Abstract
We have recently designed a new Plasmodium falciparum mouse model and documented its potential for the study of immune effector mechanisms. In order to determine its value for drug studies, we evaluated its response to existing antimalarial drugs compared to that observed in humans. Immunocompromised BXN (bg/bg xid/xid nu/nu) mice were infected with either the sensitive NF54 strain or the multiresistant T24 strain and then treated with chloroquine, quinine, mefloquine, or dihydroartemisinin. A parallelism was observed between previously reported human responses and P. falciparum-parasitized human red blood cell (huRBC)–BXN mouse responses to classical antimalarial drugs, measured in terms of speed of decrease in parasitemia and of morphological alterations of the parasites. Mice infected with the sensitive strain were successfully cured after treatment with either chloroquine or mefloquine. In contrast, mice infected with the multiresistant strain failed to be cured by chloroquine or quinine but thereafter responded to dihydroartemisinin treatment. The speed of parasite clearance and the morphological alterations induced differed for each drug and matched previously reported observations, hence stressing the relevance of the model. These data thus suggest that P. falciparum-huRBC–BXN mice can provide a valuable in vivo system and should be included in the short list of animals that can be used for the evaluation of P. falciparum responses to drugs.
In the absence of a long-awaited effective vaccine, antimalarial drugs remain the main means by which to control morbidity and mortality due to Plasmodium falciparum malaria. Although several antimalarials are available, P. falciparum has gradually developed resistance to nearly all of them. Furthermore, the prevalence and degree of resistance are increasing and the pipeline of new antimalarial compounds is drying out (26). Thus, there is an urgent need to find either new combined therapies using available compounds or to develop new antimalarial drugs to replace failing ones. However, among the various reasons for the shortage of new drugs are the small number of animal species receptive to P. falciparum and their price.
Immunodeficient mice have been widely used as xenogeneic transplantation models allowing in vivo investigations of human cells and organs. Recently, Badell et al. developed a model (1) in which P. falciparum-parasitized human red blood cells (P. falciparum-huRBC) can be grafted into immunodeficient (bg/bg xid/xid nu/nu) BXN laboratory mice (P. falciparum-huRBC–BXN). In this mouse strain, which lacks T-cell, LAKC, and T-independent B-cell functions, a main factor in the survival of P. falciparum is the concomitant decrease in innate, nonadaptive immunity. Through chemical reduction of tissue macrophages and blood leukocytes, in vivo development of medium-grade P. falciparum parasitemia can be obtained in these mice in several weeks. This new model may eventually provide a valuable tool with which to investigate the biology of this malaria parasite under in vivo conditions and therefore facilitate research in the fields of chemotherapy and vaccine development.
The goal of the present work was to investigate the potential value of this new animal model for antimalarial drug studies. This could be achieved only by using those drugs whose effects in humans are well established. Immunocompromised BXN mice were infected with the drug-sensitive NF54 strain or the multidrug-resistant T24 strain and treated with chloroquine, quinine, mefloquine, or dihydroartemisinin. We compared in vivo data obtained from the P. falciparum-huRBC–BXN mouse model with the drug sensitivity of strains determined in vitro and with the response usually observed in natural infections of humans.
MATERIALS AND METHODS
Mice.
Six 8-week-old male and female BXN mice were used. They were purchased from Charles River, kept in sterile isolators, and provided with autoclaved tap water and a γ-irradiated pelleted diet ad libitum. Mice were manipulated under pathogen-free conditions under laminar-flux hoods. All animals were treated according to laboratory animal guidelines.
Parasites.
The P. falciparum African NF54 strain and the Thailand T24 strain, obtained from the Paediatrics Service of Bangkok (8), were employed in this study. Strains were maintained at 5% hematocrit in complete culture medium at 37°C in a candle jar. This medium contained RPMI 1640 medium (Gibco BRL, Grand Island, N.Y.), 35 mM HEPES (Sigma, St Louis, Mo.), 24 mM NaHCO3, 0.5% Albumax (Gibco BRL), 1 mg of hypoxanthine (Sigma) per liter, and 5 μg of gentamicin (Gibco BRL) per ml. The cultures were synchronized twice weekly by Plasmagel (Roger Bellon, Neuilly-sur-Seine, France) floatation (20). When required, parasites were subjected to 5% sorbitol treatment (29) in order to obtain highly synchronized cultures.
huRBC.
huRBC were collected by venipuncture on either CPD anticoagulant (MacoPharma, Tourcoing, France) or sodium heparin (Sanofi Winthrop, Gentilly, France). Blood donors had no history of malaria and belonged to the AB+ or A+ blood group. Whole blood was centrifuged at 500 × g for 10 min at 20°C. The buffy coat was separated, and packaged RBC were suspended in SAGM (MacoPharma) and kept at 4°C for a maximum of 30 days. Before use, huRBC were washed three times by centrifugation at 300 × g with RPMI 1640 medium supplemented with 1 mg of hypoxanthine per liter.
Protocol of immunomodulation and grafting of P. falciparum-huRBC.
