Abstract
Background
Pulmonary sclerosing pneumocytoma is a kind of rare benign pulmonary tumor with potential malignancy. The clinical features, risk factors for prognosis, and optimal treatment have not been identified yet. This study aimed to investigate the clinical features and prognosis of pulmonary sclerosing pneumocytoma.
Methods
We retrospectively performed a review of pulmonary sclerosing pneumocytoma patients in West China Hospital from 2009 to 2019. The basic characteristics, treatment regimens, operation detail, postoperative variables, and follow-up time were recorded for each case. Differences in features between patients undergoing lobectomy and segmentectomy were compared. We also performed a case review and summarized reported clinical features in former studies.
Results
Altogether 61 pulmonary sclerosing pneumocytoma patients were retrospectively reviewed. Fifty-six patients were female and 5 were male. The patients’ median age was 51 (23-73). Seven (11.48%) patients had smoking history. Twenty tumors were located in the right lung [upper lobe (n = 7), middle (n = 2), and lower (n = 11)] and 41 in the left [upper (n = 12) and lower (n = 29)]. The median tumor size was 2 (0.9-7) cm. Thirty-six (59.02%) patients underwent sublobectomy (segmentectomy or wedge resection) whereas 25 (40.98%) underwent lobectomy. All patients recovered uneventfully, and no perioperative mortality was identified. Sublobectomy showed a trend towards reduced chest tube duration and shorter postoperative hospital stays compared with lobectomy.
Conclusions
The findings showed good prognosis of pulmonary sclerosing pneumocytoma and proved its benign characteristics. Sublobectomy showed advanced efficacy regarding chest tube duration and postoperative hospital stay compared with lobectomy.
Keywords: Pulmonary sclerosing pneumocytoma, Benign characteristic, Clinical features, Surgery
Background
Pulmonary sclerosing pneumocytoma (PSP), traditionally named pulmonary sclerosing hemangioma, is a kind of rare benign tumor with potential malignancy [1]. It was firstly described by Liebow et al. in 1956 [2]. Despite the implication by its name of a vascular neoplasm, sclerosing pneumocytoma was considered to be the tumor originated from pulmonary epithelium (type II pneumocyte) [3]. Therefore, some investigators called it as “pneumocytoma”. This kind of tumor was usually seen in the fifth-decade female [3], which was possibly attributed to the presence of progesterone receptors [1]. It was commonly presented as an asymptomatic solitary peripheral nodule [4] and an incidental lung mass on chest radiograms [5].
According to the WHO categorization of lung and pleural tumors (2018, ICD-11 for Mortality and Morbidity Statistics), pulmonary sclerosing pneumocytoma was categorized as benign, fibromatous neoplasms [6]. Nevertheless, there have been several reports on the possible malignant characteristics with lymph node metastasis [7–11] or local recurrence [12]. Although the prognosis of pulmonary sclerosing pneumocytoma seems not to be affected by these malignant potentials, there are other factors which may be related to the prognosis. The pulmonary sclerosing pneumocytoma have a large-scale range of size and could appear in different lobes of lung [13, 14]. However, whether these factors could affect the prognosis has not been discussed in a large cohort yet.
Furthermore, treatment for this kind of benign tumor remains controversial. Surgery was the main treatment for pulmonary sclerosing pneumocytoma [13, 14]. Sublobectomy, including mainly segmentectomy and wedge resection, tended to be preferred for peripheral small-sized tumor [7], while lobectomy could prevent the potential metastasis and recurrence which would worse long-term prognosis [15, 16]. However, which resection extent was optimal has not been answered well.
Herein, we retrospectively reviewed pulmonary sclerosing pneumocytoma patients admitted in our center from 2009 to 2019, aiming to investigate clinical features, risk factors, and treatment for patients with pulmonary sclerosing pneumocytoma.
Methods
Study cohort
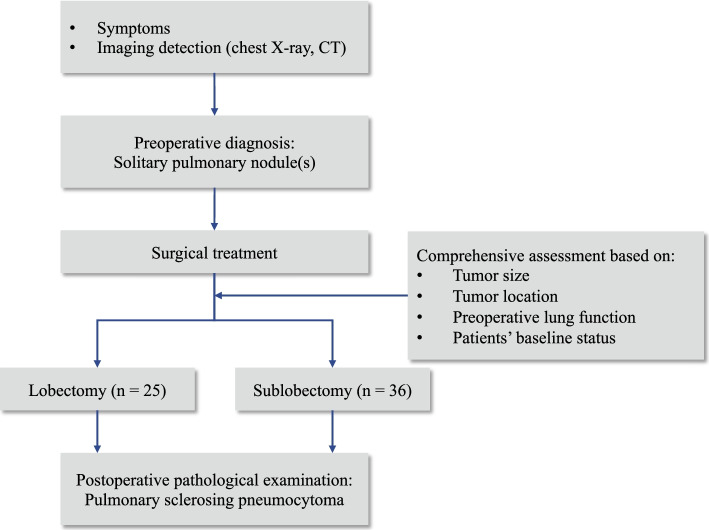
We retrospectively performed a chart review of patients admitted in West China Hospital of Sichuan University from June 2009 to August 2019. Chest computed tomography (CT) was conducted among all patients, and those who were initially diagnosed with solitary pulmonary nodule and strongly asked for surgery would receive surgical treatment (Fig. 1). Resection extent of lobectomy or sublobectomy (Fig. 2) was decided based on the following criteria: (ii) tumor size; (ii) tumor location (peripheral versus central); (iii) preoperative lung function test; (iv) patients’ baseline characteristics like age and BMI. The decision was based on comprehensive consideration of the above criteria [17, 18]. All patients underwent intraoperative frozen section analysis, and being confirmedly diagnosed by postoperative pathological examination. We performed selective lobe-specific lymph node dissection and mediastinal lymph node dissection as the intraoperative frozen section showed non-malignancy of tumors. After surgery, we inspected and recorded chest drainage volume and air leak every day until chest tube removal. The removal criteria consisted of less than 300 mL drainage fluid/day, no bubbling was observed lasting 12 h, and adequate lung inflation in chest radiology. Postoperative complications were recorded during daily patient round. Patient was discharged the next day after chest tube removal as if no accidence existed.
