Abstract
Cells in tissues experience a plethora of forces that regulate their fate and modulate development and homeostasis. Cells sense mechanical cues through localized mechanoreceptors or by influencing cytoskeletal or plasma membrane organization. Cells translate force and modulate their behavior through a process termed mechanotransduction. Cells tune their tension upon exposure to chronic force by engaging cellular machinery that modulates actin tension, which in turn stimulates matrix remodeling and stiffening and alters cell-cell adhesions until cells achieve a state of tensional homeostasis. Loss of tensional homeostasis can be induced through oncogene activity and/or tissue fibrosis, and accompanies tumor progression and associates with increased cancer risk. The mechanical stresses that develop in tumors can also foster the mesenchymal-like transdifferentiation of cells to induce a stem-like phenotype that contributes to their aggression, metastatic dissemination, and treatment resistance. Thus, strategies that ameliorate tumor mechanics may comprise an effective strategy to prevent aggressive tumor behavior.
Introduction
Cells within tissues sense and integrate mechanical cues that regulate their fate and direct tissue-specific development. Such forces also play a critical role in maintaining tissue structure, function and homeostasis. The types of forces include tension, compression and shear stress (Box 1). These forces cross length scales, ranging from the tissue to the cellular and subcellular level. At the tissue-level, such forces include the tensile stress urothelial cells experience in response to bladder filling (Merrill et al., 2016), the compression stress osteocytes within the bone endure following weight bearing (Qin et al., 2020), the shear stress endothelial cells within blood vessels experience in response to blood flow (Kutys and Chen, 2016) and the cyclic shear stress alveolar epithelial cells experience during breathing (Yang et al., 2020). Cellular-level forces include sound pressure wave-induced activation of ion channels in cochlear hair cells within the inner ear canal (Goutman, Elgoyhen and Gómez-Casati, 2015), compression stress exerted on luminal epithelial cells within the breast ducts by adjacent contracting myoepithelial cells (Adriance et al., 2005), and cell-cell forces cells exert against each other within developing expanding tissue masses (Bazellières et al., 2015). Cells within tissues also sense the viscoelastic properties of the extracellular matrix (ECM), as well as the material properties of neighboring cells, through specialized protein receptors, and respond to these physical properties by generating actomyosin-dependent tension. Cells respond to externally applied force through localized or diffuse mechanically responsive sensors that translate these cues into biochemical signals through an array of adaptor proteins and second messengers. In this review, we link these forces in directing stem cell behavior during development and tissue-specific differentiation. We review how dysregulation of tissue-level force increases risk to malignancy and promotes tumor progression, and can induce a stem-like phenotype in tumor cells to drive tumor aggression and treatment resistance. We summarize many of the key studies of tissue mechanics in stem cell fate, development and cancer (Table 1).
Box 1. Force definitions.
Force is a push or pull on an object, that causes an object with mass to change its velocity. It has magnitude and direction, making it a vectoral quantity. It is measured in newtons and represented by the symbol F.
Mechanical stress is the objects internal resistance to an external force. It is measured as force per unit area (N/m2, where N is newtons and m is meters).2 There are three types: tension, compression and shear. Tension (pull) and compression (push) stresses occur when forces act perpendicular to the area of the object. Shear stress occurs when two forces act parallel to the area of the object. Example of initial (dark blue) and final (light blue) geometries of objects following stress.
Strain is a measure of deformation to the object under force relative to its original length.
Elasticity is the ability of a deformed object to return to its original shape after removal of the force. The modulus of elasticity is defined as the ratio of stress to strain. Young’s modulus (E) describes the elasticity of an object subjected to tensile and compression stress. Shear modulus (G) describes the elasticity of an object subjected to shear stress.
Stiffness describes the elasticity of an object. It is measured in pascals (Pa). Stiffness relates to elasticity, though stiffness may change as force increases.
Viscoelasticity is the property of materials that possess both elastic and viscous properties when deformed. The strain of viscous materials is time-dependent, while strain of elastic materials is time-independent.
Table 1.
Key studies of tissue mechanics in stem cell fate, development and cancer
| Force and embryogenesis | ||
|---|---|---|
| Early development | [29, 30, 32–34] | |
| Gastrulation | [35, 38–40] | |
| Organogenesis | [44–46] | |
| Force and tissue development | ||
| Vasculature | [47, 50–54] | |
| Branching morphogenesis | [57, 58, 60] | |
| Stem cell specification and maintenance | [38, 40, 48, 49, 64, 65, 84] | |
| Force and malignant transformation | ||
| Tissue fibrosis | [86–88] | |
| Breast density and cancer risk | [89, 91] | |
| Force and tumor aggression | ||
| ECM properties | [107, 108, 117, 118] | |
| Membrane | [24, 96] | |
| Contractility | [98] | |
| Force and tumor cell fate | ||
| Cancer stem cells | [120, 121, 124] | |
| Epithelial-mesenchymal transition | [40, 124, 129, 134] | |
| Tumor aggression and metastasis | [121, 124] | |
Mechanical cues direct cell fate and shape tissue development and homeostasis. Here, Hayward et al. discuss how dysregulation of tissue forces increases risk to malignancy and promotes tumor aggression, and induces a stem-like phenotype in tumor cells to drive tumor aggression and treatment resistance.
Mechanosensing and mechanotransduction
Cells have evolved mechanisms that permit them to sense force either through protein-based mechanosensors or cytoskeletal and membrane-mediated molecular responses. Cells then translate these mechanical cues into biochemical signals that then elicit a biological response through a process termed mechanotransduction. Cells sense mechanical cues in multiple ways (Fig. 1a). For instance, ion channels such as Piezo1 and Piezo2, open in response to a variety of mechanical stimuli including stretch (Douguet and Honoré, 2019). In other cases, key mechanosensing structures are cell-surface receptors that enable cell-ECM and cell-cell interactions, such as integrins in focal adhesions (Kechagia, Ivaska and Roca-Cusachs, 2019) and cadherins in adheren junctions (Angulo-Urarte, van der Wal and Huveneers, 2020). Both of these interactions are connected to the actin cytoskeleton, allowing transmission of mechanical cues between the cell exterior and interior. Starting at the cell exterior, the first elements that encounter biophysical cues are ECM molecules. For example, forces of 80–200 pN can extend fibronectin (FN)III domains, which contain integrin-binding sites (Oberhauser et al., 2002; Oberhauser et al., 1998). This is a form of mechanotransduction as mechanical stress exposes cryptic binding sites in these domains that facilitate integrin adhesion and promote further fibronectin fibrillogenesis (Sechler et al., 2001). Similarly, fibrinogen can also undergo unfolding under forces of ∼100 pN (Brown et al., 2007). Fibrinogen unfolding can activate its integrin receptor, macrophage-1 antigen (MAC1) to promote inflammatory pathways (Deng et al., 2011). Next, mechanosensors on the cell surface modulate their structure in response to these extracellular stresses or changes to ECM stiffness. In particular, integrins undergo conformational changes that shifts the integrin from a folded-to-stretched state in response to a stiff ECM, in order to facilitate ligand binding (Fig. 1b) (Case and Waterman, 2015). Mechanical load may influence the lifetime of ECM-integrin adhesions. For instance, some integrins under increasing tensile force, such as αIIbβ3, exhibit slip-bond behavior in which bond lifetime is shortened, and occurs at unbinding forces of 50–100 pN (Litvinov et al., 2011). While others, such as α5β1, exhibit catch-bond behavior in which bond lifetime is prolonged, and occurs at forces of 10–30 pN (Kong et al., 2009; Roca-Cusachs et al., 2009). In this way, mechanosensors respond by adopting force-induced conformational changes that promote mechanically adhesive or signaling functions.
Figure 1. Mechanosensing and mechanotransduction mechanisms in cells.
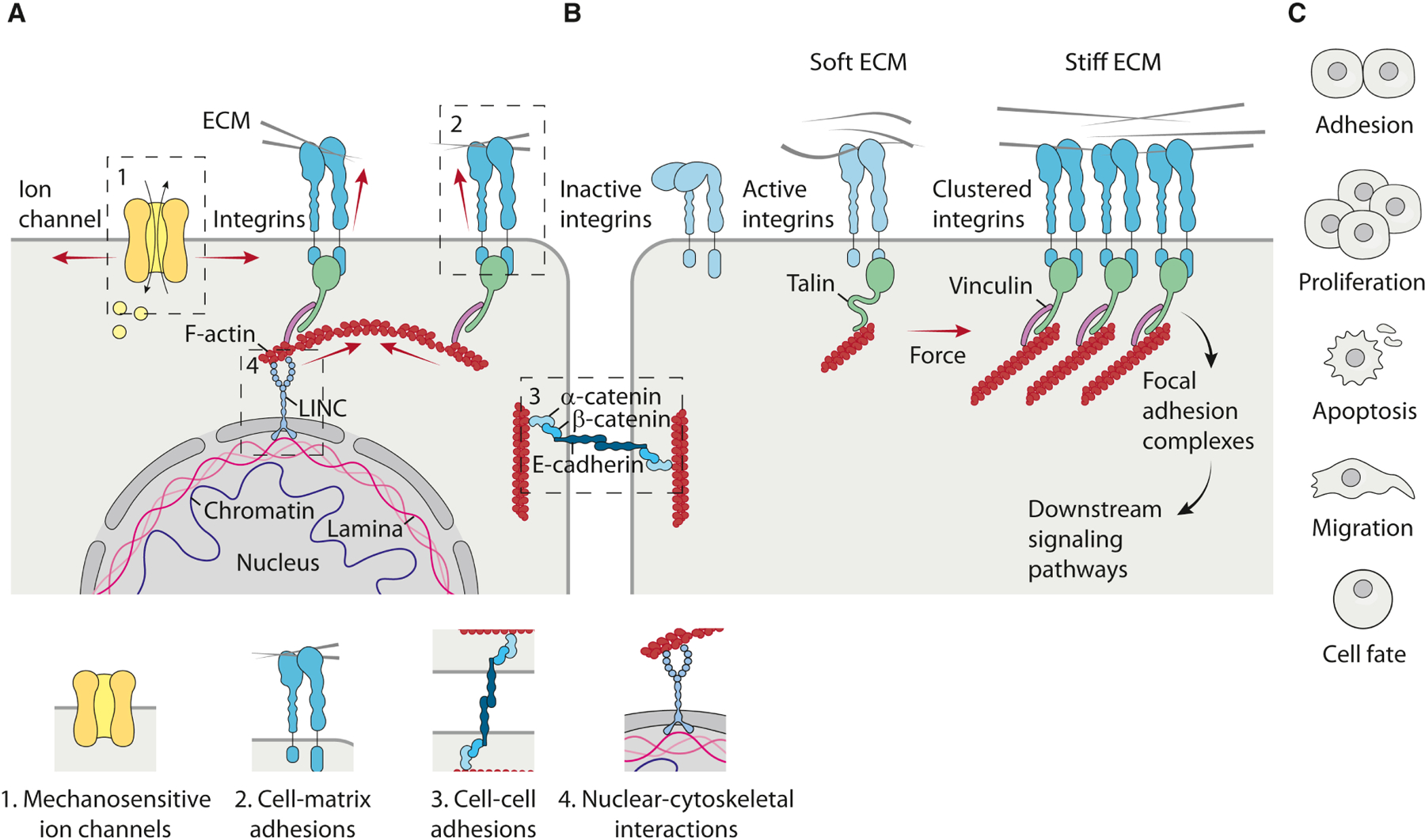
a, Cells have evolved to sense force either through protein-based mechanosensors or cytoskeletal and membrane-mediated molecular responses, in a process known as mechanosensing. Cells sense mechanical cues in multiple ways including; mechanosensitive ion channels, integrins in focal adhesions, cadherins in adheren junctions and nuclear membrane proteins in cytoskeletal interactions. b, Cells then translate these mechanical cues into biochemical signals that then elicit a biological response through a process termed mechanotransduction. In the case of focal adhesions, the initial activation and binding of integrins to the ECM can occur in the presence of low resisting forces (soft ECM), leading to the formation of a transient focal complex, where the integrin intracellular domain is weakly attached to F-actin. In the presence of high resisting forces (stiff ECM), the recruitment of adaptor proteins promotes integrin clustering, actin remodeling and myosin-mediated contraction to increase internal cellular tension. Force-bearing proteins such as talin undergo force-induced conformational changes, which promotes the local recruitment of other proteins to reinforce the linkage to actin, such as vinculin, to regulate local signaling to provide a positive feedback leading to further reinforcement and maturation of focal adhesions. Multiple mechanisms then contribute to regulate downstream signaling pathways mediating the biological responses to ECM mechanical cues. c, Examples of biological responses to ECM-derived mechanical cues occurring in different cell types. ECM cues influence cell-cell adhesions, regulates proliferation and apoptosis, directs cell migration and alters cell fate.
