Abstract
Compulsive eating is an overlapping construct with binge eating and shares many characteristics with substance abuse disorders. Compulsive eating may impact millions of Americans; presenting in some cases of binge eating disorders, overweight/obesity, and among individuals who have not yet been diagnosed with a recognized eating disorder. To study the behavioral and neurobiological underpinnings of compulsive eating, we employ a published rodent model using cyclic intermittent access to a palatable diet to develop a self-imposed binge-withdrawal cycle. Here, we further validated this model of compulsive eating in female Wistar rats, through the lens of behavioral economic analyses, and observed heightened demand intensity, inelasticity and essential value as well as increased food-seeking during extinction. Using electrophysiological recordings in the anterior insular cortex, a region previously implicated in modulating compulsive-like eating in intermittent access models, we observed functional adaptations of pyramidal neurons. Within the same neurons, application of leptin led to further functional adaptations, suggesting a previously understudied, extrahypothalamic role of leptin in modulating feeding-related cortical circuits. Collectively, the findings suggest that leptin may modulate food-related motivation or decisionmaking via a plastic cortical circuit that is influenced by intermitted access to a preferred diet. These findings warrant further study for whether the behavioral economics of compulsive eating behavior can impact disordered eating outcomes in humans, and whether there is translational relevance of a leptin-sensitive anterior insular circuit implicated in these behaviors.
Keywords: Anterior Insula, Behavioral Economics, Behavioral Neuroscience, Electrophysiology, Intermittent Access, Binge Eating
1. Introduction
Binge eating, or consuming excess food in a brief period with loss of control(American Psychiatric Association, 2013), is a hallmark of binge eating disorder, bulimia nervosa(Curtis and Davis, 2014; Wiss and Brewerton, 2017), and some obesity cases(Bak-Sosnowska, 2017; de Zwaan, 2001; McCuen-Wurst et al., 2017; Meany et al., 2014; Palavras et al., 2017). Compulsive eating, an overlapping construct(Moore et al., 2017a, b; Parylak et al., 2011), shares features of substance use disorders(Gearhardt et al., 2009, 2016; Pursey et al., 2014b; Serafine et al., 2021), including use despite adverse consequences, increased effort and time spent to obtain the substance, negative emotional symptoms with abstinence, and use for negative reinforcement(Avena et al., 2011; Davis et al., 2011; Parylak et al., 2011 ; Zorrilla and Koob, 2019). Compulsive eating distinguishes the etiology, biology, severity, prognosis, and treatment of cases of binge-disordered eating and obesity from those without compulsive eating(Avena et al., 2011; Curtis and Davis, 2014; Davis, 2017; Davis et al., 2011; Gearhardt et al., 2014; Moore et al., 2017b; Parylak et al., 2011; Pedram and Sun, 2014; Randolph, 1956; Rozin et al., 1991; Wiss and Brewerton, 2017; Zorrilla et al., 2021). Most people with binge-type eating disorders(Gearhardt et al., 2016; Pursey et al., 2014b), 15-33% of overweight/obese people(Gearhardt et al.), and many healthy weight individuals not diagnosed with eating disorders exhibit compulsive eating, as operationalized by Yale Food Addiction Scale (YFAS) criteria. Thus, the mental health burden(Agh et al., 2015; Agh et al., 2016; Erskine et al., 2016; Hay et al., 2015; Hay et al., 2017; Kornstein, 2017; Schaumberg et al., 2017) of compulsive eating affects millions.
To model compulsive binge eating, we use cycles of intermittent access to palatable food(Alboni et al., 2017; Cottone et al., 2009; Davis et al., 2007; Kreisler et al., 2017; Parylak et al., 2012; Zorrilla et al., 2021) that resemble the practice of recurrent dieting(Montani et al., 2015) from calorie-rich, palatable foods. In humans, such dieting promotes binge eating, poor metabolic outcomes, and cycling body weight(Cannon, 2007; Goldschmidt et al.; Lowe, 2015; Lowe et al., 2013; Mathes et al., 2009; Montani et al., 2015; Polivy and Herman, 1985), with similar outcomes in our model(Kreisler et al., 2017; Spierling et al., 2020; Spierling et al., 2018; Zorrilla et al., 2021). Some rats develop signs of compulsive eating, defined as persistent self-administration despite incorrect or adverse outcomes(Koob, 2013; Spierling et al., 2020; Vendruscolo et al., 2012), operationalized as qualitatively increased progressive-ratio (PR) breakpoints(Spierling et al., 2020; Spierling et al., 2018; Vendruscolo et al., 2012; Wade et al., 2015), punishment-resistant food self-administration(Spierling et al., 2020), and increased responding during non-reinforced timeout periods(Spierling et al., 2020; Spierling et al., 2018).
Behavior economic (BE) analysis(Bentzley et al., 2013; Bickel et al., 1993; Bickel et al., 1990; Bickel et al., 2014; Hursh, 1991, 2014; Hursh and Roma, 2016; Hursh and Silberberg, 2008) provides another perspective on compulsive use by dissecting self-administration into a “hedonic set point” measure of demand intensity (Q0), or intake if the commodity were “free”(Bentzley et al., 2013; Bentzley et al., 2014), vs. a dissociable measure(Oleson et al., 2011; Yates et al., 2019) of demand elasticity (α), or how sensitively intake decreases with increases in the commodity’s “unit cost”(Bentzley et al., 2013; Bentzley et al., 2014; Hursh and Silberberg, 2008). Inelasticity indicates that food demand persists despite mounting costs of consumption. A related measure, essential value (EV), determines consumption at given price levels and the amount of work emitted for that intake level. Humans demand functions are stable(Acuff and Murphy, 2017) and predict addiction-related measures, including substance use(Bruner and Johnson, 2014; Jacobs and Bickel, 1999; Manning et al., 1995; Murphy and MacKillop, 2006; Murphy et al., 2011), craving(MacKillop et al., 2010b), and aspects of severity(Gray and MacKillop, 2014; MacKillop et al., 2014; MacKillop et al., 2010a; Murphy et al., 2009). Suggesting clinical relevance, demand intensity (Q0) for energy-dense foods positively correlates with BMI in women(Epstein et al., 2018). Here, we test the hypotheses that intermittent access to palatable food increases demand intensity (Q0) and essential value (EV) and decreases demand elasticity (α). We further test whether demand measures are associated with daily intake, weight cycling or other operant motivational measures. Finally, we compare responding under extinction conditions as another index of compulsive-like eating behavior.
Towards identifying the neurobiological bases of compulsive-like eating in our model, we previously found that glutamatergic projections from the anterior insular cortex (AIC) to the ventral striatum modulate compulsive-like food self-administration, which correlates directly with circulating leptin levels(Spierling et al., 2020). Leptin, an adipocyte hormone that regulates energy balance via the mediobasal hypothalamus and modulates hippocampal plasticity, also has high receptor expression in pyramidal neurons(Baskin et al., 1999; Hakansson et al., 1998) of the AIC(Hakansson et al., 1996; Mercer et al., 1996a; Patterson et al., 2011b; Shioda et al., 1998b). The AIC subserves visceroemotional states(Craig, 2009; Critchley et al., 2004) that shape behavior as “somatic markers”(Craig, 2009) and is implicated in compulsive eating(Ding et al., 2020; Frank et al., 2013; Zorrilla E. P.; Koob, 2019). Palatable foods and their cues(Belfort-DeAguiar et al., 2016; Dodds et al., 2012; Kim et al., 2012; Pursey et al., 2014a; Tang et al., 2012; Weygandt et al., 2012) elicit insula activation in binge-type eating disorders, food addiction, obesity, Prader-Willi syndrome and hunger states(Boutelle et al., 2015; Brooks et al., 2013; Connolly et al., 2013; Imperatori et al., 2015; Jastreboff et al., 2013; Kalon et al., 2016; Ogura et al., 2013; Wood et al., 2016a; Wood et al., 2016b). Greater insula responses predict craving(Jastreboff et al., 2013; Wonderlich et al., 2017) and fat gain(Stice and Yokum, 2016), and altered insula responses to palatable food and food cues are seen in bulimia nervosa and BED(Aviram-Friedman et al., 2018; Bohon and Stice, 2011; Kessler et al., 2016; Monteleone et al., 2017). Thus, the present study also tested the hypothesis that intermittent access to palatable food increased the excitability of and decreased leptin responsivity of AIC pyramidal neurons.
2. Methods
2.1. Animals
Because disordered eating is disproportionately prevalent in women(Hudson et al., 2007; Nagl et al., 2016) and given the increased vulnerability of females in our model(Spierling et al., 2018), we studied pair-housed young adult (125–150 g on arrival) female Wistar rats (Charles River, Raleigh, NC) separated by clear, perforated Plexiglas, in wire-topped plastic cages in a temperature- (22 °C) and humidity- (60%) controlled vivarium (12:12 h reverse light cycle). Males (200-225 g on arrival) were studied to assess possible sex differences in insula leptin receptor expression. Before experiments, rats had ad libitum chow (45-mg pellets, 5TUM: ~66% carbohydrates, 24% protein, and 10% fat by kcal; TestDiet, St. Louis, MO) and water. Body weights and food intake were recorded daily for 2–5 days before experiments. Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by The Scripps Research Institute’s Institutional Care and Use Committee.
2.2. Operant self-administration training
Rats (n = 48) learned to self-administer 5TUM chow pellets in previously described operant chambers(Spierling et al., 2018) with one “active” and one “inactive” lever. Water was available ad libitum via sipper tube. After completion of a ratio requirement at the active lever, one pellet was delivered, followed by a 3.25 s post-reinforcement timeout to promote pellet intake, during which responses had no consequences and were deemed “time-out” responses(Fekete et al., 2011). Responses at the inactive lever had no consequences but were recorded. No cues or house lights were used. During training, rats were reinforced by 5TUM chow and received ad libitum 5TUM chow in their home cages. Training began with a single 24-h fixed ratio 1 (FR1) session, run with their cagemate, which accelerates performance in our laboratory. Rats then received individual FR1 training sessions, decreasing in duration from 24 hr to 3 hr to 30 min as a criterion of ≥75% discrimination of active vs. inactive levers with ≥10 pellets/session was attained. Rats then received four 30-min FR1 sessions to establish baseline performance for matching to experimental groups.
2.3. Diet schedules
Rats, matched for baseline chow intake, body weight, percent body fat (Echo-MRI 900, Houston, TX), and operant self-administration, were then assigned to 1 of 3 dietary groups: ad libitum chow access (CHOW); ad libitum access to a more preferred diet only (PREF); and intermittent access (INT) to the preferred diet for 24 h beginning at dark cycle onset for 3 nonconsecutive days/week (e.g., Monday, Wednesday, Friday) with chow access otherwise. The preferred diet, chocolate-flavored, nutritionally-complete 45-mg 5TUL pellets (Test Diets, St. Louis, MO), has similar macronutrient composition (~67% carbohydrates, 21% protein, and 13% fat by kcal) and caloric density (~3.44 kcal/g) as chow (3.30 kcal/g). 5TUL is higher in sucrose content (49.6% vs. 3.8%) and preferred over 5TUM chow (~91% mean preference) (Cottone et al., 2008). Food intake was measured each weekday and body weight twice weekly -- at the end of an access day and of a non-access day -- each of the first 5 weeks.
