Abstract
The pharmacokinetics of multiple-dose linezolid were determined following administration of five 600-mg oral doses given every 12 h to each of six healthy male volunteers. Concentrations of the drug were determined in plasma and inflammatory blister fluid using high-pressure liquid chromatography. A mean peak concentration in plasma of 18.3 μg/ml (standard deviation [SD], 6.0) was attained at a mean time of 0.7 h (SD, 0.3) after the final dose. The penetration into the inflammatory fluid was 104% (SD, 20.7). A mean peak concentration of 16.4 μg/ml (SD, 10.6) was attained in the inflammatory fluid at 3 h (SD, 0.6) after the final dose. The elimination half-life from serum and inflammatory fluid was 4.9 (SD, 1.8) and 5.7 (SD, 1.7) h, respectively. The area under the concentration-time curve in plasma and blister fluid was 140.3 (SD, 73.1) and 155.3 (SD, 80.1) μg · h/ml, respectively. These data suggest that linezolid has good tissue penetration, and we can predict that it will be successful in the treatment of a variety of gram-positive infections.
Linezolid belongs to a new class of antibiotics, the oxazolidinones. It acts by selectively inhibiting the initiation of bacterial protein synthesis and has been shown to possess in vitro and in vivo activity against gram-positive organisms, including Streptococcus pneumoniae strains that are resistant to penicillin, methicillin-resistant Staphylococcus aureus, Enterococcus faecalis, and Enterococcus faecium with phenotypes Van A, B, and C (2, 6, 9, 11, 13). The aim of this study was to assess the pharmacokinetics and tissue penetration of linezolid following multiple oral doses of 600 mg. A blister technique was used to assess tissue penetration since blister fluid mimics inflammatory exudate and hence is a model of likely pharmacokinetics at the site of infection (10).
MATERIALS AND METHODS
Subjects.
Eight healthy volunteers, with a mean age of 29.6 years (standard deviation [SD], 8.7), a mean weight of 78.6 kg (SD, 7.1), and a mean height of 180.4 cm (SD, 14.1), gave written informed consent to participate in this study. One volunteer was unable to attend the actual study day due to febrile illness; therefore, only seven participants were studied. Exclusion criteria for the trial included any history of significant illness or atopy, recent participation in another drug trial, and use of prescription or nonprescription drugs (particularly monoamine oxidase inhibitors) within 14 or 7 days of the study, respectively. All volunteers underwent a full medical history, an examination and a full blood count, clotting, renal, and liver function tests, and urinalysis and a urine drugs-of-abuse screen, all of which were normal. The hospital's ethics committee granted approval for this study.
Drug administration.
The volunteers were given one 600-mg linezolid tablet every 12 h for 2.5 days (a total of five doses). The first dose was given in the hospital with the physician present for the first hour. Doses two and three were self-administered by the volunteer at home. This was confirmed by the use of a diary card. The fourth dose was given in the hospital on the evening of day 2. At the same time two 0.2% cantharidine-impregnated plasters were placed on each subject's forearm to induce blister formation. The volunteers returned to the hospital the following morning having fasted for 12 h and ready to receive the final dose with 200 ml of water.
Sampling.
Blood samples were collected at various time points for linezolid assay as follows: prior to the first and final dose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, and 12 h following the last dose of medication. Blood samples were also taken before initiation and at the end of the study for safety laboratory evaluations. Specimens were stored at ≤−20°C from the time of collection until the analysis was completed. The stability of linezolid in plasma under these conditions has been documented for up to 1 year (N. K. Hopkins, Pharmacia & Upjohn study report a0028098, Pharmacia & Upjohn, Uppsala, Sweden, 1998).
Skin blister fluid samples (about 50 μl) were drawn just prior to and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, and 12 h following the last dose of linezolid on day 3. The blister fluids were frozen (−20°C) until assayed.
Drug assay: linezolid in human plasma.
