Abstract
Our eyes move constantly but are often inhibited momentarily in response to external stimuli (oculomotor inhibition [OMI]), depending on the stimulus saliency, anticipation, and attention. Previous studies have shown prolonged OMI for auditory oddballs; however, they required counting the oddballs, possibly reflecting voluntary attention. Here, we investigated whether the “passive” OMI response to auditory deviants can provide a quantitative measure of deviance strength (pitch difference) and studied its dependence on the inter-trial interval (ITI). Participants fixated centrally and passively listened to repeated short sequences of pure tones that contained a deviant tone either regularly or with 20% probability (oddballs). In an “active” control experiment, participants counted the deviant or the standard. As in previous studies, the results showed prolonged microsaccade inhibition and increased pupil dilation following the rare deviant tone. Earlier inhibition onset was found in proportion to the pitch deviance (the saliency effect), and a later release was found for oddballs, but only for ITI <2.5 seconds. The active control experiment showed similar results when counting the deviant but longer OMI for the standard when counting it. Taken together, these results suggest that OMI provides involuntary markers of saliency and deviance, which can be obtained without the participant's response.
Keywords: saccadic inhibition, oculomotor inhibition (OMI), ISI, saliency, anticipation, attention, microsaccade, pupil, blink, auditory deviant, oddball, passive viewing, passive listening, passive attentive
Introduction
Survival largely depends on one's ability to detect sudden changes in the environment, anticipate upcoming events, and process them optimally. Predictive computations represent one of the fundamental principles of neural processing, and a prediction mismatch may support behavioral complexity and dynamics (Bubic, von Cramon, & Schubotz, 2010). Responses to an auditory mismatch can reflect the violations of predictive assessments or adaptation to a repeated stimulus. However, a mismatched response to an omitted predicted signal, for example, is better interpreted as top-down predictive processing rather than simple stimulus adaptation (Wacongne, Labyt, van Wassenhove, Bekinschtein, Naccache, & Dehaene, 2011; Winkler, Denham, & Nelken, 2009). Although many of the studies dealing with auditory deviants used electrophysiology, similar effects can be observed by involuntary eye movement measures (Valsecchi & Turatto, 2009).
Involuntary eye movements during fixation, including microsaccades, spontaneous eye blinks, and ocular drift, occur continuously; however, the eyes tend to freeze in response to transient stimuli (oculomotor inhibition [OMI]), and the latencies of the inhibition onset and release depend on stimulus saliency (Bonneh, Adini, & Polat, 2015; Bonneh, Adini, & Polat, 2016; Rolfs, 2009; Zhao, Yum, Benjamin, Benhamou, Yoneya, Furukawa, Dick, Slaney, & Chait, 2019), attention (Fried, Tsitsiashvili, Bonneh, Sterkin, Wygnanski-Jaffe, Epstein, Polat, 2014; Meyberg, Sinn, Engbert, & Sommer, 2017), expectations, or surprise (Amit, Abeles, Carrasco, & Yuval-Greenberg, 2019). Microsaccades are rapid, small-amplitude saccades that can be executed voluntarily to desired locations, even from memory (Willeke, Tian, Buonocore, Bellet, Ramirez-Cardenas, & Hafed, 2019), but typically they occur involuntarily during fixation, with a rate of about one or two per second (Engbert, 2006), and an inhibitory pattern in response to transient stimuli (saccadic inhibition) that has been studied extensively (Amit et al., 2019; Bonneh et al., 2015; Fried et al., 2014; Hafed, Goffart, & Krauzlis, 2009; Rolfs, Kliegl, & Engbert, 2008; Valsecchi, Betta, & Turatto, 2007; Widmann, Engbert, & Schroger, 2014; Yablonski, Polat, Bonneh, & Ben-Shachar, 2017; Zhao et al., 2019). For example, the latency of the first microsaccade relative to stimulus onset, following inhibition, termed the microsaccade response time (msRT), was found to be sensitive to the contrast and spatial frequency of the stimulus; a shorter msRT occurs with more salient stimuli (Bonneh et al., 2015). Microsaccade inhibition results from a peak of activity at the fixation (central) location in the superior colliculus (SC) saccade map (Rolfs et al., 2008), which plays a role in attentive and orienting behaviors and is involved in generating microsaccades (Hafed et al., 2009). The SC activity reflects a map of the relevant behavioral goals and may correspond to sensory input from different modalities rather than only to a visual stimulus (Hafed & Krauzlis, 2008).
In recent studies, microsaccades have been found to be highly informative about cognitive processes. Whereas saliency driven by stimulus properties, such as contrast, shortens the inhibition, longer inhibition was found for oddballs in a sequence. This was found for a blue patch among frequent red patches (Valsecchi, Betta, & Turatto, 2007). However, these findings were obtained when participants had to attend to and report the deviant stimulus, possibly reflecting a prolonged inhibition to the attended stimulus rather than a perceptual surprise. In more recent studies from our laboratory, we found preliminary evidence of similar effects obtained in passive viewing without specifically attending to the oddballs. This was demonstrated for visual oddballs, such as a high-contrast patch among low-contrast patches (Bonneh, Adini, Sagi, Tsodyks, Fried, & Arieli, 2013), with similar results obtained with eye blinks (Bonneh, Polat, & Adini, 2016) and for temporal oddballs (unpredicted intervals; Bonneh et al., 2016), all showing preliminary evidence of prolonged inhibition for the deviant stimulus. Prolonged OMI for an auditory deviant tone in a sequence has been previously reported in a few studies (Valsecchi & Turatto, 2009; Widmann et al., 2014; Yuval-Greenberg & Deouell, 2011); however, the auditory oddball effect, without attending specifically to the deviant stimulus and its dependency on the deviant magnitude, were never demonstrated.
Originally, one of the purposes of this study was to develop involuntary oculomotor measures of different surprise levels similar to those obtained via event-related potential (ERP) to test non-communicating individuals (Bekinschtein, Dehaene, Rohaut, Tadel, Cohen, & Naccache, 2009). That ERP study used an auditory oddball paradigm to evaluate the violations of auditory regularities, either local in time, within a single trial (5 tone sequences with a deviant at the fifth position), or global across trials of several seconds. In our first study (local deviance-strength), we used five tone sequences, as in the ERP study, using a blocked design in which the deviant was predictable, and accompanied by a neutral visual stimulus to strengthen the fixation and enhance the OMI. In the second study, we investigated the global oddball effect, in which the oddball appeared unpredictably in 20% of the trials, along with the effect of the interstimulus interval. Because the five-tone sequences did not produce reliable results (see the explanation in the Methods), we changed to six-tone sequences with the deviant in the middle.
In the first study (local deviance-strength), we aimed to investigate the effect of the local deviance size on the OMI, with two possible outcomes: shorter as previously found for higher (more salient) visual contrast (Bonneh et al., 2015) or longer, as previously found for oddballs (Valsecchi, Dimigen, Kliegl, Sommer, & Turatto, 2009). In the second study (the global oddball effect), we aimed to investigate the global oddball (a rare deviant) effect on the OMI as a function of the inter-trial interval (ITI), expecting it to decrease with longer intervals. We assumed that the strength of the formed global regularity will depend on the temporal proximity as previously found for the ERP mismatch negativity (MMN; Sussman & Gumenyuk, 2005).
We, therefore, focused on two specific research questions: (1) Can OMI be used as an involuntary marker of auditory deviance detection?; and (2) Can OMI be used as a measure for saliency and deviance effects in auditory sequences? In addition, we investigated whether the deviance effect is reduced by a longer ITI. To address these questions, participants were asked to attend a stream of sounds in predictable (study 1) and oddball conditions (study 2) while fixating on a central visual fixation without performing a task. In this way, the methods we develop in the current study could be tested on non-communicating individuals in the future. For comparison, we also conducted control experiments that involved a counting task.
Methods
Participants
Overall, 96 participants, ages 20 to 40 years, were recruited for both studies. Study 1 (local deviance strength): Fifty-six participants, 29 women and 27 men, were recruited for the passive-attentive experiment; one participant was removed from the data analysis of the first experiment because of bad recording (gaps or blinking in 97% of the trials). In addition, 17 participants, 10 women and 7 men, were recruited for the Deviant/Standard Counting, control experiments, including 7 new participants. Study 2 (global oddball): Seventeen participants, 6 women and 11 men, were recruited for the oddball experiment. In addition, 17 participants (16 new), 8 women and 9 men were recruited for the reverse-oddball control experiment.
All participants had normal or corrected-to-normal vision and were naïve to the purpose of the study, except for the first author. The experiments were approved by the Bar-Ilan University Internal Review Board (IRB) Ethics Committee. All participants gave written informed consent, and all the experiments were conducted according to the IRB guidelines.
Apparatus
Stimuli were displayed at a distance of 0.6 meters (m) on a 24-inch LCD monitor (Eizo Foris fg2421) running at 1920 × 1080 screen resolution and at a 100 Hz refresh rate, using an in-house-developed platform for psychophysical and eye-tracking experiments (PSY) developed by Y.S. Bonneh. All experiments were administered in dim light, and the screen background was gray with 50 cd/m2 luminance. We used a remote video-based eye-tracking system (Eyelink, SR Research), with a sampling rate of 500 Hz, and recorded from a distance of 50 to 55 cm. All recordings were done binocularly, with analyses done on data from the left eye. The monocular analysis was chosen, based on our experience with the accuracy of monocular microsaccade detection (Bonneh et al., 2015; Bonneh et al., 2016; Yablonski, Polat, Bonneh, & Ben-Shachar, 2017) and to allow future testing of non-communicating patients for which binocular recording could be difficult. For the same reason, we chose a 25 mm-wide lens in a head-free mode to increase flexibility in future studies. A standard nine-point calibration was performed before each session.