2.4. Operant self-administration
Each week, rats received fixed-ratio (FR1) operant self-administration sessions with their respective diets on days that INT rats received preferred diet access. To measure the time course of escalation of the reinforcing efficacy of food, female rats not participating in subsequent behavioral economics analyses (n = 24) also received a weekly progressive ratio (PR) session in lieu of an FR1 session on one of the access days(Spierling et al., 2020). For PR sessions, the response requirement increased per the progression: response ratio = [4 × (e# of remforcer*0.075) − 3.8](Cottone et al., 2008), but with the first reinforcer requiring 3 responses. PR sessions ended when rats did not acquire a reinforcer in 14 min, with a maximum session duration of 2-h. The “breakpoint” was the last response requirement completed. After operant sessions, rats were returned to their home cage with their diet and water available ad libitum.
2.5. Behavioral economic and extinction analysis
To avoid influencing demand curve functions, female rats studied in behavioral economics analysis (n=24) only received 30-min FR1 sessions during the first 6 diet schedule weeks (i.e., no PR sessions). Then, response requirements increased to FR3 in Week 7 and doubled weekly thereafter (FR6, FR12, FR24, FR48), concluding with a single FR96 session during Week 12(Hursh and Roma, 2016), during which only 2 of the rats earned a reinforcer. Demand curves were fit to weekly averages using Demand Curve Analyzer (Gilroy et al., 2018). The parameters α and Q0 were obtained from the exponentiated demand function(Koffarnus et al., 2015).
Derived parameters include the maximum work output (Omax) across all costs, the unit price (Pmax) at maximum work output, and the reinforcer’s essential value (EV)(Hursh and Roma, 2016). An exponentiated, rather than exponential, function was used because zero reinforcers were earned by many rats at high ratio requirements(Fragale et al., 2017; Koffarnus et al., 2015).
Rats then received five 30-min FR1 sessions to reestablish stable self-administration performance. After re-establishing FR1 performance, a random subset of rats (n=16) was tested under extinction conditions for 3 days, during which no reinforcers were delivered.
2.6. Slice electrophysiology of diet schedule effects in the anterior insula
To determine the effects of diet schedule on layer V AIC pyramidal neurons, female Wistar rats were studied in slice electrophysiology experiments after chronic (9-14 weeks) exposure to CHOW (n=3), INT (n=14), or PREF (n=3) diet schedules, sacrificed within 1 hr of dark cycle onset. To explore possible effects of acute feeding state, some INT rats were sacrificed after 24 hr of access to the non-preferred chow diet at the time that they otherwise would receive self-administration testing (INT-Withdrawal [INT-WD; n=10)]), and some were sacrificed immediately after completing binge-like FR1 self-administration access to the preferred diet (INT-BINGE; n=4). Isoflurane-anesthetized rats were decapitated and the brain immediately removed into an ice-cold, high-sucrose cutting solution of the following composition (in mM): 206 sucrose; 2.5 KCl; 0.5 CaCl2; 7 MgCl2; 1.2 NaH2PO4; 26 NaHCO3; 5 glucose; 5 HEPES (Kirson et al., 2021; Tunstall et al., 2019). Coronal slices (300 μm) containing the anterior agranular insula (+2 to +3.5 mm from bregma) were prepared and incubated at 32°C for 30 minutes in 2 mM kynurenic acid containing oxygenated artificial cerebrospinal fluid (aCSF) of the following composition (in mM) (Kirson et al., 2021; Tunstall et al., 2019): 130 NaCl; 3.5 KCl; 1.25 NaH2PO4; 1.5 MgSO4•7H2O; 2.0 CaCl2; 24 NaHCO3; 10 glucose, and then continuously superfused with room temperature oxygenated aCSF. Whole-cell patch-clamp recordings were acquired at a sampling rate of 20 kHz and low-pass filtered at 10 kHz, using a Multiclamp 700B amplifier, Digidata 1440A, and pClamp 10 software (Molecular Devices, Sunnyvale, CA, USA). Current-clamp recordings of layer V pyramidal neurons in the AIC were acquired using an input-output current-voltage step protocol to measure passive and active properties. Recordings were performed in aCSF alone (Baseline), and in the presence of GABAB and ionotropic glutamate receptor antagonists (1 μM CGP 55845A, 20 μM DNQX and 30 μM DL-AP5) (Blockers) (Kirson et al., 2021; Tunstall et al., 2019). Patch pipettes (3-6 MΏ) were pulled from borosilicate glass (Warner Instruments) and filled with internal solution composed of (in mM) (Kirson et al., 2021; Tunstall et al., 2019): 145 Kgluconate; 0.5 EGTA; 2 MgCl2; 10 HEPES; 2 Mg-ATP; 0.2 Na-GTP. Data were analyzed using Clampfit (Molecular Devices) and NeuroExpress (Attila Szücs) (Khom et al., 2020; Warden et al., 2020).
2.7. Leptin receptor mRNA in the insular cortex and whole-body energy expenditure
Leptin receptor (Lepr) expression was measured in the AIC of rats trained as described above and previously reported for behavioral parameters and indirect calorimetry analysis(Spierling et al., 2018). Briefly, after chronic (7-11 weeks) of receiving the diet schedules and operant self-administration sessions on access days, rats were acclimated (48 hr) to an Oxymax Comprehensive Laboratory Animal Monitoring System (CLAMS) (Columbus Instruments, Columbus, OH) system, and tested in individual cages for respiratory exchange ratio and energy expenditure(Spierling et al., 2018). After completion of testing, they were decapitated during the first half of the dark cycle of a non-access day.
To measure Lepr mRNA expression in the AIC, isoflurane-anaesthesized male (n=18) and female rats (n=30) were rapidly decapitated. Brains were removed onto an ice-cold stage, snap-frozen in isopentane, and stored at −80°C. For dissection, frozen samples were immersed overnight in RNALater ICE solution at −20°C (ThermoFisher), and tissue punches were then obtained from cryostat sections (300 μm) spanning A/P: +1 to +2 mm bregma followed by Qiazol (Qiagen) RNA extraction, DNase I treatment, and reverse-transcription using SuperScript III First Strand Synthesis Kit (Invitrogen). Taqman qPCR for Lepr (ThermoFisher cat# 4453320) and the reference gene Gapdh (cat# 4331182) was performed using an ABI StepOne Plus.
2.8. Slice electrophysiology of leptin effects on AIC pyramidal neurons
To study the effects of leptin on insula pyramidal neurons, we initially studied experimentally-naïve, chow-fed female Wistar rats (n=6 rats) to determine an effective leptin concentration and A-P range of AIC to target. More details on this preliminary experiment are available in the Supplemental Materials. To then test the hypothesis that chronic diet schedule altered responses to leptin, effects of leptin superfusion (60 nM) for 3 min on AIC neurons (+2 to +3.5 mm from bregma) were studied in diet schedule rats (n=20 rats) after their previously-described recording under baseline and Blockers conditions.
2.9. Statistical analysis
Daily intake and body weight were analyzed using mixed-design 3-way ANOVA with Diet Schedule as a between-subjects factor and Week and Day (e.g., Access vs. Non-Access) as within-subject factors); baseline body weight was a covariate in body weight analyses. FR, PR, and extinction performance were analyzed using mixed-design 2-way ANOVA (Diet as between-subjects factor, and Week or Session as a within-subject factor). Demand curve parameters (EV, α, Q0, Omax, Pmax) and total extinction responses were analyzed by 1-way ANOVA (Diet). Fisher’s LSD test was used to interpret significant Diet effects, and paired t-tests were used to interpret within-subject changes. Elasticity (α) was log-transformed to meet assumptions of parametric analysis. Pearson correlations were used to assess the relationships between EV, log α, and Q0 with daily intake, the cycling amplitude of intake and weight from access to non-access days, as well as operant performance (stable FR1 reinforcers, timeout responses per pellet, and PR breakpoint, averaged from Weeks 4-6). Diet schedule effects in electrophysiology were analyzed by 1-way ANOVA with Tukey’s post hoc tests or unpaired t-tests (with Welch’s correction if variance differed significantly between groups) as appropriate; within-subject analyses of leptin effects were computed via paired t-tests. For qPCR data, to compare expression between sexes and diet schedule groups, we compared CT for Lepr covarying for Gapdh CT. Analyses were performed using SPSS version 25 (IBM, Armonk, NY), Prism 9 (GraphPad, La Jolla, CA) and R version 3.5.3.
3. Results
3.1. Cyclic intake, body weight and escalated operant self-administration in the model
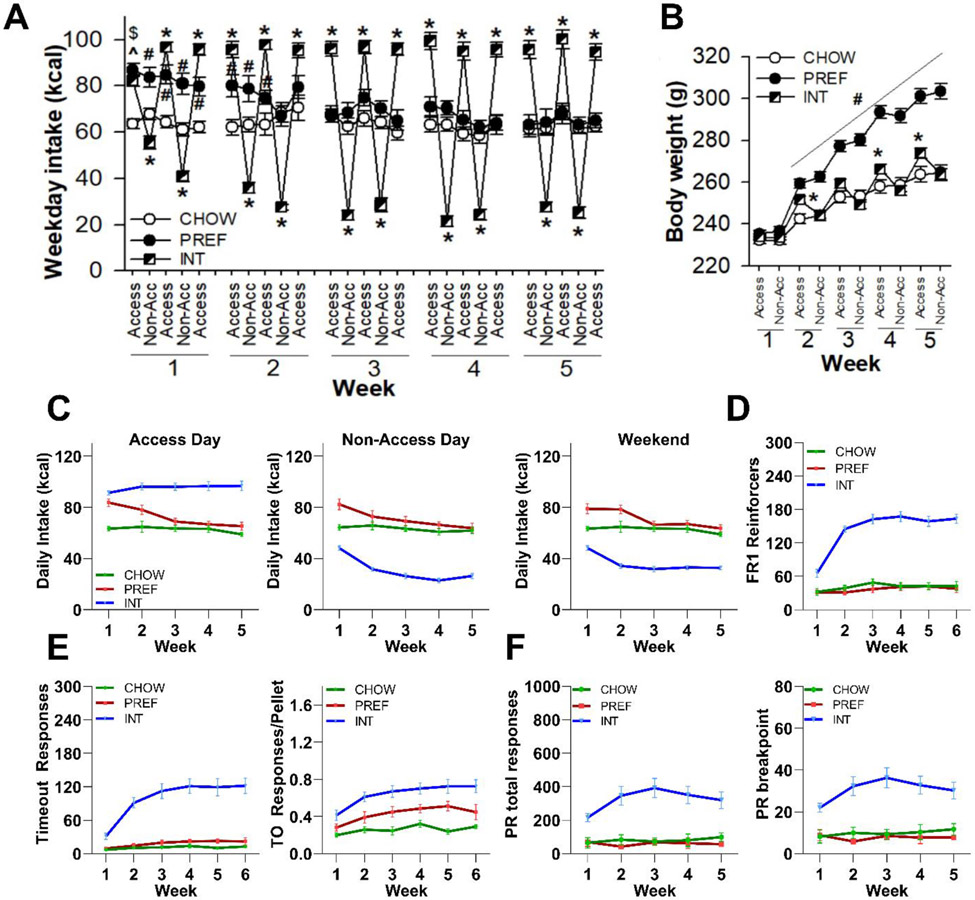
As per our previous work(Spierling et al., 2020; Spierling et al., 2018), Figure 1 shows that INT rats developed cycling daily intake (Fig. 1A; Group X Day: F(8,668)=162.23, p<0.0001; Group X Week X Day: F(32,668)=5.57, p<0.0001) and body weight (Fig. 1B; Group X Day: F(2,43)=55.56, p<0.0001; Group X Week X Day: F(8,172)=8.60, p<0.0001). They weighed significantly more than CHOW controls at the end of access, but not non-access, days by Weeks 2, 3 and 5. They lost ~10 g on days that they did not receive access to the palatable diet (p<0.0001). PREF rats weighed significantly more than both INT and CHOW rats beginning from Week 2 and further diverged thereafter (Group: F(2,43)=31.72, p<0.0001; Group X Week: F(8,172)=29.95, p<0.0001).
