Summary
Adenine base editors (ABEs), which are generally engineered adenosine deaminases and Cas variants, introduce site‐specific A‐to‐G mutations for agronomic trait improvement. However, notably varying editing efficiencies, restrictive requirements for protospacer‐adjacent motifs (PAMs) and a narrow editing window greatly limit their application. Here, we developed a robust high‐efficiency ABE (PhieABE) toolbox for plants by fusing an evolved, highly active form of the adenosine deaminase TadA8e and a single‐stranded DNA‐binding domain (DBD), based on PAM‐less/free Streptococcus pyogenes Cas9 (SpCas9) nickase variants that recognize the PAM NGN (for SpCas9n‐NG and SpGn) or NNN (for SpRYn). By targeting 29 representative targets in rice and assessing the results, we demonstrate that PhieABEs have significantly improved base‐editing activity, expanded target range and broader editing windows compared to the ABE7.10 and general ABE8e systems. Among these PhieABEs, hyper ABE8e‐DBD‐SpRYn (hyABE8e‐SpRY) showed nearly 100% editing efficiency at some tested sites, with a high proportion of homozygous base substitutions in the editing windows and no single guide RNA (sgRNA)‐dependent off‐target changes. The original sgRNA was more compatible with PhieABEs than the evolved sgRNA. In conclusion, the DBD fusion effectively promotes base‐editing efficiency, and this novel PhieABE toolbox should have wide applications in plant functional genomics and crop improvement.
Keywords: CRISPR, Cas9 variants, TadA8e, adenine base editors, single‐stranded DNA‐binding domain (DBD), rice
Introduction
Genome editing technology, especially examples developed from clustered regularly interspaced short palindromic repeats (CRISPR) and the CRISPR‐associated nuclease Cas9, can efficiently produce knockout mutants by inducing DNA double‐strand breaks (DSBs) at target sites. However, important agronomic traits of crops are often determined by single‐nucleotide mutations in one or more genes (Zhao et al., 2011). The development of base‐editing tools, including adenine base editors (ABEs) and cytosine base editors (CBEs), comprising single‐stranded DNA deaminases and Cas nickase variants has improved genome‐editing tools from crude molecular scissors that destroy target genes to correctors that change specific bases in the target sequence (Rees and Liu, 2018). We previously developed a high‐efficiency plant CBE (PhieCBE) toolbox (Zeng et al., 2020a) that can efficiently introduce C‐to‐T substitutions in plants by using the codon‐optimized evolved cytidine deaminases evoFERNY and evoCDA1. (Thuronyi et al., 2019). Compared to CBEs, ABEs derived from the Escherichia coli engineered TadA7.10 adenine deaminase yield almost no off‐target editing products (almost no non‐A‐to‐G conversions or insertions/deletions [Indels]), but show relatively low editing efficiencies in plants (Hua et al., 2019b; Molla and Yang, 2019; Rees and Liu, 2018; Zeng et al., 2020b). TadA8e, a new TadA deaminase variant evolved from TadA7.10 (with eight amino acid substitutions), exhibits higher base‐editing efficiency in mammalian cells and in rice (Oryza sativa) (Li et al., 2021; Ren et al., 2021b; Richter et al., 2020; Wei et al., 2021; Xu et al., 2021a; Yan et al., 2021). However, a systematic analysis of its stability and target preference in plants is lacking.
The widely used Streptococcus pyogenes Cas9 (SpCas9) mainly recognizes canonical NGG‐type protospacer‐adjacent motif (PAM) sites, which limits its target range in genomes (Kaya et al., 2016; Li et al., 2021). Accordingly, various Cas9 variants with altered or broadened PAM recognition features, including SpCas9‐NRRH, SpCas9‐NRTH, SpCas9‐NRCH, SpCas9‐NG, SpG, SpRY, S. canis Cas9 (ScCas9), S. aureus Cas9 (SaCas9), Nm1Cas9 and Nm2Cas9, have been adopted in ABE and CBE systems to enhance their scope (Hua et al., 2019a; Li et al., 2021; Liu et al., 2021; Ren et al., 2021b; Wang et al., 2020; Xu et al., 2021a; Yan et al., 2021; Zeng et al., 2020b). Among them, the SpG variant was engineered from Cas9‐NG to accept the same NGN PAM (PAM‐less) requirement but with enzymatic activity; further structure‐guided evolution created a novel SpRY variant with a nearly PAM‐free (unrestricted NNN PAM requirement) feature (Walton et al., 2020). However, these base editors using Cas9 variants show varying editing efficiencies across genomic sites and, in some cases, cannot edit difficult‐to‐edit sites (Li et al., 2021; Ren et al., 2021b; Zeng et al., 2020b).
Base editors generally only exert their editing activity over a narrow window (M4‐M8 relative to the PAM [at positions 21‐23], where M is A or C) near their target sequences; in particular, nucleotides close to PAM motifs are difficult to edit (Koblan et al., 2018; Komor et al., 2016). This issue is difficult to resolve by the simple replacement of editors with evolved deaminases or Cas variants (Koblan et al., 2018; Thuronyi et al., 2019). Zhang et al. (2020) reported that in mammalian cells, the inclusion of the single‐stranded DNA‐binding domain (DBD) from RADIATION SENSITIVE 51 (Rad51) in a CBE allowed efficient C‐to‐T editing and broadened the editing window (from C4–C8 to C4–C15) by increasing the exposure of the single‐stranded target DNA to the deaminase. Recently, the DBD‐containing hyper CBEs (hyCBEs) were optimized for rice editing (Xu et al., 2021b). However, whether and how the DNA‐binding domain affects ABE editing performance in plants has not yet been established.
In this study, we developed the new plant high‐efficiency ABE (PhieABE) toolbox and comprehensively evaluated the editing efficiency and compatibility of the TadA8e deaminase and DBD with the Cas9n‐NG, SpGn and SpRYn variants at 29 representative rice genomic sites linked to ten agronomic trait‐related genes. These engineered PhieABE variants, hyABE8e‐NG, hyABE8e‐SpG and hyABE8e‐SpRY, exhibited greatly enhanced adenine base‐editing activities with broadened targeting windows and low single guide RNA (sgRNA)‐dependent off‐target editing. Furthermore, SpRY‐guided TadA8e appeared to behave in a PAM‐free manner and exhibited nearly 100% editing efficiency at several tested sites. To reduce self‐editing frequency, the evolved sgRNA (esgRNA) scaffold starting with ‘GCCCC’ was used. However, we observed that the esgRNA is poorly compatible with PhieABEs and did not work at acceptable levels. Taken together, our results indicate that the PhieABE toolbox has broad potential applications in plant functional genomics research and genetic improvement of crops.
Results and discussion
Development of the PhieABE toolbox
To update our previous ABE system (Zeng et al., 2020b), we synthesized the coding sequences of evolved TadA8e and the bipartite nuclear localization signal (bpNLS), codon‐optimized them for expression in plants (rice) (Figure S1) and cloned them in frame with codon‐optimized Cas9n‐NG, SpGn and SpRYn PAM‐less variants (Figure S1) to generate three basic ABE8es: ABE8e‐Cas9n‐NG (ABE8e‐NG), ABE8e‐SpGn (ABE8e‐SpG) and ABE8e‐SpRYn (ABE8e‐SpRY) (Figure 1a). To further improve editing efficiency, we inserted the sequence encoding the DBD from Rad51 codon‐optimized for plant (rice) expression (Figure S1) between the sequences of TadA8e and the Cas9n variants, resulting in three DBD‐enhanced hyper PhieABEs: ABE8e‐DBD‐Cas9n‐NG (hyABE8e‐NG), ABE8e‐DBD‐SpGn (hyABE8e‐SpG) and ABE8e‐DBD‐SpRYn (hyABE8e‐SpRY) (Figure 1a).
Figure 1.