P. falciparum development in mice induces a strong increase in tissue macrophages, particularly in the liver, the spleen, and the peritoneal cavity, as well as in circulating polymorphonuclear neutrophils and monocytes. The number of tissue macrophages in BXN mice was reduced by using dichloromethylene diphosphonate (Cl2MDP), a gift from Roche Diagnostique, Mannheim, Germany, encapsulated in liposomes as described previously (40). The increase in polymorphonuclear neutrophils was controlled by using an NIMP-R14 monoclonal antibody (21). The hybridoma was a gift from Malcom Strath. Mice initially received an intraperitoneal (i.p) injection of NIMP-R14 (300 μg per mouse), and 2 days later they received an injection of 0.2 ml of Cl2MDP by the same route. Three days later, mice were inoculated i.p. with 1 ml of a suspension of highly synchronized ring forms (parasitemia, 0.3 to 0.6%) at 50% hematocrit in RPMI 1640 medium mixed with 300 μg of NIMP-R14. After infection, 1 ml of washed huRBC at 50% hematocrit was injected i.p. together with NIMP-R14 every 3 to 4 days, and Cl2MDP was injected every 4 to 5 days.
Thin smears of peripheral blood samples taken from the tails of the mice were prepared and stained with Giemsa. Since successfully grafted mice have 85 to 99% huRBC in their peripheral blood, parasitemia in mice was expressed as the overall percentage of P. falciparum-infected RBC observed in thin smears. Blood samples were used to determine the percentage of huRBC in the peripheral blood by the immunofluorescence technique using a fluorescein isothiocyanate-labeled anti-human glycophorin monoclonal antibody (Dako, Glostrup, Denmark).
In vitro drug sensitivity assay.
Two assays were performed to determine the 50% inhibitory concentrations (IC50) for each strain. A suspension of P. falciparum-huRBC (2% hematocrit and 0.005% parasitemia) in complete culture medium was distributed in a 96-well plate (200 μl/well) containing various concentrations of antimalarial drugs. Twofold dilutions were studied so that the final drug concentrations ranged from 5.4 to 2,870 nM for chloroquine, from 6.5 to 3,333 nM for quinine, and from 1.4 to 722.8 nM for both mefloquine and sodium artesunate. Each antimalarial range was tested in duplicate. After 48 h of culture at 37°C in a plastic candle jar, microcultured plates were frozen to stop parasite development. Parasite growth at each drug concentration was determined by the colorometric DELI method (9). Drug sensitivity was expressed as the concentration of the drug that resulted in 50% inhibition of parasite growth (IC50) compared to that of control wells without the drug. The cutoff IC50s between sensitivity and resistance were >100 nM for chloroquine, >300 nM for quinine, and >30 nM for mefloquine (4, 5).
Antimalarial treatment.
To validate our model, we tested the main antimalarial drugs used to treat P. falciparum infections, i.e., chloroquine sulfate (Rhône-Poulenc-Rorer, Vitry, France), quinine hydrochloride (Sanofi, Montpellier, France), mefloquine hydrochloride (a gift from H. Matile, Hoffmann-La Roche, Basel, Switzerland), and dihydroartemisinin (kindly supplied by P. Olliaro, World Health Organization, Geneva, Switzerland). Chloroquine sulfate was dissolved in RPMI 1640 medium and quinine was dissolved in a 10% glucose solution. A 10% solution of dimethyl sulfoxide (Sigma) in sterile water was used to dissolve mefloquine. Dihydroartemisinin was administrated as a suspension in sterile water.
In order to determine the mouse equivalents of the therapeutic regimens employed for human beings we relied on the work of Freireich et al. (12). Those authors found that the doses used for humans are approximately 1/12 of the dose used for mice, 1/9 of the dose used for hamsters, and 1/7 of the dose used for rats. Following preliminary experiments, we decided to use the coefficient for rats because initial experiments showed substantial toxicity of the doses used for mice. For mefloquine and dihydroartemisinin, which underwent recent rodent toxicity studies (whereas no such data are available for chloroquine and quinine), the doses were adjusted to the maximum acceptable based on mouse toxicity studies (27). Chloroquine, quinine, and dihydroartemisinin were administered orally (using a gastric cannula for delivery), whereas mefloquine was injected i.p. Three mice received chloroquine at a dose of 73 mg/kg/day for 2 days and 36.5 mg/kg on the third day. Mefloquine was given to two mice at a dose of 50 mg/kg/day for 2 days. Two mice received a first chloroquine treatment by the regimen described above, followed by treatment with dihydroartemisinin at a dose of 50 mg/kg/day for 2 days. Two mice treated with quinine received 73 mg/kg three times a day, i.e., 219 mg/kg/day, for 5 days.
Drugs were administered when parasitemia had been stable for at least 6 days and when ring forms were the predominant stage. For each antimalarial drug, the parasite clearance time and parasite reduction rate after 48 h of treatment were determined. These parameters led the estimation of the in vivo response to drug treatment of different strains, which were classified as S, RI, RII, and RIII, in accordance with World Health Organization nomenclature (42). Blood schizonticidal effects upon each stage and parasite morphology were also recorded in order to assess indirectly the possible modes of action of these antimalarials in our model.
RESULTS
In vitro responses of strains to drugs before and after passage in mice.