Figure 1: Intake, body weight, and fixed- and progressive-ratio (FR, PR) self-administration.
Within the first 5 weeks of diet schedules, female rats receiving chronic intermittent access to preferred diet (INT) developed significant A) cycling of food intake, with an amplitude of ~65-70 kcal between access vs. non-access (Non-Acc) days, and B) cycling body weight as compared to rats continuously fed chow (CHOW). They weighed significantly more than CHOW rats after access days during Weeks 2, 3 and 5 and lost 10 g during non-access days. PREF rats ate significantly more than CHOW rats during the first 2 weeks, but not thereafter, and showed progressively greater body weight than both INT and CHOW rats. C) INT rats developed ~50% higher average daily intake on access days, ~40% lower average daily intake on non-access weekdays, and continued to eat significantly less on chow-fed weekends as compared to CHOW and PREF rats receiving their respective diets. Across the first 6 weeks of FR1 self-administration sessions on access days, D) INT rats earned ~4-fold more reinforcers, E) and made ~10-fold more responses than controls during post-reinforcement “timeout” periods during which responses had no effect. This resulted in a significant, disproportionate increase in the number of timeout responses per pellet. F) INT rats also developed significant increases in total responses and breakpoints of weekly PR sessions. PREF and CHOW rats did not differ significantly from one another on any operant performance measures. All panels show M+SEM. Please see text for statistical detail.
Cyclic intake reflected that by Weeks 4-5, INT rats overate on access days ~50% more than both CHOW and PREF rats (p<0.0001; Fig. 1C), whereas on non-access days they ate only ~40% as much as controls (p<0.0001; Fig. 1C). Undereating continued on weekends (Group X Week: F(8,172)=4.56, p<0.0001; Fig. 1C). PREF rats ate significantly more than CHOW rats during the first 2 weeks (Figs. 1A, 1C), but they decreased to similar intake levels by Week 3.
Figure 1D shows that during FR1 operant self-administration, INT rats progressively earned ~4-fold more pellets (Group: F(2,44)=105.60, p<0.0001; Group X Week: F(10,220)=15.69, p<0.0001) than both CHOW and PREF rats. Figure 1E shows that INT rats had disproportionately increased responding during non-reinforced time out periods, with ~10-fold more timeout responses than CHOW controls (Group: F(2,44)=30.83, p<0.0001; Group X Week: F(10,220)=9.87, p<0.0001). They also had more time out responses per pellet earned (Group: F(2,44)=15.71, p<0.0001) than both CHOW and PREF rats. Figure 1F shows that INT rats developed significantly increased total active responses (Group: F(2,21)=17.24, p<0.0001) and breakpoints (Group: F(2,21)=18.34, p<0.0001) in PR self-administration vs. CHOW and PREF controls.
After 10 weeks on the diet, diet schedule effects were seen on body weight (F(2,20)=13.38, p<0.0001) and total body fat (F(2,20)=16.98, p<0.0001), but not lean mass (F(2,20)=0.10, ns), yielding effects on % body fat (F(2,20)=18.41, p<0.0001). PREF rats were significantly (ps<0.0001) heavier and fatter (M+SEM: weight: 400+48 g, fat: 128+40 g and 31.4+6.3 %) than both INT (weight: 327+24 g, fat: 63+19 g and 18.9+7.8%) and CHOW rats (weight: 308+31 g, fat: 49+19 g and 15.6+4.1%), which did not differ reliably from one another.
3.2. Demand curve analysis
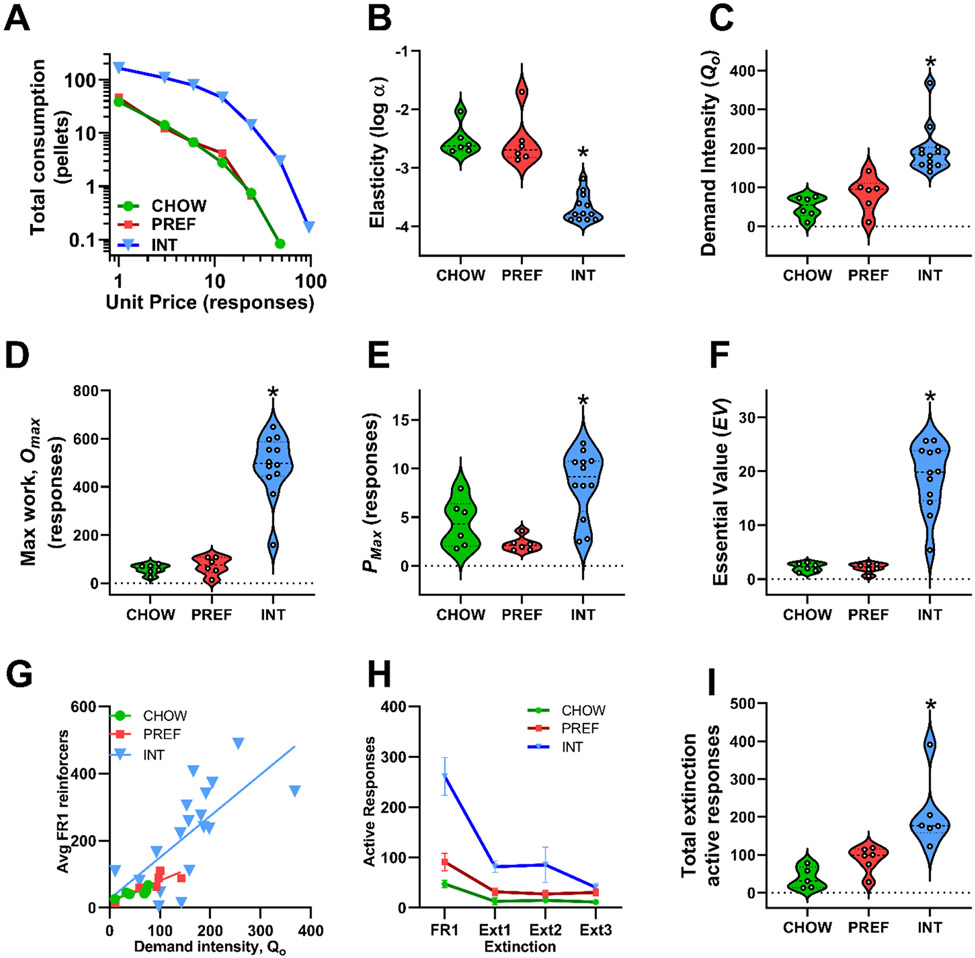
For purposes of illustration, Figure 2A shows that aggregate demand functions of INT rats differed markedly from those of CHOW and PREF rats (Fig. 2A). Demand parameters, calculated individually for each subject, revealed that INT rats showed an 11-fold decrease in elasticity (α) (F(2, 21)=44.07, p<0.001) (Fig. 2B) and a 3-fold increase in demand intensity (Q0) (F(2, 21)=19.60, p<0.001) (Fig 2C) vs. CHOW and PREF rats. INT rats also had 9-fold increased maximal work output (Omax) (F(2, 21))=38.37, p<0.001) (Fig 2D) and a higher unit price (Pmax) for food reinforcers at their maximal work output (F(2, 21)=11.23, p<0.001) (Fig 2E) of 8.2 responses vs. only 3.7 and 1.8 for CHOW and PREF rats, respectively. Finally, the essential value (EV) of food reinforcers was ~9-fold greater for INT rats (F(2, 21)=41.1, p<0.001) (Fig. 2F). CHOW and PREF rats did not differ significantly from one another on any parameters.
Figure 2: Demand function parameters and extinction responding.
Behavioral economics analysis using between-session escalation of fixed-ratio requirements (FR1, 3, 6, 12, 24, 48, 96) revealed that A) INT rats exhibit substantially different aggregate demand functions as compared to both CHOW and PREF rats. Exponentiated demand function analysis of individual subjects’ consumption as a function of increasing unit price showed that INT rats have significantly B) decreased elasticity (log α), C) increased unconstrained demand intensity (Qo), D) increased maximum work (Omax) and E) unit price (Pmax) at the level of maximum work, and F) increased essential value of the preferred diet (EV) as compared to both CHOW and PREF rats. As expected, there were G) significant correlations of demand intensity to average reinforcers earned under low effort (FR1) requirements during weeks 4-6 across all rats (r=0.88, not shown) and within INT, CHOW and PREF diet schedule groups, separately (rs=0.59, 0.84 and 0.84). H) INT rats showed significantly more active responses than CHOW rats during each of 3 extinction sessions after reestablishment of FR1 self-administration, and I) INT rats made significantly more cumulative active responses than both CHOW and PREF controls during the 3 extinction sessions. CHOW and PREF rats did not differ significantly on any parameters. Panel A shows group means and connecting lines of averages at each FR requirement of access day self-administration sessions with escalating FR requirements. Panels B-F and I show violin plots with individual scatter. The dashed line within the violin plots denotes the median and the dotted lines denote the 1st and 3rd quartile. Panel G shows a scatterplot with regression lines fit to each groups’ data; demand intensity was calculated from behavioral economics sessions from Weeks 7-14 and Pearson correlated to average reinforcers earned during FR1 sessions in Weeks 4-6. Panel H shows M+SEM of active responses from 3 consecutive extinction sessions and average active responses emitted during 5 pre-extinction FR1 sessions. *p<0.05 vs. both CHOW and PREF.
Table 1 shows that across all subjects, essential value (EV) and demand intensity (Qo) showed strong positive correlations and elasticity (log α) strong inverse correlations with daily intake on access days, hypophagia on non-access days, larger amplitude cycling of food intake and body weight, more FR1 self-administration, disproportionately increased timeout responding, and higher PR breakpoints. Demand intensity, but not elasticity, also correlated with FR1 reinforcers when analyzed separately in INT, CHOW and PREF groups (Fig. 2G; r’s=0.59, 0.84 and 0.84, respectively, p’s<0.05).
Table 1.
Correlations of demand curve parameters with cycling daily intake and operant performance
| Measure | Essential value (EV) |
Demand intensity (Qo) |
Elasticity (log α) |
|---|---|---|---|
| Access day daily intake (kcal) | 0.79 | 0.79 | −0.82 |
| Non-access day daily intake | −0.82 | −0.72 | 0.85 |
| Weekend day kcal intake | −0.84 | −0.71 | 0.84 |
| Cycling amplitude of intake | 0.83 | 0.78 | −0.86 |
| Cycling amplitude of body weight | 0.71 | 0.72 | −0.76 |
| FR1 reinforcers | 0.90 | 0.88 | −0.84 |
| Timeout responses per pellet | 0.63 | 0.60 | −0.65 |
| PR breakpoint | 0.71 | 0.74 | −0.73 |
Note: Pearson correlations (r) are shown; n = 24, all ps<0.002
During 5 sessions of reacquisition of FR1 self-administration after behavioral economic analysis, INT rats again earned more pellets than PREF and CHOW rats (M+SEM: 165.1+10.6, 66.9+12.1 and 42.6+7.1 per session, respectively; F(2,21)=37.82, p<0.0001). During 3 subsequent extinction sessions, INT rats showed significantly more active lever responses than CHOW rats on all 3 days (Fig. 2H) and more cumulative extinction responses than both CHOW and PREF rats (F(2,14)=11.01, p<0.001) (Fig. 2I).