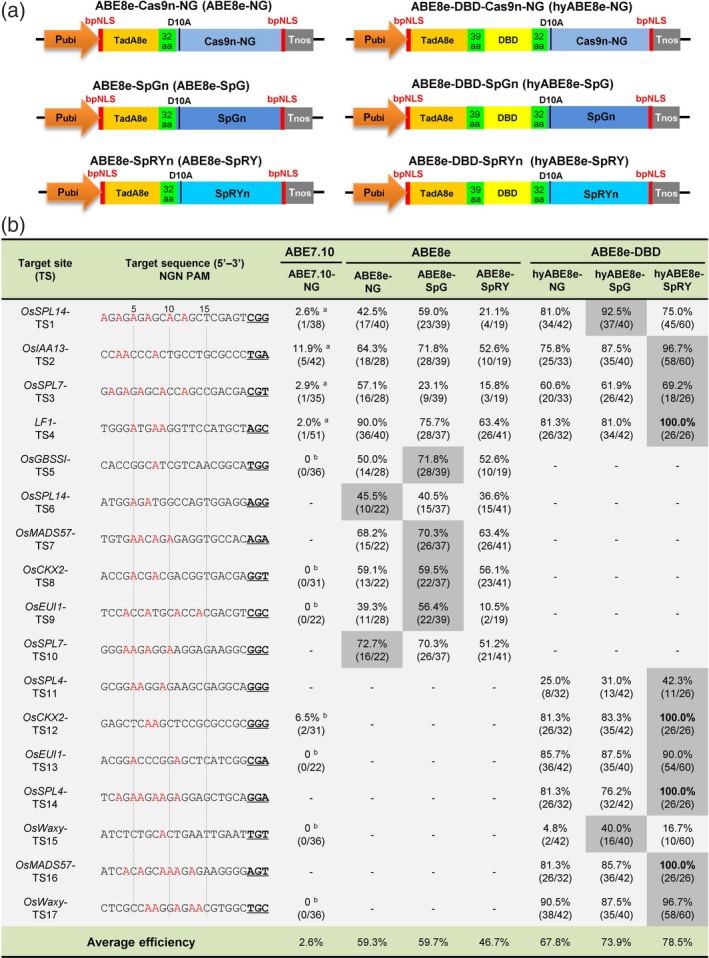
PhieABEs allow efficient A‐to‐G conversion at NGN‐PAM target sites in rice. (a) Schematic diagrams of basic ABE8es (ABE8e‐NG, ABE8e‐SpG and ABE8e‐SpRY) and PhieABE (hyABE8e‐NG, hyABE8e‐SpG and hyABE8e‐SpRY) constructs. The sequences encoding the evolved adenosine deaminase TadA8e and DBD were fused to three PAM‐less/free SpCas9 nickase variants, Cas9n‐NG, SpGn and SpRYn. bpNLS, bipartite nuclear localization signal; 39 aa and 32 aa, 39‐aa and 32‐aa linker peptides; D10A, D10A substitution in Cas9 nickases. (b) Base‐editing efficiencies of PhieABEs compared to basic ABE8es and ABE7.10‐NG at 17 NGN‐PAM targets in T0 rice plants. A bases edited to G are highlighted in red. The numbers of edited and total T0 plants are given in parentheses, and data with grey background indicate the highest efficiency for each target among editors. ‘a’ and ‘b’ indicate data from previous studies included for comparative purposes (Hua et al., 2019b; Zeng et al., 2020b).
PhieABEs improve base‐editing efficiency at NGN‐PAM target sites in rice
We selected four target sites previously shown to exhibit low editing efficiency with the ABE7.10 system (with NG‐PAM) (Hua et al., 2019b), individually targeting SQUAMOSA PROMOTER BINDING PROTEIN‐LIKE 14 (OsSPL14; TS1, NGG‐PAM), AUXIN‐INDUCED PROTEIN 13 (OsIAA13; TS2, NGA‐PAM), OsSPL7 (TS3, NGT‐PAM) and LATERAL FLORET 1 (LF1, TS4, NGC‐PAM), to test the base‐editing efficiency of these basic ABE8es and new PhieABEs in transgenic rice expressing the same sgRNAs used in earlier work (Figure 1b). Using the same sgRNA expression cassettes (Figure S2), we observed much higher editing efficiencies with the basic ABE8es and PhieABEs (with editing rates from 21.1% to 92.5% at TS1; 52.6% to 96.7% at TS2; 15.8% to 69.2% at TS3; and 63.4% to 100% at TS4) than with ABE7.10 (2.0% to 11.9%) (Figure 1b). To test whether the basic ABE8e and PhieABE systems can recognize various NG‐PAM sites and target different A•T‐distributing sites, we targeted another set of thirteen sites, seven of which (TS5, TS8, TS9, TS12, TS13, TS15 and TS17) were selected from our previous work (Zeng et al., 2020b), with the remaining six being newly designed for this study (TS6, TS7, TS10, TS11, TS14 and TS16). We employed ABE8e‐NG, ABE8e‐SpG and ABE8e‐SpRY for TS5–TS10 and hyABE8e‐NG, hyABE8e‐SpG and hyABE8e‐SpRY for TS11–TS17 (Figure 1b; Figure S2). Almost all tested targets were efficiently edited with varied mutation types (Figures S3–S9), suggesting that these basic ABE8es and new PhieABEs are highly efficient to edit sites with NGN‐PAM in rice.
The use of ABE8e and the bpNLS in ABE8e‐NG resulted in substantially higher average editing efficiency for the tested NGN target sites (59.3%) than ABE7.10‐NG (2.6%), which consisted of a wtTadA‐TadA7.10 dimer (Hua et al., 2019b; Zeng et al., 2020b), in agreement with other recent studies (Li et al., 2021; Ren et al., 2021b; Yan et al., 2021). Enhanced PhieABEs also displayed higher average editing efficiency than basic ABE8es: 67.8% for hyABE8e‐NG vs 59.3% for ABE8e‐NG, 73.9% for hyABE8e‐SpG vs 59.7% for ABE8e‐SpG and 78.5% for hyABE8e‐SpRY vs 46.7% for ABE8e‐SpRY (Figure 1b), indicating that the addition of the DBD indeed improves the base‐editing efficiency of ABEs.
Notably, the hyABE8e‐SpRY editor exhibited the highest editing efficiency (average 78.5%, ranging from 16.7% to 100%, or 1.68‐fold higher than with ABE8e‐SpRY) and showed 100% editing efficiency for the four target sites TS4, TS12, TS14 and TS16 (Figure 1b). We also obtained very high editing efficiency for hyABE8e‐SpG, hyABE8e‐NG, ABE8e‐SpG, ABE8e‐NG and ABE8e‐SpRY. Among basic ABE8es and PhieABEs, hyABE8e‐SpRY also showed the highest editing efficiency for most evaluated targets (9/11) (Figure 1b). These results suggested that the DBD is most compatible with the SpRYn variant.
Although the SpG variant previously exhibited a higher efficiency than Cas9‐NG in accepting NGN‐PAM in human cells (Walton et al., 2020), its base‐editing activity in rice appeared to be highly variable (average from 0% to 91.1%, Table S2) (Li et al., 2021; Ren et al., 2021a; Zhang et al., 2021). Indeed, our results indicated that SpGn‐guided base editing has a comparable or slightly higher efficiency than Cas9n‐NG‐guided editing, both in PhiABEs (1.09‐fold higher) and basic ABE8es (1.01‐fold higher). In most published work, SpCas9 variants exhibited a preference for NGG‐PAM sites over NGH‐PAM sites (where H is A, C, or T) (Ge et al., 2019; Ren et al., 2021b; Zeng et al., 2020a, 2020b). To better understand the PAM requirements of the ABE8e system, we analysed the average editing efficiencies of each basic ABE8e and PhieABE at the tested target sites with NGG‐ and NGH‐PAMs. Unexpectedly, although ABE8es performed well on most tested sites, they showed relatively greater base‐editing efficiencies at NGA‐ (52.6% to 100%, average of 78.1%) and NGC‐ (10.5% to 100%, average of 71.1%) PAM sites than at NGG‐ (21.1% to 100%, average of 57.3%) and NGT‐PAMs (4.8% to 100%, average of 53.1%) (Figure 2a). ABE7.10‐NG, assessed as a control, displayed very low base‐editing activity for NGT‐ and NGC‐PAM targets (Figure 1b). In addition, although the SpCas9‐NG ABE has been reported to have a preference for target sites with NG(G/A/T)‐PAM rather than NGC‐PAM (Nishimasu et al., 2018), the ABE8e‐NG and hyABE8e‐NG variants did not appear to target NGC‐PAM sites less efficiently (Figures 1b and 2a). These results indicated that the ABE8es, including basic ABE8es and PhieABEs, can compensate for the low activity of SpCas9‐based base editors on NGH‐PAM target sites. Taken together, the combination of highly efficiency adenosine deaminases with a bpNLS and Rad51 DBD into the PhieABEs hyABE8e‐NG, hyABE8e‐SpG and hyABE8e‐SpRY improved the efficiency of adenosine base editing and broadened the range of target site selection compared with basic ABEs, ABE8e‐NG, ABE8e‐SpG and ABE8e‐SpRY.