We selected the drug-sensitive NF54 strain and the multidrug-resistant T24 strain for this study. Since parasite response to drugs can be modified following cryopreservation, culture, or in vivo passage, we monitored the in vitro drug susceptibility patterns of each strain before and after passage in mice. For the NF54 strain maintained under culture conditions, the chloroquine, quinine, mefloquine, and dihydroartemisinin IC50s were 15, 100, 20, and 4 nM, respectively, while for the T24 strain, the IC50s of the same antimalarials were 1,122, 1,170, 13, and 3 nM. These results confirmed that NF54 was sensitive to the four antimalarial drugs while T24 was highly resistant to chloroquine and quinine. The in vitro drug susceptibility patterns of the T24 strain, measured after passage in two mice for 12 days when the parasitemia was stable, were similar to those previously determined for the in vitro-cultured strain. These results are indicative of absence of selection of the strain by passage in mice.
In vivo antimalarial effect upon P. falciparum in P. falciparum-huRBC–BXN.
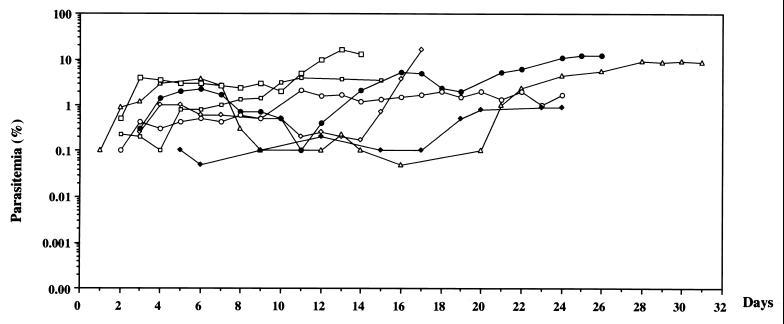
In agreement with other findings (2), the improved protocol led us to obtain consistent and sustained parasite growth in huRBC-BXN mice. As shown in Fig. 1, low to moderate levels of parasitemia were obtained. In fact, they could persist as long as uninfected huRBC were regularly transfused into mice and the immunomodulation protocol was continued. Furthermore, despite individual variations in the maximal parasitemia reached, the parasitemias observed were stable enough to allow assessment of the in vivo effect of an antimalarial agent.
FIG. 1.
Evolution of P. falciparum parasitemia in seven untreated mice. Mice were inoculated i.p. with 1 ml of highly synchronized cultures of P. falciparum at the ring stage on day 0. After infection, mice received normal RBC every 3 to 4 days. Parasitemia in mice was expressed as the percentage of P. falciparum-huRBC in the total RBC observed in thin smears. The data shown are from the first day of detectable parasitemia up to the day the mice were used for other malaria studies.
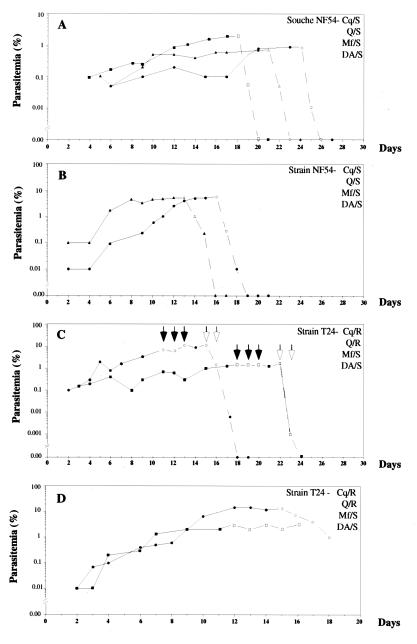
In three mice chloroquine rapidly cleared the drug-sensitive NF54 strain, as evidenced by a 20- to 40-fold decrease in parasitemia within 24 h (Fig. 2A) and full clearance within 48 h. Two mice infected with the same strain were successfully cured by mefloquine, but parasite clearance, at 3 days, was slower than that observed with chloroquine (Fig. 2B). In contrast, in two mice infected with the multiresistant T24 strain, chloroquine treatment was ineffective parasitemia levels only decreased from 7 to 6% and from 2 to 1.4%, (Fig. 2C). Two days after chloroquine treatment was discontinued, parasitemia had increased to 11.6 and 1.9% in these mice. They were therefore subsequently treated with dihydroartemisinin, which showed potent growth-inhibitory activity against the multiresistant T24 strain. Parasitemia decreased to below 0.005% within 24 h in both mice and fully disappeared within 2 days (Fig. 2C). Quinine also failed to cure two mice infected with the drug-resistant T24 strain (Fig. 2D), as there was no significant change in parasitemia over 5 days of treatment.
FIG. 2.
Evolution of parasitemia in P. falciparum-infected mice before (continuous line) and after (discontinuous line) antimalarial treatment. Open symbols indicate the days of treatment. (A) Chloroquine was administered to three mice at a dose of 73 mg/kg on the first 2 days of treatment and at 36.5 mg/kg on the last day. (B) Mefloquine was administered to two mice at a dose of 50 mg/kg. (C) After failed chloroquine treatment (closed arrows) of two mice, they were treated with dihydroartemisinin (open arrows) at 50 mg/kg. (D) Quinine at 73 mg/kg was administered three times per day to two mice.