Cells next translate these physical cues into biochemical signals through an array of adaptor proteins and second messengers. In the context of integrins, initial activation and binding to the ECM may occur in a compliant matrix (soft ECM), leading to the formation of transient focal points where the intracellular domain of the integrin is loosely attached to the actin cytoskeleton. With increasing substrate rigidity (stiff ECM), integrin clustering initiates the recruitment of focal adhesion signaling molecules to trigger signaling cascades, and cells remodel their cytoskeleton in order to adjust reciprocal intracellular tension. Many proteins within integrin adhesions undergo mechanosensitive unfolding to promote the further growth and reinforcement of focal adhesions. For example, forces of 12 pN can unfold talin to reveal cryptic binding sites and cause vinculin binding (del Rio et al., 2009), which once bound to talin promotes the recruitment of intracellular plaque proteins at the cytoplasmic tail of the β-integrins and fosters the assembly of focal adhesions (Bays and DeMali, 2017). Other focal adhesion–associated proteins also exhibit force-induced conformational changes, such as p130Cas, which is extended by mechanical stress to reveal a domain that can be phosphorylated by SRC family kinases (Sawada et al., 2006). Multiple mechanisms then contribute to the regulation of downstream signaling pathways mediating the biological response to mechanical cues.
Cells then translate these mechanosignals into either a transient response or sustained cellular behavior (Fig. 1c). For example, in response to ECM rigidity, receptor tyrosine kinase (RTK) signaling to GTPase RAS enhances the activation of MAPKs including ERK to promote the proliferation and survival of lung (Chess, Toia and Finkelstein, 2000) and mammary epithelial cells (MEC) (Paszek et al., 2005). Force-dependent integrin signaling also permits cells to rapidly respond to dynamic forces and modify their behavior accordingly. For instance, mechanically-activated ERK cooperates with other kinases, such as SRC and FAK, to induce cell proliferation or sustain cell survival (Chaturvedi, Marsh and Basson, 2007), as shown for MAPK-dependent growth of keratinocytes in response to mechanical stretch (Kippenberger et al., 2000), and the load-dependent survival of osteocytes (Plotkin et al., 2005). Cells can also generate sustained responses to mechanical stress by altering their gene expression. For example, high tensile stress can activate fibroblasts into ECM-producing and modifying cells, myofibroblasts, that remodel and stiffen the surrounding ECM (Piersma, Hayward and Weaver, 2020). The upregulation of ECM-related proteins can create a positive feedback mechanism whereby cells responding to mechanical force modify the composition, organization, and elasticity of their surrounding environment. This mechanism of mechanoreciprocity equips cells with the ability to adjust their behavior to correspond with the physical nature of the ECM.
Force, embryogenesis and tissue development
Force and embryogenesis
Force directs cell state transitions that are critical for embryogenesis. Membrane tension, facilitated by protein complexes that connect the plasma membrane to the actin cytoskeleton, controls the first fate decision of pluripotent stem cells (PSCs) as they transition from naïve to primed pluripotency (Bergert et al., 2020; De Belly et al., 2020). Decreased membrane tension associated with cell spreading promotes endocytosis-mediated ERK signaling (De Belly et al., 2020), known to drive the transition from naïve to primed pluripotency in the mouse embryo (Nichols et al., 2009). Forces are also critical for driving the cell rearrangements necessary to facilitate growth and expansion of the early embryo. Extensive work in Drosophila has revealed that multicellular actomyosin cables sustain mechanical tension and mediate the formation of rosettes to drive axis elongation (Blankenship et al., 2006; Fernandez-Gonzalez et al., 2009). In the mouse embryo, remodeling of the polar trophectoderm has been implicated in mechanically driving the rearrangement of epiblast cells that occurs during implantation (Weberling and Zernicka-Goetz, 2021). Together, these studies illustrate that forces regulate both the physical rearrangements of the early embryo and the molecular signaling events that govern pluripotent cell fate transitions.
As embryogenesis progresses, pluripotent epiblast cells undergo fate specification and physically segregate to form the three primary germ lineages during gastrulation. Atomic force microscopy (AFM) measurements of zebrafish germ layer progenitors revealed that differences in cortical tension and adhesive properties are responsible for segregation of the germ layers (Krieg et al., 2008). More recent studies have identified an evolutionarily conserved mechanism through which mechanical tension at adheren junctions promotes the release of β-catenin to stimulate mesoderm specification (Brunet et al., 2013; Röper et al., 2018; Muncie et al., 2020). Intriguingly, a tensile actomyosin ring is generated at the border of embryonic and extraembryonic tissues in avian embryos to drive the fluid tissue flows associated with gastrulation (Saadaoui et al., 2020). Taken together, these studies indicate that tensile forces may simultaneously regulate both cell fate specification and morphogenesis. In addition to cell-generated forces, the mechanical properties of the ECM also influence germ layer specification. Soft substrates (Elastic Modulus (E) = 102 Pa) prime human embryonic stem cells (hESCs) to undergo mesoderm specification by enhancing Wnt/β-catenin signaling and sequestering the transcriptional repressor Kaiso outside of the nucleus (Przybyla, Lakins and Weaver, 2016). Conversely, stiff substrates (E = 105 Pa) limit mesoderm specification by promoting β-catenin degradation and suppressing Wnt signaling. Looking forward, the emergence of a number of complementary model systems for studying gastrulation present new opportunities for exploring the role of force in early embryogenesis. Geometric confinement of hESCs on elastic substrates enables measurement and manipulation of the cell-cell and cell-ECM forces that regulate cell fate specification (Muncie et al., 2019; Muncie et al., 2020). Microfluidic control of cellular organization and signaling facilitates amnion development (Zheng et al., 2019), and may prove an ideal system for defining the mechanics of amniotic cavity formation. Unconfined aggregates of ESCs, termed gastruloids, recapitulate key features of gastrulation and somitogenesis, and are likely to unveil critical roles for force and adhesion in the allocation and organization of germ layer progenitors subsequent to gastrulation (van den Brink et al., 2020).
Later in development, forces are responsible for sculpting tissue organization during organogenesis. During neurulation in vertebrates, tissue-level forces are necessary to fold the neural plate into the neural tube (Nikolopoulou et al., 2017). Intriguingly, as the neural tube closes, multipotent neural crest cells (NCCs) arise and separate from the neuroepithelium, yet it remains unclear whether the forces driving neural tube closure affect NCC differentiation or migratory capacity. Mechanical force is also critical for multiple aspects of cardiogenesis. Following formation of a linear heart tube, tissue-level forces mediate looping of the tube to properly position the future left and right heart chambers (Ramasubramanian et al., 2013). At the cellular level, heterogeneity in actomyosin contractility between neighboring cells leads to delamination and trabeculation of the myocardium (Priya et al., 2020), a structural transformation that is essential for proper cardiac function. Moreover, there is direct feedback between the structure of the developing heart and its function. The onset of blood flow regulates cell morphology and chamber growth by modulating activity of the transcription factor Klf2a in the hemodynamic-sensitive endocardium (Dietrich et al., 2014). Mechanical forces also play an important role in adult stem cell maintenance and activation. In the muscle stem cell niche, secreted Wnt4 ligands stimulate actomyosin contractility via RhoA and YAP signaling to maintain quiescence (Eliazer et al., 2019). Similarly, the epithelial stem cells of the skin are mechanosensitive and rely on cell-cell and basement membrane interactions to maintain plasticity and ultimately carry out their barrier function (Gonzales and Fuchs, 2017). Thus, forces do not simply shape developing tissues and organs in a predetermined manner, rather, they serve as a critical and dynamic feedback mechanism that fosters the increasing complexity of the developing organism.
Force and tissue development
Force is essential for tissue-specific development. For example, mechanical stresses associated with blood flow; cyclic strain and fluid shear stress, are key factors that operate to shape the vascular tree and promote heart chamber maturation during development (Kutys and Chen, 2016). Interestingly, endothelial progenitor cells can be differentially directed toward endothelial lineages by shear stress and smooth muscle cell lineages by cyclic strain (Yamamoto et al., 2005; Shimizu et al., 2008). Similarly, fluid-filled luminal pressure promotes branching morphogenesis during lung development (Nelson et al., 2017). Not surprisingly, developmental defects that result in increased or decreased fluid-pressure in the luminal cavity can impair lung development, resulting in hyperplastic or hypoplastic lung branching, respectively (Blewett et al., 1996; Harding, Hooper and Han, 1993). These data highlight the role of mechanical stress in directing tissue-specific development.
Tissues possess a characteristic stiffness and each cell type within a tissue exhibits a unique rheology that can adapt as necessary to regulate its development and/or function, which may vary throughout its lifetime. For example, biophysical force from the stroma, along with hormonal and growth cues, directs the development and function of the mammary gland. During puberty, a branching morphogenesis program commences. In mouse mammary gland development, branching morphogenesis is characterized by the formation of large, multi-layered branch tips called terminal end buds (TEBs) that extend into the stroma of the mammary fat pad (Fig. 2a). Mammary branches are thought to be created primarily via bifurcation of multi-layered epithelial tips or from side-branching off existing ducts (Fata, Werb and Bissell, 2004; Huebner and Ewald, 2014). These branching events are influenced by the surrounding stroma. Such that, collagen fibers in the mammary fat pad align proximal to the TEBs under RHO-mediated contractility, as a patterning cue for mammary branch orientation (Brownfield et al., 2013). Indeed, inhibition of RHO-dependent cytoskeletal tension leads to defects in branching morphogenesis, and tissues exhibit a disruption to the myoepithelial cell layer that permits increased branching and poorly developed ductal elongation (Vargo-Gogola et al., 2006; Ewald et al., 2008). Branch initiation sites are regions of high ECM rigidity, predicted and measured by 3D traction force microscopy (Gjorevski and Nelson, 2010). Branching at these sites is disrupted by inhibition of FAK, and subsequent branching depends on matrix stiffness and RHO-induced cell contractility (Gjorevski and Nelson, 2010). Importantly, much of our understanding of mammary gland branching morphogenesis has come from organoid models, due to the challenge of live imaging the mammary glands in vivo. Thereby, these behaviors were studied in the absence of the native tissue microenvironment, which is known to influence tissue-specific development. Such that, modulating the amount and composition of the ECM also modulates branching morphogenesis (Goodwin and Nelson, 2020). For example, mammary ECM (mECM) isolated from adult mice and rats redirect testicular-derived cells to produce normal mammary epithelial trees within cleared mouse mammary fat pads. Conversely, ECM isolated from omental fat and lung fail to redirect testicular cells to a MEC fate, indicating the necessity of tissue specific components of the mECM (Bruno et al., 2017), demonstrating the importance of the native microenvironment for mammary gland development. Importantly, the microenvironment of the developing mammary gland is different between mice and humans, which raises the possibility that branching morphogenesis proceeds differently in each species (McNally and Stein, 2017). Thus, the pattern and magnitude of mechanical and spatial cues cooperate with biochemical signaling to direct tissue-specific development processes.
