Abstract
Objective:
To investigate the association among widefield swept-source optical coherence tomography angiography (WF SS-OCTA) metrics and systemic parameters and the occurrence of vitreous hemorrhage (VH) in eyes with proliferative diabetic retinopathy (PDR).
Design:
Prospective, observational study.
Participants:
Fifty-five eyes from 45 adults with PDR, with no history of VH, followed for at least 3 months.
Methods:
All patients were imaged with WF SS-OCTA (Montage 15×15mm and HD-51 Line scan). Images were independently evaluated by two graders for quantitative and qualitative WF SS-OCTA metrics defined a priori. Systemic and ocular parameters and WF SS-OCTA metrics were screened using Least Absolute Shrinkage and Selection Operator (LASSO) and Logistic/Cox regression for variable selection. Firth’s Bias-Reduced logistic regression models (outcome: occurrence of VH) and Cox regression models (outcome: time to occurrence of VH) were used to identify parameters associated with the occurrence of VH.
Main outcome measures:
Occurrence of VH.
Results:
Over a median follow-up of 363 days (range: 28–710 days), 13 of 55 (24%) PDR eyes developed VH during the follow-up period. Presence of extensive neovascularizations (NVs) (odds ratio [OR]=8.05, 95% confidence interval [CI]:1.43–58.56, P=0.02), defined as NVs with total area greater than 4 disc diameters, and forward NVs (OR=5.42, 95%CI:1.26–35.16, P=0.02) that traversed the posterior hyaloid face into the the vitreous were associated with the occurrence of VH. The presence of flat NVs (OR=0.25, 95%CI:0.04–1.01, P=0.05) confined to the posterior hyaloid face was associated with a lower risk of VH with borderline significance. Similarly, presence of extensive NVs (hazard ratio [HR]=18.24, 95% CI:3.51–119.47, P<0.001) and forward NVs (HR=9.50, 95%CI: 2.07–68.08, P=0.002) were significantly associated with time to development of VH.
Conclusions:
WF SS-OCTA is useful to evaluate NVs and their relationship with the vitreous. The presence of forward NVs and extensive NVs were associated with the occurrence of VH in patients with PDR. Larger samples and longer follow-up are needed to verify the risk factors and imaging biomarkers for diabetic VH.
Introduction
Diabetic retinopathy (DR) is the leading cause of visual impairment among the working age population across the world.1 Vitreous hemorrhage (VH) is a common adverse event of proliferative diabetic retinopathy (PDR) and can occur in approximately one-third of PDR eyes despite previous panretinal photocoagulation (PRP) treatment.2,3 Although VH can be cleared by pars plana vitrectomy, repeat VH may stimulate fibrosis and vitreous contraction, which can result in a tractional retinal detachment (TRD) and significantly impact quality of life. Early identification of diabetic patients at higher risk of VH could influence clinical decision-making and improve outcomes with timely treatment.4
However, established risk factors based on the eye examination and two-dimensional (2D) retinal imaging methods including color fundus photography (CFP) and fluorescein angiography (FA) are unable to capture the morphology of retinal neovascularization (NV) and the relationship with surrounding tissues (internal limiting membrane and vitreous) that may be associated with the development of VH. Although Early Treatment Diabetic Retinopathy Study 7-standard field 35-mm stereoscopic color 30º fundus photographs (7F ETDRS photos) and FA remain widely used to diagnose, grade and monitor DR in clinical practice and research, these imaging modalities have limitations and may not be optimal to predict DR progression. 7F ETDRS photos cannot identify non-perfusion areas (NPAs) and poorly differentiate intraretinal microvascular abnormalities (IRMAs) from NV, a key distinction for correct staging of DR. FA is not only invasive and time-consuming but also limited in the evaluation of retinal ischemia and assessment of NV activity due to leakage that can blur the boundary and morphology of abnormal vessels.5
With the advantage of longer wavelengths and shorter acquisition time, swept-source optical coherence tomography (SS-OCT) and optical coherence tomography angiography (SS-OCTA) allow for improved visualization of the vitreous and vitreoretinal interface, thus allowing for qualitative and quantitative assessment of the characteristics of NVs in cross-sectional B-scans and en face OCTA images.6,7 The use of these imaging modalities may expand our understanding of the pathophysiology of DR and associated complications such as VH.7–11 However, limited field of view in conventional OCT and OCTA only allows for depth-resolved assessment of the macular and optic disc area and fails to capture more peripheral pathologic features of DR. Technological improvements in widefield swept-source OCTA (WF SS-OCTA) have significantly increased the field of view from 20 ° to 50° (even 80° in some cases) and enables the evaluation of the vitreous, retinal microstructure and microvasculature not only in the posterior pole but also part of the midperiphery. We first evaluated the image quality, artifacts and segmentation errors associated with WF SS-OCTA in 136 eyes of 98 diabetic patients.12 We then reported the detection rate of NV on Angio 6×6mm imaging was half of that on WF SS-OCTA imaging (Montage 15×15mm), indicating that WF SS-OCTA offers improved detection of NV compared to conventional OCTA.13 In addition, we found that WF SS-OCTA (Montage 15×15mm) had better performance in detecting neovascularization elsewhere (NVE) and neovascularization of the optic disc (NVD) (NVE: 38% vs. 29%, P=0.015; NVD+NVE: 40% vs. 30%, P=0.007) than ultra-widefield CFP (UWF CFP) in 152 eyes of 101 diabetic patients. Despite a smaller field of view (56° vs. 200°), WF SS-OCTA demonstrated comparable detection of selected DR lesions (including NV) compared to ultra-widefield FA (UWF FA) images.14 Taken together, these results suggested that WF SS-OCTA may be useful to predict VH occurrence through the evaluation of NV and related DR lesions in PDR patients.
To our knowledge, few studies have explored predictive factors for the occurrence of VH, especially using WF SS-OCTA to identify PDR patients at higher risk of developing VH by qualitatively and quantitatively analyzing the characteristics of NV and its relationship with the vitreous. Prior work has largely focused on systemic risk factors.3,15–20
Here, we present a prospective, longitudinal observational study to explore WF SS-OCTA metrics related to NV and systemic factors that may predict VH in diabetic patients with PDR.
Methods
This prospective, longitudinal observational study was conducted at Massachusetts Eye and Ear (MEE) from December 2018 to December 2020. This study, one of a series of observational studies on the clinical application of WF SS-OCTA in DR,12–14,21 was approved by the institutional review board of MEE, and informed consent was obtained from all subjects. All procedures adhered to the tenets of the Declaration of Helsinki and Health Insurance Portability and Accountability Act regulations.
