Abstract
Moringa concanensis Nimmo (Moringaceae) belongs to the same family of M. oleifera (miracle tree) and is a medicinal plant traditionally used by Indians to treat various ailments related to diabetes, tumours, inflammation, and blood pressure. Despite its versatility, the photoprotective properties of the plant remain unclear. This study revealed the UV-protective properties of its methanol bark extract and respective subfractions, chloroform, hexane, and ethyl acetate through total phenolic and flavonoid content (TPC & TFC), antioxidant (DPPH), sun protecting factor (SPF) value, and UV absorption spectra analysis. This study also investigated on the inhibitory effect of the tested samples on collagenases and elastase, which are well-known for their role in the skin. The cytotoxic and H2O2 scavenging properties of M. concanensis in 3T3-L1 cells were explored. Finally, the phytochemical profiling of the active fraction was conducted through UPLC-QTOF/MS analysis. Among the tested fractions, the chloroform fraction of M. concanensis showed the highest TPC (30.92 ± 0.71 mg GAE/DW), TFC (29.05 ± 0.09 mg QE/DW), and antioxidant properties (IC50-6.616 ± 1.90 μgml−1). Additionally, chloroform fraction demonstrated the highest SPF value, 10.46 at 200 μgml−1, compared to the other tested fractions. All the fractions showed a broad absorption spectrum covering both UVA and UVB ranges. The chloroform fraction of M. concanensis also showed collagenase (50%) and elastase (IC50-2.95 ± 1.23 μgml−1) inhibition properties similar to the positive control. Cytotoxic results revealed that the chloroform fraction of M. concanensis prevented the H2O2-induced oxidative damage in 3T3-L1 cells even at lower concentrations (1.56 μgml−1). UPLC-QTOF/MS analysis tentatively identified the presence of bioactive flavonoids and phenolics such as astragalin, quercetin, isoquercetin, and caffeic acid in the active fraction of M. concanensis bark. Overall, it is suggested that the chloroform fraction of M. concanensis bark has the potency to be used as an active ingredient in sunscreen products.
1. Introduction
In recent times, the intensity of ultraviolet (UV) radiation reaching the earth's surface has increased due to ozone depletion by releasing human-made chemicals into the atmosphere. [1]. Three types of UV (200–400 nm) radiations are available: UVA (320–400 nm), UVB (290–320 nm), and UVC (200–290 nm). UV radiation-stimulated skin damage is highly influenced by its wavelength, intensity, and exposure time [2]. UV radiation directly interacts with the skin, which leads to the activation of several signaling cascades in the skin layers. Excessive doses of UVA and UVB rays are widely considered the leading causative agent for sunburns, early aging, DNA damage, wrinkle formation, oxidative stress, and skin cancer. [3]. In particular, UVA radiation produces immediate skin pigmentation and skin alterations related to premature aging via penetrating the deeper layers of the skin, whereas UVB is responsible for skin damage, including erythema and sunburn, by inducing late pigmentation. UVB is also associated with changes in cellular DNA and can provoke skin cancer [4]. Both rays are capable of promoting lipid peroxidation, which is strongly associated with the generation of reactive oxygen species (ROS) [5]. The excessive production of ROS leads to direct DNA damage; stimulation of proinflammatory cytokines such as IL-1 (interleukin-1), IL-6 (interleukin-6), and TNF-α (tumor necrosis factor); activation of signaling pathways; and the release of matrix metalloproteinases. Overexpression of these biomarkers could damage the matrix proteins such as elastin and collagen, which finally results in phototoxic reactions such as photoallergy, photosensitivity, photoaging, and photocarcinogenesis [6, 7].
Nowadays, sunscreen-based photoprotection is one of the essential strategies utilized to prevent UV-induced damage and other skin-related disorders. Sunscreens can protect the skin from sunburn, sun allergy rash, immune suppression, and other harmful effects induced by UV radiation. These sunscreens are made up of different organic and inorganic filters to protect the skin [3, 4]. Synthetic-based sunscreens are now becoming a serious threat to consumers and a threat to marine lives and the environment. A wide range of novel hypoallergenic cosmetics has been developed using natural products due to the adverse effect of synthetic sunscreen ingredients. Specifically, plant-based products are in high demand due to their myriad benefits such as antioxidant, anti-inflammatory, and immune enhancers [3–9]. Compared to synthetic filters, compounds, fractions, and extracts from plant sources offer a broad range of UV absorption. Several natural products are incorporated into sunscreen products. Previous research reported the usage of numerous traditional plants such as Moringa. oleifera Lam, Zanthoxylum rhetsa (Roxb.) DC., Parentucellia latifolia Caruel, and Nephelium lappaceum L., Camellia sinensis L. Kuntze, and other plant extracts or fractions that are rich in polyphenols were described to possess appreciable sunscreen properties via targeting free radicals, inflammatory pathways (NF-κB, MAPK) and cytokines, and matrix metalloproteinases (MMP1, MMP3, MMP9, etc.) [2, 3, 6, 8, 10, 11]. Though there are studies that revealed the photoprotective potency of some traditional medicinal plants, it remains limited.
