Global climate change challenges modern agriculture and food security. Intensive selection in crop breeding considerably narrows genetic diversity for climate adaption (Atherton and Rudich, 1986; Lin et al., 2014). For instance, modern cultivars approximately account for only about 5% of the total genetic variability of tomato resources (Atherton and Rudich, 1986). These challenges raise an urgent need to develop novel strategies to exploit wild species, a largely untapped source of desirable stress‐resistant traits, to accelerate breeding of climate‐smart crops. Genome editing has shown its power as a rapid and precise breeding technique, but it is still challengeable to create complex and polygenic traits underpinned by multiple quantitative loci, such as stress resistance traits (Gao, 2021). In particular, many desirable traits are difficult to create by gene editing due to its inefficient knock‐in and knock‐up property in plants. Introgression of stress‐tolerance traits from wild relatives into elite cultivars via genetic crosses can achieve such success. However, the introgression progress is often slow and labour‐consuming due to multiple hurdles presented by genetic barriers, large growth habit variations of wild species and labour cost of manual emasculation in elite cultivars. For example, while tomato seed catalogues are presently dominated by F1 hybrids, seed production in tomato is expensive and laborious because it requires manual emasculation of the seed parent line one‐by‐one and also its pollination (Atherton and Rudich, 1986).
Here, we show a ‘two‐in‐one’ strategy to accelerate breeding by combining male‐sterility production by CRISPR in elite cultivars with de novo domestication of wild species. Crossing de novo domesticated wild species into male‐sterile elites allows faster introgression of stress‐tolerance trait and selection of new germplasms because male‐sterility reduces labour and time cost for crosses and de novo domestication save time from rounds of backcrosses caused by huge differences of growth habits of wild species. New varieties can be produced by backcrosses using desirable F2 progenies as male and male‐sterile elite as female (Figure 1a).
Figure 1.
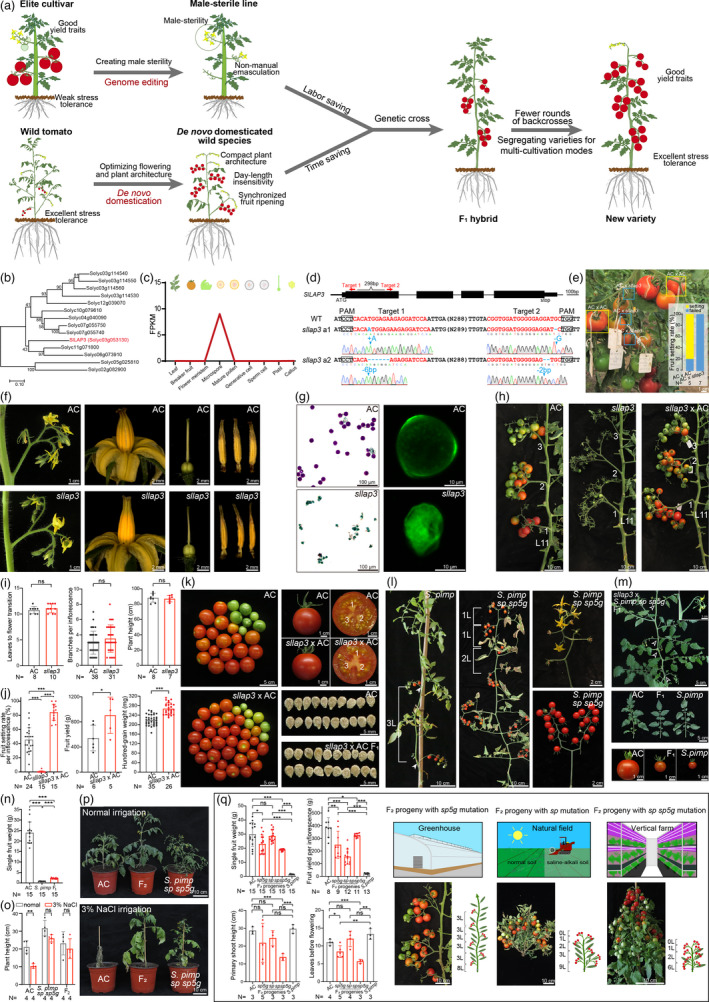
A two‐in‐one breeding strategy promotes rapid utilization of wild species and elite varieties. (a) Schematics illustrating the breeding strategy. (b) Phylogeny of tomato strictosidine synthase family proteins. (c) Expression atlas of SlLAP3. (d) The CRISPR construct for SlLAP3 and verified null mutation alleles. (e) Reciprocal crosses between AC and sllap3 confirming the male sterility. (f) Images showing reproductive organs of AC and sllap3 plants. (g) Alexander staining (left panel) and confocal imaging (right panel) showing pollen defects of sllap3 plants. (h–k) Images and quantitative data showing shoot architecture, fruits and seeds from self‐pollinated AC, sllap3 and manual‐pollinated sllap3 by AC pollens. (l) Representative images showing wild and de novo domesticated S. pimp with sp sp5g mutations. (m, n) Phenotypes and statistics of F1 progeny from cross between sllap3 and S. pimp sp sp5g. (o–p) Phenotypic comparison and quantification between parental plants and F2 progenies upon salt solution irrigation. (q) Representative plants, statistics and schematics showing F2 progenies segregated to fit three different cultivation modes. All data are shown in means ± s.d. (two‐tailed t‐test). The P values are *P < 0.05; **P < 0.01; ***P < 0.001; ns, non‐significant difference.
