Key Points
Question
How many people with a new diagnosis of HIV infection—who are at increased risk of meningococcal disease—receive the recommended meningococcal A, C, W, Y (MenACWY) vaccination in the US?
Findings
This cohort study of 1208 vaccine-eligible individuals found that, 2 years after a diagnosis of HIV infection, 16.3% were estimated to have received 1 or more doses of the MenACWY vaccine. Uptake was higher among those who received pneumococcal vaccination, attended a well-care visit, resided in the West or Midwest, and were male.
Meaning
This study suggests that poor uptake of the MenACWY vaccine among people with a new diagnosis of HIV underscores the need for education about vaccination recommendations for patients at increased risk of meningococcal disease.
Abstract
Importance
In the United States, individuals with HIV infection have been recommended to receive a 2-dose series of the meningococcal A, C, W, Y (MenACWY) vaccine since 2016 owing to their increased risk of meningococcal disease.
Objective
To examine uptake and time to receipt of the MenACWY vaccine among people with a new diagnosis of HIV.
Design, Setting, and Participants
This cohort study used health insurance data from the US Optum Research Database from January 1, 2016, through March 31, 2018, to retrospectively identify 1208 individuals aged 2 years or older with 1 or more inpatient claim or 2 or more outpatient claims evidencing a new diagnosis of HIV infection and with continuous insurance enrollment for 12 or more months before and 6 or more months after diagnosis. Follow-up was 6 to 33 months. Statistical analysis was conducted from March 7, 2019, to January 5, 2022.
Exposure
Receipt of the MenACWY vaccine.
Main Outcomes and Measures
The coprimary outcomes were uptake and time to receipt of 1 or more doses of the MenACWY vaccine after a new HIV diagnosis. Secondary outcomes included uptake and time to receipt of 2 or more doses of the MenACWY vaccine. Vaccination uptake and receipt were estimated by Kaplan-Meier analysis; factors associated with receipt of 1 or more doses of the MenACWY vaccine were identified with multivariable Cox proportional hazards regression analysis.
Results
Of 1208 individuals eligible for vaccination (1024 male patients [84.8%]; mean [SD] age, 38.8 [12.5] years; 35 [2.9%] Asian; 273 [22.6%] Black; 204 [16.9%] Hispanic; 442 [36.6%] White), 16.3% were estimated to have received a first dose of the MenACWY vaccine in the 2 years after a new HIV diagnosis. Among individuals who received a first dose, at 1 year or more of enrollment after the first dose, 66.2% were estimated to have received a second dose within 1 year of the first dose. Factors statistically significantly associated with uptake of the MenACWY vaccine included receipt of a pneumococcal vaccine (hazard ratio [HR], 23.03; 95% CI, 13.93-38.09), attendance at a well-care visit (HR, 3.67; 95% CI, 1.11-12.12), West or Midwest geographic region (West: HR, 2.24; 95% CI, 1.44-3.47; Midwest: HR, 1.78; 95% CI, 1.16-2.71), and male sex (HR, 2.72; 95% CI, 1.18-6.26), whereas age of 56 years or older was significantly associated with reduced uptake of the MenACWY vaccine (HR, 0.42; 95% CI, 0.18-0.97).
Conclusions and Relevance
This cohort study suggests that MenACWY vaccine uptake among people with a new diagnosis of HIV was low, highlighting the need to educate patients and clinicians about the recommendations for conditions such as HIV infection that increase the risk of meningococcal disease among high-risk populations.
This cohort study examines uptake and time to receipt of the meningococcal A, C, W, Y vaccine among people with a new diagnosis of HIV.
Introduction
Invasive meningococcal disease is rare but is associated with severe morbidity and mortality.1 Studies from the United States, England, and South Africa show that people infected with HIV have a 4.5- to 12.9-fold increased risk of meningococcal disease.2,3,4,5 In February 2016, the US Advisory Committee on Immunization Practices proposed recommending meningococcal serogroups A, C, W, and Y (MenACWY) vaccine for people with HIV infection.6 After a unanimous vote in June 2016,7 this recommendation was published in November 2016.8 The MenACWY vaccine is generally given as a 2-dose primary series followed by regular boosters (eTable in the Supplement).9,10
To our knowledge, there are no data on the uptake of 1 or more doses or 2 or more doses of the MenACWY vaccine and factors associated with uptake among people with a new diagnosis of HIV in the US. Therefore, the objective of the present study was to fill this knowledge gap.