Figure 2.
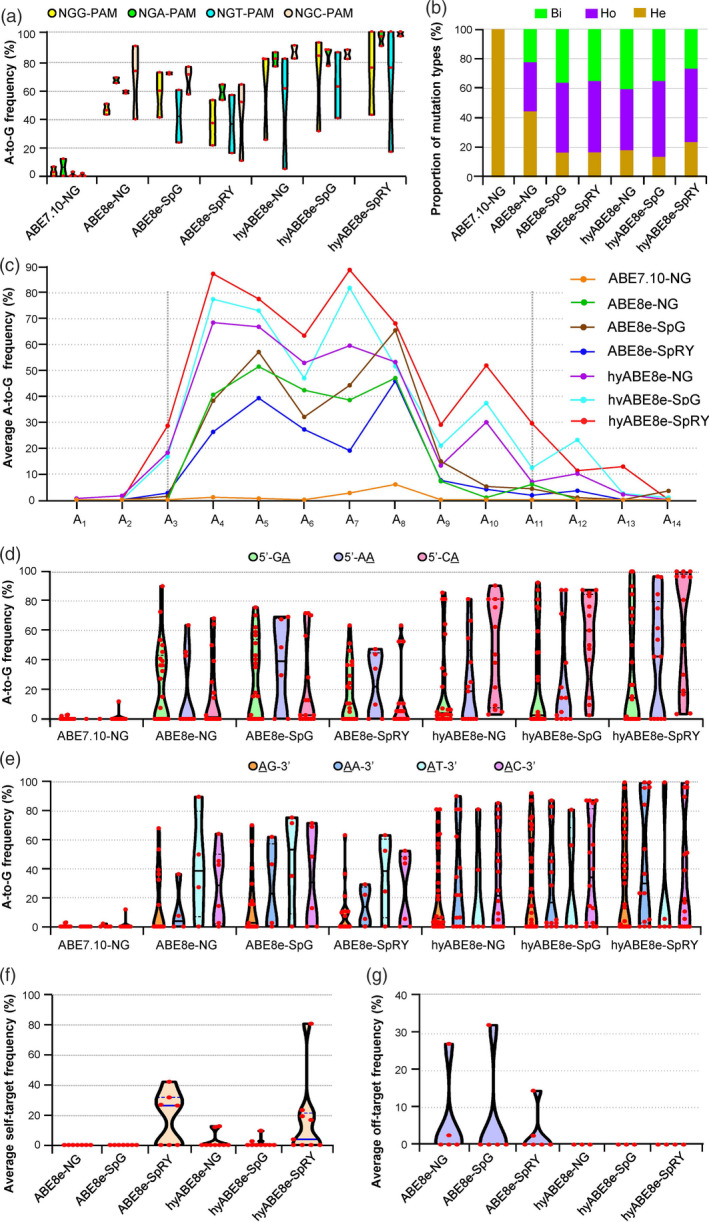
PhieABEs possess wider editing activity windows and better target sequence compatibility at NGN‐PAM sites. (a) Editing efficiencies at NGG‐, NGA‐, NGT‐ and NGC‐PAM targets in T0 plants with basic ABE8es and PhieABEs. ABE7.10‐NG (Hua et al., 2019b; Zeng et al., 2020b) was also used for comparison. (b) Proportion of mutation types induced by basic ABE8es and PhieABEs at all edited NGN‐PAM target sites (TS1–TS17). Bi, bi‐allelic mutations; Ho, homozygous; He, heterozygous. (c) Editing activity windows and efficiencies of PhieABEs, basic ABE8es and ABE7.10‐NG at TS1–TS17 sites. (d) and (e) Site preference analysis of 5’‐GA, 5’‐AA and 5’‐CA (d), and AG‐3’, AA‐3’, AT‐3’ and AC‐3’ (e) contexts for TS1–TS17 targets within the editing window A1–A14. (f) Self‐targeted editing efficiencies in the sgRNA expression cassettes at the TS1–TS17 sites. The hyABE8e‐SpRY shows weaker self‐editing activity than ABE8e‐SpRY in the T0 plants. (g) Off‐target editing frequencies of basic ABE8es and PhieABEs at sites homologous to TS1–TS4 and TS10. We observed no off‐target effects in T0 plants edited by PhieABEs.
PhieABEs produce homozygous/bi‐allelic mutation at high rates and possess wider editing activity windows and better target sequence compatibility
The mutations introduced by TadA7.10‐guided editing were almost always heterozygous in T0 plants (Hua et al., 2019b; Zeng et al., 2020b), indicative of the weaker base‐editing activity of ABE7.10 in plants. Replacing TadA7.10 with TadA8e in the ABE8e system increased the frequency of homozygous or bi‐allelic mutations, although most induced mutations remained mainly in a heterozygous state in T0 plants (Li et al., 2021; Yan et al., 2021). We thus turned to the mutation types in all tested NGN‐PAM target sites, which revealed that the frequency of homozygous (average of 45.0%, ranging from 33.1% to 51.2%) and bi‐allelic mutations (average of 32.5%, ranging from 22.3% to 40.4%) in basic ABE8e‐ and PhieABE‐edited T0 plants is much higher than that of heterozygous mutations (average of 22.5%, ranging from 13.8% to 44.6%); notably, ABE7.10‐NG only produced heterozygous mutation plants at the same targets (Figure 2b). Furthermore, in contrast to previous studies of base editing in callus cells that reported chimeric mutations (Li et al., 2021; Yan et al., 2021), we detected no such chimeric mutation events in the ABE8e‐edited plants of this study, indicating that the T0 edited plants likely regenerated from single independently edited callus cells. Among the edited sites, we identified homozygous mutations mostly at base sites with high editing efficiency, such as GA 5T in the TS4 site, CA 4C in the TS16 site and CA 7A in the TS17 site (Figures S4 and S9). Furthermore, we obtained frequencies of homozygous and bi‐allelic mutations in T0 plants of 81.6%, 86.2% and 76.3% for hyABE8e‐NG, hyABE8e‐SpG and hyABE8e‐SpRY, respectively, which were somewhat higher than or similar to those calculated for ABE8e‐NG (55.2%), ABE8e‐SpG (83.3%) and ABE8e‐SpRY (83.2%) (Figure 2b). In the tested TS1 to TS17 sites, hyABE8e‐SpG produced the highest ratio (288/334, 86.2%) of homozygous and bi‐allelic mutations, demonstrating its strong base‐editing activity. Taken together, these data indicate that ABE8es, especially PhieABEs, generate homozygous or bi‐allelic mutations more efficiently than the ABE7.10 system.
