Abstract
Purpose
To characterize the ocular hypotensive and pharmacological properties of QLS-101, a novel ATP-sensitive potassium (KATP) channel opening prodrug.
Methods
Ocular hypotensive properties of QLS-101 were evaluated by measuring IOP with a handheld rebound tonometer after daily topical ocular instillation of 0.2% (n = 5) or 0.4% QLS-101 (n = 10) in C57BL/6J mice. KATP channel specificity was characterized in HEK-293 cells stably expressing human Kir6.2/SUR2B subunits and assessed for off-target interactions using a receptor binding screen. Conversion of QLS-101 prodrug to its active moiety, levcromakalim, was evaluated in vitro using human ocular tissues and plasma samples and after incubation with human phosphatase enzymes (2.0 nM-1.0 µM).
Results
C57BL/6J mice treated once daily with 0.2% QLS-101 exhibited significant (P < 0.01) IOP reductions of 2.1 ± 0.4 mmHg after five days; however, a daily attenuation of the effect was noted by 23h post-dose. By comparison, treatment with 0.4% QLS-101 lowered IOP by 4.8 ± 0.7 mm Hg (P < 0.0001) which was sustained for 24 hours. Unlike levcromakalim, QLS-101 failed to induce KATP channel activity in HEK-Kir6.2/SUR2B cells consistent with its development as a prodrug. No off-target receptor effects were detected with either compound. In vitro ocular tissue conversion of QLS-101 prodrug was identified in human iris, ciliary body, trabecular meshwork, and sclera. Alkaline phosphatase was found to convert QLS-101 (mean Km = 630 µM, kcat = 15 min−1) to levcromakalim.
Conclusions
QLS-101 is a novel KATP channel opening prodrug that when converted to levcromakalim shows 24-hour IOP lowering after once-daily topical ocular administration.
Keywords: glaucoma, potassium channels, intraocular pressure, prodrug
Glaucoma is a leading cause of irreversible blindness, affecting more than 80 million people worldwide.1,2 Despite its prevalence, the only treatment modality available to prevent progressive glaucomatous vision loss is lowering intraocular pressure (IOP).3–6 Currently, available ocular hypotensive agents either target aqueous humor (AH) production (e.g., beta-blockers, carbonic anhydrase inhibitors) or increase AH outflow through the uveoscleral pathway (e.g., prostaglandin analogues) or through the trabecular meshwork (netarsudil, latanoprostene bunod).7 Although effective, many of these treatments are associated with adverse side effects and tachyphylaxis. These include conjunctival hyperemia, hypertrichosis, increased iris pigmentation, and skin hyperpigmentation with prostaglandins;8 respiratory and cardiac events with beta-blockers;9 and significant hyperemia and epithelial keratopathy with netarsudil.10,11 Therefore continued innovations to identify novel therapeutic options to lower IOP and prevent glaucomatous vision loss are still needed.
Adenosine triphosphate (ATP)–sensitive potassium (KATP) channels are evolutionarily conserved, membrane-bound proteins that are involved in regulation of numerous cellular functions, including vascular tone and blood pressure, through their sensitivity to changes in intracellular concentrations of ATP.12,13 KATP channels are comprised of a hetero-octameric complex of four pore-forming potassium inwardly rectifying subunits (Kir6.1 or Kir6.2) and four sulfonylurea receptor subunits (SUR1, SUR2A, or SUR2B).14 Systemically, KATP channel openers are known to be potent vasodilatory agents used to treat disorders such as hypertension, angina pectoris, and myocardial ischemia, among others.15 The ocular hypotensive properties of topical KATP channel openers, including levcromakalim, diazoxide, and nicorandil, have been described in human anterior segments and multiple normal and ocular hypertensive animal models.16–20 Studies using knockout mouse models treated with subtype-specific KATP channel openers have strongly indicated that the ocular hypotensive effect appears to occur through Kir6.2/SUR2B subunit-containing channels.19 When applied topically to the eye, KATP channel openers such as cromakalim prodrug 1 (CKLP1) have been demonstrated to lower IOP by specifically reducing episcleral venous pressure.17,20
Despite their strong ocular hypotensive properties, commercially available KATP channel openers are sparingly water-soluble and, hence, not well suited for use as a topical ocular IOP-lowering agent in the clinic. To overcome this limitation, a water-soluble phosphate ester prodrug of levcromakalim (CKLP1) was recently created.21 After topical application to the eye, endogenous phosphatases cleave the phosphate group converting CKLP1 to the active metabolite levcromakalim.16,21,22 CKLP1 was shown to retain the potent ocular hypotensive properties of levcromakalim and showed additive effects when used in combination with existing IOP-lowering drugs in normotensive animal model systems.16,17,21,22
Given the clinical potential of CKLP1, Qlaris Bio, Inc., developed a proprietary form of CKLP1 termed QLS-101 (Table 1) as a novel once-daily topical ophthalmic drop to lower IOP in patients. In the current study, we evaluated the ocular hypotensive efficacy of QLS-101 in a normotensive mouse model, examined its specificity for Kir6.2/SUR2B channels, and determined its conversion efficacy to levcromakalim in human ocular tissues.
Table 1.