First, we created male‐sterility in a classical inbred line, Ailsa Craig (AC), which has been grown over 100 years and had served as a popular part of a traditional English breakfast due to its juicy and exceptional flavour (Atherton and Rudich, 1986). Unfortunately, it is disease‐susceptible and sensitive to abiotic stresses. To produce specific male‐sterility without penalty of plant growth and yield, we screened the genes specifically involved in early stages of pollen development by analysing the public organ and tissue specific transcriptome data (Liu et al., 2018). We selected the SlLAP3 gene that specifically expressed in microspore, a tomato ortholog of Arabidopsis LESS ADHERENT POLLEN 3 (LAP3) encoding a strictosidine synthase essential for pollen exine formation (Figure 1b,c; Dobritsa et al., 2009; Du et al., 2020). We used CRISPR/Cas9 technique to target SlLAP3 and identified two independent null alleles with identical phenotypes (Figure 1d). The CR‐sllap3 mutant showed completely male‐sterile without setting any fruit and seed at all tested growth conditions, including greenhouse (Figure 1e‐h), open field and vertical farm. sllap3 plants produced flowers indistinguishable from AC, including normal morphology of stamen and stigma, but dissection of the stamen showed aborted pollens that result in the male sterility (Figure 1f,g). Notably, sllap3 plants exhibited normal vegetative growth, flowering transition, shoot and inflorescence architecture (Figure 1h,i). We manually pollinated wild‐type pollens to sllap3 and found fruit‐setting was quite well with higher setting rate than self‐pollinated AC plants. The fruit shape was normal with slightly increased yield (Figure 1j,k), indicating the rapid generation of specific sporophytic male‐sterility in tomato.
Second, we selected wild species Solanum pimpinellifolium (S. pimp) as the introgression donor, an ancestor of modern tomato with excellent stress tolerance (Lin et al., 2014). A rapid domestication of major yield traits in S. pimp, including day‐length sensitivity, shoot architecture, flower and fruit production, can quickly eliminate flowering asynchrony and trait discrepancy of wild parental lines that often require rounds of backcross in conventional breeding. We used our CRISPR/Cas9 multiplex‐editing system to simultaneously edit SELF‐PRUNING 5G (SP5G, Solyc05g053850) and SELF PRUNING (SP, Solyc06g074350) to create day‐neutral S. pimp plants with synchronized flowering and compact shoot architecture (Li et al., 2018). The Cas9‐free T1 progenies with SP5G and SP mutations flowered after producing about eight leaves in contrast to 13–15 leaves in S. pimp, comparable to AC plants. Simultaneous mutation of SP and SP5G also converted the indeterminate vine architecture of S. pimp into determinate growth with early termination of sympodial cycling, thus, resulting in compact tomato plants with intensive and almost synchronously ripening fruits (Figure 1l).
