Abstract
Background
Axillary lymph node characteristics on axillary ultrasound (US), breast MRI and 18F-FDG PET/CT are relevant at breast cancer diagnosis. Axillary lymphadenopathy after COVID-19 vaccination has been frequently reported. This may cause a diagnostic dilemma, particularly in the ipsilateral axilla in women who have a either a recent diagnosis of breast cancer or a history of breast cancer. This review provides an overview of the current evidence regarding axillary lymph node characteristics at breast cancer diagnosis versus “post-COVID-19 vaccination”.
Methods
A non-systematic narrative review was performed. Studies describing axillary lymph node characteristics per imaging modality (axillary US, breast MRI and 18F-FDG PET/CT) in breast cancer patients versus post-COVID-19 vaccination were selected and used for the current study.
Results
The morphologic characteristics and distribution of abnormal nodes on US may differ from the appearance of metastatic adenopathy since diffuse cortical thickening of the lymph nodes is the most observed characteristic after vaccination, whereas metastases show as most suspicious characteristics focal cortical thickening and effacement of the fatty hilum. Current evidence on MRI and 18F-FDG on morphologic characteristics of axillary lymphadenopathy is missing, although it was suggested that vaccine related lymphadenopathy is more likely to be present in level 2 and 3 nodes than metastatic nodes. Reported frequencies of lymphadenopathy post-COVID-19 vaccination range from 49% to 85% (US), 29% (breast MRI) and 14.5% to 53.9% (18F-FDG PET/CT). Several factors may impact the presence or extent of lymphadenopathy post-COVID-19 vaccination: injection site, type of vaccine (i.e., mRNA versus vector), time interval (days) between vaccination and imaging, previous history of COVID-19 pneumonia, and first versus second vaccine dose.
Conclusion
Although lymph node characteristics differ at breast cancer diagnosis versus post-COVID-19 vaccination, clinical information regarding injection site, vaccine type and vaccination date needs to be documented to improve the interpretation and guide treatment towards the next steps of action.
Abbreviations: cN0, patients without suspicious axillary lymph node findings; cN+, patients with suspicious axillary lymph node findings; US, ultrasound; MRI, magnetic resonance imaging; 18F-FDG, fluorodexoyglucose; PET/CT, positron emission tomography / computed tomography
Keywords: Axilla, Lymphadenopathy, Breast cancer, COVID-19 vaccination
1. Introduction
Presence of axillary lymph node metastases at time of breast cancer diagnosis is considered to be one of the most important prognostic factors, as it decreases the general five-year overall survival rate from 98% to 85% [1]. Consequently, European guidelines recommend axillary ultrasound (US) at time of breast cancer diagnosis, followed by the proper axillary treatment strategy depending on imaging findings (including biopsy results): patients without suspicious axillary lymph node findings (cN0) versus patients with suspicious axillary lymph node findings (cN+) [2], [3]. Besides axillary US, other imaging modalities like breast MRI and 18F-FDG PET/CT can be used for the evaluation of axillary lymph node status in breast cancer patients, although 18F-FDG PET/CT is only used for more advanced disease [4].
The COVID-19 pandemic created dilemmas in the evaluation of axillary lymph nodes in newly diagnosed breast cancer patients. Axillary lymphadenopathy was frequently observed in patients after the introduction of COVID-19 vaccinations, although multiple studies also confirmed occurrence of lymphadenopathy in patients diagnosed with COVID-19 pneumonia (albeit relatively uncommon when compared to other viral pneumonia), [5], [6], [7]. The COVID-19 vaccines are commonly injected intramuscularly in the deltoid muscle of the (preferably) non-dominant arm, causing relatively frequent lymph node swelling at physical examination in the ipsilateral axilla and supraclavicular region in up to 16% [8], [9], [10].
When considering a recent breast cancer diagnosis, lymphadenopathy ‘post-COVID-19 vaccination’ might create clinical dilemmas, since differentiation between benign versus malignant lymph nodes will become even more challenging, especially after breast cancer diagnosis on the ipsilateral side of the COVID-19 vaccine site [11], [12]. The aim of this report is to provide an overview of the characteristics of lymphadenopathy using axillary US, breast MRI and 18F-FDG PET in women with breast cancer and a recent history of COVID-19 vaccination and how best to approach them.
2. Methods
A non-systematic narrative review was performed. Studies describing axillary lymph node characteristics per imaging modality (axillary US, breast MRI and 18F-FDG PET/CT) in breast cancer patients were selected in accordance of all three authors, based on experience and expertise of the three authors (T.v.N., M.J., M.L.). Next, a search to select studies describing axillary lymph node characteristics ‘post-COVID-19 vaccination’ per imaging modality (axillary US, breast MRI and 18F-FDG PET/CT) was performed in the medical database of PubMed until March 30, 2022. Search terms included axilla or lymphadenopathy and COVID-19. Next, manual cross-reference search was performed to select additional studies. All studies were selected in accordance by the three authors (T.v.N., M.J., M.L.).