Methods
Study Design
This nationwide cohort study, which used administrative claims data from the Optum Research Database, included approximately 19% of the US commercially insured population in 2017 and represents individuals enrolled in managed care from one of the largest commercial health plans in the US. Medical claims and encounter data are collected from all available health care sites for all service types from a variety of geographic regions and employer groups. The data used for this study were deidentified. Institutional review board approval, waiver of authorization, and informed consent were not required for this study because the study data were deidentified in accordance with the Health Insurance Portability and Accountability Act of 1996; therefore, no protected health information was accessed. This report follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Participants
The index date was the first date with an International Classification of Diseases diagnosis code for HIV (International Classification of Diseases, Ninth Revision codes 042, V08, and 079.53; and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes B20, Z21, and B9735). Individuals were identified retrospectively from January 1, 2016, through March 31, 2018. Participants needed to (1) have 1 or more inpatient claims or 2 or more outpatient claims (≥30 days apart) with an HIV diagnosis code; (2) be 2 years of age or older in the index year (to capture postnatally acquired rather than perinatally acquired HIV infection); (3) have continuous insurance enrollment for 12 or more months before and 6 or more months after the index date; (4) have no evidence of other high-risk conditions (anatomic or functional asplenia, complement deficiency, or eculizumab treatment) on the index date; and (5) have no evidence of any high-risk condition(s) during the 12 or more months before the index date. All data available before the index date were examined to capture only people with newly diagnosed HIV (total data capture, January 1, 2005, to September 30, 2018). In addition, those who received a first MenACWY vaccine dose and had 12 or more months of continuous enrollment after the first dose were included in the analysis of uptake of 2 or more doses of the MenACWY vaccine.
Outcomes
The coprimary outcomes were uptake and time to receipt of 1 or more doses of the MenACWY vaccine (MenACWY oligosaccharide diphtheria CRM197 conjugate vaccine [MenACWY-CRM; GSK], MenACWY polysaccharide diphtheria toxoid conjugate vaccine [MenACWY-D; Sanofi Pasteur], or quadrivalent meningococcal polysaccharide vaccine [Sanofi Pasteur]) after a new HIV diagnosis. Patient data were examined until receipt of the MenACWY vaccine, disenrollment from the health plan, or the end of the study, whichever occurred first. Outcomes were assessed during up to 33 months of follow-up. Secondary outcomes included uptake and time to receipt of 2 or more doses of the MenACWY vaccine, and analysis of factors associated with receipt of 1 or more doses of the MenACWY vaccine.
As patients were included from January 2016, while the recommendation to administer the MenACWY vaccine to people with HIV infection was not published until November 20168 (despite being proposed6 in February 2016 and approved7 in June 2016), we conducted a sensitivity analysis limited to people who received a diagnosis of HIV in December 2016 or later.
Statistical Analysis
Statistical analysis was conducted from March 7, 2019, to January 5, 2022. Baseline variables are provided as numbers and percentages for categorical variables, and as mean (SD) values for continuous variables. The percentages of people who received 1 or more doses of the MenACWY vaccine and 2 or more doses of the MenACWY vaccine were estimated separately using Kaplan-Meier analysis.
Baseline variables examined in univariable analysis were age; sex; race and ethnicity (estimated by an algorithm based on US Census data zip codes [zip +4 digits] and first and last names); educational level (median for the census block group); household income; geographic region; urbanicity; health plan type; year of index date; Charlson Comorbidity Index score11; comorbidities; all-cause health care use (separately for office visits, emergency department visits, inpatient visits, and pharmacy fills); all-cause total health care, medical, and pharmacy costs; influenza vaccination; and index eligibility for the MenACWY vaccine. Time-varying covariates (from index to end of follow-up) were ever had a well-care visit, ever had a pneumococcal vaccination, and ever had a meningococcal serogroup B vaccination. All covariates (apart from race and ethnicity, educational level, and household income) were extracted from the health insurance database. Cox proportional hazards regression was used to identify characteristics associated with receipt of 1 or more doses of the MenACWY vaccine. Covariates included in the adjusted model were determined based on statistical significance (P < .05 from 2-sided statistical tests) using a variety of approaches, including univariate analyses; backward, forward, or stepwise selection; and clinical relevance. The covariates that were retained in the model were age, sex, race and ethnicity, educational level, household income, geographic region, urbanicity, health plan type, year of index date, Charlson Comorbidity Index score,11 emergency department visits, and influenza vaccination. Time-varying covariates (from index to end of follow-up) were well-care visits and pneumococcal vaccination. Uptake of the MenACWY vaccine by age and by clinician type (overall and by age group [2-18, 19-55, and ≥56 years]) is also described. Statistical analyses were conducted using SAS, version 9.4 (SAS Institute Inc).