Fig. 1.
Flowchart showing diagnosis and management of patients in this study.
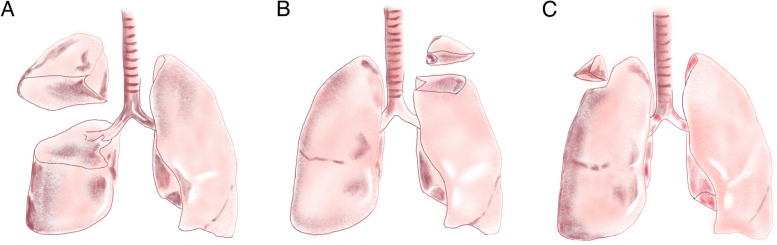
Fig. 2.
Representation image showing A lobectomy, sublobectomy: B segmentectomy and C wedge resection
The basic characteristics [demographic characteristics, smoking status, pulmonary function test outcomes, comorbidity (history of high blood pressure [HBP] and high glucose), tumor size, and location], treatment regimens, operation details (surgery duration and intraoperative blood loss), postoperative variables [length of postoperative hospital stay, chest tube duration and main postoperative complications (pulmonary infection, prolonged air leak [PAL], atelectasis, hoarseness, chylothorax, and bronchopleural fistula)] and follow-up time were collected. Tumor size was measured by the maximum diameter of tumor on preoperative CT scan. Chest tube duration was considered to be the time from chest tube placement to removal. PAL was considered as air leak lasting for more than 5 days. All indicators were defined according to the definition of the European Society of Thoracic Surgery and the Society of Thoracic Surgeons [19].
Follow-up protocol
All patients received history inquiry, physical examination, chest, abdominal, and brain CT scans or magnetic resonance imaging (MRI) every 3 months for the first 1 year after surgery, every 6 months for 2 to 3 years, and every 12 months after 3 years. Besides, bone scan was performed every year until the last follow-up. Follow-up time was defined as the time from the day of surgery to the day of death or last follow-up.
Literature review
We performed literature review on case series of PSP. We impose no limit on publication date. We summarized reported clinical features, including sex, age, tumor size, tumor location, extent of resection, and survival.
Statistical analysis
All statistical analyses were performed using IBM SPSS (version 25.0, IBM Corp., Armonk, NY). Dichotomous variables were described as number of cases and incidence, while continuous variables as median (range) or mean ± standard difference. We then compared clinical features and early postoperative outcomes between two groups of sublobectomy and lobectomy. Furthermore, we compared incidence of complications in subgroups regarding to tumor location, sex, smoking history, and history of HBP. Dichotomous variables were analyzed using the Pearson chi-squared test, while continuous variables were compared using the Student t test. All tests were two-sided. P value less than 0.05 was considered statistically significant.
Results
Clinical features
From June 2009 to August 2019, 61 pulmonary sclerosing pneumocytoma patients were included in our study who underwent curative pulmonary resection in our institution. Intraoperatively, 56/61 patients were found to be pulmonary sclerosing pneumocytoma according to frozen section analysis, while 5 patients were diagnosed with benign lung neoplasm which could not be specified. The diagnoses of pulmonary sclerosing pneumocytoma for 61 patients were confirmed by postoperative pathological examination (Figs. 1 and 2). A total of 56 patients were female and 5 patients were male. The patients’ median age was 51(23-73) and the mean BMI was 22.69 (2.48) kg/m2. Altogether, 7 patients had a smoking history. Two patients had a history of hyperglycemia and 10 had a history of HBP. The median tumor size was 2 (0.9-7) cm. Altogether, 20 tumors were located in the right lung [upper lobe (n = 7), middle lobe (n = 2), and lower lobe (n = 11)] and 41 in the left [upper lobe (n = 12) and lower lobe (n = 29)]. The incidence of complications varied among different tumor location (P = 0.007). Table 1 summarized the clinical features of all included patients.
