Abstract
Mortality historically has been the primary outcome of choice for acute and critical care clinical trials. However, undue reliance on mortality can limit the scope of trials that can be performed. Large sample sizes are usually needed for trials powered for a mortality outcome, and focusing solely on mortality fails to recognize the importance that reducing morbidity can have on patients’ lives. The COVID-19 pandemic has highlighted the need for rapid, efficient trials to rigorously evaluate new therapies for hospitalized patients with acute lung injury. Oxygen-free days (OFDs) is a novel outcome for clinical trials that is a composite of mortality and duration of new supplemental oxygen use. It is designed to characterize recovery from acute lung injury in populations with a high prevalence of new hypoxemia and supplemental oxygen use. In these populations, OFDs captures two patient-centered consequences of acute lung injury: mortality and hypoxemic lung dysfunction. Power to detect differences in OFDs typically is greater than that for other clinical trial outcomes, such as mortality and ventilator-free days. OFDs is the primary outcome for the Fourth Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-4) Host Tissue platform, which evaluates novel therapies targeting the host response to COVID-19 among adults hospitalized with COVID-19 and new hypoxemia. This article outlines the rationale for use of OFDs as an outcome for clinical trials, proposes a standardized method for defining and analyzing OFDs, and provides a framework for sample size calculations using the OFD outcome.
Key Words: acute lung injury, COVID-19, oxygen, respiratory failure
Graphical Abstract
Mortality historically has been the primary outcome of choice for efficacy trials evaluating interventions for acute respiratory failure and other severe acute medical conditions.1 Although decreasing the incidence of mortality clearly is an important and patient-centered goal, the selection of mortality as a primary outcome has limitations, including the following: (1) the potential for missing an important efficacy signal for reduced morbidity; (2) the large sample sizes needed for adequate power to detect important differences in mortality; and (3) the fact that interventions under study may impact only specific pathways toward death, whereas acutely ill patients often have many potential causes of death that may not be attributable to the intervention.2 , 3
Days alive and free of a supportive therapy (“free-day” outcomes) provide an alternative to mortality as a primary outcome in clinical trials of therapies targeted at ARDS, sepsis, and other severe illnesses.2 , 4 , 5 Free-day outcomes combine mortality with clinically relevant morbidities (eg, days receiving ventilator support,6 days receiving vasopressor support,7 days in the hospital8), creating composite outcomes reflective of both morbidity and mortality. Free-day outcomes typically are analyzed as ordinal variables and can permit trials with smaller sample sizes to identify clinically meaningful differences in patient outcomes.
The COVID-19 pandemic has revealed a further need for patient-centered outcomes that facilitate efficient rapid trials of promising therapies for patients with acute lung injury. Because of its widespread morbidity and mortality, the COVID-19 pandemic requires rapid identification of efficacious therapies and equally rapid abandonment of therapies with a low likelihood of efficacy. Thus, the pandemic demands both efficiency and rigor in clinical trial design. Using patient-centered, nonmortal primary outcomes is one method of improving clinical trial efficiency. Although several nonmortal outcomes for COVID-19 trials have been used, including time to recovery,9 clinical status scores,10 and sustained recovery,11 , 12 none has been adopted universally.
Most patients admitted to the hospital with COVID-19 experience mild to moderate lung injury, are treated with supplemental oxygen via nasal cannula on hospital wards, and do not progress to invasive mechanical ventilation or death.13, 14, 15, 16 In this setting, wherein death, invasive mechanical ventilation, and other organ support therapies are rare, a composite outcome that includes death and oxygen use may capture the key disease-related acute mortality and morbidity of interest. Oxygen-free days (OFDs), a composite ordinal outcome that includes mortality and duration of supplemental oxygen use, has been used as a key outcome measure in trials of COVID-1910 , 17 and other acute diseases.18 OFDs also serves as the primary outcome in a new platform trial evaluating host response therapies among adults hospitalized with COVID-19-associated lung injury: the Fourth Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-4) Host Tissue platform (ClinicalTrials.gov Identifier: NCT04924660). In this article, we discuss the rationale for selecting OFDs as the primary outcome in the ACTIV-4 Host Tissue platform, propose a standard definition for OFDs, outline an approach to analyzing OFDs, and demonstrate sample size calculations for OFDs using data from a recently completed COVID-19 trial.17
Rationale for OFDs as an Important Outcome Measure
The OFDs outcome is modeled after the ventilator-free days outcome, a composite of duration of invasive mechanical ventilation and death. Ventilator-free days is an established outcome measure in trials of critically ill patients receiving invasive mechanical ventilation.6 , 19, 20, 21, 22 Ventilator-free days was developed based on the notion that trials powered only on mortality frequently require very large sample sizes (or often a sacrifice in scientific rigor) and that therapies that reduce the duration of mechanical ventilation can produce meaningful improvements in health.3 , 23 Duration of mechanical ventilation alone without considering death would ignore the competing risk of death; patients who die rapidly have a short duration of mechanical ventilation, yet should not be considered to have a favorable outcome. By considering death and duration of mechanical ventilation together, the ventilator-free day outcome enables a comparison of duration of mechanical ventilation while accounting for the competing risk of death and maintenance of an accurate order of illness severity. The use of OFDs extends these concepts into a larger, less severely ill population of patients treated in the hospital with any form of supplemental oxygen, ranging from oxygen therapy by nasal cannula to invasive mechanical ventilation. Trials particularly well suited for use of OFDs as the primary outcome include those evaluating therapies for severe acute respiratory infections, such as COVID-19, influenza, and community-acquired pneumonia.