The nucleotide positions supporting efficient base editing define the activity window of ABEs, which depends on productive interactions between the deaminase and its substrate nucleotides (Anzalone et al., 2020). For precise editing of A bases at certain positions, a narrower base‐editing window is more suitable (Molla and Yang, 2019). However, for gene functional screening, saturation mutagenesis, editing regulatory elements or alternative splicing, it may be more advantageous to use base editors with wider editing activity windows (Li et al., 2020). Compared to the A4–A8 editing activity window of ABE7.10‐NG, these ABE8es, especially PhieABEs, exhibited wider editing windows, extending to A3–A11 or even to A1–A14 at the tested TS1 to TS17 sites (Figure 2c and Figures S3–S9). The nucleotide positions experiencing the highest editing frequency changed in PhieABEs (at A4 and A7 with ≥60.0% editing efficiencies) relative to basic ABE8es (at A5 and A8 with ≥40.0% editing efficiencies) (Figure 2c). We also noticed that A‐to‐G substitutions are significantly induced at A3 and A10–A12 by PhieABEs, whereas these positions were hard to edit with basic ABE8es (Figure 2c). Within the centre region (A4–A8) of the editing window, the editing activity rose 1.37‐ to 2.45‐fold upon addition of the DBD (to 60.3% vs 44.0% for Cas9n‐NG, 66.3% vs 47.4% for SpGn and 77.1% vs 31.5% for SpRYn), and it rose up to 7.98‐fold when considering the A9–A14 positions (to 10.4% vs 2.3% for Cas9n‐NG, 16.2% vs 4.8% for SpGn and 22.4% vs 2.8% for SpRYn) (Figure 2c and Figures S3–S9). Due to this broadened editing activity window, we obtained more rare edited alleles in PhieABE‐edited T0 plants than with ABE7.10 and basic ABE8es. For example, at the TS1 in OsSPL14, we detected nine distinct edited alleles produced by hyABE8e‐NG, compared to only four edited alleles from ABE8e‐NG (Figure S3).
These results were generally consistent with previous studies focussing on the TadA9‐based ABE, which exhibits an A3–A12 activity window (Yan et al., 2021). Compared to basic ABE8es, PhieABEs showed relatively high base‐editing activity within the main editing windows (A3‐A11) and presented wide activity windows at some targets previously recalcitrant to editing by the TadA9‐based ABE. Therefore, these PhieABEs are recommended for saturation mutation screens and artificial evolution of genes in plants. For the functional modification of certain sites, ABE8e‐NG, ABE8e‐SpG and ABE8e‐SpRY may be more suitable due to their narrower editing windows.
From the above analyses, it was unclear whether ABE8es have base‐editing preference for 5’‐NA or AN‐3’ positions (the editing site is underlined). To address this question, we calculated the average editing efficiency for all 5’‐GA, 5’‐AA, 5’‐CA, AG‐3’, AA‐3’, AT‐3’ and AC‐3’ motifs (the 5’‐TA motif was not represented in the targets tested here) at A1–A14 active positions in basic ABE8es‐ and PhieABEs‐edited T0 plants and determined their base preference(s). We observed no obvious differences in editing efficiency among the three types of 5’‐NA sites or the four types of AN‐3’ sites by PhieABEs, basic ABE8es or ABE7.10‐NG (for comparison) (Figure 2d and e), indicating that ABE8es, including PhieABEs, have no base preference for the 5’‐end or the 3’‐end A nucleotide within the editing windows, which may contribute to their stability and target sequence compatibility.
Adding the DBD to SpRYn reduces self‐editing frequency and prevents off‐target editing
The development of diverse Cas9 variants, especially the Cas9‐NG and SpG variants (with NG‐PAM) and the PAM‐free SpRY variant, substantially extended the targeting range of genomes and effectively alleviated the restriction imposed by the PAM (Nishimasu et al., 2018; Walton et al., 2020). However, ABEs based on the SpCas9 nickase variants, and especially SpRY, may recognize non‐canonical GTT‐PAM sites in the sgRNA cassettes harboured by the T‐DNA inserted in transgenic plants, which might result in self‐editing of the sgRNA and cause secondary off‐target effects (Qin et al., 2020; Ren et al., 2021b).
We thus PCR amplified and sequenced the sgRNA cassettes with a GTT‐PAM in all T0 plants for 12 representative targets (TS1–TS6, TS9, TS11–TS14 and TS16). Importantly, we detected no instances of self‐editing in ABE8e‐NG‐ and ABE8e‐SpG‐edited plants, and a low self‐editing frequency in the T‐DNA region for T0 plants edited with hyABE8e‐NG (0%–12.5%) and hyABE8e‐SpG (0%–9.5%) (Figure 2f and Figures S3–S9). We also observed some self‐editing (average 16.9%, ranging from 0.0% to 80.8%) in the T‐DNA of the T0 plants edited with ABE8e‐SpRY and hyABE8e‐SpRY for the target sites TS1, TS2, TS4, TS6, TS11, TS13 and TS14 (Figures S3, S4, S5, S7 and S8).
In most instances of targeting by ABE8e‐SpRY and hyABE8e‐SpRY, we detected both on‐target editing and self‐editing in the T0 plants (Figures S3–S5 and S7–S9). However, there was no clear correlation between the frequencies of on‐target editing and self‐editing. For example, hyABE8e‐SpRY produced high editing activity at both the genomic target sites (100% and 90.0%) and in the T‐DNA region (80.8% and 23.3%) for the TS4 and TS13 sites, respectively, while the same base editor displayed 100% on‐target editing at the TS12 and TS16 sites but with no self‐editing (Figures S4, S7 and S9). However, except at the TS4 site, the self‐editing frequency of hyABE8e‐SpRY (with a median of 3.8%) was generally lower than that of ABE8e‐SpRY (with a median of 26.3%) (Figure 2f); for instance, hyABE8e‐SpRY showed 0% self‐editing for TS1 and 16.7% for TS2 while ABE8e‐SpRY reached 42.1% and 26.3% respectively (Figure S3). These results suggested that the DBD reduces the self‐editing activity at sites with GTT‐PAM by SpRYn‐based ABEs. On‐target editing efficiency did not appear to be affected by high self‐editing rates, as observed with TS4 (Figure S4). These data indicated that despite self‐editing, SpRYn‐based ABEs, particularly those with the DBD, enable high‐efficiency base editing.
Compared to ABE7.10, the ABE8e system improved on‐target editing activity, but also inevitably increased the risk of off‐target events in human cells (Lapinaite et al., 2020). To assess how the ABE8e system would fare in plants, we identified the most likely off‐target sites for TS1–TS4 and TS10 (with 1‐nt or 2‐nt mismatches) with the CRISPR‐GE tool (Xie et al., 2017) and sequenced PCR amplicons covering these sites. Next‐generation sequencing detected low off‐target rates (0%–2.6%) at the TS1–TS4‐homologous sequences and relatively high rates (14.6%–32.4%) of off‐target mutations at the TS10‐homologous site with a minimum of 1‐nt mismatch (Os04g0395600/OsAFB2) in the T0 edited lines by ABE8e‐NG, ABE8e‐SpG and ABE8e‐SpRY (Figure 2g and Figure S12). In sharp contrast, we identified no mutation in the same potential homologous off‐target sites in plants edited with PhieABEs (Figure 2g and Figure S12), suggesting that DBD reduces the off‐target effect of ABEs in plants.
SpRY‐guided TadA8e enables efficient A‐to‐G editing at NHN‐PAM targets
Several studies have reported that the SpRY variant exhibits PAM‐free editing activity in human cells and in plants (Ren et al., 2021b; Walton et al., 2020; Yan et al., 2021; Zhang et al., 2021). To determine the recognition capacity of these SpRY‐guided TadA8e at non‐NGN PAMs, we tested 12 target sites with NHN‐PAM (NAN‐, NTN‐ and NCN‐) using ABE8e‐SpRY and hyABE8e‐SpRY. In the resulting T0 plants, the average base‐editing frequency was 59.5% for targets with NAN‐PAM, 36.9% for targets with NTN‐PAM and 41.7% for targets with NCN‐PAM, with 100% editing efficiency for TS20 (NAT‐PAM), TS24 (NTG‐PAM) and TS29 (NCC‐PAM) edited by hyABE8e‐SpRY (Figure 3a). These data suggested that SpRYn‐based ABEs, especially hyABE8e‐SpRY, can edit NHN‐PAM sites and reach higher editing efficiency at targets with NGN‐ and NAN‐PAM than with NCN‐ or NTN‐PAMs (Figure 3b). The editing activity windows and mutation types of ABE8e‐SpRY and hyABE8e‐SpRY at NHN‐PAM targets were similar to targets with NGN‐PAM, with a central active region at A3–A8 and high rated homozygous/bi‐allelic mutations (Figure 3c and d).
Figure 3.