Moringa concanensis Nimmo is an important traditional medicinal plant in the family of Moringaceae mainly spotted in the Western Ghats of India and also widely distributed in Asian and Arab countries [12]. This species looks almost similar to the well-known medicinal, nutritional value species M. oleifera. However, it has some distinct features such as bipinnate leaves, a strong central trunk with an extremely distinctive layer of very furrowed bark, and the petals and sepals of the flower have green patches at the tip. Moreover, the leaves and flowers of M. concanensis are larger compared to M. oleifera [13]. In Indian traditional systems of medicine, M. concanensis is mainly used to treat fertility problems. The leaves and bark of this species were used to treat skin tumours, tiredness, high blood pressure, jaundice, eye problems, diabetes, and swellings [14] with a wide range of therapeutic properties. Studies revealed that the ethanol extract of M. concanensis extract exhibited hyperglycaemic activity and showed protective activity against oxidative tissue damage in the pancreas, liver, and kidney [15]. In this study, the photoprotective properties of various solvent fractions of the M. concanensis bark were revealed for the first time using biochemical assays such as total phenolic (TPC) and flavonoid content (TFC), DPPH free radical scavenging activity, SPF value determination, and UVA/UVB absorption spectra analysis. Moreover, the inhibitory effect of the plant extract towards collagenase and elastase was also shown using gelatin digestion and antielastase assay. The cytotoxic and H2O2 damage preventive potential of the extracts and fractions were assessed through the cell culture technique using MTT assay. The potential metabolites present in the active fraction responsible for the biological properties of M. concanensis bark were identified through UPLC-QTOF/MS analysis.
2. Materials and Methods
2.1. Chemical and Reagents
Solvents such as hexane (CAS No. 110-54-3), chloroform (CAS No. 67-66-3), methanol (CAS No. 67-56-1), and ethyl acetate (CAS No. 141-78-6) used for extraction are of analytical grade obtained from Merck Millipore (Darmstadt, Germany). Collagenase from Clostridium histolyticum (CAS No. 9001-12-1), N-succinyl-ala-ala-ala-p-nitroanilide (AAAPVN, CAS No. 52299-14-6), 2,2-diphenyl-1-picrylhydrazyl (DPPH, CAS, No. 1898-66-4), gelatin (CAS No. 9000-70-8), porcine elastase (CAS No. 39445-21-1), Coomassie blue R-250 (CAS No. 6104-59-2), ascorbic acid (AA) (CAS No. 50-81-7), epigallocatechin gallate (EGCG, CAS No. 989-51-5), Tris-HCl (CAS No. 1185-53-1), agarose (CAS No. 9012-36-6), acetic acid (CAS No. 64-19-7), dimethyl sulfoxide (DMSO, CAS No. 67-68-5), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, CAS No. 57360-69-7) were purchased from Sigma-Aldrich Chemicals (St. Louis, MO, USA). Dulbecco's modified Eagle's medium (DMEM, CAT No. 11-995-073), TrypLE Express (CAT No. 12604–013), fetal bovine serum (FBS, CAT No. 26-140-079), and penicillin/streptomycin (CAT No. 15140–122) were purchased from Gibco (Life Technologies, California, USA).
2.2. Samples Collection and Extraction
The stem bark of M. concanensis was collected from Madukkarai, Coimbatore, Tamil Nadu, India. The plant specimen was authenticated, and plant identification certificate was given by Dr. G.V.S. Moorthy, Head of Office, Southern Regional Centre (Letter No. BSI/SRC/5/23/2018/Tech-437), Botanical Survey of India, Coimbatore, Tamil Nadu, India. The Voucher specimen (BUH-VA-3203/2018) was submitted to the Herbarium of Department of Botany, Bharathiar University, Coimbatore, Tamil Nadu, India. The crude methanolic extract (28 g) from dried and powdered bark (600 g) of M. concanensis was obtained using ultrasound-assisted extraction technique according to the literature [6].
2.3. Liquid-Liquid Partitioning
Various solvent fractions of the crude methanol extract of M. concanensis were subjected to liquid-liquid partitioning technique using different solvents based on their polarity. Initially, 15 g of the methanol crude extract was mixed with equal volume of hexane and water (1 : 1) and loaded into the separating funnel. After few agitations, the hexane fraction was removed and the chloroform was added to the separating funnel. Then, the same process was repeated with ethyl acetate. Finally using rotavapor, the resultant fractions such as hexane (3.1 g), chloroform (6.2 g), and ethyl acetate (1.8 g) were obtained and dried under vacuum and lyophilized [11].
2.4. Total Phenolic Content
The total phenolic content for all the solvent fractions of M. concanensis bark was determined using the Folin–Ciocalteu method [16]. Briefly, 50 μL of the sample (1 mgml−1) in methanol was mixed with 50 μL distilled water, 50 μL of 10% Folin–Ciocalteu's phenol reagent, and 50 μL of 1 M sodium carbonate solution in a 96-well microwell plate and incubated for 60 min at room temperature without exposing it to light. Methanol was used as a blank. The absorbance of the reaction mixture was measured using a microplate reader at 750 nm. The total phenolic content was determined through calibration curve using gallic acid (7.81, 15.62, 31.25, 62.5, 125, 250, and 500 μgmL−1) as standard. Results are expressed as milligram gallic acid equivalents (GAE) per gram of dry plant extract. All tests were done in triplicates.
2.5. Total Flavonoid Content
The total flavonoid content for all the fractions of M. concanensis bark was determined using spectrophotometric method [16]. 100 μL of the plant extract (1 mgmL−1) and standard solutions of quercetin (7.81, 15.62, 31.25, 62.5, 125, 250, and 500 μgmL−1) in methanol solution were mixed with 100 μl of 2% AlCl3 solution. Reaction mixtures were incubated for an hour at room temperature. The absorbance was measured using Tecan Infinite F200 Pro plate reader at λmax 415 nm. Total flavonoid contents were expressed as mg quercetin equivalent (QE) per gram of dry plant extract. Experiments were done in triplicates.
2.6. DPPH Scavenging Activity Assay
The crude extract and the fractions of M. concanensis bark were tested for their free radical scavenging properties using DPPH free radical scavenging assay [16, 17]. Briefly, 0.12 mM DPPH in methanol was prepared and mixed with various concentrations of test samples at 1 : 1 ratio (100 μL). Ascorbic acid was used as the positive control. Test samples and DPPH solution were incubated at room temperature for 30 min in the dark. After the incubation, the absorbance value of the mixture was recorded on a microwell plate reader at 570 nm. The experiment was done in triplicates. The DPPH radical scavenging activity was calculated using the formula:
| (1) |
where Abscontrol is the absorbance of DPPH radical + methanol and Abssample is the absorbance of DPPH radical + samples/positive control.