Previous work has emphasized that clinical trial outcomes should be the following: (1) important to the patient; (2) potentially modifiable by the intervention under investigation; and (3) reliably measurable.24 In this section, we review how the OFDs outcome meets these criteria.
Is a Reduction in the Duration of Supplemental Oxygen Therapy Important to Patients?
The 2019 Critical Care Trialists Workshop brought together key stakeholders for critical care clinical trial design, including patients, family members, physicians, trialists, statisticians, and regulators. A key take-away from the Critical Care Trialists Workshop was “a shared desire expressed, particularly from regulators and patient representatives, to incorporate patient-centered outcomes other than mortality, reflecting patients’ quality of life (i.e., the challenge of surviving critical illness) in future trials.”25 Ongoing need for oxygen support, both in the hospital and after discharge, represents a burden on patients and reflects important elements of recovery and the challenge of surviving a severe acute illness. Patients who chronically use supplemental oxygen at home have described it as highly burdensome.26, 27, 28 Although the burden of home oxygen therapy after acute illness has not been characterized comprehensively, during development of the ACTIV-4 Host Tissue platform, investigators elicited input about the patient’s perspective of supplemental oxygen use through discussions with ARDS survivors in the ARDS Foundation (ardsglobal.org). Key input about supplemental oxygen use from these patients highlighted chronic discomfort and nasal dryness that interfered with sleep and physical therapy, difficulty managing and transporting supplemental oxygen equipment, and being perceived by others as sick. A common experience reported by survivors of COVID-19 who are newly dependent on supplemental oxygen was a perpetual fear that they will die if their oxygen devices stop working.29
Eileen Rubin, a survivor of ARDS and president of the ARDS Foundation, recounted her own experiences and thoughts regarding supplemental oxygen use during recovery from acute illness. Her statements, which are displayed in the e-Appendix 1, emphasize the physical, mental, and financial burden that prolonged supplemental oxygen use can have on patients.
Can OFDs Be Modified by the Interventions Being Evaluated?
For OFDs to be an optimal trial outcome, the control group must experience a substantial burden of supplemental oxygen use in the 28 days after randomization and the therapies under investigation must target mechanistic pathways expected to lessen or resolve hypoxemia.
In the ACTIV-4 Host Tissue platform, all enrolled patients are hospitalized with COVID-19 and hypoxemia (saturation of peripheral oxygen < 92% on room air for patients without chronic supplemental oxygen use or a supplemental oxygen flow rate higher than baseline for patients receiving chronic oxygen therapy). Thus, nearly all trial participants are receiving supplemental oxygen therapy at randomization. In this context, OFDs measures the time to lung recovery (defined as liberation from supplemental oxygen therapy) among patients with COVID-19-associated hypoxemia, with an appropriate penalty for mortality. Therefore, interventions hastening lung recovery should increase the number of OFDs. OFDs is highly applicable to all patients enrolled in ACTIV-4 Host Tissue. In contrast, ventilator-free days likely would fail to capture a substantial amount of lung recovery because most patients with COVID-19 hospitalized with hypoxemia never progress to invasive mechanical ventilation.16 Lung injury treated with noninvasive oxygen (eg, nasal cannula) would be completely missed by a ventilator-free day outcome, and practice differences in intubation threshold may introduce noise to the outcome without reflecting recovery. Further, the distribution of ventilator-free days would be highly bimodal, with a peak at –1 day for mortality and at 28 days for patients who never progressed to invasive mechanical ventilation. By contrast, as detailed herein, the distribution of OFDs is highly dispersed across the entire continuum from –1 to 28 days.
The first three therapies being evaluated on the ACTIV-4 Host Tissue platform are TXA-127 (Constant Therapeutics),30 TRV-027 (Trevena),31 and fostamatinib (Rigel Pharmaceuticals).32 These therapies are compared with placebo in a randomized, blinded platform trial. Each of these therapies is hypothesized to improve lung function for patients with COVID-19 and hypoxemia. Thus, the measurement of OFDs should capture the key beneficial effects hypothesized to occur with each therapy. A separate protocol and statistical analysis plan will be published with additional details of the therapies under investigation.
Can OFDs Be Measured Reliably and Accurately in Clinical Trials?
As detailed in the next section, the data needed to calculate OFDs are captured reliably via medical record abstraction and simple questions posed to patients during follow-up visits after discharge. Thus, OFDs can be ascertained with standard methods routinely used in clinical trials and without the need for in-person visits after discharge.
Standardizing the Definition of OFDs
No universally accepted definition for OFDs exists. To facilitate comparisons across trials, a standardized definition for OFDs will be important. In this section, we outline the definition of OFDs being used in the ACTIV-4 Host Tissue platform as a paradigm for how the measurement of OFDs may be standardized.