The blood samples were centrifuged, and the plasma was harvested. These specimens were then quantitated for linezolid using a sensitive and selective high-performance liquid chromatographic method (Hopkins, study report a0028098; D. R. Loiselle, J. A. Borchert, A. R. Cazers, and M. J. Coon, AvTech study no. 00-3030, AvTech Laboratories, Inc., Kalamazoo, Mich., 2000). Plasma specimens (0.50 ml) were spiked with the internal standard (IS), PNU-101145 (an analogue of linezolid), and extracted using prepared Isolute C2 solid-phase extraction cartridges. Each sample was eluted from the solid-phase extraction cartridges with methanol. After evaporation of the organic material, the residue was reconstituted in acetonitrile-water and transferred to injection vials. A 0.06-ml aliquot was injected onto the chromatography system, with separation accomplished on a reversed-phase analytical column (Zorbax RX-8; Dupont). The mobile phase was composed of trifluoroacetic acid-tetrahydrofuran-methanol-water (0.1:1.2:25:73.7 [vol/vol/vol/vol]). Detection was by UV absorbance at 251 nm. Retention times of linezolid and the IS were approximately 7.0 and 10.0 min, respectively. Mean recoveries for linezolid and the IS were approximately 95.4 and 95.8%, respectively. Calibration standard (CS) responses were linear over the range of 0.01 to 20.0 μg/ml using a weighted (1/concentration) linear least-squares regression. The lower limit of quantitation was 0.01 μg/ml.
Both plasma and blister fluid assays were performed in duplicate, and the mean result was employed. The correlation coefficients obtained by linear fittings of the standards were equal to 1.0. Interday accuracy was monitored by analysis of three linezolid quality control (QC) standards with target concentrations of 0.04, 4.0, and 15.0 μg/ml. QC standard accuracy, based on duplicate QC analysis, was from 99 to 100%, with QC precision reported to be ≤2.9%.
Drug assay: linezolid in human blister fluid.
Quantitation of linezolid in human blister fluid specimens was done using a sensitive and selective high-performance liquid chromatographic system that was coupled with a triple quadrupole mass spectrometer (API 365; PE Sciex) (N. K. Hopkins, Pharmacia & Upjohn study report a0031001, Pharmacia & Upjohn, 1999). Human plasma specimens (0.10 ml) were spiked with a deuterated IS, [D3] PNU-100766 (linezolid), buffered with 13 M ammonium acetate and extracted using ethyl acetate-pentane (75:25 [vol/vol]). After the organic phase was separated and evaporated, the residue was reconstituted in 0.01 M ammonium acetate-methanol (75:25 [vol/vol]) and transferred to injection vials. Sample introduction was performed using the heated nebulizer (APCI) in the positive ion mode. Detection was by selected reaction monitoring of the product ion at m/z 296 (molecular ion at m/z 338) for linezolid and the product ion at m/z 297 (molecular ion at m/z 341) for the IS. The retention times of linezolid and the IS were approximately 2.0 min. Mean recoveries for linezolid and the IS were approximately 106.3 and 100.9%, respectively.
CS responses were linear over the range of 1.0 to 250 ng/ml using a weighted (1/concentration2) linear least-squares regression. Results below the lower limit of quantitation were reported as 0.0 ng/ml. Clinical samples whose linezolid responses exceeded the CS range were diluted with blank human plasma prior to sample extraction and were reassayed.
The blister fluid samples were assayed and reported using the validated plasma method (J. A. Borchert and A. R. Cazers, AvTech study no. 97-800.03, AvTech Laboratories, Inc., Kalamazoo, Mich., 2000). As only a limited amount of blank blister fluid was available, synthetic blister fluid (i.e., 70% plasma) was used to cross-validate with the plasma method. QCs prepared in both plasma and blister fluid were used to verify that blister fluid could be accurately quantified using this method.
Correlation coefficients were all ≥0.999. Interday accuracy and precision were monitored by analysis of three linezolid QC standards with target concentrations of 17.5, 70.0, and 175 ng/ml. Interday precision for the three QC standards was ≤4.6%, with assay accuracy ranging from 105 to 107%.