2.1. Axillary ultrasound
At breast cancer diagnosis, axillary US is performed with a high frequency linear array transducer. Normal axillary lymph nodes are oval shaped, have a well-defined margin with an hypoechoic thin cortex (with a thickness 2.3–3 mm, depending on guidelines in different countries) [4], [13]. Suspicious US characteristics include diffuse or focal cortical thickening, irregular margins, and effacement of the fatty hilum. Focal cortical thickening and effacement of the fatty hilum can be considered highly suspicious of malignancy when compared to the other US characteristics [14]. In addition, extranodal extension can be seen [15].
After administration of the COVID-19 vaccine, the most frequent US characteristic of lymphadenopathy post-COVID-19 vaccination, which can occur in the ipsi- and/or contralateral axilla of the injection site, includes diffuse cortical thickening similar to the suspicious US characteristics of axillary lymph nodes at breast cancer diagnosis [16]. Igual-Rouilleault et al. evaluated a cohort of 90 healthy volunteers with axillary ultrasound before and one week after first and second vaccines and demonstrated lymphadenopathy in 64% and 85% of these volunteers (Comirnaty, Table 1 ) [16]. Diffuse cortical thickening was the most frequent sonographic finding in this cohort. Interestingly, a significantly greater cortical thickness was observed in patients without a previous history of COVID-19 pneumonia. Park et al. demonstrated lymphadenopathy post-COVID-19 vaccination in 49% of the healthy women referred to a breast cancer unit within 12 weeks after vaccination in a cohort of 413 women [17]. Unfortunately, the true number of false positive characteristics due to post-COVID-19 vaccination remains unclear because no tissue sampling was performed. In addition, they reported significantly more frequent lymphadenopathy post-COVID-19 vaccination after mRNA vaccine (Comirnaty, Spikevax) when compared to a vector vaccine (Vaxzevria) [17]. On the other hand, in a cohort of 24 patients with lymphadenopathy at physical examination, Cocco et al. found no differences in the frequency of lymphadenopathy among different COVID-19 vaccines (Comirnaty, Vaxzevria and Spikevax, respectively) detected at US [18].
Table 1.
Overview of reported COVID-19 vaccines in current report.
| Developer | Commercial name | Type of vaccine |
|---|---|---|
| Oxford-AstraZeneca | Vaxzevria | Adenovirus vector |
| Johnson and Johnson – Jannssen Pharmaceuticals | COVID-19 Vaccine Janssen | Adenovirus vector |
| Moderna | Spikevax | mRNA |
| Pfizer-BioNTech | Comirnaty | mRNA |
To reduce the number of false positive cases of lymphadenopathy in healthy women who are scheduled for routine breast evaluation including axillary US, evaluation imaging should be performed prior to or at least 12 weeks after the vaccination date [11], [19]. In patients with newly diagnosed breast cancer, clearly, breast and axillary should be performed without any delays. The follow-up time of at least 12 weeks post-COVID-19 vaccination is in line with the studies of Mehta et al. and Nguyen et al. demonstrating normalization of axillary lymph nodes at US 12–16 weeks post-COVID-19 vaccination in most of the patients [20], [21]. There are currently no reports of persistant vaccine induced lymphadenopathy on US after a follow-up period more than 16 weeks.
2.2. Breast MRI
Breast MRI can be used for primary (intramammary) tumor staging at breast cancer diagnosis. Axillary lymph nodes can be evaluated on breast MRI as well, although lymph node evaluation should not be considered an indication to perform breast MRI. One should be aware that the complete axillary region, in particularly level II/III, is not always included in the field of view during breast MRI [22]. The appearance of normal axillary lymph nodes on MRI is similar to that on ultrasound if the nodes can be adequately seen: oval shaped nodes, thin cortex (although less appreciated at MRI when compared to US) and symmetric contralateral axillary lymph nodes and presence of fatty hilum [23]. Enhancement of lymph nodes is a poor discriminator between benign and malignant axillary lymph nodes. Suspicious nodes have cortical thickening, loss of fatty hilum, round shape, irregular margin, inhomogeneous cortex, irregular enhancement and perinodal edema [4], [24].
