Abstract
Purpose
To investigate the clinical outcomes in small incision lenticule extraction (SMILE) and EVO implantable Collamer lens (ICL)–treated high myopia.
Methods
Thirty-three SMILE-treated and 32 EVO ICL-treated patients were included and followed up for 6 months. Subjective refraction, contrast sensitivity, and disk halo size were measured preoperatively and postoperatively. Patient-reported outcomes (PROs) were obtained at the final visit.
Results
Significant differences in efficacy and safety indices were observed between the SMILE and EVO ICL groups at 6 months postoperatively (P < 0.05). In the SMILE group, the mesopic contrast sensitivity at 2.2 cycles per degree (cpd) and photopic contrast sensitivity at 0.5, 3.4, and 7.1 cpd were significantly improved. In the EVO ICL group, the mesopic contrast sensitivity at 7.1 cpd and photopic contrast sensitivity at 0.5, 7.1, and 14.6 cpd were significantly improved. The halo radii after SMILE were significantly increased at 1 week, showed a decreasing trend at 1 month, returned to baseline at 3 months, and progressed stably at 6 months. However, it was unchanged in the EVO ICL group. Regarding subjective experience, haloes were the most common disturbance with mild and little bothersomeness after EVO ICL in contrast to starbursts after SMILE.
Conclusions
EVO ICL implantation yielded better visual outcomes, improved contrast sensitivity particularly at high spatial frequencies, had a stabler disk halo size, and increased incidence of haloes, with less influence than that of SMILE.
Translational Relevance
The disk halo and PRO findings will be of benefit for consultations and evaluations in visual performance and disturbances.
Keywords: SMILE, EVO ICL, high myopia, contrast sensitivity, disk halo
Introduction
Glare disability, induced by intraocular forward light scattering, decreases contrast sensitivity and deteriorates the resolution of foveal images when a veiling light is formed on the retina.1 Glare disability is not only related to lighting conditions but is also affected by the ocular pathophysiologic status.1 Patients who undergo refractive surgery may experience glare or haloes postoperatively, which affect night driving and patient satisfaction.2,3
Regarding surgical myopia correction, femtosecond laser small incision corneal lens extraction (SMILE) and implantable Collamer lens (ICL) implantation have become increasingly popular, and postoperative visual quality has received much concern.4–7 The clinical outcomes of SMILE and ICL, especially for patients with high myopia, have been compared in several studies,8–12 and the measurements were in favor of aberrometer, optical quality analysis system, or the quality of vision (QoV) questionnaires. None of those previous observations incorporated QoV instruments and contrast sensitivity to assess the visual disturbances and functions perceived by the patients. A device allowing the simulation of glare via an induced light source and calculation of the visual angle covered by the disk halo was recently developed by Metrovision in France13 and has been applied in patients with myopia in the first 3 months after SMILE14 and V4c ICL.15 The results showed that disk halo size had returned to baseline after both procedures. However, no studies have compared the longitudinal variations of disk halo size between the procedures to our knowledge. Apart from this concern, the connection between disk halo measurement and the subjective patient experience is poorly unknown. This data would be valuable for preoperative consultations and postoperative evaluations.
Therefore, this study aimed to comprehensively investigate the clinical outcomes, including refractive outcomes, contrast sensitivity, and disk halo size, and incorporated patient-reported outcomes in SMILE and EVO ICL-treated high myopia.
Methods
Patients
This prospective, nonrandomized, controlled study was approved by the Ethics Committee of the Eye, Ear, Nose, and Throat (EENT) Hospital of Fudan University (No. 2016038) and followed the principles of the Declaration of Helsinki. All patients provided informed consent concerning participation and academic publication before the surgery.
Patients with high myopia or myopic astigmatism with a corrected distance visual acuity (CDVA) ≥16/20 and stable refractive status (annual change in myopia, ≤0.50 D) were enrolled in this study. The residual stromal bed thickness was ≥280 µm in patients who underwent SMILE, whereas in patients who underwent EVO ICL implantation, the preoperative corneal endothelial cell count was >2000/mm2, the anterior chamber depth was ≥2.8 mm, and the white-to-white diameter was >11 mm. Patients who wore soft contact lenses stopped wearing them for at least 1 week prior to surgery, and those who wore rigid gas-permeable contact lenses stopped wearing them for at least 1 month prior to surgery. Patients with ocular diseases, a history of ocular trauma or surgery, or systemic diseases were excluded from the study.
Sixty-six patients (10 men and 56 women; 66 eyes) who underwent refractive surgery at the EENT hospital of Fudan University between July 2020 and March 2021 were recruited in this study. Routine preoperative examinations were performed for all patients, including slit-lamp microscopy, intraocular pressure, dark pupil size, axial length, central corneal thickness, corneal topography (Pentacam HR; Oculus Optikgeräte, GmbH, Germany), wavefront aberration (OPD-Scan III; Nidek, Tokyo, Japan), cycloplegic refraction, fundoscopy examinations, contrast sensitivity, and disk halo size measurements (MonPack One; Metrovision, Pérenchies, France). A corneal endothelial cell count, macular optical coherence tomography, and ultrasound biomicroscopy were also performed for patients undergoing EVO ICL implantation. In this study, selection of the type of surgery for patients was based on the surgeon's references (e.g., corneal thickness or corneal topography).