2.7. SPF Value Determination
The in vitro SPF value of the crude extract and solvent fractions of M. concanensis were obtained by the method described in the literature [16]. Briefly, the absorbance value of the test sample (200 μgmL−1) was determined on a UV-Visible spectrophotometer at 5 nm intervals within the range of 290–320 nm. The SPF value was then calculated by using the formula:
| (2) |
where CF is the correction factor (= 10), EE (λ) is the erythemal effect spectrum, I (λ) is the solar intensity spectrum, Abs (λ) is the absorbance of test sample, where EE (λ) x I(λ) are constants. Methanol was used as a blank, and measurements were made in triplicate.
2.8. UVA/UVB Absorption Spectra
The UV absorption spectrum of the crude extract and solvent fractions of M. concanensis (100 μgmL−1 in methanol) were measured over a wavelength range of 200–400 nm on a UV-Visible spectrophotometer using a quartz cell (1 cm). The UV absorption spectrum of the samples tested was compared with the positive control, epigallocatechin gallate (EGCG) prepared with the same concentration.
2.9. Gelatin Digestion Assay
The gelatin digestion assay was done according to the method described by Santhanam et al. [6] with slight modifications. Agarose (2%) was prepared in a collagenase buffer (50 mM Tris-HCl, 10 mM CaCl2, 0.15 M NaCl, pH 7.8) and porcine gelatin (0.15%). The mixture was transferred into a petri dish and left at room temperature for 1 h for solidification. Later, the wells were made using a sterile 200 μL microtip. Then, 25 μL of the samples was incubated with 25 μL of bacterial collagenase-1 (0.1 mgmL−1) for 1 h. Finally, the reaction mixture (50 μL) was loaded into the well and further incubated overnight. EGCG was used as a positive control. The next day, the petri dish was visualized by the Coomassie brilliant blue staining method. The degree of gelatin digestion was determined by measuring the area of the light translucent zone formed after destaining over a blue background.
2.10. Antielastase Assay
The antielastase activity of the various solvent fractions of M. concanensis was determined according to the methods of Santhanam et al. [6], with minor modifications. The assay was performed in Tris-HCl buffer (0.2 mM, pH 8.0). The stock solution of porcine pancreatic elastase (PE–E.C. 3.4.21.36) was dissolved in sterile water. N-succinyl-ala-ala-ala-p-nitroanilide (AAAPVN) was dissolved in 1 mL Tris-HCl (0.2 mM) buffer (pH 8.0), and the samples were dissolved in buffer. Then, the test samples and elastase were incubated for 15 minutes before adding the substrate to start the reaction. The final reaction mixture contains a buffer, 0.8 mM AAAPVN, 50 μgmL−1 PE, and the test samples at various test concentrations in a total volume of 250 μL. EGCG served as the positive control while water served as the negative control. The absorbance value was measured using a Tecan Infinite F200 Pro plate reader (Tecan Group Ltd., Männedorf, Switzerland) at 410 nm.
| (3) |
2.11. Cell Culture
3T3-L1 cells were maintained in complete Dulbecco's modified Eagle's medium (DMEM) high-glucose media according to the method of Raseetha et al. [18] with slight modification. The media were supplemented with 10% fetal bovine serum (FBS) and 5% of penicillin-streptomycin in a humidified 5% CO2 incubator at 37°C. Once the cell reaches 70–80% confluence, it was utilized for seeding and treatment.
2.11.1. Cytotoxicity
Cells cytotoxicity was performed using MTT assay based on the method described by Raseetha et al. [18]. The cells were seeded at a density of 1 × 105 cells/well in 96-well plates. Once the cell reaches 80% confluence, the 100 μL of various concentrations of methanol extract and solvent fractions of M. concanensis and positive control EGCG were treated in each well. After 24 h, 20 μL of MTT was added to the cells and it was incubated at 37°C for 4 h. The medium was replaced with 100 μL DMSO, and the absorbance for each well was measured at 570 nm on Tecan Infinite F200 Pro plate reader. Cytotoxicity assay was conducted to determine the range of concentrations of the active fraction. Experiments were performed in triplicates.
2.11.2. Protective Effect of Samples against H2O2-Induced Oxidative Damage in 3T3-L1 Cells
The protective effect of M. concanensis fractions against H2O2 was performed according to Chen et al. [19] with slight modification. The 3T3-L1 cells were seeded on 96-well plates at a density of 1 × 105 (in 100 μL medium) per well and incubated at 37°C for 24 h. Once the cell reaches the confluence, the medium was replaced with 100 μl of samples with various concentrations (0–100 μg mL-1) and positive control (ascorbic acid) for 18 h. Then, the cells were treated with H2O2 (125 μM) to induce oxidative damage, and the dose concentration was fixed based on the current study. After 6 h, the medium was removed, and the cells were washed thrice with PBS. Next, the MTT solution was added and the cells were further incubated for 4 h. Finally, the formazan crystals were dissolved in DMSO (100 μL per well), and the absorbance value was measured at 540 nm using Tecan Infinite F200 Pro plate reader [20].