After administration of COVID-19 vaccine, morphological MRI characteristics of lymphadenopathy post-COVID-19 vaccination have not yet been described in detail. Interestingly, Plaza et al. suggest a different distribution of suspicious lymph nodes with abnormal high level II/III axillary lymph nodes and normal level I axillary lymph nodes post-COVID-19 vaccination when compared to the most common distribution of abnormal lymph nodes in level I / II (lower part) at breast cancer diagnosis [25]. According to the authors, this difference in nodal distribution may be due to a difference in lymphatic drainage of intramuscularly injected COVID-19 vaccine in the deltoid muscle when compared to the lymphatic drainage pattern originating from the breast.
The frequency of lymphadenopathy on MRI post-COVID-19 vaccination (Comirnaty, Spikevax, COVID-19 Vaccine Janssen) was reported by Horvat et al., 29% in a cohort of 357 patients that underwent breast MRI for screening or preoperative staging [26]. Detailed data on morphologic characteristics of the lymphadenopathy was missing in this study. 11% of vaccine induced lymphadenopathy persisted at follow-up (axillary US, breast MRI, 18F-FDG PET/CT or chest CT), performed 4–10 weeks after the second dose of the vaccine.
2.3. 18F-FDG PET/CT
18F-FDG PET/CT may be performed in patients with locally advanced breast cancer, particularly patients with stage IIB or III disease. It is rarely performed to evaluate for axillary lymphadenopathy in early-stage disease as the sensitivity for all comers is only approximately 60% [27], [28], [29]. Therefore, 18F-FDG PET/CT does not improve axillary lymph node assessment when compared to axillary US and MRI. Yet, hypermetabolic axillary lymph nodes can be considered highly suggestive of malignancy in patients in the non-infectious setting [30].