Table 1.
Clinical characteristics of patients with pulmonary sclerosing pneumocytoma (N = 61)
| Characteristics | All (n = 61) | Sublobectomy (n = 36) | Lobectomy (n = 25) | P value |
|---|---|---|---|---|
| Age, year | 0.359 | |||
| Mean ± SD | 51.90 (12.40) | 50.67 (12.13) | 53.68 (13.06) | |
| Median (range) | 51 (23-73) | 51.5 (23-69) | 55 (23-73) | |
| Sex, n (%) | 1.000 | |||
| Male | 5 (8.20) | 3 (8.33) | 2 (8) | |
| Female | 56 (91.80) | 33 (91.67) | 23 (92) | |
| BMIa, kg/m2 | 22.69 (2.48) | 22.50 (2.19) | 22.95 (2.92) | 0.494 |
| Smoking history, n (%) | 0.430 | |||
| Ever | 7(11.48) | 3 (8.33) | 4 (16) | |
| Never | 54(88.52) | 33 (91.67) | 21 (84) | |
| Comorbidity, n (%) | ||||
| Hypertension | 10 (16.39) | 5 (13.89) | 5 (20) | 0.727 |
| Hyperglycemia | 2 (3.28) | 1 (2.78) | 1 (4) | 1.000 |
| Pulmonary function | ||||
| FEV1b, L | 2.33 (0.58) | 2.27 (0.56) | 2.41 (0.61) | 0.186 |
| FEV1/FVCc (%) | 77.41 (8.49) | 76.52 (10.49) | 78.67 (4.46) | 0.278 |
| Tumor size, cm | 0.004** | |||
| Mean ± SD | 2.87 (1.29) | 2.48 (1.04) | 3.44 (1.04) | |
| Median (range) | 2 (0.9-7) | 2.2 (0.9-5) | 3 (2-7) | |
| Tumor location (lobe) | 0.294 | |||
| Left upper | 12 | 7 | 5 | |
| Left lower | 29 | 17 | 12 | |
| Right upper | 7 | 2 | 5 | |
| Right middle | 2 | 2 | 0 | |
| Right lower | 11 | 8 | 3 | |
The data was presented as mean (SD) or median (range)
**P < 0.01. Continuous variables were compared using Student’s t test and categorical variables using chi-squared test
a BMI body mass index
b FEV1: the forced expiratory volume in 1 s
c FEV1/FVC: the forced expiratory volume in 1 second (FEV1)/forced volume vital capacity (FVC) ratio
The median follow-up duration was 30 (2-95) months with a total of 3/61 patients lost follow-up. All patients recovered uneventfully, and no perioperative mortality was identified. All patients were free of local recurrence or distant metastasis during the follow-up period. The postoperative complications include pulmonary infection (1/61), prolonged air leak (2/61), and atelectasis (2/61).
Pathological features
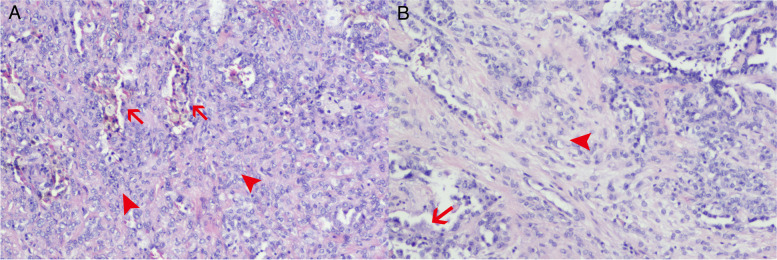
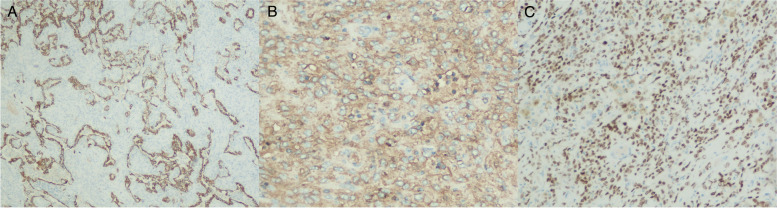
In gross, the sclerosing pneumocytoma was well circumscribed, nonencapsulated, easily shelled out, solid and firm. The cut surface might be mottled or hemorrhagic. The histological morphology of sclerosing pneumocytoma is of large diversity, while there are mainly four different pathologic patterns (Fig. 3): papillary, solid, sclerotic, and hemorrhagic. Not all cases showed the whole four patterns, but mostly at least three patterns coexisted, and often one or two patterns were predominant. There were mainly two cell types under the light microscope (Fig. 3A). The first is interstitial round cells or polygonal cells, with relatively consistent morphology, rich and light staining of cytoplasm, indistinct cell borders, fine nuclear chromatin, and rare mitoses. This type of cells could protrude into the alveolar cavity to form a papillary pattern, or diffusely proliferate to form a solid pattern. The second type is superficial cubic cells with eosinophilic cytoplasm, small and hyperchromatic nuclei, covering the surface of the papilla and lining the irregular adenoid fissures and vascular luminal surfaces in solid areas. The commonly positive marker of immunohistochemical staining included EMA and TTF-1 in both types of cells, while CK7, Napsin in surface cells, and Vimentin, PR, ER in interstitial round cells (Fig. 4).