Surgical Techniques
SMILE was performed as previously described8 using a 500-kHz VisuMax platform (Carl Zeiss Meditec AG, Jena, Germany). The cap diameter was set to 7.5 mm, cap thickness to 120 µm, lenticule diameter to 6.1 to 6.8 mm, and the side cut length to 2 mm. The incision was made at the 12-o'clock position, and the lenticule was separated and extracted after femtosecond laser scanning was completed. After surgery, all patients were routinely prescribed 0.5% levofloxacin eye drops (four times per day for 7 days), 0.1% fluorometholone eye drops (eight times per day, tapered over 24 days), and artificial tears (four times per day for 3 months).
The EVO ICL surgery was conducted as previously described10 by the same surgeon (XZ). The EVO ICL was injected through a 3-mm incision near the temporal side of the limbus, followed by a small amount of viscoelastic agent injected into the anterior chamber to protect the surface of the EVO ICL. Then, the EVO ICL was adjusted into the posterior chamber, and the viscoelastic agent was flushed out of the anterior chamber using lactated Ringer's solution. After surgery, the patient's intraocular pressure was monitored for 2 to 4 hours, and topical anti-inflammatory and infection prevention regimens were prescribed, including 1% prednisolone acetate ophthalmic suspension (Allergan Pharmaceuticals, Dublin, Ireland; four times per day for 3 days), pranoprofen eye drops (Senju Pharmaceutical Co., Ltd., OSAKA, Japan; four times per day for 14 days), moxifloxacin hydrochloride eye drops (Alcon Research LLC, Fort Worth, Texas, USA, Novartis; four times per day for 7 days), and artificial tears (four times per day for 1 to 3 months).
Follow-up
Follow-up visits were scheduled at 1 day, 1 week, 1 month, 3 months, and 6 months postoperatively. Slit-lamp microscopy, uncorrected distance visual acuity (UDVA), intraocular pressure, subjective refraction, contrast sensitivity (CS), and disk halo size measurements were conducted at each visit. Besides, the vaults and endothelial cells were monitored regularly for EVO ICL-treated patients. One eye of each patient was selected for monocular examinations for statistical purposes. Patient-reported outcomes were also obtained at the final follow-up visit.
Contrast Sensitivity Tests
The mesopic (0.08 cd/m2) and photopic (80 cd/m2) CS were tested monocularly at 2 m using MonPack One (Metrovision) at low (0.5 and 1.1 cycles per degree [cpd]), intermediate (2.2 and 3.4 cpd), and high (7.1 and 14.6 cpd) spatial frequencies, as previously described.16
Disk Halo Size Measurements
The disk halo size measurements were conducted similarly to those described previously.13–16 White LED lights with a brightness of 200,000 cd/m2 on one side of the monitor was used as a glare-inducing light source. The halo size was measured monocularly at 2.5 m from the monitor when the ipsilateral light shined. The optotype luminance was set to 1 cd/m2 and 5 cd/m2 sequentially, and the patient was required to successively read out the three-radical line of optotypes from the opposite side of the light source. The angle between the unrecognized letters and the light source was defined as the halo radius and was calculated in arc minutes (Supplementary Fig. S1; https://metrovision.fr). In this study, the halo radius was measured with optimal corrections preoperatively and without corrections postoperatively.
Quality of Vision Questionnaire
The quality of vision (QoV) questionnaire,17,18 which is specially conducted to measure the visual disturbances of postoperative patients, consists of a 10-item instrument across three subscales in terms of disturbance frequency, severity, and bothersomeness. Each item provides a 4-point scale response option. In total, each patient is required to respond to 30 questions. In this study, present subjective experiences were obtained for analysis at the final visit.
Statistical Analysis
Based on an α of 0.05, a β of 0.2, and a correlation among repeated measures of 0.5, a sample size of 60 participants to detect the difference in disk halo size was required using G*Power 3.1 software.19 However, a total sample of 66 patients was recruited, considering an estimated 10% dropout rate.
SPSS version 24 statistical software (IBM Corp., Armonk, NY, USA) was used to perform the statistical analyses. The continuous variables are presented as mean ± standard deviation and categorical or ordinal variables as percentage or frequency. The χ2 test was used to determine intergroup differences in refractive outcomes and patient-reported outcomes (e.g., percentage or frequency). The independent sample t-test was used to analyze data with normal distribution, and the Mann–Whitney U test was used to analyze data with nonnormal distribution between the SMILE and EVO ICL groups. Repeated-measures analysis of variance (ANOVA) was used to compare CS and disk halo size within and between the two groups, and Bonferroni correction was conducted for multiple comparisons. Statistical significance was set at P < 0.05.