Compared to antimalarial treatment of humans (Table 1), NF54-infected mice showed responses to chloroquine and mefloquine which were similar, in terms of parasite clearance time and parasite reduction rate, to those obtained in treated humans. Similarly, in the four mice infected with the multidrug-resistant T24 strain, chloroquine or quinine treatment led to less than 75% parasite reduction, which is indicative of an RIII level of resistance to these two antimalarials. The parasitological responses to dihydroartemisinin of this multidrug-resistant strain proved to be similar to those currently reported in humans using dihydroartemisinin or related compound in the presence of chloroquine and quinine resistance.
TABLE 1.
Parasitological responses to treatment of P. falciparum-infected humans and BXN mice
| Infected organisms | Chloroquine
|
Mefloquine
|
Dihydroartemisinin
|
|||
|---|---|---|---|---|---|---|
| Time to parasite clearance (h)a | % Reduction in parasitemia at 48 h | Time to parasite clearance (h)a | % Reduction in parasitemia at 48 h | Time to parasite clearance (h)a | % Reduction in parasitemia at 48 h | |
| Humans | 75 ± 18 | 92.1 | 88 ± 11 | 94.6 | 46 ± 23 | 100 |
| Mice | 48 | 100 | 72 | 97.9 | 48 | 100 |
Stage-dependent morphological observations.
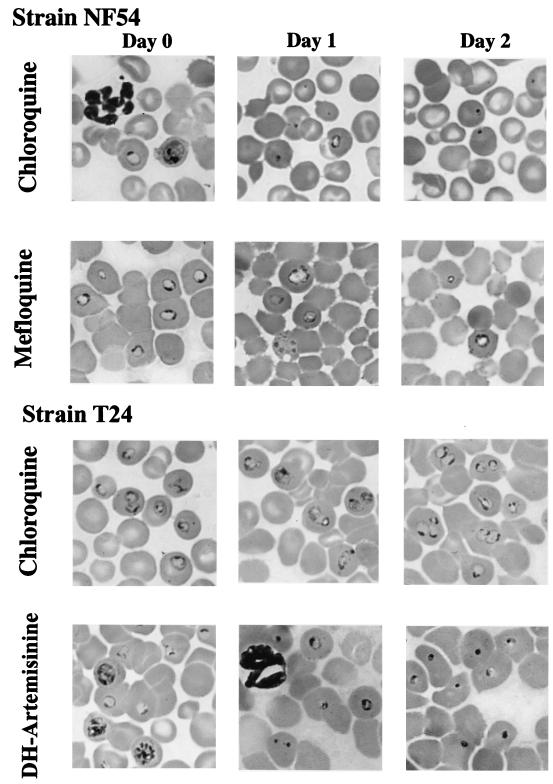
Changes in the morphology of each stage of the erythrocytic cycle following antimalarial treatment were recorded to assess indirectly the mode of action of each antimalarial drug in our model and compare it to published data. In contrast to cultures, and similar to the situation in humans, there is a trend toward spontaneous synchronization of the erythrocytic cycle in the P. falciparum-huRBC–BXN model. We decided to initiate antimalarial treatment when ring forms were predominant (Table 2). As a consequence of chloroquine treatment, schizont formation was interrupted at 24 h. Pycnotic uninucleate forms and altered trophozoites were predominant, while few healthy rings remained visible. Chloroquine-altered trophozoites were characterized by a lack of malaria pigment, compared to untreated controls (Fig. 3). At 48 h, only pycnotic forms could be seen. In contrast, the morphological effects induced by mefloquine upon NF54 parasites differed from those observed with chloroquine. At 24 h, altered trophozoites were predominant (72%) (Table 2), the majority of them presenting a cytoplasm which contained numerous vacuoles and no malaria pigment (Fig. 3). At 48 h, such atypical trophozoites could still be observed but the percentage of pycnotic forms had markedly increased (54%) (Table 2) and few apparently healthy ring forms remained. In contrast, in mice infected with the multidrug-resistant T24 strain, chloroquine or quinine induced few, if any, changes in the morphology of parasites or in the stage pattern. A low percentage of pycnotic forms could be detected (11 to 16%), but the strain still grew and developed normally over the following days (Fig. 3). Dihydroartemisinin showed a profound effect upon the T24 strain. At 24 h after initiation of the treatment, pycnotic parasites (72%) and altered trophozoites (16%) were already predominant. However, interestingly, small proportions of apparently healthy rings, trophozoites (1%), and schizonts (3%) were still observed (Table 2). At 48 h, all of the rare forms still visible were pycnotic (Fig. 3).
TABLE 2.