2.12. Ultraperformance Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (UPLC-QTOF/MS) Analysis
Phytochemical profiling in chloroform fractions of M. concanensis was conducted via ultraperformance liquid chromatography (UPLC-MS) analysis. The analysis was carried out using Waters ACQUITY ultraperformance LC system (Waters, Milford, MA, USA). Chromatographic separation of the respective extract was conducted using a selected column (ACQUITY UPLC HSS T3, 100 mm × 2.1 mm × 1.8 m, Waters, Manchester, UK) maintained at 40°C. The UPLC systems were connected to Vion IMS QTOF detector (Waters, Milford, MA, USA). The mobile phases used in the analysis were 0.1% formic acid (A) and acetonitrile (B). The composition of mobile phases was consisted of the following multistep linear gradient: 0 min, 1% B and 99% A; 0.5 min, 1% B and 99% A; 16.00 min, 35% B and 65% A; 18.00 min, 100% B and 0% A; and 20.00 min, 1% B and 99% A, respectively. The injection volume used for the sample was 1 μL while the flow rate was set at 0.6 mL/min. The data were obtained from the UHPLC system coupled with Vion IMS QTOF hybrid mass spectrometer from waters, equipped with a LockSpray ion source. The ion source was operated in negative electrospray ionization (ESI) mode by applying specific conditions such as the capillary voltage at 1.50 kV, reference capillary voltage at 3.00 kV, source temperature at 120°C, desolvation gas temperature at 550°C, desolvation gas flow at 800 L/h, and cone gas flow at 50 L/h, respectively. Nitrogen (>99.5%) was used as desolvation and cone gas. Data were acquired in high-definition MSE (HDMSE) mode ranging between m/z 50 and 1500 at 0.1 s/scan. Hence, two independent scans with different collision energies (CE) were alternatively obtained during the run: a low-energy (LE) scan at a fixed CE of 4 eV and a high-energy (HE) scan where the CE was ramped from 10 to 40 eV. Argon (99.999%) was used as collision-induced-dissociation (CID) gas. Data interpretation was carried out using Waters UNIFI Scientific Information System database. Resolved peaks were further identified with the assistance of reported values from previous literature and are shown in Table 1.
Table 1.
Compounds tentatively identified in the chloroform fraction from the methanol extract of M. concanensis bark using UPLC-QTOF/MS analysis.
| Peak no. | Tentative identification | Elemental composition | Calculated m/z [M-H]− | Observed m/z [M-H]− | Retention time | References |
|
| ||||||
| 1 | Kaempferol-3-O-rutinoside | C27H30O15 | 593.1507 | 593.1512 | 6.65 | [21] |
| 2 | Astragalin | C21H20O11 | 447.0927 | 447.0937 | 7.50 | [21] |
| 3 | Quercetin-3-O-glucuronide | C21H18O13 | 477.0670 | 477.0685 | 7.83 | — |
| 4 | Tetra-O-galloyl-glucoside | C34H28O22 | 787.0994 | 787.1005 | 8.11 | — |
| 5 | Kaempferol-3-O-glucuronosyl methyl ester | C22H20O12 | 475.0876 | 475.0867 | 8.61 | [21] |
| 6 | Isoquercetin | C21H20O12 | 463.0877 | 463.0884 | 8.63 | [21] |
| 7 | Caffeic acid | C9H8O4 | 179.0344 | 179.0352 | 9.01 | [21] |
| 8 | Hydroxy-methoxy-cinnamic acid | C10H10O4 | 193.0501 | 193.0511 | 9.38 | — |
| 9 | Sinapic acid | C11H12O5 | 223.0607 | 223.0613 | 10.04 | — |
| 10 | Kaempferol 3-O-arabinoside | C20H18O10 | 417.0822 | 417.0844 | 10.40 | — |
| 11 | Secoisolariciresinol | C20H26O6 | 361.1651 | 361.1656 | 10.43 | — |
| 12 | Kaempferol-3-O-rhamnoside | C21H20O10 | 431.0978 | 431.0995 | 10.91 | [21] |
| 13 | Quercetin-3-O-glucuronide-methyl-ester | C22H20O13 | 491.0826 | 491.0842 | 10.99 | — |
| 14 | Quercetin | C15H10O7 | 301.0348 | 301.0351 | 11.93 | [21] |
| 15 | Eugenol | C10H12O2 | 163.0759 | 163.0765 | 12.47 | [21] |
| 16 | Naringenin | C15H12O5 | 271.0607 | 271.0615 | 13.07 | — |
| 17 | Trimethoxyflavone | C18H16O5 | 311.0920 | 311.0929 | 13.32 | — |
| 18 | Demethoxycurcumin | C20H18O5 | 337.1076 | 337.1085 | 15.53 | — |
| 19 | Sanleng acid | C18H34O5 | 329.2328 | 329.2339 | 15.62 | [21] |
| 20 | Licoricone | C22H22O6 | 381.1338 | 381.1335 | 15.65 | — |
2.13. Statistical Analysis
All the data were represented as mean ± SD. Statistical analyses were performed using GraphPad Prism version 5 with one-way ANOVA followed by Dunnett's test. p values < 0.05 were considered to be statistically significant.
3. Results and Discussion
3.1. Total Phenolic and Flavonoid Contents
It is well established that phenolic compounds from various plants are strongly associated with their biological properties. Hence, it would be worthwhile to determine the total phenolic and flavonoid contents in the plant extracts [10]. The total phenolic and flavonoid contents of methanol extract and its fractions of M. concanensis bark are presented in Table 2. The total phenolic (30.920 ± 0.71 mg GAE/g DW) and flavonoid (29.054 ± 0.09 mg QE/g DW) contents were found to be higher in the chloroform fraction followed by hexane fraction. Phenolic components are regarded as powerful antioxidants and act in redox-sensitive signaling cascades to prevent DNA damage [11].
Table 2.
Total phenolic and flavonoid contents of the methanol extract and its fractions from the bark of M. concanensis.
| Fractions | Total phenolics mg GAE/g DW | Total flavonoids mg QE/g DW |
|
| ||
| Methanol | 9.858 ± 0.036d | 3.655 ± 0.061d |
| Hexane | 14.306 ± 0.073b | 9.089 ± 0.193b |
| Chloroform | 30.920 ± 0.717a | 29.054 ± 0.099a |
| Ethyl acetate | 12.154 ± 0.158c | 4.055 ± 0.056c |
Data expressed as mean ± SD, n = 3. Data with different alphabet superscript letters show significant difference at p < 0.05, among different solvents, with multiple comparisons (one-way ANOVA, followed by Dunnett's test).