Results
Baseline Characteristics
A total of 1208 people with a new diagnosis of HIV who were eligible for the MenACWY vaccine were included in the primary analyses (eFigure 1 in the Supplement). Their mean (SD) age was 38.8 (12.5) years, most (1064 [88.1%]) were aged 19 to 55 years, 1024 (84.8%) were male, and race and ethnicity was diverse (Asian, 35 [2.9%]; Black, 273 [22.6%]; Hispanic, 204 [16.9%]; and White, 442 [36.6%]) (Table 1).
Table 1. Baseline Characteristics of People With a New Diagnosis of HIV Who Were Eligible for the MenACWY Vaccine.
| Characteristic | No. (%) (N = 1208) |
|---|---|
| Age, mean (SD), y | 38.8 (12.5) |
| Age group, y | |
| 2-18 | 13 (1.1) |
| 19-55 | 1064 (88.1) |
| ≥56 | 131 (10.8) |
| Sex | |
| Male | 1024 (84.8) |
| Female | 184 (15.2) |
| Race and ethnicitya | |
| Hispanic | 204 (16.9) |
| Non-Hispanic | |
| Asian | 35 (2.9) |
| Black or African American | 273 (22.6) |
| White or Caucasian | 442 (36.6) |
| Other | 10 (0.8) |
| Multiple or unknown | 214 (17.7) |
| Geographic region of residence | |
| South | 688 (57.0) |
| Midwest | 209 (17.3) |
| West | 175 (14.5) |
| Northeast | 135 (11.2) |
| Other | 1 (0.1) |
| Health plan type | |
| Point of service | 909 (75.2) |
| Exclusive provider organization | 188 (15.6) |
| Health maintenance organization | 89 (7.4) |
| Other or unknown | 22 (1.8) |
| Year of index date | |
| 2016 | 609 (50.4) |
| 2017 | 524 (43.4) |
| 2018 | 75 (6.2) |
Abbreviation: MenACWY, meningococcal serogroups A, C, W, Y.
Estimated by an algorithm based on Census data zip codes (zip +4 digits) and first and last names. Other includes Native American, Alaska Native, and Pacific Islander.
Vaccine Uptake and Time to Receipt
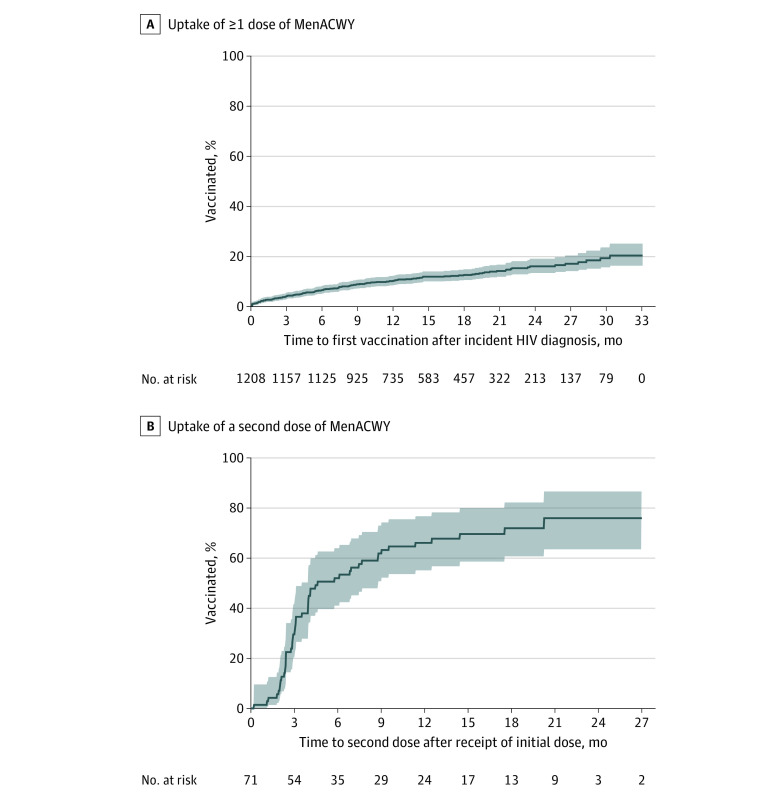
By 12, 18, and 24 months after an initial HIV diagnosis, 10.6%, 12.9%, and 16.3%, respectively, of people eligible for the MenACWY vaccine were estimated to have received 1 or more doses (Figure, A). Among the 71 individuals who received a first dose and had at least 1 year of follow-up data available, 66.2% were estimated to have received a second dose within 1 year after the first dose (Figure, B).