Structure of QLS-101 and Levcromakalim
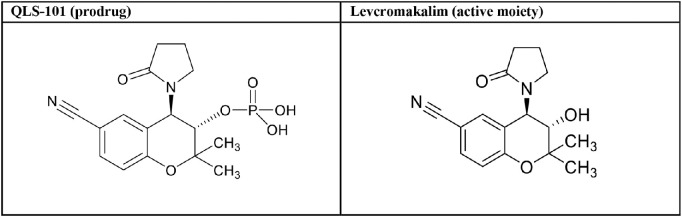
|
Methods
Reagents
QLS-101 was synthesized as a phosphate ester prodrug by Aptuit (Oxford) Ltd, an Evotec Company (Abingdon, Oxfordshire, UK) based on previously described methods.21 When needed for in vitro use, the active moiety of QLS-101, levcromakalim (Table 1), was synthesized by Aptuit. Test articles containing QLS-101 or levcromakalim were prepared and characterized consistent with United States and the Organization for Economic Co-operation and Development principles of Good Laboratory Practice. CKLP1 was synthesized in the laboratory of Peter Dosa, PhD (University of Minnesota), according to previously published methods (originally described as [3S,4R]-2).21 Tissue-nonspecific alkaline phosphatase was acquired from R&D Systems (Minneapolis, MN, USA). Acid phosphatase 1 and 5ʹ-nucleotidase were purchased from Creative Enzymes (Shirley, NY, USA). Pinacidil and glibenclamide were purchased from Millipore Sigma (St. Louis, MO, USA).
Animals and IOP Measurements
Animal experiments were pre-approved by the Mayo Clinic Institutional Animal Care and Use Committee and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Six-month-old female C57BL/6J mice (Jackson Laboratories, Bar Harbor, MA, USA) were housed five or fewer per cage at the Mayo Clinic animal facility on 12-h light/12-h dark daily cycles. Access to standard rodent chow and water was provided as desired. IOP was measured in live, conscious animals using a handheld rebound tonometer (Icare Tonolab; Colonial Medical Supply, Franconia, NH, USA) as previously described.17,18 Operators were not masked to treatment, because KATP channel openers consistently lower IOP, which becomes clear to the operator within two days of treatment.17,18,21,23 However, the same eye was always treated in all animals, inherently randomizing the treatment regimen. Before the initiation of experiments, animals were acclimatized to sham IOP measurements for three days. After acclimation, daily IOP measurements were taken at three separate time points that would correspond to one, four, and 23 hours after treatment (approximately 12:00 P.M., 3:00 P.M., and 10:00 A.M. the following day, respectively). The three measurements were averaged and recorded as the daily IOP. Preceding the experiment, baseline IOP values of all animals were measured at similar time points and averaged for three consecutive days.
In Vivo IOP Assessment of QLS-101
For topical ophthalmic use, QLS-101 was dissolved in PBS at either 0.2% (5 mM, pH 7.2) or 0.4% (10 mM, pH 6.5) doses, and CKLP1 was dissolved in PBS at a 0.2% (5 mM) dose. After baseline IOP measurements, three groups of C57BL/6J mice were treated topically in one eye with 5 µL of either 0.2% QLS-101 (n = 5, Group 1), 0.2% CKLP1 (n = 5, Group 2), or 0.4% QLS-101 (n = 10, Group 3). The contralateral eyes of Group 2 animals were treated with 5 µL of PBS and served as a vehicle control. A fourth group of animals (n = 4) treated with 0.2% CKLP1 served as a reference for Group 3. Animals were treated once daily for five consecutive days, with IOP measurements occurring one, four, and 23 hours after treatment. IOP was measured for three additional days in Groups 3 and 4 after the last daily dose, at time points that corresponded to one, four, and 23 hours after dose (approximately 12:00 P.M., 3:00 P.M., and 10:00 A.M. the following day, respectively).
KATP Channel Activity
Studies evaluating the ability of QLS-101 and levcromakalim to selectively modulate KATP channels were performed using a human embryonic kidney (HEK) cell line stably expressing human Kir6.2 and SUR2B KATP channel subunits (HEK-hKir6.2/SUR2B, n = 4-6 replicates per group). The KATP channel expressing cell line used in this study was generated at Icagen Ion Channel Technology, a division of Ligand Pharmaceuticals, Inc. (Durham, NC, USA). Briefly, cDNA constructs of Kir6.2 (from the human KCNJ11 gene) and SUR2B (from the human ABCC9 gene), both designed and synthesized at Icagen, were co-transfected into HEK-293 cells. Transfected cells were placed under selection pressure, and a single stable clonal cell line was generated. HEK-hKir6.2/SUR2B cells were cultured on 96-well plates the day before experimentation, then washed and incubated for 45 to 60 minutes with FMP-Blue (Molecular Devices, Sunnyvale, CA, USA) at room temperature protected from light. After a baseline fluorescence measurement using a fluorometric imaging plate reader (FLIPR-TETRA; Molecular Devices), cells were incubated with either QLS-101 or levcromakalim (0.003-100 µM, n = 4 replicates per group), and changes in fluorescence were monitored over a five-minute time course. The commercially available KATP channel opener pinacidil (0.003-100 µM) was used as a positive control (n = 4-6 replicates per group). After treatment, all cells were exposed to the KATP channel blocker glibenclamide (10 µM final concentration), and changes in fluorescence were monitored for an additional five minutes.
Off-Target Interactions
Potential off-target interactions of QLS-101 or levcromakalim (10 µM each) were determined using the SafetyScreen44 panel (Eurofins Panlabs, Inc., Celle l'Evescault, France) according to manufacturer's information. This assay uses a mix of competition binding assays and enzymatic inhibition assays to determine interaction with 44 selected targets belonging to the family of G protein-coupled receptors, transporters, ion channels, nuclear receptors, kinases, and other nonkinase enzymes.
Ocular Tissue Conversion Assay
A pair of eyes from a 70-year-old female donor was obtained within nine hours of death from Lions Gift of Sight (St. Paul, MN, USA). Aqueous humor was aspirated from the anterior chamber and placed on ice. Globes were bisected at the equator, and the cornea, iris, ciliary body, trabecular meshwork, sclera, optic nerve, retina, and vitreous humor were carefully dissected. Like tissues from each eye were pooled and homogenized in 50 mM Tris buffer (pH 7.1). Total protein content was quantified using a Bradford protein assay. Samples of each tissue containing 75 µg of protein (with the exception of vitreous, which contained 50 µg of protein) were incubated with QLS-101 (10 µM final concentration) at 37°C for either four or 24 hours. Reactions were immediately quenched with two volumes of acetonitrile, and samples were stored at −80°C. Contents of QLS-101 and levcromakalim in each sample were quantified by liquid chromatography followed by tandem mass spectrometry (LC-MS/MS).