3.2. Antioxidant Activity of M. concanensis Bark Fractions
3.2.1. Free Radical Scavenging Assay
The free radical scavenging capacity of the crude methanol extract and other fractions of M. concanensis bark were determined using the DPPH assay, and the results are shown in Figure 1. All the samples exhibited varying degrees of concentration-dependent DPPH radical scavenging activity. In that, the chloroform fraction showed the strongest DPPH radical scavenging activity with the IC50 value of 6.616 ± 1.90 μgmL−1 followed by ethyl acetate fraction (IC50 = 13.4 ± 2.22 μgmL−1) which is comparable to the positive control, ascorbic acid (AA). Gori et al. [22] revealed the photoprotective effect of 11 medicinal plants Atalantia ceylanica (Arn.) Oliv, Argyreia populifolia Choisy, Hibiscus furcatus Roxb. ex DC, Ipomoea mauritiana Jacq, LAsia spinosa (L.) Thw, Leucas zeylanica (L.) W.T.Aiton, Olax zeylanica Wall., Ophiorrhiza mungos Linn., Plectranthus amboinicus (Lour.), and Mollugo cerviana (L.).Ser. where the plant that showed high SPF values is closely related to its antioxidant properties. Numerous studies also suggested that the plant fraction which shows high free scavenging properties offers significant protection towards UV-induced damage [16, 22].
Figure 1.
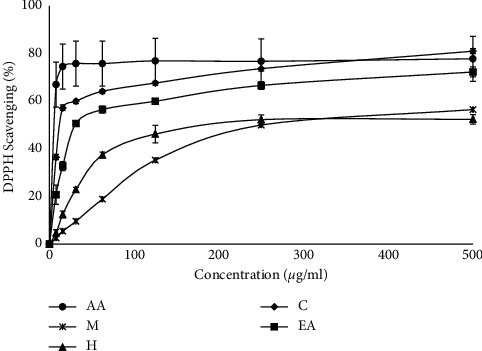
DPPH radical scavenging activity of the methanol extract and its fractions from the bark of M. concanensis. AA, ascorbic acid; M, methanol extract; H, hexane extract; C, chloroform extract; EA, ethyl acetate extract. (n = 3).
3.3. Protective Effect of the M. concanensis Fractions against UV
3.3.1. Sunscreen Protection Factor (SPF) Value
SPF value refers to the efficacy of test samples that protects the development of UV radiation-induced erythema [23]. It is a measure of UVB protection offered by sunscreen products. As the value increases, the percentage of UVB protection increases. As shown in Table 3, at the concentration of 200 μgmL−1, the chloroform fraction of M. concanensis bark exhibited the highest SPF value (10.50 ± 0.23 with >87% of UVB protection) compared to the other fractions such as ethyl acetate and hexane. The SPF value of the most active ingredient epigallocatechin gallate (EGCG) from Camellia sinensis (Green Tea) is 10.34 ± 0.21, which is almost like the SPF value of the chloroform fraction of M. concanensis bark. The literature revealed that the seed oil from M. concanensis could be a good candidate for sunscreen formulation [24]. The bark extract of medicinal plants such as Curatella americana L and Amburana cearensis (Allemao) A.C. Sm. showed SPF values 14.74 and 12.21 at 0.2 mgmL−1 which is suggested to be the promising source of future skin care products [25]. Similarly, the bark extract of M. concanensis could also be utilized as one of the encouraging ingredients in natural product sunscreen formulation.
Table 3.
The sun protection factor (SPF) value and UVB protection effect of the methanol extract and its fractions from the bark of M. concanensis.
| Samples (200 μgml−1) | SPF value | UVB protection (%) |
|
| ||
| Methanol | 4.37 ± 0.17∗∗∗ | >75 |
| Hexane | 5.49 ± 0.01∗∗∗ | >75 |
| Chloroform | 10.50 ± 0.23ns | >87 |
| Ethyl acetate | 5.18 ± 0.01∗∗∗ | >75 |
| Epigallocatechin gallate | 10.34 ± 0.21 | >87 |
Data are expressed as mean ± SD, n = 3. Data represent a significant difference at (∗∗∗) p value < 0.05, among different solvent fractions compared with the positive control (EGCG), using one-way ANOVA, followed by Dunnett's test.
3.3.2. UV Absorption Spectra
UV absorption spectra of various solvent fractions of M. concanensis and the positive control EGCG are shown in Figure 2. The results showed that all the fractions of M. concanensis have a wide range of UV absorption; however, among the other fractions, the chloroform fraction of M. concanensis showed a broad spectrum of UV absorption which covers UVA (320–400 nm), UVB (280–320 nm), and also UVC (200–280 nm). The positive control EGCG showed the absorption under the UVB range only. Similar patterns of results were observed in the active fraction of Zanthoxylum rhetsa (Roxb.) DC. bark extract which is suggested to be utilized in broad-spectrum sunscreen formulations [6]. Compared to the active fraction of Z. rhetsa, the UV absorption spectra of M. concanensis bark are high. At present, several studies and regulatory bodies recommend using broad-spectrum natural sunscreen to prevent the damages such as wrinkling and sunburns induced by both UVA and UVB, respectively [26]. The results revealed that the chloroform fraction of M. concanensis bark could be an optional ingredient to be used in a natural broad-spectrum sunscreen formulation.
Figure 2.
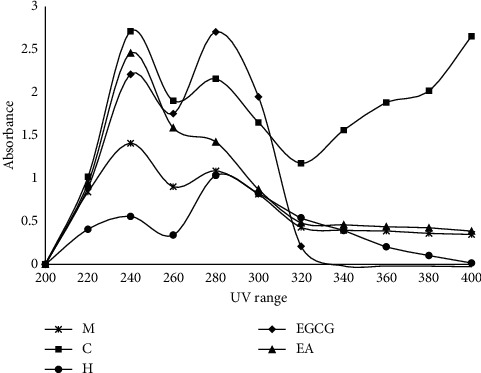
UV absorption spectra of the methanol extract and its fractions from the bark of M. concanensis. M, methanol extract; C, chloroform extract; H, hexane extract; EGCG, epigallocatechin gallate; EA, ethyl acetate extract (n = 3).