Results
Study Population
A total of 65 (10 men and 55 women) patients with a mean age of 26.63 ± 3.74 years completed the study. There was one dropout due to scheduling constraints. The mean preoperative spherical equivalent was −7.74 ± 1.02 D (range, −6.00 to −11.25 D). Thirty-three patients underwent SMILE and 32 underwent EVO ICL implantation. Except for central corneal thickness (CCT), there were no statistical differences in baseline values between the groups (Table 1). Patients in the EVO ICL group had thinner CCTs (P = 0.007).
Table 1.
Participants’ Baseline Demographics
| Characteristic | SMILE (n = 33) | EVO ICL (n = 32) | P Value |
|---|---|---|---|
| Age, y | 26.52 ± 3.21 | 26.75 ± 4.27 | 0.803 |
| Range, y | 20 to 35 | 19 to 37 | |
| Sex, male/female, n | 5/28 | 5/27 | 1.000 |
| Sphere, D | −7.11 ± 0.81 | −7.41 ± 1.05 | 0.199 |
| Range, D | −9.25 to −6.00 | −9.50 to −6.00 | |
| Cylinder, D | −0.85 ± 0.66 | −1.10 ± 0.92 | 0.206 |
| Range, D | −2.50 to 0 | −3.50 to 0 | |
| Spherical equivalent, D | −7.53 ± 0.88 | −7.96 ± 1.13 | 0.093 |
| Range, D | −10.50 to −6.00 | −11.25 to −6.00 | |
| CDVA, logMAR | −0.033 ± 0.048 | −0.013 ± 0.049 | 0.088 |
| Range, logMAR | −0.1 to 0 | −0.1 to 0.1 | |
| Axial length, mm | 26.21 ± 0.61 | 26.43 ± 1.03 | 0.301 |
| Range, mm | 24.96 to 27.58 | 24.80 to 29.29 | |
| CCT, µm | 545.82 ± 25.96 | 527.44 ± 27.54 | 0.007 |
| Range, µm | 501 to 608 | 458 to 597 | |
| Km, D | 43.61 ± 1.01 | 43.79 ± 1.47 | 0.570 |
| Range, D | 41.93 to 46.05 | 40.10 to 46.60 | |
| Mesopic pupil size, mm | 6.98 ± 0.72 | 6.98 ± 0.57 | 0.457 |
| Range, mm | 6.0 to 8.7 | 5.3 to 8.1 | |
| Halo radius @ 5 cd/m2, arc min | 78.79 ± 15.56 | 87.50 ± 23.83 | 0.088 |
| Range, arc min | 60 to 110 | 60 to 150 | |
| Halo radius @ 1 cd/m2, arc min | 225.45 ± 46.31 | 236.56 ± 58.89 | 0.400 |
| Range, arc min | 150 to 310 | 130 to 330 |
Values are presented as mean ± SD unless otherwise indicated. Km, mean keratometry; logMAR, logarithm of the minimum angle of resolution.
Visual and Refractive Outcomes
Both procedures showed favorable safety without clinically significant complications intraoperatively and postoperatively. At 6 months postoperatively, the overall safety indices (postoperative CDVA/preoperative CDVA) in the EVO ICL and SMILE groups were 1.34 ± 0.17 and 1.23 ± 0.21, respectively (Mann–Whitney U test, P = 0.037). The overall efficacy indexes (postoperative UDVA/preoperative CDVA) were 1.29 ± 0.23 and 1.18 ± 0.21, respectively (Mann–Whitney U test, P = 0.047). Therefore, the safety and efficacy indices were significantly higher in the EVO ICL group than in the SMILE group.
The refractive outcomes are shown in Figure 1. In terms of efficacy, 29 eyes (88%) in the SMILE group and 26 eyes (81%) in the EVO ICL group had postoperative UDVA ≥20/16 and 100% postoperative UDVA ≥20/25 in both groups (Fig. 1A). There were 31 eyes (94%) in the SMILE group and 32 eyes (100%) in the EVO ICL group that achieved postoperative CDVA greater than or equal to preoperative CDVA (Fig. 1B). The scatterplot shows that 94% of the SMILE-treated eyes (Fig. 1C) and 100% of the EVO ICL-treated eyes had postoperative SE within ±0.50 D (Fig. 1D). SE and residual astigmatism in all eyes lay within ±1.00 D (Figs. 1E, 1F). In terms of stability, the mean SE was +0.14 D for the SMILE group (Fig. 1G) and −0.04 D for the EVO ICL group (Fig. 1H). There were no significant differences in the above refractive outcomes between the groups, as assessed with the χ2 test (all P > 0.05).
Figure 1.
Refractive outcomes. Refractive outcomes at 6 months after SMILE and EVO ICL implantation for high myopia. (A) Comparison of efficacy between SMILE and EVO ICL. (B) Comparison of procedural safety between SMILE and EVO ICL. Refractive accuracy, attempted versus achieved spherical equivalent for SMILE (C) and EVO ICL (D). Refractive astigmatism accuracy for SMILE (E) and EVO ICL (F). Refractive stability for SMILE (G) and EVO ICL (H).