Subjects
We included adults with type 1 or type 2 diabetes and PDR in one or both eyes, and no history of VH based on clinical diagnosis and WF SS-OCTA.22 The diagnosis of PDR was based on UWF FA or UWF CFP. All subjects had follow-up at least 3 months after the baseline examination. Exclusion criteria included prior surgical procedures such as pars plana vitrectomy (PPV), other causes of VH such as trauma or retinal tears, patients with history of coronary revascularization or hemodialysis, eyes with concomitant chorioretinal disease, glaucoma and severe media opacities that interfere with image acquisition. Image quality control excluded eyes with signal strength index less than seven using the default settings of the instrument, poor image quality with severe artifacts12 preventing creation of the montage image by the device, or ungradable WF SS-OCTA images.
Study protocol
All included participants had a full ophthalmic examination at baseline and follow-up visits, including Snellen best-corrected visual acuity (BCVA), slit-lamp examination, intraocular pressure (IOP) measurement, and dilated fundus examination. Subjects were imaged at baseline with a 100 kHz WF SS-OCTA instrument (Plex® Elite 9000, Carl Zeiss Meditec Inc., Dublin, CA) that uses a laser at a central wavelength of 1060 nm with a bandwidth of 100 nm. Montage Angio (15×15mm) and HD 51 Line (12mm) scans were performed for each eye. UWF CFP or UWF FA were taken on the same day or within 1 week of the baseline visit to define the severity of DR by a single capture retinal imaging system (California, Optos, Dunfermline, UK).
Definition of End Point
The development of VH was defined as the presence of extravasated blood within the space outlined by the internal limiting membrane of the retina posteriorly and laterally, the nonpigmented epithelium of the ciliary body laterally and the lens zonular fibers and posterior lens capsule anteriorly except for the Canal of Hannover or in a space generated by a posterior vitreous detachment (retrohyaloid or subhyaloid hemorrhage) during the follow-up visit in those with no VH history at baseline.20 The duration between the baseline visit and the occurrence of VH or the most recent visit was recorded.
Assessment of Systemic and Ocular Risk Factors
Systemic and ocular parameters were obtained from electronic medical records. Systemic parameters included type and duration of diabetes, glycated hemoglobin (HbA1c), body mass index (BMI), diabetes treatment, hypertension, blood pressure and anticoagulant treatment. Baseline BCVA, IOP, history of TRD and neovascular glaucoma (NVG), history of VH in the fellow eye, lens status (pseudophakic or phakic), history of DR treatment including history of anti-vascular endothelial growth factor (anti-VEGF) treatment and PRP, diabetic macular edema (DME), as well as anti-VEGF injection during the follow-up period were also recorded as ocular parameters. The duration of diabetes was defined as the period starting from the date of the diagnosis ascertained from the medical record or reported by participants to the date of the baseline examination.
Image Processing
OCTA images were independently evaluated by two masked ophthalmologists (YC and YZ) at different time points and in different orders. A third trained grader (JBM) adjudicated all cases of discrepancy. All images were evaluated on the instrument display screen in a standardized, dimmed environment. For the SS-OCTA images, separate superior and inferior 15×9mm images were montaged to achieve an image of 50° field of view centered on the fovea using the built-in function of the device after a quality check. Treating ophthalmologists were not privy to the masked grading results of the WF SS-OCTA. These scans were not part of the medical record nor contributed to medical decision-making.
Qualitative Analysis of OCTA Images
NV was defined as an extraretinal vessel shown in the vitreoretinal interface (VRI) Angio slab, which extended from 10mm to 300mm above the internal limiting membrane (ILM). Manual segmentation was performed to correct automated segmentation errors (Figure 1). For accurate evaluation of NV, any abnormal vessels were identified in the Montage 15×15mm en face OCTA images, and then each B-scan of the Angio 15×9mm image was examined from top to bottom to differentiate the NVs from IRMAs. The characteristics of NV were evaluated using en face OCTA and cross-sectional OCTA images. First, the number of NVs in the Montage 15×15mm en face OCTA images, as well as the location of NVs (within or outside the arcades) were recorded. NV located in the optic disc or within 1 disc diameter (DD) from the margin was defined as NVD, while the rest were classified as NVE. Extensive NV was defined as NV with total area more than 4 DD including NVD, whereas others were considered local NVs. The morphology of NVs in en face OCTA images was described as exuberant vascular proliferation (EVP) or non-exuberant vascular proliferation (Figure 2).23 Secondly, tomographic features from the HD 51 Line or B-scan of Montage 15×15mm images were assessed, including NV, vitreoretinal and retinal parameters. Cross-sectional morphological classification of NVs previously described by Vaz-Pereira et al. was used, which included flat NV, forward NV and tabletop NV.9,24 Flat NV was confined to the posterior hyaloid face, whereas forward NV traversed the posterior hyaloid face into the vitreous. Tabletop NV was displaced anteriorly by vitreous traction but adhered to the retina by vascular “pegs” (Figure 3). The presence of flat NV, forward NV, tabletop NV, posterior vitreous detachment (PVD), retinal edema around NV and epiretinal membrane (ERM) were assessed for each eye. The number of flat NV, forward NV and tabletop NV was further recorded. Images were subsequently downloaded as high-quality BMP files for further quantitative analysis.
Figure 1.

Manual segmentation and image processing for quantification analysis. NVs in VRI slab (A) of Angio 15*15mm en face WF SS-OCTA image were better displayed after manual segmentation (B). After selecting region of interest, subtracting background and removing noise, the image was binarized for calculating NV area and vessel density (C). Skeletonized vessel density (D) was also calculated.
Figure 2.

Representative images of classification of NVs based on the presence or absence of exuberant vascular proliferation (EVP) on en face WF SS-OCTA images. In eyes without EVP (A,B,C), en face WF SS-OCTA VRI slabs showed the NVs with pruned vascular loops of filamentous new vessels (A: VRI slab; B: corresponding B-scan; C: retina slab). In eyes with EVP (D,E,F), NVs had irregular proliferation of fine vessels (D: VRI slab; E: corresponding B-scan; F: retina slab).
Figure 3.