3.3. Diet schedule-induced alterations in functional properties of AI pyramidal neurons
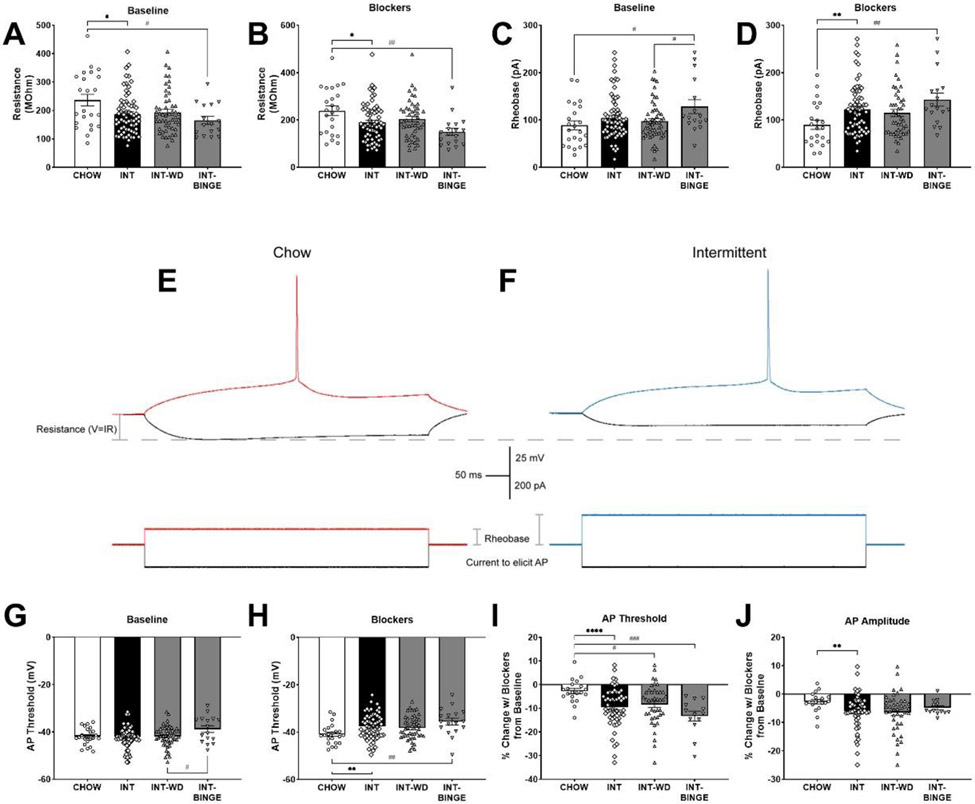
Current-clamp recordings of AIC pyramidal neurons showed that under baseline (aCSF only) conditions, Diet Schedule had a significant effect on membrane resistance (F(2,88)=4.28, p<0.05), wherein INT-BINGE rats had significantly decreased resistance compared to CHOW rats (Tukey’s p<0.05)(Fig. 3A). This diet schedule effect was also observed when using antagonists (Blockers) against ionotropic glutamate and GABAB receptors (F(2,85)=5.82, p<0.01)(Fig. 3B), as well as the decreased resistance of neurons from INT-BINGE rats compared to CHOW (p<0.01). Rheobase (the minimum current required to elicit an action potential), was significantly increased in neurons from INT-BINGE rats compared to CHOW and INT-WD rats under baseline conditions (F(2,89)=3.90, p<0.05; Tukey’s p’s<0.05) (Fig 3C). When inhibitory neurotransmission was isolated (F(2,82)=5.20, p<0.01; Tukey’s p<0.01) (Fig. 3D), rheobase in neurons from INT-BINGE rats was similarly increased compared to CHOW rats. These effects are illustrated in representative traces from a CHOW and INT rat (Fig. 3E&F, respectively). The effect of diet schedule was similarly seen for action potential threshold, with neurons from INT-BINGE rats having a depolarized AP threshold compared to INT-WD rats under baseline conditions (F(2,89)=3.12, p<0.05; Tukey’s p’s<0.05) (Fig 3G), and having a depolarized threshold from CHOW rats in Blockers (F(2,82)=5.49, p<0.01; Tukey’s p’s<0.01) (Fig. 3H).
Figure 3: Altered excitability in anterior insula pyramidal neurons following intermittent access to a preferred diet.
Following 9-14 weeks of intermittent access to preferred diet, functional adaptations were seen in the anterior insula pyramidal neurons of INT rats, especially after binge-like refeeding (INT-BINGE), as compared to those continuously fed chow, including: A) decreased resistance at baseline and B) in the presence of ionotropic glutamate and GABAB receptor blockers and C) increased rheobase at baseline and D) in the presence of blockers. E) A representative trace shows the resistance and rheobase difference in the presence of blockers in a CHOW and F) INT rat. INT-BINGE rats also showed G) increased action potential thresholds at baseline as compared to rats withdrawn to chow diet for 24 hr (INT-WD) and H) increased action potential thresholds with blockers as compared to CHOW rats. INT rats also had a I) decreased percent change in spike threshold and J) in spike amplitude from baseline in the presence of blockers as compared to CHOW controls. *: p<0.05, **: p<0.01, ***: p<0.001, ****: p<0.0001, INT vs CHOW. #: p<0.05, ##: p<0.01, ###: p<0.001, ####: p<0.0001, pairwise between CHOW, INT-WD, or INT-BINGE. n=13-69 cells per group for all measures.
To further investigate differences in responses between diet groups following application of Blockers, electrophysiological parameters were also analyzed as percent change with Blockers from baseline (aCSF only). A one-way ANOVA found significant differences in the effects of Blockers on AP threshold between the diet groups (F(2,71)=8.10, p<0.001). Tukey’s post hoc revealed both INT-BINGE (p<0.001) and INT-WD rats (p<0.05) had a greater decrease (depolarization) in AP threshold compared to the CHOW rats (Fig. 3I). Use of Blockers decreased spike amplitude in all groups, with a significantly greater decrease for all INT rats when compared to CHOW controls (t(57.47)=2.92, p<0.01) (Fig. 3J).
Unlike what was seen for INT rats, membrane resistance, rheobase, and AP threshold of AIC pyramidal neurons from PREF rats did not differ significantly from those of CHOW rats (Supplemental Table 1), implicating an effect of diet schedule rather than diet per se. AP amplitude of AIC neurons from PREF rats was modestly higher than CHOW controls (t(37.61)=2.14, p<0.05) at baseline but not when Blockers or Blockers+Leptin were applied. With these compounds, both CHOW and PREF AP amplitudes directionally changed in a similar fashion.
3.4. Leptin in the insular cortex.
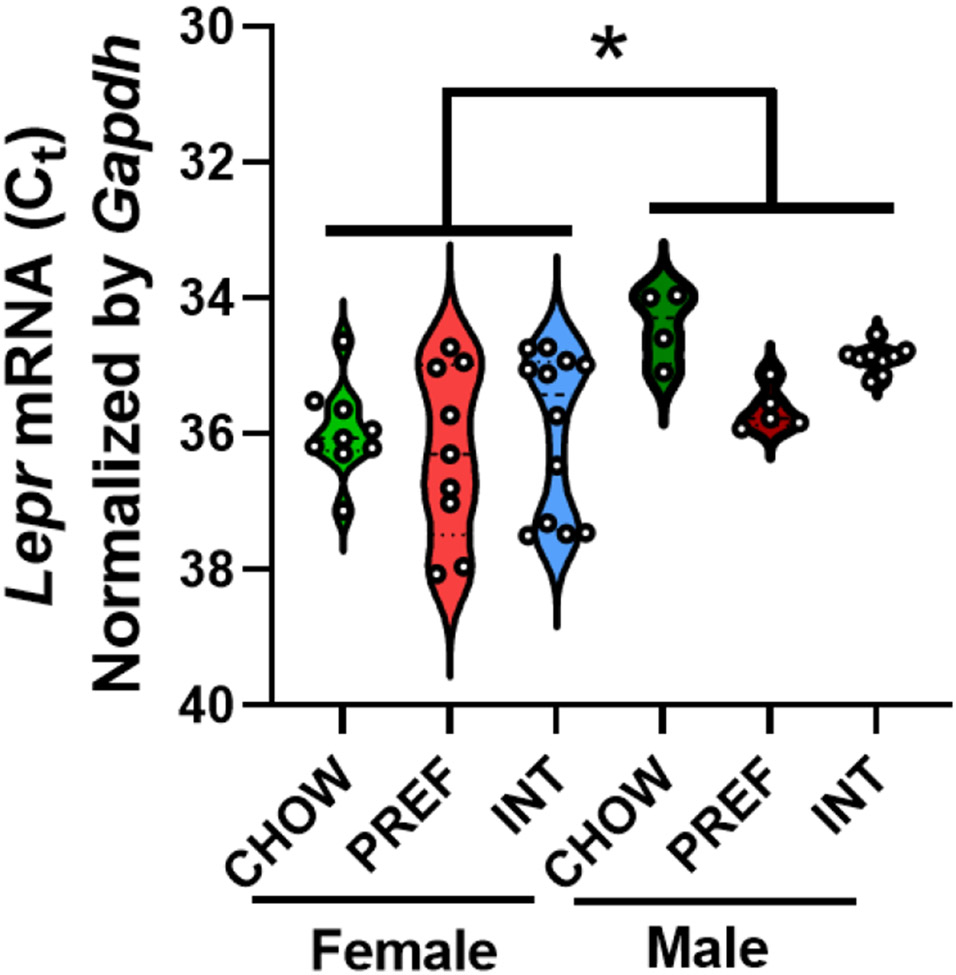
TaqMan qPCR confirmed that Lepr mRNA is expressed in the AIC of young adult male (n=18) and female Wistar rats (n=30). Male rats had significantly greater AIC Lepr mRNA expression (~1.5-2-fold) than female rats (Fig. 4). Across sexes, PREF rats showed a nonsignificant trend for increased Lepr mRNA levels vs. CHOW controls (p=0.08), with INT rats intermediate.
Figure 4: Leptin receptor expression in the anterior insula.
Violin plots with scatter show that male rats, regardless of diet schedule, had greater Lepr mRNA expression (i.e., lower Gapdh-normalized cycle thresholds [CT]) in the insula than females. Insular Lepr mRNA expression did not significantly differ with respect to diet group. The dashed line within the violin plots denotes the median and the dotted lines denote the 1st and 3rd quartile. *: p<0.05, n=4-12 per group.