Plasma Conversion Assay
Frozen plasma matrices (n = 1 per species) from Sprague Dawley rat, Dutch Belted pigmented rabbit, beagle dog, cynomolgus monkey, and human containing lithium heparin as anti-coagulant were prepared, divided, and adjusted to pH 7.4 using either 10% phosphoric acid (rat, rabbit, monkey, human) or 1N sodium hydroxide (dog). A stock solution of QLS-101 was prepared at 3 mM in dimethyl sulfoxide (DMSO) and was serially diluted to 0.3 mM and 0.03 mM. A stock solution of levcromakalim was prepared at 2 mM in DMSO and was serially diluted to 0.2 mM and 0.02 mM. These stocks were added to the plasma of each species and were used to prepare three-point standard curves for each compound for back calculating concentrations. QLS-101 was also diluted to 2 mM using DMSO and spiked into cold acetonitrile containing internal standards and matrix matched with plasma of each species to be used as reference Time Zero (T0). Stock solutions of propantheline and lovastatin (10 mM each in DMSO) were used as positive control compounds. QLS-101, propantheline, or lovastatin was added to plasma samples for a final assay concentration of 2 µM. Samples were incubated at 37°C with shaking. Duplicate aliquots of spiked plasma were transferred to a 96-well plate at timepoints of 0, 2, 4, 6, 8, and 24 hours and quenched with 300 µL of cold acetonitrile containing the internal standards. Quantity of all test compounds was analyzed by LC-MS/MS. Peak area ratios for internal standard-normalized test article and control article counts were used to calculate the percent remaining relative to the T0 value. As appropriate, results were plotted and further analyzed to calculate half-life (T1/2) values.
Phosphatase Assays
QLS-101 (5 mM) was incubated at 37°C in Tris buffer (pH 7.4) with either recombinant human alkaline phosphatase (ALP, 0.66-21 nM) (R&D Systems, Minneapolis, MN, USA), acid phosphatase (ACP, 2-62 nM) (Creative Enzymes, Shirley, NY, USA), or 5ʹ-nucleotidase (5ʹ-NT) (0.6-19 nM) (R&D Systems) for 15, 30, or 120 minutes. Phosphate cleavage was detected using the BIOMOL Green (molybdate/malachite green) phosphate detection reagent (Enzo Life Sciences, Farmingdale, NY, USA) and a Flexstation-3 multimode microplate reader (Molecular Devices, San Jose, CA, USA). Based on the results of these initial experiments, enzyme kinetic studies were performed at pH 7.4, and kcat and Km values were calculated using QLS-101 (78 µM to 5 mM) in the presence of ALP (21 nM). Cleavage reactions were also performed with ALP at pH 6.0, 7.4, or 9.0 to determine optimal pH for enzymatic activity.
Statistical Analysis
All data are expressed as mean ± standard deviation (SD). Daily IOP was calculated by taking the average of the one-, four-, and 23-hour time points. Baseline IOP was calculated for each eye by calculating the average daily IOP of the three pre-treatment days. A paired two-tailed t-test was utilized to determine statistical significance in IOP over the five-day treatment period, compared to baseline. IOP measurements between CKLP1 and QLS-101 treated groups were compared by unpaired Student's t-test. Statistical significance was ascertained at P < 0.05. Percent conversion of QLS-101 to levcromakalim was calculated by dividing the measured amount of levcromakalim by 1440 ng (theoretical QLS-101 concentration) and multiplying by 100.
Results
IOP-Lowering Efficacy
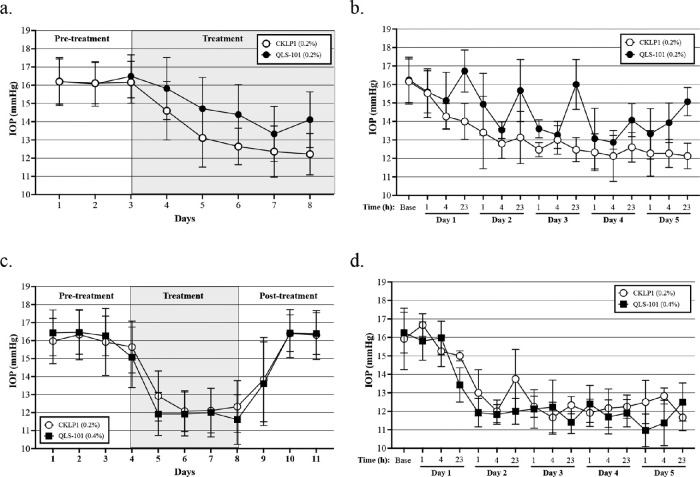
To assess the IOP lowering efficacy of QLS-101, C57BL/6J mice were topically treated in one eye with 0.2% of either QLS-101 (Group 1, n = 5) or CKLP1 (Group 2, n = 5) once daily for five days, with the contralateral eye serving as a vehicle-treated control. Mean pre-dose baseline IOP averaged 16.3 ± 0.2 mm Hg in Group 1, and 16.2 ± 0.3 mm Hg in Group 2. After five days of treatment with QLS-101, IOP readings dropped significantly to 14.5 ± 1.2 mm Hg (P = 0.01), which corresponded to a mean reduction of 13% compared to the average baseline before the start of treatment (Fig. 1a). However, these mice exhibited daily IOP fluctuations, with marked drops in pressure noted at one and four hours after dose, but with less effect noted at 23 hours (Fig. 1b). In contrast, IOPs in Group 2 mice treated with CKLP1 significantly dropped to 13.0 ± 1.0 mm Hg (P = 0.0001), corresponding to a 24% reduction from baseline at 23 hours after five days of treatment (Fig. 1a). When IOP was averaged over the five day treatment period, pressures in Group 2 treated with 0.2% CKLP1 were significantly lower from Group 1, treated with 0.2% QLS-101 (P = 0.001). In contrast, contralateral eyes treated with PBS showed no reduction of IOP compared to baseline after five days (16.3 ± 0.2 mm Hg; P = 0.094; n = 5).
