Abstract
Objective:
Venous thromboembolism (VTE) is an important cause of postoperative morbidity and mortality. However, the reported incidence after major vascular surgery has ranged from as low as 1% to >10%. Furthermore, little is known about optimal chemoprophylaxis regimens or rates of postdischarge VTE in this population. In the present study, we aimed to better characterize the rates of in-hospital and postdischarge VTE after major vascular surgery, the role of chemoprophylaxis timing, and the association of VTE with mortality.
Methods:
A single-center retrospective study of 1449 major vascular operations (2013–2020) was performed and included 189 endovascular abdominal aortic aneurysm repairs (13%), 169 thoracic endovascular aortic aneurysm repairs (12%), 318 open aortic operations (22%), 640 lower extremity bypasses (44%), and 133 femoral endarterectomies (9%). The baseline characteristics, anticoagulant and antiplatelet medications, and outcomes were abstracted from an electronic database with medical record auditing. Postoperative VTE (pulmonary embolism and deep vein thrombosis) within 90 days of surgery was classified by the location, symptoms, and treatment. A cut point analysis using Youden’s index identified the most VTE discriminating timing of chemoprophylaxis (including therapeutic vs prophylactic anticoagulant and antiplatelet medications) and Caprini score. Multivariable logistic regression was used to test the association of VTE with chemoprophylaxis timing, Caprini score, and additional risk factors. Cox proportional hazard modeling was used to measure the association between VTE and mortality.
Results:
The overall VTE incidence was 3.4% (65% deep vein thrombosis; 25% pulmonary embolism; 10% both), and 37% had occurred after discharge. The rate of symptomatic VTE was 2.4%, which was lowest for endovascular abdominal aortic aneurysm repair (0.0%) and highest for open aortic surgery (4.1%; P = .02). Those who had developed VTE had had a longer length of stay, higher rates of end-stage renal disease and prior VTE, and higher Caprini scores (8 vs 5 points; P < .01 for all). Those who had developed VTE were also more likely to have received ≥2 U of blood postoperatively, required an unplanned return to the operating room, had delayed chemoprophylaxis, anticoagulation, and/or antiplatelet initiation of >4 days postoperatively, and had increased 90-day mortality (P < .01 for all). A Caprini score of ≥7 (29% of patients) was associated with postdischarge VTE (2.6% vs 0.7%; P = .01), and chemoprophylaxis, anticoagulation, and antiplatelet timing of >4 days was associated with an increased adjusted odds of VTE (odds ratio, 2.4; 95% confidence interval, 1.1–4.9). Although no fatal VTEs were identified, VTE was an independent predictor of 90-day mortality (adjusted hazard ratio, 2.7; 95% confidence interval, 1.3–5.9).
Conclusions:
These data have shown that patients undergoing major vascular surgery are particularly prone to the development of VTE, with frequent hypercoagulable comorbidities. The earlier initiation of chemoprophylaxis was associated with a reduced risk of VTE development. Furthermore, the postdischarge VTE rates might reach thresholds warranting postdischarge chemoprophylaxis, especially for patients with a Caprini score of ≥7.
Keywords: Perioperative care, Postoperative complications, Vascular surgical procedures, Venous thromboembolism
Venous thromboembolism (VTE) remains a major public health burden that is especially relevant in the perioperative period, with an estimated 50,000 VTE-associated postoperative deaths in the United States annually.1,2 Patients undergoing vascular surgery have a high prevalence of identified hypercoagulable risk factors, increasing their risk of developing postoperative VTE.3–5 However, a paucity of evidence is available regarding the VTE incidence and prophylaxis strategies after major vascular procedures. This knowledge gap was recently highlighted by the results from a meta-analysis.6 The investigators found relatively few studies had examined VTE incidence after major vascular surgery. They also found a lack of adequately powered randomized trials assessing the effectiveness of chemoprophylaxis.6 Because of this lack of data, the American Society of Hematology has only conditionally recommended the use of pharmacologic prophylaxis after major vascular surgery, in contrast to other surgical fields in which robust evidence is available to support VTE prophylaxis guidelines.7,8
Additionally, the recognition of the incidence of postdischarge VTE has led other specialties to standardize the use of postdischarge thromboprophylaxis in clinical practice guidelines. However, data from vascular surgery are lacking.7,8 Postdischarge VTE has previously been estimated to account for as much as 40% of VTE cases after vascular surgery.9 Better characterizing the postdischarge VTE incidence could help to determine whether individuals undergoing vascular surgery could benefit from extended-duration chemoprophylaxis, in particular, those at high risk.7,8,10–12
Determining the appropriate chemoprophylaxis regimen for vascular surgery patients requires an appropriate balance between the potential benefit of a reduced risk of VTE development and the risk of postoperative bleeding. The Caprini model is one of the most used VTE risk assessment tools. However, it has not been validated specifically in vascular surgery populations.7,13 Better preoperative risk stratification for VTE could facilitate surgeons in making more informed decisions regarding the risks and benefits of initiating VTE chemoprophylaxis.
In the present study, we aimed to estimate the VTE incidence after major vascular procedures, identify the risk factors for VTE in this population, and assess the performance of the Caprini score. We hypothesized that the VTE rates would vary significantly across procedure types, with higher rates after thoracic endovascular aortic aneurysm repair (TEVAR) and open aortic operations. Additionally, we hypothesized that the Caprini score would effectively discriminate between patients with a high and low risk of VTE. Finally, we explored the association of chemoprophylaxis timing with the development of VTE and the occurrence of VTE with mortality.
METHODS
We performed a single-center, retrospective study of 1449 major vascular procedures (1245 unique individuals) from 2013 to 2020. The following procedures were included: endovascular abdominal aortic aneurysm repair (EVAR), TEVAR, open lower extremity bypass (suprainguinal or infrainguinal), femoral endarterectomy (with and without a concomitant endovascular intervention), and open aortic operations (ie, open abdominal aortic aneurysm repair or aortobifemoral bypass). Cases of complex multibranch endovascular aortic aneurysm repairs were excluded from our analysis.