Stimuli and procedures
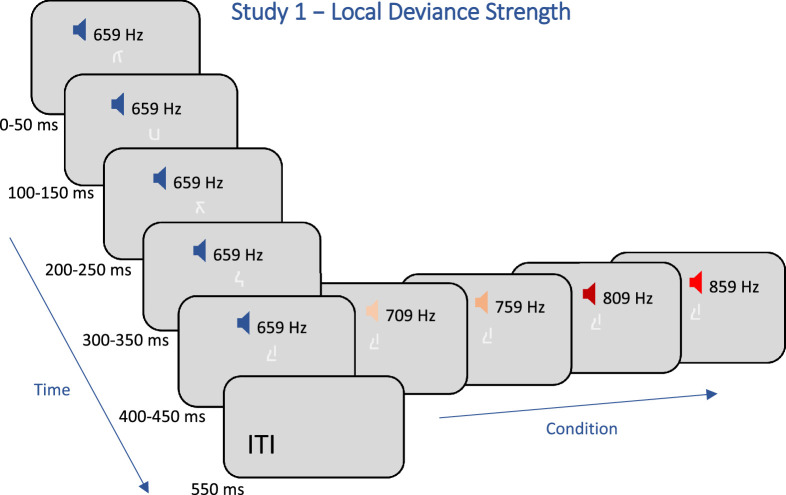
Study 1 (local deviance strength): In the passive-attentive experiment, the participants, N = 56, passively attended to a series of sounds played through headphones and watched a rapid serial visual presentation (RSVP) of small, approximately 1 degree of visual angle (dva), low-contrast, upside down Hebrew letters, presented at the center of the screen (see Figure 1). In contrast to previous oddball studies, a “passive attentive” paradigm was used (see Quirins, Marois, Valente, Seassau, Weiss, El Karoui, Hochmann, & Naccache, 2018; Silverstein, Snodgrass, Shevrin, & Kushwaha, 2015 for a similar approach), in which participants were asked to attend to all sounds without using a task to control their attention (see the Discussion for further details). Participants were only instructed to fixate on the screen center and to attend a series of sounds played through headphones. One random letter out of 27 options was presented in parallel with each sound in a series. The visual stimuli were not informative regarding the auditory conditions and were used to obtain a steady fixation without gaze wandering and a regular and strong OMI, whose modulation by the auditory stimuli was the effect we aimed to measure. The sound series consisted of rapid sequences of five identical tones (70 dB SPL, 50 ms each, with a 50 ms gap), or they contained a deviant tone at the end of the sequence (a deviant fifth tone). A schematic presentation of the paradigm is shown in Figure 1. Altogether, there were five separate conditions: standard trials with 5 identical 659 Hz pure tones and deviant trials that varied in the frequency of the fifth tone with 50 Hz steps (709, 759, 809, and 859 Hz). The total duration for each presentation of the 5 tones and letters was 450 ms, and the interval between presentations (ITI) was 550 ms; therefore, the combined stimulus rate was 1 Hz. There was no mixing between trials from different conditions (blocks of 20 trials); however, the blocks were played in random order in three separate short runs. Participants completed a total of 60 trials for each of the 5 conditions in 3 runs (20 trials per condition in a run).
Figure 1.
Schematic illustration of the trial sequence of study 1. Altogether, there were five conditions, with synchronized auditory and visual stimuli, and a fifth tone, which varied in frequency. Trials from the different conditions were played in separate blocks. In the “passive-attentive” experiment there was no mixing between conditions. However, in the “deviant-counting” and “standard-counting,” control experiments, trials from different conditions were mixed as in the classic oddball paradigm.
Deviant and standard counting control experiments
As a control experiment, we wanted to verify whether there is any difference between a “passive attentive” experiment and an “active” experiment. Because some of our former experiments in the laboratory suggested that asking the participants to count or attend a specific stimulus prolongs the OMI in response to that stimulus, we predicted that this would also apply to the current paradigm. For this reason, we performed two extra control experiments with similar stimuli and block design as in the passive-attentive experiment, but with two exceptions: (1) an “active attentive” task was adopted from previous oddball studies, with instructions of either to “count the oddballs” or “count the standards”; and (2) trials from different blocks were mixed as in the classic oddball paradigm to serve the counting task.
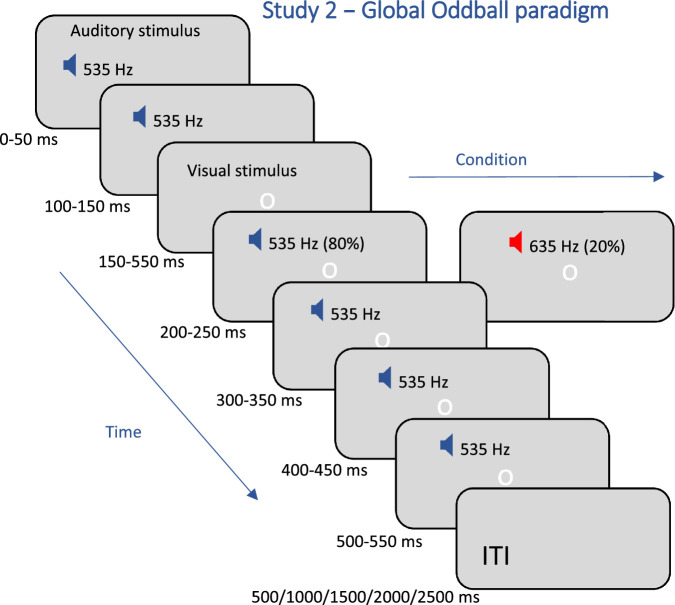
Study 2 (global oddball): As in the first study, the participants, N = 17, passively viewed and attended to a series of sounds (see Figure 2). We initially used the 5-tone sequence as in study 1, in which the deviance was predictable, and we focused on the inhibition onset; however, we did not obtain a robust effect when the deviance was unpredictable and we focused on the inhibition release. We think that the reason for this was the bimodal microsaccade distribution (see Figure 3b), with two inhibitory responses: one for the onset and another for the offset of the stimulus, which reduced the number of microsaccades and the accuracy of the second response. To address this difficulty and increase the strength of the OMI effect, we changed the sequence by adding a deviant tone at the third position. To obtain gaze fixation and regular OMI, we used a small low-contrast white circle (0.65 dva in diameter), flashed 50 ms before the deviant onset, which remained until the end of the sound series. We applied an auditory oddball paradigm with a rare deviant. There were standard trials with 6 identical 535 Hz pure tones and oddball trials with a deviant tone (635 Hz) in the third location of the sequence, 200 ms after the stimulus onset (see the schematic presentation of the paradigm in Figure 2). Stimuli from the standard and oddball conditions were played in a mixed order where the number of oddball trials constituted 20% of all trials. The sound sequence duration was 550 ms, and the interval between the sequences (ITI) varied (0.5, 1, 1.5, 2, and 2.5 seconds) in separate runs. Participants completed a total of 200 trials for each of the 5 different ITIs in 2 short runs per ITI; each included 100 mixed standard and oddball trials.
Figure 2.
Schematic illustration of the trial sequence of study 2. Oddball trials with a deviant tone were interleaved within the standard trials with a ratio of 20/80. The inter-trial interval (ITI) was set as a second factor and varied in separate runs (500/1000/1500/2000/2500 ms).
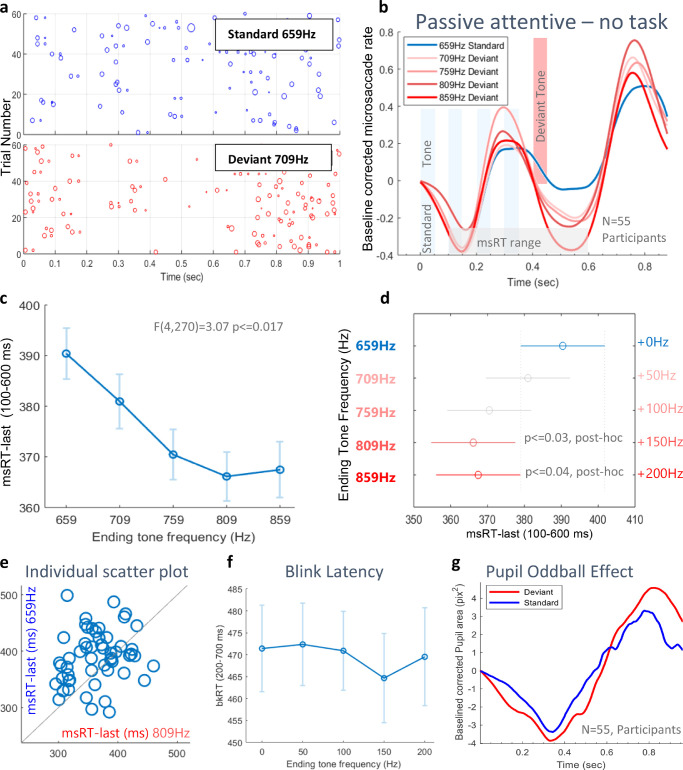
Figure 3.
“Passive attentive”: the deviance strength effect results. (a) An example of a microsaccade raster plot, with 60 epochs per condition from a single participant. Each row represents one epoch and each dot represents a microsaccade with the dot size proportional to the saccade's size. (b) Microsaccade rate modulation functions for all conditions, averaged across participants and baseline corrected. (c) The second inhibition onset estimated via msRT-last in the window (100–600 ms), denoted by the light gray block in b. The msRT values were calculated per participant, demeaned, and then averaged across participants (n = 55), with error bars denoting 1 SE across participants. (d) To determine whether msRTs in the standard condition (blue) differ from the deviant (red), 1-way ANOVA (F(4,270) = 3.07, p < = 0.017) and post hoc tests were performed. Two deviant means (809,859 Hz) significantly differ from the standard's mean; note that the bars do not overlap. The lower and upper limits of 95% confidence intervals are represented by the shortest and largest distance between the end points of the red and blue bars. (e) Scatter plot showing participants’ msRTs for the Standard condition (659 Hz) versus the deviant condition (809 Hz); note that most participants had a longer msRT for the standard condition (positioned above the symmetry line). (f) The blink latency, showing no difference between conditions. (g) A nonsignificant pupil area (camera pixels2), baseline corrected to the time of the stimulus onset, for all deviants combined, in red, compared with the standard in blue.