Figure 1.
Effect of QLS-101 on IOP. (a) Topical once-daily treatment with 0.2% QLS-101 (Group 1, closed circles, n = 5) or 0.2% CKLP1 (Group 2, open circles, n = 5) resulted in a significant reduction of IOP in female C57BL/6J mice. (b) Once-daily treatment with 0.2% QLS-101 (Group 1, closed circles, n = 5) transiently lowered pressure through the one- and four-hour time points daily, leading to observed fluctuations of IOP. By comparison, treatment with 0.2% CKLP1 (Group 2, open circles, n = 5) led to a sustained reduction of IOP which was significantly lower from 0.2% QLS-101 (P = 0.001). (c) Topical treatment once daily with 0.4% QLS-101 (Group 3, closed squares, n = 10) reduced IOP to levels that were not significantly different from 0.2% CKLP1 (Group 4, open circles, n = 4) after 5 days (P = 0.39). (d) Once-daily treatment with 0.4% QLS-101 (Group 3, closed squares, n = 10) resulted in a 24-hour sustained reduction in IOP, similar to what was observed with 0.2% CKLP1 (Group 4, open circles, n = 4). All values are mean ± SD.
To determine if IOP reduction with QLS-101 could be sustained over a 24-hour period, we increased the dose to 0.4% (Group 3, n = 10) and compared results to 0.2% CKLP1 (Group 4, n = 4). In Group 3 eyes treated with 0.4% QLS-101, pressures dropped from a pre-dose baseline of 16.4 ± 0.1 mm Hg to 11.6 ± 1.4 (29% reduction) after five days of treatment (P < 0.0001; Fig. 1c). Importantly, IOP in Group 3 mice treated with 0.4% QLS-101 remained lower at all timepoints (one, four, and 23 hours) compared to baseline (Fig. 1d). By comparison, IOP measurements in Group 4 eyes treated with 0.2% CKLP1 for five days dropped significantly (P = 0.002) from 16.1 ± 0.2 mm Hg to 12.3 ± 1.4 mm Hg (23% reduction), also at all timepoints (one, four, and 23 hours). The reduction in IOP in Group 3 mice treated with 0.4% QLS-101 was similar to that observed in Group 4 mice treated with 0.2% CKLP1 (P = 0.39). After the cessation of treatment, Group 3 and Group 4 IOPs returned to baseline values after 2 days (Fig. 1c). In contrast, vehicle-treated contralateral eyes exhibited a small but significant (P = 0.005) increase in IOP after five days of treatment (16.6 ± 0.1 mm Hg compared to average baseline of 15.9 ± 0.1 mm Hg; n = 5).
In Vitro Ion Channel Activity
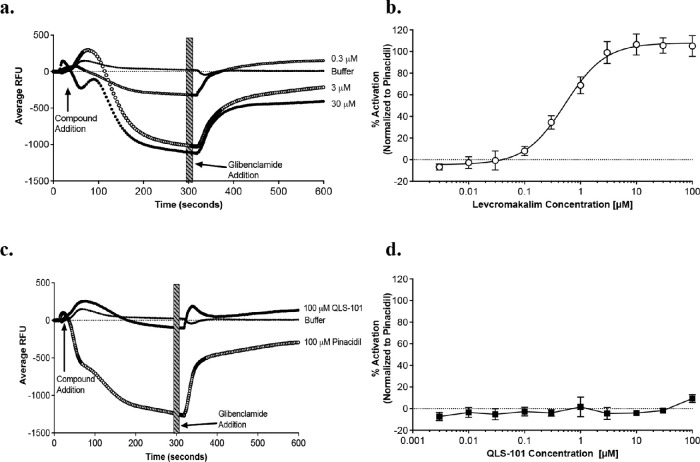
To examine the specificity of QLS-101 for KATP channels, HEK-293 cells expressing human Kir6.2 and SUR2B (HEK-hKir6.2/SUR2B) were treated with increasing concentrations of either QLS-101 or its active moiety, levcromakalim. Increasing concentrations of levcromakalim (0.003-100 µM) potently induced potassium efflux in a log dose responsive manner (Fig. 2a, Table 2). HEK-hKir6.2/SUR2B cells treated with 0.3 µM levcromakalim demonstrated moderate membrane hyperpolarization, which peaked at 3 µM (EC50 = 0.534 ± 0.05; Fig. 2b). In the presence of glibenclamide, a potent KATP channel antagonist, levcromakalim-mediated hyperpolarization was markedly attenuated, suggesting specificity for levcromakalim with the Kir6.2/SUR2B channel (Fig. 2b). In contrast, treatment with QLS-101 did not induce any detectable membrane hyperpolarization, nor was any dose-response curve or EC50 value detected (Fig. 2c-d, Table 2). These results are consistent with the inert nature of QLS-101 as a prodrug in the absence of converting enzymes. By comparison, positive control pinacidil potently induced membrane hyperpolarization in HEK-hKir6.2/SUR2B with corresponding EC50 values of 5.49 ± 0.99 µM, respectively (Table 2).
Figure 2.
