Abstract
In both scid and BALB/c mouse-Leishmania donovani models, hexadecyphosphocholine (miltefosine) and AmBisome had similar levels of activity. In contrast, sodium stibogluconate (Pentostam) was significantly less active against L. donovani in scid mice than in BALB/c mice. The in vitro anti-leishmanial activity of miltefosine was similar in peritoneal macrophages derived from both scid and BALB/c mice, whereas Pentostam and AmBisome were significantly more active in the latter.
Visceral leishmaniasis (VL) is caused by the obligate intracellular protozoan parasites Leishmania donovani, Leishmania infantum, and Leishmania chagasi. Currently, there are an estimated 500,000 new cases of VL/annum, with particularly important foci in northeast India and Sudan (World Health Organization). The recommended drugs for the treatment of VL are the pentavalent antimonials (SbVs) sodium stibogluconate (Pentostam) and meglumine antimonate (Glucantime), with amphotericin B (Fungizone), lipid formulations of amphotericin B, and paromomycin (aminosidine) used as alternative therapies (3, 9). All these drugs have limitations of the need for parenteral administration and long courses of treatment, toxicity, and/or cost. There is increased concern over the rising number of cases of VL in India that fail to respond to standard antimonials (28) and the emergence of VL as an opportunistic infection in human immunodeficiency virus (HIV)-infected and AIDS patients (1), especially in the L. infantum focus in southwestern Europe (10). Treatment of Leishmania-HIV coinfections is problematic, as the conventional antimonial treatment is less effective in immunocompromised patients (1). Courses of high-dose antimonial regimens and liposomal amphotericin B have limited efficacy, most patients relapse, and maintenance therapy is necessary (1, 9).
Recently, hexadecylphosphocholine (HPC; miltefosine), an alkylphosphocholine originally developed as an anticancer drug (29), has proved to be an effective treatment for VL in clinical trials in India (15, 27). Oral treatment with HPC at 100 mg/day for 4 weeks was effective in treating 114 of 120 VL cases (15) and cases that had not responded to prior antimonial therapy (27). These clinical studies followed experimental studies that demonstrated that alkylphosphocholines, including HPC, are active against L. donovani in in vitro macrophage and mouse models of infection (7) and by oral administration against murine VL (8, 16, 17, 30). The molecular mechanism of action of HPC against cancer cells has been linked to apoptosis as well as different lipid-dependent cell signaling pathways (2), but its mode of action against Leishmania parasites remains unclear. It has also been suggested that HPC has immunomodulatory properties (12, 13, 31); however, some studies have shown that HPC retains its antitumor properties in immunodeficient mice, suggesting that activity is not dependent on a T-cell-mediated immune response, although increases in macrophage, T-cell, and B-cell numbers were observed (25). Recently, the antileishmanial activity of HPC was shown to be retained in mouse models deficient in T-cell, endogenous gamma interferon (IFN-γ), and macrophage killing (reactive nitrogen and oxygen radicals) mechanisms (18). As part of a project on the antileishmanial activities of alkyllysophospholipids, we have examined the activity of HPC in scid mice which are functionally deficient in T and B cells (4) and compared it with those of AmBisome and sodium stibogluconate, two drugs frequently used in the treatment of VL in immunosuppressed individuals.