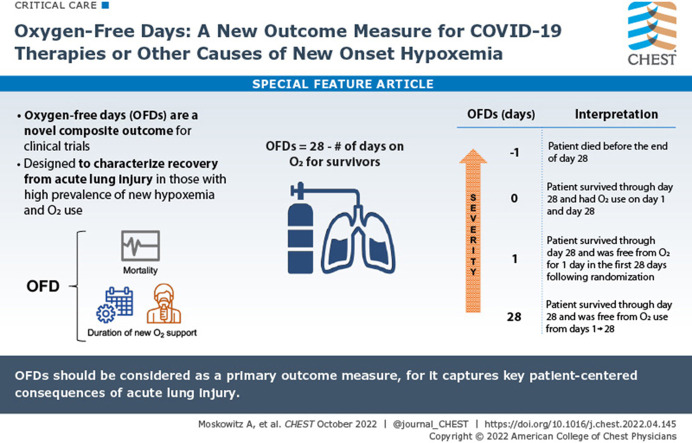
OFDs is a composite ordinal outcome incorporating death and duration of supplemental oxygen use onto the same scale, with death coded as the worst possible outcome. A follow-up period of 28 days for ascertaining OFDs is being used in the ACTIV-4 Host Tissue platform based on precedent in the field for measuring morbidity and mortality in acute care trials20, 21, 22 and recent data from COVID-19 studies demonstrating that approximately 75% of patients hospitalized with COVID-19 treated with new oxygen therapy have died or been liberated from oxygen by day 28.17 However, the duration of follow-up can be modified to fit priorities. For example, it may be preferable to extend the follow-up period to 60 or 90 days for trials of patients with more persistent acute lung injury or those evaluating patients with chronic lung disease.
Using a 28-day follow-up period, OFDs is calculated as the number of calendar days during the first 28 days after randomization during which the patient was alive and not receiving new supplemental oxygen therapy (OFDs = 28 minus the number of days of supplemental oxygen therapy for survivors). Patients who were not treated chronically with oxygen before the acute illness are coded as receiving supplemental oxygen therapy whenever they are receiving any of the following at any oxygen flow rate: oxygen by nasal cannula, oxygen by face mask, high-flow nasal cannula, noninvasive ventilation (except as a treatment for sleep apnea only), invasive mechanical ventilation, or extracorporeal membrane oxygenation. The day of randomization is day 0. Starting with calendar day 1 (the day after randomization) and continuing for 28 days, oxygen use is ascertained daily, with any duration of oxygen use on a calendar day designating that day as an oxygen use day (ie, not an OFD). OFDs is calculated using a first-on-last-off methodology. All days between the first initiation of oxygen use and the last liberation from oxygen use are classified as oxygen use days. As an example, if a patient is weaned off oxygen for a day but then is reinitiated on oxygen the next day, the single day without oxygen use between 2 days with oxygen use is not considered an OFD. This first-on-last-off approach is used so that the outcome captures the final liberation of supplemental oxygen therapy, which is considered more clinically meaningful than transient pauses in oxygen therapy.
Patients who chronically used supplemental oxygen before the acute illness are considered oxygen free when they return to the same level of oxygen support they had been using before the acute illness. For example, a patient who chronically used supplemental oxygen at 4 L/min via nasal cannula before the acute illness and then escalated oxygen use during the illness would be considered oxygen free when the patient returned to oxygen support via nasal cannula at 4 L/min or less.
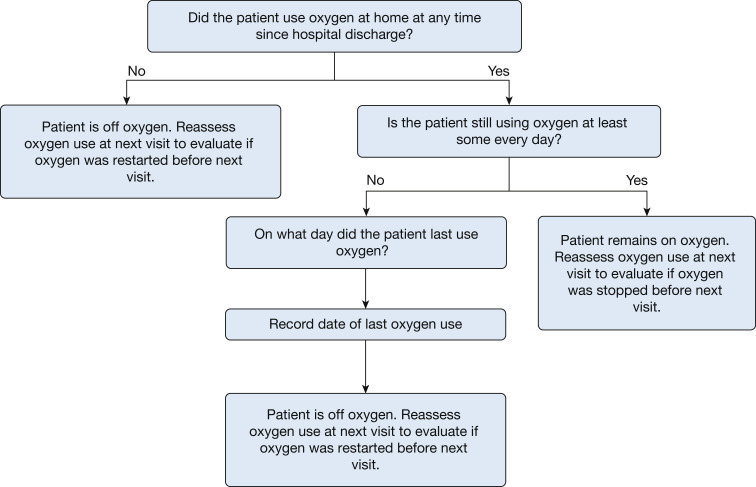
Capturing oxygen use both in the hospital and after hospital discharge up to day 28 is important to characterize morbidity after discharge. OFDs is an all-location outcome and not limited to the index hospitalization. Ascertaining oxygen use while the patient is in the hospital typically is straightforward by reviewing medical records. Ascertaining oxygen use after discharge can be achieved through periodic contacts with the patient or surrogate via telephone calls, text messaging, survey links, conference calls, or e-mail communication. In the ACTIV-4 Host Tissue platform, patients and surrogates are contacted after hospital discharge on days 1, 3, 7, 14, 21, and 28 using the standardized script detailed in Figure 1 . Using this script, oxygen use on each calendar day up to day 28 can be coded based on knowing when a patient last used new supplemental oxygen.