3.4. Gelatin Digestion Assay
Collagenases are one of the important matrix metalloproteinases responsible for the breakdown of collagen present in the skin layers. Studies revealed that continuous exposure of UV radiation could increase the level of collagenases that might damage the matrix proteins and cause wrinkle formation as well as lead to premature aging [27]. The collagenase inhibitory activity of extract and various solvent fractions of M. concanensis are presented in Table 4. Compared to the extract and other fractions, the chloroform fraction showed appreciable collagenase inhibitory activity of 50% at the concentration of 200 μgmL−1 which is comparable to that of EGCG. However, the hexane and ethyl acetate fractions exhibited a weaker collagenase inhibition activity. Studies revealed that various parts of medicinal plants such as leaves extracts (methanol) of Aegle marmelos (L.) Correa, Acalypha indica Linn, Calotropis gigantea (L.) W.T. Aiton, Nerium oleander L., and Nyctanthes arbor-tristis Linn and a rhizome extract of Acorus calamus. Linn at the concentration of 1 mgmL−1 showed 50% collagenase inhibitory activity [28], whereas the ethyl acetate fraction of the bark extract of Z. rhetsa showed 50% collagenase inhibition at the concentration of 500 μgmL−1 [6].
Table 4.
Gelatin digestion activity of the methanol extract and its fractions from the bark of M. concanensis.
| Samples (200 μgmL−1) | Zone inhibition (mm) | Enzyme inhibition (%) |
|
| ||
| Control | 20 ± 0.81 | 0 |
| EGCG | 10 ± 0.47∗∗∗ | 50 |
| Chloroform | 10 ± 1.24∗∗∗ | 50 |
| Hexane | 16 ± 0.47∗∗∗ | 20 |
| Ethyl acetate | 16 ± 0.47∗∗∗ | 20 |
| Methanol | 18 ± 0.81 | 10 |
Data are expressed as mean ± SD, n = 3. Data represent significant differences at (∗∗∗) p value < 0.05, among different solvent fractions compared with negative control (water), using one-way ANOVA, followed by Dunnett's test.
3.5. Antielastase
Elastase is a serine protease, responsible for the breakdown of elastin, an important protein responsible for skin elasticity [29]. The inhibitory activity of methanol extract and its fractions of M. concanensis on elastase enzyme were examined. Figure 3 and Table 5 show the effect of methanol extract and its fractions of M. concanensis bark against elastase enzyme activity. Among different fractions tested, the chloroform fraction exhibited remarkable elastase inhibition activity with the IC50 value of 2.957 ± 1.23 μgmL−1 than the standard EGCG (IC50-39.32 ± 1.41 μgmL−1). These findings demonstrated that chloroform fraction of M. concanensis may have antiaging potentials by inhibiting elastase enzyme production and delaying the degradation of elastin fibers. Researchers suggested that the plant fraction that is rich in phenolics and flavonoids is reported to possess significant antielastase activity. The ethyl acetate fraction of Garcinia daedalanthera Pierre. stem bark extract possesses 43.96 ± 12.53% elastase inhibitory effect at the concentration of 100 ppm [30]. In this study, the chloroform fraction of M. concanensis bark exhibited >50% of elastase inhibitory effect at the concentration of 31.25 μgmL−1.
Figure 3.
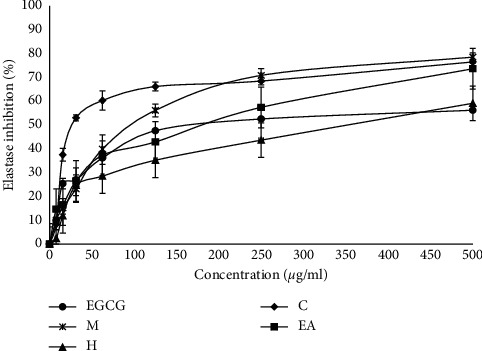
Antielastase activity of the methanol extract and its fractions from the bark of M. concanensis. Data are expressed as mean ± SD, n = 3.
Table 5.
IC50 values of elastase inhibition activity of the methanol extract and its fractions from the bark of M. concanensis.
| Samples | IC50 (μgmL−1) |
|
| |
| EGCG | 39.32 ± 1.41 |
| Methanol | 88.53 ± 1.95∗∗ |
| Hexane | 78.11 ± 1.72∗ |
| Chloroform | 2.957 ± 1.23∗∗ |
| Ethyl acetate | 176.7 ± 2.20∗∗∗ |
Data expressed as mean ± SD, n = 3. Data represent significant differences at p value < 0.05, among different solvent fractions compared with the positive control (EGCG), using one-way ANOVA, followed by Dunnett's test.