The baseline demographics, comorbidities, anticoagulant and/or antiplatelet medications, and outcomes were abstracted from an electronic database with medical record auditing. The Caprini scores were calculated using the modified Caprini risk assessment model and preoperative patient International Classification of Diseases (ICD), 9th and 10th revision, codes to determine the presence of each risk factor, as previously described.7,13 The time to the initiation of antiplatelet medications (aspirin or clopidogrel) and anticoagulant drugs (heparin, enoxaparin, or oral anticoagulant agents) was determined by calculating the interval from surgery to the first dose administered, starting with postoperative day 0. The use of heparin and enoxaparin was further classified as therapeutic or prophylactic. Postoperative VTE (pulmonary embolism [PE] and deep vein thrombosis [DVT]) within 90 days of surgery was identified by ICD-9 and −10 codes. All patients with a corresponding ICD-9 or −10 code for VTE were further reviewed to determine the VTE type and location (DVT vs PE), symptomatic vs incidental, and treatment. A VTE diagnosis was confirmed only if diagnostic imaging findings had documented the diagnosis. Cases of documented chronic VTE and acute VTE diagnosed before surgery were excluded from the definition of VTE for the purposes of the present study. Major bleeding was defined as the transfusion of ≥2 U of blood in the 7 days postoperatively or if the patient had required a return to the operating room for management of hemorrhage consistent with the established definitions of major bleeding.14
The patient characteristics and outcomes were compared between those with and without VTE using t tests for continuous and normally distributed variables, Wilcoxon rank sum tests for continuous and skewed variables, and χ2 tests for categorical variables. A cut point analysis using Youden’s index identified the thresholds most discriminating for VTE development for the interval to chemoprophylaxis initiation (including therapeutic or prophylactic anticoagulant and antiplatelet medications) and Caprini scores.15 For the analyses performed in the present study, each vascular procedure included (n = 1449) was considered an independent event. Bivariate analysis and multivariable logistic regression were used to test the association of VTE with the Caprini score, chemoprophylaxis timing, and additional risk factors, including the length of stay, end-stage renal disease (chronic kidney disease stage 5 [CKD-5], including dialysis-dependent and non–dialysis-dependent patients), major bleeding, and coronary artery disease. The multivariable logistic regression model was optimized by testing previously described risk factors for VTE in a manual stepwise fashion, optimizing the model fit assessed using the Akaike information criterion.13,16 Cox proportional hazard modeling was used to assess the association between VTE and 90-day mortality. The institutional review board at the University of California, San Francisco, approved the present study (study no. 20–30624) and waived the requirement for patient informed consent owing the retrospective and minimal risk nature of the research.
RESULTS
Overall, 1449 procedures, including 169 TEVARs, 189 EVARs, 318 open aortic operations (208 open aortic aneurysm repairs and 110 aortobifemoral bypasses), 640 lower extremity bypasses, and 133 femoral endarterectomies, were evaluated. Postoperative VTE was identified in 49 patients (3.4%). Symptomatic VTE was identified in 35 patients (2.4%), and postdischarge VTE was identified in 18 patients (1.2%). VTE frequency varied by procedure type and was more frequently diagnosed after TEVAR and open aortic operations (5.3% and 6.5% respectively), less frequently after lower extremity bypass and femoral endarterectomy (range, 2.7%–3.0%), and not detected after EVAR (Fig 1). Most cases of VTE were DVT (n = 37), followed by 17 cases of PE, of which 5 were concurrent with DVT. Among the TEVAR patients, however, seven of the VTE cases were PE (64%), and three of the seven (43%) were symptomatic. In contrast, 9 of the 10 cases of PE (90%) in the non-TEVAR patients were symptomatic (Fig 1). Nearly one half of the 18 cases of DVT (49%) were located in the upper extremity. Of the 18 upper extremity DVTs, 12 were associated with the central venous catheter. In contrast, only 1 of the 19 lower extremity DVTs were associated with the central venous catheter (Supplementary Table I, online only).