Figure 1.
Standardized script used in the Fourth Accelerating COVID-19 Therapeutic Interventions and Vaccines Host Tissue Platform to ascertain oxygen use after hospital discharge for the calculation of oxygen-free days for patients not receiving oxygen at baseline (before the acute illness). For patients who did use oxygen at baseline, similar questions are asked to ascertain oxygen use above baseline. These questions are asked by telephone, text message, or another telecommunication method during study visits after the patient has been discharged from the hospital. Answers to these questions are used to understand if the patient used oxygen after hospital discharge, and if so, when the final liberation from oxygen occurred.
For the calculation of OFDs, a patient who dies before day 28 (either in the hospital or after discharge) is coded as having –1 OFD regardless of the number of days of oxygen use before death. Hence, OFDs is an ordinal outcome with 30 possible levels (range, –1 to 28). The ordinal levels are ordered so that lower numbers indicate a worse outcome (Table 1 ). However, the difference between levels is not implied to be equal across the scale. For example, the clinical difference between death (–1 OFD) and 0 OFDs is not the same as the difference between 10 OFDs and 11 OFDs. After their original introduction into clinical trials, free-day outcomes often coded death as 0.3 More recently, and consistent with the approach outlined herein, investigators have been coding death as –1 to distinguish death from organ support for the full follow-up period.4 , 5 , 23 , 33 This is especially important for the OFD, where ongoing oxygen need at the end of the follow-up period is likely to be a far preferable option to death. As with all composite outcomes, it is important to report results for each component of OFDs—duration of oxygen use in survivors and 28-day mortality—to present trial findings clearly.
Table 1.
Interpretation of OFDs
| Severity | OFDs (d) | Interpretation |
|---|---|---|
| More severe | –1 | Patient died before the end of day 28. |
| 0 | Patient survived through day 28 and received oxygen on days 1 and 28. | |
| 1 | Patient survived through day 28 and was free of oxygen use for 1 d in the first 28 d after randomization. | |
| 10 | Patient survived through day 28 and was free of oxygen use for 10 d in the first 28 d after randomization. | |
| 25 | Patient survived through day 28 and was free of oxygen use for 25 d in the first 28 d after randomization. | |
| Less severe | 28 | Patient survived through day 28 and was free of oxygen use on every calendar day from days 1 to 28. |
OFD = oxygen-free day.
Comparison of OFDs With Other Clinical Trial Outcomes
During the COVID-19 pandemic, several outcomes for trials evaluating in-hospital therapies have been advanced as investigators have sought to capture the key patient-centered concepts that may be amenable to new therapies. The World Health Organization (WHO) COVID-19 Clinical Progression ordinal scale has been used as a primary outcome in several trials.34 This scale, initially conceived to track the progress of patients with COVID-19 through the health care system, has a number of strengths and is a recommended outcome measure for respiratory failure in COVID-19 core outcome sets.35 It comprises mortality and levels of respiratory support. It is broadly applicable to the full range of COVID-19 disease severity, is relatively simple to measure, and can be abstracted easily from medical records. However, the WHO Clinical Progression scale has limitations. Although the scale may be measured serially and analyzed with longitudinal models, it typically is used to measure clinical status at a discrete cross-sectional point in time, such as at 14 or 28 days after randomization. This is not consistent with how lungs recover from acute injury, which is usually a gradual process that occurs over weeks. One advantage of OFDs is that this measure captures lung recovery over the entire 28-day follow-up period, enabling a more detailed characterization of lung recovery over time. Comparison of OFDs and the eight-level WHO COVID-19 ordinal scale is shown in Table 2 .
Table 2.
Comparison Between OFDs and the 8-Level WHO COVID-19 Clinical Progress Ordinal Scale34
| WHO Scale Category | Category Description | Notes for OFD Calculation |
|---|---|---|
| 1 | Not hospitalized without limitation in daily activity | Classified as OFD |
| 2 | Not hospitalized with limitation in daily activity or home oxygen use | Days with home oxygen use are classified as days with supplemental oxygen use. Days at home with limitations in daily activity, but with no home oxygen, use are classified as OFDs. |
| 3 | Hospitalized not with supplemental oxygen | Classified as OFD |
| 4 | Hospitalized with standard supplemental oxygen via nasal cannula or mask | Classified as day with supplemental oxygen use |
| 5 | Hospitalized with high-flow nasal cannula or noninvasive ventilation | Classified as day with supplemental oxygen use |
| 6 | Hospitalized with invasive mechanical ventilation without other organ support | Classified as day with supplemental oxygen use |
| 7 | Hospitalized with invasive mechanical ventilation and other organ support (including vasopressors, RRT, or ECMO) | Classified as day with supplemental oxygen use |
| 8 | Death | Death at any time before day 28 is coded as –1 OFDs |
ECMO = extracorporeal membrane oxygenation; OFD = oxygen-free day; WHO = World Health Organization.