Figure. Uptake of the Meningococcal A, C, W, Y (MenACWY) Vaccine.
A, Kaplan-Meier curve of uptake of 1 or more doses of the MenACWY vaccine. B, Kaplan-Meier curve of uptake of a second dose of the MenACWY vaccine among those who received a first dose and had 12 or more months of continuous enrollment after the first dose. Shaded areas indicate 95% CIs, estimated using the pointwise method.
Vaccination by Age
During all available follow-up, 157 of 1208 people (13.0%) received the MenACWY vaccine. However, uptake varied by age group (2-18 years, 4 of 13 [30.8%]; 19-55 years, 147 of 1064 [13.8%]; ≥56 years, 6 of 131 [4.6%]).
Vaccination Clinician Types
The MenACWY vaccine was administered by a wide range of clinician types overall (25.5% [40 of 157] infectious disease specialist, 20.4% [32 of 157] internal medicine, 17.2% [27 of 157] primary care, 2.6% [4 of 157] pediatrician, 31.2% [49 of 157] other specialist, and 3.2% [5 of 157] unknown). Pediatricians administered all vaccinations for the 4 children aged 11 to 18 years, and internal medicine, infectious disease specialists, and other specialists each provided vaccinations for 2 people aged 56 years or older. Only 9.6% of vaccinations (15 of 157) were given at well-care visits.
Factors Associated With Vaccine Receipt
By multivariable Cox regression, factors that were statistically significantly associated with receipt of the MenACWY vaccine were receipt of pneumococcal vaccine (hazard ratio [HR], 23.03; 95% CI, 13.93-38.09), attendance at a well-care visit (HR, 3.67; 95% CI, 1.11-12.12), male sex (HR, 2.72; 95% CI, 1.18-6.26), and West or Midwest geographic location (West: HR, 2.24; 95% CI, 1.44-3.47; Midwest: HR, 1.78; 95% CI, 1.16-2.71) (Table 2). However, age of 56 years or older (HR, 0.42; 95% CI, 0.18-0.97) and baseline emergency department visit (HR, 0.54; 95% CI, 0.37-0.79) were statistically significantly associated with a reduced incidence of receipt of the MenACWY vaccine.
Table 2. Factors Statistically Significantly Associated With Likelihood of Receipt of 1 or More Doses of the MenACWY Vaccine in Cox Proportional Hazards Regression Analyses.
| Variable | Univariable analysisa | Multivariable analysisb | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age ≥56 yc | 0.31 (0.14-0.71) | .005 | 0.42 (0.18-0.97) | .04 |
| Male sex | 4.63 (2.05-10.53) | <.001 | 2.72 (1.18-6.26) | .02 |
| Geographic region | ||||
| Westd | 2.63 (1.76-3.94) | <.001 | 2.24 (1.44-3.47) | <.001 |
| Midwestd | 2.54 (1.71-3.76) | <.001 | 1.78 (1.16-2.71) | .008 |
| Baseline ED visit | 0.52 (0.36-0.75) | <.001 | 0.54 (0.37-0.79) | .001 |
| Well-care visite,f | 13.37 (4.22-42.38) | <.001 | 3.67 (1.11-12.12) | .03 |
| PCV13 vaccine or PPSV23 vaccinee | 31.75 (19.32-52.18) | <.001 | 23.03 (13.93-38.09) | <.001 |
Abbreviations: ED, emergency department; HR, hazard ratio; MenACWY, meningococcal serogroups A, C, W, Y; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
Additional variables examined in univariable analysis were race and ethnicity, educational level, household income, urbanicity, health plan type, year of index date, baseline Charlson Comorbidity Index score, baseline comorbidities, baseline health care use and costs, baseline influenza vaccine exposure, index MenACWY vaccine eligibility, and meningococcal serogroup B vaccination.