After administration of COVID-19 vaccine, hypermetabolic axillary lymph nodes on 18F-FDG PET/CT can be detected representing lymphadenopathy post-COVID-19 vaccination [31]. This phenomenon was described as uncommon finding in earlier studies with vaccines such as influenza and human papillomavirus [32], [33]. The frequency of hypermetabolic axillary lymphadenopathy on 18F-FDG PET/CT post-COVID-19 vaccination was investigated by Bernstine in a cohort of 650 cancer patients with recent Comirnaty vaccination: 14.5% after first dose and 43.3% after second dose [34]. Besides the difference in frequency of hypermetabolic axillary lymphadenopathy, there was no difference in amount of metabolic uptake between the two vaccination doses. The increased frequency of hypermetabolic lymphadenopathy post-COVID-19 vaccination (Comirnaty) after the second dose when compared to the first dose was confirmed by Cohen et al. in a cohort of 728 patients that underwent 18F-FDG PET/CT: 36.4% versus 53.9% [35].
Persistence of hypermetabolic lymphadenopathy was detected at least 70 days after latest vaccination (Comirnaty) in 19% of the 205 cases [36]. This is different from earlier studies demonstrating normalization of initial hypermetabolic lymphadenopathy within 40 days after vaccination for influenza [37].
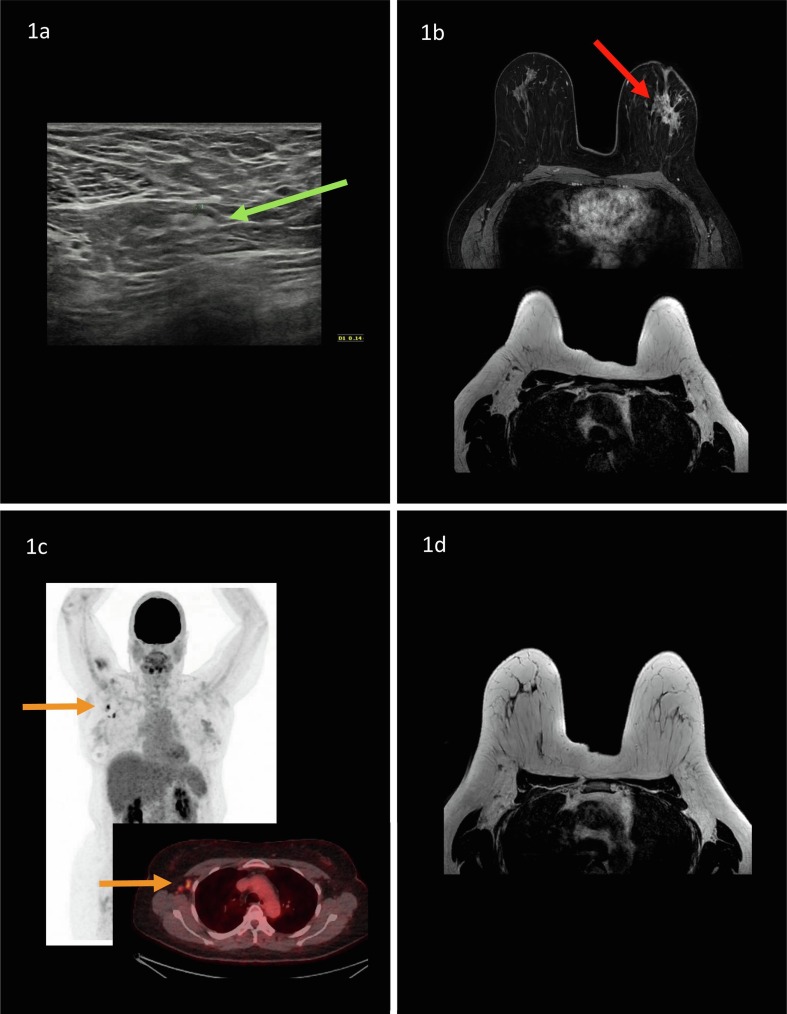
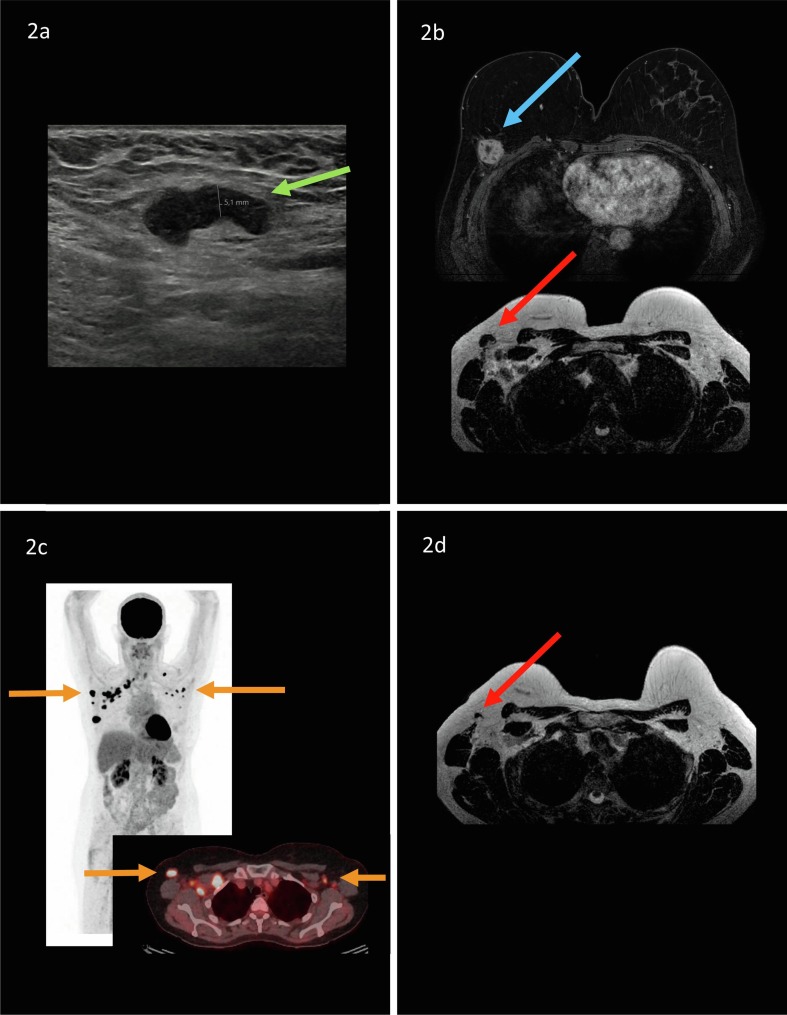
Fig. 1, Fig. 2 represent two example case of patients with recent breast cancer diagnosis, including findings of hypermetabolic axillary lymphadenopathy on 18F-FDG PET/CT.
Fig. 1.