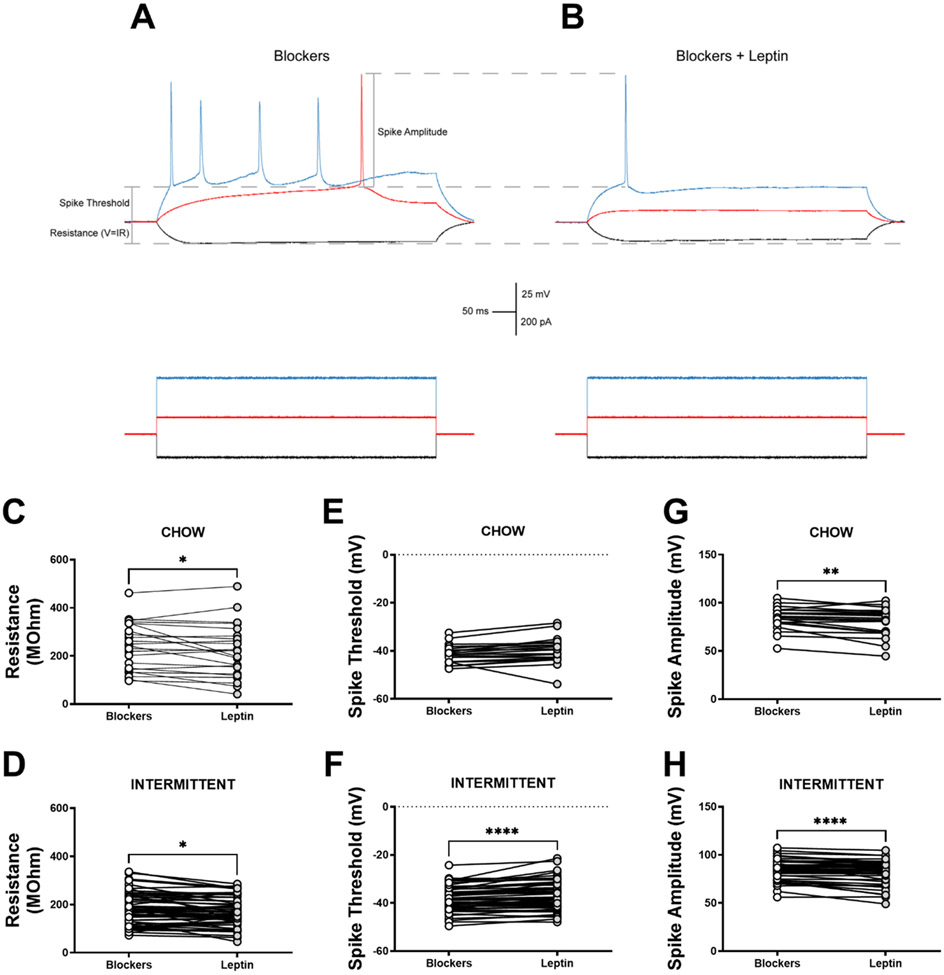
To test leptin action in rats with chronic diet schedule histories, current-clamp recordings were taken from INT and CHOW rats in baseline conditions, in the presence of Blockers, and then following superfusion of leptin. Representative traces of a neuron in Blockers from an INT rat (Fig. 5A) and then with the addition of leptin (Fig. 5B) reveal that in paired comparisons within the INT group, leptin decreased resistance (t(56)=2.46, p<0.05) (Fig 5D), significantly increased AP threshold (t(50)=5.20, p<0.0001) (Fig. 5F). and decreased AP amplitude (t(49)=4.97, p<0.0001) (Fig. 5H). Some of these findings seem to be general effects of leptin, as leptin had similar effects in AIC neurons from CHOW rats to decrease resistance (t(22)=2.10, p<0.05) (Fig. 5C) and decrease AP amplitude significantly (t(20)=3.16, p<0.01) (Fig. 5H). Leptin did not significantly increase AP threshold in CHOW rats (p=0.058) (Fig. 5E).
Figure 5: Functional effect of leptin in the anterior insula in rats with intermittent access to a preferred diet.
A) Representative trace of a layer V anterior insula pyramidal neuron showing resistance, AP threshold, and AP amplitude in the presence of GABAB and ionotropic glutamate receptor blockers and depicts how these parameters change in B) the same neuron following superfusion of leptin. Paired comparisons of each recorded cell of ad libitum chow-fed controls and intermittent access rats show a reduction in resistance for both groups (C,D), no effect on threshold for CHOW (E) rats, a significant depolarization in threshold for INT (F) rats, and a significant decrease in amplitude for both CHOW and INT rats (G,H) when leptin is added to the blockers. *: p<0.05, **: p<0.01, ***: p<0.001, ****: p<0.0001, paired comparison Leptin vs Blockers. n=21-57 cells for paired comparison measures.
4. Discussion
The present analysis in female rats shows that intermittent access to a more preferred food, rather than the preferred food per se, increased the essential value of the food by an order of magnitude. Increases in the intensity and inelasticity of demand for the food were seen, accompanied by marked escalation in the maximum total work emitted, in the equilibrium unit price of food at the maximum work level, and in greater food-seeking behavior under non-reinforced, extinction conditions. In parallel, intermittent access altered the resistance, rheobase and spiking properties of pyramidal neurons in the anterior insula, projections of which to the nucleus accumbens have been implicated in compulsive-like food self-administration of high-responding INT rats(Spierling, 2016). Consistent with previous reports(Hakansson et al., 1996; Mercer et al., 1996b; Patterson et al., 2011a; Shioda et al., 1998a), the same medial, anterior region of the insula was found to express functional leptin receptors by both qPCR and electrophysiology; here, greater AIC Lepr expression was seen in females than in males. Leptin application decreased neuron excitability via reduced resistance and increased AP threshold of AIC pyramidal neurons, an effect that was evident when GABAB and ionotropic glutamate receptors were blocked with antagonists. While leptin had similar effects on neurons from both CHOW and INT rats, the effects were greater in INT rats. These results suggest that intermittent access to preferred food may drive functional adaptation in leptin-regulated AIC pyramidal neurons in association with increased intensity and inelasticity of food demand. The findings are consistent with the hypothesis that leptin may modulate food-related motivation or decision-making via an AIC circuit that is malleable to intermittent availability of preferred food.
4.1. Functional adaptations in the anterior insula following intermittent diet schedule
The AIC putatively represents salient visceroemotional states(Craig, 2009; Critchley et al., 2004), or “somatic markers”(Craig, 2009), that guide behavior in a manner to promote survival and homeostasis. Accordingly, the AIC plays a role in addictive behavior(Abdolahi et al., 2015a, b, 2017; Hefzy et al., 2011; Naqvi and Bechara, 2009; Schrand, 2010; Seif et al., 2013; Suner-Soler et al., 2012; Yousefzadeh-Fard et al., 2013), perhaps by representing visceral states of use, craving and withdrawal/abstinence that motivate drug-taking. Relatedly, the AIC has been hypothesized to play a role in compulsive and disordered eating(Aviram-Friedman et al., 2018; Bohon and Stice, 2011; Ding et al., 2020; Frank et al., 2013; Kessler et al., 2016; Monteleone et al., 2017; Shott et al., 2016; Wagner et al., 2008; Zorrilla E. P.; Koob, 2019) via altered representation of interoceptive states associated with exposure to palatable food or its cues, craving and hunger(Belfort-DeAguiar et al., 2016; Boutelle et al., 2015; Brooks et al., 2013; Connolly et al., 2013; Dodds et al., 2012; Imperatori et al., 2015; Jastreboff et al., 2013; Kalon et al., 2016; Kim et al., 2012; Ogura et al., 2013; Pursey et al., 2014a; Tang et al., 2012; Weygandt et al., 2012; Wonderlich et al., 2017; Wood et al., 2016a; Wood et al., 2016b). Consistent with this view, we previously found that optoinhibition of AIC to NAc projections in this intermittent access model of compulsive eating modulated progressive-ratio and punished food self-administration in a subset of high-responding INT rats. The present findings support the hypothesis that intermittent access to preferred food promotes AIC neuroadaptation. After chronic diet schedules, pyramidal neurons of INT rats showed significantly decreased resistance compared to those of CHOW control rats, which did not differ from PREF rats fed the palatable diet ad libitum. Differences were seen under both baseline conditions and when antagonizing GABAB and ionotropic glutamate receptors. These effects were significant in INT rats studied following binge-like access to the preferred diet, and slightly, but not significantly, less so in INT rats studied following 24-hr withdrawal to chow. A decrease in resistances is potentially consistent with more ion channels being open in the INT insular neurons. Because the glutamate blockers did not diminish this effect and resistance is generally measured from hyperpolarizing current injections, the decreased resistance is hypothesized to be due to either increased proportion of open GABAA channels or an increased opening of channels that help set the resting membrane potential, such as voltage-gated potassium channels.
Rheobase was increased in INT rats studied after binge-like access to the preferred diet under baseline conditions as well as after blocking GABAB and ionotropic glutamate receptors. Rheobase was less affected in INT rats studied following access to chow, suggesting a role for recent preferred diet availability. The increased rheobase is consistent with the interpretation of more open channels in that voltage changes are smaller in magnitude for a given current injection when resistance is decreased, so more current is necessary to reach the voltage threshold for an action potential. In the presence of glutamate blockers, INT rats studied after binge-like access to the preferred diet had a depolarized action potential threshold. When analyzed as the effect of blockers relative to baseline (% change), INT rats showed decreased (depolarized) spike threshold and decreased spike amplitude compared to CHOW rats. Threshold is typically determined by the interplay between voltage-gated sodium and potassium channels, and antagonism of ionotropic glutamate receptors is less likely to be involved in this effect. However, metabotropic GABAB receptors may be involved, as they can couple to ion channels that alter the excitability and spike characteristics of neurons, including G-protein coupled inward rectifying potassium channels (GIRK)(Padgett and Slesinger, 2010).
Suggesting a potential link between AIC pyramidal neuron action potential threshold and feeding behavior, within the INT-BINGE group, spike threshold correlated inversely with the amplitude of cyclic oscillations in food intake (r = −0.61, p<0.05) and body weight (r = −0.42, p<0.001) between access and non-access days and directly with more persistent responding during non-reinforced timeout periods during self-administration sessions (r = 0.58, p<0.05) (Supplemental Figure 2). These correlations were feeding state-dependent, as they were absent in Chow and INT-WD rats.
4.2. Intermittent access to preferred food increases compulsive-like food demand
The increases reported here in demand intensity, inelasticity, essential value, and extinction responding as well as the replication(Kreisler et al., 2017; Spierling et al., 2020; Spierling et al., 2018) of increases in non-reinforced timeout responding, escalated PR breakpoints and FR1 self-administration, and cyclic dependence of food intake and body weight on the available diet reinforce that compulsive-like eating develops with intermittent access to a preferred diet. Such changes have been proposed to resemble diagnostic signs of substance use disorder, including food reward tolerance, escalation of intake, increased effort to obtain food, and outcome-independent food-seeking behavior(Gearhardt et al., 2009, 2016; Zorrilla E. P.; Koob, 2019). Escalated intake and operant performance were not seen in rats receiving continuous access to the preferred diet. Rather, PREF rats decreased their intake to chow-like levels over 2 weeks of access as seen previously(Kreisler et al., 2017; Spierling et al., 2020; Spierling et al., 2018). The findings emphasize the key role of intermittency and not only current diet characteristics per se in the genesis of compulsive eating. Demand function measures also strongly correlated with daily intake of preferred diet, rejection of alternative chow, and operant measures of motivation and compulsive-like responding, consistent with other data(Epstein et al., 2018) suggesting potential translational relevance for human eating outcomes.
Female rats were studied here due to the greater prevalence of diagnoses of compulsive-like eating pathologies in women. Perhaps accordingly, most rats in the INT group met the previously-published criterion of ‘INT-HIGH’ in our model -- PR breakpoints greater than 2 standard deviations above the mean of rats continuously fed the chow or preferred diet(Spierling et al., 2020; Spierling et al., 2018). Because so few rats met the INT-LOW classification, there was not adequate power to test whether changes in demand functions or AIC pyramidal neuron resistance, rheobase and spike properties are differential in INT-HIGH rats. It also is unclear whether male rats develop the same (magnitude) changes. Future studies can investigate similar measures in male rats, in which we would expect a more balanced distribution of INT-LOW vs. INT-HIGH rats for subgroup analyses(Spierling et al., 2018).