Fig 1.
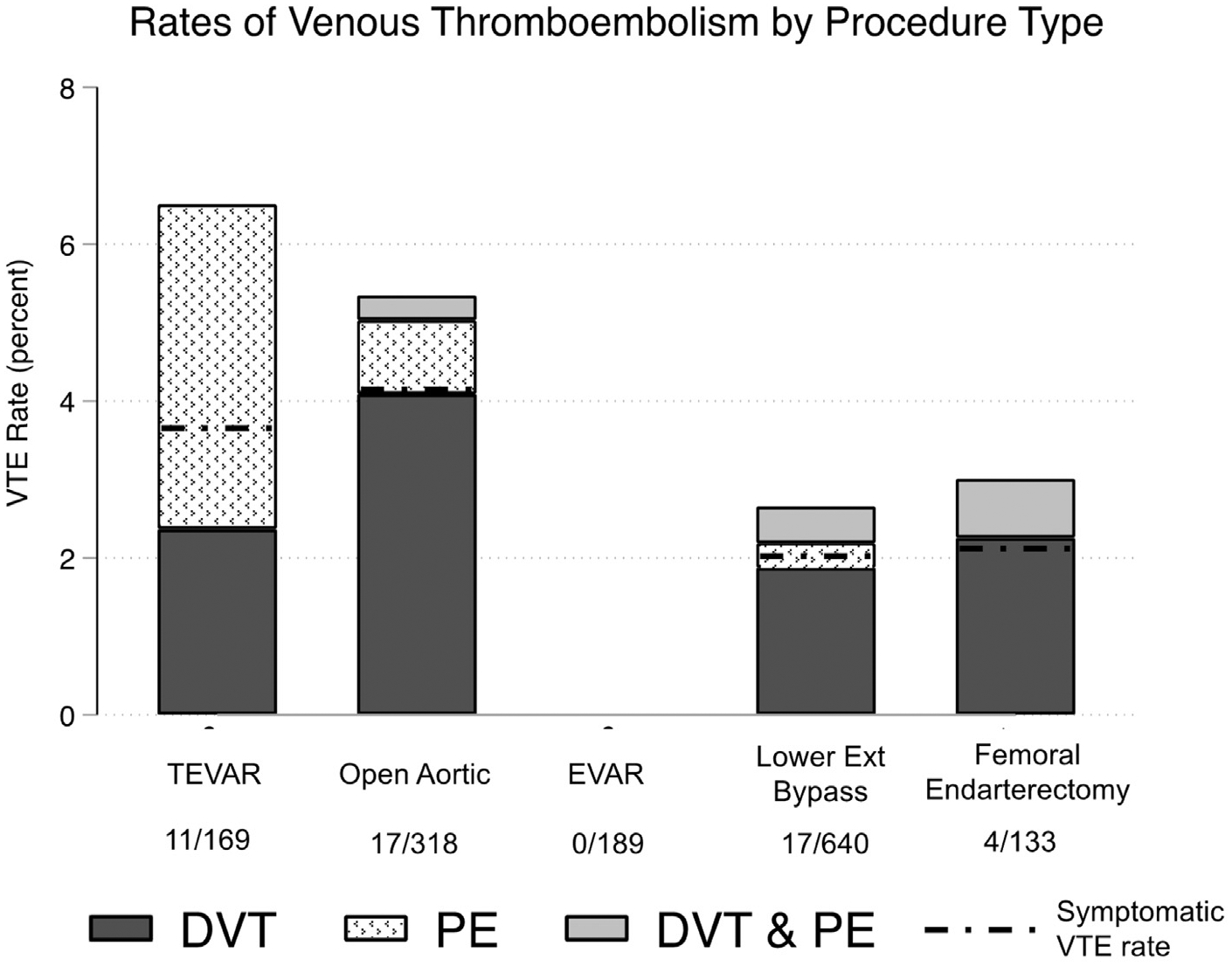
Rates of postoperative venous thromboembolism (VTE) across procedure types. Y-axis: VTE rate includes all VTEs (symptomatic and asymptomatic); black dotted line represents symptomatic VTE rate for each procedure. Data below each bar denote number of VTE events of total in each operative category. EVAR, Endovascular aortic aneurysm repair; Lower Ext Bypass, open lower extremity bypass; Open Aortic, open abdominal aortic aneurysm repair (n = 208) or aortobifemoral bypass (n = 110); TEVAR, thoracic endovascular aortic aneurysm repair.
The 49 patients who had developed VTE were younger (65 vs 68 years; P = .09), had required a longer length of stay (16 vs 7 days; P < .01), and were more likely to be admitted nonelectively (60% vs 45%; P = .04; Table I). They were also more likely to have a history of end-stage renal disease (CKD-5; 37% vs 14%), central venous catheter placement (37% vs 7%), a prior VTE (26% vs 4%), and a recent diagnosis (within 1 month) of myocardial infarction, sepsis, pneumonia, or severe lung disease (Table I; P < .05 for all). Other factors associated with VTE included delayed initiation of an anticoagulant (prophylactic or therapeutic dose) or antiplatelet agent of >4 days after surgery, transfusion of ≥2 U of blood or a return to the operating room for bleeding, and non-home discharge (P < .05 for all; Table I). Finally, patients with VTE experienced increased mortality at 30 (10.4% vs 3.5%) and 90 (20.8% vs 4.8%) days (P < .05 for both; Table I).
Table I.
Baseline characteristics stratified by postoperative VTE
| VTE | ||||
|---|---|---|---|---|
| Characteristic | No (n = 1400; 96.6%) | Yes (n = 49; 3.4%) | Total (N = 1449; 100%) | P value |
| Age | 68 ± 13 | 65 ± 14 | 68 ± 13 | .09 |
| Male sex | 66 | 68 | 66 | .88 |
| BMI, kg/m2 | .49 | |||
| <19 | 6 | 6 | 6 | |
| 19–30 | 70 | 77 | 70 | |
| >30 | 24 | 17 | 24 | |
| Length of stay, days | 7 (4–12) | 16 (10–26) | 7 (4–12) | <.01 |
| Nonelective admission | 45 | 60 | 46 | .04 |
| CKD-5 | 14 | 37 | 15 | <.01 |
| CAD | 34 | 24 | 34 | .17 |
| DM | 31 | 27 | 31 | .53 |
| COPD | 19 | 20 | 19 | .99 |
| Smoking history | 30 | 24 | 30 | .43 |
| Impaired mobility | 2 | 8 | 2 | .03 |
| Malignancy | 0.6 | 2.0 | 0.7 | .29 |
| Hypercoagulable disorder | 11 | 16 | 11 | .16 |
| Varicose veins | 3.3 | 2.0 | 3.2 | .99 |
| Inflammatory bowel disease | 0.9 | 2.0 | 0.9 | .39 |
| Central venous catheter placed during admission | 7 | 37 | 8 | <.01 |
| Sepsis (≤1 month) | 6 | 29 | 7 | <.01 |
| Acute MI (≤1 month) | 5 | 12 | 5 | .03 |
| Acute stroke (≤1 month) | 5 | 10 | 6 | .22 |
| Pneumonia or severe lung disease (≤1 month) | 9 | 27 | 9 | <.01 |
| Prior VTE | 4 | 26 | 4 | <.01 |
| Preoperative creatinine, mg/dL | 1.0 (0.8–1.3) | 0.9 (0.7–1.4) | 1.0 (0.8–1.3) | .39 |
| Caprini score | 5 (4–7) | 8 (6–10) | 5 (5–7) | <.01 |
| Caprini score ≥7 | 27 | 65 | 29 | <.01 |
| Interval to chemoprophylaxis,a days | .07 | |||
| <3 | 40 | 35 | 39 | |
| >3 | 11 | 22 | 12 | |
| None | 49 | 43 | 49 | |
| Interval to chemoprophylaxis or anticoagulation, days | .20 | |||
| <2 | 33 | 20 | 32 | |
| 2–4 | 23 | 26 | 23 | |
| >4 or nonea | 45 | 53 | 45 | |
| Interval to chemoprophylaxis, anticoagulation, antiplatelet, days | <.01 | |||
| <2 | 68 | 45 | 67 | |
| 2–4 | 15 | 22 | 16 | |
| >4 or nonea | 17 | 33 | 17 | |
| ≥2 pRBCs transfused postoperatively (days 1–7) | 14 | 29 | 14 | <.01 |
| Return to OR for bleeding (30 days) | 0.8 | 4.1 | 0.9 | .07 |
| Return to OR bleeding or ≥2 pRBCs transfused postoperatively (days 1–7) | 14 | 31 | 15 | <.01 |
| Nonhome discharge | 29 | 49 | 30 | <.01 |
| Mortality at 30 days | 3.5 | 10.4 | 4.7 | .05 |
| Mortality at 90 days | 4.8 | 20.8 | 5.3 | <.01 |
BMI, Body mass index; CAD, coronary artery disease; CKD-5, chronic kidney disease stage 5; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; MI, myocardial infarction; OR, operating room; pRBCs, packed red blood cells; VTE, venous thromboembolism.