Example of a 51-year old woman who presented with a palpable lump in her left breast. After mammography and ultrasound with tissue sampling, invasive lobular cancer (ER+,PR+,HER2+) was confirmed. Axillary ultrasound demonstrated no suspicious lymph nodes, a lymph node (green arrow) with a maximum cortical thickness of 1.4 mm was observed and considered benign (Fig. 1a). Breast MRI demonstrated a multicentric mass of approximately 7 cm in her left breast (red arrow). No suspicious axillary, internal mammary or periclavicular lymph nodes were detected bilateral (Fig. 1b). 18F-FDG PET/CT was requested by the clinician to rule out distant metastasis. Yet, six days prior to 18F-FDG PET/CT, after ultrasound and breast MRI, the patient received COVID-19 Vaccine Janssen in her right arm. 18F-FDG PET/CT demonstrated hypermetabolic axillary lymph nodes in the contralateral axilla (orange arrows), which were considered lymphadenopathy post-COVID-19 vaccination (Fig. 1c). Patient was treated with neoadjuvant systemic therapy (9 cycles of TCHP regimen (docetaxel, carboplatin, trastuzumab and pertuzumab). Breast MRI, performed mid-way and after neoadjuvant systemic therapy prior to surgical treatment, demonstrated no suspicious axillary lymph nodes bilateral (Fig. 1d). Patient underwent a left-sided mastectomy with sentinel lymph node biopsy. Three ipsilateral sentinel lymph nodes were removed, demonstrating no lymph node metastases at final histopathology. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Example of a 57-year old woman who presented with a palpable lump in her right axillary region. Patient underwent a right sided mastectomy and autologous breast reconstruction six years earlier because of breast cancer diagnosis. After recent ultrasound examination of the reconstructed breast with tissue sampling, invasive carcinoma of no special type (ER+,PR-,HER2-) was confirmed. Axillary ultrasound demonstrated a suspicious lymph node with a diffuse enlarged cortical thickness of 5,1 mm (green arrow; Fig. 2a). Ultrasound-guided tissue sampling was performed, axillary lymph node metastasis was confirmed. Breast MRI demonstrated an unifocal mass in the upper outer quadrant of the reconstructed breast (blue arrow) and multiple suspicious axillary lymph nodes in the right axillary region (red arrow; Fig. 2b). 18F-FDG PET/CT was requested by the clinician to rule out distant metastasis. Four days prior to 18F-FDG PET/CT, after ultrasound and breast MRI, the patient received Spikevax in her (contralateral) left arm. 18F-FDG PET/CT demonstrated bilateral hypermetabolic lymph nodes (orange arrows; Fig. 2c). Ultrasound-guided biopsy of the most suspicious axillary lymph node in the left axillary region after 18F-FDG PET/CT demonstrated no metastasis with visible lymphoid tissue after histopathological evaluation. Patient is currently treated with neoadjuvant systemic therapy (8 cycles of dose-dense AC-P (doxorubicine, cyclofosfamide and paclitaxel). Mid-way neoadjuvant systemic therapy breast MRI was performed. The axillary lymph nodes in the left axillary region were normalized, which can be explained by normalization of lymphadenopathy post-COVID-19 vaccination. The axillary lymph nodes in the right axillary region remained suspicious, though a reduced cortical thickness was observed when compared to the previous breast MRI, which can be explained by lymphadenopathy due to breast cancer diagnosis (red arrow; Fig. 2d). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3. Summary
Morphologic characteristics of axillary lymph nodes on axillary US might differ when considering suspicious lymph nodes at breast cancer diagnosis versus lymphadenopathy post-COVID-19 vaccination, since after vaccination the most frequent characteristic includes diffuse cortical thickening of the lymph nodes in contrast to the most suspicious findings of focal cortical thickening or effacement of the fatty hilum in breast cancer patients [14]. However, regardless of the type of nodal abnormality or vaccination history, any axillary nodal abnormality on the ipsilateral side of a woman presenting with breast cancer should be biopsied. Current evidence on morphologic characteristics on breast MRI and 18F-FDG PET/CT is missing. Yet, the distribution of affected axillary lymph nodes may be different due to different lymphatic drainage patterns: level I/II (lower part) at breast cancer diagnosis and high level II/III post-COVID-19 vaccination [25] (Table 2 ).
Table 2.
Overview of reported axillary lymph node characteristics and findings per imaging modality.
| Imaging modality | Breast cancer | ‘Post-COVID-19 vaccination’ |
|---|---|---|
| Axillary US | Most suspicious characteristics: Focal cortical thickening, effacement of fatty hilum | Most reported characteristics: Diffuse cortical thickening |
| – | Reported frequencies of lymphadenopathy: 49–85% | |
| Breast MRI | Distribution pattern of suspicious lymph nodes: Abnormal level I/II, normal level III | Distribution pattern of suspicious lymph nodes: abnormal high level II/III and normal level I |
| Suspicious characteristics: Cortical thickening, loss of fatty hilum, round shape, irregular margin, inhomogeneous cortex, irregular enhancement, perinodal edema | – | |
| Reported frequency of lymphadenopathy: 29% | ||
| 18F-FDG PET/CT | Suspicious characteristics: Hypermetabolic axillary lymph nodes (non-infectious setting) | – |
| – | Reported frequencies of lymphadenopathy: 14.5–53.9%, increased after second vaccination dose when compared to first vaccination dose |
Reported frequencies of lymphadenopathy post-COVID-19 vaccination range from 49% to 85% (US), 29% (breast MRI) and 14.5% to 53.9% (18F-FDG PET/CT). Several items were considered relevant to increase frequency and/or extent of lymphadenopathy post-COVID-19: injection site, type of vaccine (mRNA versus vector), interval between vaccination date and imaging examination date, previous history of COVID-19 pneumonia, first versus second vaccine dose. Consequently, in the case of lymphadenopathy potentially caused after vaccination clinical information needs to be documented in each patient to improve the interpretation and guide treatment towards the next steps of action. In patients with a recent breast cancer diagnosis and recent COVID-19 vaccination with ipsilateral lymphadenopathy, tissue sampling is advised to determine lymph node status. In the case of contralateral lymphadenopathy after recent breast cancer diagnosis and recent COVID-19 vaccination, follow-up in 12–16 weeks can be considered rather than immediate tissue sampling.