Figure 2. Tissue forces in mammary gland development and function.
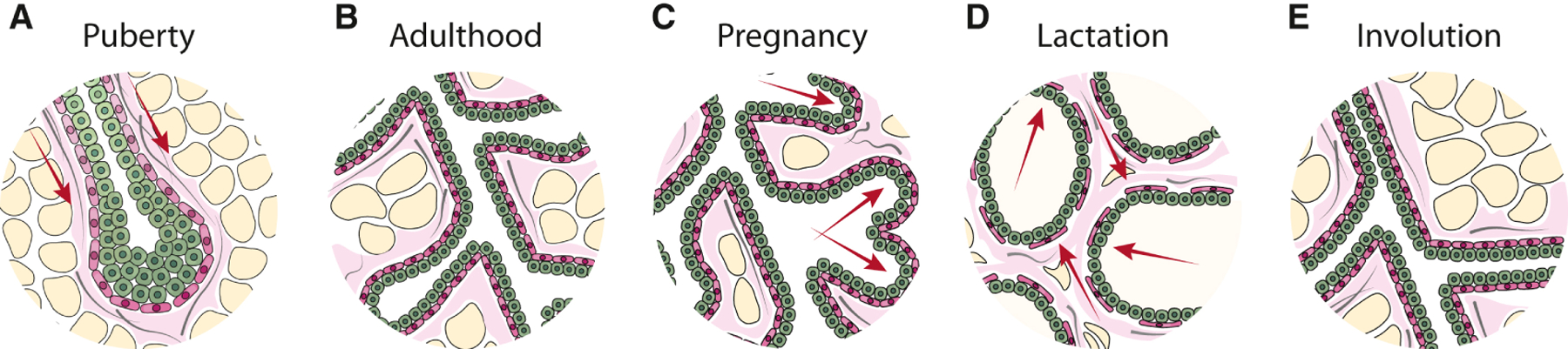
The mammary gland is subjected to a number of biophysical forces (red arrows) that facilitate its development and function. a, During puberty, branching is marked by the ductal outgrowth of the mammary epithelium into the adipocyte-rich (yellow) stroma of the fat pad by directing the collective migration of mammary epithelial cells (MEC) led by the terminal end bud (TEB). Branch orientation is regulated by the bundling of type I collagen fibers (grey) proximal to the TEB. b, The developed breast ducts comprise an inner layer of oriented luminal epithelial cells (green) and an outer layer of contractile myoepithelial cells (pink). c, During pregnancy, hormonal cues direct the expansion of alveolar cells that mature into milk-secreting cells. The alveoli expand out from the ducts filling the majority of the fat pad and the stroma is remodeled to accommodate the expanding epithelium. d, During lactation, the accumulation of milk and distension of ducts applies compressive stress on the surrounding cells and basement membrane. Upon suckling-mediated oxytocin stimulation, epithelial cells encounter inward tensile stress and the myoepithelium contracts to force milk out of the alveolar sacs. e, During involution, the mammary gland undergoes extensive remodeling of the cellular and extracellular stroma to the pre-pregnancy architecture. The remodeled stroma in these stages consequently alters the signals and forces encountered by MECs within the ducts and by doing so, sets the stage for subsequent cycles of proliferation, differentiation or involution.
The composition, organization and biomechanical features of the extracellular stroma can also direct tissue-specific differentiation by modulating cell differentiation. For instance, mesenchymal stem cells (MSC) can be directed toward different lineages in response to the elasticity of their underlying matrix. Soft matrices promote adipogenic and neurogenic cell fates, whereas stiffer matrices favor the formation of myogenic and osteogenic lineages (Engler et al., 2006). However, cells are not passive responders to applied force, in fact they modulate their shape and behavior through molecular mechanisms that include RHO-dependent actomyosin contractility. For example, MSCs are directed towards adipocyte or osteogenic fate depending on low or high RHO-mediated activity, respectively (McBeath et al., 2004). Furthermore, tuning substrate geometry (Kilian et al., 2010), topography (Abagnale et al., 2015), and viscoelasticity (Cameron, Frith and Cooper-White, 2011) direct MSC differentiation. Not surprisingly, the biomechanical properties of the ECM have also been implicated in inducing the stem-like phenotype of cells and has been leveraged to induce pluripotent stem (iPS) cells in culture. For instance, soft matrices have been used to favor the reprogramming of MSCs into iPS cells (Gerardo et al., 2019). Nevertheless, while intriguing, further studies are needed to improve the mechanically-directed reprogramming efficiency of iPS cells before the clinical utility of this approach can be realized.
Force, tissue function and homeostasis
Force and tissue function
Force is essential for the function of differentiated tissues. For example, genetic diseases resulting in defective ECM synthesis of collagen in osteogenesis imperfecta, lowers bone rigidity and subsequently causes bone fragility (Vanleene et al., 2012). Mechanical force can adapt over the lifetime of a tissue; the mammary gland is an example of adaptive function in response to force, as it undergoes functional changes during pregnancy, lactation and involution (Fig. 2b-e). In these stages, MEC progenitors undergo differentiation, proliferation or apoptosis in response to stimuli, giving rise to significant remodeling of the epithelial tissue architecture and surrounding stroma (Inman et al., 2015). During lactation, the breast is subjected to compressive stress on the epithelium and basement membrane due to the accumulation of milk and distension of ducts, which is facilitated by the highly compliant, relaxed collagen matrix surrounding the differentiated acini. Upon suckling, the luminal epithelial cells encounter an inward projecting tensile stress as the myoepithelial cells contract in response to oxytocin, in order to expulse milk out of the alveolar sacs and into the ducts (Adriance et al., 2005). In the absence of the suckling stimulus, continued milk accumulation and gland distension exerts an outwardly projecting compressive force of increasing magnitude on the surrounding epithelium. With prolonged milk stasis, this compressive force eventually compromises the integrity of the tight junctions between luminal secretory alveolar cells (Stelwagen and Singh, 2014), and the gland undergoes involution accompanied by extensive remodeling of the cellular and extracellular stroma (Watson, 2006). The remodeled stroma consequently alters the signals and the force encountered by the MECs within the ducts and by so doing sets the stage for a subsequent round of epithelial proliferation and differentiation. For example, MECs form polarized acini with an endogenous basement membrane and differentiate in response to lactogenic hormones when embedded in a compliant matrix, but form invasive mesenchymal-like structures when grown in a stiffer matrix (Barcellos-Hoff et al., 1989; Paszek et al., 2005). Similarly, MECs fail to express one of the major milk proteins, β-casein, unless they interact with a compliant matrix (Li et al., 1987; Alcaraz et al., 2008). Thereby, force is essential for development and function of tissues.
Force and tissue homeostasis
Forces must be balanced in order to maintain adult tissue homeostasis. For instance, mechanical forces are crucial for the maintenance of force-producing and force-bearing tissues such as muscles and bones (Felsenthal and Zelzer, 2017). These tissues undergo remodeling in response to changing mechanical loads, such that extended periods of reduced mechanical loading in immobilization with unilateral lower limb suspension or in microgravity, result in loss of bone mineral density and consequently, bone strength (Rittweger et al., 2006; Lang et al., 2004). Accordingly, diseases such as osteoporosis and osteoarthritis have been linked to altered mechanoregulation of bone-remodeling cells. Moreover, mechanical loading associated with exercise leads to an increased proteoglycan content in joint cartilage to maintain joint mobility (Bird et al., 2000). Conversely, a loss of proteoglycan content worsens osteoarthritis joint degeneration (Ni et al., 2016). In particular, mechanical stress induces the expression of the proteoglycan PRG4, or lubricin, (Ogawa et al., 2014), that may be a marker of joint cartilage progenitor cells that undergo expansion in response to mechanical loading (Kozhemyakina et al., 2015). The regulation of progenitor and multipotent stem cell populations in tissues is an essential mechanism by which tissues adapt to tensional homeostasis. Tissue rigidity directs the dynamic control of their self-renewal and differentiation required to restore tissue structure and function. For instance, the self-renewal of muscle stem cells is maintained in vitro using substrates with a stiffness that mimic the elasticity of muscle, and only cells propagated in this manner contribute to muscle regeneration when subsequently transplanted into mice (Gilbert et al., 2010). In a different example, mechanical load-bearing strategies that simulate intrinsic mechanisms of bone tissue regeneration have been exploited to expedite stem cell–initiated bone healing (Ng et al., 2014).
Disruption of force homeostasis in cancer risk and aggression
Force in malignant transformation
Disruption of tensional homeostasis is associated with a broad range of pathological conditions, including neurological defects, inflammatory diseases, and tumorigenesis. Tumors are often associated with an increase in stiffness, as with breast and pancreatic cancer (~4kPa), raising the possibility that chronic disruption to tensional homeostasis may act as a precursor to malignant transformation. Indeed, pathologic conditions of chronically elevated stiffness such as cystic fibrosis and cirrhosis of the liver, which often present with extensive ECM accumulation, are associated with increased risk of malignancy (Neglia et al., 1995; Bataller and Brenner, 2005). Similarly, chronic pancreatitis, which is characterized by significant fibrosis, as well as age-associated liver fibrosis, elevates an individual’s overall risk for subsequent tumor formation (Bataller and Brenner, 2005; Raimondi et al., 2010). Moreover, dense breast tissues are fibrotic, and display more abundant collagen that is more oriented, fibrillar and stiffer (Northey et al., 2020). Women with dense breasts have an increased lifetime risk for breast cancer development (Boyd et al., 2011). Moreover, these dense breast tissues also exhibit an increased pro-tumorigenic immune infiltrate (Huo et al., 2018), raising the possibility that women with high mammographic density are predisposed to chronic inflammation that may account for their increased risk to malignancy. Such that, anti-inflammatory drugs have been shown to inhibit tumor progression in a murine model of pregnancy-associated breast cancer (O’Brien et al., 2011). While, tamoxifen treatment is an effective chemoprevention strategy that can reduce breast density, it has significant adverse side effects (Cuzick et al., 2011). This suggests that alternatives, such as the use of anti-inflammatory drugs to modulate breast density may prove an effective strategy for breast cancer prevention.
Aberrant ECM deposition may be done by other cell types, in addition to CAFs. For example, matrix deposited by adipose cells from obese mice induces mechanosignaling and nuclear localization of Yes-associated protein 1 (YAP1)/WW Domain Containing Transcription Regulator 1 (WWTR1; commonly referred to as TAZ) to enhance the tumorigenesis of premalignant human breast epithelial cells (Seo et al., 2015). Moreover, obese adipose tissue produces a stiffer matrix compared with adipose tissue from non-obese animals. These data represent an intriguing area for further investigation given obesity as a well-known risk factor for cancer (Fukumura et al., 2016). A stiffened and crosslinked ECM also promotes the loss of polarity and invasion of mammary epithelial cells (Levental et al., 2009). Mechanical feedback is stimulated not only by cell-ECM contacts, but mechanical stresses are also propagated within cells. For instance, disruption of cell–cell adhesions through overexpression of active NOTCH resulted in hyperproliferation of cells in the colon crypts of mice, which increased mechanical stress and β-catenin signaling in adjacent nontumorous epithelial cells to drive hyperplasia and tumor formation in crypt foci (Fernández-Sánchez et al., 2015). Moreover, the stimulation of cell-intrinsic force generation through ROCK-mediated actomyosin contractility in the epidermis caused an increase in the incidence, growth, and progression of spontaneous carcinogen-induced papilloma (Samuel et al., 2011). This suggests that strategies to prevent ECM stiffening or aberrant cell contractility may prove effective as strategies for cancer prevention. Together, these data suggest that patient screening for disruption to tissue tensional homeostasis may aid in the identification of patients at high risk for future cancer development.