Concentration-dependent membrane hyperpolarization of HEK-hKIR6.2/SUR2B cells by various compounds. (A, C) Averaged FLIPR traces of membrane potential response to (A) levcromakalim (n = 4), or (C) QLS-101 (n = 4) compared to the buffer control as indicated. Arrows indicate time of either addition of test compound or the KATP channel blocker glibenclamide (10 µM). Thick striped bar indicates the time range that was exported for EC50 calculation. The KATP channel opener pinacidil (100 µM) was included as a positive control in the QLS-101 assay as indicated (n = 4-6 replicates per group). (B, D) Concentration dependence of activation-averaged data across replicate testing days. Data points for individual batches are mean ± SD for six replicates recorded across two separate experimental days.
Table 2.
EC50 Values for Activation of Kir6.2/SUR2B
| Test Compound | EC50 (µM) (Mean ± SEM) |
|---|---|
| Levcromakalim | 0.534 ± 0.05 |
| QLS-101 | >100 ± NA |
| Pinacidil | 5.49 ± 0.99 |
N = 4-6 replicates.
To confirm KATP channel specificity, we investigated the ability of QLS-101 and levcromakalim (10 µM each) to bind to G protein-coupled receptors, neurotransmitter transporters, ion channels, nuclear steroid receptors, kinases, and non-kinase enzymes. Using SafetyScreen44, a screening assay designed to investigate receptor or enzymatic specificity, treatment with either QLS-101 or levcromakalim demonstrated no marked (>25%)24 off-target effects, indicating the specificity of both entities for KATP channels (data not shown).
QLS-101 Conversion
Having established the necessity of QLS-101 conversion to levcromakalim for KATP channel activation, we next assessed the ability of human ocular tissues to catalyze this conversion in vitro. Tissues harvested from a pair of human 70-year-old female donor eyes were incubated with QLS-101 (10 µM) for either four or 24 hours at 37°C. After four hours, only iris demonstrated modest conversion of QLS-101 to levcromakalim (0.90% conversion, Table 3). In contrast, at 24 hours, levcromakalim was detected in iris (3.93%), ciliary body (2.55%), sclera (1.61%), trabecular meshwork (1.60%), optic nerve (0.89%), cornea (0.77%), and retina (0.74%) (Table 3). No detectable conversion of QLS-101 was noted in aqueous humor or vitreous humor.
Table 3.
Conversion of QLS-101 to Levcromakalim in Various Human Ocular Tissues
| Tissue | Time Point (h) | QLS-101 (ng/mL) | Levcromakalim (ng/mL) | Percent Conversion* |
|---|---|---|---|---|
| Ciliary body | ||||
| 4 | 1496.77 | BLQ | NA | |
| 24 | 1428.45 | 36.67 | 2.55% | |
| Optic nerve | ||||
| 4 | 1517.06 | BLQ | NA | |
| 24 | 1399.47 | 12.82 | 0.89% | |
| Aqueous humor | ||||
| 4 | 1602.95 | BLQ | NA | |
| 24 | 1568.22 | BLQ | NA | |
| Vitreous humor | ||||
| 4 | 1500.30 | BLQ | NA | |
| 24 | 1440.22 | BLQ | NA | |
| Iris | ||||
| 4 | 1425.37 | 12.92 | 0.90% | |
| 24 | 1459.07 | 56.66 | 3.93% | |
| Sclera | ||||
| 4 | 1391.67 | BLQ | NA | |
| 24 | 1435.49 | 23.16 | 1.61% | |
| Retina | ||||
| 4 | 1539.14 | BLQ | NA | |
| 24 | 1472.86 | 10.69 | 0.74% | |
| Cornea | ||||
| 4 | 1378.18 | BLQ | NA | |
| 24 | 1414.45 | 11.04 | 0.77% | |
| Trabecular meshwork | ||||
| 4 | 1629.65 | BLQ | NA | |
| 24 | 1696.07 | 22.93 | 1.60% |
BLQ, below the limit of quantitation;
NA, not applicable.
N = 2 human eyes
Percent conversion was calculated by dividing the amount of levcromakalim by the initial theoretical QLS-101 concentration (1440 ng/mL) and multiplying by 100.
The preferable administration of QLS-101 to humans would be through topical administration as eyedrops. As is the case with all eyedrops, after topical instillation, some drug will enter systemic circulation. To begin examining the systemic bioavailability of QLS-101, conversion of QLS-101 to levcromakalim was quantified in plasma isolated from multiple species (Table 4). Overall, QLS-101 was largely stable in plasma, with 97.3% of QLS-101 remaining unconverted in Dutch-belted rabbit plasma after 24 hour incubation at 37°C. Order of decreasing QLS-101 stability in plasma was as follows: Dutch-belted rabbit >Sprague Dawley rat>beagle dog>human>cynomolgus monkey. All plasmas showed significantly more stability with QLS-101 than with propantheline and lovastatin, two drugs readily converted in plasma (Table 4). In all species, no levcromakalim formation was observed in plasma after 24 hours.
Table 4.