Data presented as mean ± standard deviation, percentages, or median (interquartile range).
Including patients who started anticoagulation or antiplatelet therapy after a VTE diagnosis.
The median Caprini score was 8 vs 5 points in the VTE vs no VTE groups (P < .01), and a cut point analysis identified an inflection point for the risk of VTE at a score of ≥7 points (P < .01; Table I). Caprini scores of 2 to 6 were associated with the lowest VTE and postdischarge VTE rates (1.6% and 0.7%, respectively; Fig 2). A marked stepwise increase in the VTE and postdischarge VTE rates was found for those with Caprini scores of 7 to 9 (5.6% and 2.1%, respectively) and ≥10 (14.7% and 4.2%, respectively; Fig 2). This corresponded to odds ratios for VTE and postdischarge VTE of 3.6 (95% confidence interval [CI], 1.8–7.0) and 3.3 (95% CI, 1.1–9.4) for the group with Caprini scores of 7 to 9 and 10.9 (95% CI, 4.9–21.7) and 6.4 (95% CI, 1.9–22.4) for the group with Caprini scores of ≥10 (vs 2–6 points as the reference in the univariate logistic regression models with good accuracy; C-statistic: VTE, 0.71; postdischarge VTE, 0.68; P < .05 for both; Fig 2; Supplementary Table II, online only).
Fig 2.
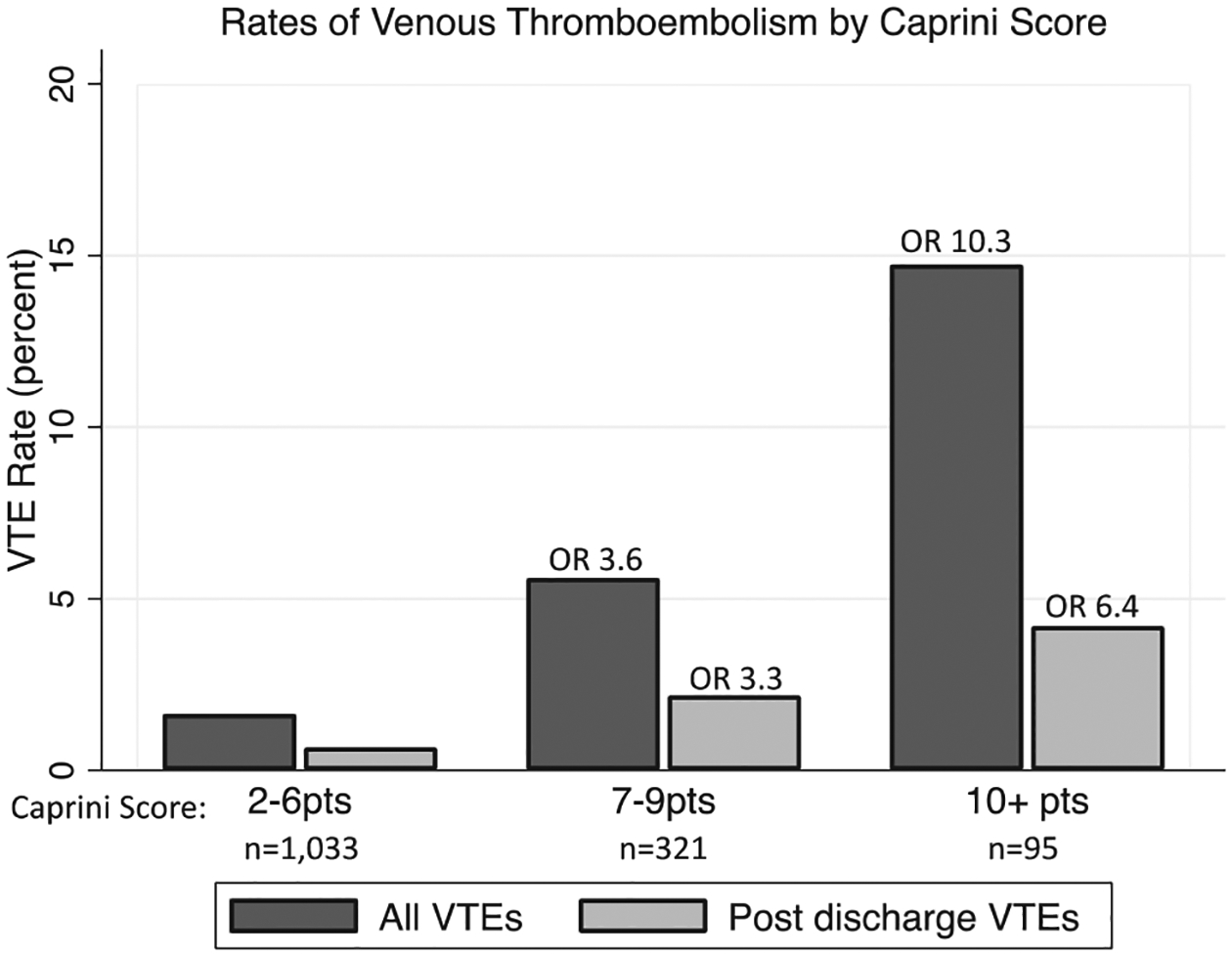
Rates of venous thromboembolism (VTE) stratified by Caprini score group. Odds ratios for association of Caprini score 7 to 9 and ≥10 groups with each outcome (all VTE and postdischarge VTE) shown at top of bars, with 2- to 6-point group as reference. Logistic regression results presented in Supplementary Table I (online only). P < .05 for all odds ratios displayed.