Contrast Sensitivity
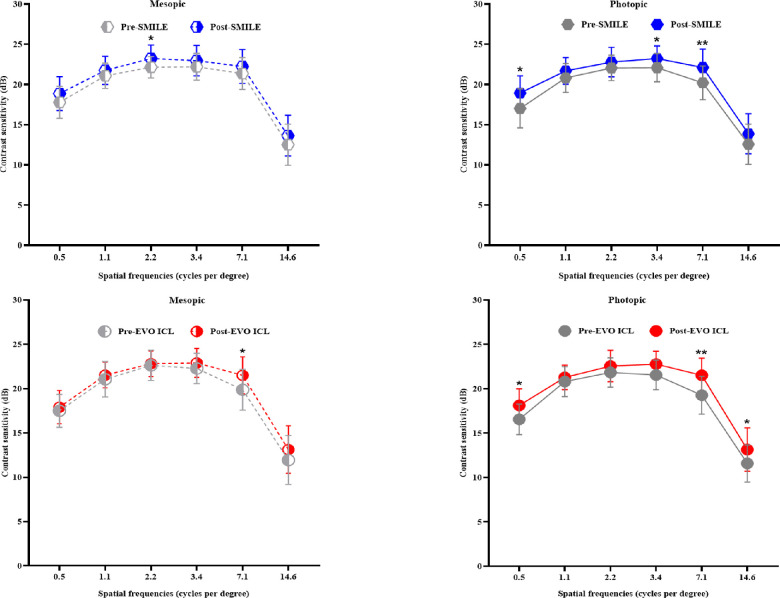
Repeated-measures ANOVA revealed that compared to baseline, the variations of CS at all spatial frequencies at 6 months postoperatively were not significantly different between the groups and also not significantly different across the spatial frequencies within either the SMILE or the EVO ICL group (all P > 0.05). Overall, the postoperative CS was recovered and even better than the baseline values in both groups. Bonferroni's multiple comparison tests also found that mesopic CS at 2.2 cpd (t = 2.931, P = 0.029) and photopic CS at 0.5, 3.4, and 7.1 cpd (t = 3.400, P = 0.007; t = 2.814, P = 0.039; t = 3.541, P = 0.005, respectively) were significantly improved in the SMILE group. In the EVO ICL group, by contrast, mesopic CS at 7.1 cpd (t = 2.957, P = 0.026) and photopic CS at 0.5, 7.1, and 14.6 cpd (t = 3.089, P = 0.025; t = 4.220, P = 0.001; t = 2.832, P = 0.048) were significantly improved (Fig. 2).
Figure 2.
Mesopic and photopic contrast sensitivity. The changes in mesopic and photopic contrast sensitivity before and 6 months after SMILE and EVO ICL implantation are shown (*P < 0.05; **P < 0.01).
Disk Halo Size
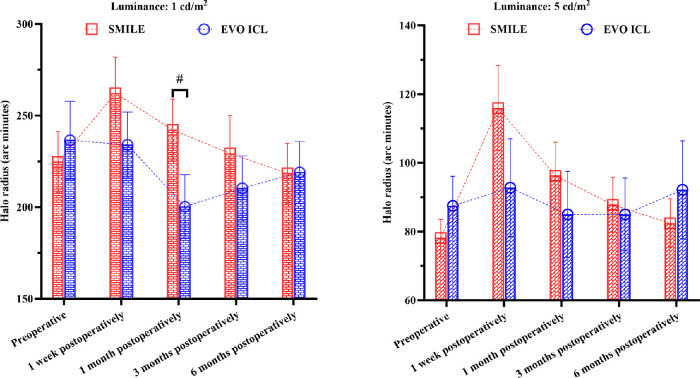
The preoperative and postoperative disk halo size at 1 and 5 cd/m2 luminance levels is shown in Table 2. Repeated-measures ANOVA revealed that there was a significant time effect in the SMILE group at 1 and 5 cd/m2 (both P < 0.01) but not in the EVO ICL group (both P > 0.05).
Table 2.
Repeated-Measures ANOVA of Disk Halo Size under Different Luminance Conditions in High Myopes before and after Surgery
| Halo Radius, arc min | |||||||
|---|---|---|---|---|---|---|---|
| Luminance | Preoperative | One Week Postoperatively | One Month Postoperatively | Three Months Postoperatively | Six Months Postoperatively | F | P |
| 1 cd/m2 | |||||||
| SMILE | 224.69 ± 46.83 | 262.19 ± 55.87 | 242.19 ± 47.23 | 231.21 ± 58.08 | 218.48 ± 46.11 | 5.318 | 0.0008 |
| ICL | 236.56 ± 58.89 | 234.06 ± 49.77 | 200.31 ± 48.49a | 209.69 ± 50.64 | 219.06 ± 46.72 | ||
| 5 cd/m2 | |||||||
| SMILE | 78.13 ± 15.33 | 115.94 ± 34.91 | 96.25 ± 27.56 | 88.48 ± 22.66 | 82.42 ± 20.16 | 6.999 | 0.0003 |
| ICL | 87.50 ± 23.83 | 92.87 ± 39.45 | 85.00 ± 34.73 | 84.06 ± 29.39 | 92.19 ± 39.49 | ||
Data are presented as mean ± SD.
aHalo radius comparison between SMILE and ICL groups at 1 cd/m2 luminance, P = 0.0032.