4.3. Leptin action in the anterior insula
Given our previous finding of a causal role for the AIC and correlations of leptin levels with compulsive-like self-administration in this model and reports of discrete leptin receptor expression in the medial AIC(Hakansson et al., 1996; Mercer et al., 1996b; Patterson et al., 2011a; Shioda et al., 1998a), we hypothesized that leptin might act on AIC neurons that influence food-motivated behavior. Regardless of previous diet exposure, we found that male rats had ~1.5-2-fold greater Lepr expression in the anterior insula compared to female rats. There were no significant diet schedule-related differences in Lepr expression, though PREF rats showed a trend towards increased levels vs. CHOW rats. To understand the functional role of leptin, we first studied effects of bath application of leptin on CamKIIa-expressing layer V AIC neurons of chow-fed rats. Under these conditions, leptin acutely increased spike frequency, including when blockers were used to isolate GABAA neurotransmission. This finding is consistent with the hypothesis that depolarizing effects of leptin on Layer V AIC pyramidal neurons may reflect disinhibition due to inhibiting presynaptic GABAergic input from interneurons(Murayama et al., 2019), similar to its mode of action to depolarize pro-opiomelanocortin (POMC) neurons in the arcuate nucleus(Liu et al., 2012; Pinto et al., 2004; Vong et al., 2011). Alternatively, leptin may be modulating K+ conductance directly(Baver et al., 2014; Lee et al., 2018). Within both 24-hr fasted and fed animals, leptin reduced mean rheobase in the presence of blockers in many, but not all, layer V neurons across the anterior-posterior extent of the AIC, suggesting that leptin can increase excitability throughout the AIC at least partly independent of current energy state. These findings contrast from studies of leptin effects on posterior insular layer 2/3 pyramidal neurons that showed energy state-dependent excitability(Murayama et al., 2019).
Unlike these findings, we observed in layer V AIC pyramidal neurons of chronic schedule INT rats that leptin decreased resistance, increased spike threshold, and decreased spike amplitude; the resistance and amplitude findings also were seen in age-matched CHOW rats. These findings resemble more the previously reported effects of leptin in the posterior insula, where leptin increased GABAergic activity onto layer 2/3 pyramidal neurons, decreasing excitability(Murayama et al., 2019). In this chronic study, we again did not observe significant differences in actions of leptin on pyramidal neurons in the AIC depending on whether INT rats were sacrificed following 24 hr withdrawal to chow or following binge-like refeeding. We cannot rule out that we were underpowered to detect such an effect, or that a greater or more chronic difference in energy state may have revealed differential leptin actions.
Suggesting a potential link between the response to leptin and feeding behavior, within the INT-BINGE group only, the effect of leptin on resistance correlated with average daily intake. Specifically, greater insensitivity to leptin correlated with significantly greater undereating of non-preferred chow on non-access weekdays (r = −0.65, p<0.01) and across the weekend (r = −0.66, p<0.01), during which rats only had chow and not the preferred diet (Supplemental Figure 3). Conversely, greater insensitivity to leptin tended to associate with greater overeating of preferred diet when it was available (r = 0.38). Thus, rats that consumed less on chow days and binged more on preferred diet access days were less sensitive to the effects of leptin (r = 0.51, p=0.0509 to amplitude of food intake cycling), but only when sacrificed immediately after a binge session. These correlations were feeding state-dependent, as they were absent in INT-WD rats.
Several procedural differences may account for the apparently disparate effects of leptin between studies. First, leptin may have varying effects within the heterogeneous population of insula neurons based on local microcircuitry and projection-specific functionality, similar to contrasting effects of leptin on POMC- vs. agouti-related protein (AgRP)-expressing neurons within the arcuate nucleus(Baver et al., 2014; Lee et al., 2018; Liu et al., 2012; Pinto et al., 2004; Vong et al., 2011). Studies may have sampled different neuron populations by chance or because the chronic schedule study focused on the most anterior aspect of the AIC (+2 to +3.5 mm), whereas our initial study extended posterior to bregma. Second, animals studied in the chronic diet study were months older and had higher adiposity than those studied at 2-3 months of age in our initial study; age and long-term energy state(Petervari et al., 2014; Scarpace et al., 2000) are known to be associated with changes in leptin signaling and sensitivity. Similarly, in the chronic diet schedule studies, we recorded from unlabeled layer V pyramidal neurons, which may include, but not recapitulate, the subset of layer V pyramidal neurons that were labeled by CamKII- promoter-driven virus in our initial study. In addition, we cannot rule out the possibility that leptin may have effects in brain regions other than the AIC that could contribute to the intermittent access to preferred food-induced binge eating behavior.
Overall, though, the results are consistent in showing that leptin modulates properties and excitability of AIC pyramidal neurons that change in relation to intermittent availability of a preferred diet and that it still does so in the presence of blockers that isolate GABAA current.
5. Conclusion
In conclusion, we further show that intermittent access to preferred food, rather than access to a preferred food per se, increases compulsive-like eating. Resulting measures of heightened demand intensity, inelasticity and essential value as well as increased food-seeking during extinction may have translational relevance for predicting disordered eating outcomes in humans. Intermittent access to preferred food induced functional adaptations in AIC pyramidal neurons, seen as increased resistance, decreased rheobase and altered spiking properties. Within the same neurons, leptin reduced resistance, increased spike threshold and decreased spike amplitude, pointing to a previously understudied, extrahypothalamic role of leptin in modulating feeding-related cortical circuits. Future studies should determine if plasticity in this leptin-sensitive AIC circuit also occurs in human conditions with varying availability of preferred food, such as food insecurity, recurrent dieting, and high cultural dietary variety, leading to altered food-related motivation and decision-making.
Supplementary Material
Acknowledgments
The authors thank Ashley L. Scott and Syed Taimur Rehan for their assistance.
Funding
Support for this study was provided by the National Institutes of Health through National Institute of Mental Health grant R21 MH124036, National Institute on Drug Abuse grant R21 DA046865, National Institute on Alcohol Abuse and Alcoholism grants R01 AA028879, AA027700, AA017447, P60 AA006420, K99/R00 AA026638, the NIAAA Institutional Training Grant T32 AA007456, the National Institutes of Health Clinical Translational Science Award (NIH CTSA) STSI TL1 Training Program TR002551, and the Pearson Center for Alcoholism and Addiction Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no competing interests.
Abbreviations:
- AIC
Anterior Insular Cortex
- FR
Fixed Ratio
- PR
Progressive Ratio
- BE
Behavioral Economics
- BED
Binge Eating Disorder
- GABA
gamma-Aminobutyric acid
- CHOW
ad libitum standard chow fed
- PREF
ad libitum preferred-diet fed
- INT
intermittent access to preferred diet
- Lepr
Leptin receptor
- AP
action potential
References:
- Abdolahi A, Williams GC, Benesch CG, Wang HZ, Spitzer EM, Scott BE, Block RC, van Wijngaarden E, 2015a. Damage to the insula leads to decreased nicotine withdrawal during abstinence. Addiction 110, 1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdolahi A, Williams GC, Benesch CG, Wang HZ, Spitzer EM, Scott BE, Block RC, van Wijngaarden E, 2015b. Smoking cessation behaviors three months following acute insular damage from stroke. Addict Behav 51, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdolahi A, Williams GC, Benesch CG, Wang HZ, Spitzer EM, Scott BE, Block RC, van Wijngaarden E, 2017. Immediate and Sustained Decrease in Smoking Urges After Acute Insular Cortex Damage. Nicotine Tob Res 19, 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuff SF, Murphy JG, 2017. Further examination of the temporal stability of alcohol demand. Behav Processes 141, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agh T, Kovacs G, Pawaskar M, Supina D, Inotai A, Voko Z, 2015. Epidemiology, health-related quality of life and economic burden of binge eating disorder: a systematic literature review. Eat Weight Disord 20, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agh T, Kovacs G, Supina D, Pawaskar M, Herman BK, Voko Z, Sheehan DV, 2016. A systematic review of the health-related quality of life and economic burdens of anorexia nervosa, bulimia nervosa, and binge eating disorder. Eat Weight Disord 21, 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alboni S, Micioni Di Bonaventura MV, Benatti C, Giusepponi ME, Brunello N, Cifani C, 2017. Hypothalamic expression of inflammatory mediators in an animal model of binge eating. Behavioural Brain Research 320, 420–430. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders, Washington, DC. [Google Scholar]
- Avena NM, Bocarsly ME, Hoebel BG, Gold MS, 2011. Overlaps in the nosology of substance abuse and overeating: the translational implications of "food addiction". Curr Drug Abuse Rev 4, 133–139. [DOI] [PubMed] [Google Scholar]
- Aviram-Friedman R, Astbury N, Ochner CN, Contento I, Geliebter A, 2018. Neurobiological evidence for attention bias to food, emotional dysregulation, disinhibition and deficient somatosensory awareness in obesity with binge eating disorder. Physiol Behav 184, 122–128. [DOI] [PubMed] [Google Scholar]
- Bak-Sosnowska M, 2017. Differential criteria for binge eating disorder and food addiction in the context of causes and treatment of obesity. Psychiatr Pol 51, 247–259. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Schwartz MW, Seeley RJ, Woods SC, Porte D Jr., Breininger JF, Jonak Z, Schaefer J, Krouse M, Burghardt C, Campfield LA, Burn P, Kochan JP, 1999. Leptin receptor long-form splice-variant protein expression in neuron cell bodies of the brain and co-localization with neuropeptide Y mRNA in the arcuate nucleus. J Histochem Cytochem 47, 353–362. [DOI] [PubMed] [Google Scholar]
- Baver SB, Hope K, Guyot S, Bjorbaek C, Kaczorowski C, O'Connell KM, 2014. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J Neurosci 34, 5486–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort-DeAguiar R, Seo D, Naik S, Hwang J, Lacadie C, Schmidt C, Constable RT, Sinha R, Sherwin R, 2016. Food image-induced brain activation is not diminished by insulin infusion. Int J Obes (Lond) 40, 1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston-Jones G, 2013. The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology (Berl) 226, 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G, 2014. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci U S A 111, 11822–11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST, 1993. Behavioral economics: a novel experimental approach to the study of drug dependence. Drug Alcohol Depend 33, 173–192. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST, Hughes JR, 1990. Behavioral economics of drug self-administration. I. Functional equivalence of response requirement and drug dose. Life Sci 47, 1501–1510. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Johnson MW, Koffarnus MN, MacKillop J, Murphy JG, 2014. The Behavioral Economics of Substance Use Disorders: Reinforcement Pathologies and Their Repair. Annu Rev Clin Psychol 10, 641–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohon C, Stice E, 2011. Reward abnormalities among women with full and subthreshold bulimia nervosa: a functional magnetic resonance imaging study. Int J Eat Disord 44, 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutelle KN, Wierenga CE, Bischoff-Grethe A, Melrose AJ, Grenesko-Stevens E, Paulus MP, Kaye WH, 2015. Increased brain response to appetitive tastes in the insula and amygdala in obese compared with healthy weight children when sated. Int J Obes (Lond) 39, 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, Cedernaes J, Schioth HB, 2013. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS One 8, e60393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner NR, Johnson MW, 2014. Demand curves for hypothetical cocaine in cocaine-dependent individuals. Psychopharmacology (Berl) 231, 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon G, 2007. Dieting. Makes you fat? British Journal of Nutrition 93, 569–570. [DOI] [PubMed] [Google Scholar]