Parasitic pattern in P. falciparum-huRBC–BXN model after treatment with conventional antimalarial drugs
| Drug | Strain | Day 0
|
Day 1
|
Day 2
|
Day 3
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % of rings | % of trophozoites | % of schizonts | % of rings | % of altered trophozoites | % of pycnotic forms | % of rings | % of altered trophozoites | % of pycnotic forms | % of rings | % of altered trophozoites | % of pycnotic forms | ||
| Chloroquine | NF54 | 78 | 4 | 18 | 10 | 26 | 64 | 0 | 0 | 100 | 0 | 0 | 100 |
| Mefloquine | NF54 | 59 | 5 | 36 | 7 | 72 | 21 | 3 | 43 | 54 | 0 | 0 | 100 |
| Dihydroartemisinin | T24 | 51a | 0a | 22a | 4b | 16b | 76b | 0 | 0 | 100 | 0 | 0 | 100 |
The percentages of pycnotic forms and altered trophozoites after chloroquine treatment were 16 and 11%, respectively.
The percentages of trophozoites and schizonts were 1 and 3%, respectively.
FIG. 3.
Morphological changes induced in vivo by chloroquine, mefloquine, and dihydroartemisinin (DH-Artemisinine) in multiresistant strain T24 and sensitive strain NF54.
DISCUSSION
The present study shows that the P. falciparum-huRBC–BXN model, in which BXN mice are grafted with huRBC infected with two different strains of P. falciparum, can be employed as a tool with which to evaluate in vivo responses to antimalarial drugs, as demonstrated by the blood schizonticidal effects of chloroquine, quinine, mefloquine, and dihydroartemisinin.
The effects of these antimalarial drugs have been widely documented in P. falciparum-infected humans, providing a positive control with which to determine the value of the P. falciparum-huRBC–BXN model for antimalarial chemotherapy (3, 4, 6, 7, 10, 11, 13–16, 18, 22–24, 30, 31, 35–39, 41). As summarized in Table 2, the preliminary results suggest that responses to treatment observed in P. falciparum-huRBC–BXN mice parallel those reported in humans infected with P. falciparum in terms of parasite clearance and parasite reduction rate. This applies to strains which are susceptible and those which are not.
The effects of these conventional antimalarial drugs on parasite morphology or targeted stages of development were also compared with those obtained from treated humans. However, due to sequestration of maturing forms in humans, the results also had to be compared with data from in vitro studies. Chloroquine seems to act on dividing forms, and this is a likely explanation for the absence of schizonts following chloroquine treatment. This finding may be related to the fact that the drug can affect both protein synthesis and DNA replication (25), leading to schizont destruction. After treatment with mefloquine, we observed a loss of structural integrity, resulting in multiple vacuoles, which is in keeping with the in vitro findings reported by Jacobs et al. (17). Similarly, we found that dihydroartemisinin was highly effective against all stages of the parasites (34), and Kombila et al. (19) observed that 24 h after the treatment of infected children with artemether, all of their parasites were abnormal. Thus, the similarity of effects on parasite morphology and parasitic stages in our model or in treated humans and in vitro studies suggests that the modes of action of these antimalarial drugs are also similar.
Taken together, the available data on the speed of clearance and targeted stage tend to validate the use of this model for chemotherapy studies of P. falciparum malaria.
A major practical problem in drug discovery studies is the scarcity of animal models receptive to P. falciparum, due to the strict host specificity of the parasite. This explains the current emphasis on in vitro cultures of the intraerythrocytic stages of P. falciparum, despite their limitations. The most evident limitation is their inability to detect the antimalarial activity of prodrugs, e.g., proguanil, that require in vivo metabolism to reach an active form. Another limiting factor is that the assessment of a given compound only by in vitro methods is imprecise, as it does not supply any information about its pharmacokinetics, an essential parameter of its potential.
In this context, the P. falciparum-huRBC–BXN model provides a totally new and interesting opportunity to work with the most relevant parasite target, P. falciparum, under in vivo conditions. Thus, it is important to note that now P. falciparum infections can be obtained not only in South American monkeys (32, 33) but also in small experimental animals. Whereas mice are easy to reproduce under laboratory conditions, monkeys are in limited supply and expensive. Therefore, only a limited number of monkeys can be used in each experiment, which indeed limits the number of new compounds being studied in vivo and the number of parameters investigated, such as dose finding. Monkeys are difficult to handle, they require a dedicated staff and setup, and they can be infected with only a small number of adapted P. falciparum strains, which restricts the scope of genetic diversity studies. In contrast, huRBC-BXN mice have the advantage of susceptibility to apparently any parasite, either derived from in vitro cultures or sampled from infected humans, without the need for prior adaptation (the growth of three freshly collected isolates could be obtained in BXN mice, whereas these failed to be adapted under in vitro conditions). Our results suggest that the passage of the strain in P. falciparum-huRBC–BXN mice does not induce a detectable change in their drug susceptibility patterns or in the genetic makeup of the complex mixture of clones that constitute an isolate or a strain. (This was determined after 2 months of parasitemia in BXN mice [i] by PCR amplification of polymorphic regions of MSA1, MSA2, CS, and TRAP which showed the same clonal content as the strain initially inoculated and [ii] by the stability of the IC50s of four antimalarial drugs.)
The P. falciparum-huRBC–BXN model also differs from the P. berghei rodent model commonly used to assess the bioavailability of antimalarial compounds (28, 42). However, use of the P. berghei model following primary in vitro screening upon P. falciparum, in fact, introduces two variables at once (host and parasite species); hence, interpretation of the results obtained is difficult. The information obtained from such studies cannot be reliably extrapolated to P. falciparum in humans. In contrast, the P. falciparum-huRBC–BXN model offers the opportunity to evaluate bioavailability at an early stage, on a large scale, and by working with the same target parasite as the one used in vitro.