Specimens were stored at ≤−20°C at collection but were inadvertently allowed to thaw for approximately 24 h during shipment. Linezolid has documented stability for this length of time as well as for up to three freeze-thaw cycles. The long-term stability of frozen samples has been documented for up to 1 year (Hopkins, study report a0028098).
Pharmacokinetic analysis.
Standard noncompartmental, non-steady state analysis was used to determine the pharmacokinetic parameters. The maximum concentration of linezolid in plasma or blister fluid (Cmax) and time to Cmax (Tmax), the area under the plasma or skin blister fluid concentration curve up to the last measurable concentration (AUClast), the area under the plasma or skin blister fluid concentration curve extrapolated to infinity (AUC0–∞), and the elimination half-life (t1/2) in plasma or skin blister fluid were calculated by the noncompartmental model 200 of WinNonlin, version 4.2a (Scientific Consulting Inc., Apex, N.C.). The percentage of penetration of the drug into inflammatory fluid was calculated by comparing the AUC0–∞ taken from the blister fluid data with that from the plasma data.
RESULTS
Three subjects suffered adverse events during the trial. One patient developed oral thrush a few days after the trial which subsequently subsided with oral nystatin, another volunteer was noted to have a transiently elevated alanine transaminase level that was only marginally higher than the upper limit of normal, and one subject was admitted to the hospital for monitoring over night. The last volunteer developed symptoms within 2 h of taking the first dose of linezolid. His heart rate increased from 88 to 120 beats per min and his blood pressure increased marginally from 150/88 mm Hg at baseline to a maximum of 163/90 mm Hg. It was felt that anxiety had played a major role in this episode, but the possibility of a drug-related adverse event could not be discounted. He was subsequently excluded from the study.
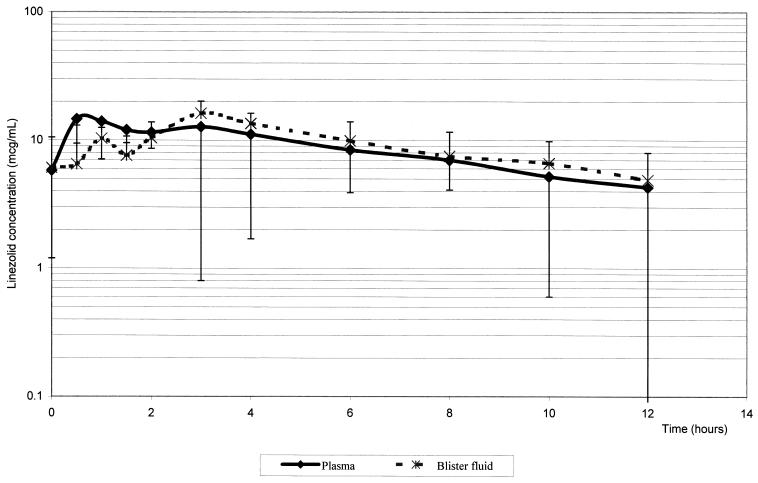
The mean concentration-time profiles found in plasma and inflammatory fluid following the last of the five 600-mg doses of linezolid are shown in Fig. 1, and the derived pharmacokinetic parameters are summarized in Table 1.
FIG. 1.
Concentration of linezolid in plasma and inflammatory fluid versus time after final dose.
TABLE 1.
Pharmacokinetic parameters for linezolid in plasma and inflammatory fluid after five doses of 600-mg tablets at 12-h intervals
| Source of fluid | Mean ± SD (range [minimum–maximum])
|
|||||
|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Tmax (h) | t1/2 (h) | AUClast (μg · h/ml) | AUC0–∞ (μg · h/ml) | % Penetration | |
| Plasma | 18.3 ± 6.0 (12.2–27.5) | 0.7 ± 0.3 (0.5–1.0) | 4.9 ± 1.8 (2.9–7.9) | 107.5 ± 40.6 (64.5–271.4) | 140.3 ± 73.1 (60.3–171.6) | |
| Blister | 16.4 ± 10.6 (6.8–36.8) | 3.0 ± 0.6 (2.0–4.0) | 5.7 ± 1.7 (4.6–8.6) | 101.6 ± 63.0 (57.4–224.5) | 155.3 ± 80.1 (79.5–283.8) | 104 ± 20.7 (80–130) |
The mean Cmax of linezolid in plasma was 18.3 μg/ml, with a Tmax of 0.7 h after administration of the fifth oral dose. The mean t1/2 from plasma was 4.9 h (range, 2.9 to 7.9 h). The AUClast and AUC0–∞ were 107.5 and 140.3 μg · h/ml, respectively.