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronins KA. SEER Cancer Statistics Review, 1975–2010, National Cancer Institue. Bethesda, MD, <https://seer.cancer.gov/archive/csr/1975_2010/>, based on November 2012 SEER data submission, posted to the SEER website, April 2013.
- 2.Cardoso F., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rubio I.T., Zackrisson S., Senkus E., Committee E.G. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019;30(10):1674. doi: 10.1093/annonc/mdz189. [DOI] [PubMed] [Google Scholar]
- 3.A. Evans, R.M. Trimboli, A. Athanasiou, C. Balleyguier, P.A. Baltzer, U. Bick, J. Camps Herrero, P. Clauser, C. Colin, E. Cornford, E.M. Fallenberg, M.H. Fuchsjaeger, F.J. Gilbert, T.H. Helbich, K. Kinkel, S.H. Heywang-Kobrunner, C.K. Kuhl, R.M. Mann, L. Martincich, P. Panizza, F. Pediconi, R.M. Pijnappel, K. Pinker, S. Zackrisson, G. Forrai, F. Sardanelli, w.l.r.b.E.D.-T.E.B.C.C. European Society of Breast Imaging, Breast ultrasound: recommendations for information to women and referring physicians by the European Society of Breast Imaging, Insights Imag. 9(4) (2018) 449–461. [DOI] [PMC free article] [PubMed]
- 4.Chang J.M., Leung J.W.T., Moy L., Ha S.M., Moon W.K. Axillary nodal evaluation in breast cancer: state of the art. Radiology. 2020;295(3):500–515. doi: 10.1148/radiol.2020192534. [DOI] [PubMed] [Google Scholar]
- 5.Bai H.X., Hsieh B., Xiong Z., Halsey K., Choi J.W., Tran T.M.L., Pan I., Shi L.B., Wang D.C., Mei J., Jiang X.L., Zeng Q.H., Egglin T.K., Hu P.F., Agarwal S., Xie F.F., Li S., Healey T., Atalay M.K., Liao W.H. Performance of radiologists in differentiating COVID-19 from non-COVID-19 viral pneumonia at chest CT. Radiology. 2020;296(2):E46–E54. doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X.i., Yu C., Qu J., Zhang L., Jiang S., Huang D., Chen B., Zhang Z., Guan W., Ling Z., Jiang R., Hu T., Ding Y., Lin L., Gan Q., Luo L., Tang X., Liu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging. 2020;47(5):1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta N., Sales R.M., Babagbemi K., Levy A.D., McGrath A.L., Drotman M., Dodelzon K. Unilateral axillary Adenopathy in the setting of COVID-19 vaccine. Clin. Imag. 2021;75:12–15. doi: 10.1016/j.clinimag.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn R.W., Mootz A.R., Brewington C.C., Abbara S. Axillary lymphadenopathy after mRNA COVID-19 vaccination. Radiol. Cardiothorac. Imag. 2021;3(1):e210008. doi: 10.1148/ryct.2021210008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bekker L.G., Garrett N., Goga A., Fairall L., Reddy T., Yende-Zuma N., Kassanjee R., Collie S., Sanne I., Boulle A., Seocharan I., Engelbrecht I., Davies M.A., Champion J., Chen T., Bennett S., Mametja S., Semenya M., Moultrie H., de Oliveira T., Lessells R.J., Cohen C., Jassat W., Groome M., Von Gottberg A., Le Roux E., Khuto K., Barouch D., Mahomed H., Wolmarans M., Rousseau P., Bradshaw D., Mulder M., Opie J., Louw V., Jacobson B., Rowji P., Peter J.G., Takalani A., Odhiambo J., Mayat F., Takuva S., Corey L., Gray G.E., Sisonke Protocol T. Sisonke study, effectiveness of the Ad26.COV2.S vaccine in health-care workers in South Africa (the Sisonke study): results from a single-arm, open-label, phase 3B, implementation study. Lancet. 2022;399(10330):1141–1153. doi: 10.1016/S0140-6736(22)00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiaffino S., Pinker K., Magni V., Cozzi A., Athanasiou A., Baltzer P.A.T., Camps Herrero J., Clauser P., Fallenberg E.M., Forrai G., Fuchsjager M.H., Helbich T.H., Kilburn-Toppin F., Kuhl C.K., Lesaru M., Mann R.M., Panizza P., Pediconi F., Pijnappel R.M., Sella T., Thomassin-Naggara I., Zackrisson S., Gilbert F.J., Sardanelli F. Axillary lymphadenopathy at the time of COVID-19 vaccination: ten recommendations from the European Society of Breast Imaging (EUSOBI) Insights Imag. 2021;12(1):119. doi: 10.1186/s13244-021-01062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehman C.D., Lamb L.R., D'Alessandro H.A. Mitigating the impact of coronavirus disease (COVID-19) vaccinations on patients undergoing breast imaging examinations: a pragmatic approach. AJR Am. J. Roentgenol. 2021;217(3):584–586. doi: 10.2214/AJR.21.25688. [DOI] [PubMed] [Google Scholar]