Additional variables included in multivariable analysis were race and ethnicity, educational level, household income, urbanicity, health plan type, year of index date, baseline Charlson Comorbidity Index score,11 and baseline influenza vaccine exposure.
Compared with 19 to 55 years for univariable analysis; compared with 2 to 55 years for multivariable analysis.
Compared with South or other for univariable analysis; compared with South for multivariable analysis.
Time-varying covariate.
Excluding Current Procedural Terminology code 90734 (meningococcal vaccine administered).
Sensitivity Analysis
When the start of the identification period was limited to December 2016 and later, uptake of the MenACWY vaccine among 642 people with a new diagnosis of HIV was estimated to be 11.6% at 1 year and 13.9% at 18 months.
Discussion
In this nationwide cohort study, only 16.3% of retrospectively identified people with a new diagnosis of HIV were estimated to have received the MenACWY vaccine during the 24 months after their diagnosis. This rate lies between the rates found in similar studies of people with newly diagnosed complement deficiency12,13 and asplenia,14 with all 3 rates being suboptimal. The rate of uptake of a second dose within 1 year was, however, considerably higher among those with newly diagnosed HIV (66.2%) than those with asplenia (9.7%).14 This finding could be due to patient reminders from HIV clinics, as has been reported for other recommended vaccines for people with HIV, such as hepatitis B.15 Uptake of a second dose of the MenACWY vaccine was considerably higher than uptake of a first dose, perhaps because people who received an initial vaccination were visiting clinicians who are more likely to vaccinate in general and/or indicating that people with HIV are more likely to complete a vaccine series than to start it.
The vaccination rate among adults in our study was somewhat higher than for meningococcal C vaccination among adults with HIV in a study conducted in an AIDS outpatient clinic in Brazil in 2015 and 2016 (first dose coverage, 8.2%).16 However, the rate among those aged 2 to 18 years in our study (30.8%) was much lower than in a retrospective study among children with HIV in Spain in the late 1990s and early 2000s (meningococcal C vaccine uptake, 80.8%),17 although the Spanish study included infant meningococcal C vaccination, which is routinely recommended in Spain.18
To our knowledge, no US studies have reported coverage of the MenACWY vaccine among people with HIV, although 2 US studies have reported coverage among men who have sex with men (MSM),19,20 with results according to HIV status. Holloway et al20 reported that 48.9% of HIV-positive MSM and 36.2% of HIV-negative MSM self-reported receiving the MenACWY vaccine (adjusted odds ratio, 1.12; 95% CI, 0.43-2.91). Similarly, Phillips et al19 reported that 60.0% of HIV-positive MSM and 46.3% of HIV-negative MSM self-reported meningococcal vaccination (odds ratio, 1.73; 95% CI, 1.10-2.73). Vaccination rates in these 2 studies19,20 were likely higher than our study, as they were conducted during meningococcal disease outbreaks, during which MSM were recommended to receive meningococcal vaccination. Also, both studies19,20 assessed “ever vaccinated,” while we assessed vaccination after receiving a new diagnosis of HIV. Nevertheless, the higher coverage among HIV-positive MSM indicates some knowledge—among clinicians and MSM—of the increased risk of meningococcal infection among people with HIV.
Similar to a previous study of patients with a new diagnosis of asplenia,14 the factors that were most correlated with higher uptake of the MenACWY vaccine after a new diagnosis of HIV infection included receipt of pneumococcal vaccination and attendance at a well-care visit. In a US survey of MSM, factors associated with meningococcal vaccination among those who were HIV-positive included advice from a health care professional and low or no cost of the vaccine; barriers included lack of insurance, fear of adverse events, and fatalism; and intervention strategies included improving awareness and incentivizing vaccination.21 Overall, both health care practitioners and HIV-positive individuals require education about the importance of the MenACWY vaccination.