Multivariable logistic regression for VTE was then performed, controlling for the length of stay and bleeding events (significant transfusions and return to the operating room for bleeding). The Caprini score remained the strongest risk factor for VTE with an odds ratio of 2.4 (95% CI, 1.2–5.0) and 5.2 (95% CI, 2.3–12.0) for Caprini scores of 7 to 9 and ≥10, respectively (Table II). Additionally, a delayed initiation of anticoagulation (therapeutic or prophylactic dose) or antiplatelet agents of >4 days after surgery was associated with an odds ratio for VTE of 2.4 (95% CI, 1.1–4.9; Table II). A similar increased risk was associated with a diagnosis of CKD-5, although a history of coronary artery disease was associated with a reduced risk of VTE (Table II). The incorporation of these additional risk factors improved model accuracy compared with the Caprini score alone, with a C-statistic of 0.81 (Table II).
Table II.
Multivariable logistic regression results for risk factors predictive of postoperative VTEa
| Predictive factor | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Caprini score | |||
| <7 points (reference) | 1.0 | NA | NA |
| 7–9 points | 2.4 | 1.2–5.0 | .03 |
| ≥10 points | 5.2 | 2.3–12.0 | <.01 |
| Interval to anticoagulation (therapeutic or prophylactic) or antiplatelet initiation,b days | 1.0 | ||
| <2 (reference) | NA | NA | NA |
| 2–4 | 1.5 | 0.7–3.3 | .30 |
| ≥5 | 2.4 | 1.1–4.9 | .02 |
| CKD-5 | 2.0 | 1.0–4.0 | .04 |
| Length of stay (per 5 days) | 1.4 | 1.2–1.7 | <.01 |
| Major bleedingc | 1.4 | 0.7–2.8 | .18 |
| CAD | 0.5 | 0.2–1.0 | .05 |
CAD, Coronary artery disease; CI, confidence interval; CKD-5, chronic kidney disease stage 5; NA, not applicable.
Total number of patients, 1293, excluding those with a length of stay of ≤2 days; n = 49 for VTE; C-statistic = 0.81.
Chemoprophylaxis included patients receiving prophylactic or therapeutic dose anticoagulant therapy; antiplatelet included single and dual antiplatelet therapy.
Return to operating room for bleeding or transfusion of ≥2 pRBCs between postoperative days 1 and 7.
Although no identified VTEs were fatal, VTE was an independent risk factor for 90-day survival on Cox proportional hazard modeling (adjusted hazard ratio for VTE, 2.7; 95% CI, 1.3–5.9; P = .01; Fig 3; Supplementary Table III, online only).
Fig 3.
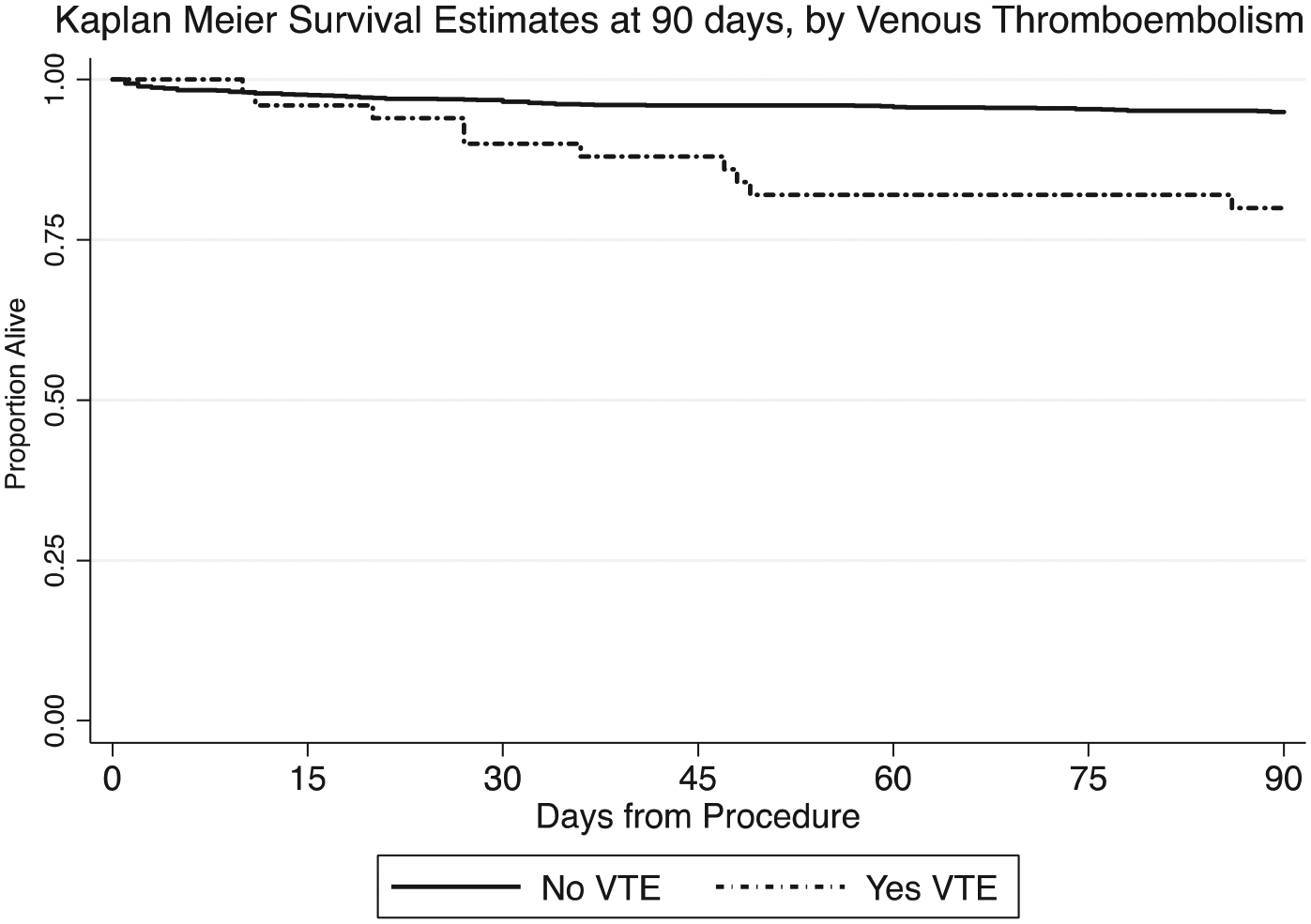
Kaplan-Meier curves comparing survival ≤90 days for those with and without postoperative venous thromboembolism (VTE). Solid line indicates no VTE group (n = 1400; deaths, n = 67); dotted line, VTE group (n = 49; deaths, n = 10). Adjusted hazard ratio for VTE was 2.8 (P = .01). The results from the full Cox proportional hazard model are presented in Supplementary Table II (online only).
DISCUSSION
In the present study, we characterized the VTE incidence, timing, and risk factors in a large single-center cohort of patients who had undergone inpatient major open vascular and endovascular surgery. Our findings underscore the high prevalence of hypercoagulable risk factors and greater VTE incidence among vascular surgery patients compared with average risk general surgery populations. We identified postoperative VTE in 3.4% of cases, of which most (71%) were symptomatic and more than one third (37%) had occurred after discharge (≤90 days). These rates are higher than those reported in large analyses of multicenter databases.3,9,17 However, these data registries might be more prone to underreporting, especially for postdischarge VTE, because many only examined events ≤30 days postoperatively. In contrast, several prior single-center reports have demonstrated much higher rates of VTE (≥10%), especially after open aortic operations and TEVAR.18–21 These discrepancies could also be accounted for by the use of more inclusive VTE definitions and the duplex ultrasound screening practices included in the protocols of studies with the highest reported rates of VTE. Postoperative screening for DVT was not performed as a part of routine clinical practice for the patients included in the present study. Because of these interstudy differences, direct comparisons of absolute VTE rates can be difficult to interpret and a comparison of relative trends might be more informative.