Fig. 3.
Histological section of pulmonary sclerosing pneumocytoma. A Hemorrhagic pattern (arrow) and solid pattern (arrowhead). B Papillary pattern (arrow) and sclerotic pattern (arrowhead). The arrow in A also pointed to superficial cubic cells
Fig. 4.
Immunohistochemical staining of pulmonary sclerosing hemangioma. A CK-7 positive in superficial cubic cells. B EMA immunolabeling positive in the interstitial round cells. C TTF-1 positive in both type of cells
Outcomes related to different surgical regimen
There are 36 (59.02%) patients underwent sublobectomy (segmentectomy or wedge resection), whereas 25 (40.98%) patients received lobectomy. The mean tumor size was 2.48 cm in sublobectomy group, while 3.44 cm in lobectomy group.
The difference of basic demographic characteristics between the sublobectomy and lobectomy group was well distributed regarding age, sex, BMI, preoperative comorbidities, smoking status, pulmonary function, and tumor location. The sublobectomy group had shorter surgery time than lobectomy group did (88.17 ± 26.49 minutes vs. 125.40 ± 41.64 min, P < 0.001). The less intraoperative blood loss was also noticed in the sublobectomy group compared with lobectomy group (47.92 vs. 79.20 mL, P = 0.006). All the surgical procedures were uneventful with no intraoperative severe bleeding or mortality. All the early postoperative outcomes of the 2 groups were showed in Table 2. Patients undergoing sublobectomy showed a trend towards reduced time to chest tube removal compared with lobectomy group [2(1-7) days, vs. 3(2-6) days, P < 0.001]. The median length of postoperative hospital stay in sublobectomy group was significantly shorter than that in the lobectomy group [4(2-10) days vs. 6(4-9) days, P = 0.003]. With respect to postoperative complications including pulmonary infection, prolonged air leak, and atelectasis, no significant difference was summarized between the sublobectomy and lobectomy groups. The median follow-up time was 28.5(7-95) months in sublobectomy group and 38.28(2-92) months in lobectomy group, no significant statistical difference was identified (P = 0.351).
Table 2.
Clinical outcomes of patients with pulmonary sclerosing pneumocytoma (N = 61)
| Characteristics | Sublobectomy (n = 36) |
Lobectomy (n = 25) |
P value |
|---|---|---|---|
| Surgery time, min | 88.17 (26.49) | 125.40 (41.64) | < 0.001*** |
| Intraoperative blood loss, ml | 47.92 (29.36) | 79.20 (47.52) | 0.006** |
| Chest tube duration, day | 2 (1-7) | 3 (2-6) | < 0.001*** |
| Postoperative hospital stays, day | 4 (2-10) | 6 (4-9) | 0.003** |
| Complications, n (%) | 2 (5.56) | 2 (8) | 1.000 |
| Pulmonary infection | 0 | 1 | 0.410 |
| Prolonged air leak | 2 | 0 | 0.508 |
| Atelectasis | 1 | 1 | 1.000 |
| Follow-up time, months | 28.5 (7-95) | 38.28 (2-92) | 0.351 |
The data was presented as mean (SD) or median (range)
**P < 0.01
***P < 0.001. Continuous variables were compared using Student’s t test and categorical variables using chi-squared test
Results of literature review
Altogether 27 articles reporting on case series of PSP were identified (Table 3). Age and sex were reported in all articles. The mean age in all reported patients was 49 (14.51) and ranged from 10 to 78 years old. The ratio for female was 81.7% (201/246). The mean (SD) of tumor size was 2.75 (1.95) cm among all reported patients and ranged from 0.5 to 12 cm.
Table 3.
