-vaccination during the outcomes observation period in December 2021), and the unvaccinated group.
Abbreviations:
BNT162b2 = Pfizer-BioNTech; CI = confidence intervals; RT-PCR = reverse transcriptase-PCR; RTK-Ag = rapid antigen; VE = vaccine effectiveness.
In the secondary analysis (limited to all tests performed in December 2021), VEs against SARS-CoV-2 infections over time were comparable (60.6% [95% CI 23.7, 81.5], 56.9% [95% CI 51.1, 62.0], 65.7% [95% CI 59.8, 70.7] for those fully vaccinated in September, October, and November, respectively). When restricted to only RT-PCR tests, VE estimates were also comparable (76.2% [95% CI 41.5, 92.1], 62.8% [95% CI 56.4, 68.3], 70.2% [95% CI 64.0, 75.3] for those fully vaccinated in September, October, and November, respectively).
Discussion
Our study observed a VE of 65.7% (95% CI 64.4, 66.9) against SARS-CoV-2 infections among fully vaccinated adolescents over at least three months duration. There was no evidence of waning VE in an analysis restricted to tests in December 2021. SARS-CoV-2 infections in this study were predominantly of the Delta variant. Although unable to capture data from all adolescents, the cohort of public school-going adolescents represents approximately 80% of this age group's population and has a similar risk environment for SARS-CoV-2 infections.
Limitations include under-testing because of the increasing use of unsupervised self-test kits, although supervised testing rates with RT-PCR and RTK-Ag remain high. We included all supervised testing during the study period for analysis but could not distinguish between the reasons for testing. The waning of VE against infection among adolescents after three to five months (Chiew et al., 2022; Prunas et al., 2022) and lower VE against the Omicron variant (Dorabawila et al., 2022; Klein et al., 2022) were described elsewhere, but not investigated here because of the limited study period. Further inspection is therefore prudent.
Acknowledgments
Declarations of competing interest
The authors declare that they have no conflicts of interest
Funding Source
No funding
Ethical Consideration
This work was conducted as part of The Real-World Evaluation of COVID-19 Vaccines under the Malaysia National COVID-19 Immunisation Programme (RECoVaM) study registered in the National Medical Research Register (NMRR-21-1660-60697). Ethical approval was granted by the Medical Research and Ethics Committee (MREC), Ministry of Health, Malaysia.
Acknowledgement
We would like to thank the Director-General of Health Malaysia for permission to publish this article. We further acknowledge Maheshwara Rao Appannan, Hazlina Yahaya, Shahanizan Mohd Zin and Faizah Muhamad Zin from the Ministry of Health Malaysia for their work with data collection, management, and sharing arrangements.
Authors Contributions
All authors conceived and designed the study. MH, JLS, and TT performed the data curation, analysis and visualisation. All authors interpreted the data. MH and PSKT wrote the original draft of the manuscript. MH, JLS, and TT accessed and verified the data. BHT, KMP, and SS supervised the study. All authors had full access to all data in the studies and had final responsibility for the decision to submit for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.04.053.
Appendix. Supplementary materials
References
- Chiew CJ, Wei WE, Ong B, et al. Vaccine Effectiveness against COVID-19 Infection among 12 to 18 year olds in Singapore. SSRN. Preprint posted online January 8, 2022. doi: 10.2139/ssrn.3996796
- Dorabawila V, Hoefer D, Bauer UE, et al. Effectiveness of the BNT162b2 vaccine among children 5-11 and 12-17 years in New York after the Emergence of the Omicron Variant. medRxiv. Preprint posted online February 28, 2022. doi: 10.1101/2022.02.25.22271454
- Frenck RW, Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 COVID-19 vaccine in adolescents. N Engl J Med. 2021;385:239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein NP, Stockwell MS, Demarco M, et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA vaccination in preventing COVID-19–associated emergency department and urgent care encounters and hospitalisations among nonimmunocompromised children and adolescents aged 5-17 years - VISION Network, 10 States, April 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:352–358. doi: 10.15585/mmwr.mm7109e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SM, Newhams MM, Halasa NB, et al. Effectiveness of BNT162b2 vaccine against critical COVID-19 in adolescents. N Engl J Med. 2022;386:713–723. doi: 10.1056/NEJMoa2117995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunas O, Weinberger DM, Pitzer VE, et al. Waning Effectiveness of the BNT162b2 Vaccine Against Infection in Adolescents. medRxiv. Preprint posted online January 5, 2022. doi:. 10.1101/2022.01.04.22268776
- Reis BY, Barda N, Leshchinsky M, et al. Effectiveness of BNT162b2 vaccine against delta variant in adolescents. N Engl J Med. 2021;385:2101–2103. doi: 10.1056/NEJMc2114290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suah JL, Tok PSK, Ong SM, et al. PICK-ing Malaysia's epidemic apart: effectiveness of a diverse COVID-19 vaccine portfolio. Vaccines (Basel) 2021;9:1381. doi: 10.3390/vaccines9121381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suah JL, Husin M, Tok PSK, et al. Waning COVID-19 vaccine effectiveness for BNT162b2 and CoronaVac in Malaysia: an observational study. Int J Infect Dis. 2022;119:69–76. doi: 10.1016/j.ijid.2022.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Strategy to achieve global COVID-19 vaccination by mid-2022, [cited 2022, Apr 4]. Available from: https://cdn.who.int/media/docs/default-source/immunization/covid-19/strategy-to-achieve-global-covid-19-vaccination-by-mid-2022.pdf?sfvrsn=5a68433c_5, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.