HPC (Sigma, Poole, United Kingdom), Pentostam and sodium stibogluconate (GlaxoWellcome, Dartford, United Kingdom), Fungizone (E. R. Squibb & Sons, Hounslow, United Kingdom), and AmBisome (generously donated by R. Proffitt, Gilead Biosciences, San Dimas, Calif.) were used in the study. Drugs were tested in either C.B-17 scid mice (from a colony maintained at the London School of Hygiene and Tropical Medicine) or BALB/c mice (Charles River Ltd., Margate, United Kingdom). As described previously (7) 6- to 8-week-old mice were infected intravenously with 2 × 107 L. donovani MHOM/ET/67/L82 amastigotes derived from a hamster and randomly sorted into groups of five. In the first experiment mice were dosed 7 days after infection with 30 mg of HPC per kg of body weight per dose (orally [p.o.]) or 45 mg of SbV as sodium stibogluconate per kg per dose (subcutaneously [s.c.]) for 5 consecutive days. In a second experiment, groups of mice were dosed 14 days after infection with HPC at 30, 10, 3, or 1 mg/kg/dose (p.o.) or with sodium stibogluconate at 45, 15, and 5 mg of SbV/kg/dose (s.c.) for 5 consecutive days. The activity of AmBisome was compared with that of Fungizone in the two mouse models in two experiments by using 5, 1, and 0.2 mg of AmBisome per kg per dose in a single dose given intravenously (i.v.) and Fungizone at 1 mg/kg/dose as a standard in the first experiment and at 5, 1, and 0.2 mg/kg/dose three times on alternate days in the second experiment. In all experiments, mice were weighed and necropsied 3 days after the completion of treatment. Impression smears, prepared from weighed livers, were methanol fixed and Giemsa stained. Drug activity was determined by comparing the number of amastigotes per 500 liver cells × organ weight (in milligrams) (Leishman Donovan unit [LDU]) in mice from the treated and the untreated groups. The 50% effective doses (ED50s) and ED90s were calculated by sigmoidal regression analysis (Msxlfit; ID Business Solution, Guilford, United Kingdom). The activities of these drugs were further tested in vitro with infected peritoneal macrophages (PMφ) from scid mice and BALB/c mice. PMφ were obtained by abdominal lavage with Dulbecco's minimal essential medium (DMEM; Life Technologies, Paisley, United Kingdom). A total of 106 cells/ml were plated in 16-well Lab-tek tissue culture slides (Life Technologies) and allowed to adhere for 16 h at 37°C in a 5% CO2–95% air mixture in DMEM with 10% heat-inactivated fetal calf serum (Harlan Sera-Lab, Loughborough, United Kingdom). Adherent PMφ were infected with L. donovani amastigotes at a ratio of 10 parasites:1 macrophage. After 12 h, nonphagocytosed parasites were removed by washing with serum-free DMEM. Infected cultures were incubated for 72 h with the drugs in a threefold dilution series in quadruplicate at each concentration. Drug activity was determined microscopically by counting the percentage of infected cells in methanol-fixed and Giemsa-stained preparations. The ED50s and ED90s were calculated and analyzed as described above.
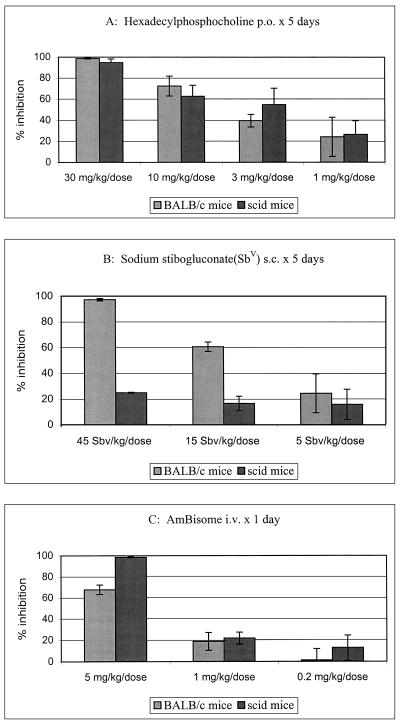
Treatment with HPC at 30 mg/kg/dose p.o. for 5 days was previously shown to be effective against L. donovani in BALB/c mice (8, 16, 17). In an initial study with this dose, HPC proved to be equally active in BALB/c and scid mice, with >95% parasite inhibition during the second week of infection (data not shown). In comparison, sodium stibogluconate at a similarly chosen effective dose (45 mg of SbV/kg/dose for five days) was significantly (P < 0.05) more active in BALB/c mice (87.35% ± 9.15% parasite inhibition) than in scid mice (41.00% ± 14.35% parasite inhibition) (data not shown). HPC, AmBisome, and sodium stibogluconate were then tested over a dose range during the 3rd week of infection, a period in which the parasite burden in the liver has been shown to be similar in both strains of mice (11). The LDUs before treatment (2 weeks after infection) were 1,804 ± 85 and 2,088 ± 78 in scid and BALB/c mice, respectively. At the end of the treatment (3 