Results of literature review
| Author | Years | Patients | Female | Age | Size, cm | Lobe locationa | Extent of resection | Survival status | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RUL | RML | RLL | LUL | LLL | Lobectomy | Sub-lobectomy | Lost to follow-up | Dead | Alive | ||||||
| K.W. Chan [20] | 1982 | 14 | 14 | 50 (16.21) | 2.33 (0.64) | 0 | 2 | 5 | 4 | 1 | 9 | 1 | 6 | 1 | 7 |
| A.S Fassina [21] | 1986 | 6 | 3 | 59 (9.72) | 1.83 (0.59) | 4 | 2 | 1 | 5 | ||||||
| N.P Ohori [22] | 1991 | 14 | 14 | 45 (14.65) | 3 | 3 | 8 | ||||||||
| A.C. Chan [23] | 2000 | 1 6 | 15 | 52 (14.13) | 2.39 (1.65) | 1 | 5 | 4 | 0 | 6 | |||||
| J.E. Nam [24] | 2002 | 2 | 2 | 50 (16.26) | 2.25 (1.06) | 0 | 0 | 0 | 0 | 2 | |||||
| M.C. Aubry [25] | 2002 | 16 | 16 | 50 (12.65) | 2.96 (2.35) | 1 | 10 | 1 | 1 | 14 | |||||
| Y.C. Cheung [26] | 2003 | 6 | 5 | 35 (14.58) | 6 | 2 | |||||||||
| K. Yamazaki [27] | 2004 | 7 | 7 | 54 (14.30) | 4.50 (3.22) | ||||||||||
| S.D. Sak [28] | 2007 | 26 | 16 | 53 (9.76) | 2.08 (1.31) | 5 | 2 | 3 | 2 | 14 | |||||
| G. Sartori [29] | 2007 | 11 | 10 | 47 (11.00) | 3.09 (0.80) | 1 | 2 | 0 | 4 | 4 | 5 | 6 | 11 | ||
| S. Islam [30] | 2009 | 6 | 3 | 56 (9.75) | 1.92 (0.59) | 1 | 1 | ||||||||
| K.H. Lin [31] | 2011 | 6 | 5 | 50 (11.29) | 2.87 (1.55) | 0 | 1 | 1 | 1 | 3 | |||||
| Q.B. Wang [32] | 2011 | 16 | 14 | 53 (11.66) | 1 | 9 | 4 | 2 | 1 | ||||||
| Lee, E [33]. | 2013 | 26 | 23 | 44 (11.47) | 2.47 (1.26) | 2 | 6 | 6 | 2 | 8 | |||||
| C.Y. Wu [34] | 2016 | 14 | 14 | 53 (15.12) | 2.26 (1.41) | 2 | 2 | 2 | 3 | 5 | |||||
| A. Lovrenski [35] | 2019 | 6 | 5 | 51 (9.00) | 1.75 (0.50) | 0 | 0 | 1 | 2 | 3 | 6 | ||||
| J. Xu [36] | 2019 | 22 | 12 | 54 (12.76) | 2.59 (1.38) | ||||||||||
| Q. Gao [37] | 2020 | 32 | 23 | 41 (18.57) | 4.77 (3.11) | 4 | 1 | 9 | 6 | 12 | |||||
| Overall | 246 | 201 | 49 (14.51) | 2.75 (1.95) | 16 | 30 | 35 | 26 | 59 | 26 | 22 | 11 | 5 | 51 | |
| Median (range) | 51(10-78) | 2.20 (0.50-1.20) | |||||||||||||
The data was presented as mean (SD) or median (range)
a LUL left upper lobe, LLL left lower lobe, RLL right lower lobe, RML, right middle lobe, RLL right lower lobe
Discussion
In most cases, pulmonary sclerosing pneumocytoma has a benign behavior. Shibata and colleagues [38] reported a patient with pulmonary sclerosing pneumocytoma that progressed into severe exertional dyspnea 47 years after detection of abnormal shadow through X-ray. The tumor measured 20 × 16 × 15 cm, weighed 2.3 kg, and occupied the whole left thoracic cavity. This case indicated that pulmonary sclerosing pneumocytoma was not self-limiting despite its benign nature. Some pulmonary sclerosing pneumocytoma cases have been reported with multiple lung involvement [39], lymph node metastasis [8, 11, 16], and distant metastasis [40]. Those elucidated the potential malignant nature of pulmonary sclerosing pneumocytoma. There were theories that lymph node metastasis of pulmonary sclerosing pneumocytoma was mediated through air space pattern [11]. Dantis and colleagues [11] reported that PET scan could help guide lymph node dissection, since the cases with SUV max uptake of more than 2.5 mostly had positive mediastinal lymph node metastasis. Wei and colleagues [4] reported a pulmonary sclerosing pneumocytoma case with local recurrence 10 years after initial wedge resection. This patient was subjected to a second wedge resection to completely remove the recurrent lesion. Iyoda and colleagues [12] also reported a pulmonary sclerosing pneumocytoma case with local recurrence 4 years after initial resection and therefore subjected to a second resection. However, these cases did not indicate a dismal prognosis. In our case series, all patients recovered uneventfully, and no perioperative mortality, postoperative recurrence, or metastasis was identified. In the review of prior studies, among 67 patients who reported survival status, 5 were reported of death. Overall, the biological behavior of pulmonary sclerosing pneumocytoma is obscure.
The molecular alterations in the sclerosing pneumocytoma were one of the study focuses and showed diagnostic value. AKT1 mutation was the most commonly reported gene mutation and was speculated to be the genetic hallmark of sclerosing pneumocytoma [11, 41, 42]. AKT1 mutation might induce cells proliferation and morphology changing, but would not induce progress to malignancy [41]. Beta-catenin was the secondly most common gene mutation in the sclerosing pneumocytoma [11, 41], which might also play a role in producing a benign tumor but not a malignant one. Mutations in other tumor-related genes were also identified in the sclerosing pneumocytoma, like PTEN, BRAF V600E, BLM, KMT2D, but with relatively smaller incidence than AKT1 and β-catenin [11, 41, 43].
