Abstract
Nano silver is one of the most widely used engineering nanomaterials with antimicrobial activity against bacteria, fungi, and viruses. However, the widespread application of nano silver preparations in daily life raises concerns about public health. Although several review articles have described the toxicity of nano silver to specific major organs, an updated comprehensive review that clearly and systematically outlines the harmful effects of nano silver is lacking. This review begins with the routes of exposure to nano silver and its distribution in vivo. The toxic reactions are then discussed on three levels, from the organ to the cellular and subcellular levels. This review also provides new insights on adjusting the toxicity of nano silver by changing their size and surface functionalization and their combination with other materials to form a composite formulation. Finally, future development, challenges, and research directions are discussed.
Keywords: nano silver, exposure routes, distribution, toxicity, mechanism
Graphical Abstract
Introduction
Nano silver refers to silver particles that have at least one dimension <100 nm on a three-dimensional scale.1 It presents unique physical and chemical properties such as its nano scale, high specific surface area, strong surface reactivity and strong interaction between particles,2 which makes nano silver widely used in various fields, such as imaging, diagnostics, and medicine,3 as well as in paints for the production and preservation of artistic work,4 in cosmetics to improve product safety and stability, in the processing industry as a packaging material to improve food freshness and prolonged release of biologically active ingredients,5 in agrifoods sector to fight against agricultural pest and pathogen, and support food production, in poultry industry sector to product vaccine, control animal skin infections, stimulate immune responses and diagnose.6–8 Compared to ordinary silver, nano silver has unique biological properties, such as stronger antibacterial activity. Nano silver can be added to toothpaste to achieve an oral sterilization activity, and can be prepared in gel form to treat cervicitis.9 Nano silver has quietly become more common in daily life, and people are increasingly exposed to products that contain nano silver. Nevertheless, individuals are not fully aware of the toxic effects of nano silver, the mechanisms involved in its toxic effects, and potential approaches to modify its toxicity profile are limited. Therefore, this article summarizes recent data to elaborate on these issues to provide a better understanding of the properties of nano silver and to provide insight into its real-life applications.
The methodology adopted to search and summarize this literature review was as follows: (1) an initial search based on key words, including “AgNPs”, “nano silver”, “silver nanoparticles”, “metal nanoparticles”, “toxicity”, “safety issues” and “hazard effects”, in PubMed, Science Direct, Crossref and other databases; (2) preliminary screening of literature according to the title, keywords, and guideline; (3) addition of new references like a snow ball from original references; and (4) summary and organization of literatures.
Different Routes of Exposure to Nano Silver and Its Distribution in the Body
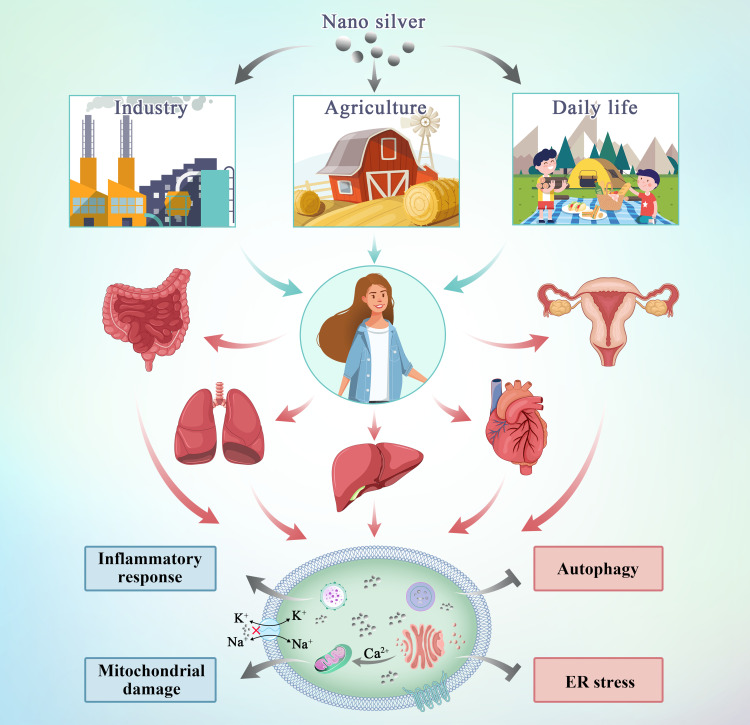
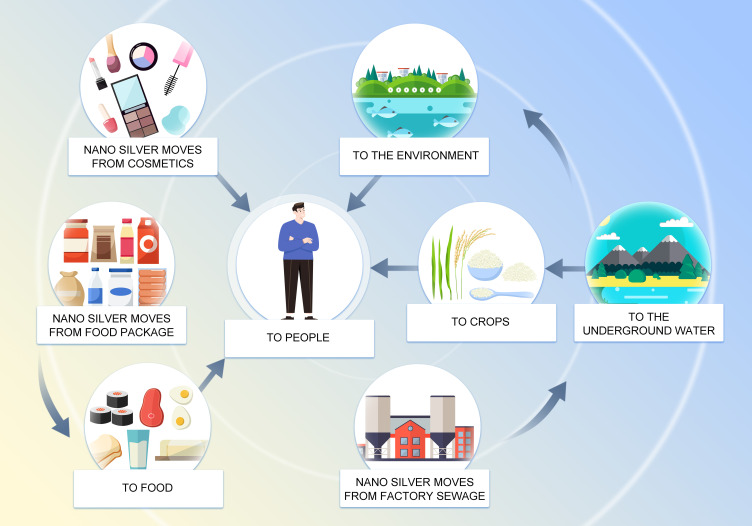
Due to the widespread use of nano silver in the environment and everyday products, individuals encounter these nanoparticles in a variety of ways. Nano silver mainly enters the human body via ingestion, inhalation, skin contact, and may directly enter the systemic circulation through intraperitoneal or intravenous injection.10 Silver nanomaterials are used in industrial production processes, resulting in a great amount of silver in the form of nanoparticles being discharged into groundwater with the release of industrial wastewater. Urban and industrial effluents enter the aquatic ecosystem and accumulate along trophic chains, which results in unconscious intake of nano silver.
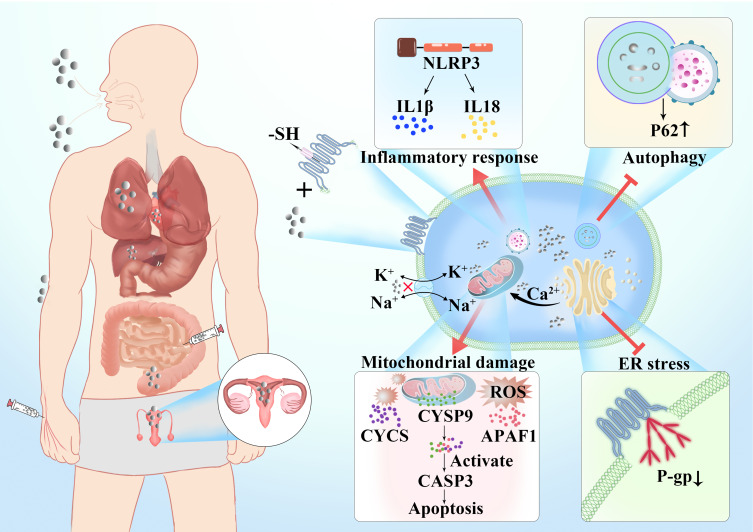
There are several ways for nano silver to enter the human body and exert its activity (Figure 1). After oral intake, nano silver is absorbed and distributed to organs.11 Studies have shown that after silver nanoparticles enter the body through the respiratory tract, they mainly accumulate in the lungs. After passing through the lung epithelial mucosal system, because of their small particle diameter, the nanoparticles are transported from the lungs to other tissues and diffuse throughout the body.12 The skin is the first barrier between the internal environment of the human body and the external environment as it is directly exposed to the air.13 Nanoparticles are able to penetrate both damaged and healthy skin. Nano silver penetrates the epidermis, diffuses to the dermis, and even the underlying structure of the skin such as the subcutaneous tissue.14 Therefore, there is a strong possibility that nano silver present in cosmetic wound dressings and antibacterial textiles would diffuse through the skin in large amounts. Nano silver injected through the abdominal cavity or intravenously enters the systemic circulation directly. After entering the systemic circulation, they are distributed to the heart, liver, kidney, brain, testes, and ovary and cause organ-specific pathophysiological effects.15
Figure 1.