Classification of NVs based on the morphology on cross-sectional WF SS-OCTA (A,D,G) and Angio 15*15mm en face WF SS-OCTA images (B,E,H: VRI slabs; C,F,I: retina slabs). Flat NV was confined to the posterior hyaloid face (A,B,C). Forward NV showed posterior hyaloid face traversal (D,E,F). Tabletop NV was displaced anteriorly by vitreous traction but tethered to the retinal by one or more vascular membranes (G,H,I).
Quantitative Analysis of Images
Fiji software (National Institutes of Health, Maryland, USA) was utilized for quantitative analysis of images. Two investigators (YC and RL) independently measured NV area, vessel density (VD), skeletonized vessel density (SD) and NPAs using en face OCTA images imported into Fiji. NV area, SD and VD of NV were analyzed in the VRI layer after manual segmentation (Figure 1). NV selected area was manually outlined using the freehand selection tool and expressed as square millimeters based on the scale set for pixel distance. The area measured in pixels was converted to square millimeters using the formula: NV selected area (mm2) = NV selected area (pixel) / total surface area (pixel) × total surface area (mm2). The density of NV was quantified using VD and SD in the VRI layer with subsequent subtraction of the background, adjustment of the brightness/contrast, and binarization using the automated local thresholding method. A skeletonized vessel map was further created for each image, in which every vessel had a width of one pixel, and NV density after skeletonization was obtained (Figure 1). Flow area of NVs was calculated using the formula: Flow area of NVs (mm2) = selected area of all NVs (mm2) × VD or SD of NVs. Flow area of NVs was calculated using both VD and SD. NPAs were measured manually by 2 graders using the OCTA en face whole retina layer. Ischemia index was calculated by dividing NPAs (pixel) by the total image area (pixel). The mean measurement of NV area, VD, SD and ischemia index between the two graders was used for further statistical analysis.
Statistical Methods
Statistical analyses were performed using STATA version 16.0 (STATACorp,Texas, USA) and R software (the R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics were used to characterize the demographics of the included study population. Baseline WF SS-OCTA metrics, ocular and systemic parameters were considered as predictors. The Least Absolute Shrinkage and Selection Operator (LASSO) regression was applied for variable selection to minimize the potential collinearity of variables measured from the same patients and over-fitting of variables.25 These regression models penalize the absolute size of the coefficients of a regression model based on the value of λ. With larger penalties, the estimates of weaker factors shrink toward zero, so that only the strongest predictors remain in the model. The most predictive covariates were selected by the minimum (λ min). Subsequently, variables identified by LASSO-Logistic regression analysis were entered into multivariable Firth’s bias-reduced Logistic regression models which were used to determine factors associated with the development of VH (binomial outcome: VH yes or no). Similarly, multivariable Firth’s bias-reduced Cox regression models were used to explore risk factors related to time to development of VH, after variable selection using LASSO-Cox regression analysis. All statistical tests were 2-sided and a P value less than 0.05 was considered statistically significant.
Results
Characteristics of the Study Cohort
A total of 137 eligible eyes of 78 patients with PDR had a baseline examination. Among these, 82 eyes were excluded for the following reasons: exclusion criteria (37 eyes), loss to follow-up (18 eyes), poor image quality (27 eyes) (Figure 4). Fifty-five eyes from 45 patients were included. Demographic and systemic parameters, ocular characteristics and WF SS-OCTA metrics of study participants in the cohort are presented in Table 1–3. Over a median follow-up of 363 days (range: 28–710), 13 of 55 (24%) PDR eyes without VH history at baseline developed VH.
Figure 4.
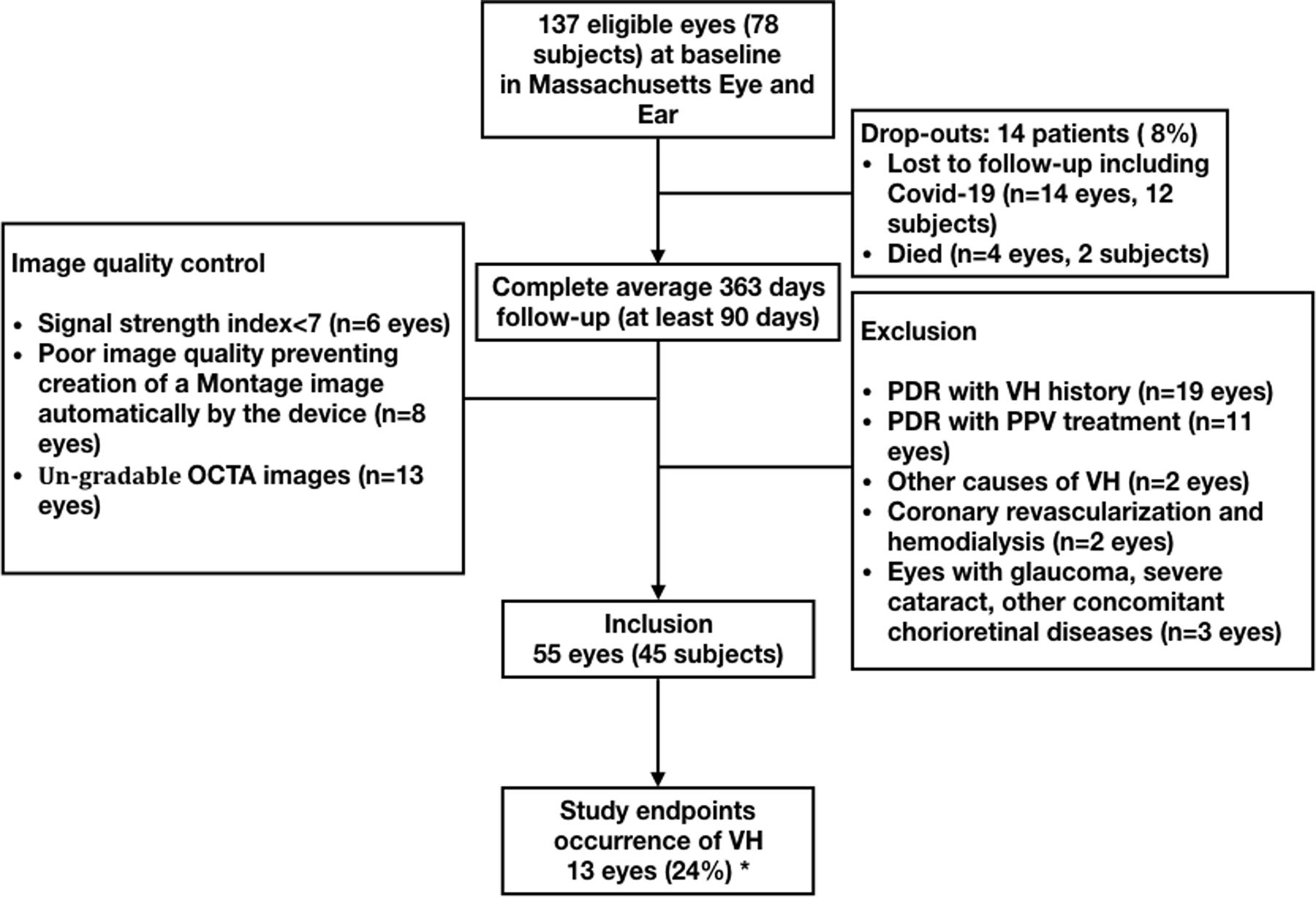
Study flowchart. VH=vitreous hemorrhage; PDR=proliferative diabetic retinopathy; PPV: pars plana vitrectomy. *In assessment of the occurrence of VH, eyes with VH at the baseline visit were excluded, leaving 55 eyes of 45 subjects eligible for analysis.