Another possible advantage of the P. falciparum-huRBC–BXN model is that chronic, stable, and long-lasting parasitemia can be obtained without killing the animal. This situation is closer to that of humans than the fast-rising and high parasitemia seen in monkeys, as well as in other rodent models. Indeed, as shown in our study, successive therapies can be tested in the same animal, depending on the susceptibility of the strain. In this manner, the individual and combined effects of several compounds could be evaluated so as to define new drug combinations, taking into account the pharmacokinetics of each component. Although the aim of this work was to study the relevance of this model in chemotherapy studies, its use is not limited to this area. The similarity of responses to antimalarial treatment in the P. falciparum-huRBC–BXN model and in infected human beings suggests that this model will also be valuable for studying the biology of human Plasmodium infections and immune responses to this parasite (2). Major advances in the development of this model have been made since our first report (1). The course of parasitaemia is reproducible in ca. two-thirds of the mice succesfully grafted, and the number of mice which cannot be grafted has been reduced. The parasitemia is uninterrupted for several weeks once it has started, except for the rare deaths. The model remains time-consuming to handle, and a moderate failure rate can still be observed, which is a constraint and a partial limitation to scaling up. However, it is also clear that this model is still in its infancy and recent observations suggest that it will be possible to further improve it in the near future. Moreover, the number of mice which can be enrolled in such studies is almost unlimited, as opposed to primates susceptible to human malaria.
Finding effective antimalarial treatments is a world health priority. The present study demonstrates that the P. falciparum-huRBC–BXN model offers a number of significant advantages over previous models. (i) This is the first rodent model in which P. falciparum can be maintained. (ii) Rodents, not monkeys, are needed to examine the large number of compounds involved in initial drug development. (iii) This model may accept a wide range of clinical isolates. (iv) The real advantage of this model is that it is the first rodent model in which the drug susceptibility of a human parasite, pharmacokinetics, and toxicology can all be determined. Therefore, it should be included in the very short list of models in which P. falciparum drug responses can be evaluated.
ACKNOWLEDGMENTS
Thanks to Jean-Louis Pérignon and Christine Müller-Graf for reviewing the manuscript.
This work was supported by World Health Organization grant ID 960 617.
REFERENCES
- 1.Badell E, Pasquetto V, Van Rooijen N, Druilhe P. A mouse model for human malaria erythrocytic stages. Parasitol Today. 1995;11:235–237. doi: 10.1016/0169-4758(95)80147-2. [DOI] [PubMed] [Google Scholar]
- 2.Badell E, Oeuvray C, Moreno A, Soe S, Van Rooijen N, Bouzidi A, Druilhe P. Human malaria in immunocompromised mice: an in vivo model to study defense mechanisms against Plasmodium falciparum. J Exp Med. 2000;192(11):1653–1660. doi: 10.1084/jem.192.11.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baptista J L, Das Neves I, D'Alessandro U, Hendrix L, Wery M. Plasmodium falciparum chloroquine and quinine sensitivity in asymptomatic and symptomatic children in Sao Tome Island. Trop Med Int Health. 1997;2:582–588. doi: 10.1046/j.1365-3156.1997.d01-325.x. [DOI] [PubMed] [Google Scholar]
- 4.Brasseur P, Druilhe P, Kouamouo J, Brandicourt O, Danis M, Moyou S R. High level of sensitivity to chloroquine of 72 Plasmodium falciparum isolates from southern Cameroon in January 1985. Am J Trop Med Hyg. 1986;35:711–716. doi: 10.4269/ajtmh.1986.35.711. [DOI] [PubMed] [Google Scholar]
- 5.Brasseur P, Kouamouo J, Brandicourt O, Moyou-Somo R, Druilhe P. Patterns of in vitro resistance to chloroquine, quinine, and mefloquine of Plasmodium falciparum in Cameroon, 1985–1986. Am J Trop Med Hyg. 1988;39:166–172. doi: 10.4269/ajtmh.1988.39.166. [DOI] [PubMed] [Google Scholar]
- 6.Chongsuphajaisiddhi T, Sabchareon A, Chantavanich P, Singhasivanon V, Attanath P, Wernsdorfer W H, Sheth U K. A phase-III clinical trial of mefloquine in children with chloroquine-resistant falciparum malaria in Thailand. Bull W H O. 1987;65:223–226. [PMC free article] [PubMed] [Google Scholar]