- Connolly L, Coveleskie K, Kilpatrick LA, Labus JS, Ebrat B, Stains J, Jiang Z, Tillisch K, Raybould HE, Mayer EA, 2013. Differences in brain responses between lean and obese women to a sweetened drink. Neurogastroenterol Motil 25, 579–e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP, 2008. Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. Am J Physiol Regul Integr Comp Physiol 295, R1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP, 2009. Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food. Psychoneuroendocrinology 34, 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, 2009. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 10, 59–70. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ, 2004. Neural systems supporting interoceptive awareness. Nat Neurosci 7, 189–195. [DOI] [PubMed] [Google Scholar]
- Curtis C, Davis C, 2014. A qualitative study of binge eating and obesity from an addiction perspective. Eat Disord 22, 19–32. [DOI] [PubMed] [Google Scholar]
- Davis C, 2017. A commentary on the associations among 'food addiction', binge eating disorder, and obesity: Overlapping conditions with idiosyncratic clinical features. Appetite 115, 3–8. [DOI] [PubMed] [Google Scholar]
- Davis C, Curtis C, Levitan RD, Carter JC, Kaplan AS, Kennedy JL, 2011. Evidence that 'food addiction' is a valid phenotype of obesity. Appetite 57, 711–717. [DOI] [PubMed] [Google Scholar]
- Davis JF, Melhorn SJ, Shurdak JD, Heiman JU, Tschop MH, Clegg DJ, Benoit SC, 2007. Comparison of hydrogenated vegetable shortening and nutritionally complete high-fat diet on limited access-binge behavior in rats. Physiol Behav 92, 924–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zwaan M, 2001. Binge eating disorder and obesity. Int J Obes Relat Metab Disord 25 Suppl 1, S51–55. [DOI] [PubMed] [Google Scholar]
- Ding Y, Ji G, Li G, Zhang W, Hu Y, Liu L, Wang Y, Hu C, von Deneen KM, Han Y, Cui G, Wang H, Wiers CE, Manza P, Tomasi D, Volkow ND, Nie Y, Wang GJ, Zhang Y, 2020. Altered Interactions Among Resting-State Networks in Individuals with Obesity. Obesity (Silver Spring) 28, 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds CM, O'Neill B, Beaver J, Makwana A, Bani M, Merlo-Pich E, Fletcher PC, Koch A, Bullmore ET, Nathan PJ, 2012. Effect of the dopamine D3 receptor antagonist GSK598809 on brain responses to rewarding food images in overweight and obese binge eaters. Appetite 59, 27–33. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Paluch RA, Carr KA, Temple JL, Bickel WK, MacKillop J, 2018. Reinforcing value and hypothetical behavioral economic demand for food and their relation to BMI. Eat Behav 29, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine HE, Whiteford HA, Pike KM, 2016. The global burden of eating disorders. Curr Opin Psychiatry 29, 346–353. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Zhao Y, Szucs A, Sabino V, Cottone P, Rivier J, Vale WW, Koob GF, Zorrilla EP, 2011. Systemic urocortin 2, but not urocortin 1 or stressin 1-A, suppresses feeding via CRF2 receptors without malaise and stress. Br J Pharmacol 164, 1959–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragale JE, Beck KD, Pang KC, 2017. Use of the Exponential and Exponentiated Demand Equations to Assess the Behavioral Economics of Negative Reinforcement. Front Neurosci 11, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Kullmann S, Veit R, 2013. Food related processes in the insular cortex. Front Hum Neurosci 7, 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Boswell RG, White MA, 2014. The association of "food addiction" with disordered eating and body mass index. Eat Behav 15, 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Corbin WR, Brownell KD, 2009. Preliminary validation of the Yale Food Addiction Scale. Appetite 52, 430–436. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Corbin WR, Brownell KD, 2016. Development of the Yale Food Addiction Scale Version 2.0. Psychol Addict Behav 30, 113–121. [DOI] [PubMed] [Google Scholar]
- Gilroy SP, Kaplan BA, Reed DD, Koffarnus MN, Hantula DA, 2018. The Demand Curve Analyzer: Behavioral economic software for applied research. J Exp Anal Behav 110, 553–568. [DOI] [PubMed] [Google Scholar]
- Goldschmidt AB, Wall M, Loth KA, Le Grange D, Neumark-Sztainer D, Which Dieters Are at Risk for the Onset of Binge Eating? A Prospective Study of Adolescents and Young Adults. Journal of Adolescent Health 51, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JC, MacKillop J, 2014. Interrelationships among individual differences in alcohol demand, impulsivity, and alcohol misuse. Psychol Addict Behav 28, 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister B, 1998. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci 18, 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson ML, Hulting AL, Meister B, 1996. Expression of leptin receptor mRNA in the hypothalamic arcuate nucleus--relationship with NPY neurones. Neuroreport 7, 3087–3092. [DOI] [PubMed] [Google Scholar]
- Hay P, Girosi F, Mond J, 2015. Prevalence and sociodemographic correlates of DSM-5 eating disorders in the Australian population. J Eat Disord 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay P, Mitchison D, Collado AEL, Gonzalez-Chica DA, Stocks N, Touyz S, 2017. Burden and health-related quality of life of eating disorders, including Avoidant/Restrictive Food Intake Disorder (ARFID), in the Australian population. J Eat Disord 5, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefzy H, Silver RW, Silver B, 2011. The no smoking sign--insular infarction. J Neuroimaging 21, e169–170. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG Jr., Kessler RC, 2007. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry 61, 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, 1991. Behavioral economics of drug self-administration and drug abuse policy. J Exp Anal Behav 56, 377–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, 2014. Behavioral economics and the analysis of consumption and choice. In: McSweeney FK, Murphy ES, (Eds), The Wiley Blackwell handbook of operant and classical conditioning. Wiley Blackwell. [Google Scholar]
- Hursh SR, Roma PG, 2016. Behavioral economics and the analysis of consumption and choice. Managerial & Decision Economics 37, 224–238. [Google Scholar]
- Hursh SR, Silberberg A, 2008. Economic demand and essential value. Psychol Rev 115, 186–198. [DOI] [PubMed] [Google Scholar]
- Imperatori C, Fabbricatore M, Innamorati M, Farina B, Quintiliani MI, Lamis DA, Mazzucchi E, Contardi A, Vollono C, Della Marca G, 2015. Modification of EEG functional connectivity and EEG power spectra in overweight and obese patients with food addiction: An eLORETA study. Brain Imaging Behav 9, 703–716. [DOI] [PubMed] [Google Scholar]
- Jacobs EA, Bickel WK, 1999. Modeling drug consumption in the clinic using simulation procedures: demand for heroin and cigarettes in opioid-dependent outpatients. Exp Clin Psychopharmacol 7, 412–426. [DOI] [PubMed] [Google Scholar]
- Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Potenza MN, 2013. Neural correlates of stress- and food cue-induced food craving in obesity: association with insulin levels. Diabetes Care 36, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalon E, Hong JY, Tobin C, Schulte T, 2016. Psychological and Neurobiological Correlates of Food Addiction. Int Rev Neurobiol 129, 85–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RM, Hutson PH, Herman BK, Potenza MN, 2016. The neurobiological basis of binge-eating disorder. Neurosci Biobehav Rev 63, 223–238. [DOI] [PubMed] [Google Scholar]
- Khom S, Wolfe SA, Patel RR, Kirson D, Hedges DM, Varodayan FP, Bajo M, Roberto M, 2020. Alcohol Dependence and Withdrawal Impair Serotonergic Regulation of GABA Transmission in the Rat Central Nucleus of the Amygdala. J Neurosci 40, 6842–6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KR, Ku J, Lee JH, Lee H, Jung YC, 2012. Functional and effective connectivity of anterior insula in anorexia nervosa and bulimia nervosa. Neurosci Lett 521, 152–157. [DOI] [PubMed] [Google Scholar]
- Kirson D, Khom S, Rodriguez L, Wolfe SA, Varodayan FP, Gandhi PJ, Patel RR, Vlkolinsky R, Bajo M, Roberto M, 2021. Sex Differences in Acute Alcohol Sensitivity of Naive and Alcohol Dependent Central Amygdala GABA Synapses. Alcohol Alcohol 56, 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Franck CT, Stein JS, Bickel WK, 2015. A modified exponential behavioral economic demand model to better describe consumption data. Exp Clin Psychopharmacol 23, 504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2013. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci 13, 3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornstein SG, 2017. Epidemiology and Recognition of Binge-Eating Disorder in Psychiatry and Primary Care. J Clin Psychiatry 78 Suppl 1, 3–8. [DOI] [PubMed] [Google Scholar]
- Kreisler AD, Garcia MG, Spierling SR, Hui BE, Zorrilla EP, 2017. Extended vs. brief intermittent access to palatable food differently promote binge-like intake, rejection of less preferred food, and weight cycling in female rats. Physiol Behav 177, 305–316. [DOI] [PubMed] [Google Scholar]
- Lee S, Lee J, Kang GM, Kim MS, 2018. Leptin directly regulate intrinsic neuronal excitability in hypothalamic POMC neurons but not in AgRP neurons in food restricted mice. Neurosci Lett 681, 105–109. [DOI] [PubMed] [Google Scholar]
- Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL, Lowell BB, 2012. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron 73, 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MR, 2015. Dieting: proxy or cause of future weight gain? Obesity Reviews 16, 19–24. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Doshi SD, Katterman SN, Feig EH, 2013. Dieting and restrained eating as prospective predictors of weight gain. Front Psychol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Acker J, Gray JC, Brown CL, Murphy JG, Ray LA, Sweet LH, 2014. The neuroeconomics of alcohol demand: an initial investigation of the neural correlates of alcohol cost-benefit decision making in heavy drinking men. Neuropsychopharmacology 39, 1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Miranda R Jr., Monti PM, Ray LA, Murphy JG, Rohsenow DJ, McGeary JE, Swift RM, Tidey JW, Gwaltney CJ, 2010a. Alcohol demand, delayed reward discounting, and craving in relation to drinking and alcohol use disorders. J Abnorm Psychol 119, 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, O'Hagen S, Lisman SA, Murphy JG, Ray LA, Tidey JW, McGeary JE, Monti PM, 2010b. Behavioral economic analysis of cue-elicited craving for alcohol. Addiction 105, 1599–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning W, Blumberg L, Moulton LH, 1995. The demand for alcohol: The differential response to price. Journal of Health Economics 14, 123–148. [DOI] [PubMed] [Google Scholar]
- Mathes WF, Brownley KA, Mo X, Bulik CM, 2009. The biology of binge eating. Appetite 52, 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCuen-Wurst C, Ruggieri M, Allison KC, 2017. Disordered eating and obesity: associations between binge-eating disorder, night-eating syndrome, and weight-related comorbidities. Ann N Y Acad Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meany G, Conceicao E, Mitchell JE, 2014. Binge eating, binge eating disorder and loss of control eating: effects on weight outcomes after bariatric surgery. Eur Eat Disord Rev 22, 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P, 1996a. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett 387, 113–116. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P, 1996b. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Letters 387, 113–116. [DOI] [PubMed] [Google Scholar]