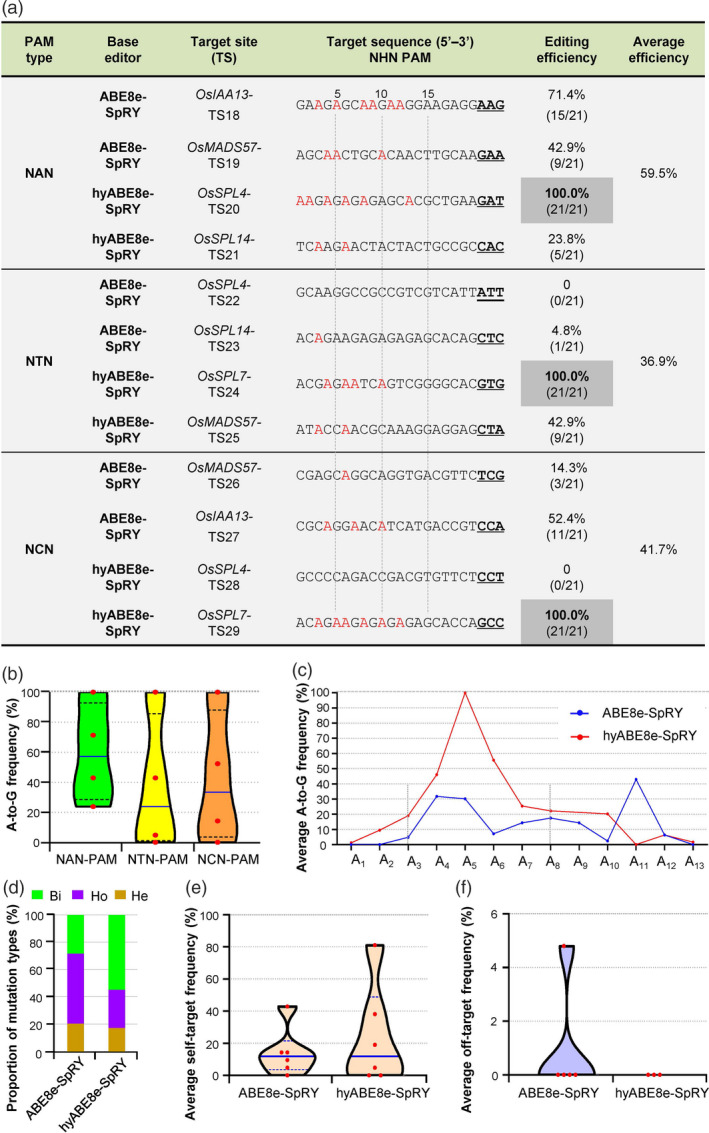
SpRY‐guided TadA8e allows efficient A‐to‐G editing at NHN‐PAM sites in rice. (a) Base‐editing efficiencies of ABE8e‐SpRY and hyABE8e‐SpRY at 12 NHN‐PAM (where H is A, T or C) sites (TS18–TS29) in T0 rice plants. A bases edited to G are highlighted in red. The number of edited and total T0 plants is given in parentheses. (b) Median editing efficiencies at NAN‐, NTN‐ and NCN‐PAM targets in the T0 plants edited by ABE8e‐SpRY or hyABE8e‐SpRY. (c) Editing activity windows and efficiencies of ABE8e‐SpRY and hyABE8e‐SpRY at the TS18–TS29 sites. (d) Proportion of mutation types induced by ABE8e‐SpRY and hyABE8e‐SpRY at the TS18–TS29 sites. (e) Self‐targeted editing efficiencies of the sgRNA expression cassettes at the TS18–TS29 sites. Both ABE8e‐SpRY and hyABE8e‐SpRY showed obvious self‐editing activities in the T0 plants. (f) Frequency of off‐target mutations at sites homologous to TS19, TS20, TS23, TS26 and TS29 induced by ABE8e‐SpRY or hyABE8e‐SpRY. No off‐target effect was detected in T0 plants edited by hyABE8e‐SpRY.
The hyABE8e‐SpRY and ABE8e‐SpRY showed comparable self‐editing efficiencies (both with a median frequency of 11.9%) at NHN‐PAM sites (Figure 3e). Unexpectedly, at all the NYN‐PAM sites tested (ATT, CTC, GTG, CTA, TCG, CCA, CCT and GCC), we observed no clear correlation between self‐editing frequency (0%, 14.3%, 19.0%, 0%, 14.3%, 9.5%, 4.8% and 81.0% respectively) and editing at the intended target sites (0%, 4.8%, 100%, 42.9%, 14.3%, 52.4%, 0% and 100% respectively) (Figures S10 and S11), unlike in a previous report (Ren et al., 2021b). Furthermore, we turned to an analysis of homologous off‐target sites for the five target sites (TS19, TS20, TS23, TS26 and TS29) identical to or with 1‐base differences from their target sequences using CRISPR‐GE. Off‐target site 1 of TS20, which had the same PAM type (CAT) as TS20 (GAT), was characterized by the same editing efficiency as TS20 (both 100%) catalysed by hyABE8e‐SpRY, whereas off‐target site 1 and site 2 of TS23, which had different PAM types (CTG and TTG respectively) from TS23 (CTC), showed stronger editing activity than TS23 (28.6%, 28.6% vs 4.8%) by ABE8e‐SpRY (Figure S13). All other homologous sites with 1‐base variations were associated with off‐target editing frequencies between 0% and 4.8% (Figure S13). For these sgRNA‐dependent off‐target sites with 1‐base variation, hyABE8e‐SpRY exhibited lower off‐target frequency (0%) than ABE8e‐SpRY (0%–4.8%) (Figure 3f). Overall, these data indicate that the SpRY‐guided TadA8e‐based base editors, especially hyABE8e‐SpRY, are potent tools for PAM‐free editing with minimal sgRNA‐dependent off‐target effects. Therefore, since the SpRYn variant can also recognize NNN‐PAM, we recommend selecting different 20‐bp target sequences, even if on‐target and off‐target sites have different PAMs.
The evolved sgRNA is incompatible with DBD‐containing PhieABEs
Recently, Qin et al. (2020) described an evolved sgRNA (esgRNA) scaffold starting with ‘GCCCC’ and containing a longer stem domain (Figure 4a) that retains high genome on‐target editing activity while alleviating the self‐editing of Cas9‐NG. We thus tested whether PhieABEs are compatible with the esgRNA scaffold to edit the TS11, TS12, TS14 and TS16 sites in rice. Compared to the editing efficiency obtained with the original sgRNA scaffold starting with ‘GUUUU’ (Figure 4a), editing efficiency declined for the two targets with NGG‐PAM when tested with all three PhieABEs, while they were comparable or slightly lower for the other two sites (GGA‐ and AGT‐PAMs) (Figure 4b and c). Adenosine base‐editing efficiency was about 1.23–2.70‐fold lower for esgRNA‐guided editing relative to the original sgRNA backbone (Figure 4b). However, the mutation types and editing activity windows were similar for sgRNA‐guided and esgRNA‐guided editing events (Figure 4d and e), indicating that target site preference is not affected by the use of esgRNA.
Figure 4.