Strengths and Limitations
This study includes some strengths, including that we took steps to include only people with newly diagnosed HIV—so that we could study time to vaccination—and that our cohort consisted of individuals with a wide US geographic distribution. Furthermore, the variable observation period maximized both sample size and observation time.
However, this study also has some limitations. We included patients from January 2016, but the official recommendations on the MenACWY vaccine for people with HIV were not published until November 2016.8 However, the uptake rates of the MenACWY vaccine in our sensitivity analysis (which excluded people diagnosed before December 2016) were only marginally higher than in the original analysis. This finding could be because, from January to October 2016, people were vaccinated during follow-up after the recommendation was published in November 2016.8 It may also be because some clinicians started following the MenACWY vaccine recommendation after the proposal in February 20166 or the approval in June 2016.7
The requirement for 6 or more months of continuous enrollment after index could have introduced survivor bias, although any effect of this is likely to be small.22 We also assumed that vaccine receipt was independent of enrollment status change, and we did not perform a competing risk analysis (eg, death or meningococcal disease).
Some individuals may have received the MenACWY vaccine that was not submitted to their health plan for reimbursement, which could have resulted in an underestimation of vaccine coverage. Conversely, MenACWY vaccination rates were considerably higher among those aged 2 to 18 years vs those aged 19 years or older (30.8% vs 12.8%), so some adolescent vaccinations could have been coincidental routine age-related vaccinations (MenACWY vaccination is recommended at the ages of 11-12 years and 16 years9). Additionally, as the data were from patients with US commercial insurance, these results may not be generalizable to uninsured patient populations, patients covered by other types of insurance, or those who receive HIV-related care through programs such as the Ryan White Program.23 Health plan type was not significantly correlated with vaccine uptake, but this may have been owing to the predominance of one plan type.
Conclusions
This cohort study found that uptake of the MenACWY vaccine among people with a new diagnosis of HIV infection was low and the time to receipt of first vaccination was long. These data highlight the need for better education of patients and clinicians about the risk of meningococcal disease and the need for vaccination.10 eFigure 2 in the Supplement contains a summary of the context, novelty, and objective of the study in a format that can be shared with patients.
eTable. ACIP Recommendations for MenACWY Vaccination for People with HIV Infection
eReferences.
eFigure 1. Study Flow Chart
eFigure 2. Plain Language Summary
References
- 1.Wang B, Santoreneos R, Giles L, Haji Ali Afzali H, Marshall H. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine. 2019;37(21):2768-2782. doi: 10.1016/j.vaccine.2019.04.020 [DOI] [PubMed] [Google Scholar]
- 2.Simmons RD, Kirwan P, Beebeejaun K, et al. Risk of invasive meningococcal disease in children and adults with HIV in England: a population-based cohort study. BMC Med. 2015;13:297. doi: 10.1186/s12916-015-0538-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller L, Arakaki L, Ramautar A, et al. Elevated risk for invasive meningococcal disease among persons with HIV. Ann Intern Med. 2014;160(1):30-37. doi: 10.7326/0003-4819-160-1-201401070-00731 [DOI] [PubMed] [Google Scholar]
- 4.Harris CM, Wu HM, Li J, et al. Meningococcal disease in patients with human immunodeficiency virus infection: a review of cases reported through active surveillance in the United States, 2000-2008. Open Forum Infect Dis. 2016;3(4):ofw226. doi: 10.1093/ofid/ofw226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen C, Singh E, Wu HM, et al. ; Group for Enteric, Respiratory and Meningeal Disease Surveillance in South Africa (GERMS-SA) . Increased incidence of meningococcal disease in HIV-infected individuals associated with higher case-fatality ratios in South Africa. AIDS. 2010;24(9):1351-1360. doi: 10.1097/QAD.0b013e32833a2520 [DOI] [PubMed] [Google Scholar]
- 6.Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention . Summary report. February 24, 2016. Accessed January 19, 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2016-02.pdf