3.6. Cytotoxicity of M. concanensis Fractions in Cell Culture (3T3-L1 Cell Lines)
3.6.1. MTT Assay
Cytotoxic effect of various solvent fractions of M. concanensis was tested in 3T3-L1 cells with a wide range of concentration (0–500 μgmL−1). Results revealed that all the fractions of M. concanensis bark except the chloroform fraction were nontoxic to the cells up to 250 μgmL−1. However, the chloroform fraction of M. cocanensis was toxic to the cells at the concentration >25 μgmL−1, where it showed the strongest cytotoxic activity against 3T3-L1 cells by reducing the cell viability to 16.5% at the concentration of 62.5 μgmL−1, Figure 4. This is the first study to reveal the cytotoxic effect of various solvent fractions of M. concanensis bark against the preadipocyte cells. Previously, the ethanol extract of the leaves of M. concanensis was also reported to be nontoxic to 3T3-L1 adipocytes up to 100 μgmL−1 until 24 h of treatment [15]. Balamurugan et al. [31] tested the crude ethanol extract of M. concanensis leaves and bark against HepG2 cell lines and revealed that both parts of M. concanensis strongly inhibit the growth of cancerous cells in a dose-dependent manner. In another study, the methanolic root bark extract of M. concanensis was found to be toxic against HepG2 cells, whereas it does not induce toxic effect against A549 and HT-29 cell line which demonstrates its selective cytotoxicity [21]. In this study, the crude methanol extract of M. concanensis bark does not induce any toxicity against 3T3-L1 cells up to 500 μgmL−1. Since the other solvent fractions of M. concanensis bark have toxic effect >250 μgmL−1 and the chloroform fraction has toxic effect >25 μgmL−1, on an average, we reduced the concentration range of all the extracts/fractions to 0–100 μgmL−1 for further assay.
Figure 4.
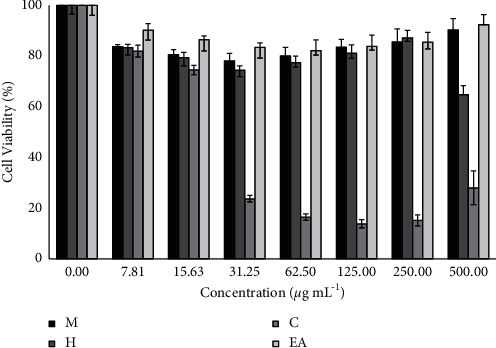
Cytotoxic effect of the methanol extract and various solvent fractions of M. concanensis bark treated with 3T3 mouse fibroblasts for 24 h data expressed as mean ± SD, n = 3.
3.6.2. H2O2-Induced Damage
The protective effect of various solvent fractions of M. concanensis bark against H2O2-induced oxidative damage in 3T3-L1 was determined and shown in Figure 5. From the results, it has been clear that the H2O2 (125 μM) reduced the viability of cells to 15.35% after 6 h of treatment, whereas the cells pretreated with various solvent fractions of M. concanensis bark prevented the cells from oxidative damage. However, the level of protection by each fraction varies and it depends on the concentration and constituents present in each sample. The chloroform fraction of M. concanensis bark offers significant protection against H2O2-induced damage even at low concentration, where the viability of cells treated with 1.56 μgmL−1 protects 56% of cells which was comparable to that of positive control, AA (cell viability—57%). As the concentration increases, up to 12.5 μgmL−1, the chloroform fraction prevented the cells up to 72% where AA prevented up to 69%. However, at the concentration of 25 μgmL−1 and above, the viability of cells treated with chloroform fraction got drastically reduced. This might be due to the overdose of chemical constituents present in it. Similar patterns of results were obtained in the cytotoxicity assay, and at low concentration (<15 μgmL−1), the chloroform fraction was nontoxic to 3T3-L1 cells. As the concentration increases, the chloroform fraction is toxic. As reported, the extracts and fractions of various parts of M. concanensis are cytotoxic to different cancer cell lines like HepG2 [31, 32], and the chloroform fraction of M. concanensis bark could offer a significant cytotoxic effect in other cancerous cell lines. Further research is needed to evaluate the selective cytotoxic effect of chloroform fraction of M. concanensis bark.
Figure 5.
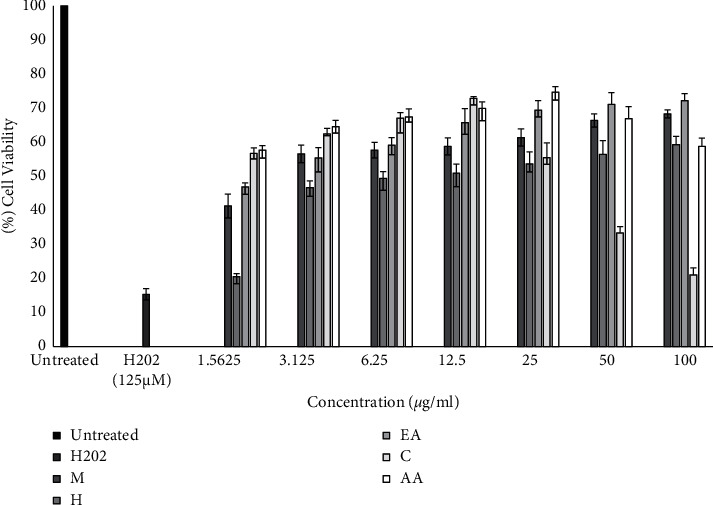
Protective effect of various solvent fractions of M. concanensis bark against H2O2-induced oxidative damage in 3T3-L1 cells treated for 24 h. Data are expressed as mean ± SD, n = 3. Data represent significant difference at p value < 0.05, among different solvent fractions of M. concanensis bark (various concentrations) compared with the H2O2 (125 μM), using one-way ANOVA, followed by Dunnett's test.
From this study, it has been evident that compared to other fractions, the chloroform fraction of M. concanensis bark has a high sunscreen protection factor value, broad-spectrum UV absorption, appreciable DPPH scavenging, anticollagenase, antielastase, high phenolic, and high flavonoid content. Additionally, it also prevents the H2O2-induced oxidative damage in 3T3-L1 cells which was comparable to that of positive control, AA.