Our findings demonstrating a significant differential risk of VTE across major open and endovascular procedures are similar to those described previously. We detected the highest VTE rates after TEVAR (6.5%) and open aortic operations (5.3%). These findings are consistent with other studies demonstrating high rates of VTE after TEVAR (≤9%) and open abdominal aortic aneurysm repair (as high as 18% when routine screening for VTE was performed).18,19 The high rates of PE after TEVAR might, in part, be attributable to ascertainment bias owing to the frequent use of postoperative computed tomography of the chest for endograft surveillance and was reflected in the higher rate of asymptomatic PE in the TEVAR group. TEVAR patients do have specific VTE risk factors to consider, including more frequent immobilization postoperatively, especially in the setting of spinal drain use, which can also necessitate a delay or interruption in thromboprophylaxis. In contrast to TEVAR and open aortic operations, the VTE incidence after EVAR and lower extremity bypass have been estimated to be lower, ranging from ~1% to 3%.9,17,22 Similarly, we found that VTE was less frequent after lower extremity bypass (2.7%), with no cases detected after EVAR (0.0%). These findings should be considered when assessing the VTE risk for vascular surgery patients, especially because the Caprini risk assessment model does not assign differential risk scores to specific vascular surgical procedures—all major surgical procedures >45 minutes are assigned an equal number of points in the model.
Despite the lack of vascular procedure specificity in the Caprini score, it remained predictive of VTE. The Caprini score classifies patients as having very low (0 points), low (1–2 points), moderate (3–4 points), and high (≥5 points) risk, with the corresponding predicted rates of VTE ranging from <0.5% to 6% for the very low to high risk group.7 According to this stratification, 75% of the patients in the present study would have been in the highest risk group. Our analysis suggests that different cutoffs might be relevant for patients undergoing vascular surgery. The identification of VTE for those with a score of 2 to 6 (71% of patients) was low at 1.7% but increased substantially to 5.4% for those with a score of 7 to 9 (22% of patients) and 15% for the smaller subset with a score of ≥10 (7% of patients). A similar trend was noted for the rates of postdischarge VTE, with a rate <1% in the 2- to 6-point group vs 2% and 4% in the 7- to 9- and ≥10-point group. Given these differences, a more intensive VTE prophylaxis strategy might be warranted for patients with a Caprini score of ≥7 when the bleeding risk is acceptable. These patients might benefit from an extended duration chemoprophylaxis given that their rate of postdischarge VTE was >1%, a cutoff point above which postdischarge chemoprophylaxis has been shown to be cost-effective and beneficial.23,24 Prospective studies are needed to delineate the role and choice of agent for extended duration chemoprophylaxis after vascular surgery, especially in the setting of concomitant antiplatelet therapy.