weeks after infection), untreated control mice from both groups presented with similar liver parasite burdens, with LDUs of 2,335 ± 185 (scid mice) and 2,375 ± 270 (BALB/c mice). HPC showed similar dose-response effects in both BALB/c and scid mice (Fig. 1A), with ED50s and ED90s of 3.98 and 27.13 mg/kg/dose and 4.53 and 42.66 mg/kg/dose, respectively, with no significant difference between the values (P > 0.45). In contrast, sodium stibogluconate had a significantly (P > 0.05) higher level of activity in BALB/c mice than in scid mice, with ED50s and ED90s of 20.26 and 56.53 mg of SbV/kg/dose and >45 and >45 mg of SbV/kg/dose, respectively (Fig. 1B). The percentages of parasite killing by HPC at 30 mg/kg were similar in both BALB/c and scid mice (98.68 and 94.7%, respectively); this is in contrast to the results of treatment with sodium stibogluconate at 45 mg of SbV/kg, with which there was a higher percentage of parasite killing in BALB/c mice than in scid mice (96.26 and 28.8%, respectively). The drugs were well tolerated by the mice at the top doses, and no weight reductions were recorded in the mice in the treated groups. AmBisome at a single dose was active in both models (Fig. 1C), with ED50s and ED90s of 2.91 and >5 mg/kg/dose and 1.51 and 3.1 mg/kg/dose in BALB/c and scid mice, respectively. There was no significant difference between the two models in the present study (P > 0.5). The standard amphotericin B formulation (Fungizone) was inactive at 1 mg/kg/dose i.v. in both models. In a second experiment, multiple dosing (on 3 alternate days) with AmBisome gave lower ED50s and ED90s in both models: <0.2 and <0.2 mg/kg/dose, respectively, in BALB/c mice (98.5% inhibition at the lowest dose of 0.2 mg/kg) and 0.3 and 0.19 mg/kg/dose, respectively, in scid mice. Multiple doses of Fungizone at 1 mg/kg gave 65.5% inhibition in scid mice and 79.5% inhibition in BALB/c mice.
FIG. 1.
Effects of HPC (A), sodium stibogluconate (B), and AmBisome (C) against L. donovani amastigotes in the livers of BALB/c and scid mice.
To extend these in vivo observations that suggested the T- and B-cell independence of the antileishmanial activities of HPC and AmBisome and the immunodependence of sodium stibogluconate, all compounds were tested in vitro against L. donovani-infected PMφ. In two experiments, no difference was observed between the activities of HPC in scid and BALB/c mouse PMφ (Table 1). In contrast, significant differences (P > 0.05) were observed in the antileishmanial activity of Pentostam, with ED50s for scid mouse-derived infected PMφ being approximately threefold higher than those for BALB/c mouse-derived infected PMφ. AmBisome was significantly more active in BALB/c mouse PMφ than in scid mouse PMφ (P < 0.05). At 0.25 μM, AmBisome caused 97.18% ± 0.97% parasite inhibition in infected BALB/c mouse PMφ, whereas it caused 76.22% ± 1.51% parasite inhibition in infected PMφ from scid mice (Table 1). In the two studies the levels of infection in untreated control PMφ were 92 and 95%, respectively, at 72 h. The ED50s and ED90s for BALB/c mouse-derived macrophages are higher in the present study than the values reported previously (8) due to the 3-day drug exposure in the present study compared to the 5 days used in the screening study.
TABLE 1.
Activities of HPC, AmBisome, and Pentostam against L. donovani in resident peritoneal macrophages from BALB/c and scid mice
| Compound | BALB/c mice
|
scid mice
|
||
|---|---|---|---|---|
| ED50a | ED90a | ED50 | ED90 | |
| HPC | 7.48 (7.29)b | 10.85 (10.31) | 7.47 (11.12)c | 11.42 (20.30) |
| Pentostam | 7.58 (10.5) | 13.15 (>25) | 20.69 (>25)d | >25 (>25) |
| AmBisome | 0.05 | 0.2 | 0.11d | >0.25 |
| Fungizone | 0.03 | 0.061 | 0.06d | >0.12 |
The ED50s and ED90s are in micromolar for HPC, AmBisome, and Fungizone and are in micrograms of SbV per milliliter for Pentostam.
The values parentheses are the results of a second experiment. P values were calculated for the drug activity after 72 h, in infected PMφ from BALB/c and scid mice.
P = 0.25.
P > 0.05.