Pulmonary sclerosing pneumocytoma is more common in females and could occur in all ages. In our case series, female patients occupied 91% and patient age ranged from 23 to 73, while in the literature ratio of female was 81% and the age ranged from 10 to 78 years old. Previous studies have shown that the sclerosing pneumocytoma has no predilection for a particular lobe of the lung [14]. And there have not been any studies focusing on the prognostic effect of the tumor location. Our study showed that the incidence of complications varied among different tumor location (P = 0.007). Tumor located on right middle lobe showed a trend toward higher incidence of complications. We also evaluated other factors which may affect the early postoperative outcomes of the tumor, including sex, smoking history, and history of HBP; however, none of them showed relationship with prognosis.
There were few studies having a discussion on whether sublobectomy or lobectomy has better oncological outcomes for pulmonary sclerosing pneumocytoma [44]. We performed a comparison between the outcomes of the two surgical regimens in this case series. The distribution of age, preoperative morbidities, and lung function between patients with the two surgical regimens were well balanced. The sublobectomy (segmentectomy or wedge resection) showed a trend towards a better clinical outcome, with reduced time to chest tube removal and length of postoperative hospital stay compared with lobectomy for pulmonary sclerosing pneumocytoma patients. It meant that we could consider sublobectomy more for pulmonary sclerosing pneumocytoma, for it could not only remove the tumor completely, but also preserve lung function more.
This study had several limitations. First, the follow-up duration was limited. However, the long-term survival outcomes on pulmonary sclerosing pneumocytoma remained unclear, thus the optimal follow-up duration required was still uncertain. This exploratory study might provide the reference for follow-up in the further study. Considering its possible malignant characteristics, future studies could conduct a longer follow-up procedure (> 3 years) to observe outcomes (recurrence or metastasis) effectively. Second, limited sample size might restrict the sufficiency and efficiency of the conclusions. Researches with larger sample size was warranted. Third, as the potential selecting bias existed in comparison between two groups of patients with different surgical regimens (lobectomy or sublobectomy), the conclusion on outcomes different surgical regimen should be referenced cautiously. Larger clinical trials are expected to provide further analysis.
Conclusion
Pulmonary sclerosing pneumocytoma showed benign behavior both in our case series and literature review. All patients in our case series recovered uneventfully without metastasis and recurrence. Both sublobectomy and lobectomy could achieve radical resection and present promising clinical outcomes. Sublobectomy showed a trend towards reduced chest tube duration and shorter postoperative hospital stay compared with lobectomy.
Acknowledgements
None declared.
Abbreviations
- PSP
Pulmonary sclerosing pneumocytoma
- CT
Computed tomography
- HBP
High blood pressure
- PAL
Prolonged air leak
- MRI
Magnetic resonance imaging
Authors’ contributions
Conception and design: FL, QZ, JZ. Acquisition of data: FL, SM, GL, ZL. Data analysis and interpretation: QZ, JZ, BC, TW. Drafting of manuscript: All authors. Final approval of manuscript: All authors.
Funding
This work was supported by the Key Research and Development Program, Department of Science and Technology of Sichuan Province (2019YFS0335) (to Dr. Feng Lin), Post-Doctor Research Project, West China Hospital, Sichuan University (2020HXBH109) (to Dr. Jian Zhou).
Availability of data and materials
The datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.
Declarations
Ethic approval and consent to participate
The study was approved by Institutional Ethic Committee for Clinical Research of West China Hospital, Sichuan University [NO.2019 (445)] and individual consent for this retrospective analysis was waived.
Consent for publication
The study contained no individual person’s data and individual consent for this retrospective analysis was waived.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Quan Zheng and Jian Zhou contributed equally to this work.
References
- 1.Devouassoux-Shisheboran M, Hayashi T, Linnoila RI, et al. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol. 2000;24:906–916. doi: 10.1097/00000478-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Liebow AA, Hubbell DS. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer. 1956;9:53–75. doi: 10.1002/1097-0142(195601/02)9:1<53::AID-CNCR2820090104>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.Baysak A, Oz AT, Moğulkoç N, et al. A rare tumor of the lung: pulmonary sclerosing hemangioma (pneumocytoma) Respir Med. 2013;107:448–450. doi: 10.1016/j.rmed.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Wei S, Tian J, Song X, et al. Recurrence of pulmonary sclerosing hemangioma. Thorac Cardiovasc Surg. 2008;56:120–122. doi: 10.1055/s-2007-989280. [DOI] [PubMed] [Google Scholar]
- 5.Chien NC, Lin CW, Tzeng JE. Sclerosing haemangioma with lymph node metastasis. Respirology. 2009;14:614–616. doi: 10.1111/j.1440-1843.2009.01523.x. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. 2018. https://icd.who.int/browse11/l-m/en. Accessed 2 Apr 2019.