Reversed oddball control experiment
We also ran a reversed oddball control experiment, similar to the oddball experiment, but it involved new participants. We used a single optimal ITI of 1.5 seconds. Here, the deviant sequence (AABAAA) was played in 80% of all trials, and the standard sequence (AAAAAA) was rare; it was played in 20% of the trials.
The design of the two experiments was based on the idea of using a strong and predictable visual OMI (characterized by a typical robust “rebound” effect) that is modulated by an auditory stimulus, where this modulation depends only on the deviance strength or the oddball effect that we measured. The choice of temporal predictability was based on the preliminary experiments, which showed less consistent OMI effects when the timing was unpredictable, presumably due to the additional and irrelevant temporal uncertainty. In study 2, the deviant tone timing within the sequence was predictable for the reason explained above, but the appearance of the oddball sequence was unpredictable.
Data analysis
Microsaccade and blink detection
For the microsaccade detection, we used the algorithm introduced by Engbert and Kliegl (Engbert & Kliegl, 2003), which is based on eye movement velocity and has been implemented in our recent study (Yablonski et al., 2017). Raw data were first smoothed using the LOWESS method with a window of 15 ms to optimize microsaccade extraction, especially for noisy recordings (Engbert & Kliegl, 2003). Because microsaccades are ballistic movements as saccades, they show a high correlation between the peak velocity and the amplitude. A velocity range of 8 degrees/s to 150 degrees/s and an amplitude range of 0.08 to 1 degree were allowed. We also rejected eye movements with a duration smaller than 9 ms. Eye blinks were detected as in our previous study (Bonneh et al., 2016). We first defined periods with zero pupil size and then extended them by estimating the eyes’ closed and open times, based on the vertical eye movement that typically precedes the blink (Yablonski et al., 2017). The blink rate was calculated per session and condition as the percentage of epochs (equal in time for all conditions) containing a blink, averaged within participants, demeaned, and adjusted by adding the grand mean of all conditions and participants. Finally, the mean and standard error were recalculated across participants. In study 1, the grand mean was 17.2% of all trials, SD = 14.9%, and in study 2 it was 20.3%, SD = 17.9%. Epochs were extracted, triggered by the stimulus onset in a range of -0.1 seconds to 1.1 seconds relative to this trigger with one epoch per experimental trial. Periods of missing data within an epoch, for example, during an eye blink, were discarded from analysis with an additional margin of 50 ms, without discarding the whole epoch. The rejection rate varied across recordings and was typically 5% to 25%. Besides discarding the missing data points during blinks, we also verified that the blinks did not affect the OMI results in both experiments. For that purpose, we calculated the blink latencies (bkRT) to check for overlaps between the msRT and bkRT. Only in the first study was there overlap between the blink latencies and the time period used to calculate the microsaccade latencies; therefore, we verified that the blink rate and bkRT did not differ between conditions (Figure 3).
Microsaccade rate function calculation
The microsaccade rate modulation function was calculated to compute the event-related modulation of eye movements (Bonneh, Donner, Sagi, Fried, Cooperman, Heeger, & Arieli., 2010) as in Yablonski et al. (2017) and Rosenzweig & Bonneh (2019); it was calculated for the raw microsaccade onsets (see Figure 3a for an example of a raster plot of microsaccade occurrences), and it is described here briefly. For each epoch, the rate function was computed by convolving an assumed estimate of one microsaccade per sample duration (equivalent to 500/seconds for the 500 Hz sampling rate) with a Gaussian window and with a sigma of 50 ms at the time of the microsaccade onset. The rate functions were then averaged across the epochs within participants separately for each condition and demeaned by subtracting the participants’ mean and adjusted by adding the total average for all conditions and all the participants. Finally, the mean and standard error were recalculated across participants. Sometimes (specified in each condition) we used the baseline correction of the average rate modulation within participant and condition by subtracting the rate value at time zero (the stimulus onset) or subtracting the average value of the 100 ms before time zero.
Statistical assessment
To assess the significance of the microsaccade rate results, we used a Monte-Carlo cluster-based nonparametric permutation test (as in Widmann et al., 2014; see also Maris & Oostenveld, 2007) to determine the difference between conditions. We first looked for a significant continuous cluster between the two conditions by performing paired t-tests at each time point. Then, we randomized the condition labels of the participants’ means at each time point and recalculated the group averages to create 1000 permutation tests, then repeated the first step. We then computed the p value as the fraction of permutations in which the original test statistic was exceeded by the permuted data.
Microsaccade RT and pupil peak calculation
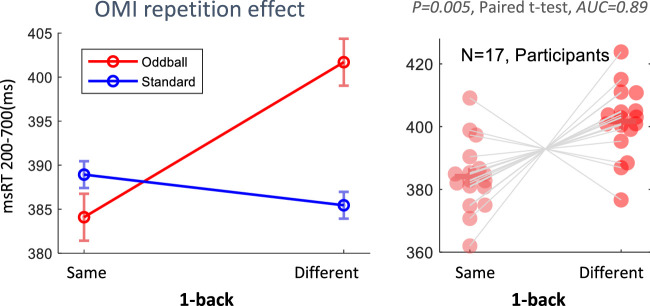
The msRT was calculated for each epoch relative to the stimulus onset in predefined time windows. The choice of the range was made to accommodate the variability between participants while focusing on the region of interest derived from the rate modulation functions (a region that shows a difference across conditions). To estimate the onset of the microsaccade inhibition, msRT-last was computed as the latency of the last microsaccade in the selected window. In study 1, an early window was chosen that starts a few hundred ms before the deviant stimulus onset because the microsaccade probability tends to decrease before this onset if the stimulus timing is predictable (Amit et al., 2019). In study 2, msRT-first was computed to estimate the onset of the release from the microsaccade inhibition (because the oddball effect prolongs the OMI) as the latency of the first microsaccade in a late window, as was done in our previous study (Bonneh et al., 2015). Epochs without microsaccades within the selected windows were excluded from the average, typically around 30% to 50%. The microsaccade RTs were averaged across the epochs of each condition within participants and demeaned by subtracting the participants’ mean, then averaged across participants, and, finally, the total average for all conditions and participants was added (Cousineau & Morey's method; Morey, 2008, see also Bonneh et al., 2015). This normalization procedure affected only the error bars and did not alter the averages. In computing the error bars for the RT values averaged across subjects, we applied the Cousineau method (multiplied by Morey's correction factor: √ (n/ (n−1)), which controls the between-subject variance and allows a better representation of within-subject effects (Cousineau & Morey's method; Morey, 2008, see also Bonneh et al. Bonneh et al., 2015). The pupil peak was detected for every epoch in a time window of 0 to 1 second relative to the stimulus onset for all conditions. It was converted to the percentage change in the pupil area from the average of a 100 ms period pre-stimulus onset. To test the effect of repetition on the standard and oddball trials in study 2, we conducted an additional analysis for the trial history and tagged trials with similar preceding stimuli as “same” and non-similar stimuli as “different” (see Figure 7).
Figure 7.
Oddball repetition effect. (a) OMI results for the standard (blue) and the oddball (red), comparing repeating stimuli (same) versus a change in stimulus (different). (b) Comparison between repeating (same, light red) and surprising (different, red) Oddball trials’ msRT, yielding a significant difference (p = 0.005, Paired t-test) and an area under the ROC curve yielding AUC = 0.89 (see the Methods). The results show a nonlocal effect of a significantly prolonged inhibition (20 ms) of a change (oddball after standard), compared with repetition (oddball after oddball).
Statistical assessment
Statistical analysis of variance (ANOVA) and multiple comparison post hoc tests were performed using MATLAB 2018b (1-way ANOVA in study 1 and 2-way ANOVA in study 2, the Tukey method). We first verified that the msRT or the pupil peak distributions of different conditions come from normal distributions with equal variance. For the msRT statistics in study 2, after acquiring the significance, we ran 2-tailed paired t-tests and applied the Bonferroni correction method for multiple comparisons. To assess the reliability of individual results, we performed a receiver operating characteristic (ROC) analysis, which shows the balance between the true and false positive rates, and we calculated the “area under the curve” (AUC), which is a popular tool for assessing the classification performance.