The concept of time to liberation from oxygen therapy also has been used extensively within time-to-recovery outcomes. For example, the primary outcome for the first Adaptive COVID-19 Treatment Trial (ACTT-1) was time to recovery, defined as the time between randomization and the earlier of hospital discharge or discontinuation of oxygen therapy and other in-hospital therapies for COVID-19.9 Weaknesses of time to recovery as an outcome include the competing risk of death, which is not incorporated into the outcome, and the truncation of outcome assessment at hospital discharge. Limiting outcome ascertainment to the in-hospital setting is particularly problematic for duration of oxygen use in the COVID-19 pandemic because the practice of discharging patients with supplemental oxygen has been evolving throughout the pandemic. OFDs builds on the concept of time to recovery, while strengthening it by combining death and duration of oxygen use into a composite outcome and measuring oxygen use in all locations for 28 days.
Potential Disadvantages of OFDs
All clinical trial outcomes have potential disadvantages and limitations. Several limitations of OFDs should be considered. First, OFDs is an outcome designed for trials evaluating patients with lung injury or at high risk of lung injury and for therapies that impact lung function. OFDs is unlikely to be a useful outcome in trials in which a substantial proportion of the population is not treated with oxygen therapy or in trials of interventions not directly targeted at improving lung function. For example, some future SARS-CoV-2 variants may not result in significant enough lung injury to warrant clinical trials of interventions aimed at treating acute lung injury or use of OFDs as an outcome. Second, the duration of supplemental oxygen use not only is impacted by the patient’s lung function, but also by the clinical practice of weaning oxygen therapy, which may vary by provider and by time. Variation in oxygen weaning practices could interfere with the ability of OFDs to represent lung function accurately. Variations in the practice of weaning oxygen may be exacerbated in pandemic settings, during which factors such as hospital strain or the availability of oxygen may result in unusually early weaning of oxygen. Conversely, factors such as the inability of patients to receive timely care after hospital discharge may lead to fewer opportunities to wean patients off oxygen and unusually long oxygen use after hospital discharge. Concerns about variations in oxygen weaning practices can be attenuated somewhat through stratification of randomization by site. Third, the output for OFDs does not have inherent meaning that is immediately clinically interpretable. For example, a difference of 2 OFDs between an intervention group and a placebo group could represent an improvement in mortality without an effect on the duration of oxygen use, a 2-day reduction in the duration of oxygen use among survivors without an effect on mortality, or combined effects on both mortality and duration of oxygen use. Reporting each component of the composite OFD outcome is key to representing trial results fully in the most interpretable way. Fourth, although unlikely, it is possible for an intervention to result in higher mortality and shorter duration of oxygen use among survivors, such that OFDs demonstrates an overall benefit, despite higher mortality in the intervention group. To safeguard against such a scenario, investigators can specify that OFDs will be used to support the efficacy of an intervention only if mortality is not significantly worse in the intervention group compared with the control group.
Importantly, OFDs is a better reflection of illness duration as opposed to peak illness severity. For instance, a patient who is treated with invasive mechanical ventilation and a total duration of oxygen use of 14 days will have the same number of OFDs as a patient who is treated with supplemental oxygen by nasal cannula for 14 days, despite the first patient having higher peak severity of illness. That OFDs does not fully reflect illness severity has implications both for statistical power (ie, some information is lost regarding the potential impact of an intervention by not differentiating the duration of different intensities of respiratory support) and for the patient centeredness of the outcome because individuals likely would prefer to avoid a period of more invasive support even if the overall duration were similar. Alternative outcomes that incorporate both duration of oxygen support and illness severity using a ranking system are technically possible; however, they would be substantially more complex and could be difficult to interpret from a clinical perspective. Secondary outcomes assessing peak illness severity can be used to complement OFDs and to attenuate the above concerns.
Analyzing OFDs
In the ACTIV-4 Host Tissue platform, OFDs is analyzed as an ordinal outcome with 30 possible levels (range, –1 to 28 days). The number of OFDs in intervention and control groups are summarized with histograms, medians values, and interquartile ranges. The primary analysis is conducted using a proportional odds model comparing the distribution of OFDs in an intervention group vs a concurrent placebo group. This method is appropriate for ordinal outcomes like OFDs, where the levels are ordered, but the relative differences between levels are not defined. The statistical model includes covariates for key baseline characteristics, including age, sex, and WHO COVID-19 ordinal score. The model produces an OR that represents the covariate-adjusted effect of intervention on the odds of more OFDs for a participant in the active group compared with the placebo group. For example, an adjusted OR of 1.2 means that a participant has 20% greater odds in the intervention group compared with the control group of having more than x OFDs. The proportional odds assumption means this interpretation applies for each possible value of x. An adjusted OR of > 1.0 indicates a greater number of OFDs (benefit) in the active group compared with the placebo group. In the event that the proportional odds assumption is violated, the estimated ORs remain interpretable and reflect a global assessment of treatment effectiveness. Alternative methods, such as 0-1 inflated β-binomial regression, also may be used for modeling the distribution of OFDs. The method used to simulate treatment effects using the proportional odds model is described in e-Appendix 2 and e-Figure 1; this appendix includes R script (R Foundation for Statistical Computing) for the simulation.