Content of QLS-101 and Positive Control Compounds in Plasma From Multiple Species
| Matrix | Compound | T1/2 (min) | Remaining at 24 Hours |
|---|---|---|---|
| Sprague Dawley rat | |||
| QLS-101 | 4085 | 93.70% | |
| Propantheline | 577 | 14.60% | |
| Lovastatin | < 120 | 0.00% | |
| Dutch belted rabbit | |||
| QLS-101 | 6012 | 97.30% | |
| Propantheline | 18.8 | 0.10% | |
| Lovastatin | <120 | 0.00% | |
| Beagle dog | |||
| QLS-101 | 4192 | 95.40% | |
| Propantheline | 224 | 1.00% | |
| Lovastatin | 279 | 1.70% | |
| Cynomolgus monkey | |||
| QLS-101 | 2737 | 80.30% | |
| Propantheline | 272 | 2.60% | |
| Lovastatin | 413 | 0.00% | |
| Human | |||
| QLS-101 | 19.33 | 81.30% | |
| Propantheline | 25.5 | 0.00% | |
| Lovastatin | 258 | 0.00% |
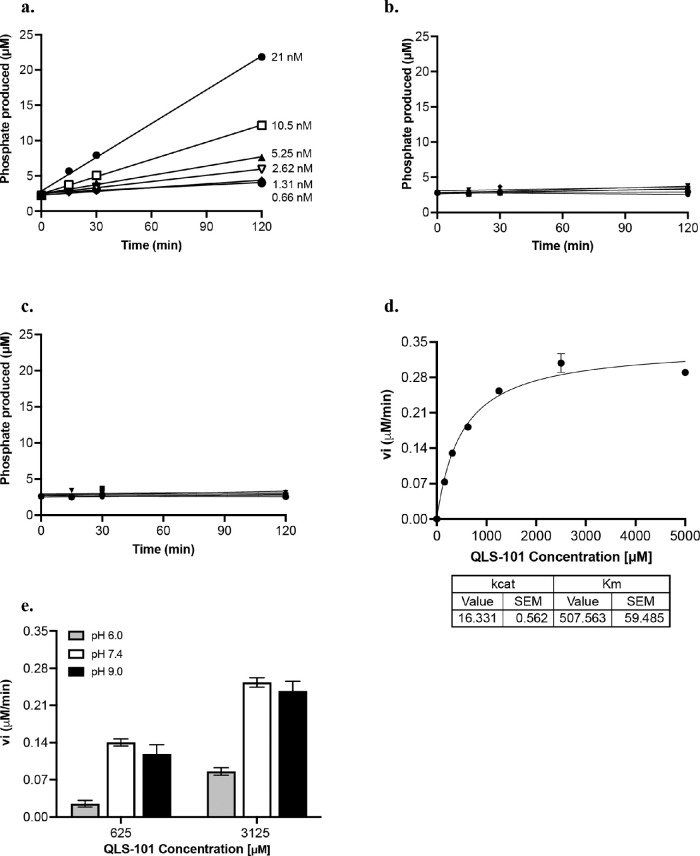
Based on the structure of QLS-101 as a phosphate prodrug, we evaluated several phosphatases known to be present in the anterior chamber of the eye for their ability to convert QLS-101 to levcromakalim. To investigate this, QLS-101 conversion at physiological salt and pH conditions was evaluated in the presence of purified recombinant human alkaline phosphatase (ALP), acid phosphatase (ACP), or 5ʹ-nucleotidase (5ʹ-NT) enzymes. Although ACP (Fig. 3b) and 5’-NT (Fig. 3c) did not demonstrate any conversion of QLS-101 to levcromakalim, ALP did convert QLS-101 to levcromakalim in a dose-dependent manner (Fig. 3a). Enzyme kinetic analysis done in triplicate determined the average kcat and Km values of ALP for QLS-101 to be 15 min(-1) and 630 µM, respectively, in a non-phosphate buffer at physiological pH (Fig. 3d). Cleavage of QLS-101 at 625 µM (Km) and 3125 µM (5X Km) by ALP (21 nM) was most robust at either pH 7.4 or 9.0, but it was markedly lower at pH 6.0 (Fig. 3e). This indicates that QLS-101 is more readily cleaved at either physiological or alkaline pH by ALP, consistent with its known activity.
Figure 3.
Enzymatic cleavage of QLS-101 in vitro. (A) The ability of alkaline phosphatase (ALP) to cleave QLS-101 at physiological salt and pH was quantified at five minutes, 30 minutes, and two hours in a single experiment. (B, C) The ability of acid phosphatase (B) or 5ʹnucleotidase (C) to cleave QLS-101 at physiological salt and pH was quantified at five minutes, 30 minutes, and two hours in a single experiment. (D) Representative graph of three separate experiments quantifying ALP kinetics when incubated with QLS-101 in a non-phosphate physiological buffer (n = 2-3 per experiment). (E) Effect of pH on activity of ALP (21 nM) for QLS-101 was determined at pH 6.0 in MES buffer, as well as 7.4 and 9.0 in Tris buffer (n = 3). Data shown (D, E) are depicted as mean ± SD.
Discussion
Current medical treatments for glaucoma can be limited by side effects and tachyphylaxis; hence, there is a need for additional new and novel therapies to lower IOP with improved tolerability. To attain this goal, QLS-101 is being developed as a novel water-soluble phosphate ester prodrug of levcromakalim. In the current study, we demonstrate that once-daily topical ocular dosing of QLS-101 significantly lowers IOP in normotensive C57BL/6J mice. To activate KATP channels and subsequently lower IOP, QLS-101 must be converted to its active moiety, levcromakalim, which can occur within 24 hours in human ocular tissues, and most likely by alkaline phosphatases.
A number of previous studies have demonstrated the potent ocular hypotensive effects of KATP channel openers in normotensive and ocular hypertensive mice.17,18,20,21,23 Topical application of levcromakalim reduced IOP in wild-type C57BL/6J mice by 3.3 ± 0.4 mm Hg over a treatment period of five days.18 By comparison, the KATP channel openers diazoxide and nicorandil lowered IOP in wild-type C57BL/6J mice by 3.9 ± 0.6 mm Hg and 3.4 ± 0.4 mm Hg, respectively.19 More recently, CKLP1 was shown to lower IOP in wild-type C57BL/6J mice by 3.9 ± 0.6 mm Hg.17 Similar IOP-lowering results were noted with 0.2% CKLP1 in the current study (Fig. 1); however, only QLS-101 at the 0.4% dose was able to match the ocular hypotensive efficacy of CKLP1 (Fig. 1c-d). At the 0.2% dose, the IOP-lowering effect of QLS-101 was only noted at the one- and four-hour post-dose time points, with an attenuation of the effect noted by 23 hours after dose. This indicated that a higher dose of QLS-101 was required to achieve a sustained 24-hour IOP reduction. Indeed, mice treated with the 0.4% dose did exhibit a reduction of IOP over a 24-hour period, similar to what was seen with CKLP1. Furthermore, 0.4% QLS-101 provided a greater IOP-lowering effect when compared to the 0.2% dose, and pressures remained markedly lower than baseline for 24 hours after cessation of treatment (Fig. 1d).