Results
Study 1
Passive-attentive experiment
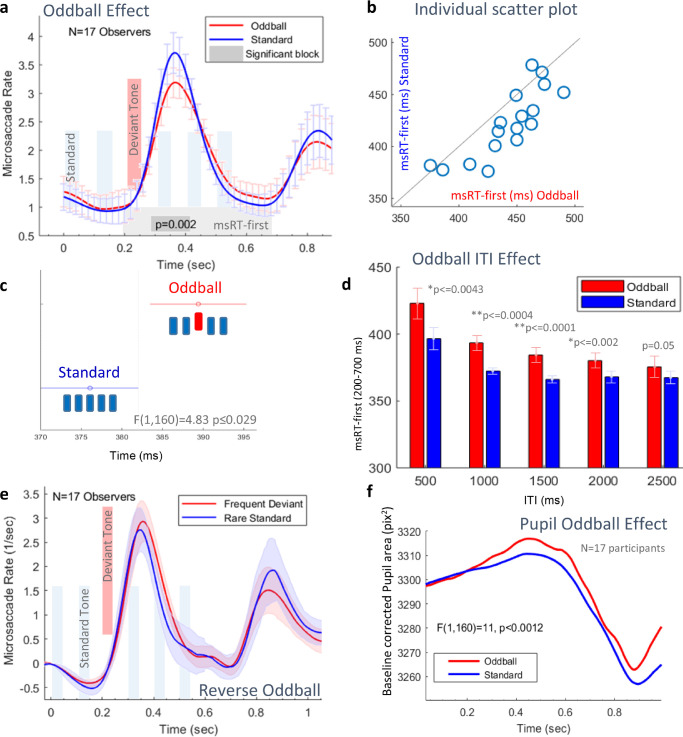
In this experiment, we investigated whether the OMI in the “passive attentive” paradigm depends on the magnitude of the auditory pitch deviance. We hypothesized two possible outcomes: (1) a prolonged OMI after the deviant tone as in previous oddball studies; or (2) early-onset of OMI even before the deviant tone, due to anticipation, resulting from the predictable block design. To test this, we extracted epochs triggered by stimulus onset in a range of -0.1 seconds to 1.1 seconds relative to this trigger. We first calculated the microsaccade rate modulation averaged across participants (N = 55), and the baseline corrected to the value at the stimulus onset time (see the Methods). We then compared the five conditions: a standard stimulus with five equal tones (659 Hz) and four deviant stimulus conditions, with a deviant tone in the fifth location and with different magnitudes (frequency differences) relative to the standard (659 + 50/100/150/200 Hz). Figure 3b shows a typical microsaccade inhibition and release in response to the combined visual and auditory, bimodal stimulus onset (the letters and the sound sequence) and a second inhibition in response to the stimulus offset, which describes the different conditions because it starts around the time of the deviant tone. The onset of this second inhibition is measured by the last microsaccade in a window of 100 ms and 600 ms after stimulus onset; it is termed “msRT-last” (see Figure 3c). This time window was selected by visually inspecting the microsaccade rate function and finding a region of interest that shows a difference across conditions. We found that a larger deviant produced a faster onset of inhibition (F(4,270) = 3.07, p < = 0.017), which confirms our second initial prediction. The msRT-first (release from inhibition), with a time range of 500 to 1000 ms, to account for a possible surprise effect, did not reach significance (F(4,270) = 0.27, p = 0.9) and contradicted our first expected outcome. The individual scatter plot in Figure 3e shows that most participants had longer microsaccade latencies in the standard condition, compared with the 809 Hz (+150 Hz) deviant condition, which was found to be significantly different in the post hoc tests (see Figure 3d). The blink latency (Figure 3f), measured in the time range of 200 to 700 ms, did not differ between conditions and therefore, it did not affect the msRT results. The pupil dilated more in the deviant conditions relative to the standard conditions (an insignificant effect, Figure 3g).
Control experiments
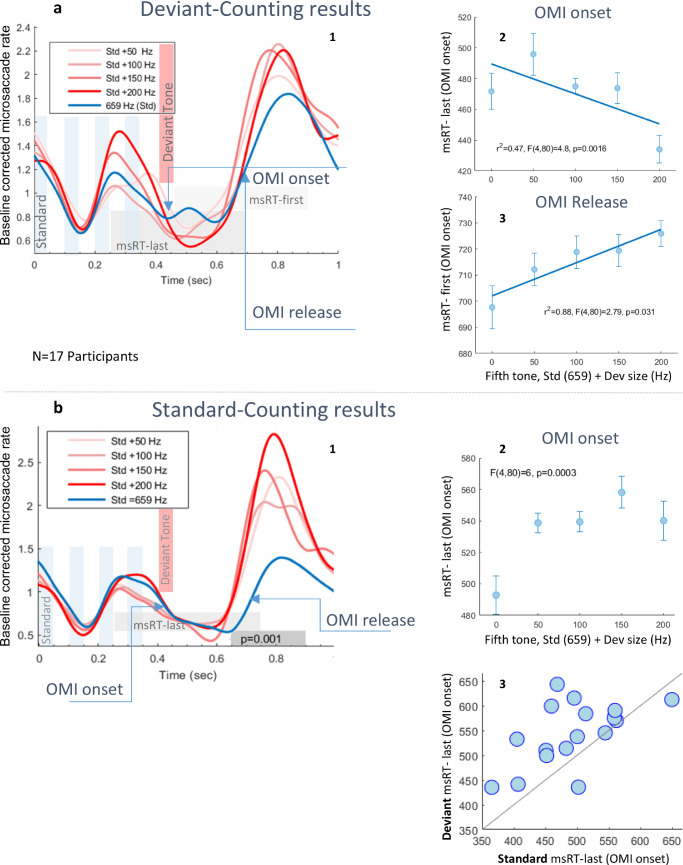
Figure 4 describes the results of the two additional “active attentive” control experiments. The results for the “deviant-counting” experiment are shown in Figure 4a. The microsaccade rate modulation plot (Figure 4a-1), averaged across participants (N = 17) and baseline corrected (see the Methods), shows a very similar pattern of inhibition as in the “passive-attentive” experiment (see Figure 3b), but with a stronger OMI in response to the deviant tone versus the standard tone. A faster inhibition onset for the larger deviance (Figure 4a-2) is shown via the latency of the last microsaccade (msRT-last) in a time window of 250 to 700 ms around the deviant onset, p = 0.0016, one-way ANOVA. The results also show longer inhibition for the deviants via the latency of the first microsaccade (msRT-first) in a time window of 450 to 900 ms around the deviant onset, p = 0.031, one-way ANOVA (Figure 4a-3), as in previous oddball studies (see also experiment 2 Figure 5) that indicates prolonged sustained attention with larger deviants (Pastukhov & Braun, 2010). Overall, these results are very similar to the results of the passive experiment, indicating that the participants were indeed attending, perhaps involuntarily, to the deviants in the passive experiment. The results for the “standard-counting” experiment, which we anticipated would reverse the OMI outcome, are shown in Figure 4b. Our purpose was to test whether the attended stimuli that are not necessarily oddballs induce prolonged inhibition (OMI). A microsaccade rate plot (Figure 4b-1), illustrating the time course of microsaccades in response to auditory standard and oddball stimuli, averaged across participants (N = 17), and baseline corrected (see the Methods) show, as hypothesized, a longer OMI in response to the standards. Significance was assessed using the Monte-Carlo permutation test (see the Methods), yielding a region having a significant difference (p = 0.001), around a time of 650 to 900 ms from the stimulus onset. This implies that previous findings of prolonged OMI for oddballs could result from the task (counting the oddballs). The latency of the last microsaccade (msRT-last) in a time window 250 to 700 ms, plotted for all the conditions, demeaned, and averaged across participants, shows a faster inhibition onset for the standards, compared with the deviant conditions. This is the opposite of the control experiment “deviant-counting” results (Figure 4b-2). The effect of attending the standard (via the counting instruction) is further demonstrated by a diagonal scatter plot on participants showing that all participants but two had an earlier OMI onset for the attended standard, reflected by a longer msRT-last in response to all the deviant conditions combined (Figure 4b-3).
Figure 4.
“Active attentive” control experiments. (a) Participants (N = 17) were instructed to count the appearance of a higher pitch sound (oddball). (a1) “Count the oddball” control experiment MS-rate plot, baseline corrected and averaged across participants; the auditory periods are denoted by faded bars. The OMI onset and release times are denoted by arrows. (a2,3) The latency of the last microsaccade (msRT-last) in a time window around the deviant onset (250–700 ms, denoted by the light gray block in 1). This denotes the OMI onset and the first microsaccade (msRT-first) in a late time window (450–900 ms, denoted by the light green block in 2), indicating the release from inhibition, plotted for all conditions, demeaned, and averaged across participants. The results are similar to those in the “passive-attentive” paradigm, but with a faster inhibition onset in response to the larger deviant tone (p = 0.0016, one-way ANOVA) and significantly prolonged inhibition for the deviant (p = 0.031, one-way ANOVA), because of the task, which did not reach significance in the original experiment. (b) “Standard-counting” control experiment. (b1) Microsaccade rate plot illustrating the time course of microsaccades in response to auditory standard (blue) and oddball (red) stimuli, averaged across participants. Significance was assessed using the Monte-Carlo permutation test (see the Methods), yielding a significantly different region (p = 0.001), around a time of 650 to 900 ms from the stimulus onset. The OMI onset and release times are denoted by arrows. (b2) The latency of the last microsaccade (msRT-last, denoted by the light gray block in 1) in a time window 250 to 750 ms, plotted for all conditions, demeaned, and averaged across participants. The results show a faster inhibition onset for the attended standards, compared with the deviant conditions, which are the opposite from the “deviant-counting” control experiment. (b3) Diagonal scatter plot (one symbol per participant), comparing OMI onset, via msRT-last, for the combined deviant conditions (Y-axis) versus the attended standard (X-axis). As shown, all participants but two had an earlier OMI onset for the attended standard.
Figure 5.
Results for the auditory oddball in different inter-trial intervals (ITIs; study 2). (a) The microsaccade rate modulation for the rare oddball (red), compared with the standard (blue), with all ITIs combined and averaged across participants (N = 17). A time segment with a statistically significant difference (in gray) was found between 300 and 420 ms after stimulus onset (p = 0.002, Monte-Carlo, nonparametric permutation test). The faded bars denote the sound sequence timing. (b) A diagonal scatter plot of individual participants’ msRT (first, denoted by the light gray block in a), for the oddball (X-axis) versus the standard (Y-axis) conditions, with all ITIs combined. Note the consistently faster (below the diagonal) msRT for the Standard. (c) Two-way ANOVA results for the oddball versus standard in all the ITIs combined. The lower and upper limits of 95% confidence intervals are represented by the shortest and largest distance between the endpoints of the red and blue bars. (d) The group average msRT (first) for the standard and oddball conditions for different ITIs (0.5–2.5 seconds). Error bars denote 1 SE across participants. Multiple comparisons were run using 2-tailed paired t-tests (Bonferroni correction was applied). A significant difference in msRT was found for the rare oddball effect in the short ITIs (ITI <2.5 seconds). In all conditions, the msRT was calculated in a time window of 200 to 700 ms post-stimulus onset. (e) Results of the reversed combination of deviant and standard tones. Microsaccade rate modulation for the frequent deviant (AABAAA) is in red and the rare Standard (AAAAAA) is in blue. (f) Pupil area (camera pixels2) for the oddball in red, compared with the standard in blue, baseline corrected to the time of the stimulus onset (F(1,160) = 11, p < 0.0012, two-way ANOVA).