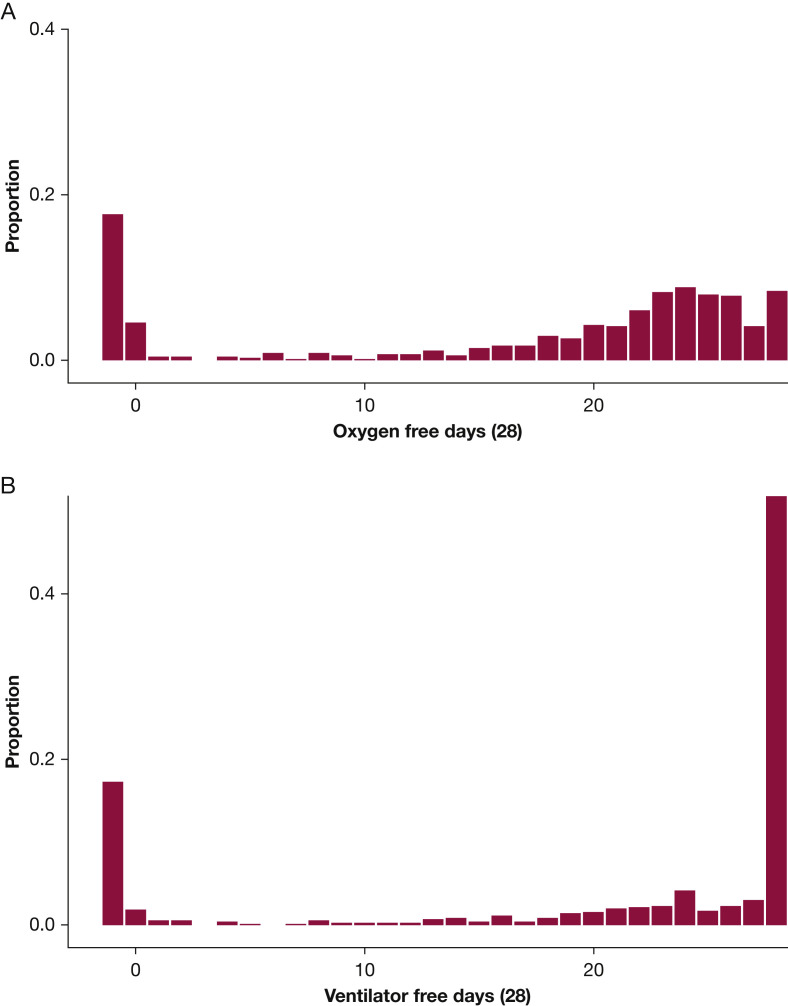
The statistical design of the ACTIV-4 Host Tissue platform was informed by OFDs observed in a recently completed trial of COVID-19 convalescent plasma called Passive Immunity Trial for Our Nation (PassITON; ClinicalTrials.gov Identifier: NCT04362176).17 PassITON was a masked, randomized clinical trial comparing COVID-19 convalescent plasma with placebo among adults hospitalized with hypoxemia as a result of COVID-19 in 25 US hospitals. Eligibility criteria were nearly identical to those being used in the ACTIV-4 Host Tissue platform. The distribution of OFDs among the first 698 patients enrolled in PassITON is shown in Figure 2 A. Median OFDs were 22 days, with an interquartile range of 8 to 25 days. Approximately 17.6% of participants died before day 28, and median OFDs among survivors were 23 days (interquartile range, 19-25 days).
Figure 2.
A, B, Histograms showing (A) oxygen-free days and (B) ventilator-free days among the first 698 participants in the Passive Immunity Trial for Our Nation trial, a randomized trial of COVID-19 convalescent plasma vs placebo among adults hospitalized with COVID-19 and hypoxemia in the United States.
Power to Detect Differences in OFDs
Although the minimum clinically important difference in OFDs has not been described definitively, using a detectable difference of about 2 OFDs for sample size calculations is consistent with approaches used for other free-day and time-to-recovery outcomes21 , 22 and expert opinion on meaningful changes in duration of organ support.36 Table 3 shows the relationship between differences in OFDs in an intervention and control group and the OR generated from a proportional odds model based on PassITON data. An OR of 1.55 corresponds to a mean difference of 2.3 OFDs (16.8 OFDs in the placebo group and 19.1 OFDs in the intervention group), including a 5.5% absolute reduction in mortality (17.6% mortality in the placebo group and 12.1% mortality in the intervention group).
Table 3.