Although our findings have confirmed the utility of the Caprini score for risk stratifying VTE risk after vascular surgery, additional patient and clinical factors not included in the model are relevant to consider. In our study, no patient with a length of stay of ≤2 days had developed VTE (0 of 159 patients). We additionally identified CKD-5 as a significant risk factor for VTE, also not included in the Caprini model. Although patients with CKD-5 are known to have impaired platelet function in the setting of uremia, CKD-5 is also known to cause deficiencies in fibrinolysis and natural anticoagulant pathways, which predispose patients to thrombotic complications.25,26
To explore whether specific cutoffs for initiating prophylaxis are associated with VTE, we incorporated the time to chemoprophylaxis initiation in our multivariable models examining the risk factors for VTE. Studying the chemoprophylaxis practices used for vascular surgery patients is complicated by the near ubiquitous use of intraoperative heparin, frequent use of therapeutic postoperative anticoagulation, and high rates of antiplatelet therapy. To explore the use of both anticoagulant (prophylactic and therapeutic dose) and antiplatelet therapy, we accounted for the time to postoperative initiation of any anticoagulant or antiplatelet therapy, including all patients in the model with a length of stay of >2 days. We found a significant association between the time to the initiation of any anticoagulant or antiplatelet therapy and those who developed VTE. Patients who had received anticoagulant or antiplatelet therapy initiated early after surgery (within 24 hours) had had a significantly reduced odds of VTE compared with those who had had antithrombotic medications initiated >4 days postoperatively, with a nonsignificant decrease compared with patients with anticoagulant or antiplatelet therapy initiated between 2 and 4 days postoperatively. These findings are supported by a prior multicenter retrospective analysis that examined the association of chemoprophylaxis timing with VTE development after vascular surgery in which patients receiving VTE prophylaxis within 24 hours had a decreased incidence of postoperative VTE after lower extremity bypass.17 The potential role of antiplatelet therapy in mitigating the VTE risk in vascular patients is also supported by our findings of decreased VTE risk associated with coronary artery disease. That decreased risk can be explained by the greater rates of dual antiplatelet therapy among these patients. Antiplatelet therapy is known to be an effective VTE prevention strategy for orthopedic surgery patients but has not been recommended for nonorthopedic surgical populations for chemoprophylaxis except when low dose molecular weight heparin and unfractionated heparin are contraindicated.7,8,27
Finally, we found that VTE was independently associated with increased 90-day mortality after controlling for age, procedure type, nonelective admission, a history of VTE, CKD-5, and length of stay, although none of the VTEs identified were directly fatal. This finding suggests that VTE is also a marker for underlying disease severity or the development of other postoperative complications. VTE is considered the leading cause of preventable death in hospitalized patients, although it might only be identified on autopsy in many cases, especially when imaging studies cannot be obtained in the case of rapid physiologic compromise or death.28–30 Prior studies have also demonstrated increased mortality associated with VTE in vascular surgery patients, underscoring the potential clinical effects of improving VTE prevention strategies for this population.9,18
Study limitations.
Our study had some important limitations. First, given its retrospective design, prospective studies are warranted to further corroborate our findings, including further validation and application of the Caprini score for vascular surgery patients. Additionally, we were unable to account for some important VTE risk factors, including procedure duration and American Society of Anesthesiologists scores, both of which are known to be associated with VTE.7,31 Although the risk of major bleeding associated with prophylactic dose anticoagulant therapy is thought to be low, it is difficult ascertain this risk retrospectively. Modeling controlled for transfusions and a return to the operating room for bleeding, although these might not be the most sensitive measures of bleeding or bleeding risk. Also, approximately one fourth of VTEs were asymptomatic in our analysis; however, we believe these still to be clinically relevant, especially because most (88%) were subsequently treated with therapeutic anticoagulation therapy, and VTE was associated with worse overall survival. It is possible that our reported VTE rate was underestimated if some VTEs had occurred that had not been classified by the ICD codes owing to loss to follow-up or other reasons. Although our findings suggest a role for postdischarge VTE chemoprophylaxis for vascular patients at a high risk of VTE, prospective, randomized studies are needed to determine whether this practice is effective and safe after major vascular procedures.
CONCLUSIONS
The present comprehensive, retrospective, single-center study of VTE incidence, timing, and risk factors after major open vascular and endovascular surgery has demonstrated a high disease burden, underscoring the importance of VTE prophylaxis and awareness. Vascular surgery patients remain at high risk of the development of postoperative VTE; thus, additional study of the optimal chemoprophylaxis practices tailored to individual patient risk might improve outcomes. Future trials of vascular surgery patients should consider reporting VTE as a secondary outcome to further improve our understanding of the epidemiology of this important cause of postoperative morbidity and mortality. Finally, prospective, randomized trials of VTE prophylaxis, including extended duration VTE prophylaxis, for vascular surgery patients are warranted, given the high burden of postdischarge VTE.
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research: A single-center, retrospective cohort study
Key Findings: The rate of venous thromboembolism (VTE) was 3.4% after 1449 major vascular operations, with one third occurring after discharge. A Caprini score of ≥7 discriminated patients at high risk of VTE (7.7% vs 1.6%). Additional risk factors included chronic kidney disease stage 5 and a delay of the initiation of chemoprophylaxis of >4 days.
Take Home Message: For patients undergoing major vascular surgery, the preoperative assessment of the Caprini score can risk stratify for the development of VTE and inform postoperative chemoprophylaxis decision-making.
Footnotes
Author conflict of interest: none.
Presented at the Vascular Annual Meeting of the Society for Vascular Surgery, San Diego, Calif, August 18–21, 2021.
Additional material for this article may be found online at www.jvsvenous.org.