Study 2
Oddball experiment
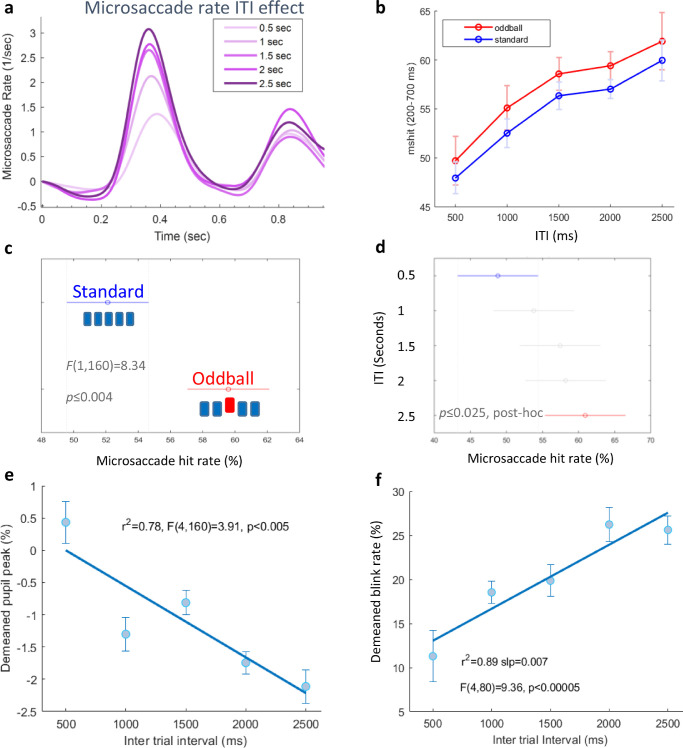
In the oddball experiment, we tested whether the time interval between the auditory sequences had an impact on the OMI oddball effect in a “passive attentive” paradigm. We estimated a decline in the oddball effect with longer temporal separation, because the sensory memory of the preceding item decays, and the oddball is defined by its relationship to the preceding items. The results are shown in Figures 5 and 6. Microsaccade rate modulations, to illustrate the oddball effect, averaged across participants with all ITIs combined (p ≤ 0.002, Monte-Carlo, permutation test; see the Methods), are shown in Figure 5a. As in experiment 1, the rate shows a typical response for stimulus onset and offset, but here, the first inhibition provides more information about the difference between the standard and oddball conditions, determined by the earlier onset of the deviant tone. Here, the visual stimulus was timed 50 ms before the oddball effect, to obtain a strong OMI at the oddball time (see the Methods). To quantify the OMI after stimulus onset for the different conditions, we computed “msRT-first,” which is calculated for the latency of the first microsaccade released from the inhibition in a window of 200 to 700 ms after stimulus onset. This time window was selected by visually inspecting the microsaccade rate function and estimating a time region that shows a difference across conditions (see the Methods for further details). Thirteen out of 17 participants had a longer msRT in the oddball condition (Figure 5b; The trials from all ITIs were combined). The msRT results were analyzed using 2-way ANOVA (Figure 5c). Oddball msRTs were significantly longer (F(1,160) = 4.83, p < = 0.029), but no interaction was found with the ITI factor. We used Bonferroni corrected, two-tailed paired t-tests to assess the significance for multiple comparisons of the standard and oddball conditions in each ITI separately. Figure 5d shows the standard and oddball conditions in all five different ITIs (0.5–2.5 seconds). The results show a significant difference between the standard and oddball msRTs in ITIs shorter than 2.5 sec. Pupil measurements showed an oddball effect (Figure 5f) as well as an ITI effect (Figure 6e). Significance was assessed using the pupil peak (% change in the pupil area), detected for every epoch in a time window of 0 to 1 second relative to stimulus onset, for all conditions (see the Methods). A 2-way ANOVA showed significant effects; F(4,160) = 3.91, p < 0.005 for the oddball factor, and F(1,160) = 11, p < 0.0012 for the ITI factor.
Figure 6.
The effect of the inter-trial interval (ITI). (a) Baseline-corrected microsaccade rate modulation for different ITIs, standard and oddball combined. (b) Percentage of trials with microsaccade (msHit) at a window of 200 to 700 ms after stimulus onset for all conditions. (c) Two-way ANOVA and post hoc tests results for the oddball versus standard in all the ITIs (0.5, 1, 1.5, 2, and 2.5 seconds) combined (F(1,160) = 8.34, p < = 0.004)). (d) Two-way ANOVA (F(4,160) = 2.62, p < = 0.037) and post hoc tests results showing a significant difference between the ITI = 0.5 seconds and ITI = 2.5 seconds conditions. (e) Pupil area peak (% change relative to the 100 ms pre-stimulus) measurements, demeaned and re-adjusted with the grand average (see the Methods), as a function of the inter-trial interval, showing a negative correlation (F(4,160) = 3.91, p < 0.005). (f) Blink rate (%), demeaned and readjusted with the grand average, showing a positive correlation with ITI (F(4,80) = 9.36, p < 0.00005).
Reverse-oddball experiment
We tested the reverse combination of standard and oddball and did not observe a significant oddball effect for the rare standard trials interleaved with the frequent deviant trials (Figure 5e), as we found for counting the standards in study 1 (Figure 4b). The reverse-oddball effect was tested only with the optimal 1.5 second ITI, which did not reach significance.
The results for the inter-trial interval effect are shown in Figure 6, showing higher microsaccade rates for longer ITIs. We then calculated the “microsaccade hit rate” (denoted here as “msHit”) corresponding to the percentage of trials with at least one microsaccade occurring within a time window of 200 to 700 ms after stimulus onset. We found that (1) oddball trials had significantly higher microsaccade hit rates at that window for all ITIs (Figure 6b,c; F(1,160) = 8.34, p ≤ 0.004) and that (2) longer ITIs also produced a significant increase in the rates (Figure 6b,d; F(4,160) = 2.62, p ≤ 0.037). The msHit results were very robust and did not depend at all on the time range. We got similar results using a longer time range of 300 to 1000 ms (1000 is the maximum for the short ITI, and 300 is the time of the deviant). A significant decrease in the pupil area (F(4,160) = 3.91, p < 0.005; Figure 6e) and an increase in the blink rates (F(4,80) = 9.36, p < 0.00005) was also observed with longer ITIs (Figure 6f). It was calculated at a 0 to 1 second time range for all conditions (see the Methods).
We also wanted to determine whether the OMI oddball effect was affected by the inter trial dynamics and confirm that it not only stems from the local deviance (see the Methods), as implied by the results of the reverse-oddball experiment, which did not show a significant difference. The results of this analysis are shown in Figure 7. Repeating the oddball trials indicated that msRT was similar to that of the standards; however, oddballs preceded by standards showed a significantly prolonged OMI, displaying a non-local change effect (see Figure 7a), p = 0.005 in a paired t-test, and the “area under the curve” of the ROC function (see the Methods), AUC = 0.89 (Figure 7b).
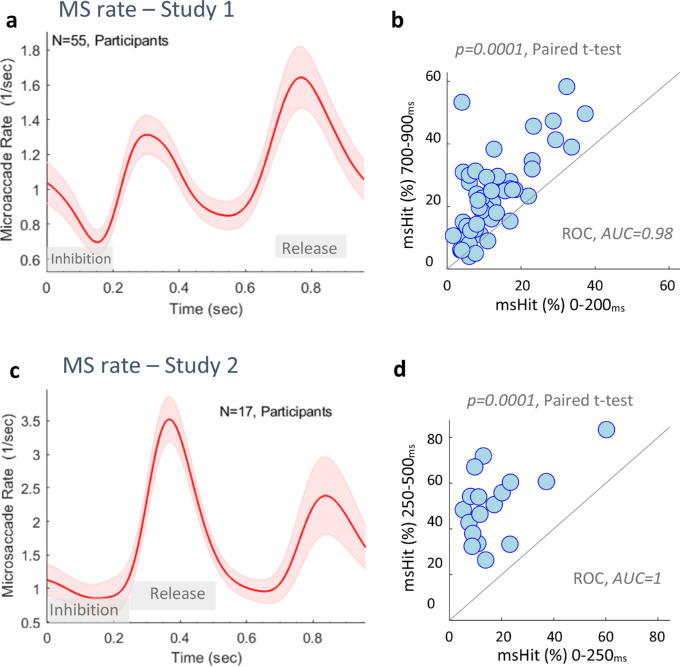
Finally, we tested the reliability of the OMI measures per individual in the passive experiments of both studies for possible future clinical applications to non-communicating individuals whose response to any external stimulus is uncertain. For that purpose, we assessed the reliability of the basic OMI effect across all conditions combined, regardless of the deviance. We compared the percentage of microsaccade occurrences (msHit) in the inhibition and release windows: 0 to 200 and 700 to 900 ms for study 1 (Figure 8a), and 0 to 250 and 250 to 500 ms for study 2 (Figure 8c). The results, shown in Figure 8, show a robust difference between the inhibition and release, which occurred in 51 of 55 participants (93%) in study 1 (AUC = 0.98; Figure 8b) and for all participants in study 2 (AUC = 1; Figure 8d).