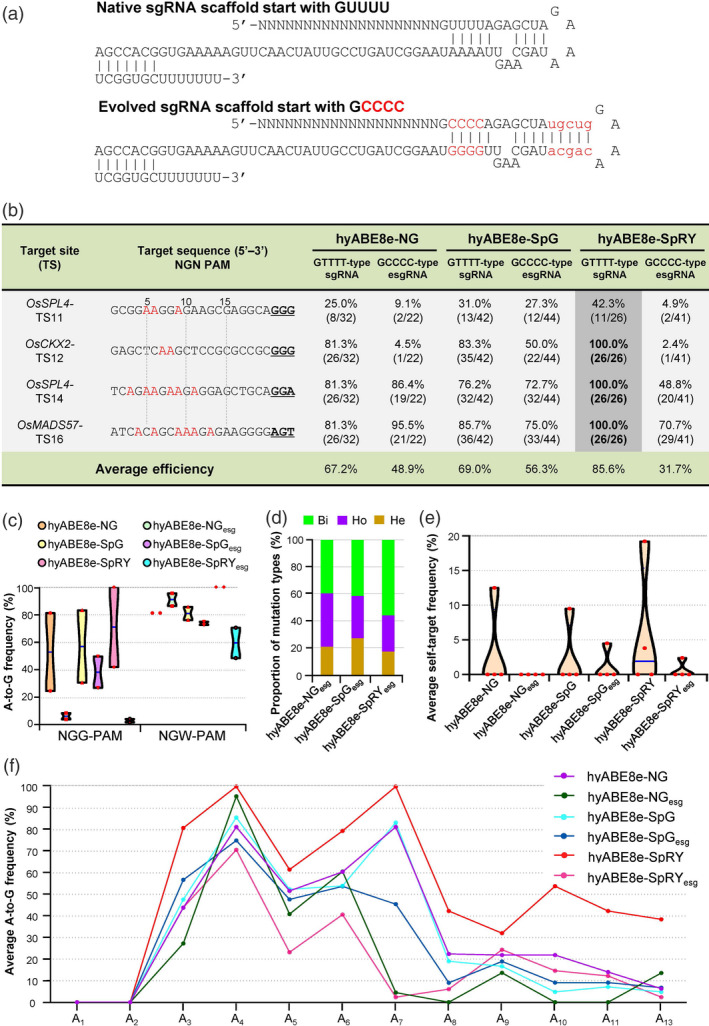
Evolved sgRNA esgRNA does not work well with PhieABEs. (a) Schematic diagram of the original sgRNA and evolved sgRNA (esgRNA). The esgRNA scaffold starting with ‘GCCCC’ and containing a longer stem domain was used in hyABE8e‐NG, hyABE8e‐SpG and hyABE8e‐SpRY. The replaced nucleotides are shown in red and uppercase font; the additional nucleotides are shown in red and lowercase font. (b) Comparison of sgRNA‐ and esgRNA‐guided base‐editing efficiency at TS11, TS12, TS14 and TS16 sites. GUUUU‐type sgRNA, native sgRNA; GCCCC‐type sgRNA, esgRNA (c) Editing efficiencies at NGG‐ and NGW‐PAM (where W is A or T) targets in the T0 plants edited by sgRNA‐ and esgRNA‐guided PhieABEs. PhieABEs with ‘esg’ (also in d–f) indicate results obtained with esgRNAs. (d) Proportion of mutation types induced by esgRNA‐guided PhieABEs. (e) Self‐targeted editing efficiencies in the sgRNA expression cassettes of esgRNA‐guided PhieABEs. Self‐targeted frequencies were much lower when esgRNAs were used, probably due to their lower editing activity with PhieABEs. (f) Editing activity windows and efficiencies with sgRNA‐ and esgRNA‐guided PhieABEs.
For the four tested sites, esgRNA appeared to induce lower self‐targeting than the original sgRNA by the PhieABEs (Figure 4e and Figures S7–S9), in agreement with a previous study (Qin et al., 2020). In summary, although the engineered esgRNA significantly lowered self‐editing efficiency, it also affected the editing activity of PhieABEs, resulting in a great reduction in overall on‐target editing. We speculate that the different ABE8e variants fused to the DBD are not compatible with the esgRNA scaffold.
Conclusion
Base editors are important tools for plant functional genomics research and crop improvement. Recently, several updated ABE8e systems using the evolved adenosine deaminase TadA8e have been reported to increase A‐to‐G editing efficiency in plants (Li et al., 2021; Ren et al., 2021b; Wei et al., 2021; Yan et al., 2021). Nonetheless, whether these ABEs are fully optimized and whether other components (e.g. DBD and sgRNA types) can further enhance ABE activity is unclear. In this study, we developed the novel PhieABE toolbox by fusing the resulting TadA8e deaminase and a DBD with the three PAM‐less/free SpCas9 variants, Cas9n‐NG, SpGn and SpRYn. By systematic testing of editing at 17 NGN‐PAM and 12 NHN‐PAM target sites (Table S1) by PhieABEs and basic ABE8es, we confirmed that TadA8e and the DBD are compatible with these Cas9 variants and result in highly efficient adenosine base editing in rice using the original sgRNA scaffold starting with GUUUU but not esgRNA. PhieABEs display excellent editing activity compared to current plant TadA8e‐based ABE8es (Table S2). In particular, hyABE8e‐SpRY has the highest editing efficiency at NGN‐PAM sites (nearly 100% adenosine base‐editing efficiency at some target sites) with an almost PAM‐free target‐recognition capacity, reduced self‐editing and off‐target frequency and wider editing windows as well as better target sequence compatibility.
According to the previous study, the TadA8e domain engages with the exposed single‐stranded region of the PAM distal non‐target strand (Lapinaite et al., 2020). The high active but sequence‐independent feature of TadA8e may cause a non‐specific transient melting of DNA during double‐stranded DNA surveillance by SpCas9n, resulting off‐target and self‐editing effect (Lapinaite et al., 2020). In our study, the DBD not only enhanced on‐targeting activity but also decreased off‐target and self‐editing effect. It suggested that the fused DBD may influence the spatial structure of the DNA‐bound ABE8e complex and improve the interaction between SpCas9n and TadA8e domain, which make the deamination process activated until the target sequence and PAM were strictly matched by SpCas9n. Overall, these PhieABEs with superior base‐editing properties are potent tools for plant functional genomics research and crop improvement.
Experimental procedures
Vector construction
The nucleotide sequences encoding TadA8e, bpNLS, DBD and linker peptides (Richter et al., 2020; Thuronyi et al., 2019; Zhang et al., 2020) were codon‐optimized for rice and synthesized by GeneCreate (Wuhan, China) (Figure S1). The gene Cas9n‐NG (Zeng et al., 2020b) was generated by introducing the D10A mutation into the Cas9p coding sequence codon‐optimized for rice (Ma et al., 2015). The codon sequences for SpGn (with point mutations R1111L/V1135L/S1136W/R1218K/ F1219Q/R1322A/V1335Q) and SpRYn (with point mutations A61R/V1135L/ S1136W/R1218K/F1219Q/N1317R/R1333P/V1335Q) (Walton et al., 2020) (Figure S1) were generated by PCR with primers containing the point mutations, using Cas9n‐NG as template. The coding sequences for TadA8e and DBD were linked to those of the Cas9n‐NG/SpGn/SpRYn variants by overlapping PCR. The resulting DNA fragments were then cloned into the pYLCRISPR/Cas9Pubi‐H vector (Ma et al., 2015) (digested with Pst I and BamH I) by the modified Gibson cloning method (Zhu et al., 2014), to produce the binary vectors for basic ABEs and PhieABEs (ABE8e‐NG, ABE8e‐SpG, ABE8e‐SpRY, hyABE8e‐NG, hyABE8e‐SpG and hyABE8e‐SpRY). The pYLesgRNA‐OsU3 construct was amplified with the primer pair esgRNA F/esgRNA R using pYLsgRNA‐OsU3 (Ma et al., 2015) as template and then linked by Gibson assembly assay. All primers used in this study are listed in Table S3.
Target design and assembly of the sgRNA expression cassette
To systematically analyse the base‐editing efficiency of different ABE variants among ABE8es, 29 target sites (TS1–TS29) located at 10 genes (Table S1) were selected from previous studies using ABE7.10‐NG (Hua et al., 2019b; Zeng et al., 2020b) or using the online webtool CRISPR‐GE (http://skl.scau.edu.cn/) (Xie et al., 2017). Among the sites, TS1–TS4 were used for testing the editing efficiencies of the three PhieABEs compared to ABE7.10 and the three basic ABE8es. Sites TS5–TS17 with various A‐base distributions and NGN‐PAM types were used to assess the editing window and PAM preference of ABE8es. Sites TS18–TS29 with non‐canonical NHN‐PAMs were used determine the PAM preference of SpRY‐based ABEs. Predicted off‐target sites with 1–2‐nucleotide mismatches with sgRNAs were selected using CRISPR‐GE. The assembly of expression cassettes with multiple sgRNAs for multiplex editing was performed using Golden‐Gate cloning (Ma et al., 2015); the combinations of sgRNA expression cassettes assembled into PhieABEs and basic ABE8es are listed in Figure S2.