Force in tumor aggression
Alterations to the physical properties of cells and their ECM develop prior to and coincident with malignant transformation and increase as a function of tumor progression to create a microenvironment that facilitates tumor cell growth, survival and invasion (Pickup, Mouw and Weaver, 2014). Tumors display an altered tensional homeostasis that develops in part through solid stress exerted by an expanding tumor mass. For instance, in ductal carcinoma in situ (DCIS), the expansion of cells confined within the duct exerts force on the surrounding myoepithelial-basement membrane barrier (Fig. 3). The solid stress induced by tumor expansion can also promote tumor progression. For example, pancreatic tumors display a disorganized and thinning laminin and type IV collagen basement membrane, that when combined with an outward projecting compression force, facilitates tumor cell invasion (Ingber, Madri and Jamieson, 1981). Furthermore, compressive stress can activate cancer-associated fibroblasts (CAF) to secrete ECM proteins including type I collagen, to enhance pancreatic cancer cell migration (Kalli et al., 2018). Solid stress can also compress the tumor vasculature, lymphatics and interstitial space, causing leaky vasculature and impaired lymphatic drainage. In parallel, interstitial pressure can accumulate due to compression of the vasculature and lymphatics, which can impair anticancer drug delivery (Swartz and Lund, 2012). Together, these forces induce angiogenesis and generate regions of hypoxia, whereby activation of hypoxia-inducible factor (HIF)1α can induce an epithelial-to-mesenchymal (EMT) transition in colorectal cancer and hepatocellular carcinoma cells (Zhang et al., 2013; Zhang et al., 2015). Moreover, hypoxic tumor cells isolated from xenografts contain increased subpopulations of tumor cells with cancer stem cell (CSC)-like characteristics (Kim et al., 2018). Compression force can also shrink the intercellular space surrounding cells, which increases the local concentration of growth factors and cytokines that may promote tumor growth (Tschumperlin et al., 2004).
Figure 3. Disruption of tissue mechanics in breast cancer.
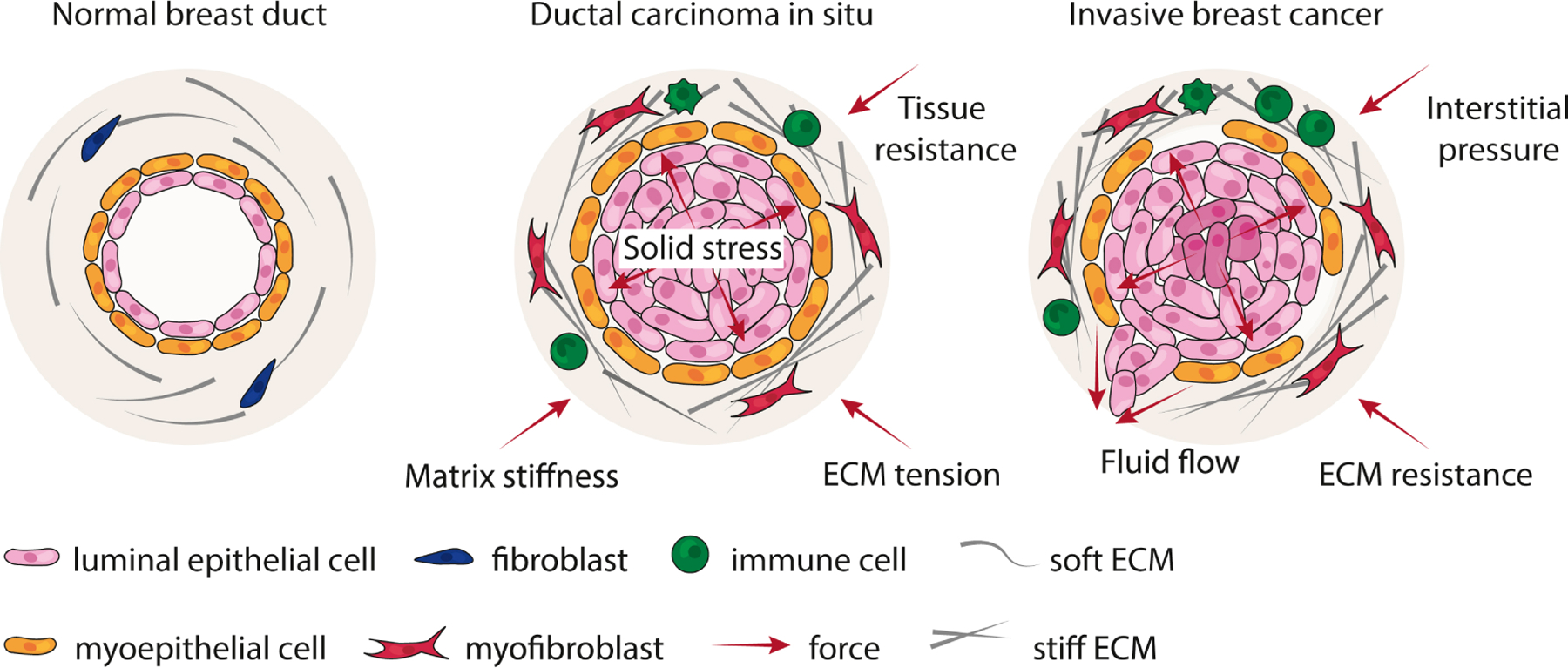
In the normal breast, forces between the epithelium and stroma maintain a state of tensional homeostasis. Normal ducts are surrounded by a compliant and soft ECM. In ductal carcinoma in situ (DCIS), epithelial cells proliferate within the lumen of the duct, exerting an outward projective compressive force on the adjacent myoepithelium and basement membrane (solid stress). These forces are countered by an inward projecting resistance force from the ECM. Accompanying DCIS progression is the remodeling, crosslinking and stiffening of the ECM by myofibroblasts and immune infiltrate. In parallel, interstitial pressure accumulates due to impaired lymphatic drainage and vessel compression. Together, these forces can generate regions of hypoxia within a tumor, which can induce epithelial-to-mesenchymal or stem-like transition in tumor cells. Neoplastic epithelial cells exhibit modified tensional homeostasis and respond to these accumulating forces. At some point, the myoepithelial-basement membrane barrier is breached, and linearized and stiffened ECM tracks surrounding ducts facilitate tumor cell invasion and metastasis.
Tumors develop a desmoplastic response that is characterized by increased levels, remodeling and crosslinking of the ECM, that impedes the expansion of the tumor mass and compromises lymphatic drainage, thereby providing a resistant force to solid stress. For example, the stroma surrounding DCIS lesions exhibits higher levels of linearized and thickened collagen fibers (Acerbi et al., 2015). These collagen fibers begin to align parallel to the tumor boundary, which associates with a higher propensity for progression to invasive breast cancer (Provenzano et al., 2006). Collagen abundance in breast tumors is also a significant risk factor for patient mortality (Hasebe et al., 1997). Two-photon imaging has revealed that the tumors that develop in breast cancer patients with thickened and linearized collagen fibers that are oriented radially surrounding the tumor mass are more aggressive (Egeblad, Rasch and Weaver, 2010). Consistently, the presence of linearized, and stiffened collagen bundles is predictive of poor breast cancer patient prognosis (Conklin et al., 2011), which is likely due to these tracks facilitating cancer cell migration and invasion. Indeed, linearized collagen bundles surrounding the tumor serve as migratory tracks to facilitate fibroblast, immune, and tumor cell migration and invasion (Egeblad, Rasch and Weaver, 2010). Moreover, ECM stiffening fosters the aggressive behavior of cancer cells, such that a variety of cell types migrate faster on stiffer substrates and their persistent migration can be directed up a stiffness gradient, through a process termed durotaxis (Kai, Laklai and Weaver, 2016). Similarly, cancer cells exhibit a haptotactic response in which cell migration is guided by gradients of surface-bound molecules such as the ECM (Oudin and Weaver, 2016). For example, human breast cancer cells tend to migrate toward a higher concentration of fibronectin (Oudin et al., 2016). Similarly, pancreatic cancer cells exhibit haptotactic behavior toward higher concentration of type I collagen (Lu et al., 2014). As the tumor stroma shows increased levels of ECM proteins, that are progressively remodeled and stiffened, durotaxis and haptotaxis may account for stiffer tumors showing higher infiltration of immune cells (Acerbi et al., 2015) and a higher frequency of fibroblasts (Piersma, Hayward and Weaver, 2020) at the invasive front. Thus, the remodeled, stiffened ECM that develops in tumors can directly foster tumor cell invasion and promotes tumor progression by activating stromal fibroblasts and immune cell infiltration.
Progression to an invasive carcinoma is accompanied by further ECM remodeling and collagen crosslinking and stiffening. For instance, inhibition or blocking of lysyl oxidase (LOX)-mediated crosslinking and stiffening delayed the transformation, frequency of invasive carcinomas and decreased tumor grade in a HER2/Neu model of murine mammary cancer and a KRAS/p53-induced model of murine pancreatic cancer (Levental et al., 2009; Miller et al., 2015). Similarly, inhibiting collagen crosslinking and reducing ECM stiffening also inhibited polyoma middle T–induced mammary tumor metastasis. Indeed, breast cancer patients with high levels of LOX have a higher probability of metastasis (Pickup et al., 2013). Moreover, aggressive subtypes of human breast cancer (HER2-enriched and triple-negative), exhibit the stiffest invasive stroma (Acerbi et al., 2015), and display the greatest abundance of collagen crosslinks (Maller et al., 2020). Importantly, stromal cells express the highest levels of collagen crosslinking enzymes, which was found to correlate significantly with disease specific mortality (Maller et al., 2020). Furthermore, a stiff ECM may additionally facilitate invasion by promoting a mesenchymal-like phenotype. Cancer cells with this phenotype are more contractile and deformable, which facilitate their migration through a dense and stiffened ECM (Mekhdjian et al., 2017). Indeed, basal-like and triple-negative breast cancers (TNBC), which have the worst prognostic outcome and stiffest ECM also display mesenchymal markers, as well as a stem-like molecular signature that associates with therapeutic resistance (Sotiriou et al., 2003). Poor patient prognosis and a less differentiated mesenchymal phenotype were also correlated with increased collagen deposition in patients with pancreatic cancer (Laklai et al., 2016). Moreover, glioblastoma multiforme (GMB), which is an aggressive brain tumor (Barnes, Przybyla and Weaver, 2017), displays a greater abundance of mesenchymal and stem-like tumor cells that exhibit elevated therapeutic resistance relative to bulk glioma cells (Bao et al., 2006). These mesenchymal-like GBM tumors are stiffer and present with higher levels of glycoproteins that enhances their glycocalyx (Barnes et al., 2018). Since a large proportion of these bulky glycoproteins, such as tenascin-C (TNC), are also stem markers, upregulation of glycoproteins and their modulators enhances GBM stem-like behavior. These data suggest that a crosslinked and stiffened ECM may induce a mesenchymal and stem-like transition in tumor cells that promotes their aggressive behavior.