Blisters were successfully raised in six volunteers, but only five blisters provided enough inflammatory fluid throughout the day to allow adequate data for full pharmacokinetic analysis (i.e., at least three data points in the elimination phase). Linezolid penetrated into the inflammatory fluid moderately rapidly, the mean Tmax being 3 h (range, 2 to 4 h). The mean Cmax in the inflammatory fluid was 16.4 μg/ml (range, 6.8 to 36.8 μg/ml). The mean t1/2 of linezolid from the inflammatory exudate was 5.7 h, slightly greater than that from plasma. The mean percentage of penetration of linezolid into inflammatory fluid was 104% (range, 80 to 130%).
DISCUSSION
The pharmacokinetic data presented here are similar to those previously documented in unpublished studies by Pharmacia & Upjohn (S. D. Pawsey, J. D. Harry, P. Blood, P. T. Daley-Yates, and J. D. Harry, Pharmacia & Upjohn technical report 1424-95-004, Pharmacia & Upjohn, 1996; S. D. Pawsey, J. D. Harry, P. Blood, P. T. Daley-Yates, and J. D. Harry, Pharmacia & Upjohn technical report 1424-96-001, Pharmacia & Upjohn, 1996).
In our study the mean penetration of linezolid into inflammatory exudate was found to be 104%. The degree of protein binding of an antimicrobial can play a major role in determining the level of tissue penetration (4). The plasma protein binding of linezolid is relatively low, at 31% (1). The degree of drug penetration into well-perfused areas of the body is also related to the volume of distribution. The steady-state volume of distribution for linezolid has been shown to be approximately 50 liters (5; Pawsey et al., technical reports 1424-95-004 and 1424-96-001, 1996).
The mean 12-h concentration of linezolid in the inflammatory fluid was 4.9 mg/liter and the MICs at which 90% of the isolates were inhibited for S. aureus (methicillin sensitive and resistant), streptococci, and enterococci have been demonstrated to be less than 4 μg/ml (1). This suggests that a wide range of organisms should be amenable to treatment with this antibiotic in clinical practice.
The data produced in this study can be compared with the various pharmacodynamic and pharmacokinetic relationships that can be used to predict clinical outcome (12). The AUC24/MIC ratio (the ratio of the AUC over 24 h to the MIC) is said to reflect clinical efficacy, at least in fluoroquinolones and aminoglycosides (4, 8). AUC24/MIC ratios of >100 are desirable and values of >125 have been associated with optimal antibacterial activity (3). Linezolid's AUC24/MIC ratios for S. aureus and S. pneumoniae, for example, are 215 and 107.5, respectively. The Cmax/MIC ratio is thought to be relevant in the prevention of the emergence of resistance (4, 7, 8, 12). Cmax/MIC ratios of 8 to 10 have been associated with a low likelihood of emergence of antimicrobial-resistant organisms. However, these data refer only to fluoroquinolones, aminoglycosides, and to a lesser extent to β-lactams and have not yet been verified for oxazolidinones. For linezolid, this ratio is 9.1. A third predictor of clinical efficacy for some antimicrobial agents is the time that concentrations of the drug in plasma remain above the MIC for a particular organism. There is preliminary evidence from a mouse thigh model (D. Andes, M. L. van Ogtrop, and W. A. Craig, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-9, 1998) that the time that concentrations of the drug in plasma remain above the MIC may be the relevant parameter for linezolid, the goal being for 40% of the time of the dosing interval to be above the MIC. This study on linezolid shows that this parameter is at least 12 h, as seen in the graph, which is about 100% of the dosing interval when a twice-daily regimen is employed.