Table 1.
Baseline demographic and systemic characteristics
| Demographic and systemic characteristics | Number (percent, %)/Median(interquartile range,IQR) | |
|---|---|---|
| Number of patients | 45 | |
| Age, years | 55.0 (40.0–58.5) | |
| Gender,male (%) | 26 (57.8%) | |
| BMI | Normal ⩾18.5, and <25 | 13 (29.6%) |
| Abnormal ⩾25 | 31 (70.5%) | |
| Type of Diabetes, (%) | T1DM | 15 (33.3%) |
| T2DM | 30 (66.7%) | |
| Diabetes duration, years | 17.0(11.5–26.0) | |
| HbA1c (%), baseline | 8.2 (7.0–10.3) | |
| Diabetes treatment,yes (%) | 44 (97.8%) | |
| Insulin treatment,yes (%) | 40 (88.9%) | |
| Hypertension,yes (%) | 38 (84.4%) | |
| SBP, mmHg | <140 mmHg | 20 (44.4%) |
| ⩾140 mmHg | 25 (55.6%) | |
| DBP, mmHg | <90 mmHg | 34 (75.6%) |
| ⩾90 mmHg | 11 (24.4%) | |
| Anti-coagulant,yes (%) | 26 (57.8%) | |
| BUN (mg/dl)# | 19 (15.0–25.8) | |
| Creatinine (mg/dl)# | 1.0 (0.8–1.2) | |
BMI=body mass index; T1DM=type 1 diabetes mellitus; T2DM=type 2 diabetes mellitus; HbA1c=glycated hemoglobin; SBP=systolic blood pressure; DBP=diastolic blood pressure; BUN=blood urea nitrogen
BUN data missing for 1 eye with VH occurrence and 9 eyes without VH occurrence;
Creatinine data missing for 1 eye with VH occurrence and 9 eyes VH occurrence.
Table 3.
Baseline WF SS-OCTA characteristics
| WF SS-OCTA characteristics | Number (percent, %)/Median(interquartile range,IQR) |
|---|---|
| Total number of NVs | 1 (1–3) |
| Number of NVs located within the arcades | 1 (0–2) |
| The number of NVs Located in mid periphery, and within the panoramic of Montage 15mm×15mm | 0 (0–2) |
| NVE, yes (%) | 43 (78.2%) |
| NVD, yes (%) | 23 (41.8%) |
| Extensive NV, yes (%) | 8 (14.6%) |
| EVP, yes (%) | 34 (61.8%) |
| Number of NV with EVP | 1 (0–3) |
| Flat NV, yes (%) | 25 (45.5%) |
| Number of flat NVs | 0(0–1) |
| Forward NV, yes (%) | 29 (52.7%) |
| Number of forward NVs | 1 (0–1) |
| Tabletop NV, yes (%) | 16 (29.1%) |
| Number of tabletop NVs | 0 (0–1) |
| Selected area of all NVs (mm2), per eye | 0.7 (0.2–4.2) |
| Vessel density of all NVs, per eye | 0.5 (0.4–0.7) |
| Skeletonized vessel density of all NVs, per eye | 0.2 (0.1–0.2) |
| Flow area of NVs (selected area of all NVs × vessel density of NVs), per eye | 0.3 (0.1–1.9) |
| Flow area of NVs (selected area of all NVs× skeletonized vessel density of NVs) (mm2)#, per eye | 0.1 (0.0–0.5) |
| Ischemia index* | 0.1 (0.1–0.3) |
| PVD, yes (%) | 34 (61.8%) |
| Retina edema around NVE, yes (%) | 14 (25.5%) |
| ERM, yes (%) | 33 (60.0%) |
NV=neovascularization; NVE=neovascularization elsewhere; NVD=neovascularization elsewhere;EVP=exuberant vascular proliferation; PVD=posterior vitreous detachment; ERM=epiretinal membrane;
Ischemia index=non-perfusion areas/total area.
Factors associated with development of VH
A total of 38 WF SS-OCTA metrics, ocular and systemic parameters (Table 1–3) were included in the LASSO regression. After LASSO-Logistic regression selection, 8 variables remained as significant predictors of VH, including diabetes duration, HbA1c, the presence of extensive NVs, flat NV and forward NV, the number of forward NVs and flow area of NVs (selected area of all NVs × VD of NVs, selected area of all NVs × SD of NVs). Inclusion of these 8 variables in a multivariable Firth’s bias-reduced Logistic regression model resulted in the presence of extensive NV (odds ratio [OR]=8.05, 95% confidence interval [CI]:1.43–58.56, P=0.02) and forward NV (OR=5.42, 95%CI:1.26–35.16, P=0.02) as parameters significantly associated with the occurrence of VH. The presence of flat NV (OR=0.25, 95%CI:0.04–1.01, P=0.05) was associated with a lower risk of VH with borderline significance. Representative images of eyes with and without VH at follow-up are shown (Figure 5 and 6).
Figure 5.