Agrobacterium tumefaciens‐mediated rice transformation
All constructs were transformed into the japonica rice cultivar Zhonghua 11 (ZH11) by Agrobacterium (Agrobacterium tumefaciens)‐mediated transformation with strain EHA105 (Nishimura et al., 2006). PCR‐positive transgenic (T0) plants for the hygromycin phosphotransferase gene HPT and Cas9 were used for further analyses.
Detection of on‐target and self‐targeted editing in T0 plants
Genomic DNA for all T0 plants was extracted by the cetyltrimethylammonium bromide method. The genomic regions flanking sgRNA target sites and the sgRNA construct harboured by the T‐DNA inserted in each T0 plant were amplified using specific primers (Table S3). The resulting PCR products (~220–230 bp) were sequenced by next‐generation sequencing (NGS) and analysed with the Hi‐TOM platform (Liu et al., 2019). The filter threshold of Hi‐TOM assay was set as 15%.
Analysis of off‐target events
Using the CRISPR‐GE tool (Xie et al., 2017), potential homologous off‐target sites (≤2‐nucleotide mismatches) of ten target sites (Figures S12 and S13) were identified for off‐targeting analysis. The potential off‐target regions were PCR‐amplified from the T0 plants carrying the corresponding on‐target sgRNA expression cassettes using site‐specific primers (Table S3). The PCR products were subjected to NGS; off‐target mutation frequencies were analysed by the Hi‐TOM platform with a filter threshold of 15% (Liu et al., 2019).
Conflict of interest
The authors declare that they have no conflicts of interest.
Author contributions
Q.Z. conceived and supervised the project. J.T., D.Z., Y.Z., Y.W., T.L., S.L., Y.X., Y.L. and X.X. performed the experiments. J.T., D.Z., Y.Z., L.C., Y.‐G.L. and Q.Z. analysed data. Q.Z., J.T. and Y.‐G.L. wrote and revised this paper.
Supporting information
Figure S1. Nucleotide sequences encoding the TadA8e adenosine deaminases, bpNLSs, DBD peptide and SpCas9 nickase variants codon‐optimized for rice.
Figure S2. T‐DNA structures of the ABE binary vectors used for multiplex base editing.
Figure S3. Base editing of OsSPL 14‐TS1 and OsIAA13‐TS2 in rice using the basic ABE8e and PhieABE toolbox.
Figure S4. Base editing of OsSPL7‐TS3 and LF1‐TS4 in rice using the basic ABE8e and PhieABE toolbox.
Figure S5. Base editing of OsGBSSI‐TS5, OsSPL14‐TS6 and OsMADS57‐TS7 in rice using the basic ABE8e toolbox.
Figure S6. Base editing of OsCKX2‐TS8, OsEUI1‐TS9 and OsSPL7‐TS10 in rice using the basic ABE8e toolbox.
Figure S7. Base editing of OsSPL4‐TS11, OsCKX2‐TS12 and OsEUI1‐TS13 in rice using the PhieABE toolbox.
Figure S8. Base editing of OsSPL4‐TS14 and OsWaxy‐TS15 in rice using the PhieABE toolbox.
Figure S9. Base editing of OsMADS57‐TS16 and OsWaxy‐TS17 in rice using the PhieABE toolbox.
Figure S10. Base editing of OsIAA13‐TS18, ‐TS27, ‐TS19, OsMADS57‐TS26, OsSPL4‐TS22 and OsSPL14‐TS23 in rice using ABE8e‐SpRY.
Figure S11. Base editing of OsSPL4‐TS20, ‐TS28, OsSPL14‐TS21, OsSP7‐TS24, ‐TS29 and OsMADS57‐TS25 in rice using hyABE8e‐SpRY.
Figure S12. Off‐target analysis of the basic ABE8e and PhieABE toolbox at TS1–TS4‐ and TS10‐homologous sites.
Figure S13. Off‐target analysis of ABE8e‐SpRY and hyABE8e‐SpRY at TS19‐, TS20‐, TS23‐, TS26‐ and TS29‐homologous sites with NHN‐PAMs.
Table S1. Rice genes used for adenine base editing in this study.
Table S2. Comparison of PhieABEs and the very recently reported ABE8e systems.
Table S3. List of primer used in this study.
Acknowledgements
This work was supported by grants from the Major Program of Guangdong Basic and Applied Research (2019B030302006), the National Natural Science Foundation of China (31991222; 31971915), the Guangdong special support program of young top‐notch talent in science and technology innovation (2019TQ05N147) and the China Postdoctoral Science Foundation (2020M682728; 2020M682729).
Tan, J. , Zeng, D. , Zhao, Y. , Wang, Y. , Liu, T. , Li, S. , Xue, Y. , Luo, Y. , Xie, X. , Chen, L. , Liu, Y.‐G. and Zhu, Q. (2022) PhieABEs: a PAM‐less/free high‐efficiency adenine base editor toolbox with wide target scope in plants. Plant Biotechnol. J., 10.1111/pbi.13774
References
- Anzalone, A.V. , Koblan, L.W. and Liu, D.R. (2020) Genome editing with CRISPR‐Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38, 824–844. [DOI] [PubMed] [Google Scholar]
- Ge, Z. , Zheng, L. , Zhao, Y. , Jiang, J. , Zhang, E.J. , Liu, T. , Gu, H. et al. (2019) Engineered xCas9 and SpCas9‐NG variants broaden PAM recognition sites to generate mutations in Arabidopsis plants. Plant Biotechnol. J. 17, 1865–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, K. , Tao, X. and Zhu, J.K. (2019a) Expanding the base editing scope in rice by using Cas9 variants. Plant Biotechnol. J. 17, 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, K. , Tao, X. , Han, P. , Wang, R. and Zhu, J.K. (2019b) Genome engineering in rice using Cas9 variants that recognize NG PAM sequences. Mol. Plant, 12, 1003–1014. [DOI] [PubMed] [Google Scholar]
- Kaya, H. , Mikami, M. , Endo, A. , Endo, M. and Toki, S. (2016) Highly specific targeted mutagenesis in plants using Staphylococcus aureus Cas9. Sci. Rep. 6, 26871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblan, L.W. , Doman, J.L. , Wilson, C. , Levy, J.M. , Tay, T. , Newby, G.A. , Maianti, J.P. et al. (2018) Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 36, 843–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor, A.C. , Kim, Y.B. , Packer, M.S. , Zuris, J.A. and Liu, D.R. (2016) Programmable editing of a target base in genomic DNA without double‐stranded DNA cleavage. Nature, 533, 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapinaite, A. , Knott, G.J. , Palumbo, C.M. , Lin‐Shiao, E. , Richter, M.F. , Zhao, K.T. , Beal, P.A. et al. (2020) DNA capture by a CRISPR‐Cas9‐guided adenine base editor. Science, 369, 566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. , Zhang, R. , Meng, X. , Chen, S. , Zong, Y. , Lu, C. , Qiu, J.L. et al. (2020) Targeted, random mutagenesis of plant genes with dual cytosine and adenine base editors. Nat. Biotechnol. 38, 875–882. [DOI] [PubMed] [Google Scholar]
- Li, J. , Xu, R. , Qin, R. , Liu, X. , Kong, F. and Wei, P. (2021) Genome editing mediated by SpCas9 variants with broad non‐canonical PAM compatibility in plants. Mol. Plant, 14, 352–360. [DOI] [PubMed] [Google Scholar]
- Liu, Q. , Wang, C. , Jiao, X. , Zhang, H. , Song, L. , Li, Y. , Gao, C. et al. (2019) Hi‐TOM: a platform for high‐throughput tracking of mutations induced by CRISPR/Cas systems. Sci. China Life Sci. 62, 1–7. [DOI] [PubMed] [Google Scholar]
- Liu, T. , Zeng, D. , Zeng, Z. , Lin, Z. , Xue, Y. , Li, T. , Xie, X. et al. (2021) The ScCas9++ variant expands the CRISPR toolbox for genome editing in plants. J. Integr. Plant Biol. 63, 1611–1619. [DOI] [PubMed] [Google Scholar]