Force and tumor cell fate
Tumor heterogeneity remains a considerable obstacle as it impinges on prognosis, response to therapy and metastasis. Genetic, phenotypic and functional diversity exists among tumors from different patients (intertumor heterogeneity), and even within an individual tumor (intratumor heterogeneity). The intertumor heterogeneity is typically characterized by gene expression profiles that associate with patient outcome and prediction of treatment success. For example, examination of receptor status (ER/PR/HER2) in breast cancers has led to the identification of molecular subtypes; luminal A, luminal B, basal-like, HER2-enriched and normal-like, that associate with different clinical prognosis (Perou et al., 2000) and to some extent, treatment response (Troester et al., 2004). Furthermore, these detrimental characteristics are confounded by the distinct subpopulations of cells that exist within a tumor. CSCs have been proposed to account for intratumor heterogeneity. CSCs are a low-abundance population of tumor cells with stem-like properties due to their ability to self-renew and generate the diverse cells that comprise a tumor, implicating CSCs in tumor origination (Visvader and Lindeman, 2008). Evidence also supports a role for CSCs in aggressive tumor behaviors such as disease relapse and metastatic spread due to their resistance to anticancer drugs. Furthermore, characterization of CSC gene expression and function has revealed that they possess properties similar to cells that have undergone EMT (Mani et al., 2008). For example, disseminated tumor cells from HER2-enriched breast cancers display a stem cell phenotype and acquire EMT markers such as TWIST1, SNAI1 and ZEB1 (Reuben et al., 2011).
Increasing evidence suggests mechanical forces influence tumor heterogeneity and aggression. While the stroma within a tumor is stiffer than that of a healthy tissue, this stiffness is not uniform and they display considerable heterogeneity in compliance and rigidity throughout the tumor itself (Acerbi et al., 2015; Laklai et al., 2016; Lopez et al., 2011). Indeed, tumors show the stiffest regions at the invasive front, and aggressive subtypes display the highest number of stiff regions (Acerbi et al., 2015). Moreover, these aggressive breast cancer subtypes which have the stiffest stroma and worst patient prognosis, display elevated mesenchymal markers as well as a stem-like molecular signature, which have been associated with detrimental tumor characteristics such as resistance to diverse forms of anticancer therapy, seeding recurrent tumors and ability to disseminate to and colonize distant tissues (Park, Choi and Nam, 2019; Sotiriou et al., 2003). These data suggest that disruption to tensional homeostasis may promote mesenchymal or stem-like tumor cells. Mechanical force may act to reprogram differentiated tumor cells to exhibit mesenchymal and stem-like behaviors. For instance, ECM stiffness can promote the development of stem-like properties in breast cancer cells by modulating levels and activity of integrin-linked kinase (ILK) (Pang et al., 2016). Similarly, a stem-like phenotype is exhibited in pancreatic cancers that exhibit increased collagen accumulation and elevated expression of YAP1 (Laklai et al., 2016). Changes to tissue rigidity may also induce the proliferative expansion of premalignant progenitor cells or transformed stem cells, though these potential mechanisms require further investigation.
Conclusions and future perspectives
Clearly, biophysical cues have an important role in regulating cell fate and directing tissue-specific development. With increasing experimental tools available to investigate the physical properties of tissues and cells (Box 2; reviewed in (Roca-Cusachs, Conte and Trepat, 2017)), there is expanding potential to identify unique therapeutic interventions that may normalize the tensional microenvironment in order to ameliorate aggressive tumor behavior and even prevent malignant transformation. Certainly, more extensive characterization of mechanical niches within tumors that influence the behavior of stem-like tumor cells, including their quiescence, self-renewal and differentiation behaviors, will be critical for developing strategies aimed at suppressing their development and persistence in cancer. Nevertheless, in order to pertain clinical utility, many questions in the field must be better understood.
Box 2. Tools for studying cell and tissue forces.
a, Traction force microscopy (TFM) maps stresses at the cell surface by measuring deformation of the surrounding material. Typically, fluorescent beads are embedded within gels with tuneable elasticity, and cells may be cultured on top (2D) or within (3D) the gels. Traction forces generated by cell contractile forces result in deformation of the substrate, which can be quantified by the displacement of fluorescent beads. A variety of computational methods are used to generate traction force maps from these displacement measurements.
b, Atomic force microscopy (AFM) maps the stiffness of a material by measuring the displacement of the cantilever-tip. Cantilevers are elongated elastic materials which are fixed at one end and free at the other. The force exerted on the free end by probing the material causes the cantilever to bend. This allows for the stiffness of the material to be readily measured from the displacement of the cantilever. Again, a variety of computational methods are used to generate AFM stiffness maps for cells and tissues.
c, Fluorescence resonance energy transfer (FRET) is used to quantify forces by deforming individual molecules. As nanoscale deformation of individual molecules cannot be resolved optically, they are measured indirectly through fluorescence microscopy. Typically, a cassette containing a mechanically calibrated linker flanked by two different fluorophores is encoded into a protein of interest. Force application to the molecule stretches the linker, thereby altering the FRET between the two fluorophores. This is used to understand the forces experienced by specific molecules, including talin and vinculin.
d, Laser ablation uses focused laser pulse energy to obliterate biological structures that transmit forces at the subcellular, cellular or tissue level. Laser ablation generally expands the targeted structure, indicating the structure and its surroundings were previously under tension in the opposite direction to the expansion. Force estimations conducted through laser ablation are limited by the assumption that materials are constant and uniform across experimental conditions, though these may be determined independently.
e, Magnetic or optical tweezers can be used to apply force to or measure the rheological properties of cells. Either a dielectric or a magnetic bead is functionalized with molecules to facilitate cell attachment. Then, either a focused beam of light or magnetic field is used to apply force to the bead, which is transmitted to the cell. Precise calibration of these systems allows simultaneous measurement of the cellular resistance to the applied force, from which rheological properties of the cell can be calculated.
f, Bulk rheology characterizes the flow or deformation of a material of interest and can be used to measure the viscoelastic properties of biological materials, such as biopolymer hydrogels or tissues. Rheology measurements are performed using an instrument called a rheometer, of which there are two types: controlled-stress and controlled-strain rheometers. Viscoelastic properties (i.e., storage, loss, and elastic moduli) are determined by examining the relationship between stress and strain for different modalities of applied stress or strain. The main challenge associated with bulk rheology is typically sample preparation – as viscoelastic materials can be inherently difficult to stabilize and load into the rheometer.
Considerable data supports mechanically initiated cell signaling in modulating transcription factor activation (Wei et al., 2015), yet it is also appreciated that mechanical cues control chromosome organization through cytoskeletal-nuclear links, and hence gene expression. Nuclear mechanotransduction has been proposed to be one process regulating stem cell fate including self-renewal, lineage specification, and differentiation; however, how chromosome configurations are altered in response to changes in nuclear mechanics following microenvironmental cues is not well understood. One possibility is that the cellular cytoskeleton transmits force directly to the nucleus through specific physical linkages such as those mediated by Linker of the Nucleoskeleton and Cytoskeleton (LINC) complex to the nuclear lamina (Davidson and Lammerding, 2014). These links apply differential mechanical force by bridging the cytoskeleton and chromatin. Specifically, KASH domain proteins (nesprins) on the outer nuclear membrane link with actin, and in turn, are connected to the inner nuclear membrane through SUN domain proteins, which are linked to nuclear lamina and the chromatin (Davidson and Lammerding, 2014). Studies have revealed that the specific locations of chromosomes in the nucleus depends on the mechanical state of the nucleus (Wang et al., 2017). While gene-rich chromosomes are located towards the interior, gene-poor chromosomes are located towards the nuclear periphery anchored by specific laminin-associated domains (LAD) (Chen et al., 2015; Kind et al., 2015). This may be an important mechanism in regulating cell fate decisions, as stem cells display highly dynamic chromatin structures resulting from a more plastic nucleus, while differentiated cells show chromosomes arrangements and a stiffer cell nucleus (Maharana et al., 2016). Similarly, ovarian CSCs exhibit a softer elastic modulus than more differentiated neoplastic precursors and aggressive ovarian cancer cells due to changes in their cytoskeletal architecture (Babahosseini et al., 2014). It is conceivable that alterations in nuclear morphology and cytoskeletal-nuclear links in cancer may result in rearrangement of chromosomes to activate differential gene expression programs that may facilitate aggressive tumor-phenotypes. Moreover, while alterations in nuclear morphometrics have long been recognized as biomarkers of cancer diagnostics, machine-based high-resolution imaging approaches provide promising prognostic tools based on such nuclear alterations (Hayward et al., 2020). Further development of digital image analysis techniques combined with genomic analysis may prove effective in early cancer diagnostics.
Cell metabolism has also emerged as an important process regulated by mechanical cues (Romani et al., 2021). For example, ECM stiffness activates glycolysis and glutamine metabolism in both squamous cell carcinoma cells and CAFs. A stiff ECM induced a metabolic cross talk between tumor cells and CAFs, whereby CAF-derived aspartate facilitated tumor cell proliferation, while tumor cell-derived glutamate supported CAF ECM remodeling activity (Bertero et al., 2019). In this manner, ECM stiffness can rewire cell metabolism to promote cell proliferation and ECM remodeling to foster tumor growth and aggression. In turn, metabolism also directly influences tissue mechanics. Indeed, one study showed that breast cancer cells rely on pyruvate to support crosslinking, and subsequent stiffening of collagen at the metastatic niche, and the inhibition of pyruvate metabolism impaired breast cancer-derived lung metastases (Elia et al., 2017). Importantly, metabolism may also influence cell fate. As cells differentiate the metabolic state of the cell shifts energy production from oxidative phosphorylation to glycolysis. For example, ESCs in the most naïve, undifferentiated state, exhibit high levels of oxidative phosphorylation. As they differentiate toward a primed state, they shift their metabolism program to glycolysis for energy and biomass production (Zhou et al., 2012). It is plausible that this metabolic shift occurs prior to induction of gene expression reprogramming, implying it is a prerequisite to cell fate decisions. Together, these data suggest that mechanics and metabolism are intertwined, and mechanically induced metabolic rewiring could facilitate cell fate decisions. These research areas, among others, require further investigation in order to develop unique therapeutic strategies to target stem-like cells in cancer prevention and treatment.
Acknowledgements
V.M. Weaver is supported by US National Institutes of Health/National Cancer Institute grants R01CA192914, 1R01CA222508–01 and 1R35CA242447–01A1. Thanks to Roger Oria-Fernandez for his helpful insight and comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
V.M. Weaver is a member of the Advisory Board for Developmental Cell but did not participate in the editorial process of this manuscript.