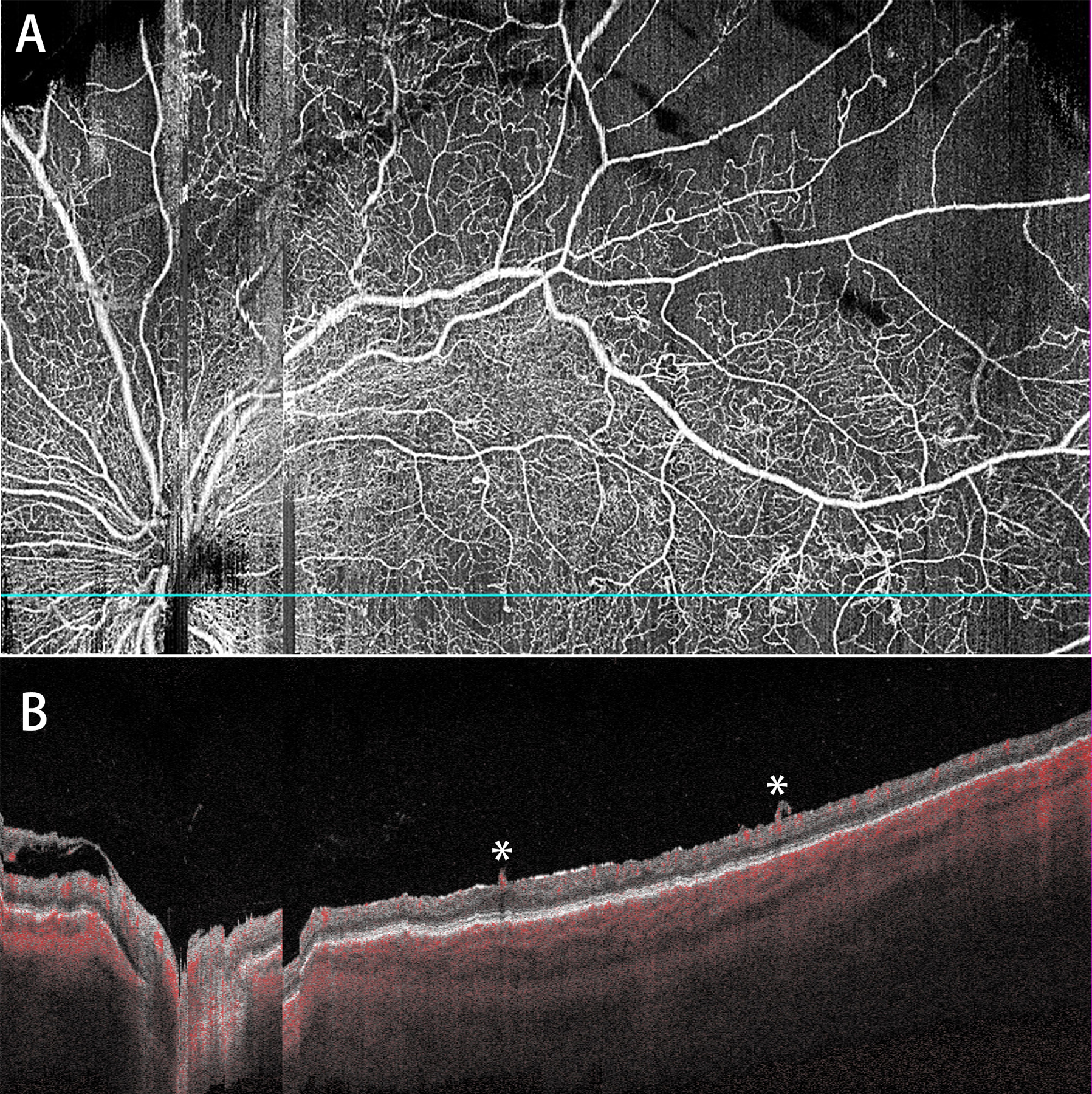
Representative WF SS-OCTA images of a PDR eye with the occurrence of VH during the follow-up (28 days) (A: whole retina slab; B: corresponding B-scan). Two forward NVs (*) are shown in B.
Figure 6.

Representative WF SS-OCTA images of a stable PDR eye without vitreous hemorrhage over one year of follow-up (A,B,C: VRI slabs; D,E,F: corresponding B-scans; G,H,I: retina slabs; J,K,L: retina depth encoded images). Images of baseline (A,D,G,J), 6 month follow-up (B,E,H,K) and 12 month follow-up (C,F,I,L) are shown. Only 1 NV was observed in the superior retina.
Factors associated with time to development of VH
After LASSO-Cox regression selection using the same 38 parameters, 10 variables remained significantly associated with time to development of VH, including diabetes duration, treatment of diabetes, the presence of extensive NV and forward NV, the absence of local NV and flat NV, the number of forward NV, selected area of all NVs, SD of NVs and flow area of NVs (selected area of all NVs × SD of NVs). Inclusion of these 10 variables in a multivariable Firth’s bias-reduced Cox regression model resulted in the presence of extensive NV (hazard ratio [HR]=18.24, 95% CI:3.51–119.47, P<0.001) and forward NV (HR=9.50, 95%CI:2.07–68.08, P=0.002) as parameters significantly associated with time to development of VH. Anticoagulant use, history of anti-VEGF therapy and hypertension were not related to VH occurrence.
Discussion
To our knowledge, this is the first prospective study using WF SS-OCTA metrics, ocular and systemic parameters to explore risk factors that may predict the development of VH in patients with PDR. This study provides new longitudinal clues to elucidate risk factors and imaging biomarkers for the occurrence of VH. Our results demonstrated that the presence of extensive NV and NV with forward cross-sectional morphology were associated with both the occurrence of VH and the time to occurrence of VH in patients with PDR. These findings highlight the importance of the characteristics of NV and its relationship with the posterior vitreous and may help identify PDR patients at higher risk of developing VH according to NV characteristics on WF SS-OCTA.
Prior studies investigated systemic parameters of diabetic patients associated with VH.15,17,18,26 Kleinmann et al. identified early onset and long duration of disease as risk factors for developing VH in patients with PDR and severe NPDR of type 1 diabetes mellitus (T1DM), and shorter follow-up and fewer angiographic examinations as risk factors in patients with type 2 diabetes mellitus (T2DM) during an average follow-up of 26 months after completion of initial PRP.26 Motoda et al. found that longer duration of vitrectomy, higher fasting blood glucose before surgery, no treatment with antiplatelet drugs and treatment with antihypertensive agents increased the risk of postoperative VH in 72 eyes with DR.15 Few studies have investigated the role of hypertension16 and oral anticoagulants17–20 in the occurrence of VH.