Intergroup analysis showed that in contrast to baseline values, at 1 cd/m2, the postoperative halo radii in the SMILE group exhibited a significantly increasing trend at 1 week (adjusted P < 0.05), following recovery at 1 month (adjusted P > 0.05), then stable progression at 3 and 6 months. The halo radii at 1 week, 1 month, and 3 months postoperatively were not significantly different from each other (all P > 0.05), but the halo radii at 1 week were larger than those at 6 months (adjusted P < 0.05). Similarly, at 5 cd/m2, the postoperative halo radii in the SMILE group exhibited a significantly increasing trend at 1 week (adjusted P < 0.0001) and 1 month (adjusted P < 0.05), a reduction and return to baseline at 3 months (P > 0.05), and then stable progression at 6 months (P > 0.05). Compared to postoperative halo radii at 1 week, significant differences were not observed at 1 month (P > 0.05) but were found at 3 and 6 months (both P < 0.001). The halo radii did not differ significantly from one another at 1 month, 3 months, and 6 months postoperatively (P > 0.05). By contrast, the halo radii in the EVO ICL group remained unchanged at 1 and 5 cd/m2 (both P > 0.05).
Intergroup comparisons demonstrated that at 1 cd/m2 luminance, the disk halo size at 1 month postoperatively was significantly larger in the SMILE than in the EVO ICL group (adjusted P < 0.01). However, at 5 cd/m2 luminance, significant differences in disk halo size were not detected between the groups at any follow-up visit (P > 0.05) (Fig. 3).
Figure 3.
Disk halo size findings. Disk halo size changes after SMILE and EVO ICL implantation for high myopia correction (means with 95% confidence intervals, #P < 0.01).
Patient-Reported QoV Outcomes
Table 3 summarizes the patient-reported outcomes. At 6 months postoperatively, haloes, starbursts, glare, and focusing difficulty were the most common disturbances for both groups. Halo disturbances were perceived significantly more often by EVO ICL patients than SMILE patients (χ2 = 16.100, P = 0.000) in terms of severity (χ2 = 13.069, P = 0.000) and bothersomeness (χ2 = 10.773, P = 0.000). However, starbursts perceived by SMILE patients were significantly higher in frequency only than those by EVO ICL patients (χ2 = 17.525, P < 0.01). There was no significant difference in the other eight visual disturbances between the groups.
Table 3.
Comparison of Patient-Reported Outcomes at the 6-Month Postoperative Visit
| Frequency | Severity | Bothersomeness | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Symptoms | SMILE (n = 33), % | EVO ICL (n = 32), % | P | SMILE (n = 33), % | EVO ICL (n = 32), % | P | SMILE (n = 33), % | EVO ICL (n = 32), % | P |
| Glare | 12.1 | 15.6 | 0.240 | 12.1 | 12.5 | 0.963 | 0 | 9.4 | 0.072 |
| Haloes | 21.3 | 68.8 | 0.000 | 21.2 | 65.6 | 0.000 | 0 | 28.1 | 0.001 |
| Starburst | 54.5 | 43.8 | 0.000 | 51.5 | 43.8 | 0.539 | 6.1 | 9.4 | 0.616 |
| Hazy vision | 0 | 0 | / | 0 | 0 | / | 0 | 0 | / |
| Blurred vision | 0 | 0 | / | 0 | 0 | / | 0 | 0 | / |
| Distortion | 0 | 0 | / | 0 | 0 | / | 0 | 0 | / |
| Double vision | 3 | 3.1 | 0.982 | 3 | 3.1 | 0.982 | 3 | 0 | 0.321 |
| Fluctuation in vision | 9.1 | 0 | 0.218 | 6.1 | 0 | 0.157 | 3 | 0 | 0.321 |
| Focusing difficulty | 15.1 | 3.1 | 0.230 | 12.1 | 3.1 | 0.174 | 3 | 0 | 0.321 |
| Difficulty with depth perception | 0 | 0 | / | 0 | 0 | / | 0 | 0 | / |
The χ2 test was conducted for intergroup comparison. /, not applicable.