Figure 8.
Individual OMI reliability. (a) Microsaccade rate for all study 1 conditions combined, averaged across participants, showing a double OMI pattern. The gray and green blocks denote the inhibition and release periods examined. (b) Participant scatter plot comparing the percentage of microsaccade occurrences (msHit) during the first (stronger) inhibition period (X-axis) and in the later (stronger) rebound period (Y-axis), demonstrating microsaccade inhibition across individuals, p = 0.0001, Paired t-test and AUC = 0.98. (c) Microsaccade rate for study 2, the same as (a). (d) Participant scatter plot like (b) for study 2, p = 0.0001, paired t-test and AUC = 1. The results show a very robust effect of microsaccade inhibition.
Discussion
In the current study, we conducted two main experiments with auditory pitch deviance and measured the involuntary OMI response. We found that when the deviant was frequent and therefore predictable, the microsaccade inhibition onset was faster as a function of the deviant size (higher pitch), and when it was rare, the inhibition was prolonged. This was achieved in a passive way, without the participants’ response. Moreover, when participants had to perform a counting task in our control experiments, the results indicate that attended stimuli induce prolonged inhibition regardless of the stimulus-specific properties. This raises the question whether previous studies indeed measured the predictability effects or rather, at least partially, the effect of voluntary attention towards a specific stimulus. Next, we will discuss the different aspects of these findings and their interpretation.
Auditory deviance and OMI: Salience versus surprise
Given that an auditory oddball effect was found to increase the period of saccadic inhibition (i.e. postpone its release), at least when a task was involved (Valsecchi & Turatto, 2009; Widmann et al., 2014), one would expect that this inhibition will be prolonged with larger deviance. On the other hand, because the OMI is also known to be faster and shorter with the saliency of the stimuli (e.g. for higher visual contrast; Bonneh et al., 2015), one would expect faster and shorter inhibition with a larger deviance that appears perceptually more salient.
In study 1 with the “passive attentive” paradigm, the stimulus was a repeated short sequence of five identical tones, or it contained a fifth deviant tone. The deviant varied between conditions, but there was no mix of trials from different conditions; thus, the deviant was frequent and entirely predictable. We found that the OMI was sensitive to auditory pitch deviance and was affected by its magnitude. In response to larger deviance, the OMI was faster (it started earlier), as evident by the difference in the rate modulation function (see Figure 3b) and by the earlier inhibition onset around the time of the deviant tone presentation (see Figure 3c). Because the deviant was predictable, the results resemble those of the OMI in response to visual contrast stimuli (Bonneh et al., 2015) that show a faster inhibition onset for higher contrast. Here, the effect of the deviant tone did not stem from an unpredictable change or surprise, but instead, from its contrast with the four preceding Standard tones, hence, the similarity to the effect of visual contrast. We therefore can conclude that the OMI measured in this experiment reflects the perceived stimulus saliency. Widmann et al. (2014) already found prolonged OMI for pitch as well as for location oddball. However, we show here, for the first time, that OMI depends on the pitch deviance magnitude, which can be interpreted here as a marker of saliency. Two additional “active attentive” control experiments, “deviant-counting” and “standard-counting,” indicate that voluntarily counting a specific stimulus biases attention toward that stimulus and therefore may prolong the inhibition (see Figure 4). In the “passive-attentive” experiment, there was no top-down bias, and the stimulus was predictable; therefore, the results are likely to reflect the change detection bottom-up processes in the auditory domain, biasing attention away from the common stimulus. Stimulus specific adaptation (SSA) reflects the habituation to a recurring stimulus, spanning several time scales ranging from milliseconds to tens of seconds (Ulanovsky, Las, Farkas, & Nelken, 2004). The mismatch negativity (MMN) is an ERP that is evoked when a train of “standard” stimuli is interrupted by an oddball or “deviant” stimulus that differs from the standards. It reflects the brains’ response to a sudden change in stimulus, peaking at about 150 ms (Giard, Perrin, Pernier, & Bouchet, 1990; Jääskeläinen, Ahveninen, Bonmassar, Dale, Ilmoniemi, Levänen, Lin, May, Melcher, Stufflebeam, Tiitinen, & Belliveau, 2004; Näätänen, Gaillard, & Mäntysalo, 1978). SSA and MMN are not entirely dissociated; they share a common origin in the auditory cortex; some studies suggest that SSA provides a neuronal correlate of MMN (May & Tiitinen, 2010; Nelken, Fishbach, Las, Ulanovsky, & Farkas, 2003). Based on these known processes, we considered the following explanation for our results: the early inhibition onset that preceded the stimulus offset indicates preparation and is associated with temporal anticipation due to the paradigm's design. The higher sensory saliency of the deviant sequence is caused by habituation of the repetitive reference tone (SSA) and a fresh response to the deviant tone (depending on its deviance; Yarden & Nelken, 2017), resulting in faster inhibition onsets. In addition, the mismatched deviant tone signals a prediction error relative to the size of the difference, leading to a better adjustment of a temporal model (Friston, 2005).
In study 2, we used an oddball paradigm, where an infrequent sequence of tones was presented randomly among 6 repeated identical tone sequences at a ratio of 20/80. As in study 1 (see Figure 3b), we observed a bimodal distribution of the microsaccade latencies (see Figure 5a), with the first inhibition triggered by the stimulus onset and the second by its disappearance. In the second study, we investigated the properties of the first OMI response, because it is modulated by the occurrence of the rare deviant tone right before the typical rebound of microsaccades, around 250 to 400 ms post-stimulus onset. We expected a prolonged OMI, along with a reduction in the effect as a function of the interval, because the strength of the formed global regularity depends on the second order temporal proximity (Sussman & Gumenyuk, 2005). The results showed that a rare auditory pattern induced a significantly prolonged OMI as in previous oddball studies (Valsecchi & Turatto, 2009; Widmann et al., 2014; Yuval-Greenberg & Deouell, 2011). With a long ITI of two and a half seconds, this effect became nonsignificant (p = 0.05, 2-tailed paired t-test; see Figure 5d), possibly due to the slow pace of the experiment and the lack of any attentive task, making participants less alert, which is discussed next. Interestingly, we observed a surprising increase in the microsaccade rate in the oddball condition (see Figures 6b, 6c). This might be related to the recent finding of an exploratory oculomotor search response following surprise signals by neurons in the supplementary eye field (SEF; Kawaguchi, Sakamoto, Saito, Furusawa, Tanji, Aoki, & Mushiake, 2015). It has also been suggested that some microsaccades in humans are exploratory (Shelchkova, Tang, & Poletti, 2019), suggesting that surprise might trigger exploration.
Our results of study 2 indicate a deviance response reflected by prolonged inhibition triggered by a violation of temporal expectations, as previously found (Amit et al., 2019). This is explicitly shown in Figure 7, with the serial dependency analysis of the preceding events, where an oddball sequence, preceded by a standard sequence (global change), induces a longer OMI, compared with an oddball sequence preceded by another oddball sequence (repetition). This result is consistent with previous findings of serial dependency effects in which previous stimuli were shown to bias the current processing (Kiyonaga, Scimeca, Bliss, & Whitney, 2017). Our results of shorter inhibition for repetition of the oddball are also consistent with a previous finding of a shorter inhibition for a more frequent visual target when its frequency was manipulated, 20%, 50%, and 80% of all trials (Valsecchi et al., 2009), because a higher frequency increases the incidence of repetition.
The oddball paradigm has been traditionally used for P300 ERP measures (Valsecchi et al., 2009), which are associated with attentional shifts and reflect the top-down response to violations of expectations and decision making (Duncan-Johnson & Donchin, 1977). It was also reported that microsaccade direction and its inhibition are related to spatial and temporal attention (Engbert & Kliegl, 2003; Kingstone & Klein, 1993; Meyberg, Sommer, & Dimigen, 2017; White & Rolfs, 2016). However, because the experiment did not involve a task, longer inhibition could therefore be associated with early change detection processes as well. Our “reverse-oddball” control experiment did not result in prolonged inhibition for the rare standards, unlike the study 1 “standard-counting” control results (see Figure 4b), suggesting less top-down intervention (see Figure 5e). Other studies suggest earlier categorization of sound. Widman et al. reported an early oculomotor marker of an auditory oddball, found at 80 to 100 ms after the deviant onset and derived from the microsaccade rate difference between targets and nontargets (Widmann et al., 2014); this is earlier than suggested by MMN ERP studies. Animal studies provide evidence for early and low-level mechanisms that could be related to the effects we have found. There is evidence for sound categorization in the rat inferior colliculus (IC; Malmierca, Niño-Aguillón, Nieto-Diego, Porteros, Pérez-González, & Escera, 2019) and also for inhibitory inputs from the external nucleus of the IC to the SC that can affect eye movements (Appell & Behan, 1990).
Comparison with previous oddball studies
Previous OMI studies of auditory oddballs (Valsecchi & Turatto, 2009) involved a task that could have influenced the results by generating an attention effect. For example, our preliminary results from a serial dependency study, in which participants were asked to count a colored patch from a group of red and green patches, showed a significantly longer OMI for the attended stimuli (Bonneh et al., 2013). A task such as counting the oddballs (Valsecchi & Turatto, 2009) involves additional processing time to hold the current number of oddballs within working memory (WM; Dalmaso, Castelli, Scatturin, & Galfano, 2017) and to make a decision involving target discrimination. Thus, it could have prolonged the saccadic inhibition for targets regardless of whether or not these targets were oddballs.