REFERENCES
- 1.Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med 2003;163:1711–7. [DOI] [PubMed] [Google Scholar]
- 2.Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrom J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost 2007;5: 692–9. [DOI] [PubMed] [Google Scholar]
- 3.Aziz F, Patel M, Ortenzi G, Reed AB. Incidence of postoperative deep venous thrombosis is higher among cardiac and vascular surgery patients as compared with general surgery patients. Ann Vasc Surg 2015;29:661–9. [DOI] [PubMed] [Google Scholar]
- 4.White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost 2003;90:446–55. [DOI] [PubMed] [Google Scholar]
- 5.Sweetland S, Green J, Liu B, Berrington de Gonzalez A, Canonico M, Reeves G, et al. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ 2009;339:b4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toth S, Flohr TR, Schubart J, Knehans A, Castello MC, Aziz F. A meta-analysis and systematic review of venous thromboembolism prophylaxis in patients undergoing vascular surgery procedures. J Vasc Surg Venous Lymphat Disord 2020;8:869–81.e2. [DOI] [PubMed] [Google Scholar]
- 7.Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(Suppl):e227S–77S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson DR, Morgano GP, Bennett C, Dentali F, Francis CW, Garcia DA, et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv 2019;3:3898–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramanan B, Gupta PK, Sundaram A, Lynch TG, MacTaggart JN, Baxter BT, et al. In-hospital and postdischarge venous thromboembolism after vascular surgery. J Vasc Surg 2013;57:1589–96. [DOI] [PubMed] [Google Scholar]
- 10.Naik R, Mandal I, Hampson A, Lane T, Adshead J, Rai BP, et al. The role of extended venous thromboembolism prophylaxis for major urological cancer operations. BJU Int 2019;124:935–44. [DOI] [PubMed] [Google Scholar]
- 11.Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008;372:31–9. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson M, Chan N, Bhagirath V, Ginsberg J. Prevention of venous thromboembolism in 2020 and beyond. J Clin Med 2020;9:2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahl V, Hu HM, Henke PK, Wakefield TW, Campbell DA Jr, Caprini JA. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg 2010;251:344–50. [DOI] [PubMed] [Google Scholar]
- 14.Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W, et al. Definition of major bleeding in clinical investigations of anti-hemostatic medicinal products in surgical patients. J Thromb Haemost 2010;8:202–4. [DOI] [PubMed] [Google Scholar]
- 15.Fluss R, Faraggi D, Reiser B. Estimation of the Youden index and its associated cutoff point. Biom J 2005;47:458–72. [DOI] [PubMed] [Google Scholar]
- 16.Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon 2005;51:70–8. [DOI] [PubMed] [Google Scholar]
- 17.Sutzko DC, Georgoff PE, Obi AT, Healy MA, Osborne NH. The association of venous thromboembolism chemoprophylaxis timing on venous thromboembolism after major vascular surgery. J Vasc Surg 2018;67:262–71.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grobben RB, Frima C, Nathoe HM, Leiner T, Kwakkel-van Erp JM, van Klei WA, et al. Pulmonary embolism after endovascular aortic repair, a retrospective cohort study. Eur J Vasc Endovasc Surg 2019;57:304–10. [DOI] [PubMed] [Google Scholar]
- 19.de Maistre E, Terriat B, Lesne-Padieu AS, Abello N, Bouchot O, Steinmetz EF. High incidence of venous thrombosis after surgery for abdominal aortic aneurysm. J Vasc Surg 2009;49:596–601. [DOI] [PubMed] [Google Scholar]
- 20.Pawlaczyk K, Gabriel M, Dzieciuchowicz L, Stanisic M, Begier-Krasinska B, Gabriel Z, et al. Post-operative venous thromboembolism in patients operated on for aorto-iliac obstruction and abdominal aortic aneurysm, and the application of pharmacological thromboprophylaxis. Eur J Vasc Endovasc Surg 2016;51:121–6. [DOI] [PubMed] [Google Scholar]
- 21.Hollyoak M, Woodruff P, Muller M, Daunt N, Weir P. Deep venous thrombosis in postoperative vascular surgical patients: a frequent finding without prophylaxis. J Vasc Surg 2001;34:656–60. [DOI] [PubMed] [Google Scholar]
- 22.Passman MA, Farber MA, Marston WA, Carlin RE, Owens LV, Burnham CB, et al. Prospective screening for postoperative deep venous thrombosis in patients undergoing infrainguinal revascularization. J Vasc Surg 2000;32:669–75. [DOI] [PubMed] [Google Scholar]
- 23.Iannuzzi JC, Young KC, Kim MJ, Gillespie DL, Monson JR, Fleming FJ. Prediction of postdischarge venous thromboembolism using a risk assessment model. J Vasc Surg 2013;58:1014–20.e1. [DOI] [PubMed] [Google Scholar]
- 24.Iannuzzi JC, Rickles AS, Kelly KN, Fleming FJ, Dolan JG, Monson JR, et al. Defining high risk: cost-effectiveness of extended-duration thromboprophylaxis following major oncologic abdominal surgery. J Gastrointest Surg 2014;18:60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molino D, De Lucia D, Gaspare De Santo N. Coagulation disorders in uremia. Semin Nephrol 2006;26:46–51. [DOI] [PubMed] [Google Scholar]
- 26.Assouad M, Eknoyan G. Does the choice of renal replacement therapy adversely affect the hypercoagulability associated with renal disease? Am J Nephrol 1998;18:175–8. [DOI] [PubMed] [Google Scholar]
- 27.Pulmonary Embolism Prevention (PEP) Trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: pulmonary embolism prevention (PEP) trial. Lancet 2000;355:1295–302. [PubMed] [Google Scholar]
- 28.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(Suppl):7S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandler DA, Martin JF. Autopsy proven pulmonary embolism in hospital patients: are we detecting enough deep vein thrombosis? J R Soc Med 1989;82:203–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michota FA. Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process. J Gen Intern Med 2007;22:1762–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bani-Hani M, Titi M, Al-Khaffaf H. Deep venous thrombosis after arterial surgery: a literature review. Eur J Vasc Endovasc Surg 2008;36: 565–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