- 13.Net J.M., Mirpuri T.M., Plaza M.J., Escobar C.A., Whittington E.E., Collado-Mesa F., Yepes M.M. Resident and fellow education feature: US evaluation of axillary lymph nodes. Radiographics. 2014;34(7):1817–1818. doi: 10.1148/rg.347140081. [DOI] [PubMed] [Google Scholar]
- 14.Bedi D.G., Krishnamurthy R., Krishnamurthy S., Edeiken B.S., Le-Petross H., Fornage B.D., Bassett R.L., Jr., Hunt K.K. Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: in vitro sonographic study. AJR Am. J. Roentgenol. 2008;191(3):646–652. doi: 10.2214/AJR.07.2460. [DOI] [PubMed] [Google Scholar]
- 15.Neal C.H., Daly C.P., Nees A.V., Helvie M.A. Can preoperative axillary US help exclude N2 and N3 metastatic breast cancer? Radiology. 2010;257(2):335–341. doi: 10.1148/radiol.10100296. [DOI] [PubMed] [Google Scholar]
- 16.Igual-Rouilleault A.C., Soriano I., Quan P.L., Fernandez-Montero A., Elizalde A., Pina L. Unilateral axillary adenopathy induced by COVID-19 vaccine: US follow-up evaluation. Eur. Radiol. 2021 doi: 10.1007/s00330-021-08309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park J.Y., Lee J.Y., Yi S.Y. Axillary lymphadenopathy on ultrasound after COVID-19 vaccination and its influencing factors: a single-center study. J Clin Med. 2022;11(1):238. doi: 10.3390/jcm11010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cocco G., Delli Pizzi A., Fabiani S., Cocco N., Boccatonda A., Frisone A., Scarano A., Schiavone C. Lymphadenopathy after the Anti-COVID-19 vaccine: multiparametric ultrasound findings. Biology (Basel) 2021;10(7):652. doi: 10.3390/biology10070652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.L. Grimm, S. Destounis, B. Dogan, B. Nicholson, B. Dontchos, E. Sonnenblick, H. Milch, J. Pushkin, J. Benson, K. Dodelzon, N. Modi, R. Yang, V. Dialani, P. Vidushani, SBI Recommendations for the management of axillary adenopathy in patients with recent COVID-19 vaccination, Committee SoBIPCaD, editor, 2021, Page 3.
- 20.Mehta N., Sales R.M., Babagbemi K., Levy A.D., McGrath A.L., Drotman M., Dodelzon K. Unilateral axillary adenopathy in the setting of COVID-19 vaccine: follow-up. Clin. Imag. 2021;80:83–87. doi: 10.1016/j.clinimag.2021.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen D.L., Ambinder E.B., Myers K.S., Mullen L.A., Panigrahi B., Oluyemi E. COVID-19 vaccine-related axillary adenopathy on breast imaging: follow-up recommendations and histopathologic findings. AJR Am. J. Roentgenol. 2021 doi: 10.2214/AJR.21.27162. [DOI] [PubMed] [Google Scholar]
- 22.van Nijnatten T.J.A., Ploumen E.H., Schipper R.J., Goorts B., Andriessen E.H., Vanwetswinkel S., Schavemaker M., Nelemans P., de Vries B., Beets-Tan R.G.H., Smidt M.L., Lobbes M.B.I. Routine use of standard breast MRI compared to axillary ultrasound for differentiating between no, limited and advanced axillary nodal disease in newly diagnosed breast cancer patients. Eur. J. Radiol. 2016;85(12):2288–2294. doi: 10.1016/j.ejrad.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 23.Ecanow J.S., Abe H., Newstead G.M., Ecanow D.B., Jeske J.M. Axillary staging of breast cancer: what the radiologist should know. Radiographics. 2013;33(6):1589–1612. doi: 10.1148/rg.336125060. [DOI] [PubMed] [Google Scholar]
- 24.Baltzer P.A.T., Dietzel M., Burmeister H.P., Zoubi R., Gajda M., Camara O., Kaiser W.A. Application of MR mammography beyond local staging: is there a potential to accurately assess axillary lymph nodes? evaluation of an extended protocol in an initial prospective study. AJR Am. J. Roentgenol. 2011;196(5):W641–W647. doi: 10.2214/AJR.10.4889. [DOI] [PubMed] [Google Scholar]