In our study, we performed experiments that did not involve a task. Instead, the participants were asked to attend to all sounds without any request to pay specific attention to the oddballs. We also performed additional control experiments that involved counting a specific stimulus. Our “active attentive” control experiments indicate that attended stimuli induce prolonged inhibition regardless of the stimulus-specific properties. When participants were asked to count the oddballs, prolonged OMI was found for the oddballs (see Figure 4a), in addition to a faster OMI onset, also shown in passive viewing (see Figure 3c). This might result from the counting task. Although mixing the trials was the other factor that changed, the “oddballs” constituted 80% of all trials; thus, they were highly predictable and were unlikely to cause a surprise effect. Our “reverse-oddball” passive control experiment did not result in prolonged inhibition for the rare standards (see Figure 5e), and together with the finding of prolonged OMI for the standards (see Figure 4b), it suggests that the active counting task is the main factor contributing to suppress these saccadic movements.
Unlike previous ERP studies that investigated the MMN, we did not aim to bias attention away from the auditory stimuli by engaging the participants in a visual task. We instructed them to pay attention to the sounds (see the Methods for the specific instruction), but there was no control over their attention. We show here, for the first time, the OMI effects for auditory oddballs in a passive attentive paradigm (see Bekinschtein et al., 2009; Quirins et al., 2018; Silverstein et al., 2015 for a similar passive-attentive paradigm). However, these OMI effects appear smaller than those obtained with attended stimuli (Valsecchi & Turatto, 2009) or in response to visual stimuli as a function of contrast (Bonneh et al., 2015).
Our study implements an auditory oddball paradigm similar to that of Bekinschtein et al. (Bekinschtein et al., 2009), which measured the ERP markers of violations of auditory regularities, either “local” in time, within a single trial (similar to our study 1), or “global” across trials of several seconds (similar to our study 2). Their local-global paradigm suggests the existence of a hierarchical organization consisting of at least two levels of perceptual prediction mechanisms: (1) an early mechanism, reflected in the MMN signal, which is effective only in a limited time window for changes that are “local” in time (Pegado, Bekinschtein, Chausson, Dehaene, Cohen, & Naccache, 2010), and (2) a later, more distributed predictive mechanism, reflected by P3b (a subcomponent of P300) response to more “global” violations of expectations (Wacongne et al., 2011). They report a global effect as a marker of awareness for a rare auditory pattern with an ITI of approximately 1.5 seconds, measured by P3b when participants were asked to count the oddballs. In contrast, when participants were engaged in mind-wandering or in an active visual target detection of letters, the P3b magnitude for the surprise sounds decreased dramatically (Bekinschtein et al., 2009). Thus, it follows that, in this study, the P3b signal could have resulted from counting rather than as a marker of predictive violation, because P3b is also related to context updating and is associated with memory operations (Polich, 2007), such as holding the number of oddball occurrences in working memory. When we compared our results to this ERP study (Bekinschtein et al., 2009), we found both similarities and differences. Unlike Bekinschtein et al., we did not find an OMI effect for the reversed combination of a global standard (AABAAA) and a global deviant (AAAAAA) without a counting task, which implies a strong contribution of early mechanisms to the oddball OMI effect (see Figure 5e). Our participants reported being aware of the oddballs when asked after the experiment; however, their level of engagement and its contribution to the OMI is unknown. It is therefore impossible to distinguish between the contribution of an automatic change detection process and a higher-level predictive mechanism.
The effect of inter trial intervals on the oculomotor response
We observed a significant increase in the microsaccade and blink rates as a function of ITI (see Figure 6). This could be explained by reduced alertness due to a lower stimulus rate, resulting in reduced inhibition in the longer ITIs. Such an explanation is supported by the results of the pupil response (see Figure 6e). Alertness induced more pupil dilation (Gabay, Pertzov, & Henik, 2011; Joshi, Li, Kalwani, & Gold, 2016) in response to oddballs (Preuschoff, 't Hart, & Einhäuser, 2011). Figure 5f illustrates the pupil response to standard and oddball sequences, showing an oddball effect as previously found by Quirins et al. (2018). The pupil first dilated and then constricted after stimulus presentation. We found that the relative constriction was negatively correlated with the ITI (see Figure 6e), possibly reflecting a stronger dilation effect for the short intervals, which counters the constriction due to increased alertness. The pupil response and blink rates were calculated within a similar time range for all ITIs. The link between alertness and microsaccade inhibition was demonstrated, for example, in the finding of reduced inhibition in attention deficit hyperactivity disorder (ADHD) in a continuous performance task, which was recovered by administering a stimulating medication (Fried et al., 2014). It was also reported that higher attentional loads, as in the shorter ITIs with increased alertness, are associated with a lower microsaccade rate (Pastukhov & Braun, 2010). An alternative explanation may be related to the microsaccade preparation time; less time to prepare with shorter intervals results in fewer microsaccades.
Finally, as one of the purposes of this study was to develop involuntary oculomotor measures to test non-communicating individuals, we tested the reliability of the OMI itself as a marker of stimulus reactivity per individual. The results indicated that basic OMI is very reliable (see Figure 8) and the overall OMI pattern across the current experiments, as well as previous studies (Hicheur, Zozor, Campagne, & Chauvin, 2013; Rolfs et al., 2008; Rosenzweig & Bonneh, 2019; Widmann et al., 2014; Yablonski et al., 2017) suggest that its length is related to the cortical processing time of the stimulus. Moreover, we demonstrated that the attended item prolongs the OMI even more than a sensory oddball (see Figure 4), which suggests that the OMI could be used for communication by attending one item in a multiple-choice sequence.
Summary and conclusions
In measuring OMI for microsaccades in response to auditory deviant tones passively, we found a shorter inhibition onset in proportion to the pitch deviance (the local saliency effect), and a longer inhibition release latency for rare deviants (the global effect), as long as the inter-trail interval was shorter than 2.5 seconds. Longer ITIs induced higher microsaccade and blink rates, together with smaller pupil dilation, indicating reduced alertness. We also found increased pupil dilation for the rare deviant tone, as previously reported. These results were obtained with a passive attentive paradigm and without an active task to direct attention to or away from the deviant sound sequences. We found that even under these conditions, the basic OMI effect was robust and was found in almost all participants. With a counting task, we found a similar saliency effect and prolonged OMI to the attended stimuli, standards, or deviants, regardless of their predictability. This stands in contrast to previous oddball studies. Thus, the use of involuntary ocular measures for assessing saliency and deviance could serve as a valuable tool in cognitive assessment and rehabilitation, especially for unresponsive individuals.
Acknowledgments
Author Contributions: O.K. and Y.S.B. designed the experiments. O.K. collected the data. O.K. and Y.S.B. developed the software used for running the experiments and the data analysis. O.K. analyzed the data and wrote the manuscript. Y.S.B. reviewed the manuscript.
Data Availability: The experimental datasets generated during the current study will be available from the corresponding author upon reasonable request.
Commercial relationships: none.
Corresponding author: Yoram S. Bonneh.
Email: yoram.bonneh@gmail.com.
Address: School of Optometry and Vision Science, Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan 5290002, Israel.
References
- Amit, R., Abeles, D., Carrasco, M., & Yuval-Greenberg, S. (2019). Oculomotor inhibition reflects temporal expectations. NeuroImage, 184(March 2018), 279–292. [DOI] [PubMed] [Google Scholar]
- Appell, P. P., & Behan, M. (1990). Sources of subcortical GABAergic projections to the superior colliculus in the cat. Journal of Comparative Neurology, 302(1), 143–158. [DOI] [PubMed] [Google Scholar]
- Bekinschtein, T. A., Dehaene, S., Rohaut, B., Tadel, F., Cohen, L., & Naccache, L. (2009). Neural signature of the conscious processing of auditory regularities. Proceedings of the National Academy of Sciences, 106(5), 1672–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneh, Y., Adini, Y., Sagi, D., Tsodyks, M., Fried, M., & Arieli, A. (2013). Microsaccade latency uncovers stimulus predictability: Faster and longer inhibition for unpredicted stimuli. Journal of Vision, 13(9), 1342–1342. [Google Scholar]
- Bonneh, Y. S., Adini, Y., & Polat, U. (2015). Contrast sensitivity revealed by microsaccades. Journal of Vision, 15(9), 11. [DOI] [PubMed] [Google Scholar]
- Bonneh, Y. S., Adini, Y., & Polat, U. (2016). Contrast sensitivity revealed by spontaneous eyeblinks: Evidence for a common mechanism of oculomotor inhibition. Journal of Vision, 16(7), 1. [DOI] [PubMed] [Google Scholar]
- Bonneh, Y. S., Donner, T. H., Sagi, D., Fried, M., Cooperman, A., Heeger, D. J., & Arieli, A. (2010). Motion-induced blindness and microsaccades: cause and effect. Journal of Vision, 10(14), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneh, Y., Polat, U., & Adini, Y. (2016). The buildup of temporal anticipation revealed by microsaccades and eye-blinks. Journal of Vision, 16(12), 935. [Google Scholar]