The immunomodulatory effects of HPC have been described previously, including its activity as a costimulatory signal for T-cell and macrophage activation in vitro (12, 25, 31), an enhancer of IFN-γ production and granulocyte-macrophage colony-stimulating factor expression from peripheral mononuclear human cells in combination with interleukin-2 (13, 31), and an inducer of nitric oxide when used in a liposomal HPC preparation on the human histiocyte cell line U937 (12) or peritoneal macrophages after they are triggered with lipopolysaccharide (33). The stimulatory effect of HPC on the hematopoietic system has also been reported; an increased number of white blood cells and platelets were observed after oral treatment of humans with HPC (23). Additionally, oral treatment with high doses of HPC (45 mg/kg/dose for 5 days) led to significant increases in the levels of recruitment of endothelial cells, macrophages, T helper cells, cytotoxic T cells, and B cells in the spleens of nude mice, suggesting an effect on the development of immune tissues (25). In contrast, our study has confirmed and extended observations that a functional T- and B-cell response is not necessary for the activity of HPC against L. donovani, as the dose-responses were similar in both scid mice and the standard BALB/c mouse model. This is in agreement with the results of Murray and Delph-Etiene (18), who showed that HPC retained antileishmanial activity in T-cell-deficient mice and transgenic mice with impaired macrophage killing mechanisms. Additionally, a topical formulation of HPC effective in reducing cutaneous lesions caused by Leishmania mexicana in susceptible mice did not induce a protective immune response or IFN-γ production by T cells (26). Our study has extended the observations on L. donovani infections through the use of T- and B-cell-defective scid mice (4), the matching of the parasite loads in both strains of mice, and the dose-response analysis.
AmBisome also had similar levels of activity against L. donovani in both scid and BALB/c mice, confirming that a functional T-cell and/or B-cell response is not necessary for the activity of this formulation and adding to the previous report of the activity of amphotericin B against L. donovani in athymic (nude) BALB/c mice (20). The immunomodulatory effects of amphotericin B have been reported previously (6, 24), but they do not appear to play a role in the antileishmanial activity of this drug (20). In contrast to the similar activities of HPC and AmBisome in scid and BALB/c mice, sodium stibogluconate was significantly less active in scid mice. The highest dose of drug used in this study (45 mg/kg/dose for 5 days s.c.) caused only a 20% reduction in liver amastigote numbers in scid mice, whereas it caused a 98% reduction in BALB/c mice. The roles of T cells, cytokines, and macrophage activation by IFN-γ and tumor necrosis factor alpha in the antileishmanial effect of sodium stibogluconate in vivo have been described previously (19, 22). More recently, it has been demonstrated that IL-12 regulates the in vivo effect of sodium stibogluconate by regulation of IFN-γ production by T cells (21). To extend these studies and confirm that the immune dependency of drug activity was at the T- or B-cell level, the activities were also determined in vitro against amastigotes in PMφ from both strains. Whereas the activity of HPC was similar in both macrophage models, sodium stibogluconate was significantly more active against PMφ from BALB/c mice than those from scid mice. This result suggests that macrophage type or status has a role in the activity of this drug, as there are no intrinsic differences between macrophages from both species of mice (14, 19). Interestingly, AmBisome was also significantly more active against L. donovani in PMφ from BALB/c mice than against L. donovani in PMφ from scid mice (P > 0.05). Variations in the activities of amphotericin B formulations against Leishmania in macrophage models have been reported previously, including differences between the activities in peritoneal and THP-1-derived macrophages (32).
There have been increasing numbers of patients with L. infantum and HIV coinfections, especially in Mediterranean countries, during the past decade (1, 10). These immunocompromised patients generally have a poor response to antimonials, and failure or relapse rates of 52% within 1 to 36 months are commonly reported (10). Experimental studies with mice previously suggested that Fungizone could be an effective antileishmanial agent in immunocompromised patients. However, this drug has not been so successful, even in lipid formulations, for the treatment of patients with L. infantum and HIV coinfections (10). The dissemination of parasites in these patients away from the usual sites of infection does not favor the pharmacokinetic profile of amphotericin B or its lipid formulations. However, HPC is well absorbed following oral administration, is distributed throughout the body (5), and offers opportunities for further study of the treatment of VL in immunocompromised patients.
Acknowledgments
Patricia Escobar is supported by the Instituto Colombiano para el Desarrollo de la Ciencia y la Tecnologia “Francisco Jose de Caldas” COLCIENCIAS and by the University Industrial de Santander, Bucaramanga, Colombia. This work was supported by a grant from the EC INCO-DC programme (grant IC18CT96-0084).