These data suggest that linezolid will be successful in the treatment of gram-positive infections and that it is possible that resistance is less likely to emerge than with less active established agents.
ACKNOWLEDGMENT
We thank Pharmacia and Upjohn Limited for its financial support of this study and for undertaking the assays.
REFERENCES
- 1.Ford C, Hamel J, Stapert D, Moerman J, Hutchinson H, Barbachyn M, Zurenko G. Oxazolidinones: a new class of antimicrobials. Infect Med. 1999;16:435–445. [Google Scholar]
- 2.Ford C W, Hamel J C, Wilson D M, Moerman J K, Stapert D, Yancey R J, et al. In vivo activities of U-100 592 and U-100 766, novel oxazolidinone antimicrobial agents against experimental bacterial infections. Antimicrob Agents Chemother. 1996;40:1508–1513. doi: 10.1128/aac.40.6.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forrest A, Nix D E, Ballow C H, Goss T F, Birmingham M C, Schentag J J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyatt J M, McKinnon P S, Zimmer G S, Schentag J J. The importance of pharmacokinetic/pharmacodynamic surrogate markers of outcome. Focus on antibacterial agents. Clin Pharmacokinet. 1995;28:143–160. doi: 10.2165/00003088-199528020-00005. [DOI] [PubMed] [Google Scholar]
- 5.Lasher-Sisson T, Jungbluth G, Hopkins N K. A pharmacokinetic evaluation of concomitant administration of linezolid and aztreonam. J Clin Pharmacol. 1999;39:1277–1282. doi: 10.1177/00912709922011962. [DOI] [PubMed] [Google Scholar]
- 6.Mason E O, Jr, Lamberth L B, Kaplan S L. In vitro activities of oxazolidinones U-100592 and U-100766 against penicillin-resistant and cephalosporin-resistant strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:1039–1040. doi: 10.1128/aac.40.4.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nix D E, Schentag J J. The quinolones: an overview and comparative appraisal of their pharmacokinetics and pharmacodynamics. J Clin Pharmacol. 1988;28:169–178. doi: 10.1002/j.1552-4604.1988.tb05740.x. [DOI] [PubMed] [Google Scholar]
- 8.Schentag J J, Nix D E, Adelman M H. Mathematical examination of dual individualization principles. I Relationships between AUC above MIC and area under the inhibitory curve for cefmenoxime, ciprofloxacin and tobramycin. DICP Ann Pharmacother. 1991;25:1050–1057. doi: 10.1177/106002809102501003. [DOI] [PubMed] [Google Scholar]
- 9.Slee A M, Wuonola M A, McRipley R J, Zajac I, Zawada M J, Bartholomew T, et al. Oxazolidinones, a new class of synthetic antibacterials: in vitro and in vivo activities of DuP105 and DuP721. Antimicrob Agents Chemother. 1987;31:1791–1797. doi: 10.1128/aac.31.11.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wise R, Gillett A P, Cadge B, Durham R, Baker S. The influence of protein binding upon tissue fluid levels of six β-lactam antibiotics. J Infect Dis. 1980;142:77–82. doi: 10.1093/infdis/142.1.77. [DOI] [PubMed] [Google Scholar]
- 11.Wise R, Andrews J M, Boswell F J, Ashby J P. The in-vitro activity of linezolid (U-100766) and tentative breakpoints. J Antimicrob Chemother. 1998;42:721–728. doi: 10.1093/jac/42.6.721. [DOI] [PubMed] [Google Scholar]
- 12.Wise R. Clinical efficacy and antimicrobial pharmacodynamics. Hosp Med. 2000;61:24–30. doi: 10.12968/hosp.2000.61.1.1268. [DOI] [PubMed] [Google Scholar]
- 13.Zurenko G E, Yagi B H, Schaadt R D, Allison J W, Kilburn J O, Glickman S E, et al. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob Agents Chemother. 1996;40:839–845. doi: 10.1128/aac.40.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]