References
- Abagnale G, Steger M, Nguyen VH, Hersch N, Sechi A, Joussen S, Denecke B, Merkel R, Hoffmann B, Dreser A, Schnakenberg U, Gillner A and Wagner W (2015) ‘Surface topography enhances differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages’, Biomaterials, 61, pp. 316–26. [DOI] [PubMed] [Google Scholar]
- Acerbi I, Cassereau L, Dean I, Shi Q, Au A, Park C, Chen YY, Liphardt J, Hwang ES and Weaver VM (2015) ‘Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration’, Integr Biol (Camb), 7(10), pp. 1120–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriance MC, Inman JL, Petersen OW and Bissell MJ (2005) ‘Myoepithelial cells: good fences make good neighbors’, Breast Cancer Res, 7(5), pp. 190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz J, Xu R, Mori H, Nelson CM, Mroue R, Spencer VA, Brownfield D, Radisky DC, Bustamante C and Bissell MJ (2008) ‘Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia’, EMBO J, 27(21), pp. 2829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo-Urarte A, van der Wal T and Huveneers S (2020) ‘Cell-cell junctions as sensors and transducers of mechanical forces’, Biochim Biophys Acta Biomembr, 1862(9), pp. 183316. [DOI] [PubMed] [Google Scholar]
- Babahosseini H, Ketene AN, Schmelz EM, Roberts PC and Agah M (2014) ‘Biomechanical profile of cancer stem-like/tumor-initiating cells derived from a progressive ovarian cancer model’, Nanomedicine, 10(5), pp. 1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN (2006) ‘Glioma stem cells promote radioresistance by preferential activation of the DNA damage response’, Nature, 444(7120), pp. 756–60. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG and Bissell MJ (1989) ‘Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane’, Development, 105(2), pp. 223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JM, Kaushik S, Bainer RO, Sa JK, Woods EC, Kai F, Przybyla L, Lee M, Lee HW, Tung JC, Maller O, Barrett AS, Lu KV, Lakins JN, Hansen KC, Obernier K, Alvarez-Buylla A, Bergers G, Phillips JJ, Nam DH, Bertozzi CR and Weaver VM (2018) ‘A tension-mediated glycocalyx-integrin feedback loop promotes mesenchymal-like glioblastoma’, Nat Cell Biol, 20(10), pp. 1203–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JM, Przybyla L and Weaver VM (2017) ‘Tissue mechanics regulate brain development, homeostasis and disease’, J Cell Sci, 130(1), pp. 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller R and Brenner DA (2005) ‘Liver fibrosis’, J Clin Invest, 115(2), pp. 209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays JL and DeMali KA (2017) ‘Vinculin in cell-cell and cell-matrix adhesions’, Cell Mol Life Sci, 74(16), pp. 2999–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazellières E, Conte V, Elosegui-Artola A, Serra-Picamal X, Bintanel-Morcillo M, Roca-Cusachs P, Muñoz JJ, Sales-Pardo M, Guimerà R and Trepat X (2015) ‘Control of cell-cell forces and collective cell dynamics by the intercellular adhesome’, Nat Cell Biol, 17(4), pp. 409–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergert M, Lembo S, Sharma S, Russo L, Milovanović D, Gretarsson KH, Börmel M, Neveu PA, Hackett JA, Petsalaki E and Diz-Muñoz A (2020) ‘Cell Surface Mechanics Gate Embryonic Stem Cell Differentiation’, Cell Stem Cell [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertero T, Oldham WM, Grasset EM, Bourget I, Boulter E, Pisano S, Hofman P, Bellvert F, Meneguzzi G, Bulavin DV, Estrach S, Feral CC, Chan SY, Bozec A and Gaggioli C (2019) ‘Tumor-Stroma Mechanics Coordinate Amino Acid Availability to Sustain Tumor Growth and Malignancy’, Cell Metab, 29(1), pp. 124–140.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird JL, Platt D, Wells T, May SA and Bayliss MT (2000) ‘Exercise-induced changes in proteoglycan metabolism of equine articular cartilage’, Equine Vet J, 32(2), pp. 161–3. [DOI] [PubMed] [Google Scholar]
- Blankenship JT, Backovic ST, Sanny JS, Weitz O and Zallen JA (2006) ‘Multicellular rosette formation links planar cell polarity to tissue morphogenesis’, Dev Cell, 11(4), pp. 459–70. [DOI] [PubMed] [Google Scholar]
- Blewett CJ, Zgleszewski SE, Chinoy MR, Krummel TM and Cilley RE (1996) ‘Bronchial ligation enhances murine fetal lung development in whole-organ culture’, J Pediatr Surg, 31(7), pp. 869–77. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Martin LJ, Yaffe MJ and Minkin S (2011) ‘Mammographic density and breast cancer risk: current understanding and future prospects’, Breast Cancer Res, 13(6), pp. 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AE, Litvinov RI, Discher DE and Weisel JW (2007) ‘Forced unfolding of coiled-coils in fibrinogen by single-molecule AFM’, Biophys J, 92(5), pp. L39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownfield DG, Venugopalan G, Lo A, Mori H, Tanner K, Fletcher DA and Bissell MJ (2013) ‘Patterned collagen fibers orient branching mammary epithelium through distinct signaling modules’, Curr Biol, 23(8), pp. 703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet T, Bouclet A, Ahmadi P, Mitrossilis D, Driquez B, Brunet AC, Henry L, Serman F, Béalle G, Ménager C, Dumas-Bouchiat F, Givord D, Yanicostas C, Le-Roy D, Dempsey NM, Plessis A and Farge E (2013) ‘Evolutionary conservation of early mesoderm specification by mechanotransduction in Bilateria’, Nat Commun, 4, pp. 2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RD, Fleming JM, George AL, Boulanger CA, Schedin P and Smith GH (2017) ‘Mammary extracellular matrix directs differentiation of testicular and embryonic stem cells to form functional mammary glands in vivo’, Sci Rep, 7, pp. 40196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron AR, Frith JE and Cooper-White JJ (2011) ‘The influence of substrate creep on mesenchymal stem cell behaviour and phenotype’, Biomaterials, 32(26), pp. 5979–93. [DOI] [PubMed] [Google Scholar]
- Case LB and Waterman CM (2015) ‘Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch’, Nat Cell Biol, 17(8), pp. 955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi LS, Marsh HM and Basson MD (2007) ‘Src and focal adhesion kinase mediate mechanical strain-induced proliferation and ERK1/2 phosphorylation in human H441 pulmonary epithelial cells’, Am J Physiol Cell Physiol, 292(5), pp. C1701–13. [DOI] [PubMed] [Google Scholar]
- Chen H, Chen J, Muir LA, Ronquist S, Meixner W, Ljungman M, Ried T, Smale S and Rajapakse I (2015) ‘Functional organization of the human 4D Nucleome’, Proc Natl Acad Sci U S A, 112(26), pp. 8002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess PR, Toia L and Finkelstein JN (2000) ‘Mechanical strain-induced proliferation and signaling in pulmonary epithelial H441 cells’, Am J Physiol Lung Cell Mol Physiol, 279(1), pp. L43–51. [DOI] [PubMed] [Google Scholar]
- Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A and Keely PJ (2011) ‘Aligned collagen is a prognostic signature for survival in human breast carcinoma’, Am J Pathol, 178(3), pp. 1221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, Forbes JF and Warren RM (2011) ‘Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study’, J Natl Cancer Inst, 103(9), pp. 744–52. [DOI] [PubMed] [Google Scholar]
- Davidson PM and Lammerding J (2014) ‘Broken nuclei--lamins, nuclear mechanics, and disease’, Trends Cell Biol, 24(4), pp. 247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Belly H, Stubb A, Yanagida A, Labouesse C, Jones PH, Paluch EK and Chalut KJ (2020) ‘Membrane Tension Gates ERK-Mediated Regulation of Pluripotent Cell Fate’, Cell Stem Cell [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM and Sheetz MP (2009) ‘Stretching single talin rod molecules activates vinculin binding’, Science, 323(5914), pp. 638–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng ZJ, Liang M, Monteiro M, Toth I and Minchin RF (2011) ‘Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation’, Nat Nanotechnol, 6(1), pp. 39–44. [DOI] [PubMed] [Google Scholar]
- Dietrich AC, Lombardo VA, Veerkamp J, Priller F and Abdelilah-Seyfried S (2014) ‘Blood flow and Bmp signaling control endocardial chamber morphogenesis’, Dev Cell, 30(4), pp. 367–77. [DOI] [PubMed] [Google Scholar]
- Douguet D and Honoré E (2019) ‘Mammalian Mechanoelectrical Transduction: Structure and Function of Force-Gated Ion Channels’, Cell, 179(2), pp. 340–354. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Rasch MG and Weaver VM (2010) ‘Dynamic interplay between the collagen scaffold and tumor evolution’, Curr Opin Cell Biol, 22(5), pp. 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia I, Broekaert D, Christen S, Boon R, Radaelli E, Orth MF, Verfaillie C, Grünewald TGP and Fendt SM (2017) ‘Proline metabolism supports metastasis formation and could be inhibited to selectively target metastasizing cancer cells’, Nat Commun, 8, pp. 15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliazer S, Muncie JM, Christensen J, Sun X, D’Urso RS, Weaver VM and Brack S (2019) ‘Wnt4 from the Niche Controls the Mechano-Properties and Quiescent State of Muscle Stem Cells’, Cell Stem Cell, 25(5), pp. 654–665.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL and Discher DE (2006) ‘Matrix elasticity directs stem cell lineage specification’, Cell, 126(4), pp. 677–89. [DOI] [PubMed] [Google Scholar]
- Ewald AJ, Brenot A, Duong M, Chan BS and Werb Z (2008) ‘Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis’, Dev Cell, 14(4), pp. 570–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Werb Z and Bissell MJ (2004) ‘Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes’, Breast Cancer Res, 6(1), pp. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenthal N and Zelzer E (2017) ‘Mechanical regulation of musculoskeletal system development’, Development, 144(23), pp. 4271–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Simoes S. e. M., Röper JC, Eaton S and Zallen JA (2009) ‘Myosin II dynamics are regulated by tension in intercalating cells’, Dev Cell, 17(5), pp. 736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Sánchez ME, Barbier S, Whitehead J, Béalle G, Michel A, Latorre-Ossa H, Rey C, Fouassier L, Claperon A, Brullé L, Girard E, Servant N, Rio-Frio T, Marie H, Lesieur S, Housset C, Gennisson JL, Tanter M, Ménager C, Fre S, Robine S and Farge E (2015) ‘Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure’, Nature, 523(7558), pp. 92–5. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Incio J, Shankaraiah RC and Jain RK (2016) ‘Obesity and Cancer: An Angiogenic and Inflammatory Link’, Microcirculation, 23(3), pp. 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardo H, Lima A, Carvalho J, Ramos JRD, Couceiro S, Travasso RDM, Pires das Neves R and Grãos M (2019) ‘Soft culture substrates favor stem-like cellular phenotype and facilitate reprogramming of human mesenchymal stem/stromal cells (hMSCs) through mechanotransduction’, Sci Rep, 9(1), pp. 9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP and Blau HM (2010) ‘Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture’, Science, 329(5995), pp. 1078–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorevski N and Nelson CM (2010) ‘Endogenous patterns of mechanical stress are required for branching morphogenesis’, Integr Biol (Camb), 2(9), pp. 424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales KAU and Fuchs E (2017) ‘Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche’, Dev Cell, 43(4), pp. 387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin K and Nelson CM (2020) ‘Branching morphogenesis’, Development, 147(10). [DOI] [PubMed] [Google Scholar]