A stacked percentage histogram giving a comprehensive representation of the frequency, severity, and bothersomeness of the 10-item disturbances is presented in Figure 4. Starbursts were the most reported with mild to moderate severity and no bothersome present symptoms in SMILE patients. In contrast to an incidence rate of 54.5% in SMILE patients, only 43.8% of EVO ICL patients reported starbursts, which was the second most perceived disturbance in this group. In EVO ICL patients, haloes were the leading visual disturbance reported by 68.8% of patients. In SMILE patients, however, haloes showed an incidence rate of only 21.3%. After EVO ICL implantation, haloes were also the leading disturbance in terms of severity and bothersomeness, with 28.1% of EVO ICL patients feeling bothered due to haloes. In contrast, no one felt bothered by haloes after SMILE.
Figure 4.
Patient-reported outcomes. Patient-reported outcomes measured by the QoV questionnaire after SMILE and EVO ICL implantation. Bars are arranged separately for frequency, severity, and bothersomeness and ranked in descending order of incidences. (A) Frequency after SMILE. (B) Severity after SMILE. (C) Bothersomeness after SMILE. (D) Frequency after EVO ICL. (E) Severity after EVO ICL. (F) Bothersomeness after EVO ICL.
Discussion
Visual performance and disturbances after surgical myopia correction are common concerns in clinical practice. This study is the first to comprehensively analyze and compare the clinical results and patient-reported QoV outcomes after SMILE and EVO ICL implantation for high myopia correction.
Both procedures showed favorable safety without clinically significant complications, and none of the eyes in either group lost two or more lines of CDVA during the follow-up. Nevertheless, EVO ICL implantation manifested a slight superior safety and efficacy profile, which was consistent with the visual outcomes reported by Siedlecki et al.9 These superior visual results may be directly related to the technique's superior refractive accuracy observed in the present study. Another explanation may be attributed to less induced higher-order aberrations after ICL than SMILE, which has been verified in V4 or V4c ICL in comparison to SMILE.8,9
CS reflects the fine resolution of the visual system. In the present study, our data demonstrated that the postoperative mesopic and photopic CS at 6 months were recovered and even better than the preoperative baseline values in both groups. Compared to SMILE patients, EVO ICL patients exhibited more favorable improvements in CS at high spatial frequencies. Sekundo et al.7 first investigated the mesopic and photopic CS at 3, 6, and 12 months after SMILE and did not observe any significant differences compared to the preoperative baseline values, suggesting that CS fully recovered by 3 months postoperatively. Similarly, Vestergaard et al.20 detected unchanged CS at 6 months after SMILE. However, the efficacy and UDVA achieved in SMILE patients in the current study were superior to that achieved in those studies, which may account for the above differences. Likewise, Reinstein et al.21 have also reported a significant improvement in mesopic CS (measured by CSV-1000) 3 months after V4c ICL. These results are consistent with the findings observed in the current study, and the improvements may be related to the optical imaging effect of EVO ICL in the eye.22
Our data demonstrated that the disk halo size showed a significantly increasing trend at 1 week, following a reduction and return to baseline at 3 months, then stable progression after SMILE, in contrast to a profile of stable disk halo size after EVO ICL. The halo size at 6 months for both procedures was consistent with the values of patients without night vision disturbance (SE: −1.00 D to −10.38 D).16 Also, the halo size at 1 month postoperatively was larger in SMILE than in EVO ICL at 1 cd/m2 luminance level. Although the relationship between pupil size and glare has not been clinically confirmed in patients after laser vision correction,23,24 ICL is thought to result in more favorable optical zone and intraocular scattering results than SMILE. The empirically calculated effective optical zone at the corneal plane for myopia within −10.0 D and/or astigmatism within 6.0 D is typically >6.9 mm (Supplementary Tables S1 and S2)25,26; by contrast, it was <6.9 mm for SMILE in the current study. Additionally, the intraocular scattering value is positively correlated with halo size27,28 and recovers faster after ICL than SMILE.11 Furthermore, the miotic effect may lead to reduced glare in the early stages after ICL.29 These findings from previous studies may explain the intergroup discrepancy in disk halo size.
The patient-reported QoV outcomes revealed that haloes and starbursts were the leading disturbances for both groups. Specifically, the frequency, severity, and bothersomeness of haloes were higher in EVO ICL than SMILE. By contrast, the frequency of starbursts was significantly higher after SMILE than EVO ICL. Our findings are similar to results from a matched comparative study9 but different from the results reported by Wei et al.,8 in which the most common symptoms after SMILE and V4c ICL were blurred vision, glare, and haloes. These differences may be relevant to the fact that QoV questionnaire outcomes are determined by multiple factors.30 In addition, only mild and less bothersome were reported for perceived dysphotopsia in this study, which echoed the findings of disk halo size at 6 months postoperatively.