- 7.De Vries P J, Tran K D, Nguyen X K, Le Nguyen B, Pham T Y, Dao D D, Van Boxtel C J, Kager P A. The pharmacokinetics of a single dose of artemisinin in patients with uncomplicated falciparum malaria. Am J Trop Med Hyg. 1997;56:503–507. doi: 10.4269/ajtmh.1997.56.503. [DOI] [PubMed] [Google Scholar]
- 8.Druilhe P, Brandicourt O, Chongsuphajaisiddhi T, Berthe J. Activity of a combination of three cinchona bark alkaloids against Plasmodium falciparum in vitro. Antimicrob Agents Chemother. 1988;32:250–254. doi: 10.1128/aac.32.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Druilhe, P., A. Moreno, C. Blanc, P. Brasseur, and P. Jacquier. A colorimetric in vitro sensitivity assay for Plasmodium falciparum based on a highly sensitive double-site pLDH antigen capture ELISA. Am. J. Trop. Med. Hyg., in press. [DOI] [PubMed]
- 10.Elueze E I, Osisanya J O, Edafiogho I O. Sensitivity to chloroquine in vivo and in vitro of Plasmodium falciparum in Sokoto, Nigeria. Trans R Soc Trop Med Hyg. 1990;84:45. doi: 10.1016/0035-9203(90)90375-o. [DOI] [PubMed] [Google Scholar]
- 11.Fowler V G, Jr, Lemnge M, Irare S G, Malecela E, Mhina J, Mtui S, Mashaka M, Mtoi R. Efficacy of chloroquine on Plasmodium falciparum transmitted at Amani, eastern Usambara Mountains, north-east Tanzania: an area where malaria has recently become endemic. J Trop Med Hyg. 1993;96:337–345. [PubMed] [Google Scholar]
- 12.Freireich E J, Gehan E A, Rall D P, Schmidt L H, Skipper H E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50:219–244. [PubMed] [Google Scholar]
- 13.Fryauff D J, Baird J K, Candradikusuma D, Masbar S, Sutamihardja M A, Leksana B, Tuti S, Marwoto H, Richie T, Romzan A. Survey of in vivo sensitivity to chloroquine by Plasmodium falciparum and P. vivax in Lombok, Indonesia. Am J Trop Med Hyg. 1997;56:241–244. doi: 10.4269/ajtmh.1997.56.241. [DOI] [PubMed] [Google Scholar]
- 14.Ha V, Nguyen N H, Tran T B, Bui M C, Nguyen H P, Tran T H, Phan T Q, Arnold K. Severe and complicated malaria treated with artemisinin, artesunate or artemether in Viet Nam. Trans R Soc Trop Med Hyg. 1997;91:465–467. doi: 10.1016/s0035-9203(97)90287-x. [DOI] [PubMed] [Google Scholar]
- 15.Harinasuta T, Bunnag D, Wernsdorfer W H. A phase II clinical trial of mefloquine in patients with chloroquine-resistant falciparum malaria in Thailand. Bull W H O. 1983;61:299–305. [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan Alin M, Ashton M, Kihamia C M, Mtey G J, Bjorkman A. Multiple dose pharmacokinetics of oral artemisinin and comparison of its efficacy with that of oral artesunate in falciparum malaria patients. Trans R Soc Trop Med Hyg. 1996;90:61–65. doi: 10.1016/s0035-9203(96)90480-0. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs G H, Aikawa M, Milhous W K, Rabbege J R. An ultrastructural study of the effects of mefloquine on malaria parasites. Am J Trop Med Hyg. 1987;36:9–14. doi: 10.4269/ajtmh.1987.36.9. [DOI] [PubMed] [Google Scholar]
- 18.Jiang J B, Li G Q, Guo X B, Kong Y C, Arnold K. Antimalarial activity of mefloquine and qinghaosu. Lancet. 1982;ii:285–288. doi: 10.1016/s0140-6736(82)90268-9. [DOI] [PubMed] [Google Scholar]
- 19.Kombila M, Duong T H, Dufillot D, Koko J, Guiyedi V, Guiguen C, Ferrer A, Richard-Lenoble D. Light microscopic changes in Plasmodium falciparum from Gabonese children treated with artemether. Am J Trop Med Hyg. 1997;57:643–645. doi: 10.4269/ajtmh.1997.57.643. [DOI] [PubMed] [Google Scholar]
- 20.Lambros C, Vanderberg J P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 21.Lopez A F, Strath M, Sanderson C J. Differentiation antigens on mouse eosinophils and neutrophils identified by monoclonal antibodies. Br J Haematol. 1984;57:489–494. doi: 10.1111/j.1365-2141.1984.tb02923.x. [DOI] [PubMed] [Google Scholar]
- 22.Luxemburger C, Nosten F, Raimond S D, Chongsuphajaisiddhi T, White N J. Oral artesunate in the treatment of uncomplicated hyperparasitemic falciparum malaria. Am J Trop Med Hyg. 1995;53:522–525. doi: 10.4269/ajtmh.1995.53.522. [DOI] [PubMed] [Google Scholar]
- 23.Masaba S C, Spencer H C. Sensitivity of Plasmodium falciparum to chloroquine in Busia District, Kenya. Trans R Soc Trop Med Hyg. 1982;76:314–316. doi: 10.1016/0035-9203(82)90178-x. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen-Dinh P, Schwartz I K, Sexton J D, Egumb B, Bolange B, Ruti K, Nkuku-Pela N, Wery M. In vivo and in vitro susceptibility to chloroquine of Plasmodium falciparum in Kinshasa and Mbuji-Mayi, Zaire. Bull W H O. 1985;63:325–330. [PMC free article] [PubMed] [Google Scholar]