Relationship Between OFDs Expressed on a –1 to 28 scale and an OR From a Proportional Odds Model Comparing OFDs in an Intervention Group and Placebo Group
| OFD Characteristic | Placebo Group | Intervention Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (intervention vs placebo) | 0.67 | 0.80 | 1.40 | 1.45 | 1.50 | 1.55 | 1.60 | 1.65 | 1.70 | |
| Mean OFDs | 16.8 | 14.5 | 15.5 | 18.6 | 18.8 | 19.0 | 19.1 | 19.3 | 19.5 | 19.5 |
| Median OFDs | 22 | 19 | 20 | 23 | 23 | 23 | 23 | 23 | 23 | 24 |
| Proportion at OFD level | ||||||||||
| -1 (death) | 0.176 | 0.242 | 0.211 | 0.133 | 0.129 | 0.125 | 0.121 | 0.118 | 0.115 | 0.112 |
| 0 | 0.046 | 0.056 | 0.052 | 0.037 | 0.036 | 0.035 | 0.034 | 0.033 | 0.033 | 0.032 |
| 1 | 0.004 | 0.005 | 0.005 | 0.004 | 0.004 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 |
| — | — | — | — | — | — | — | — | — | — | — |
| 27 | 0.041 | 0.030 | 0.034 | 0.053 | 0.054 | 0.056 | 0.057 | 0.058 | 0.060 | 0.061 |
| 28 | 0.084 | 0.058 | 0.068 | 0.114 | 0.117 | 0.121 | 0.124 | 0.128 | 0.131 | 0.135 |
The data for the placebo group come from the Passive Immunity Trial for Our Nation.17 In the placebo group, mean OFDs were 16.8 days, 17.6% of participants died, 4.6% of participants received supplemental oxygen for at least 28 days (0 OFDs), and 8.4% of participants were weaned off oxygen before the day after randomization (28 OFDs). The table demonstrates the distribution of OFDs for hypothetical intervention groups corresponding to various OR. OFD = oxygen-free day.
Power calculations for the ACTIV-4 Host Tissue platform were conducted using these data from PassITON. Enrollment of 300 participants in an intervention group and 300 patients in a placebo group (600 total participants) provides 85% power to detect an OR of 1.55 for OFDs. Thus, each treatment arm in the ACTIV-4 Host Tissue platform will target 300 participants per group. Detection of ORs of 1.40 (difference of 1.8 OFDs), 1.45 (difference of 2.0 OFDs), and 1.50 (difference of 2.2 OFDs) with 85% power would require 510, 392, and 346 participants per group, respectively.
The power to detect clinically meaningful changes was substantially higher for OFDs than for ventilator-free days and mortality. The distribution of ventilator-free days in PassITON is shown in Figure 2B, demonstrating reduced variability and a higher proportion of the population experiencing 28 free days compared with OFDs. An OR of 1.55 in PassITON corresponded to approximately a mean 2.1-day improvement in ventilator-free days. A sample size of 300 participants per group provided 72% power to detect an OR of 1.55 for ventilator-free days. For a 28-day mortality dichotomous outcome, 300 participants per group provided 47% power to detect a 5.5% absolute difference in mortality between the intervention and control groups.
Summary
OFDs is an emerging clinical trials outcome that is a composite of mortality and duration of new supplemental oxygen use. This outcome is designed to characterize recovery from acute lung injury in populations with a high prevalence of supplemental oxygen use and relatively low prevalence of invasive mechanical ventilation. In these populations, OFDs captures key patient-centered consequences of acute lung injury (including mortality and clinically meaningful lung dysfunction) and increases statistical power to detect meaningful differences. OFDs is an important addition to the set of outcome measures that investigators can use to characterize a patient’s course of acute lung injury and for evaluating therapies aimed at improving morbidity and mortality for these patients.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. d. W. reports grant support from the PETAL network. M. N. G. reports personal consulting fees for serving on the scientific advisory panel for Endpoint Health and Philips Healthcare, support for attending ATS board meetings, and participation on a data safety monitoring board for Regeneron. A. A. G. reports grant support from Faron Pharmaceuticals and AbbVie and participation on data safety monitoring boards for studies at Vanderbilt University Medical Center and the University of Wisconsin. S. M. B. serves as the chair of the data safety monitoring board for Hamilton Ventilators. C. J. L. reports grant support from bioMerieux, Entegrion, Inc., Endpoint Health, and AbbVie; patents issued to Cincinnati Children’s Hospital Medical Center for risk stratification in sepsis and septic shock; participation on data safety monitoring boards for clinical trials unrelated to the current work, stock options in Bioscape Digital (unrelated to the current work), and leadership roles (executive committee, immediate past president, member, board of directors) for the Association for Clinical and Translational Science. S. P. C. reports personal consulting fees from Vir Biotechnology. None declared (A. M., M. S. S., K. W. G., M. H., Y. R., J. T., L. H. M., D. C. F., K. H., B. T. T., D. J. D., E. R., M. M. J., L. W., G. R. B., M. W. S., W. H. S.).
Funding/support: This research was funded in part by the National Heart, Lung and Blood Institute, National Institutes of Health [Grants 42-312-0217571-66406L and 10T2HL156812].
Role of sponsors: Investigators from the sponsor (National Heart, Lung, and Blood Institute) participated in the design of this study, the analysis of data, and the preparation of the manuscript.
Disclaimer: The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the National Institutes of Health, the National Heart, Lung, and Blood Institute, or the US Department of Health and Human Services.
Additional information: The e-Appendixes and e-Figure are available online under “Supplementary Data.”