Various routes of exposure to nano silver in human body.
Toxicity of Nano Silver to Organs
Nano silver enters the biological system through various ways. Routes of exposure and time, size and state of aggregation, and doses of silver nanoparticles link to their bioavailability, biodistribution, and pathological symptoms. To explore the toxicity of nano silver to organs, different animal models are established and employed (Table 1). 16–19
Table 1.
Toxicity of Nano Silver in Different Organs
| Animals | Dose | End-Point | Toxic Effect | Related Organs | Mechanism | Ref. |
|---|---|---|---|---|---|---|
| Healthy adult male mice | 2 mg·kg−1 | 35 days | Alterations in the ultrastructure of the liver; focal hepatocytes necrosis and apoptosis | Liver | Free radical production and oxidative stress induction | [16] |
| Healthy female New Zealand rabbits | 0.1 g·kg−1 | 24 and 72 h | Ultrastructural pathological changes and the promoted cytotoxic reactions | Generative organ | - | [17] |
| Zebrafish | 8, 45, and 70 µg·L−1 | 30 days | Reversible damage to the mucosal epithelium of the gills, and to a lesser degree to the intestinal tissue | Gut, liver and gills | - | [18] |
| Drosophila melanogaster | 50 mg·L−1 | 10, 20, and 30 days | Behavioral abnormalities and altered metabolic activity at early larval stage | Fat body and wing imaginal disc | Impaired essential metabolic components, and increased reactive oxygen species | [19] |
Intestine and Liver
Compared to ordinary materials, nano-silver materials have better barrier function, antibacterial ability, and higher mechanical strength, and are widely used in various daily necessities and packaging materials.20 After oral intake, silver nanoparticles reach the stomach rapidly, where they dissolve under acidic conditions. After passing through the intestine, the properties of nano silver are affected. Once absorbed by the intestinal mucosa, nano silver reaches the liver.21
Studies have shown that after a 24-hour intravenous injection of nano silver in rats, higher levels of silver can be detected in the liver, feces, and colon.22 Approximately 30 to 99% of the nano-silver dose will accumulate and sequester in the liver after being administered to the body. This leads to a decrease in delivery to the target diseased tissues and potentially an increase in toxicity at the hepatocyte level.23
Research by Jia et al found that nano silver increased the level of protein phosphorylation of normal human colonic epithelial cells NCM460 and human colorectal cancer HCT116 and promoted the expression of the p53 and Bcl-2-associated X protein (Bax). When the exposure to nano silver was higher than 15 µg·mL−1, the survival rate of both cell types began to decrease. The study also showed that nano silver can promote the downregulation of B cell lymphoma/leukemia-2 (Bcl-2), leading to an increase in the Bax/Bcl-2 ratio and activation of p21, further accelerating cell death.24 D’Arcy et al showed that silver nanoparticles can induce focal hepatocyte necrosis and apoptosis.25 The apoptosis induced in the liver of mice treated with 10-nm silver nanoparticles indicates that nano silver may induce intercellular stress leading to cell death. Silver nanoparticles may also lead to the destruction of the endoplasmic reticulum (ER) and partial degranulation, causing severe liver tissue and ultrastructural changes that affect the metabolism and function of the liver and other important organs.16
Lungs
Animal and human studies have shown that inhaled nanoparticles are less efficiently eliminated by macrophage removal mechanisms than other large particles. Nano silver is retained in the lungs and causes damage, or is transported through the circulation, nervous system, and to distal tissues and organs.26 The lung and liver are the main target tissues after exposure to silver nanoparticles via inhalation for 90 days, and the resulting toxicity is dose-dependence.27
The chemical characterization of silver nanoparticles endows them with redox ability. The reaction involves the elements Ag and H2O2 to generate hydroxyl and oxidize silver ions.28 This mechanism allows silver nanoparticles to induce oxidative stress, and this interaction with cellular matter interacts to produce oxidants.29 Surface oxidation of silver nanoparticles may contribute to the release of silver ions, thus amplifying toxicity. Mitochondrial function is impaired when lung epithelial cells are exposed to nano silver. In the process, NADPH oxidase (NOX) activity increases, leading to damage to oxidative stress. Tight junction proteins in the lung epithelium are a known target of oxidative stress damage, which alters epithelial transport processes and damages the homeostasis and integrity of the lung epithelial barrier.30
Heart
Lin et al evaluated the physiological toxicity of nano silver for the heart and concluded that nano silver acts quickly and inhibits the activity of rectifying the inward potassium current (IK1) and inward sodium current (INa) channels of cardiomyocytes, leading to rapid collapse of cardiac cell transmembrane potential (TMP) with subsequent loss of excitability. Toxic effects of nano silver on similar channels of the cardiac conduction system and autonomic nerves can also be expected, but the exact mechanism of action needs further study.31
Recombinant myosin heavy chain 6 (MYH6) is a cardiomyocyte marker gene that encodes the alpha heavy chain subunit of cardiac myosin.32 The treatment of silver nanoparticles triggers abnormal changes in ISL1, MYH6, and alpha heavy chain subunits, which seriously damage the process of embryogenesis, germ layer, and heart development. The steps of nano silver to sabotage cardiomyocytes are as follows: (1) silver ions are slowly released from silver nanoparticles; (2) protein crowns are formed by the combination of silver nanoparticles with different serum proteins; and (3) changes occur in the total surface charge of silver nanoparticles, which will disrupt the ion balance in the body and affect the electrophysiology of cardiomyocytes.33
Reproductive Organs
The rapid development of the nanotechnology industry has brought many potential risks that are of serious concern. In order to safely use nanomaterials in consumer products and pharmaceuticals, regulatory health risk assessment of such particles should be mandatory, including the potential impact on reproduction and fertility.34
Silver nanoparticles are able to cross the blood-testis barrier and locate directly in the testes after intraperitoneal or intravenous injection.35 The human testicular embryonic carcinoma cell line (NT2) Ntera2 and primary testicular cells from C57BL6 mice were used as cell models to simulate the repair state and oxidative damage of human testicular cells exposed to silver nanoparticles of 20 and 200 nm in size. Nano silver exhibited strong cytotoxicity and cytostatic properties, causing apoptosis, necrosis, and reduction of proliferation in a concentration- and time-dependent manner. Silver nanoparticles with a size of 200 nm even caused DNA strand breaks in NT2 cells.36
Toxicity of Nano Silver to Cells
At the cellular level, nano silver generates a large amount of reactive oxygen species (ROS) by activating the inhibitory kappa B kinase/transcription factor nuclear factor-kappa B (IKK/NF-κB) signaling pathway, destroying the cytoskeleton and DNA, damaging DNA repair enzymes, and upregulating autophagy to activate p53-dependent or mitochondrial-dependent apoptosis pathways to induce cell apoptosis and exert its cytotoxic effects.37 At the genetic level, a lower dose of silver nanoparticles will lead to changes in human skin fibroblast energy metabolism, oxidative stress, changes in the cell cycle, and in other related genes. Even very low doses of nano silver are capable of causing structural or functional damage to target cells.38 As shown in Table 2, the following mainly describes the cytotoxicity of silver nanoparticles based on the progressive effect induced on cell layers.24,30–32,36 Figure 2 shows the potential mechanisms of nano silver-induced cytotoxicity in the cell.