REFERENCES
- 1.Alvar J, Canavate C, Gutierrez-Solar B, Jimenez M, Laguna F, Lopez-Velez R, Molina R, Moreno J. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev. 1997;10:298–319. doi: 10.1128/cmr.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur G, Bittman R. The inhibition of cell signaling pathways by antitumor ether lipids. Biochim Biophys Acta. 1998;1390:85–102. doi: 10.1016/s0005-2760(97)00163-x. [DOI] [PubMed] [Google Scholar]
- 3.Berman J D. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 4.Bosma G C, Custer R P, Bosma M J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 5.Breiser A, Kim D J, Fleer E A, Damenz W, Drube A, Berger M, Nagel G A, Eibl H, Unger C. Distribution and metabolism of hexadecylphosphocholine in mice. Lipids. 1987;22:925–926. doi: 10.1007/BF02535556. [DOI] [PubMed] [Google Scholar]
- 6.Chia J K, Pollack M. Amphotericin B induces tumor necrosis factor production by murine macrophages J. Infect Dis. 1989;159:113–116. doi: 10.1093/infdis/159.1.113. [DOI] [PubMed] [Google Scholar]
- 7.Croft S L, Neal R A, Pendergast W, Chan J H. The activity of alkyl phosphorylcholines and related derivatives against Leishmania donovani. Biochem Pharmacol. 1987;36:2633–2636. doi: 10.1016/0006-2952(87)90543-0. [DOI] [PubMed] [Google Scholar]
- 8.Croft S L, Snowdon D, Yardley V. The activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei. J Antimicrob Chemother. 1996;38:1041–1047. doi: 10.1093/jac/38.6.1041. [DOI] [PubMed] [Google Scholar]
- 9.Davidson R N. Visceral leishmaniasis in clinical practice. J Infect. 1999;39:112–116. doi: 10.1016/s0163-4453(99)90001-4. [DOI] [PubMed] [Google Scholar]
- 10.Desjeux P. Global control and Leishmania HIV co-infection. Clin Dermatol. 1999;17:317–325. doi: 10.1016/s0738-081x(99)00050-4. [DOI] [PubMed] [Google Scholar]
- 11.Engwerda, C. R., S. Smelt, and P. M. Kaye. An in vivo analysis of cytokine production during Leishmania donovani infection in scid mice. Exp. Parasitol. 84:195–202. [DOI] [PubMed]
- 12.Eue I, Zeising R, Arndt D. Alkylphosphocholine-induced production of nitric oxide and tumor necrosis factor alpha by U 937 cells. J Cancer Res Clin Oncol. 1995;121:350–356. doi: 10.1007/BF01225687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hochhuth C H, Vehmeyer K, Eibl H, Unger C. Hexadecylphosphocholine induces interferon-gamma secretion and expression of GM-CSF mRNA in human mononuclear cells. Cell Immunol. 1992;141:161–168. doi: 10.1016/0008-8749(92)90135-c. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim M E, Hag-Ali M, el-Hassan A M, Theander T G, Kharazmi A. Leishmania resistant to sodium stibogluconate: drug-associated macrophage-dependent killing. Parasitol Res. 1994;80:569–574. doi: 10.1007/BF00933004. [DOI] [PubMed] [Google Scholar]
- 15.Jha T K, Sundar S, Thakur C P, Bachmann P, Karbwang J, Fischer C, Voss A, Berman J. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N Engl J Med. 1999;341:1795–1800. doi: 10.1056/NEJM199912093412403. [DOI] [PubMed] [Google Scholar]
- 16.Kuhlencord A, Maniera T, Eibl H, Unger C. Hexadecylphosphocholine: oral treatment of visceral leishmaniasis in mice. Antimicrob Agents Chemother. 1992;36:1630–1634. doi: 10.1128/aac.36.8.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Fichoux Y, Rousseau D, Ferrua B, Ruette S, Lelievre A, Grousson D, Kubar J. Short- and long-term efficacy of hexadecylphosphocholine against established Leishmania infantum infection in BALB/c mice. Antimicrob Agents Chemother. 1998;42:654–658. doi: 10.1128/aac.42.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray H W, Delph-Etiene S. Visceral leishmanicidal activity of hexadecylphosphocholine (miltefosine) in mice deficient in T cells and activated macrophage microbicidal mechanisms. J Infect Dis. 2000;181:795–799. doi: 10.1086/315268. [DOI] [PubMed] [Google Scholar]
- 19.Murray H W, Granger A M, Mohanty S K. Response to chemotherapy in experimental visceral leishmaniasis: T cell-dependent but interferon-gamma-and interleukin-2-independent. J Infect Dis. 1991;163:622–624. doi: 10.1093/infdis/163.3.622. [DOI] [PubMed] [Google Scholar]