- Goutman JD, Elgoyhen AB and Gómez-Casati ME (2015) ‘Cochlear hair cells: The sound-sensing machines’, FEBS Lett, 589(22), pp. 3354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R, Hooper SB and Han VK (1993) ‘Abolition of fetal breathing movements by spinal cord transection leads to reductions in fetal lung liquid volume, lung growth, and IGF-II gene expression’, Pediatr Res, 34(2), pp. 148–53. [DOI] [PubMed] [Google Scholar]
- Hasebe T, Tsuda H, Tsubono Y, Imoto S and Mukai K (1997) ‘Fibrotic focus in invasive ductal carcinoma of the breast: a histopathological prognostic parameter for tumor recurrence and tumor death within three years after the initial operation’, Jpn J Cancer Res, 88(6), pp. 590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward MK, Jones JL, Hall A, King L, Ironside AI, Nelson AC, Hwang ES and Weaver VM 2020. Derivation of a nuclear heterogeneity image index to grade DCIS. Computational and Structural Biology Journal [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner RJ and Ewald AJ (2014) ‘Cellular foundations of mammary tubulogenesis’, Semin Cell Dev Biol, 31, pp. 124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo CW, Hill P, Chew G, Neeson PJ, Halse H, Williams ED, Henderson MA, Thompson EW and Britt KL (2018) ‘High mammographic density in women is associated with protumor inflammation’, Breast Cancer Res, 20(1), pp. 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE, Madri JA and Jamieson JD (1981) ‘Role of basal lamina in neoplastic disorganization of tissue architecture’, Proc Natl Acad Sci U S A, 78(6), pp. 3901–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman JL, Robertson C, Mott JD and Bissell MJ (2015) ‘Mammary gland development: cell fate specification, stem cells and the microenvironment’, Development, 142(6), pp. 1028–42. [DOI] [PubMed] [Google Scholar]
- Kai F, Laklai H and Weaver VM (2016) ‘Force Matters: Biomechanical Regulation of Cell Invasion and Migration in Disease’, Trends Cell Biol, 26(7), pp. 486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalli M, Papageorgis P, Gkretsi V and Stylianopoulos T (2018) ‘Solid Stress Facilitates Fibroblasts Activation to Promote Pancreatic Cancer Cell Migration’, Ann Biomed Eng, 46(5), pp. 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kechagia JZ, Ivaska J and Roca-Cusachs P (2019) ‘Integrins as biomechanical sensors of the microenvironment’, Nat Rev Mol Cell Biol, 20(8), pp. 457–473. [DOI] [PubMed] [Google Scholar]
- Kilian KA, Bugarija B, Lahn BT and Mrksich M (2010) ‘Geometric cues for directing the differentiation of mesenchymal stem cells’, Proc Natl Acad Sci U S A, 107(11), pp. 4872–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lin Q, Glazer PM and Yun Z (2018) ‘The hypoxic tumor microenvironment in vivo selects the cancer stem cell fate of breast cancer cells’, Breast Cancer Res, 20(1), pp. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind J, Pagie L, de Vries SS, Nahidiazar L, Dey SS, Bienko M, Zhan Y, Lajoie B, de Graaf CA, Amendola M, Fudenberg G, Imakaev M, Mirny LA, Jalink K, Dekker J, van Oudenaarden A and van Steensel B (2015) ‘Genome-wide maps of nuclear lamina interactions in single human cells’, Cell, 163(1), pp. 134–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippenberger S, Bernd A, Loitsch S, Guschel M, Müller J, Bereiter-Hahn J and Kaufmann R (2000) ‘Signaling of mechanical stretch in human keratinocytes via MAP kinases’, J Invest Dermatol, 114(3), pp. 408–12. [DOI] [PubMed] [Google Scholar]
- Kong F, García AJ, Mould AP, Humphries MJ and Zhu C (2009) ‘Demonstration of catch bonds between an integrin and its ligand’, J Cell Biol, 185(7), pp. 1275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhemyakina E, Zhang M, Ionescu A, Ayturk UM, Ono N, Kobayashi A, Kronenberg H, Warman ML and Lassar AB (2015) ‘Identification of a Prg4-expressing articular cartilage progenitor cell population in mice’, Arthritis Rheumatol, 67(5), pp. 1261–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg M, Arboleda-Estudillo Y, Puech PH, Käfer J, Graner F, Müller DJ and Heisenberg CP (2008) ‘Tensile forces govern germ-layer organization in zebrafish’, Nat Cell Biol, 10(4), pp. 429–36. [DOI] [PubMed] [Google Scholar]
- Kutys ML and Chen CS (2016) ‘Forces and mechanotransduction in 3D vascular biology’, Curr Opin Cell Biol, 42, pp. 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laklai H, Miroshnikova YA, Pickup MW, Collisson EA, Kim GE, Barrett AS, Hill RC, Lakins JN, Schlaepfer DD, Mouw JK, LeBleu VS, Roy N, Novitskiy SV, Johansen JS, Poli V, Kalluri R, Iacobuzio-Donahue CA, Wood LD, Hebrok M, Hansen K, Moses HL and Weaver VM (2016) ‘Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression’, Nat Med, 22(5), pp. 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T, LeBlanc A, Evans H, Lu Y, Genant H and Yu A (2004) ‘Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight’, J Bone Miner Res, 19(6), pp. 1006–12. [DOI] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL and Weaver VM (2009) ‘Matrix crosslinking forces tumor progression by enhancing integrin signaling’, Cell, 139(5), pp. 891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ML, Aggeler J, Farson DA, Hatier C, Hassell J and Bissell MJ (1987) ‘Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells’, Proc Natl Acad Sci U S A, 84(1), pp. 136–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov RI, Barsegov V, Schissler AJ, Fisher AR, Bennett JS, Weisel JW and Shuman H (2011) ‘Dissociation of bimolecular αIIbβ3-fibrinogen complex under a constant tensile force’, Biophys J, 100(1), pp. 165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JI, Kang I, You WK, McDonald DM and Weaver VM (2011) ‘In situ force mapping of mammary gland transformation’, Integr Biol (Camb), 3(9), pp. 910–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhou S, Siech M, Habisch H, Seufferlein T and Bachem MG (2014) ‘Pancreatic stellate cells promote hapto-migration of cancer cells through collagen I-mediated signalling pathway’, Br J Cancer, 110(2), pp. 409–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharana S, Iyer KV, Jain N, Nagarajan M, Wang Y and Shivashankar GV (2016) ‘Chromosome intermingling-the physical basis of chromosome organization in differentiated cells’, Nucleic Acids Res, 44(11), pp. 5148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller O, Drain AP, Barrett AS, Borgquist S, Ruffell B, Zakharevich I, Pham TT, Gruosso T, Kuasne H, Lakins JN, Acerbi I, Barnes JM, Nemkov T, Chauhan A, Gruenberg J, Nasir A, Bjarnadottir O, Werb Z, Kabos P, Chen YY, Hwang ES, Park M, Coussens LM, Nelson AC, Hansen KC and Weaver VM (2020) ‘Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression’, Nat Mater [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J and Weinberg RA (2008) ‘The epithelial-mesenchymal transition generates cells with properties of stem cells’, Cell, 133(4), pp. 704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K and Chen CS (2004) ‘Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment’, Dev Cell, 6(4), pp. 483–95. [DOI] [PubMed] [Google Scholar]
- McNally S and Stein T (2017) ‘Overview of Mammary Gland Development: A Comparison of Mouse and Human’, Methods Mol Biol, 1501, pp. 1–17. [DOI] [PubMed] [Google Scholar]
- Mekhdjian AH, Kai F, Rubashkin MG, Prahl LS, Przybyla LM, McGregor AL, Bell ES, Barnes JM, DuFort CC, Ou G, Chang AC, Cassereau L, Tan SJ, Pickup MW, Lakins JN, Ye X, Davidson MW, Lammerding J, Odde DJ, Dunn AR and Weaver VM (2017) ‘Integrin-mediated traction force enhances paxillin molecular associations and adhesion dynamics that increase the invasiveness of tumor cells into a three-dimensional extracellular matrix’, Mol Biol Cell, 28(11), pp. 1467–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill L, Gonzalez EJ, Girard BM and Vizzard MA (2016) ‘Receptors, channels, and signalling in the urothelial sensory system in the bladder’, Nat Rev Urol, 13(4), pp. 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BW, Morton JP, Pinese M, Saturno G, Jamieson NB, McGhee E, Timpson P, Leach J, McGarry L, Shanks E, Bailey P, Chang D, Oien K, Karim S, Au A, Steele C, Carter CR, McKay C, Anderson K, Evans TR, Marais R, Springer C, Biankin A, Erler JT and Sansom OJ (2015) ‘Targeting the LOX/hypoxia axis reverses many of the features that make pancreatic cancer deadly: inhibition of LOX abrogates metastasis and enhances drug efficacy’, EMBO Mol Med, 7(8), pp. 1063–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncie JM, Ayad NME, Lakins JN, Xue X, Fu J and Weaver VM (2020) ‘Mechanical Tension Promotes Formation of Gastrulation-like Nodes and Patterns Mesoderm Specification in Human Embryonic Stem Cells’, Dev Cell, 55(6), pp. 679–694.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncie JM, Falcón-Banchs R, Lakins JN, Sohn LL and Weaver VM (2019) ‘Patterning the Geometry of Human Embryonic Stem Cell Colonies on Compliant Substrates to Control Tissue-Level Mechanics’, J Vis Exp, (151). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neglia JP, FitzSimmons SC, Maisonneuve P, Schöni MH, Schöni-Affolter F, Corey M and Lowenfels AB (1995) ‘The risk of cancer among patients with cystic fibrosis. Cystic Fibrosis and Cancer Study Group’, N Engl J Med, 332(8), pp. 494–9. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Gleghorn JP, Pang MF, Jaslove JM, Goodwin K, Varner VD, Miller E, Radisky DC and Stone HA (2017) ‘Microfluidic chest cavities reveal that transmural pressure controls the rate of lung development’, Development, 144(23), pp. 4328–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng MH, Duski S, Tan KK, Yusof MR, Low KC, Rose IM, Mohamed Z, Bin Saim A and Idrus RB (2014) ‘Repair of segmental load-bearing bone defect by autologous mesenchymal stem cells and plasma-derived fibrin impregnated ceramic block results in early recovery of limb function’, Biomed Res Int, 2014, pp. 345910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni GX, Zhou YZ, Chen W, Xu L, Li Z, Liu SY, Lei L and Zhan LQ (2016) ‘Different responses of articular cartilage to strenuous running and joint immobilization’, Connect Tissue Res, 57(2), pp. 143–51. [DOI] [PubMed] [Google Scholar]
- Nichols J, Silva J, Roode M and Smith A (2009) ‘Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo’, Development, 136(19), pp. 3215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulou E, Galea GL, Rolo A, Greene ND and Copp AJ (2017) ‘Neural tube closure: cellular, molecular and biomechanical mechanisms’, Development, 144(4), pp. 552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northey JJ, Barrett AS, Acerbi I, Hayward MK, Talamantes S, Dean IS, Mouw JK, Ponik SM, Lakins JN, Huang PJ, Wu J, Shi Q, Samson S, Keely PJ, Mukhtar RA, Liphardt JT, Shepherd JA, Hwang ES, Chen YY, Hansen KC, Littlepage LE and Weaver VM (2020) ‘Stiff stroma increases breast cancer risk by inducing the oncogene ZNF217’, J Clin Invest, 130(11), pp. 5721–5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J, Hansen K, Barkan D, Green J and Schedin P (2011) ‘Non-steroidal anti-inflammatory drugs target the pro-tumorigenic extracellular matrix of the postpartum mammary gland’, Int J Dev Biol, 55(7–9), pp. 745–55. [DOI] [PubMed] [Google Scholar]
- Oberhauser AF, Badilla-Fernandez C, Carrion-Vazquez M and Fernandez JM (2002) ‘The mechanical hierarchies of fibronectin observed with single-molecule AFM’, J Mol Biol, 319(2), pp. 433–47. [DOI] [PubMed] [Google Scholar]