Table 2.
Toxicity of Nano Silver in Different Cells
| Cells | Dose (μg·mL−1) | End-Point | Toxic Effect | Ref. |
|---|---|---|---|---|
| NCM460 | 3–60 | 24 h | Increased intracellular ROS content | [24] |
| HCT116 | ||||
| A549 cells | 0.5–5 | Oxidative damage | [30] | |
| Cardiomyocytes | 0.001–1 | 1 h | Rapid collapses of TMP and loss of excitability | [31] |
| Human embryonic stem cells | 0.001–0.1 | 18 days | Changes in endoderm-derived hepatocyte differentiation | [32] |
| NTERA-2 | 10–100 | 24, 48, and 72 h | Cytotoxicity and cell inhibition, DNA damage | [36] |
| Primary testicular cells |
Figure 2.
Mechanisms of entry of silver nanoparticles into the organism and potential mechanisms of nano silver-induced cytotoxicity in the cell.
Effects on the Cell Membrane
Silver nanoparticles can interact with membrane proteins and activate signaling pathways, thereby inhibiting cell proliferation. They directly interact with the macromolecular structure of living cells and affect cellular metabolism.39 Nano silver interferes with Na and K ion channels on the cell membrane, causing an imbalance in the cell membrane potential, or reacts with sulfhydryl (-SH) protein on the cell membrane destroying the barrier function and the material exchange function of the cell membrane, resulting in direct cell necrosis.40 Gunawan et al used attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy to detect the toxic mechanism of silver nanoparticles in bacteria. The results showed that nanoparticles caused major structural changes in the cell membrane components and interfered with the peptides and lipid chains (phospholipids) as well as sugar and phosphate groups leading to the breakdown of the cell structure.41
Anuj et al explored a scheme to improve the bactericidal effect of linezolid on gram-negative bacteria with nano silver. The change in the zeta-potential caused by the interaction between nano silver and bacterial membrane protein enhanced the permeability of the bacterial cell membrane and the alteration of integrity, which allowed linezolid to penetrate into the cell, thereby increasing the cytoplasmic concentration of linezolid to an effective level. This study demonstrated that silver nanoparticles can change the permeability of the cell membrane, causing the leakage of intracellular material or the entry of extracellular material to cause cell death.42
Effects on Endocytosis
The cell membrane only allows for free diffusion of oxygen, carbon dioxide, water, small hydrophobic or non-polar molecules, and 10–30 nm particles. Various particles enter the cell through different cell internalization pathways. These internalization pathways are classified as endocytosis. The endocytosis mechanism includes phagocytosis and pinocytosis.43 Depending on the size of the vesicles and the proteins involved in the formation of the vesicle, pinocytosis can be further divided into four mechanisms, which include (1) macropinocytosis; (2) clathrin-mediated endocytosis; (3) caveolae-mediated endocytosis; and (4) non-clathrin- and non-caveolin-mediated endocytosis.44
Once the nano silver is internalized, it will migrate to the mitochondria and nucleus and induce changes in cell morphology, oxidative stress, DNA damage, inflammation, genotoxicity, mitochondrial dysfunction, and subsequent apoptosis or necrosis.45
Free nano silver in the extracellular fluid causes only a limited release of ROS in the cell.46 Silver nanoparticles that enter the cell through endocytosis were then transferred to the lysosome. Under the action of the acidic environment of the lysosome, the oxidative dissolution releases silver ions, and the cell itself degrades and releases nano silver, causing a higher degree of ROS release, thereby destroying the lysosome. In the cell membrane, particles escape from the lysosomal sequestration into the cytosol, and then target other subcellular compartments, resulting in a higher degree of cytotoxicity.47 Bouallegui et al used the uptake inhibitor amantadine to evaluate the effects of blocking clathrin-mediated endocytosis on nano silver protein-induced toxicity in mussel gills and digestive glands. Blocking clathrin-mediated endocytosis may protect cells from nano silver toxicity, which indicates that this uptake of clathrin-mediated endocytosis is a key mechanism for silver nanoparticles to exert their toxic effects.48
In a recent study, using 15, 50, and 100 nm silver nanoparticles, Chen et al showed that the smallest 15 nm silver nanoparticles exerted the strongest cytotoxicity. The 100-nm silver nanoparticles aggregate and cannot pass through the plasma membrane, and thus cannot be captured by endocytosis or cause toxicity to the cell.49
Effects Mediated by Autophagy
Autophagy is a mechanism in which cellular materials are delivered to lysosomes for degradation, leading to the basic turnover of cellular components, and providing energy and macromolecular precursors.50 Autophagy is activated at the basic level under normal physiological conditions, selectively removing stress-mediated protein aggregates, and removing damaged organelles. Autophagy also actively participates in the elimination of cell invaders and maintaining intracellular balance. Studies have shown that exposure of cells to silver nanoparticles activates the cellular defense mechanism defined as autophagy. However, silver nanoparticle-activated autophagy results in defective autophagosome-lysosome fusion, which leads to autophagy defects and increases cell toxicity.51
Ubiquitination confers autophagy selectivity and regulates the stabilization, activation, and transport of proteins involved in the autophagy pathway.52 Silver nanoparticles have been shown to increase the level of enzymes involved in ubiquitination processes or weaken ubiquitination.53 The reactivity of silver nanoparticles can interfere with the formation of ubiquitin. The interference of silver nanoparticles on ubiquitination may be the cause of autophagy defects and cytotoxicity caused by silver nanoparticles.54,55 As a multi-domain adaptor protein, p62 binds microtubule-associated protein 1 light chain 3 (LC3) and ubiquitin. The accumulation of the p62 subunit caused by defective autophagy may also be a potential cause of silver nanoparticle cytotoxicity.56
Lee et al showed for the first time in vitro that nano silver led to the formation of numerous cytoplasmic acid vesicle organelles (AVOs) (autophagosomes and autolysates). In addition, exposure to nano silver resulted in a dose-dependent increase in the conversion of LC3-I to LC3-II and a dose-dependent accumulation of p62 protein, indicating that although nano silver activates autophagy, it may eventually lead to the interruption of autophagy flow.50
Subcellular Cytotoxicity
Effects on Mitochondria
Previous investigations have shown that exposure of cells to silver nanoparticles can cause mitochondrial damage. Silver nanoparticles are capable of inducing mitochondrial swelling, increasing intracellular ROS levels, and disrupting mitochondrial membrane potentials, whose breakdown leads to mitochondrial pathway-induced apoptosis.57,58 Silver nanoparticles induce changes in the morphology and structure of mitochondria. The expression of nuclear fission-related protein 1 (p-Drp1) (Ser616) was significantly up-regulated, and the expression of mitochondrial biogenesis protein (PGC-1α) in cells treated with nano silver decreased, indicating that silver nanoparticles induce cytotoxicity by targeting mitochondria, leading to the destruction of mitochondrial function and the damage to the mitochondrial structure and morphology that interferes with mitochondrial dynamics and biogenesis.59
The mitochondrial respiratory chain is the main source of ROS in cells. Under normal circumstances, ROS are balanced by the mitochondrial antioxidant system. In the process of cellular stress, mitochondria may malfunction, with increased ROS production, leading to cell damage and cell death.60
Holmila et al studied the effects of silver nanoparticles and ionizing radiation on the mitochondrial redox state and function in lung cell lines (A549, BEAS-2B, Calu-1, and NCI-H358). In Calu-2 cells, exposure to nano silver reduced cell proliferation by inducing cell cycle arrest. Nano silver increased mitochondrial reactive oxygen and protein oxidation in sensitive cell lines in a time- and dose-dependent manner, but did not significantly change mitochondrial respiration mechanisms.61
To demonstrate that nano silver would induce cell death through both the apoptotic p53 pathway and the independent p53 pathway, a model system containing two osteosarcoma cell lines was used and the cell response after nano silver administration was tested.62 Loss of mitochondrial membrane potential, increased leakage of cytochrome C protein into the cytoplasm, and increased ROS levels were detected in both U2OS cells harboring sufficient levels of p53 and in Saos-2 cells lacking functional p53, indicating that nano silver in both cell lines induced mitochondrial stress.63,64 Although nano-silver treatment activates p53 in p53-containing osteosarcoma cells, the main property of nano silver is to induce mitochondrial stress, thus driving cancer cell p53-independent apoptosis.