- 25.Olliaro P, Castelli F, Caligaris S, Druilhe P, Carosi G. Ultrastructure of Plasmodium falciparum “in vitro”. II. Morphological patterns of different quinolines effects. Microbiologica. 1989;12:15–28. [PubMed] [Google Scholar]
- 26.Olliaro P, Cattani J, Wirth D. Malaria, the submerged disease. J Am Med Assoc. 1996;275:230–233. [PubMed] [Google Scholar]
- 27.Peters W. Chemotherapy and drug resistance in malaria. New York, N.Y: Academic Press, Inc.; 1970. pp. 94–99. [Google Scholar]
- 28.Peters W. The chemotherapy of rodent malaria. XXII. The value of drug-resistant strains of P. berghei in screening for blood schizontocidal activity. Ann Trop Med Parasitol. 1975;69:155–171. [PubMed] [Google Scholar]
- 29.Reese R T, Langreth S G, Trager W. Isolation of stages of the human parasite Plasmodium falciparum from culture and from animal blood. Bull W H O. 1979;57:53–61. [PMC free article] [PubMed] [Google Scholar]
- 30.Roche J, Benito A, Ayecaba S, Amela C, Molina R, Alvar J. Resistance of Plasmodium falciparum to antimalarial drugs in Equatorial Guinea. Ann Trop Med Parasitol. 1993;87:443–449. doi: 10.1080/00034983.1993.11812794. [DOI] [PubMed] [Google Scholar]
- 31.Salako L A, Sowunmi A, Laoye O J. Evaluation of the sensitivity in vivo and in vitro of Plasmodium falciparum malaria to quinine in an area of full sensitivity to chloroquine. Trans R Soc Trop Med Hyg. 1988;82:366–368. doi: 10.1016/0035-9203(88)90120-4. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt L H. Plasmodium falciparum and Plasmodium vivax infections in the owl monkey (Aotus trivirgatus). II. Responses to chloroquine, quinine, and pyrimethamine. Am J Trop Med Hyg. 1978;27:703–717. doi: 10.4269/ajtmh.1978.27.703. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt L H. Plasmodium falciparum and Plasmodium vivax infections in the owl monkey (Aotus trivirgatus). III. Methods employed in the search for new blood schizonticidal drugs. Am J Trop Med Hyg. 1978;27:718–737. doi: 10.4269/ajtmh.1978.27.718. [DOI] [PubMed] [Google Scholar]
- 34.Skinner T S, Manning L S, Johnston W A, Davis T M. In vitro stage-specific sensitivity of Plasmodium falciparum to quinine and artemisinin drugs. Int J Parasitol. 1996;26:519–525. doi: 10.1016/0020-7519(96)89380-5. [DOI] [PubMed] [Google Scholar]
- 35.Smithuis F M, van Woensel J B, Nordlander E, Vantha W S, ter Kuile F O. Comparison of two mefloquine regimens for treatment of Plasmodium falciparum malaria on the northeastern Thai-Cambodian border. Antimicrob Agents Chemother. 1993;37:1977–1981. doi: 10.1128/aac.37.9.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sowunmi A, Oduola A M, Salako L A, Ogundahunsi O A, Laoye O J, Walker O. The relationship between the response of Plasmodium falciparum malaria to mefloquine in African children and its sensitivity in vitro. Trans R Soc Trop Med Hyg. 1992;86:368–371. doi: 10.1016/0035-9203(92)90221-w. [DOI] [PubMed] [Google Scholar]
- 37.Sowunmi A, Salako L A, Walker O, Ogundahunsi O A. Clinical efficacy of mefloquine in children suffering from chloroquine-resistant Plasmodium falciparum malaria in Nigeria. Trans R Soc Trop Med Hyg. 1990;84:761–764. doi: 10.1016/0035-9203(90)90067-o. [DOI] [PubMed] [Google Scholar]
- 38.Spencer H C, Masaba S C, Kiaraho D. Sensitivity of Plasmodium falciparum isolates to chloroquine in Kisumu and Malindi, Kenya. Am J Trop Med Hyg. 1982;31:902–906. doi: 10.4269/ajtmh.1982.31.902. [DOI] [PubMed] [Google Scholar]
- 39.Tin F, Hlaing N, Lasserre R. Single-dose treatment of falciparum malaria with mefloquine: field studies with different doses in semi-immune adults and children in Burma. Bull W H O. 1982;60:913–917. [PMC free article] [PubMed] [Google Scholar]
- 40.Van Rooijen N. The liposome-mediated macrophage ‘suicide’ technique. J Immunol Methods. 1989;124:1–6. doi: 10.1016/0022-1759(89)90178-6. [DOI] [PubMed] [Google Scholar]
- 41.Walker O, Salako L A, Obib P O, Bademosi K, Sodeinde O. The sensitivity of Plasmodium falciparum to chloroquine and amodiaquine in Ibadan, Nigeria. Trans R Soc Trop Med Hyg. 1984;78:782–784. doi: 10.1016/0035-9203(84)90019-1. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. Chemotherapy of malaria and resistance to antimalarials. WHO Tech Rep Ser. 1973;529:1–121. [PubMed] [Google Scholar]