- 20.Murray H W, Hariprashad J, Fichtl R E. Treatment of experimental visceral leishmaniasis in a T-cell-deficient host: response to amphotericin B and pentamidine. Antimicrob Agents Chemother. 1993;37:1504–1505. doi: 10.1128/aac.37.7.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray H W, Montelibano C, Peterson R, Sypek J P. Interleukin-12 regulates the response to chemotherapy in experimental visceral leishmaniasis. J Infect Dis. 2000;182:1497–1502. doi: 10.1086/315890. [DOI] [PubMed] [Google Scholar]
- 22.Murray H W, Oca M J, Granger A M, Schreiber R D. Requirement for T cells and effect of lymphokines in successful chemotherapy for an intracellular infection. Experimental visceral leishmaniasis. J Clin Investig. 1989;83:1253–1257. doi: 10.1172/JCI114009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pronk L C, Planting A S, Oosterom R, Drogendijk T E, Stoter G, Verweij J. Increases in leucocyte and platelet counts induced by the alkyl phospholipid hexadecylphosphocholine. Eur J Cancer. 1994;30:1019–1022. doi: 10.1016/0959-8049(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 24.Reyes E, Cardona J, Prieto A, Bernstein E D, Rodriguez-Zapata M, Pontes M J, Alvarez-Mon M. Liposomal amphotericin B and amphotericin B-deoxycholate show different immunoregulatory effects on human peripheral blood mononuclear cells J. Infect Dis. 2000;181:2003–2010. doi: 10.1086/315517. [DOI] [PubMed] [Google Scholar]
- 25.Safa O, Parkin S, Matthew A M, Bibby M C. Morphological and immunological observations on the effects of hexadecylphosphocholine (HPC) in nude mice bearing MT-1 breast cancer xenografts. Anticancer Res. 1997;17:37–43. [PubMed] [Google Scholar]
- 26.Schmidt-Ott R, Klenner T, Overath P, Aebischer T. Topical treatment with hexadecylphosphocholine (Miltex®) efficiently reduces parasite burden in experimental cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1999;93:85–90. doi: 10.1016/s0035-9203(99)90192-x. [DOI] [PubMed] [Google Scholar]
- 27.Sundar S, Rosenkainmer F, Makharia M K, Goyal A K, Mandal A K, Voss A, Hilgard P, Murray H W. Trial of oral miltefosine for visceral leishmaniasis. Lancet. 1998;352:1821–1823. doi: 10.1016/S0140-6736(98)04367-0. [DOI] [PubMed] [Google Scholar]
- 28.Thakur C P, Sinha G P, Pandey A K, Kumar N, Kumar P, Hassan S M, Narain S, Roy R K. Do the diminishing efficacy and increasing toxicity of sodium stibogluconate in the treatment of visceral leishmaniasis in Bihar, India, justify its continued use as a first-line drug? An observational study of 80 cases. Ann Trop Med Parasitol. 1998;92:561–569. doi: 10.1080/00034989859258. [DOI] [PubMed] [Google Scholar]
- 29.Unger C, Damenz W, Fleer E A, Kim D J, Breiser A, Hilgard P, Engel J, Nagel G, Eibl H. Hexadecylphosphocholine, a new ether lipid analogue. Studies on the antineoplastic activity in vitro and in vivo. Acta Oncol. 1989;28:213–217. doi: 10.3109/02841868909111249. [DOI] [PubMed] [Google Scholar]
- 30.Unger C, Maniera T, Kaufmann-Kolle P, Eibl H. In vivo antileishmanial activity of hexadecylphosphocholine and other alkylphosphocholines. Drugs Today. 1998;34:133–140. [Google Scholar]
- 31.Vehmeyer K, Scheurich P, Eibl H, Unger C. Hexadecylphosphocholine-mediated enhancement of T-cell responses to interleukin 2. Cell Immunol. 1991;137:232–238. doi: 10.1016/0008-8749(91)90072-j. [DOI] [PubMed] [Google Scholar]
- 32.Yardley V, Croft S L. A comparison of the activities of three amphotericin B lipid formulations against experimental visceral and cutaneous leishmaniasis. Int J Antimicrob Agents. 2000;13:243–248. doi: 10.1016/s0924-8579(99)00133-8. [DOI] [PubMed] [Google Scholar]
- 33.Zeisig R, Rudolf M, Eue I, Arndt D. Influence of hexadecyl phosphocholine on the release of tumor necrosis factor and nitroxide from peritoneal macrophages in vitro. J Cancer Res Clin Oncol. 1995;121:69–75. doi: 10.1007/BF01202215. [DOI] [PMC free article] [PubMed] [Google Scholar]