Effects on the Endoplasmic Reticulum
The ER is a multifunctional subcellular compartment in charge of protein synthesis, assembly and modification, lipid biosynthesis, protein output, calcium ion storage and its regulation and release to the cytoplasm, and redox signals.65 A series of protein-related activities are extremely susceptible to events that interfere with ER homeostasis, leading to accumulation of unfolded and misfolded proteins in the ER. During the process of solving protein folding defects and restoring ER homeostasis, an unfolded protein response is activated, involving three signal branches: RNA-dependent protein kinase-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE 1) and X box binding protein-1 (XBP-1), and activation of transcription factor 6 (ATF6). Many studies have shown that exposure of the body to metal nanoparticles induces the ER stress signaling pathway.
P-glycoprotein (P-gp) is an ATP-binding cassette transporter located on the plasma membrane, which is intrinsically linked to the occurrence of multidrug-resistant cancer.66 Silver nanoparticles of 75 nm in size induce stress in the endoplasmic reticulum in drug-resistant cells, reducing the number of correctly folded P-gp of the plasma membrane. The endoplasmic reticulum cavity is rich in calcium, which is essential for the sustained effect of endoplasmic reticulum protein quality control mechanisms, such as the calnexin/calreticulin cycle. Treatment of drug-resistant cells with 75-nm silver particles will deplete the calcium levels of the endoplasmic reticulum, which may be the cause of the induction of endoplasmic reticulum stress.67
Prolonged exposure of human neuroblastoma cell line (SH-SY5Y) to nano silver has been reported to increase the length of the ER-mitochondria contact site. The expression of phosphatase and tensin homolog deleted on chromosome ten (PTEN) protein in ER and mitochondria-associated membranes (MAMs) is enhanced, and the function of inositol-3-phosphate receptor (IP3R) is altered. Transfer of Ca2+ from the endoplasmic reticulum to the mitochondria increases, and finally the overload of mitochondrial Ca2+ triggers cell death through the mitochondrial apoptosis pathway.68
Effects on Lysosomes
Lysosomes contain a variety of acid hydrolases, such as cathepsins, which are involved in autophagy and phagocytosis. Autophagy is related to the removal of intracellular (endogenous) debris, and phagocytosis digests exogenous substances.69
The release of silver ions induces only a modest generation of ROS; in contrast, the simultaneous release of silver nanoparticles and silver ions (oxidative dissolution of silver nanoparticles in an acid lysosome environment) induces higher levels of ROS.70 The generation of a large amount of ROS destroys the integrity of the lysosomal membrane and allows the release of silver nanoparticles from the enclosed vesicle into the cytosol. Lysosomal dysfunction due to loss of integrity of the lysosomal membrane or reduced acidity also leads to the release of silver nanoparticles and is closely associated with impaired autophagosome-lysosome fusion.71
Subcytotoxic concentrations of silver nanoparticles (≤10 μg·mL−1) induce lysosomal dysfunction in liver cancer cells, leading to activation of NOD-like receptor protein 3 (NLRP3) inflammasome-dependent caspase-1. The activation of inflammatory mediators is a biological response induced by silver nanoparticles. NLRP3 inflammatory mediators directly or indirectly interact with nano silver to produce a cellular inflammatory response that leads to cytotoxicity.72
Transcription factor EB (TFEB) plays a key role in the regulation of lysosomal function.73 The activity of TFEB is regulated by its subcellular location. Under certain conditions, such as starvation or lysosomal dysfunction, TFEB transfers to the nucleus and activates the transcription of its target genes. After A549 cells were exposed to nano silver, the gene and protein levels of TFEB binding protein in the cytosol and nucleus decreased, indicating that TFEB expression was transcriptionally inhibited and affected the normal activity of lysosomes.72
Promising Methods to Overcome Nano Silver-Induced Cytotoxicity
The cytotoxicity of nano silver is associated with the available concentration of silver nanoparticles, the duration of activity, the size of the particle, the presence or absence of stabilizers, the type of stabilizer, and the pH of the environment. In addition, the toxic reactions of different types of body cells to nano silver also differ. Below are several approaches that have been proposed to overcome the cytotoxicity induced by nano silver based on the research progress in the recent years.
Particle Size
The toxicity of nano silver is closely related to the size of the particles. Most silver nanoparticles are toxic to the human body, and it is precisely because of their small particle size that they can penetrate human tissues. Zhang et al studied two sizes of nano silver to examine the differences in neurotoxic effects of (20- and 70-nm silver nanoparticles). The results show that 20-nm and 70-nm silver nanoparticles significantly reduce neuronal cell viability, and 20-nm silver nanoparticles exert stronger toxic effects than 70-nm-silver nanoparticles.74
Zhang et al studied the effects of two sizes of silver nanoparticles (10- and 50-nm) on the nitrogen fixation of Azotobacter vinelandii. The marked decrease in the number of bacterial cells associated with the smaller silver nanoparticles indicated nano silver with smaller particle size exerted higher toxicity. Cytometry analysis further confirmed this finding. At the same concentration of 10 mg·L−1 for 12 h of incubation, the apoptotic rates of cells treated with 10- and 50-nm silver nanoparticles were 20.23% and 3.14%, respectively. Observation under the scanning electron microscope of cells revealed obvious damage to the cell structure, indicating that the toxicity of silver nanoparticles was size dependent.75 Given the above findings and to ensure the desired effects of silver nanoparticles, the influence of the size of silver nanoparticles on their toxicity was briefly summarized in Table 3.16–18,74,75
Table 3.
The Influence of Size Distribution of Silver Nanoparticles on Their Toxicity
| Cells | Size (nm) | Time | Toxic Effect | Ref. |
|---|---|---|---|---|
| Kupffer cells | 10 | 35 days | Destruction and reduction of ER, mitochondrial swelling, and cytoplasmic vacuolation | [16] |
| Mucosa epithelial cells | 20–40 | 24 and 72 h | Loose and detached | [17] |
| 50 | 30 days | Sub-epithelial edema, hyperplasia, lamellar fusion, and the reduction of the length of intestinal villi | [18] | |
| Neuronal cells | 20, 70 | 7 days, 24 h | Inhibition in dopamine efflux in both mature and developing neurons | [74] |
| Azotobacter vinelandii | 10, 50 | 12 h | Production of ROS | [75] |
Surface Functionalization
Surface modification of nanoparticles is an effective way to reduce the toxicity of nanoparticles.76 Studies have shown that coated and modified nanoparticles do not lose their original characteristics; however, by modifying the surface of the nanoparticles, the inherent toxicity of the nanoparticles could be reduced, and the biocompatibility of the nanoparticles could be improved at the same time.77,78 The surface functionalization may enable further applications of nano silver in various fields.