- 7.Park JS, Kim K, Shin S, et al. Surgery for Pulmonary Sclerosing Hemangioma: Lobectomy versus Limited Resection. Korean J Thorac Cardiovasc Surg. 2011;44:39–43. doi: 10.5090/kjtcs.2011.44.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katakura H, Sato M, Tanaka F, et al. Pulmonary sclerosing hemangioma with metastasis to the mediastinal lymph node. Ann Thorac Surg. 2005;80:2351–2353. doi: 10.1016/j.athoracsur.2004.06.099. [DOI] [PubMed] [Google Scholar]
- 9.Kim KH, Sul HJ, Kang DY. Sclerosing hemangioma with lymph node metastasis. Yonsei Med J. 2003;44:150–154. doi: 10.3349/ymj.2003.44.1.150. [DOI] [PubMed] [Google Scholar]
- 10.Kocaman G, Yenigün MB, Ersöz CC, et al. Pulmonary sclerosing pneumocytoma with mediastinal lymph node metastasis: a case report. Gen Thorac Cardiovasc Surg. 2021;69:142–146. doi: 10.1007/s11748-020-01431-1. [DOI] [PubMed] [Google Scholar]
- 11.Dantis KD, Gupta AKD, Kashyap NKD, et al. Pulmonary sclerosing pneumocytoma masquerading adenocarcinoma with co-existing BRAF V600E and PTEN mutation. Cancer Treat Res Commun. 2021;28:100429. doi: 10.1016/j.ctarc.2021.100429. [DOI] [PubMed] [Google Scholar]
- 12.Iyoda A, Hiroshima K, Shiba M, et al. Clinicopathological analysis of pulmonary sclerosing hemangioma. Ann Thorac Surg. 2004;78:1928–1931. doi: 10.1016/j.athoracsur.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 13.Kuo KT, Hsu WH, Wu YC, et al. Sclerosing hemangioma of the lung: an analysis of 44 cases. J Chin Med Assoc. 2003;66:33–38. [PubMed] [Google Scholar]
- 14.Lei Y, Yong D, Jun-Zhong R, et al. Treatment of 28 patients with sclerosing hemangioma (SH) of the lung. J Cardiothorac Surg. 2012;7:34. doi: 10.1186/1749-8090-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeda R, Isowa N, Miura H, et al. Bilateral multiple sclerosing hemangiomas of the lung. Gen Thorac Cardiovasc Surg. 2009;57:667–670. doi: 10.1007/s11748-009-0452-y. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Zhang L, Wang Y, et al. Sclerosing pneumocytoma with metastasis to the mediastinal and regional lymph nodes. Indian J Pathol Microbiol. 2018;61:407–409. doi: 10.4103/IJPM.IJPM_98_17. [DOI] [PubMed] [Google Scholar]
- 17.Khullar OV, Liu Y, Gillespie T, et al. Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol. 2015;10:1625–1633. doi: 10.1097/JTO.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raman V, Jawitz OK, Voigt SL, et al. The Effect of Tumor Size and Histologic Findings on Outcomes After Segmentectomy vs Lobectomy for Clinically Node-Negative Non-Small Cell Lung Cancer. Chest. 2021;159:390–400. doi: 10.1016/j.chest.2020.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez FG, Falcoz PE, Kozower BD, et al. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg. 2015;99:368–376. doi: 10.1016/j.athoracsur.2014.05.104. [DOI] [PubMed] [Google Scholar]
- 20.Chan KW, Gibbs AR, Lo WS, et al. Benign sclerosing pneumocytoma of lung (sclerosing haemangioma) Thorax. 1982;37:404–412. doi: 10.1136/thx.37.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fassina AS, Rugge M, Scapinello A, et al. Plasma cell granuloma of the lung (inflammatory pseudotumor) Tumori. 1986;72:529–534. doi: 10.1177/030089168607200515. [DOI] [PubMed] [Google Scholar]
- 22.Ohori NP, Yousem SA, Sonmez-Alpan E, et al. Estrogen and progesterone receptors in lymphangioleiomyomatosis, epithelioid hemangioendothelioma, and sclerosing hemangioma of the lung. Am J Clin Pathol. 1991;96:529–535. doi: 10.1093/ajcp/96.4.529. [DOI] [PubMed] [Google Scholar]
- 23.Chan AC, Chan JK. Pulmonary sclerosing hemangioma consistently expresses thyroid transcription factor-1 (TTF-1): a new clue to its histogenesis. Am J Surg Pathol. 2000;24:1531–1536. doi: 10.1097/00000478-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Nam JE, Ryu YH, Cho SH, et al. Air-trapping zone surrounding sclerosing hemangioma of the lung. J Comput Assist Tomogr. 2002;26:358–361. doi: 10.1097/00004728-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Aubry MC, Myers JL, Colby TV, et al. Endometrial stromal sarcoma metastatic to the lung: a detailed analysis of 16 patients. Am J Surg Pathol. 2002;26:440–449. doi: 10.1097/00000478-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Cheung YC, Ng SH, Chang JW, et al. Histopathological and CT features of pulmonary sclerosing haemangiomas. Clin Radiol. 2003;58:630–635. doi: 10.1016/S0009-9260(03)00177-6. [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki K. Type-II pneumocyte differentiation in pulmonary sclerosing hemangioma: ultrastructural differentiation and immunohistochemical distribution of lineage-specific transcription factors (TTF-1, HNF-3 alpha, and HNF-3 beta) and surfactant proteins. Virchows Arch. 2004;445:45–53. doi: 10.1007/s00428-004-1023-3. [DOI] [PubMed] [Google Scholar]