- 25.Plaza M.J., Wright J., Fernandez S. COVID-19 vaccine-related unilateral axillary lymphadenopathy: pattern on screening breast MRI allowing for a benign assessment. Clin. Imag. 2021;80:139–141. doi: 10.1016/j.clinimag.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horvat J.V., Sevilimedu V., Becker A.S., Perez-Johnston R., Yeh R., Feigin K.N. Frequency and outcomes of MRI-detected axillary adenopathy following COVID-19 vaccination. Eur. Radiol. 2022 doi: 10.1007/s00330-022-08655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper K.L., Harnan S., Meng Y., Ward S.E., Fitzgerald P., Papaioannou D., Wyld L., Ingram C., Wilkinson I.D., Lorenz E. Positron emission tomography (PET) for assessment of axillary lymph node status in early breast cancer: a systematic review and meta-analysis. Eur. J. Surg. Oncol. 2011;37(3):187–198. doi: 10.1016/j.ejso.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Wahl R.L., Siegel B.A., Coleman R.E., Gatsonis C.G., P.E.T.S. Group Prospective multicenter study of axillary nodal staging by positron emission tomography in breast cancer: a report of the staging breast cancer with PET Study Group. J. Clin. Oncol. 2004;22(2):277–285. doi: 10.1200/JCO.2004.04.148. [DOI] [PubMed] [Google Scholar]
- 29.Heusner T.A., Kuemmel S., Hahn S., Koeninger A., Otterbach F., Hamami M.E., Kimmig K.R., Forsting M., Bockisch A., Antoch G., Stahl A. Diagnostic value of full-dose FDG PET/CT for axillary lymph node staging in breast cancer patients. Eur. J. Nucl. Med. Mol. Imag. 2009;36(10):1543–1550. doi: 10.1007/s00259-009-1145-6. [DOI] [PubMed] [Google Scholar]
- 30.Groheux D., Espié M., Giacchetti S., Hindié E. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013;266(2):388–405. doi: 10.1148/radiol.12110853. [DOI] [PubMed] [Google Scholar]
- 31.Eifer M., Eshet Y. Imaging of COVID-19 vaccination at FDG PET/CT. Radiology. 2021;299(2):E248. doi: 10.1148/radiol.2020210030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirone N., Shinkai T., Yamane T., Uto F., Yoshimura H., Tamai H., Imai T., Inoue M., Kitano S., Kichikawa K., Hasegawa M. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann. Nucl. Med. 2012;26(3):248–252. doi: 10.1007/s12149-011-0568-x. [DOI] [PubMed] [Google Scholar]
- 33.Tu W., Gierada D.S., Joe B.N. COVID-19 vaccination-related lymphadenopathy: what to be aware of. Radiol Imag. Cancer. 2021;3(3):e210038. doi: 10.1148/rycan.2021210038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernstine H., Priss M., Anati T., Turko O., Gorenberg M., Steinmetz A.P., Groshar D. Axillary lymph nodes hypermetabolism after BNT162b2 mRNA COVID-19 vaccination in cancer patients undergoing 18F-FDG PET/CT: a cohort study. Clin. Nucl. Med. 2021;46(5):396–401. doi: 10.1097/RLU.0000000000003648. [DOI] [PubMed] [Google Scholar]
- 35.Cohen D., Krauthammer S.H., Wolf I., Even-Sapir E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: incidence assessed by [(18)F]FDG PET-CT and relevance to study interpretation. Eur. J. Nucl. Med. Mol. Imag. 2021;48(6):1854–1863. doi: 10.1007/s00259-021-05314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eshet Y., Tau N., Alhoubani Y., Kanana N., Domachevsky L., Eifer M. Prevalence of increased FDG PET/CT axillary lymph node uptake beyond 6 weeks after mRNA COVID-19 vaccination. Radiology. 2021;300(3):E345–E347. doi: 10.1148/radiol.2021210886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomassen A., Lerberg Nielsen A., Gerke O., Johansen A., Petersen H. Duration of 18F-FDG avidity in lymph nodes after pandemic H1N1v and seasonal influenza vaccination. Eur. J. Nucl. Med. Mol. Imag. 2011;38(5):894–898. doi: 10.1007/s00259-011-1729-9. [DOI] [PubMed] [Google Scholar]