Borowik et al synthesized silver nanoparticles using thiobarbituric acid and 11-mercaptoundecanoic acid residues (MUA). Silver nanoparticles coated with MUA were compatible with acridine mutagens. Interaction with ICR-191 could regulate cell viability by influencing mutagens in cells.79
Das et al compared the effect of silver nanoparticles, polyethylene glycol (PEG)-coated silver nanoparticles and bovine serum albumin (BSA) functionalized silver nanoparticles on peripheral blood mononuclear cells in vitro, and found that compared with silver nanoparticles, PEG-coated silver nanoparticles and BSA-functionalized silver nanoparticles produced fewer superoxide anions, nitric oxide, intracellular ROS, reduced glutathione (GSH), oxidized glutathione, and NADPH oxidase. Further surface functionalized silver nanoparticles exhibited less toxicity than unmodified silver nanoparticles.80 Hamilton et al adsorbed silver nanoparticles onto carbon nanotubes and graphene oxide. In vitro cellular experiments showed that silver-carbon nanotube-hydroxyapatite and silver-graphene oxide are less toxic than silver nano particles.81
Compound Preparations
Nano silver has many excellent properties, but premature release and potential toxicity due to accumulation restrain its further application.82 To make better use of this nanomaterial of great potential, nano silver composite preparations that are in combination with other materials have been proposed. However, most of the studies in this field are focused on the functionality of silver nanoparticle preparations; meanwhile, the human safety of silver nanoparticle composite preparations has not drawn much attention. The formulation of silver nanoparticle composite preparations may also be an approach to overcome the toxicity of silver nanoparticles.83,84
Although nano silver is almost nontoxic at low concentrations, the accumulation of silver nanoparticles in mammalian cells may cause side effects and infections, such as silver burns and silver poisoning, by interacting with different organelles and subcellular components of the body.85 Thus, to overcome this problem, the synthesis of nanocomposite materials has been proposed, which consist of loading silver nanoparticles on a magnetic core. Magnetic core-based nanocomposite materials allow to effectively recover residual particles from the medium. In addition, modification of silver nanoparticles on magnetic particles can also provide stability as a result of their magnetic dispersion. After the silver nanoparticles are deposited on a cobalt core, the cell survival rate is improved, and the toxicity of the nanocomposite particles is even lower than that of the silver nanoparticles.86
Madla-Cruz et al synthesized a nano-silver/carboxymethyl cellulose composite using a green synthesis method and then used MTT reduction assay to evaluate the effects of the silver nanoparticles/carboxymethyl cellulose composite on the viability of normal human gingival fibroblasts (HGF). The viability of HGF was not affected at the experimental concentration that inhibits the growth of microorganisms or reduces the area of the biofilm. When the concentration of the composite is less than 15 g·mL−1, there were no significant toxic effects on HGF cells.87
To overcome the diffusion of nano silver when injected locally at the target site during positioning and labeling therapy, Lee et al combined silver nanoparticles with porous materials to inhibit the diffusion of the nanoparticles and enhance their biocompatibility to iodine. The mixed complex of cesium-nano silver-pSiMP, and subsequent immunotoxicity experiments showed that no hepatotoxicity was observed in mice treated with nano silver-pSiMP, and the main inflammatory cytokine TNF-α level in serum did not change significantly. At 8 and 24 h after injection, the nano silver-pSiMPs treatment group did not present activated lymphocytes or histological changes.88
Yu et al synthesized a composite material of cellulose silver nanoparticles. Even when the concentration of the composite treatment reached 1000 µg·mL−1, the number of viable cells did not decrease significantly. Compared to the control group, the cell viability of normal epithelial cells (FHC) of the human colon incubated with the cellulose nanofibrils (CNF)/AgNP complex (50–1000 µg·mL−1) did not decrease significantly. These results indicate that the CNF/AgNP complex was not toxic to human cells within 24 h.89
Summary and Outlook
This review introduces the in vivo toxicity of nano silver under different exposure routes, and introduces the mechanism induced by silver nanoparticle cytotoxicity from the outer to inner cell structures. Nano silver is introduced to the human body in by different routes, causing damage to various body systems, including the digestive system, respiratory system, and reproductive system.90 At present, most studies on the toxicity of silver nanoparticles are carried out through in vitro cell tests and animal tests, and there are still some challenges. For example, it is not clear to what extent the intact nano silver itself is absorbed by the human body, or whether the nano silver is altered when exposed to the physiological environment, whether the silver ions released from the nano silver are absorbed, or whether the observed effect is induced by the nano silver itself.91 An inflammatory reaction is caused by ions released by the nano silver or nanoparticle itself. Thus, there is no clear approach to elucidate toxicity mechanisms specific to nano silver.
To effectively evaluate the functional effects of nano silver, a variety of related technologies could be employed to characterize silver nanoparticles and to attempt to overcome the limitations of using a single particle characterization method alone.92 The interaction between nano silver and biological fluids will inevitably change the physical characteristics and uptake or absorption of silver nanoparticles. To determine the potential long-term effects of nano silver in a more realistic situation, the characteristics of silver nanoparticles should be evaluated in an appropriate medium. Multigenerational studies are needed to evaluate intergenerational effects in higher mammalian systems.
Because of the versatility of silver nanoparticle compounds in terms of size, physical properties, and the ability to interact and bind with other compounds, their applicability in different fields is immeasurable. These properties also led to some critical issues such as toxicity to human and animal cells, safe use, long-term exposure, and environmental safety. In future nanotoxicology research, persistent in-depth research will be requested to reveal the ultimate mystery of the toxicity mechanisms induced by nano silver. These findings will help to promote the future applications and development of nano silver-loaded preparations and allow for the use of preventive measures against the toxic risks.
Acknowledgments