A strength of this study is its comprehensive methodologic aim to compare several aspects of clinical outcome after refractive surgery, including objective and subjective outcomes. The disk halo findings match the subjective patient experience indicating that haloes could be clinically insignificant at 6 months after both procedures. The gender ratio was skewed within each group, but they are comparable between SMILE and ICL groups. In addition, disk halo size was independent of gender,13 and the gender disparity should not lead to individual difference of contrast sensitivity.31 Hence, our results were not influenced by the gender disparity. However, this study is not without limitations. There was a statistically significant difference in preoperative CCT between EVO ICL and SMILE groups. More patients should be recruited to match the CCT in the two groups to achieve true randomization and draw more reliable conclusions. In addition, patients with high myopia over −10.00 D were not included due to the restriction for SMILE correction. Furthermore, the long-term clinical outcomes between SMILE and EVO ICL warrant further study.
In conclusion, our data indicated better visual outcomes, improved CS (particularly at high spatial frequencies), but unchanged disk halo size in EVO ICL, in comparison to SMILE for high myopia correction. With regard to subjective experience, haloes were the primary disturbance with little bothersomeness after EVO ICL in contrast to starbursts after SMILE. Our findings will be of benefit for preoperative consultations and postoperative evaluations in terms of visual performance and disturbances.
Supplementary Material
Acknowledgments
Supported by the National Natural Science Foundation of China (Grant No. 81770955), Project of Shanghai Science and Technology (Grant No. 20410710100), Clinical Research Plan of SHDC (SHDC2020CR1043B), Project of Shanghai Xuhui District Science and Technology (2020-015), Project of Shanghai Xuhui District Science and Technology (XHLHGG202104), Shanghai Engineering Research Center of Laser and Autostereoscopic 3D for Vision Care (20DZ2255000), and Construction of a 3D Digital Intelligent Prevention and Control Platform for the Whole Life Cycle of Highly Myopic Patients in the Yangtze River Delta (21002411600).
Disclosure: W. Zhao, None; J. Zhao, None; T. Han, None; J. Wang, None; Z. Zhang, None; X. Zhou, None
References
- 1. Mainster MA, Turner PL.. Glare's causes, consequences, and clinical challenges after a century of ophthalmic study. Am J Ophthalmol . 2012; 153(4): 587–593. [DOI] [PubMed] [Google Scholar]
- 2. Schallhorn SC, Venter JA, Teenan D, et al.. Patient-reported outcomes 5 years after laser in situ keratomileusis. J Cataract Refract Surg . 2016; 42(6): 879–889. [DOI] [PubMed] [Google Scholar]
- 3. Ieong A, Hau SC, Rubin GS, Allan BD.. Quality of life in high myopia before and after implantable Collamer lens implantation. Ophthalmology. 2010; 117(12): 2295–2300. [DOI] [PubMed] [Google Scholar]
- 4. Gyldenkerne A, Ivarsen A, Hjortdal J.. Optical and visual quality after small-incision lenticule extraction. J Cataract Refract Surg. 2019; 45(1): 54–61. [DOI] [PubMed] [Google Scholar]
- 5. Montés-Micó R, Pastor-Pascual F, Artiaga-Elordi E, Ruiz-Mesa R, Tañá-Rivero P.. In vivo optical quality of posterior-chamber phakic implantable Collamer lenses with a central port. Eye Vis (Lond). 2021; 8(1): 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martínez-Plaza E, López-Miguel A, López-de la Rosa A, McAlinden C, Fernández I, Maldonado MJ.. Effect of the EVO+ Visian phakic implantable Collamer lens on visual performance and quality of vision and life. Am J Ophthalmol. 2021; 226: 117–125. [DOI] [PubMed] [Google Scholar]
- 7. Sekundo W, Gertnere J, Bertelmann T, Solomatin I.. One-year refractive results, contrast sensitivity, high-order aberrations and complications after myopic small-incision lenticule extraction (ReLEx SMILE). Graefes Arch Clin Exp Ophthalmol. 2014; 252(5): 837–843. [DOI] [PubMed] [Google Scholar]
- 8. Wei R, Li M, Zhang H, Aruma A, et al.. Comparison of objective and subjective visual quality early after implantable Collamer lens V4c (ICL V4c) and small incision lenticule extraction (SMILE) for high myopia correction. Acta Ophthalmol . 2020; 98(8): e943–e950. [DOI] [PubMed] [Google Scholar]
- 9. Siedlecki J, Schmelter V, Mayer WJ, et al.. SMILE versus implantable Collamer lens implantation for high myopia: a matched comparative study [published correction appears in J Refract Surg. 2020; 36(5):352]. J Refract Surg. 2020; 36(3): 150–159. [DOI] [PubMed] [Google Scholar]