- 28.Sak SD, Koseoglu RD, Demirag F, et al. Alveolar adenoma of the lung. Immunohistochemical and flow cytometric characteristics of two new cases and a review of the literature. Apmis. 2007;115:1443–1449. doi: 10.1111/j.1600-0463.2007.00762.x. [DOI] [PubMed] [Google Scholar]
- 29.Sartori G, Bettelli S, Schirosi L, et al. Microsatellite and EGFR, HER2 and K-RAS analyses in sclerosing hemangioma of the lung. Am J Surg Pathol. 2007;31:1512–1520. doi: 10.1097/PAS.0b013e318032c8cc. [DOI] [PubMed] [Google Scholar]
- 30.Islam S, Roustan Delatour NL, Salahdeen SR, et al. Cytologic features of benign solitary pulmonary nodules with radiologic correlation and diagnostic pitfalls: a report of six cases. Acta Cytol. 2009;53:201–210. doi: 10.1159/000325126. [DOI] [PubMed] [Google Scholar]
- 31.Lin KH, Chang CP, Liu RS, et al. F-18 FDG PET/CT in evaluation of pulmonary sclerosing hemangioma. Clin Nucl Med. 2011;36:341–343. doi: 10.1097/RLU.0b013e31820aa00c. [DOI] [PubMed] [Google Scholar]
- 32.Wang QB, Chen YQ, Shen JJ, et al. Sixteen cases of pulmonary sclerosing haemangioma: CT findings are not definitive for preoperative diagnosis. Clin Radiol. 2011;66:708–714. doi: 10.1016/j.crad.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Lee E, Park CM, Kang KW, et al. 18F-FDG PET/CT features of pulmonary sclerosing hemangioma. Acta Radiol. 2013;54:24–29. doi: 10.1258/ar.2011.110474. [DOI] [PubMed] [Google Scholar]
- 34.Wu CY, Wang J, Chang NY. A Comparative Study of Intraoperative Cytology and Frozen Sections of Sclerosing Pneumocytoma. Int J Surg Pathol. 2016;24:600–606. doi: 10.1177/1066896916648448. [DOI] [PubMed] [Google Scholar]
- 35.Lovrenski A, Vasilijević M, Panjković M, et al. Sclerosing Pneumocytoma: A Ten-Year Experience at a Western Balkan University Hospital. Medicina. 2019;55:27. [DOI] [PMC free article] [PubMed]
- 36.Xu J, Dong Y, Yin G, et al. (18) F-FDG PET/CT imaging: A supplementary understanding of pulmonary sclerosing pneumocytoma. Thorac Cancer. 2019;10:1552–1560. doi: 10.1111/1759-7714.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao Q, Zhou J, Zheng Y, et al. Clinical and histopathological features of pulmonary sclerosing pneumocytoma with dense spindle stromal cells and lymph node metastasis. Histopathology. 2020;77:718–727. doi: 10.1111/his.14159. [DOI] [PubMed] [Google Scholar]
- 38.Shibata R, Mukai M, Okada Y, et al. A case of sclerosing hemangioma of the lung presenting as a gigantic tumor occupying the left thoracic cavity. Virchows Arch. 2003;442:409–411. doi: 10.1007/s00428-003-0777-3. [DOI] [PubMed] [Google Scholar]
- 39.Komatsu T, Fukuse T, Wada H, et al. Pulmonary sclerosing hemangioma with pulmonary metastasis. Thorac Cardiovasc Surg. 2006;54:348–349. doi: 10.1055/s-2005-872976. [DOI] [PubMed] [Google Scholar]
- 40.Kim MK, Jang SJ, Kim YH, et al. Bone metastasis in pulmonary sclerosing hemangioma. Korean J Intern Med. 2015;30:928–930. doi: 10.3904/kjim.2015.30.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung SH, Kim MS, Lee SH, et al. Whole-exome sequencing identifies recurrent AKT1 mutations in sclerosing hemangioma of lung. Proc Natl Acad Sci U S A. 2016;113:10672–10677. doi: 10.1073/pnas.1606946113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeh YC, Ho HL, Wu YC, et al. AKT1 internal tandem duplications and point mutations are the genetic hallmarks of sclerosing pneumocytoma. Mod Pathol. 2020;33:391–403. doi: 10.1038/s41379-019-0357-y. [DOI] [PubMed] [Google Scholar]
- 43.Jiang G, Zhang M, Tan Q, et al. Identification of the BRAF V600E mutation in a patient with sclerosing pneumocytoma: A case report. Lung Cancer. 2019;137:52–55. doi: 10.1016/j.lungcan.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Ng WK, Fu KH, Wang E, et al. Sclerosing hemangioma of lung: a close cytologic mimicker of pulmonary adenocarcinoma. Diagn Cytopathol. 2001;25:316–320. doi: 10.1002/dc.2162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.