This work was supported by the Jiangxi Provincial Department of Science and Technology (20212ACB206004, 20202ACBL216015 and 20202BABL206157), the National Natural Science Foundation of China (No. 81760639), Young Jinggang Scholar of Jiangxi Province (Jing Zhang) and New Century Talents Project of Jiangxi Province (2017082, Xiang Li and 2020028, Jing Zhang), Jiangxi University of Chinese Medicine 1050 Youth Talent Project (Jing Zhang and Xiang Li), and Jiangxi University of Chinese Medicine Science and Technology Innovation Team Development Program.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bakand S, Hayes A. Toxicological considerations, toxicity assessment, and risk management of inhaled nanoparticles. Int J Mol Sci. 2016;17(6):929. doi: 10.3390/ijms17060929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Matteis V. Exposure to inorganic nanoparticles: routes of entry, immune response, biodistribution and in vitro/in vivo toxicity evaluation. Toxics. 2017;5(4):29. doi: 10.3390/toxics5040029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SH, Jun BH. Silver nanoparticles: synthesis and application for nanomedicine. Int J Mol Sci. 2019;20(4):865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sendra M, Yeste MP, Gatica JM, Moreno-Garrido I, Blasco J. Direct and indirect effects of silver nanoparticles on freshwater and marine microalgae (Chlamydomonas reinhardtii and Phaeodactylum tricornutum). Chemosphere. 2017;179:279–289. doi: 10.1016/j.chemosphere.2017.03.123 [DOI] [PubMed] [Google Scholar]
- 5.Zhang XF, Liu ZG, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17(9):1534. doi: 10.3390/ijms17091534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang C, Mission EG, Ahmad Fuaad AA-H, Shaalan M. Nanoparticle tools to improve and advance precision practices in the agrifoods sector towards sustainability - A review. J Clean Prod. 2021;293:126063. [Google Scholar]
- 7.Bansod SD, Bawaskar MS, Gade AK, Rai MK. Development of shampoo, soap and ointment formulated by green synthesised silver nanoparticles functionalised with antimicrobial plants oils in veterinary dermatology: treatment and prevention strategies. IET Nanobiotechnol. 2015;9(4):165–171. doi: 10.1049/iet-nbt.2014.0042 [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Yuying X, Tang M. Research progress on internal toxicity and the toxic mechanism of silver nanoparticles. Asian J Ecotoxicol. 2018;1:5060. [Google Scholar]
- 9.Abd El-Ghany WA, Shaalan M, Salem HM. Nanoparticles applications in poultry production: an updated review. Worlds Poult Sci J. 2021;77(4):1001–1025. doi: 10.1080/00439339.2021.1960235 [DOI] [Google Scholar]
- 10.Rezvani E, Rafferty A, McGuinness C, Kennedy J. Adverse effects of nanosilver on human health and the environment. Acta Biomater. 2019;94:145–159. doi: 10.1016/j.actbio.2019.05.042 [DOI] [PubMed] [Google Scholar]
- 11.Ferdous Z, Nemmar A. Health impact of silver nanoparticles: a review of the biodistribution and toxicity following various routes of exposure. Int J Mol Sci. 2020;21(7):2375. doi: 10.3390/ijms21072375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gambardella C, Costa E, Piazza V, et al. Effect of silver nanoparticles on marine organisms belonging to different trophic levels. Mar Environ Res. 2015;111:41–49. doi: 10.1016/j.marenvres.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 13.Wong R, Geyer S, Weninger W, Guimberteau JC, Wong JK. The dynamic anatomy and patterning of skin. Exp Dermatol. 2016;25(2):92–98. doi: 10.1111/exd.12832 [DOI] [PubMed] [Google Scholar]
- 14.George R, Merten S, Wang TT, Kennedy P, Maitz P. In vivo analysis of dermal and systemic absorption of silver nanoparticles through healthy human skin. Australas J Dermatol. 2014;55(3):185–190. doi: 10.1111/ajd.12101 [DOI] [PubMed] [Google Scholar]
- 15.Sarhan OM, Hussein RM. Effects of intraperitoneally injected silver nanoparticles on histological structures and blood parameters in the albino rat. Int J Nanomedicine. 2014;9:1505–1517. doi: 10.2147/IJN.S56729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Doaiss A, Jarrar Q, Moshawih S. Hepatic histopathological and ultrastructural alterations induced by 10 nm silver nanoparticles. IET Nanobiotechnol. 2020;14(5):405–411. doi: 10.1049/iet-nbt.2020.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen D, Yang Z. Tissue toxicity following the vaginal administration of nanosilver particles in rabbits. Regen Biomater. 2015;2(4):261–265. doi: 10.1093/rb/rbv016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pecoraro R, Marino F, Salvaggio A, et al. Evaluation of chronic nanosilver toxicity to Adult Zebrafish. Front Physiol. 2017;8:1011. doi: 10.3389/fphys.2017.01011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Raj A, Shah P, Agrawal N. Sedentary behavior and altered metabolic activity by AgNPs ingestion in Drosophila melanogaster. Sci Rep. 2017;7(1):15617. doi: 10.1038/s41598-017-15645-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schluesener JK, Schluesener HJ. Nanosilver: application and novel aspects of toxicology. Arch Toxicol. 2013;87(4):569–576. doi: 10.1007/s00204-012-1007-z [DOI] [PubMed] [Google Scholar]
- 21.Ishizaka T, Nagano K, Tasaki I, et al. Optimization and evaluation of pretreatment method for sp-ICP-MS to reveal the distribution of silver nanoparticles in the body. Nanoscale Res Lett. 2019;14(1):180. doi: 10.1186/s11671-019-3016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juling S, Bachler G, von Gotz N, et al. In vivo distribution of nanosilver in the rat: the role of ions and de novo-formed secondary particles. Food Chem Toxicol. 2016;97:327–335. doi: 10.1016/j.fct.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 23.Zhang YN, Poon W, Tavares AJ, McGilvray ID, Chan WCW. Nanoparticle-liver interactions: cellular uptake and hepatobiliary elimination. J Control Release. 2016;240:332–348. doi: 10.1016/j.jconrel.2016.01.020 [DOI] [PubMed] [Google Scholar]
- 24.Jia M, Zhang W, He T, et al. Evaluation of the genotoxic and oxidative damage potential of silver nanoparticles in human NCM460 and HCT116 cells. Int J Mol Sci. 2020;21(5):1618. doi: 10.3390/ijms21051618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43(6):582–592. doi: 10.1002/cbin.11137 [DOI] [PubMed] [Google Scholar]
- 26.Jo MS, Kim JK, Kim Y, et al. Mode of silver clearance following 28-day inhalation exposure to silver nanoparticles determined from lung burden assessment including post-exposure observation periods. Arch Toxicol. 2020;94(3):773–784. doi: 10.1007/s00204-020-02660-2 [DOI] [PubMed] [Google Scholar]
- 27.Sung JH, Ji JH, Park JD, et al. Subchronic inhalation toxicity of silver nanoparticles. Toxicol Sci. 2009;108(2):452–461. doi: 10.1093/toxsci/kfn246 [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Li S, Fang Y, Zhu Z. Boosting antibacterial activity with mesoporous silica nanoparticles supported silver nanoclusters. J Colloid Interface Sci. 2019;555:470–479. doi: 10.1016/j.jcis.2019.08.009 [DOI] [PubMed] [Google Scholar]
- 29.Abdal Dayem A, Hossain MK, Lee SB, et al. The role of Reactive Oxygen Species (ROS) in the biological activities of metallic nanoparticles. Int J Mol Sci. 2017;18(1):120. doi: 10.3390/ijms18010120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garces M, Magnani ND, Pecorelli A, et al. Alterations in oxygen metabolism are associated to lung toxicity triggered by silver nanoparticles exposure. Free Radic Biol Med. 2021;166:324–336. doi: 10.1016/j.freeradbiomed.2021.02.008 [DOI] [PubMed] [Google Scholar]
- 31.Lin CX, Yang SY, Gu JL, Meng J, Xu HY, Cao JM. The acute toxic effects of silver nanoparticles on myocardial transmembrane potential, INa and IK1 channels and heart rhythm in mice. Nanotoxicology. 2017;11(6):827–837. doi: 10.1080/17435390.2017.1367047 [DOI] [PubMed] [Google Scholar]
- 32.Szaraz P, Librach M, Maghen L, et al. In vitro differentiation of first trimester human umbilical cord perivascular cells into contracting cardiomyocyte-like cells. Stem Cells Int. 2016;2016:7513252. doi: 10.1155/2016/7513252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu B, Yin N, Yang R, Liang S, Liang S, Faiola F. Silver nanoparticles (AgNPs) and AgNO3 perturb the specification of human hepatocyte-like cells and cardiomyocytes. Sci Total Environ. 2020;725:138433. doi: 10.1016/j.scitotenv.2020.138433 [DOI] [PubMed] [Google Scholar]