- 7.Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention . Advisory Committee on Immunization Practices (ACIP) summary report: June 22-23, 2016, Atlanta, Georgia. Accessed January 19, 2022. https://stacks.cdc.gov/view/cdc/45578
- 8.MacNeil JR, Rubin LG, Patton M, Ortega-Sanchez IR, Martin SW. Recommendations for use of meningococcal conjugate vaccines in HIV-infected persons—Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(43):1189-1194. doi: 10.15585/mmwr.mm6543a3 [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention . Child and adolescent immunization schedule: recommendations for ages 18 years or younger, United States, 2022. Accessed January 31, 2022. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html
- 10.Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69(9):1-41. doi: 10.15585/mmwr.rr6909a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-682. doi: 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 12.Bengtson L, Marshall GS, Buikema AR, Koep E, Novy P, Hogea C. Meningococcal vaccination among patients newly diagnosed at high-risk for meningococcal disease in the United States. Open Forum Infect Dis. 2019;6(suppl 2):S959-S960. doi: 10.1093/ofid/ofz360.2403 [DOI] [Google Scholar]
- 13.Ghaswalla P, Bengtson L, Marshall GS, et al. Factors associated with meningococcal vaccination among patients with newly diagnosed high-risk conditions. Accessed March 26, 2021. https://www.eventscribe.net/2020/IDWeek/searchGlobal.asp?mode=posters&SearchQuery=ghaswalla
- 14.Ghaswalla PK, Bengtson LGS, Marshall GS, et al. Meningococcal vaccination in patients with newly diagnosed asplenia in the United States. Vaccine. 2021;39(2):272-281. doi: 10.1016/j.vaccine.2020.11.068 [DOI] [PubMed] [Google Scholar]
- 15.Rock C, de Barra E, Sadlier C, et al. Impact of a new vaccine clinic on hepatitis B vaccine completion and immunological response rates in an HIV-positive cohort. J Infect Public Health. 2013;6(3):173-178. doi: 10.1016/j.jiph.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 16.Pinto Neto LFDS, Vieira JV, Ronchi NR. Vaccination coverage in a cohort of HIV-infected patients receiving care at an AIDS outpatient clinic in Espírito Santo, Brazil. Braz J Infect Dis. 2017;21(5):515-519. doi: 10.1016/j.bjid.2017.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernández-Ibieta M, Ramos-Amador JT, Auñón-Martín I. HIV-infected children vaccination coverage and safety in a Western European cohort: a retrospective study. Int J STD AIDS. 2007;18(5):351-353. doi: 10.1258/095646207780749763 [DOI] [PubMed] [Google Scholar]
- 18.Comité Asesor de Vacunas de la Asociación Española de Pediatría . Childhood immunization schedule of the Spanish Association of Pediatrics 2003. Article in Spanish. An Pediatr (Barc). 2003;58(3):257-262. doi: 10.1016/S1695-4033(03)78047-3 [DOI] [PubMed] [Google Scholar]
- 19.Phillips G II, Johnson AK, Adames CN, Mustanski B. Meningitis vaccination, knowledge, and awareness among YMSM in Chicago. Health Educ Behav. 2018;45(4):607-615. doi: 10.1177/1090198117752786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holloway IW, Wu ESC, Gildner J, et al. Quadrivalent meningococcal vaccine uptake among men who have sex with men during a meningococcal outbreak in Los Angeles county, California, 2016-2017. Public Health Rep. 2018;133(5):559-569. doi: 10.1177/0033354918781085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson AK, Adames CN, Phillips G II. A qualitative exploration of facilitators and barriers to meningitis vaccination uptake among men who have sex with men. Prev Med Rep. 2018;13:41-47. doi: 10.1016/j.pmedr.2018.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanes S, Quinlan SC, Mast TC, Greenland S, Holick CN. Assessing bias in administrative database studies of RotaTeq vaccine completion due to exclusion of subjects with incomplete follow-up. Emerg Themes Epidemiol. 2015;12:5. doi: 10.1186/s12982-015-0027-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sood N, Juday T, Vanderpuye-Orgle J, et al. HIV care providers emphasize the importance of the Ryan White Program for access to and quality of care. Health Aff (Millwood). 2014;33(3):394-400. doi: 10.1377/hlthaff.2013.1297 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. ACIP Recommendations for MenACWY Vaccination for People with HIV Infection
eReferences.
eFigure 1. Study Flow Chart
eFigure 2. Plain Language Summary