- Oberhauser AF, Marszalek PE, Erickson HP and Fernandez JM (1998) ‘The molecular elasticity of the extracellular matrix protein tenascin’, Nature, 393(6681), pp. 181–5. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Kozhemyakina E, Hung HH, Grodzinsky AJ and Lassar AB (2014) ‘Mechanical motion promotes expression of Prg4 in articular cartilage via multiple CREB-dependent, fluid flow shear stress-induced signaling pathways’, Genes Dev, 28(2), pp. 127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudin MJ, Jonas O, Kosciuk T, Broye LC, Guido BC, Wyckoff J, Riquelme D, Lamar JM, Asokan SB, Whittaker C, Ma D, Langer R, Cima MJ, Wisinski KB, Hynes RO, Lauffenburger DA, Keely PJ, Bear JE and Gertler FB (2016) ‘Tumor Cell-Driven Extracellular Matrix Remodeling Drives Haptotaxis during Metastatic Progression’, Cancer Discov, 6(5), pp. 516–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudin MJ and Weaver VM (2016) ‘Physical and Chemical Gradients in the Tumor Microenvironment Regulate Tumor Cell Invasion, Migration, and Metastasis’, Cold Spring Harb Symp Quant Biol, 81, pp. 189–205. [DOI] [PubMed] [Google Scholar]
- Pang MF, Siedlik MJ, Han S, Stallings-Mann M, Radisky DC and Nelson CM (2016) ‘Tissue Stiffness and Hypoxia Modulate the Integrin-Linked Kinase ILK to Control Breast Cancer Stem-like Cells’, Cancer Res, 76(18), pp. 5277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Choi JH and Nam JS (2019) ‘Targeting Cancer Stem Cells in Triple-Negative Breast Cancer’, Cancers (Basel), 11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA and Weaver VM (2005) ‘Tensional homeostasis and the malignant phenotype’, Cancer Cell, 8(3), pp. 241–54. [DOI] [PubMed] [Google Scholar]
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO and Botstein D (2000) ‘Molecular portraits of human breast tumours’, Nature, 406(6797), pp. 747–52. [DOI] [PubMed] [Google Scholar]
- Pickup MW, Laklai H, Acerbi I, Owens P, Gorska AE, Chytil A, Aakre M, Weaver VM and Moses HL (2013) ‘Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-β-deficient mouse mammary carcinomas’, Cancer Res, 73(17), pp. 5336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup MW, Mouw JK and Weaver VM (2014) ‘The extracellular matrix modulates the hallmarks of cancer’, EMBO Rep, 15(12), pp. 1243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piersma B, Hayward MK and Weaver VM (2020) ‘Fibrosis and cancer: A strained relationship’, Biochim Biophys Acta Rev Cancer, 1873(2), pp. 188356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC and Bellido T (2005) ‘Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs’, Am J Physiol Cell Physiol, 289(3), pp. C633–43. [DOI] [PubMed] [Google Scholar]
- Priya R, Allanki S, Gentile A, Mansingh S, Uribe V, Maischein HM and Stainier DYR (2020) ‘Tension heterogeneity directs form and fate to pattern the myocardial wall’, Nature, 588(7836), pp. 130–134. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG and Keely PJ (2006) ‘Collagen reorganization at the tumor-stromal interface facilitates local invasion’, BMC Med, 4(1), pp. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla L, Lakins JN and Weaver VM (2016) ‘Tissue Mechanics Orchestrate Wnt-Dependent Human Embryonic Stem Cell Differentiation’, Cell Stem Cell, 19(4), pp. 462–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu W, Cao H and Xiao G (2020) ‘Molecular mechanosensors in osteocytes’, Bone Res, 8, pp. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P and Pezzilli R (2010) ‘Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection’, Best Pract Res Clin Gastroenterol, 24(3), pp. 349–58. [DOI] [PubMed] [Google Scholar]
- Ramasubramanian A, Chu-Lagraff QB, Buma T, Chico KT, Carnes ME, Burnett KR, Bradner SA and Gordon SS (2013) ‘On the role of intrinsic and extrinsic forces in early cardiac S-looping’, Dev Dyn, 242(7), pp. 801–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben JM, Lee BN, Gao H, Cohen EN, Mego M, Giordano A, Wang X, Lodhi A, Krishnamurthy S, Hortobagyi GN, Cristofanilli M, Lucci A and Woodward WA (2011) ‘Primary breast cancer patients with high risk clinicopathologic features have high percentages of bone marrow epithelial cells with ALDH activity and CD44⁺CD24lo cancer stem cell phenotype’, Eur J Cancer, 47(10), pp. 1527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittweger J, Winwood K, Seynnes O, de Boer M, Wilks D, Lea R, Rennie M and Narici M (2006) ‘Bone loss from the human distal tibia epiphysis during 24 days of unilateral lower limb suspension’, J Physiol, 577(Pt 1), pp. 331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Cusachs P, Conte V and Trepat X (2017) ‘Quantifying forces in cell biology’, Nat Cell Biol, 19(7), pp. 742–751. [DOI] [PubMed] [Google Scholar]
- Roca-Cusachs P, Gauthier NC, Del Rio A and Sheetz MP (2009) ‘Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction’, Proc Natl Acad Sci U S A, 106(38), pp. 16245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani P, Valcarcel-Jimenez L, Frezza C and Dupont S (2021) ‘Crosstalk between mechanotransduction and metabolism’, Nat Rev Mol Cell Biol, 22(1), pp. 22–38. [DOI] [PubMed] [Google Scholar]
- Röper JC, Mitrossilis D, Stirnemann G, Waharte F, Brito I, Fernandez-Sanchez ME, Baaden M, Salamero J and Farge E (2018) ‘The major β-catenin/E-cadherin junctional binding site is a primary molecular mechano-transductor of differentiation’, Elife, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadaoui M, Rocancourt D, Roussel J, Corson F and Gros J (2020) ‘A tensile ring drives tissue flows to shape the gastrulating amniote embryo’, Science, 367(6476), pp. 453–458. [DOI] [PubMed] [Google Scholar]
- Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P, Munro J, Schröder E, Zhou J, Brunton VG, Barker N, Clevers H, Sansom OJ, Anderson KI, Weaver VM and Olson MF (2011) ‘Actomyosin-mediated cellular tension drives increased tissue stiffness and β-catenin activation to induce epidermal hyperplasia and tumor growth’, Cancer Cell, 19(6), pp. 776–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S and Sheetz MP (2006) ‘Force sensing by mechanical extension of the Src family kinase substrate p130Cas’, Cell, 127(5), pp. 1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechler JL, Rao H, Cumiskey AM, Vega-Colón I, Smith MS, Murata T and Schwarzbauer JE (2001) ‘A novel fibronectin binding site required for fibronectin fibril growth during matrix assembly’, J Cell Biol, 154(5), pp. 1081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo BR, Bhardwaj P, Choi S, Gonzalez J, Andresen Eguiluz RC, Wang K, Mohanan S, Morris PG, Du B, Zhou XK, Vahdat LT, Verma A, Elemento O, Hudis CA, Williams RM, Gourdon D, Dannenberg AJ and Fischbach C (2015) ‘Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis’, Sci Transl Med, 7(301), pp. 301ra130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N, Yamamoto K, Obi S, Kumagaya S, Masumura T, Shimano Y, Naruse K, Yamashita JK, Igarashi T and Ando J (2008) ‘Cyclic strain induces mouse embryonic stem cell differentiation into vascular smooth muscle cells by activating PDGF receptor beta’, J Appl Physiol (1985), 104(3), pp. 766–72. [DOI] [PubMed] [Google Scholar]
- Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL and Liu ET (2003) ‘Breast cancer classification and prognosis based on gene expression profiles from a population-based study’, Proc Natl Acad Sci U S A, 100(18), pp. 10393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelwagen K and Singh K (2014) ‘The role of tight junctions in mammary gland function’, J Mammary Gland Biol Neoplasia, 19(1), pp. 131–8. [DOI] [PubMed] [Google Scholar]
- Swartz MA and Lund AW (2012) ‘Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity’, Nat Rev Cancer, 12(3), pp. 210–9. [DOI] [PubMed] [Google Scholar]
- Troester MA, Hoadley KA, Sørlie T, Herbert BS, Børresen-Dale AL, Lønning PE, Shay JW, Kaufmann WK and Perou CM (2004) ‘Cell-type-specific responses to chemotherapeutics in breast cancer’, Cancer Res, 64(12), pp. 4218–26. [DOI] [PubMed] [Google Scholar]
- Tschumperlin DJ, Dai G, Maly IV, Kikuchi T, Laiho LH, McVittie AK, Haley KJ, Lilly CM, So PT, Lauffenburger DA, Kamm RD and Drazen JM (2004) ‘Mechanotransduction through growth-factor shedding into the extracellular space’, Nature, 429(6987), pp. 83–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brink SC, Alemany A, van Batenburg V, Moris N, Blotenburg M, Vivié J, Baillie-Johnson P, Nichols J, Sonnen KF, Martinez Arias A and van Oudenaarden A (2020) ‘Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids’, Nature, 582(7812), pp. 405–409. [DOI] [PubMed] [Google Scholar]
- Vanleene M, Porter A, Guillot PV, Boyde A, Oyen M and Shefelbine S (2012) ‘Ultra-structural defects cause low bone matrix stiffness despite high mineralization in osteogenesis imperfecta mice’, Bone, 50(6), pp. 1317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargo-Gogola T, Heckman BM, Gunther EJ, Chodosh LA and Rosen JM (2006) ‘P190-B Rho GTPase-activating protein overexpression disrupts ductal morphogenesis and induces hyperplastic lesions in the developing mammary gland’, Mol Endocrinol, 20(6), pp. 1391–405. [DOI] [PubMed] [Google Scholar]
- Visvader JE and Lindeman GJ (2008) ‘Cancer stem cells in solid tumours: accumulating evidence and unresolved questions’, Nat Rev Cancer, 8(10), pp. 755–68. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nagarajan M, Uhler C and Shivashankar GV (2017) ‘Orientation and repositioning of chromosomes correlate with cell geometry-dependent gene expression’, Mol Biol Cell, 28(14), pp. 1997–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ (2006) ‘Involution: apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ’, Breast Cancer Res, 8(2), pp. 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weberling A and Zernicka-Goetz M (2021) ‘Trophectoderm mechanics direct epiblast shape upon embryo implantation’, Cell Rep, 34(3), pp. 108655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, Chen AC, Sah RL, Taylor SS, Engler AJ and Yang J (2015) ‘Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway’, Nat Cell Biol, 17(5), pp. 678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Sokabe T, Watabe T, Miyazono K, Yamashita JK, Obi S, Ohura N, Matsushita A, Kamiya A and Ando J (2005) ‘Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro’, Am J Physiol Heart Circ Physiol, 288(4), pp. H1915–24. [DOI] [PubMed] [Google Scholar]
- Yang J, Pan X, Wang L and Yu G (2020) ‘Alveolar cells under mechanical stressed niche: critical contributors to pulmonary fibrosis’, Mol Med, 26(1), pp. 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Huang G, Li X, Zhang Y, Jiang Y, Shen J, Liu J, Wang Q, Zhu J, Feng X, Dong J and Qian C (2013) ‘Hypoxia induces epithelial-mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor −1α in hepatocellular carcinoma’, BMC Cancer, 13, pp. 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Shi X, Peng Y, Wu M, Zhang P, Xie R, Wu Y, Yan Q, Liu S and Wang J (2015) ‘HIF-1α Promotes Epithelial-Mesenchymal Transition and Metastasis through Direct Regulation of ZEB1 in Colorectal Cancer’, PLoS One, 10(6), pp. e0129603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Xue X, Shao Y, Wang S, Esfahani SN, Li Z, Muncie JM, Lakins JN, Weaver VM, Gumucio DL and Fu J (2019) ‘Controlled modelling of human epiblast and amnion development using stem cells’, Nature, 573(7774), pp. 421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Choi M, Margineantu D, Margaretha L, Hesson J, Cavanaugh C, Blau CA, Horwitz MS, Hockenbery D, Ware C and Ruohola-Baker H (2012) ‘HIF1α induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition’, EMBO J, 31(9), pp. 2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]