- 34.Elsharkawy EE, Abd El-Nasser M, Kamaly HF. Silver nanoparticles testicular toxicity in rat. Environ Toxicol Pharmacol. 2019;70:103194. doi: 10.1016/j.etap.2019.103194 [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Qu G, Su L, et al. Evaluation of the biological fate and the transport through biological barriers of nanosilver in mice. Curr Pharm Des. 2013;19(37):6691–6697. doi: 10.2174/1381612811319370012 [DOI] [PubMed] [Google Scholar]
- 36.Asare N, Instanes C, Sandberg WJ, et al. Cytotoxic and genotoxic effects of silver nanoparticles in testicular cells. Toxicology. 2012;291(1–3):65–72. doi: 10.1016/j.tox.2011.10.022 [DOI] [PubMed] [Google Scholar]
- 37.Danila OO, Berghian AS, Dionisie V, et al. The effects of silver nanoparticles on behavior, apoptosis and nitro-oxidative stress in offspring Wistar rats. Nanomedicine. 2017;12(12):1455–1473. doi: 10.2217/nnm-2017-0029 [DOI] [PubMed] [Google Scholar]
- 38.Shati AA, Elsaid FG. Biosynthesized silver nanoparticles and their genotoxicity. J Biochem Mol Toxicol. 2020;34(1):e22418. doi: 10.1002/jbt.22418 [DOI] [PubMed] [Google Scholar]
- 39.McShan D, Ray PC, Yu H. Molecular toxicity mechanism of nanosilver. J Food Drug Anal. 2014;22(1):116–127. doi: 10.1016/j.jfda.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dos Santos CA, Seckler MM, Ingle AP, et al. Silver nanoparticles: therapeutical uses, toxicity, and safety issues. J Pharm Sci. 2014;103(7):1931–1944. doi: 10.1002/jps.24001 [DOI] [PubMed] [Google Scholar]
- 41.Gunawan C, Faiz MB, Mann R, et al. Nanosilver targets the bacterial cell envelope: the link with generation of reactive oxygen radicals. ACS Appl Mater Interfaces. 2020;12(5):5557–5568. doi: 10.1021/acsami.9b20193 [DOI] [PubMed] [Google Scholar]
- 42.Anuj SA, Gajera HP, Hirpara DG, Golakiya BA. Bacterial membrane destabilization with cationic particles of nano-silver to combat efflux-mediated antibiotic resistance in Gram-negative bacteria. Life Sci. 2019;230:178–187. doi: 10.1016/j.lfs.2019.05.072 [DOI] [PubMed] [Google Scholar]
- 43.Zhang T, Wang L, Chen Q, Chen C. Cytotoxic potential of silver nanoparticles. Yonsei Med J. 2014;55(2):283–291. doi: 10.3349/ymj.2014.55.2.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panzarini E, Mariano S, Carata E, Mura F, Rossi M, Dini L. Intracellular transport of silver and gold nanoparticles and biological responses: an update. Int J Mol Sci. 2018;19(5):1305. doi: 10.3390/ijms19051305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao BH, Tsai JC, Chen CW, Yan SJ, Wang YJ. Mechanisms of silver nanoparticle-induced toxicity and important role of autophagy. Nanotoxicology. 2016;10(8):1021–1040. doi: 10.1080/17435390.2016.1189614 [DOI] [PubMed] [Google Scholar]
- 46.Zhang B, Liu H, Huang X, Dong C, Ren J. Size distribution of nanoparticles in solution characterized by combining resonance light scattering correlation spectroscopy with the maximum entropy method. Anal Chem. 2017;89(22):12609–12616. doi: 10.1021/acs.analchem.7b04166 [DOI] [PubMed] [Google Scholar]
- 47.Chen LQ, Fang L, Ling J, Ding CZ, Kang B, Huang CZ. Nanotoxicity of silver nanoparticles to red blood cells: size dependent adsorption, uptake, and hemolytic activity. Chem Res Toxicol. 2015;28(3):501–509. doi: 10.1021/tx500479m [DOI] [PubMed] [Google Scholar]
- 48.Bouallegui Y, Ben Younes R, Oueslati R, Sheehan D. Redox proteomic insights into involvement of clathrin-mediated endocytosis in silver nanoparticles toxicity to Mytilus galloprovincialis. PLoS One. 2018;13(10):e0205765. doi: 10.1371/journal.pone.0205765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528–542. doi: 10.1038/nrc.2017.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee YH, Cheng FY, Chiu HW, et al. Cytotoxicity, oxidative stress, apoptosis and the autophagic effects of silver nanoparticles in mouse embryonic fibroblasts. Biomaterials. 2014;35(16):4706–4715. doi: 10.1016/j.biomaterials.2014.02.021 [DOI] [PubMed] [Google Scholar]
- 51.Xue Y, Wang J, Huang Y, et al. Comparative cytotoxicity and apoptotic pathways induced by nanosilver in human liver HepG2 and L02 cells. Hum Exp Toxicol. 2018;37(12):1293–1309. doi: 10.1177/0960327118769718 [DOI] [PubMed] [Google Scholar]
- 52.Chen RH, Chen YH, Huang TY. Ubiquitin-mediated regulation of autophagy. J Biomed Sci. 2019;26(1):80. doi: 10.1186/s12929-019-0569-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verano-Braga T, Miethling-Graff R, Wojdyla K, et al. Insights into the cellular response triggered by silver nanoparticles using quantitative proteomics. ACS Nano. 2014;8(3):2161–2175. doi: 10.1021/nn4050744 [DOI] [PubMed] [Google Scholar]
- 54.Mangini V, Dell’Aglio M, De Stradis A, et al. Amyloid transition of ubiquitin on silver nanoparticles produced by pulsed laser ablation in liquid as a function of stabilizer and single-point mutations. Chemistry. 2014;20(34):10745–10751. doi: 10.1002/chem.201402934 [DOI] [PubMed] [Google Scholar]
- 55.Sharma HS, Muresanu DF, Lafuente JV, Sjoquist PO, Patnaik R, Sharma A. Nanoparticles exacerbate both ubiquitin and heat shock protein expressions in spinal cord injury: neuroprotective effects of the proteasome inhibitor carfilzomib and the antioxidant compound H-290/51. Mol Neurobiol. 2015;52(2):882–898. doi: 10.1007/s12035-015-9297-9 [DOI] [PubMed] [Google Scholar]
- 56.Lippai M, Low P. The role of the selective adaptor p62 and ubiquitin-like proteins in autophagy. Biomed Res Int. 2014;2014:832704. doi: 10.1155/2014/832704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skalska J, Dabrowska-Bouta B, Frontczak-Baniewicz M, Sulkowski G, Struzynska L. A low dose of nanoparticulate silver induces mitochondrial dysfunction and autophagy in adult rat brain. Neurotox Res. 2020;38(3):650–664. doi: 10.1007/s12640-020-00239-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yakop F, Abd Ghafar SA, Yong YK, et al. Silver nanoparticles Clinacanthus Nutans leaves extract induced apoptosis towards oral squamous cell carcinoma cell lines. Artif Cells Nanomed Biotechnol. 2018;46(sup2):131–139. doi: 10.1080/21691401.2018.1452750 [DOI] [PubMed] [Google Scholar]
- 59.Li J, Zhang B, Chang X, et al. Silver nanoparticles modulate mitochondrial dynamics and biogenesis in HepG2 cells. Environ Pollut. 2020;256:113430. doi: 10.1016/j.envpol.2019.113430 [DOI] [PubMed] [Google Scholar]
- 60.Jezek J, Cooper KF, Strich R. Reactive oxygen species and mitochondrial dynamics: the yin and yang of mitochondrial dysfunction and cancer progression. Antioxidants. 2018;7(1). doi: 10.3390/antiox7010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holmila RJ, Vance SA, King SB, Tsang AW, Singh R, Furdui CM. Silver nanoparticles induce mitochondrial protein oxidation in lung cells impacting cell cycle and proliferation. Antioxidants. 2019;8(11). doi: 10.3390/antiox8110552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kovacs D, Igaz N, Keskeny C, et al. Silver nanoparticles defeat p53-positive and p53-negative osteosarcoma cells by triggering mitochondrial stress and apoptosis. Sci Rep. 2016;6(1):27902. doi: 10.1038/srep27902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim S, Choi JE, Choi J, et al. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol In Vitro. 2009;23(6):1076–1084. doi: 10.1016/j.tiv.2009.06.001 [DOI] [PubMed] [Google Scholar]