The pathophysiology of retinal NVs plays a crucial role in the development of severe complications of PDR such as VH and TRD, yet this complex process remains incompletely understood. By evaluating the characteristics of NV in cross-sectional OCT B-scans and en face OCTA Angio images, we found that the presence of forward NVs and extensive NV were associated with a higher risk of VH occurrence in patients with PDR. Although the number, location, area and VD of NVs can be assessed using CFP or FA, the relationship between the morphology of NV with the vitreous (posterior hyaloid face) is unable to be captured by conventional 2D retinal imaging modalities. In addition, the structural information provided by OCT alone could be helpful for NV configuration, but these new vessels are most often located outside of the traditional OCT scan areas and a large montage of multiple small scans would be time consuming and may still miss lesions. It is also impractical to locate NVs through hundreds of B-scans on traditional OCT. The advent of WF SS-OCTA not only allows for non-invasive determination of the number, location and morphology of NVs and the relationship with vitreoretinal interface changes, but also overcomes the problem of limited field of view in conventional OCT/OCTA, which usually covers macular and optic disc area but fails to capture more peripheral pathologic features of DR. Few studies used OCTA metrics to predict progression of DR and DME27 let alone identify risk factors for VH by analyzing the characteristics of NV using WF SS-OCTA.
There are a number of reasons why OCTA has not been used routinely in both retina and general ophthalmology clinical practice. Additional research needs to demonstrate improvements in medical decision making and added diagnostic information beyond that which is already provided by the fundus examination or more routine imaging tests. In DR, many clinicians correctly believe they can classify disease stage and risk of progression simply from the fundus exam or photos, and certainly from FA, and thus have no need for OCTA. However, data provided by different imaging modalites could supplement each other and increase the accuracy of DR grading. We specifically set out to show that the additional imaging information gained from WF SS-OCTA can be used to predict DR-related complications (VH in the present study).
This study found NVs that traverse the posterior hyaloid face into the vitreous and extensive NVs with total area greater than 4 DD may characterize PDR eyes at greater risk of VH. In contrast, flat NVs confined to the posterior hyaloid face may be more stable and less likely to result in VH. While the mechanism is unclear, forward NVs with an upright configuration and greater vertical component on B-scan may be more fragile and prone to resulting in VH. Further investigation is warranted to determine if forward NV is a biomarker for the development of VH in PDR patients. Notably, our findings do not align with the previously proposed mechanism of VH involving localized traction from the posterior hyaloid face and contraction of the fibrous element of the fibrovascular complex as potential contributors to VH.20,28 Although tabletop NVs showed anterior displacement due to vitreous traction, and a similar mechanism may underlie the formation of VH, increased number of tabletop NVs was not associated with a higher risk of VH in this study. Similarly, PVD and ERM were not associated with VH development. In addition, morphological classification of NVs based on en face OCTA, which may correlate with NV activity level,23 was not associated with the occurrence of VH. Longer follow-up time and a larger cohort are needed to further explore the mechanism of VH in PDR patients.
PDR is a chronic sight-threatening disease that requires long-term monitorings. Although FA can identify the activity of NV based on leakage, it isitt is invasive and not ideal for repeated use at each follow-up visit to evaluate the morphology and evolution of NV, which is crucial for understanding the progression of PDR and its related complications (VH and TRD) in clinical practice.10,23 Prior observational studies have described the characteristics of retinal NV in eyes with PDR using conventional OCT/OCTA.9,10,23,24 In a previous study using WF SS-OCTA, we longitudinally evaluated NV morphology with an average follow-up time of 104±60 days.21 Among 13 PDR eyes with follow-up imaging, 5 new NVs not seen on baseline images developed in the follow-up period. These results indicate the potential utility of frequent monitoring of PDR eyes using WF SS-OCTA to detect new NVs and improve our understanding of the evolution of NV.
Ishibazawa et al.23 observed that new vessels with EVP were converted to pruned new vessels with residual, larger vascular loops after PRP in 40 eyes of 33 patients with PDR using en face SD-OCTA images (3×3mm and 4.5×4.5mm), suggesting that NV morphology may correlate with its activity level. Interestingly, our previous study found no changes in cross-sectional morphology of 32 NVs (flat, forward, tabletop configuration) in PDR eyes with and without interval laser and anti-VEGF therapy over a short-term follow-up period (104±60 days) using WF SS-OCTA B-scan.21 These findings suggested that cross-sectional morphological characteristics of NVs may be stable post-treatment and could serve as reliable parameters in predicting VH.
Few studies have investigated whether anticoagulation affects the occurrence of VH and a clear consensus has not been reached.17,20,29,30 Some studies demonstrated a larger proportion of intraocular hemorrhage (retinal, choroidal, or vitreous) linked to warfarin or new oral anticoagulant use, while other studies found no association between oral anticoagulation and VH.17,29 The ETDRS study demonstrated that aspirin does not increase the risk of VH or have harmful effects on the progression of DR.31 In 97 diabetic eyes undergoing pars plana vitrectomy over a 30-month period, Brown et al. observed that patients on anticoagulation undergoing diabetic vitrectomy did not exhibit a higher risk of intraoperative or postoperative vitreous hemorrhage.30 The present study found that anticoagulation was not correlated with the development of VH.
This is the first study to explore imaging biomarkers for VH occurrence using qualitative and quantitative analysis of NV-related WF SS-OCTA metrics that cannot be adequately evaluated with CFP, FA or conventional OCTA. This study does have some limitations. First, although LASSO regression and Firth’s bias-reduced Logistic/Cox regression models were applied, a relatively small sample size with a limited number of events (occurrence of VH) and a wide range of predictors explored may have introduced selection bias and limited the generalizability of results. Second, not all NVs were analyzed due to the limited field of view of Montage 15×15mm images, which covered 50 to 60 degrees of retina. However, NVEs are reported to be more likely located in the posterior pole, which can be covered by WF SS-OCTA, than the mid-periphery or far-periphery on UWF FA.32 In our DR cohort, there was one eye with NV detected on UWF FA but not captured by the panoramic OCTA image (Montage 15×15mm).33 Furthermore, right censoring was present in the survival analysis as a result of the limited duration of follow-up and incomplete observation of VH in all eyes.
In conclusion, WF SS-OCTA is a useful non-invasive tool to evaluate NVs and their relationship with the vitreous, and to identify imaging biomarkers associated with the development of VH in eyes with PDR. In particular, the presence of forward NVs and extensive NVs may predict the occurrence of VH in these patients. Larger sample size and longer follow-up time are needed to verify the imaging biomarkers proposed in this study. Future work is warranted to develop a predictive model or risk score to stratify PDR patients based on the likelihood of VH development, thereby allowing patients to better understand their individualized risk of severe PDR complications and helping clinicians determine appropriate follow-up and treatment plans.