- Bubic, A., von Cramon, D. Y., & Schubotz, R. I. (2010). Prediction, cognition and the brain. Frontiers in Human Neuroscience, 4(March), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmaso, M., Castelli, L., Scatturin, P., & Galfano, G. (2017). Working memory load modulates microsaccadic rate. Journal of Vision, 17(3), 6. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson, C. C., & Donchin, E. (1977). On quantifying surprise: the variation of event-related potentials with subjective probability. Psychophysiology, 14, 456–467. [DOI] [PubMed] [Google Scholar]
- Engbert, R. (2006). Chapter 9 Microsaccades: a microcosm for research on oculomotor control, attention, and visual perception. Progress in Brain Research, 154(Suppl. A), 177–192. [DOI] [PubMed] [Google Scholar]
- Engbert, R., & Kliegl, R. (2003). Microsaccades uncover the orientation of covert attention. Vision Research, 43(9), 1035–1045. [DOI] [PubMed] [Google Scholar]
- Fried, M., Tsitsiashvili, E., Bonneh, Y. S., Sterkin, A., Wygnanski-Jaffe, T., Epstein, T., & Polat, U. (2014). ADHD subjects fail to suppress eye blinks and microsaccades while anticipating visual stimuli but recover with medication. Vision Research, 101, 62–72. [DOI] [PubMed] [Google Scholar]
- Friston, K. (2005). A theory of cortical responses. Philosophical Transactions of the Royal Society B: Biological Sciences, 360(1456), 815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay, S., Pertzov, Y., & Henik, A. (2011). Orienting of attention, pupil size, and the norepinephrine system. Attention, Perception, and Psychophysics, 73(1), 123–129. [DOI] [PubMed] [Google Scholar]
- Giard, M. -H, Perrin, F., Pernier, J., & Bouchet, P. (1990). Brain Generators Implicated in the Processing of Auditory Stimulus Deviance: A Topographic Event-Related Potential Study. Psychophysiology, 27, 627–640. [DOI] [PubMed] [Google Scholar]
- Hafed, Z. M., Goffart, L., & Krauzlis, R. J. (2009). A neural mechanism for microsaccade generation in the primate superior colliculus. Science (New York, N.Y.), 323(5916), 940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed, Z. M., & Krauzlis, R. J. (2008). Goal representations dominate superior colliculus activity during extrafoveal tracking. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 28(38), 9426–9439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicheur, H., Zozor, S., Campagne, A., & Chauvin, A. (2013). Microsaccades are modulated by both attentional demands of a visual discrimination task and background noise. Journal of Vision, 13(13), 1–20. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen, I. P., Ahveninen, J., Bonmassar, G., Dale, A. M., Ilmoniemi, R. J., Levänen, S., et al. (2004). Human posterior auditory cortex gates novel sounds to consciousness. Proceedings of the National Academy of Sciences of the United States of America, 101(17), 6809–6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, S., Li, Y., Kalwani, R. M., & Gold, J. I. (2016). Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex Article Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron, 89(1), 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi, N., Sakamoto, K., Saito, N., Furusawa, Y., Tanji, J., Aoki, M., & Mushiake, H. (2015). Surprise signals in the supplementary eye field : rectified prediction errors drive exploration-exploitation transitions. Journal of Neurophysiology, 113(3), 1001–1014. [DOI] [PubMed] [Google Scholar]
- Kingstone, A., & Klein, R. M. (1993). Visual offsets facilitate saccadic latency: Does predisengagement of visuospatial attention mediate this gap effect? Journal of Experimental Psychology: Human Perception and Performance, 19(6), 1251–1265, 10.1037//0096-1523.19.6.1251 [DOI] [PubMed] [Google Scholar]
- Kiyonaga, A., Scimeca, J. M., Bliss, D. P., & Whitney, D. (2017). Serial Dependence across Perception, Attention, and Memory. Trends in Cognitive Sciences, 21(7), 493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca, M. S., Niño-Aguillón, B. E., Nieto-Diego, J., Porteros, Á., Pérez-González, D., & Escera, C. (2019). Pattern-sensitive neurons reveal encoding of complex auditory regularities in the rat inferior colliculus. NeuroImage, 184(October 2018), 889–900. [DOI] [PubMed] [Google Scholar]
- Maris, E., & Oostenveld, R. (2007). Nonparametric statistical testing of EEG- and MEG-data. Journal of Neuroscience Methods, 164(1), 177–190. [DOI] [PubMed] [Google Scholar]
- May, P. J. C., & Tiitinen, H. (2010). Mismatch negativity (MMN), the deviance-elicited auditory deflection, explained. Psychophysiology, 47(1), 66–122. [DOI] [PubMed] [Google Scholar]
- Meyberg, S., Sinn, P., Engbert, R., & Sommer, W. (2017). Revising the link between microsaccades and the spatial cueing of voluntary attention. Vision Research, 133, 47–60. [DOI] [PubMed] [Google Scholar]
- Meyberg, S., Sommer, W., & Dimigen, O. (2017). How microsaccades relate to lateralized ERP components of spatial attention: A co-registration study. Neuropsychologia, 99, 64–80. [DOI] [PubMed] [Google Scholar]
- Morey, R. D. (2008). Confidence Intervals from Normalized Data: A correction to Cousineau (2005). Tutorials in Quantitative Methods for Psychology, 4(2), 61–64. [Google Scholar]
- Näätänen, R., Gaillard, A. W. K., & Mäntysalo, S. (1978). Early selective-attention effect on evoked potential reinterpreted. Acta Psychologica, 42(4), 313–329. [DOI] [PubMed] [Google Scholar]
- Nelken, I., Fishbach, A., Las, L., Ulanovsky, N., & Farkas, D. (2003). Primary auditory cortex of cats: Feature detection or something else? Biological Cybernetics, 89(5), 397–406. [DOI] [PubMed] [Google Scholar]
- Pastukhov, A., & Braun, J. (2010). Rare but precious: Microsaccades are highly informative about attentional allocation. Vision Research, 50(12), 1173–1184. [DOI] [PubMed] [Google Scholar]
- Pegado, F., Bekinschtein, T., Chausson, N., Dehaene, S., Cohen, L., & Naccache, L. (2010). Neuropsychologia Probing the lifetimes of auditory novelty detection processes. Neuropsychologia, 48(10), 3145–3154. [DOI] [PubMed] [Google Scholar]
- Polich, J. (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology, 118(10), 2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff, K., 't Hart, B. M., & Einhäuser, W. (2011). Pupil dilation signals surprise: Evidence for noradrenaline's role in decision making. Frontiers in Neuroscience, 5(Sep), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirins, M., Marois, C., Valente, M., Seassau, M., Weiss, N., El Karoui, I., et al. (2018). Conscious processing of auditory regularities induces a pupil dilation. Scientific Reports, 8(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfs, M. (2009). Microsaccades: Small steps on a long way. Vision Research, 49(20), 2415–2441. [DOI] [PubMed] [Google Scholar]
- Rolfs, M., Kliegl, R., & Engbert, R. (2008). Toward a model of microsaccade generation: The case of microsaccadic inhibition. Journal of Vision, 8(11), 1–23. [DOI] [PubMed] [Google Scholar]
- Rosenzweig, G., & Bonneh, Y. S. (2019). Familiarity revealed by involuntary eye movements on the fringe of awareness. Scientific Reports, 9(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelchkova, N., Tang, C., & Poletti, M. (2019). Task-driven visual exploration at the foveal scale. Proceedings of the National Academy of Sciences of the United States of America, 116(12), 5811–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein, B. H., Snodgrass, M., Shevrin, H., & Kushwaha, R. (2015). P3b, consciousness, and complex unconscious processing. Cortex, 73, 216–227. [DOI] [PubMed] [Google Scholar]
- Sussman, E. S., & Gumenyuk, V. (2005). Organization of sequential sounds in auditory memory. NeuroReport, 16(13), 1519–1523. [DOI] [PubMed] [Google Scholar]
- Ulanovsky, N., Las, L., Farkas, D., & Nelken, I. (2004). Multiple Time Scales of Adaptation in Auditory Cortex Neurons. Journal of Neuroscience, 24(46), 10440–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsecchi, M., Betta, E., & Turatto, M. (2007). Visual oddballs induce prolonged microsaccadic inhibition. Experimental Brain Research, 177(2), 196–208. [DOI] [PubMed] [Google Scholar]
- Valsecchi, M., Dimigen, O., Kliegl, R., Sommer, W., & Turatto, M. (2009). Microsaccadic inhibition and P300 enhancement in a visual oddball task. Psychophysiology, 46(3), 635–644. [DOI] [PubMed] [Google Scholar]
- Valsecchi, M., & Turatto, M. (2009). Microsaccadic responses in a bimodal oddball task. Psychological Research, 73(1), 23–33. [DOI] [PubMed] [Google Scholar]
- Wacongne, C., Labyt, E., van Wassenhove, V., Bekinschtein, T., Naccache, L., & Dehaene, S. (2011). Evidence for a hierarchy of predictions and prediction errors in human cortex. Proceedings of the National Academy of Sciences, 108(51), 20754–20759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, A. L., & Rolfs, M. (2016). Oculomotor inhibition covaries with conscious detection. Journal of Neurophysiology, 116(3), 1507–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann, A., Engbert, R., & Schroger, E. (2014). Microsaccadic Responses Indicate Fast Categorization of Sounds: A Novel Approach to Study Auditory Cognition. Journal of Neuroscience, 34(33), 11152–11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeke, K. F., Tian, X., Buonocore, A., Bellet, J., Ramirez-Cardenas, A., & Hafed, Z. M. (2019). Memory-guided microsaccades. Nature Communications, 10(1), 3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler, I., Denham, S. L., & Nelken, I. (2009). Modeling the auditory scene: predictive regularity representations and perceptual objects. Trends in Cognitive Sciences, 13(12), 532–540. [DOI] [PubMed] [Google Scholar]
- Yablonski, M., Polat, U., Bonneh, Y. S. Y. S., & Ben-Shachar, M. (2017). Microsaccades are sensitive to word structure: A novel approach to study language processing. Scientific Reports, 7(1), 3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden, T. S., & Nelken, I. (2017). Stimulus-specific adaptation in a recurrent network model of primary auditory cortex. PLoS Computational Biology, 13, e1005437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuval-Greenberg, S., & Deouell, L. Y. (2011). Scalp-recorded induced gamma-band responses to auditory stimulation and its correlations with saccadic muscle-activity. Brain Topography, 24(1), 30–39. [DOI] [PubMed] [Google Scholar]
- Zhao, S., Yum, N. W., Benjamin, L., Benhamou, E., Yoneya, M., Furukawa, S., … Chait, M. (2019). Rapid Ocular Responses Are Modulated by Bottom-up-Driven Auditory Salience. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 39(39), 7703–7714. [DOI] [PMC free article] [PubMed] [Google Scholar]