- 64.Avalos A, Haza AI, Mateo D, Morales P. Cytotoxicity and ROS production of manufactured silver nanoparticles of different sizes in hepatoma and leukemia cells. J Appl Toxicol. 2014;34(4):413–423. doi: 10.1002/jat.2957 [DOI] [PubMed] [Google Scholar]
- 65.McCaffrey K, Braakman I. Protein quality control at the endoplasmic reticulum. Essays Biochem. 2016;60(2):227–235. doi: 10.1042/EBC20160003 [DOI] [PubMed] [Google Scholar]
- 66.Mollazadeh S, Sahebkar A, Hadizadeh F, Behravan J, Arabzadeh S. Structural and functional aspects of P-glycoprotein and its inhibitors. Life Sci. 2018;214:118–123. doi: 10.1016/j.lfs.2018.10.048 [DOI] [PubMed] [Google Scholar]
- 67.Gopisetty MK, Kovacs D, Igaz N, et al. Endoplasmic reticulum stress: major player in size-dependent inhibition of P-glycoprotein by silver nanoparticles in multidrug-resistant breast cancer cells. J Nanobiotechnology. 2019;17(1):9. doi: 10.1186/s12951-019-0448-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li L, Cui J, Liu Z, et al. Silver nanoparticles induce SH-SY5Y cell apoptosis via endoplasmic reticulum- and mitochondrial pathways that lengthen endoplasmic reticulum-mitochondria contact sites and alter inositol-3-phosphate receptor function. Toxicol Lett. 2018;285:156–167. doi: 10.1016/j.toxlet.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 69.Liu N, Tang M. Toxic effects and involved molecular pathways of nanoparticles on cells and subcellular organelles. J Appl Toxicol. 2020;40(1):16–36. doi: 10.1002/jat.3817 [DOI] [PubMed] [Google Scholar]
- 70.Lacave JM, Vicario-Pares U, Bilbao E, et al. Waterborne exposure of adult zebrafish to silver nanoparticles and to ionic silver results in differential silver accumulation and effects at cellular and molecular levels. Sci Total Environ. 2018;642:1209–1220. doi: 10.1016/j.scitotenv.2018.06.128 [DOI] [PubMed] [Google Scholar]
- 71.Lin YX, Wang Y, Qiao SL, et al. pH-sensitive polymeric nanoparticles modulate autophagic effect via lysosome impairment. Small. 2016;12(21):2921–2931. doi: 10.1002/smll.201503709 [DOI] [PubMed] [Google Scholar]
- 72.Miyayama T, Fujiki K, Matsuoka M. Silver nanoparticles induce lysosomal-autophagic defects and decreased expression of transcription factor EB in A549 human lung adenocarcinoma cells. Toxicol In Vitro. 2018;46:148–154. doi: 10.1016/j.tiv.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 73.Nnah IC, Wang B, Saqcena C, et al. TFEB-driven endocytosis coordinates MTORC1 signaling and autophagy. Autophagy. 2019;15(1):151–164. doi: 10.1080/15548627.2018.1511504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang B, Liu N, Liu QS, Zhang J, Zhou Q, Jiang G. Silver nanoparticles induce size-dependent and particle-specific neurotoxicity to primary cultures of rat cerebral cortical neurons. Ecotoxicol Environ Saf. 2020;198:110674. doi: 10.1016/j.ecoenv.2020.110674 [DOI] [PubMed] [Google Scholar]
- 75.Zhang L, Wu L, Si Y, Shu K. Size-dependent cytotoxicity of silver nanoparticles to Azotobacter vinelandii: growth inhibition, cell injury, oxidative stress and internalization. PLoS One. 2018;13(12):e0209020. doi: 10.1371/journal.pone.0209020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yan L, Zhao F, Li S, Hu Z, Zhao Y. Low-toxic and safe nanomaterials by surface-chemical design, carbon nanotubes, fullerenes, metallofullerenes, and graphenes. Nanoscale. 2011;3(2):362–382. doi: 10.1039/C0NR00647E [DOI] [PubMed] [Google Scholar]
- 77.Kuchur OA, Tsymbal SA, Shestovskaya MV, Serov NS, Dukhinova MS, Shtil AA. Metal-derived nanoparticles in tumor theranostics: potential and limitations. J Inorg Biochem. 2020;209:111117. doi: 10.1016/j.jinorgbio.2020.111117 [DOI] [PubMed] [Google Scholar]
- 78.Pham XH, Kim J, Jun BH. Silver nano/microparticles: modification and applications 2.0. Int J Mol Sci. 2020;21(12):4395. doi: 10.3390/ijms21124395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borowik A, Butowska K, Konkel K, et al. The impact of surface functionalization on the biophysical properties of silver nanoparticles. Nanomaterials. 2019;9(7):973. doi: 10.3390/nano9070973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Das B, Tripathy S, Adhikary J, et al. Surface modification minimizes the toxicity of silver nanoparticles: an in vitro and in vivo study. J Biol Inorg Chem. 2017;22(6):893–918. doi: 10.1007/s00775-017-1468-x [DOI] [PubMed] [Google Scholar]
- 81.Hamilton RF, Wu Z, Thakkar M, Holian A, Mitra S. Modification of nano-silver bioactivity by adsorption on carbon nanotubes and graphene oxide. Inhal Toxicol. 2018;30(11–12):429–438. doi: 10.1080/08958378.2018.1547334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Z, Liu C, Bai J, et al. Silver nanoparticle gated, mesoporous silica coated gold nanorods (AuNR@MS@AgNPs): low premature release and multifunctional cancer theranostic platform. ACS Appl Mater Interfaces. 2015;7(11):6211–6219. doi: 10.1021/acsami.5b00368 [DOI] [PubMed] [Google Scholar]
- 83.Chen X, Huang X, Zheng C, Liu Y, Xu T, Liu J. Preparation of different sized nano-silver loaded on functionalized graphene oxide with highly effective antibacterial properties. J Mater Chem B. 2015;3(35):7020–7029. doi: 10.1039/C5TB00280J [DOI] [PubMed] [Google Scholar]
- 84.Cao W, Zhang Y, Wang X, et al. Development of a novel resin-based dental material with dual biocidal modes and sustained release of Ag(+) ions based on photocurable core-shell AgBr/cationic polymer nanocomposites. J Mater Sci Mater Med. 2017;28(7):103. doi: 10.1007/s10856-017-5918-3 [DOI] [PubMed] [Google Scholar]
- 85.Fu CW, Horng JL, Tong SK, et al. Exposure to silver impairs learning and social behaviors in adult zebrafish. J Hazard Mater. 2021;403:124031. doi: 10.1016/j.jhazmat.2020.124031 [DOI] [PubMed] [Google Scholar]
- 86.Kanwal Z, Raza MA, Riaz S, et al. Synthesis and characterization of silver nanoparticle-decorated cobalt nanocomposites (Co@AgNPs) and their density-dependent antibacterial activity. R Soc Open Sci. 2019;6(5):182135. doi: 10.1098/rsos.182135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Madla-Cruz E, De la Garza-ramos M, Romo-Saenz CI, et al. Antimicrobial activity and inhibition of biofilm formation in vitro and on human dentine by silver nanoparticles/carboxymethyl-cellulose composites. Arch Oral Biol. 2020;120:104943. doi: 10.1016/j.archoralbio.2020.104943 [DOI] [PubMed] [Google Scholar]
- 88.Lee EM, Lee J, Kim Y, et al. Hybrid composite of silver nanoparticle-porous silicon microparticles as an image-guided localization agent for computed tomography scan of the lungs. ACS Biomater Sci Eng. 2020;6(8):4390–4396. doi: 10.1021/acsbiomaterials.0c00611 [DOI] [PubMed] [Google Scholar]
- 89.Yu Z, Wang W, Kong F, Lin M, Mustapha A. Cellulose nanofibril/silver nanoparticle composite as an active food packaging system and its toxicity to human colon cells. Int J Biol Macromol. 2019;129:887–894. doi: 10.1016/j.ijbiomac.2019.02.084 [DOI] [PubMed] [Google Scholar]
- 90.Volker C, Oetken M, Oehlmann J. The biological effects and possible modes of action of nanosilver. Rev Environ Contam Toxicol. 2013;223:81–106. doi: 10.1007/978-1-4614-5577-6_4 [DOI] [PubMed] [Google Scholar]
- 91.Cameron SJ, Hosseinian F, Willmore WG. A current overview of the biological and cellular effects of nanosilver. Int J Mol Sci. 2018;19(7):2030. doi: 10.3390/ijms19072030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li J, Zhu Z, Liu F, et al. DNA-mediated morphological control of silver nanoparticles. Small. 2016;12(39):5449–5487. doi: 10.1002/smll.201601338 [DOI] [PubMed] [Google Scholar]