In the human conventional aqueous humor outflow pathway, KATP channel subunits Kir6.1, Kir6.2, SUR2A, and SUR2B have been shown to be present in the trabecular meshwork, Schlemm's canal, and in cells distal to the outer wall of Schlemm's canal.19,25 Because previous studies have demonstrated that the Kir6.2/SUR2B KATP channel was essential for IOP reduction with KATP channel openers,19 we investigated the specificity of QLS-101 in HEK-293 cells expressing these subunits. Because QLS-101 was developed as an inert prodrug that when applied topically, lowered IOP in mice, it was anticipated that conversion to its active moiety levcromakalim would be required prior to activating the Kir6.2/SUR2B KATP channel. These speculations were confirmed in HEK-hKir6.2/SUR2B cells, where QLS-101 elicited no channel activity at the highest concentrations tested. In contrast, the sensitivity of the human Kir6.2/SUR2B channel to levcromakalim was potently dose-dependent, and markedly higher than that of positive control pinacidil. These data in combination with the demonstrated ocular hypotensive ability of QLS-101 allows one to speculate that QLS-101 lowers IOP only after it is converted to its active moiety levcromakalim by phosphatases such as alkaline phosphatase.
QLS-101 and levcromakalim activity on KATP channels was highly specific since no off-target effects were found on several G-protein coupled receptors, enzymes, neurotransmitters, ion channels, or transporters (data not shown). While some targets such as 5-HT1A, 5-HT1B, 5-HT3, COX1, and COX2 did demonstrate minimal activity in response to QLS-101 or levcromakalim treatment, their activation was considered insignificant as per current standards.24 These results indicate that QLS-101 and levcromakalim do not have any common off-target effectors.
While the observed reduction in IOP following topical administration of QLS-101 to the eye strongly indicated that conversion to levcromakalim occurred in mouse eyes, the question remained as to which tissues exhibited the highest levels of converting activity. Utilizing human ocular tissues for this investigation, the results demonstrated that QLS-101 can be converted in multiple human ocular tissues within 24 hours in vitro. Although conversion in this assay was modest, the QLS-101 molecule is known to be sterically hindered from rapid conversion near the phosphate ester.21 Collectively, the level of conversion when taking into account all tissues is more significant, approaching 10% to 15%. Because of the fact that all tissues in the anterior chamber are connected by aqueous humor, it is conceivable that levcromakalim may flow toward the trabecular outflow pathway, which is the therapeutic target.
It is also feasible to consider that the slow conversion rate may be a positive attribute for QLS-101. One of the functions of KATP channel openers is that they induce vasodilatory activity.17 Two current treatments for glaucoma (latanoprostene bunod and netarsudil) also have been associated with vasodilation. However, although latanoprostene bunod is prodrug-like in that it requires esterase-dependent conversion to yield its vasodilatory moiety,26 netarsudil is vasoactive on the ocular surface, which is likely responsible for its significant association with hyperemia. In vivo studies performed to enable the Investigational New Drug (IND) application for QLS-101 demonstrated a minimal side effect profile in larger animal models, including mild and sporadic hyperemia in animals treated with 3.2 mg/kg/eye (8% QLS-101, which is 20 times greater than the highest dose used in the current study), suggesting that the slow conversion of QLS-101 to levcromakalim may be ideal to achieve a balance between potent efficacy and excellent tolerability. Nevertheless, it is important to consider that even with a slow rate of conversion, QLS-101 was able to elicit a significant reduction in IOP comparable to other KATP channel openers already reported.
These studies also showed that ALP contributes to the conversion of QLS-101 to its active moiety at a physiological pH of 7.4. The results are encouraging based on the known expression of ALP in the cornea and aqueous humor of glaucoma patients27,28 and that the drug can be administered near to a physiological pH, which would minimize adverse events such as burning and stinging on application, which are typically noted with acidic or alkaline solutions.
In summary, these studies demonstrate that once-daily topical dosing of QLS-101, a novel water-soluble phosphate ester prodrug of levcromakalim, lowers IOP in normotensive mice. QLS-101 is converted to levcromakalim in multiple anterior segment tissues, most likely by alkaline phosphatases. Collectively, these data indicate that QLS-101 is a novel topical IOP-lowering agent that may be considered as a future therapeutic for the treatment of glaucoma.