- Montani J-P, Schutz Y, Dulloo AG, 2015. Dieting and weight cycling as risk factors for cardiometabolic diseases: who is really at risk? Obesity Reviews 16, 7–18. [DOI] [PubMed] [Google Scholar]
- Monteleone AM, Castellini G, Volpe U, Ricca V, Lelli L, Monteleone P, Maj M, 2017. Neuroendocrinology and brain imaging of reward in eating disorders: A possible key to the treatment of anorexia nervosa and bulimia nervosa. Prog Neuropsychopharmacol Biol Psychiatry. [DOI] [PubMed] [Google Scholar]
- Moore CF, Sabino V, Koob GF, Cottone P, 2017a. Neuroscience of Compulsive Eating Behavior. Front Neurosci 11, 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CF, Sabino V, Koob GF, Cottone P, 2017b. Pathological Overeating: Emerging Evidence for a Compulsivity Construct. Neuropsychopharmacology 42, 1375–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama S, Yamamoto K, Fujita S, Takei H, Inui T, Ogiso B, Kobayashi M, 2019. Extracellular glucose-dependent IPSC enhancement by leptin in fast-spiking to pyramidal neuron connections via JAK2-PI3K pathway in the rat insular cortex. Neuropharmacology 149, 133–148. [DOI] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J, 2006. Relative reinforcing efficacy of alcohol among college student drinkers. Exp Clin Psychopharmacol 14, 219–227. [DOI] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J, Skidmore JR, Pederson AA, 2009. Reliability and validity of a demand curve measure of alcohol reinforcement. Exp Clin Psychopharmacol 17, 396–404. [DOI] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J, Tidey JW, Brazil LA, Colby SM, 2011. Validity of a demand curve measure of nicotine reinforcement with adolescent smokers. Drug Alcohol Depend 113, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagl M, Jacobi C, Paul M, Beesdo-Baum K, Hofler M, Lieb R, Wittchen HU, 2016. Prevalence, incidence, and natural course of anorexia and bulimia nervosa among adolescents and young adults. Eur Child Adolesc Psychiatry 25, 903–918. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A, 2009. The hidden island of addiction: the insula. Trends Neurosci 32, 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura K, Fujii T, Abe N, Hosokai Y, Shinohara M, Fukuda H, Mori E, 2013. Regional cerebral blood flow and abnormal eating behavior in Prader-Willi syndrome. Brain Dev 35, 427–434. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Richardson JM, Roberts DC, 2011. A novel IV cocaine self-administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology (Berl) 214, 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett CL, Slesinger PA, 2010. GABAB receptor coupling to G-proteins and ion channels. Adv Pharmacol 58, 123–147. [DOI] [PubMed] [Google Scholar]
- Palavras MA, Hay P, Filho CA, Claudino A, 2017. The Efficacy of Psychological Therapies in Reducing Weight and Binge Eating in People with Bulimia Nervosa and Binge Eating Disorder Who Are Overweight or Obese-A Critical Synthesis and Meta-Analyses. Nutrients 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parylak SL, Cottone P, Sabino V, Rice KC, Zorrilla EP, 2012. Effects of CB1 and CRF1 receptor antagonists on binge-like eating in rats with limited access to a sweet fat diet: lack of withdrawal-like responses. Physiol Behav 107, 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parylak SL, Koob GF, Zorrilla EP, 2011. The dark side of food addiction. Physiol Behav 104, 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CM, Leshan RL, Jones JC, Myers MG, 2011a. Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells. Brain Res 1378c, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CM, Leshan RL, Jones JC, Myers MG Jr., 2011b. Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells. Brain Res 1378, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram P, Sun G, 2014. Hormonal and dietary characteristics in obese human subjects with and without food addiction. Nutrients 7, 223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petervari E, Rostas I, Soos S, Tenk J, Miko A, Furedi N, Szekely M, Balasko M, 2014. Age versus nutritional state in the development of central leptin resistance. Peptides 56, 59–67. [DOI] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL, 2004. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304, 110–115. [DOI] [PubMed] [Google Scholar]
- Polivy J, Herman CP, 1985. Dieting and binging. A causal analysis. Am Psychol 40, 193–201. [DOI] [PubMed] [Google Scholar]
- Pursey KM, Stanwell P, Callister RJ, Brain K, Collins CE, Burrows TL, 2014a. Neural responses to visual food cues according to weight status: a systematic review of functional magnetic resonance imaging studies. Front Nutr 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursey KM, Stanwell P, Gearhardt AN, Collins CE, Burrows TL, 2014b. The prevalence of food addiction as assessed by the Yale Food Addiction Scale: a systematic review. Nutrients 6, 4552–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph TG, 1956. The descriptive features of food addiction; addictive eating and drinking. Q J Stud Alcohol 17, 198–224. [PubMed] [Google Scholar]
- Rozin P, Levine E, Stoess C, 1991. Chocolate craving and liking. Appetite 17, 199–212. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Moore RL, Tumer N, 2000. Impaired leptin responsiveness in aged rats. Diabetes 49, 431–435. [DOI] [PubMed] [Google Scholar]
- Schaumberg K, Welch E, Breithaupt L, Hubel C, Baker JH, Munn-Chernoff MA, Yilmaz Z, Ehrlich S, Mustelin L, Ghaderi A, Hardaway AJ, Bulik-Sullivan EC, Hedman AM, Jangmo A, Nilsson IAK, Wiklund C, Yao S, Seidel M, Bulik CM, 2017. The Science Behind the Academy for Eating Disorders' Nine Truths About Eating Disorders. Eur Eat Disord Rev 25, 432–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrand JR, 2010. Does insular stroke disrupt the self-medication effects of nicotine? Med Hypotheses 75, 302–304. [DOI] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW, 2013. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci 16, 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafine KM, O'Dell LE, Zorrilla EP, 2021. Converging vulnerability factors for compulsive food and drug use. Neuropharmacology 196, 108556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda S, Funahashi H, Nakajo S, Yada T, Maruta O, Nakai Y, 1998a. Immunohistochemical localization of leptin receptor in the rat brain. Neurosci Lett 243, 41–44. [DOI] [PubMed] [Google Scholar]
- Shioda S, Funahashi H, Nakajo S, Yada T, Maruta O, Nakai Y, 1998b. Immunohistochemical localization of leptin receptor in the rat brain. Neurosci Lett 243, 41–44. [DOI] [PubMed] [Google Scholar]
- Shott ME, Pryor TL, Yang TT, Frank GK, 2016. Greater Insula White Matter Fiber Connectivity in Women Recovered from Anorexia Nervosa. Neuropsychopharmacology 41, 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierling S, de Guglielmo G, Kirson D, Kreisler A, Roberto M, George O, Zorrilla EP, 2020. Insula to ventral striatal projections mediate compulsive eating produced by intermittent access to palatable food. Neuropsychopharmacology 45, 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierling SR, Kreisler AD, Williams CA, Fang SY, Pucci SN, Kines KT, Zorrilla EP, 2018. Intermittent, extended access to preferred food leads to escalated food reinforcement and cyclic whole-body metabolism in rats: Sex differences and individual vulnerability. Physiol Behav 192, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierling SR K. AD; Zorrilla EP, 2016. Intermittent access to palatable food leads to compulsive self-administration and hyperirritability during withdrawal in rats. The Society for the Study of Ingestive Behavior, Porto, Portugal. [Google Scholar]
- Stice E, Yokum S, 2016. Gain in Body Fat Is Associated with Increased Striatal Response to Palatable Food Cues, whereas Body Fat Stability Is Associated with Decreased Striatal Response. J Neurosci 36, 6949–6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suner-Soler R, Grau A, Gras ME, Font-Mayolas S, Silva Y, Davalos A, Cruz V, Rodrigo J, Serena J, 2012. Smoking cessation 1 year poststroke and damage to the insular cortex. Stroke 43, 131–136. [DOI] [PubMed] [Google Scholar]
- Tang DW, Fellows LK, Small DM, Dagher A, 2012. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav 106, 317–324. [DOI] [PubMed] [Google Scholar]
- Tunstall BJ, Kirson D, Zallar LJ, McConnell SA, Vendruscolo JCM, Ho CP, Oleata CS, Khom S, Manning M, Lee MR, Leggio L, Koob GF, Roberto M, Vendruscolo LF, 2019. Oxytocin blocks enhanced motivation for alcohol in alcohol dependence and blocks alcohol effects on GABAergic transmission in the central amygdala. PLoS Biol 17, e2006421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW Jr., Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF, 2012. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci 32, 7563–7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S Jr., Lowell BB, 2011. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF, 2015. Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology 40, 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A, Aizenstein H, Mazurkewicz L, Fudge J, Frank GK, Putnam K, Bailer UF, Fischer L, Kaye WH, 2008. Altered insula response to taste stimuli in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology 33, 513–523. [DOI] [PubMed] [Google Scholar]
- Warden AS, Wolfe SA, Khom S, Varodayan FP, Patel RR, Steinman MQ, Bajo M, Montgomery SE, Vlkolinsky R, Nadav T, Polis I, Roberts AJ, Mayfield RD, Harris RA, Roberto M, 2020. Microglia Control Escalation of Drinking in Alcohol-Dependent Mice: Genomic and Synaptic Drivers. Biol Psychiatry 88, 910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weygandt M, Schaefer A, Schienle A, Haynes JD, 2012. Diagnosing different binge-eating disorders based on reward-related brain activation patterns. Hum Brain Mapp 33, 2135–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiss DA, Brewerton TD, 2017. Incorporating food addiction into disordered eating: the disordered eating food addiction nutrition guide (DEFANG). Eat Weight Disord 22, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonderlich JA, Breithaupt LE, Crosby RD, Thompson JC, Engel SG, Fischer S, 2017. The relation between craving and binge eating: Integrating neuroimaging and ecological momentary assessment. Appetite 117, 294–302. [DOI] [PubMed] [Google Scholar]
- Wood AG, Chen J, Moran C, Phan T, Beare R, Cooper K, Litras S, Srikanth V, 2016a. Brain Activation during Memory Encoding in Type 2 Diabetes Mellitus: A Discordant Twin Pair Study. J Diabetes Res 2016, 3978428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SM, Schembre SM, He Q, Engelmann JM, Ames SL, Bechara A, 2016b. Emotional eating and routine restraint scores are associated with activity in brain regions involved in urge and self-control. Physiol Behav 165, 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Bardo MT, Beckmann JS, 2019. Environmental enrichment and drug value: a behavioral economic analysis in male rats. Addict Biol 24, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh-Fard Y, Gharedaghi MH, Esmaeili S, Pourbakhtyaran E, Sadaghiani MS, Ghorbani A, Sahraian MA, 2013. Stroke modifies drug consumption in opium addicts: role of the insula. Basic Clin Neurosci 4, 307–314. [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP; Koob G, 2019. The Dark Side of Compulsive Eating and Food Addiction: Affective Dysregulation, Negative Reinforcement, and Negative Urgency Compulsive Eating Behavior and Food Addiction. Elsevier. [Google Scholar]
- Zorrilla EP, Koob GF, 2019. Impulsivity Derived From the Dark Side: Neurocircuits That Contribute to Negative Urgency. Front Behav Neurosci 13, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Kreisler AD, Bagsic SR, 2021. Intermittent extended access rodent models of compulsive eating. In: Avena NM, (Ed), Animal Models of Eating Disorders. Humana Press, New York, pp. 133–162. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.