Table 2.
Baseline ocular characteristics
| Ocular characteristics | Number (percent, %)/Median(interquartile range,IQR) | |
|---|---|---|
| Number of eyes | 55 | |
| TRD history, yes (%) | 4 (7.3%) | |
| NVG history, yes (%) | 5 (9.1%) | |
| VH history in the fellow eye, yes (%) | 10 (18.2%) | |
| Occurrence of VH in fellow eye, yes (%) | 4 (7.3%) | |
| Pseudophakic, yes (%) | 14 (25.5%) | |
| History of DR treatment, yes (%) | 42 (76.4%) | |
| Anti-VEGF treatment history, yes (%) | 23 (41.8%) | |
| PRP treatment history, yes (%) | 35 (63.6%) | |
| DME, yes (%) | 31 (56.4%) | |
| BCVA (LogMAR),baseline | 0.2 (0.0–0.3) | |
| IOP (mmHg), baseline (%) | Normal, ⩽21mmHg | 51 (92.7%) |
| Increased,>21 mmHg | 4 (7.3%) | |
PDR=proliferative diabetic retinopathy; TRD=tractional retinal detachment; NVG=neovascular glaucoma; VH=vitreous hemorrhage;Anti-VEGF=anti-vascular endothelial growth factor; PRP=panretinal photocoagulation; DME=diabetic macular edema; BCVA=best corrected visual acuity;IOP=intraocular pressure.
Table 4.
Multivariate Firth’s Bias-Reduced Logistic Regression Analyses to Predict the Occurrence of Vitreous Hemorrhage
| Rate of VH Occurrence (eyes) | odds ratio (OR) | 95% CI for OR |
P value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| NV extensive | |||||
| Yes | 4/8 | 8.05 | 1.43 | 58.56 | 0.02 |
| No | 9/47 | 1.00 | |||
|
| |||||
| Forward NV | |||||
| Yes | 10/29 | 5.42 | 1.26 | 35.16 | 0.02 |
| No | 3/26 | 1.00 | |||
|
| |||||
| Flat NV | |||||
| Yes | 3/25 | 0.25 | 0.04 | 1.01 | 0.05 |
| No | 10/30 | 1.00 | |||
NV=neovascularization
Table 5.
Multivariate Firth’s Bias-Reduced Cox Regression Analyses to Predict the Occurrence of Vitreous Hemorrhage
| Rate of VH Occurrence (eyes) | hazard ratio (HR) | 95% CI for OR | P value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| NV extensive | |||||
| Yes | 4/8 | 18.24 | 3.51 | 119.47 | <0.001 |
| No | 9/47 | 1.00 | |||
|
| |||||
| Forward NV | |||||
| Yes | 10/29 | 9.60 | 2.07 | 68.08 | 0.002 |
| No | 3/26 | 1.00 | |||
NV=neovascularization;
Financial Support:
Lions International Fund (Grant 530125).
Footnotes
Conflicts of Interest:
D.E. is a consultant for Alcon, Aldeyra, Dutch Ophthalmic, Genentech, Glaukos.
J.W.M. is a consultant for Genetech/Roche, Sunovion, KalVista Pharmaceuticals, and ONL Therapeutics; reports honorarium from Bausch + Lomb, and Heidelberg Engineering; and has received grants from Lowy Medical Research Institute, Ltd. In addition, Dr. Miller has patents US 7,811,832 with royalties paid by ONL Therapeutics to Mass. Eye and Ear, and patents US 5,798,349; US 6,225,303; US 6,610,679; CA 2,185,644; CA 2,536,069 with royalties paid by Valeant Pharmaceuticals to Mass. Eye and Ear.
J.B.M. is a consultant for Alcon, Allergan, Heidelberg Engineering, Carl Zeiss, Sunovion, and Genentech; and has received grants from Lions International Fund.
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Ting DSW, Cheung GCM, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol 2016;44(4):260–277. doi: 10.1111/ceo.12696 [DOI] [PubMed] [Google Scholar]
- 2.Spraul CW, Grossniklaus HE. Vitreous hemorrhage. Surv Ophthalmol 1997;42(1):3–39. doi: 10.1016/S0039-6257(97)84041-6 [DOI] [PubMed] [Google Scholar]
- 3.Kleinmann G, Hauser D, Schechtman E, Landa G, Bukelman A, Pollack A. Vitreous hemorrhage in diabetic eyes previously treated with panretinal photocoagulation. Int Ophthalmol 2008. doi: 10.1007/s10792-007-9106-1 [DOI] [PubMed] [Google Scholar]
- 4.A phase 3, Double-masked, Randomized Study of the Efficacy and Safety of Intravitreal (IVT) Aflibercept Injection in patients with Moderately Severe to Severe Nonproliferative Diabetic Retinopathy https://clinicaltrials.gov/ct2/show/NCT02718326. Published 2016.