Supplementary Data
References
- 1.Moss M., Huang D.T., Brower R.G., et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yehya N., Harhay M.O., Curley M.A.Q., Schoenfeld D.A., Reeder R.W. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200(7):828–836. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoenfeld D.A., Bernard G.R. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Goligher E.C., Bradbury C.A., McVerry B.J., et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385(9):777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawler P.R., Goligher E.C., Berger J.S., et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385(9):790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiedemann H.P., Wheeler A.P., Bernard G.R., et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 7.Laterre P.F., Berry S.M., Blemings A., et al. Effect of selepressin vs placebo on ventilator- and vasopressor-free days in patients with septic shock: the SEPSIS-ACT Randomized Clinical Trial. JAMA. 2019;322(15):1476–1485. doi: 10.1001/jama.2019.14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Self W.H., Semler M.W., Wanderer J.P., et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819–828. doi: 10.1056/NEJMoa1711586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Self W.H., Semler M.W., Leither L.M., et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(21):2165–2176. doi: 10.1001/jama.2020.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundgren J.D., Grund B., Barkauskas C.E., et al. A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N Engl J Med. 2021;384(10):905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2021 doi: 10.1016/s1473-3099(21)00751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stokes E.K., Zambrano L.D., Anderson K.N., et al. Coronavirus disease 2019 case surveillance—United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(24):759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 15.Cummings M.J., Baldwin M.R., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenforde M.W., Self W.H., Adams K., et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021;326(20):2043–2054. doi: 10.1001/jama.2021.19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Self W.H., Stewart T.G., Wheeler A.P., et al. Passive Immunity Trial for Our Nation (PassITON): study protocol for a randomized placebo-control clinical trial evaluating COVID-19 convalescent plasma in hospitalized adults. Trials. 2021;22(1):221. doi: 10.1186/s13063-021-05171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dylla L., Douin D.J., Anderson E.L., et al. A multicenter cluster randomized, stepped wedge implementation trial for targeted normoxia in critically ill trauma patients: study protocol and statistical analysis plan for the Strategy to Avoid Excessive Oxygen (SAVE-02) trial. Trials. 2021;22(1):784. doi: 10.1186/s13063-021-05688-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomazini B.M., Maia I.S., Cavalcanti A.B., et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX Randomized Clinical Trial. JAMA. 2020;324(13):1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simonis F.D., Serpa Neto A., Binnekade J.M., et al. Effect of a low vs intermediate tidal volume strategy on ventilator-free days in intensive care unit patients without ARDS: a randomized clinical trial. JAMA. 2018;320(18):1872–1880. doi: 10.1001/jama.2018.14280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sevransky J.E., Rothman R.E., Hager D.N., et al. Effect of vitamin C, thiamine, and hydrocortisone on ventilator- and vasopressor-free days in patients with sepsis: the VICTAS Randomized Clinical Trial. JAMA. 2021;325(8):742–750. doi: 10.1001/jama.2020.24505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackle D., Bellomo R., Bailey M., et al. Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med. 2020;382(11):989–998. doi: 10.1056/NEJMoa1903297. [DOI] [PubMed] [Google Scholar]
- 23.Novack V., Beitler J.R., Yitshak-Sade M., et al. Alive and ventilator free: a hierarchical, composite outcome for clinical trials in the acute respiratory distress syndrome. Crit Care Med. 2020;48(2):158–166. doi: 10.1097/CCM.0000000000004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall J.C., Vincent J.L., Guyatt G., et al. Outcome measures for clinical research in sepsis: a report of the 2nd Cambridge Colloquium of the International Sepsis Forum. Crit Care Med. 2005;33(8):1708–1716. doi: 10.1097/01.ccm.0000174478.70338.03. [DOI] [PubMed] [Google Scholar]
- 25.Harhay M.O., Casey J.D., Clement M., et al. Contemporary strategies to improve clinical trial design for critical care research: insights from the First Critical Care Clinical Trialists Workshop. Intensive Care Med. 2020;46(5):930–942. doi: 10.1007/s00134-020-05934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dakkak J., Tang W., Smith J.T., et al. Burden and unmet needs with portable oxygen in patients on long-term oxygen therapy. Ann Am Thorac Soc. 2021;18(9):1498–1505. doi: 10.1513/AnnalsATS.202005-487OC. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs S.S., Krishnan J.A., Lederer D.J., et al. Home oxygen therapy for adults with chronic lung disease. An Official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(10):e121–e141. doi: 10.1164/rccm.202009-3608ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs S.S., Krishnan J.A. Patients choose hypoxemia over social isolation. Ann Am Thorac Soc. 2021;18(9):1460–1461. doi: 10.1513/AnnalsATS.202106-676ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shyong F. After hospitalizations, COVID-19 patients need oxygen. Los Angeles Times. Accessed February 2, 2022.
- 30.Constant Therapeutics. Hompage. https://www.constanttherapeutics.com
- 31.Trevena. Homepage. https://www.trevena.com/
- 32.Rigel Pharmaceuticals Homepage. https://www.rigel.com/
- 33.Beitler J.R., Sarge T., Banner-Goodspeed V.M., et al. Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-FiO2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2019;321(9):846–857. doi: 10.1001/jama.2019.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO Working Group on the Clinical Characterisation and Management of COVID-19 Infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong A., Baumgart A., Evangelidis N., et al. Core outcome measures for trials in people with coronavirus disease 2019: respiratory failure, multiorgan failure, shortness of breath, and recovery. Crit Care Med. 2021;49(3):503–516. doi: 10.1097/CCM.0000000000004817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nichol G., Brown S.P., Perkins G.D., et al. What change in outcomes after cardiac arrest is necessary to change practice? Results of an international survey. Resuscitation. 2016;107:115–120. doi: 10.1016/j.resuscitation.2016.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.