- Ma, X. , Zhang, Q. , Zhu, Q. , Liu, W. , Chen, Y. , Qiu, R. , Wang, B. et al. (2015) A robust CRISPR/Cas9 system for convenient, high‐efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant, 8, 1274–1284. [DOI] [PubMed] [Google Scholar]
- Molla, K.A. and Yang, Y. (2019) CRISPR/Cas‐mediated base editing: technical considerations and practical applications. Trends Biotechnol. 37, 1121–1142. [DOI] [PubMed] [Google Scholar]
- Nishimasu, H. , Shi, X.I. , Ishiguro, S. , Gao, L. , Hirano, S. , Okazaki, S. , Noda, T. et al. (2018) Engineered CRISPR‐Cas9 nuclease with expanded targeting space. Science, 361, 1259–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, A. , Aichi, I. and Matsuoka, M. (2006) A protocol for Agrobacterium‐mediated transformation in rice. Nat. Protoc. 1, 2796–2802. [DOI] [PubMed] [Google Scholar]
- Qin, R. , Li, J. , Liu, X. , Xu, R. , Yang, J. and Wei, P. (2020) SpCas9‐NG self‐targets the sgRNA sequence in plant genome editing. Nat. Plants, 6, 197–201. [DOI] [PubMed] [Google Scholar]
- Rees, H.A. and Liu, D.R. (2018) Base editing: precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 19, 770–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, J. , Meng, X. , Hu, F. , Liu, Q. , Cao, Y. , Li, H. , Yan, C. et al. (2021a) Expanding the scope of genome editing with SpG and SpRY variants in rice. Sci. China Life Sci. 64, 1784–1787. [DOI] [PubMed] [Google Scholar]
- Ren, Q. , Sretenovic, S. , Liu, S. , Tang, X.U. , Huang, L. , He, Y. , Liu, L.I. et al. (2021b) PAM‐less plant genome editing using a CRISPR‐SpRY toolbox. Nat. Plants, 7, 25–33. [DOI] [PubMed] [Google Scholar]
- Richter, M.F. , Zhao, K.T. , Eton, E. , Lapinaite, A. , Newby, G.A. , Thuronyi, B.W. , Wilson, C. et al. (2020) Phage‐assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat. Biotechnol. 38, 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuronyi, B.W. , Koblan, L.W. , Levy, J.M. , Yeh, W.‐H. , Zheng, C. , Newby, G.A. , Wilson, C. et al. (2019) Continuous evolution of base editors with expanded target compatibility and improved activity. Nat. Biotechnol. 37, 1070–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton, R.T. , Christie, K.A. , Whittaker, M.N. and Kleinstiver, B.P. (2020) Unconstrained genome targeting with near‐PAMless engineered CRISPR‐Cas9 variants. Science, 368, 290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Xu, Z. , Gosavi, G. , Ren, B. , Cao, Y. , Kuang, Y. , Zhou, C. et al. (2020) Targeted base editing in rice with CRISPR/ScCas9 system. Plant Biotechnol. J. 18, 1645–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, C. , Wang, C. , Jia, M. , Guo, H.X. , Luo, P.Y. , Wang, M.G. , Zhu, J.K. et al. (2021) Efficient generation of homozygous substitutions in rice in one generation utilizing an rABE8e base editor. J. Integr. Plant Biol. 63, 1595–1599. [DOI] [PubMed] [Google Scholar]
- Xie, X. , Ma, X. , Zhu, Q. , Zeng, D. , Li, G. and Liu, Y.G. (2017) CRISPR‐GE: a convenient software toolkit for CRISPR‐based genome editing. Mol. Plant, 10, 1246–1249. [DOI] [PubMed] [Google Scholar]
- Xu, R. , Qin, R. , Xie, H. , Li, J. , Liu, X. , Zhu, M. , Sun, Y. et al. (2021) Genome editing with type II‐C CRISPR‐Cas9 systems from Neisseria meningitidis in rice. Plant Biotechnol. J. 10.1111/pbi.13716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z. , Kuang, Y. , Ren, B. , Yan, D. , Yan, F. , Spetz, C. , Sun, W. et al. (2021) SpRY greatly expands the genome editing scope in rice with highly flexible PAM recognition. Genome Biol. 22, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, D. , Ren, B. , Liu, L. , Yan, F. , Li, S. , Wang, G. , Sun, W. et al. (2021) High‐efficiency and multiplex adenine base editing in plants using new TadA variants. Mol. Plant, 14, 722–731. [DOI] [PubMed] [Google Scholar]
- Zeng, D. , Li, X. , Huang, J. , Li, Y. , Cai, S. , Yu, W. , Li, Y. et al. (2020b) Engineered Cas9 variant tools expand targeting scope of genome and base editing in rice. Plant Biotechnol. J. 18, 1348–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, D. , Liu, T. , Tan, J. , Zhang, Y. , Zheng, Z. , Wang, B. , Zhou, D. et al. (2020a) PhieCBEs: plant high‐efficiency cytidine base editors with expanded target range. Mol. Plant, 13, 1666–1669. [DOI] [PubMed] [Google Scholar]
- Zhang, C. , Wang, Y. , Wang, F. , Zhao, S. , Song, J. , Feng, F. , Zhao, J. et al. (2021) Expanding base editing scope to near‐PAMless with engineered CRISPR/Cas9 variants in plants. Mol. Plant, 14, 191–194. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Chen, L. , Zhu, B. , Wang, L. , Chen, C. , Hong, M. , Huang, Y. et al. (2020) Increasing the efficiency and targeting range of cytidine base editors through fusion of a single‐stranded DNA‐binding protein domain. Nat. Cell Biol. 22, 740–750. [DOI] [PubMed] [Google Scholar]
- Zhao, K. , Tung, C.‐W. , Eizenga, G.C. , Wright, M.H. , Ali, M.L. , Price, A.H. , Norton, G.J. et al. (2011) Genome‐wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa . Nat. Commun. 2, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Q.L. , Yang, Z.F. , Zhang, Q.Y. , Chen, L.T. and Liu, Y.G. (2014) Robust multi‐type plasmid modifications based on isothermal in vitro recombination. Gene, 548, 39–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Nucleotide sequences encoding the TadA8e adenosine deaminases, bpNLSs, DBD peptide and SpCas9 nickase variants codon‐optimized for rice.
Figure S2. T‐DNA structures of the ABE binary vectors used for multiplex base editing.
Figure S3. Base editing of OsSPL 14‐TS1 and OsIAA13‐TS2 in rice using the basic ABE8e and PhieABE toolbox.
Figure S4. Base editing of OsSPL7‐TS3 and LF1‐TS4 in rice using the basic ABE8e and PhieABE toolbox.
Figure S5. Base editing of OsGBSSI‐TS5, OsSPL14‐TS6 and OsMADS57‐TS7 in rice using the basic ABE8e toolbox.
Figure S6. Base editing of OsCKX2‐TS8, OsEUI1‐TS9 and OsSPL7‐TS10 in rice using the basic ABE8e toolbox.
Figure S7. Base editing of OsSPL4‐TS11, OsCKX2‐TS12 and OsEUI1‐TS13 in rice using the PhieABE toolbox.
Figure S8. Base editing of OsSPL4‐TS14 and OsWaxy‐TS15 in rice using the PhieABE toolbox.
Figure S9. Base editing of OsMADS57‐TS16 and OsWaxy‐TS17 in rice using the PhieABE toolbox.
Figure S10. Base editing of OsIAA13‐TS18, ‐TS27, ‐TS19, OsMADS57‐TS26, OsSPL4‐TS22 and OsSPL14‐TS23 in rice using ABE8e‐SpRY.
Figure S11. Base editing of OsSPL4‐TS20, ‐TS28, OsSPL14‐TS21, OsSP7‐TS24, ‐TS29 and OsMADS57‐TS25 in rice using hyABE8e‐SpRY.
Figure S12. Off‐target analysis of the basic ABE8e and PhieABE toolbox at TS1–TS4‐ and TS10‐homologous sites.
Figure S13. Off‐target analysis of ABE8e‐SpRY and hyABE8e‐SpRY at TS19‐, TS20‐, TS23‐, TS26‐ and TS29‐homologous sites with NHN‐PAMs.
Table S1. Rice genes used for adenine base editing in this study.
Table S2. Comparison of PhieABEs and the very recently reported ABE8e systems.
Table S3. List of primer used in this study.