- 5.Kornblau IS, El-Annan JF. Adverse reactions to fluorescein angiography: A comprehensive review of the literature. Surv Ophthalmol 2019;64(5):679–693. doi: 10.1016/j.survophthal.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 6.Akiyama H, Li D, Shimoda Y, Matsumoto H, Kishi S. Observation of neovascularization of the disc associated with proliferative diabetic retinopathy using OCT angiography. Jpn J Ophthalmol 2018;62(3):286–291. doi: 10.1007/s10384-018-0571-z [DOI] [PubMed] [Google Scholar]
- 7.Muqit MMK, Stanga PE. Swept-source optical coherence tomography imaging of the cortical vitreous and the vitreoretinal interface in proliferative diabetic retinopathy: Assessment of vitreoschisis, neovascularisation and the internal limiting membrane. Br J Ophthalmol 2014;98(7):994–997. doi: 10.1136/bjophthalmol-2013-304452 [DOI] [PubMed] [Google Scholar]
- 8.Russell JF, Shi Y, Hinkle JW, et al. Longitudinal Wide-Field Swept-Source OCT Angiography of Neovascularization in Proliferative Diabetic Retinopathy after Panretinal Photocoagulation. Ophthalmol Retin 2019;3(4):350–361. doi: 10.1016/j.oret.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaz-Pereira S, Dansingani KK, Chen KC, Cooney MJ, Klancnik JM, Engelbert M. TOMOGRAPHIC RELATIONSHIPS between RETINAL NEOVASCULARIZATION and the POSTERIOR VITREOUS in PROLIFERATIVE DIABETIC RETINOPATHY. Retina 2017;37(7):1287–1296. doi: 10.1097/IAE.0000000000001336 [DOI] [PubMed] [Google Scholar]
- 10.Pan J, Chen D, Yang X, et al. Characteristics of Neovascularization in Early Stages of Proliferative Diabetic Retinopathy by Optical Coherence Tomography Angiography. Am J Ophthalmol 2018;192:146–156. doi: 10.1016/j.ajo.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 11.Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res 2018;64:1–55. doi: 10.1016/j.preteyeres.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui Y, Zhu Y, Wang JC, et al. Imaging artifacts and segmentation errors with wide-field swept-source optical coherence tomography angiography in diabetic retinopathy. Transl Vis Sci Technol 2019;8(6). doi: 10.1167/tvst.8.6.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Y, Cui Y, Wang JC, et al. Different Scan Protocols Affect the Detection Rates of Diabetic Retinopathy Lesions by Wide-field Swept-Source Optical Coherence Tomography Angiography. Am J Ophthalmol 2020. doi: 10.1016/j.ajo.2020.03.004 [DOI] [PubMed] [Google Scholar]
- 14.Cui Y, Zhu Y, Wang JC, et al. Comparison of widefield swept-source optical coherence tomography angiography with ultra-widefield colour fundus photography and fluorescein angiography for detection of lesions in diabetic retinopathy. Br J Ophthalmol June 2020. doi: 10.1136/bjophthalmol-2020-316245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motoda S, Shiraki N, Ishihara T, et al. Predictors of postoperative bleeding after vitrectomy for vitreous hemorrhage in patients with diabetic retinopathy. J Diabetes Investig 2018;9(4):940–945. doi: 10.1111/jdi.12791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lean JS, Gregor Z. The acute vitreous haemorrhage. Br J Ophthalmol 1980;64(7):469–471. doi: 10.1136/bjo.64.7.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talany G, Guo M, Etminan M. Risk of intraocular hemorrhage with new oral anticoagulants. Eye 2017;31(4):628–631. doi: 10.1038/eye.2016.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feman SS, Bartlett RE, Roth AM, Foos RY. Intraocular Hemorrhage and Blindness Associated with Systemic Anticoagulation. JAMA J Am Med Assoc 1972;220(10):1354–1355. doi: 10.1001/jama.1972.03200100066014 [DOI] [PubMed] [Google Scholar]
- 19.Wang CY, Cheang WM, Hwang DK, Lin CH. Vitreous haemorrhage: A population-based study of the incidence and risk factors in Taiwan. Int J Ophthalmol 2017;10(3):461–466. doi: 10.18240/ijo.2017.03.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor G. Vitreous hemorrhage. Ophthalmic Pract 2000;18(3):130–132. doi: 10.5005/jp/books/12566_27 [DOI] [Google Scholar]
- 21.Lu ES, Cui Y, Le R, et al. Detection of neovascularisation in the vitreoretinal interface slab using widefield swept-source optical coherence tomography angiography in diabetic retinopathy. Br J Ophthalmol Published online December 21, 2020. doi: 10.1136/bjophthalmol-2020-317983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkinson CP, Ferris FL, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003. doi: 10.1016/S0161-6420(03)00475-5 [DOI] [PubMed] [Google Scholar]
- 23.Ishibazawa A, Nagaoka T, Yokota H, et al. Characteristics of retinal neovascularization in proliferative diabetic retinopathy imaged by optical coherence tomography angiography. Investig Ophthalmol Vis Sci 2016;57(14):6247–6255. doi: 10.1167/iovs.16-20210 [DOI] [PubMed] [Google Scholar]
- 24.Vaz-Pereira S, Zarranz-Ventura J, Sim DA, et al. Optical coherence tomography features of active and inactive retinal neovascularization in proliferative diabetic retinopathy. Retina 2016;36(6):1132–1142. doi: 10.1097/IAE.0000000000000869 [DOI] [PubMed] [Google Scholar]
- 25.Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med 2020. doi: 10.1001/jamainternmed.2020.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleinmann G, Hauser D, Schechtman E, Landa G, Bukelman A, Pollack A. Vitreous hemorrhage in diabetic eyes previously treated with panretinal photocoagulation. Int Ophthalmol 2008;28(1):29–34. doi: 10.1007/s10792-007-9106-1 [DOI] [PubMed] [Google Scholar]
- 27.Sun Z, Tang F, Wong R, et al. OCT Angiography Metrics Predict Progression of Diabetic Retinopathy and Development of Diabetic Macular Edema: A Prospective Study. Ophthalmology 2019;126(12):1675–1684. doi: 10.1016/j.ophtha.2019.06.016 [DOI] [PubMed] [Google Scholar]
- 28.Jaafar El Annan MD, Petros E. Carvounis, MD FRCS. Current Management of Vitreous Hemorrhage due to Proliferative Diabetic Retinopathy. Int Ophthalmol Clin 2014;54(2):141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim KE, Yang PS, Jang E, Kim S, Joung B. Antithrombotic medication and the risk of vitreous hemorrhage in atrial fibrillation: Korean national health insurance service national cohort. Yonsei Med J 2019;60(1):65–72. doi: 10.3349/ymj.2019.60.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown JS, Mahmoud TH. Anticoagulation and clinically significant postoperative vitreous hemorrhage in diabetic vitrectomy. Retina 2011;31(10):1983–1987. doi: 10.1097/IAE.0b013e31821800cd [DOI] [PubMed] [Google Scholar]
- 31.Effects of Aspirin Treatment on Diabetic Retionopathy: ETDRS Report Number 8. Ophthalmology 1991. doi: 10.1016/S0161-6420(13)38010-5 [DOI] [PubMed] [Google Scholar]
- 32.Fan W, Nittala MG, Velaga SB, et al. Distribution of Nonperfusion and Neovascularization on Ultrawide-Field Fluorescein Angiography in Proliferative Diabetic Retinopathy (RECOVERY Study): Report 1. Am J Ophthalmol 2019;206:154–160. doi: 10.1016/j.ajo.2019.04.023 [DOI] [PubMed] [Google Scholar]
- 33.Cui Y, Zhu Y, Wang JC, et al. Comparison of widefield swept-source optical coherence tomography angiography with ultra-widefield colour fundus photography and fluorescein angiography for detection of lesions in diabetic retinopathy. Br J Ophthalmol 2020:bjophthalmol-2020–316245. doi: 10.1136/bjophthalmol-2020-316245 [DOI] [PMC free article] [PubMed] [Google Scholar]