Acknowledgments
Disclosure: C.L. Pervan-Steel, Qlaris Bio, Inc (E); U. Roy Chowdhury, Qlaris Bio, Inc (N); H.K. Sookdeo, Qlaris Bio, Inc (E); R.A. Casale, Qlaris Bio, Inc (E); P.I. Dosa, Qlaris Bio, Inc. (P, C); T.M. Htoo, Qlaris Bio, Inc (E); M.P. Fautsch Qlaris Bio, Inc (F, C, P); B.M. Wirostko, Qlaris Bio, Inc (E)
References
- 1. Quigley HA, Broman AT.. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY.. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014; 121: 2081–2090. [DOI] [PubMed] [Google Scholar]
- 3. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998; 126: 487–497. [DOI] [PubMed] [Google Scholar]
- 4. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000; 130: 429–440. [DOI] [PubMed] [Google Scholar]
- 5. Heijl A, Leske MC, Bengtsson B, et al.. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002; 120: 1268–1279. [DOI] [PubMed] [Google Scholar]
- 6. Kass MA, Heuer DK, Higginbotham EJ, et al.. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120: 701–713; discussion 829-730. [DOI] [PubMed] [Google Scholar]
- 7. Mehta AA, Kanu LN, Sood-Mendiratta S, et al.. Experience with netarsudil 0.02% and latanoprostene bunod 0.024% as adjunctive therapy for glaucoma. Eur J Ophthalmol. 2021;1120672121998913. [DOI] [PubMed] [Google Scholar]
- 8. Denis P. Adverse effects, adherence and cost-benefits in glaucoma treatment. European Ophthalmic Review. 2011; 5: 116–122. [Google Scholar]
- 9. Nelson WL, Fraunfelder FT, Sills JM, Arrowsmith JB, Kuritsky JN.. Adverse respiratory and cardiovascular events attributed to timolol ophthalmic solution, 1978-1985. Am J Ophthalmol. 1986; 102: 606–611. [DOI] [PubMed] [Google Scholar]
- 10. Ramakrishnan MS, Addis VM, Lehman AY, Sankar PS.. Netarsudil-associated epithelial keratopathy. Am J Ophthalmol Case Rep. 2020; 19: 100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kahook MY, Serle JB, Mah FS, et al.. Long-term Safety and Ocular Hypotensive Efficacy Evaluation of Netarsudil Ophthalmic Solution: Rho Kinase Elevated IOP Treatment Trial (ROCKET-2). Am J Ophthalmol. 2019; 200: 130–137. [DOI] [PubMed] [Google Scholar]
- 12. Babenko AP, Aguilar-Bryan L, Bryan J.. A view of sur/KIR6.X, KATP channels. Annu Rev Physiol. 1998; 60: 667–687. [DOI] [PubMed] [Google Scholar]
- 13. Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT.. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989; 245: 177–180. [DOI] [PubMed] [Google Scholar]
- 14. Tinker A, Aziz Q, Li Y, Specterman M.. ATP-Sensitive Potassium Channels and Their Physiological and Pathophysiological Roles. Compr Physiol. 2018; 8: 1463–1511. [DOI] [PubMed] [Google Scholar]
- 15. Roy Chowdhury U, Dosa PI, Fautsch MP. ATP sensitive potassium channel openers: A new class of ocular hypotensive agents. Exp Eye Res. 2017; 158: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roy Chowdhury U, Kudgus RA, Rinkoski TA, et al. Pharmacological and pharmacokinetic profile of the novel ocular hypotensive prodrug CKLP1 in Dutch-belted pigmented rabbits. PLoS One. 2020; 15: e0231841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roy Chowdhury U, Rinkoski TA, Bahler CK, et al. Effect of Cromakalim Prodrug 1 (CKLP1) on Aqueous Humor Dynamics and Feasibility of Combination Therapy With Existing Ocular Hypotensive Agents. Invest Ophthalmol Vis Sci. 2017; 58: 5731–5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roy Chowdhury U, Bahler CK, Holman BH, Dosa PI, Fautsch MP. Ocular Hypotensive Effects of the ATP-Sensitive Potassium Channel Opener Cromakalim in Human and Murine Experimental Model Systems. PLoS One. 2015; 10: e0141783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chowdhury UR, Holman BH, Fautsch MP.. ATP-sensitive potassium (K(ATP)) channel openers diazoxide and nicorandil lower intraocular pressure in vivo. Invest Ophthalmol Vis Sci. 2013; 54: 4892–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roy Chowdhury U, Millar JC, Holman BH, et al. Effect of ATP-sensitive Potassium Channel Openers on Intraocular Pressure in Ocular Hypertensive Animal Models. Invest Ophthalmol Vis Sci. 2022; 63: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roy Chowdhury U, Viker KB, Stoltz KL, Holman BH, Fautsch MP, Dosa PI. Analogs of the ATP-Sensitive Potassium (KATP) Channel Opener Cromakalim with in Vivo Ocular Hypotensive Activity. J Med Chem. 2016; 59: 6221–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roy Chowdhury U, Kudgus RA, Holman BH, et al. Pharmacological Profile and Ocular Hypotensive Effects of Cromakalim Prodrug 1, a Novel ATP-Sensitive Potassium Channel Opener, in Normotensive Dogs and Nonhuman Primates. J Ocul Pharmacol Ther. 2021; 37: 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roy Chowdhury U, Bahler CK, Holman BH, Fautsch MP. ATP-sensitive potassium (KATP) channel openers diazoxide and nicorandil lower intraocular pressure by activating the Erk1/2 signaling pathway. PLoS One. 2017; 12: e0179345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kers JA, Sharp RE, Defusco AW, et al.. Mutacin 1140 Lantibiotic Variants Are Efficacious Against Clostridium difficile Infection. Front Microbiol. 2018; 9: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chowdhury UR, Bahler CK, Hann CR, et al.. ATP-sensitive potassium (KATP) channel activation decreases intraocular pressure in the anterior chamber of the eye. Invest Ophthalmol Vis Sci. 2011; 52: 6435–6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cavet ME, DeCory HH.. The Role of Nitric Oxide in the Intraocular Pressure Lowering Efficacy of Latanoprostene Bunod: Review of Nonclinical Studies. J Ocul Pharmacol Ther. 2018; 34: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Latarya G, Mansour A, Epstein I, et al.. Human aqueous humor phosphatase activity in cataract and glaucoma. Invest Ophthalmol Vis Sci. 2012; 53: 1679–1684. [DOI] [PubMed] [Google Scholar]
- 28. Cejkova J, Bolkova A.. A study on alkaline phosphatase in cornea of various animals with special regard to keratocytes. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1977; 204: 209–214. [DOI] [PubMed] [Google Scholar]