Third, we crossed de novo domesticated S. pimp lines with male‐sterile AC plants. The F1 progenies exhibited typical intermediate phenotypes (Figure 1m,n). We then screened desirable F2 individuals that carry stress‐tolerant traits from S. pimp and yield traits from AC. As the S. pimp accession we de novo domesticated is salt‐tolerant, we first examined if this trait has been introgressed. We irrigated F2 progenies that phenotypically resemble AC plants with 3% NaCl solution, the salt‐stress treatment optimized for screening S. pimp accessions (Li et al., 2018). After 1‐week irrigation, in contrast to the withering to death of AC plants, selected F2 progenies and S. pimp sp sp5g parental plants kept growing and flowering, albeit some bottom leaves showed early senescence (Figure 1o,p), suggesting the stress‐tolerant traits had been successfully introduced into AC only after two generations. Strikingly, three types of plant architecture, day‐neutral (sp5g), determinate (sp) and double‐determinate (sp sp5g) segregated in F2 population, which fit three currently widely used cultivation modes, including protected cultivation, open fields and vertical farming (Figure 1q).
Together, we devised a ‘two‐in‐one’ breeding strategy that boosts rapid utilization of wild relatives and elite germplasms for stress‐tolerant and multi‐scenario cultivation breeding. Advances of CRISPR technology and de novo domestication will promote application of ‘two‐in‐one’ breeding strategy in different major and orphan crops.
Conflict of interest
The authors declare no competing interest.
Authors’ contributions
C. X. designed the research; Y. X, T. Z. and X. H. performed the experiments; C. X. wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (U1903202), Strategic Priority Research Program of Chinese Academy of Sciences (XDA24030503), Key project at central government level: The ability establishment of sustainable use for valuable Chinese medicine resources (2060302), and National Key R&D Program of China (2018YFA0900600) to C.X.
Xie, Y. , Zhang, T. , Huang, X. and Xu, C. (2022) A two‐in‐one breeding strategy boosts rapid utilization of wild species and elite cultivars. Plant Biotechnol. J., 10.1111/pbi.13788
References
- Atherton, J.G. and Rudich, J. (eds). (1986) The Tomato Crop: A Scientific Basis for Improvement. London: Springer Nature. [Google Scholar]
- Dobritsa, A.A. , Nishikawa, S. , Preuss, D. , Urbanczyk‐Wochniak, E. , Sumner, L.W. , Hammond, A. , Carlson, A.L. et al. (2009) LAP3, a novel plant protein required for pollen development, is essential for proper exine formation. Sex Plant Reprod. 22, 167–177. [DOI] [PubMed] [Google Scholar]
- Du, M. , Zhou, K.E. , Liu, Y. , Deng, L. , Zhang, X. , Lin, L. , Zhou, M. et al. (2020) A biotechnology‐based male‐sterility system for hybrid seed production in tomato. Plant J. 102, 1090–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, C. (2021) Genome engineering for crop improvement and future agriculture. Cell, 184, 1621–1635. [DOI] [PubMed] [Google Scholar]
- Li, T. , Yang, X. , Yu, Y. , Si, X. , Zhai, X. , Zhang, H. , Dong, W. et al. (2018) Domestication of wild tomato is accelerated by genome editing. Nat. Biotechnol. 36, 1160–1163. [DOI] [PubMed] [Google Scholar]
- Lin, T. , Zhu, G. , Zhang, J. , Xu, X. , Yu, Q. , Zheng, Z. , Zhang, Z. et al. (2014) Genomic analyses provide insights into the history of tomato breeding. Nat. Genet. 46, 1220–1226. [DOI] [PubMed] [Google Scholar]
- Liu, L. , Lu, Y. , Wei, L. , Yu, H. , Cao, Y. , Li, Y. , Yang, N. et al. (2018) Transcriptomics analyses reveal the molecular roadmap and long non‐coding RNA landscape of sperm cell lineage development. Plant J. 96, 421–437. [DOI] [PubMed] [Google Scholar]