- 10. Niu L, Miao H, Tian M, Fu D, Wang X, Zhou X.. One-year visual outcomes and optical quality of femtosecond laser small incision lenticule extraction and Visian Implantable Collamer Lens (ICL V4c) implantation for high myopia. Acta Ophthalmol . 2020; 98(6): e662–e667. [DOI] [PubMed] [Google Scholar]
- 11. Qin Q, Bao L, Yang L, He Z, Huang Z.. Comparison of visual quality after EVO-ICL implantation and SMILE to select the appropriate surgical method for high myopia. BMC Ophthalmol . 2019; 19(1): 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moshirfar M, Somani AN, Motlagh MN, et al.. Comparison of FDA-reported visual and refractive outcomes of the Toric ICL lens, SMILE, and topography-guided LASIK for the correction of myopia and myopic astigmatism. J Refract Surg. 2019; 35(11): 699–706. [DOI] [PubMed] [Google Scholar]
- 13. Puell MC, Pérez-Carrasco MJ, Barrio A, Antona B, Palomo-Alvarez C.. Normal values for the size of a halo produced by a glare source. J Refract Surg . 2013; 29(9): 618–622. [DOI] [PubMed] [Google Scholar]
- 14. Han T, Zhao F, Chen X, Miao H, Chen Z, Zhou X.. Evaluation of disk halo size after small incision lenticule extraction (SMILE). Graefes Arch Clin Exp Ophthalmol . 2019; 257(12): 2789–2793. [DOI] [PubMed] [Google Scholar]
- 15. Chen X, Han T, Zhao F, Miao H, Wang X, Zhou X.. Evaluation of disk halo size after implantation of a Collamer lens with a central hole (ICL V4c). J Ophthalmol . 2019; 2019: 7174913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao W, Zhao J, Han T, Li M, Wang J, Zhou X.. Evaluation of disk halo size and identification of correlated factors in myopic adults. Front Med (Lausanne). 2022; 9: 743543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McAlinden C, Skiadaresi E, Gatinel D, Cabot F, Huang J, Pesudovs K.. The Quality of Vision questionnaire: subscale interchangeability. Optom Vis Sci . 2013; 90(8): 760–764. [DOI] [PubMed] [Google Scholar]
- 18. McAlinden C, Pesudovs K, Moore JE.. The development of an instrument to measure quality of vision: the Quality of Vision (QoV) questionnaire. Invest Ophthalmol Vis Sci. 2010; 51(11): 5537–5545. [DOI] [PubMed] [Google Scholar]
- 19. Faul F, Erdfelder E, Lang AG, Buchner A.. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007; 39(2): 175–191. [DOI] [PubMed] [Google Scholar]
- 20. Vestergaard AH, Grauslund J, Ivarsen AR, Hjortdal JØ.. Efficacy, safety, predictability, contrast sensitivity, and aberrations after femtosecond laser lenticule extraction. J Cataract Refract Surg. 2014; 40(3): 403–411. [DOI] [PubMed] [Google Scholar]
- 21. Reinstein DZ, Vida RS, Archer TJ.. Visual outcomes, footplate position and vault achieved with the Visian implantable Collamer lens for myopic astigmatism. Clin Ophthalmol . 2021; 15: 4485–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rubin ML. Optics for Clinicians. 2nd ed. Gainesville, FL: TRIAD Scientific Publishers; 1977. [Google Scholar]
- 23. D Myung, S Schallhorn, Manche E E.. Pupil size and LASIK: a review. J Refract Surg. 2013; 29: 734–741. [DOI] [PubMed] [Google Scholar]
- 24. Schallhorn S, Brown M, Venter J, Hettinger K, Hannan S.. The role of the mesopic pupil on patient-reported outcomes in young patients with myopia 1 month after wavefront-guided LASIK. J Refract Surg. 2014; 30(3): 159–165. [DOI] [PubMed] [Google Scholar]
- 25. Alió JL, Perez-Santonja JJ.. Refractive Surgery with Phakic IOLs: Fundamentals and Clinical Practice. New Delhi, India: Jaypee Brothers Medical Publishers Ltd.; 2013. [Google Scholar]
- 26. Lovisolo CF, Pesando PM.. The Implantable Contact Lens (ICL) and Other Phakic IOLs. Canelli, Italy: Fabione Editore s.r.l.; 1999.
- 27. Puell MC, Pérez-Carrasco MJ, Palomo-Alvarez C, Antona B, Barrio A.. Relationship between halo size and forward light scatter. Br J Ophthalmol. 2014; 98(10): 1389–1392. [DOI] [PubMed] [Google Scholar]
- 28. Yao L, Xu Y, Han T, et al.. Relationships between haloes and objective visual quality in healthy eyes. Transl Vis Sci Technol. 2020; 9(10): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhu Y, He T, Zhu H, Chen J, Zhou J.. Static and dynamic pupillary characteristics in high myopic eyes with two implantable Collamer lenses. J Cataract Refract Surg. 2019; 45(7): 946–951. [DOI] [PubMed] [Google Scholar]
- 30. Schmelter V, Dirisamer M, Siedlecki J, et al.. Determinants of subjective patient-reported quality of vision after small-incision lenticule extraction. J Cataract Refract Surg. 2019; 45(11): 1575–1583. [DOI] [PubMed] [Google Scholar]
- 31. Bartholomew AJ, Lad EM, Cao D, Bach M, Cirulli ET.. Individual differences in scotopic visual acuity and contrast sensitivity: genetic and non-genetic influences. PLoS One. 2016; 11(2): e0148192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.