3.7. UPLC-QTOF/MS Analysis
From this study, it has been identified that the chloroform fraction is the bioactive fraction of M. concanensis bark. UPLC-QTOF/MS analysis was found to be a convenient method to determine the presence of possible phytoconstituents in the studied fraction through the exact mass detection. Polyphenolic compounds such as phenolic acids and flavonoids are naturally abundant and commonly found in the genus Moringa. Bioactive flavonols such as quercetin, hyperoside, astragalin, isoquercetin, kaempferol-3-O-rutinoside, and rutin were reported previously in this genus especially in the species, Moringa oleifera [20]. In this study, 20 different phytoconstituents were identified in the chloroform fraction obtained from the methanol extract of M. concanensis bark using UPLC-QTOF/MS analysis, Table 1. The result indicated that the chloroform fraction contains a complex mixture of bioactive tentative annotated phenolics such as kaempferol-3-O-rutinoside, astragalin, quercetin-3-O-glucuronide, kaempferol 3-O-glucuronopyranosyl methyl ester, isoquercetin, kaempferol-3-O-arabinoside, kaempferol-3-O-rhamnoside, quercetin-3-O-glucuronide-methyl ester, eugenol, quercetin, and caffeic acid. The assignations were carried out on a prediction basis and detailed phytochemistry studies required to be carried out on this species for the affirmation of the presence of the predicted metabolites in the studied fraction/extract in the future. This comprehensive phytochemical profiling analysis has revealed the structural diversity of phenolics and their similar structural patterns as well as the biological potency of the studied fractions.
Moreover, the findings are in agreement with the results obtained from the total phenolic and total flavonoids evaluation conducted on the respective fractions. The existence of flavonoids, especially flavonols, could possibly be the main contributors to photoprotective properties especially in collagenase and elastase inhibitory activities through a synergistic effect [33]. Furthermore, the presence of carbonyl and hydroxyl moieties in the main scaffold of the flavonols might be played the role in giving the inhibitory effects which allow them to bind to metalloenzymes like collagenase and elastase that could alter or inhibit metabolic pathways [34]. However, further studies are required in relation to the detailed identification and isolation of bioactive metabolites from the bark of M. concanensis as well as molecular mechanisms of their photoprotective potentials.
4. Conclusion
In summary, the result obtained from the phytochemical analysis showed that chloroform fractions of M. concanensis have the highest DPPH, TPC, and TFC values. The photoprotective study also demonstrated a high SPF value for chloroform fractions and gave the same UVB protection as its positive control, EGCG. Besides that, the chloroform fractions have a much broader spectrum of UV absorption that covers both UVA and UVB when compared to others. The inhibitory effect of M. concanensis chloroform fractions towards elastase and collagenase proved that the plant extract has potential biological properties that can reduce the breakdown of collagen and elastin within the skin. In vitro study showed that some of the fractions possessed low cytotoxicity towards the 3T3-L1 cell line even at higher concentrations (methanol, hexane, and ethyl acetate). However, chloroform fractions obtained from the plant extract exhibit high cytotoxic activity even at lower concentrations (ranging from 31.25 to 500 μgmL−1). All the fractions displayed protective effect against H2O2-induced oxidative damage in 3T3-L1 cells, with methanol, hexane, and ethyl acetate having a higher cell viability percentage even at higher concentrations (50 and 100 μgmL−1). Various metabolites especially bioactive flavonols that were tentatively identified using UPLC-QTOF/MS analysis might be the source for the high photoprotective activity. Based on these results, M. concanensis has the potential to be a new applicant as an active component in pharmaceutical and cosmetics formulation. However, detailed preclinical research is needed to demonstrate the individual or synergistic effect of the bioactive components involved in the photoprotective mechanism of M. concanensis.
Acknowledgments
The authors would like to thank Universiti Malaysia Terengganu and Universiti Sains Malaysia for the facilities provided. This research was funded by Universiti Malaysia Terengganu, Malaysia, under the Talent and Publication Enhancement Research Grant (TAPE-RG) scheme. Vote Number: 55256.
Contributor Information
Rameshkumar Santhanam, Email: ramesh@umt.edu.my.
Thiruventhan Karunakaran, Email: thiruventhan@usm.my.
Data Availability
Data used to support the findings of this study are obtained from the corresponding author.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
R.S., K.S., and T.K. conceptualized the study; R.S. was responsible for methodology; W.I.W.I., R.S., and T.K. validated the data; M.F.Z., R.S., and T.K. performed formal analysis and investigation; A.V. was responsible for resources; R.S., M.F.Z., and G.K.M. performed experiments; R.S., K.S., T.K., M.F.Z., and V.S. prepared the original draft and reviewed and edited the manuscript; R.S. and W.I.W.I. supervised the study and were responsible for funding acquisition; all authors have read and agreed to the published version of the manuscript.
References
- 1.Verma A., Kushwaha H. N., Srivastava A. K., et al. Piperine attenuates UV-R induced cell damage in human keratinocytes via NF-kB, Bax/Bcl-2 pathway: an application for photoprotection. Journal of Photochemistry and Photobiology B: Biology . 2017;172:139–148. doi: 10.1016/j.jphotobiol.2017.05.018. [DOI] [Google Scholar]
- 2.Wang Y.-S., Zhou S.-S., Shen C.-Y., Jiang J.-G. Isolation and identification of four antioxidants from Rhodiola crenulata and evaluation of their UV photoprotection capacity in vitro. Journal of Functional Foods . 2020;66 doi: 10.1016/j.jff.2020.103825.103825 [DOI] [Google Scholar]
- 3.Radice M., Manfredini S., Ziosi P., et al. Herbal extracts, lichens and biomolecules as natural photo-protection alternatives to synthetic UV filters. A systematic review. Fitoterapia . 2016;114:144–162. doi: 10.1016/j.fitote.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Schneider G., Figueroa F. L., Vega J., et al. Photoprotection properties of marine photosynthetic organisms grown in high ultraviolet exposure areas: cosmeceutical applications. Algal Research . 2020;49 doi: 10.1016/j.algal.2020.101956.101956 [DOI] [Google Scholar]
- 5.Sies H. Nutritional protection against skin damage from sunlight. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics . 2006;15:462–463. [Google Scholar]
- 6.Santhanam R. K., Fakurazi S., Ahmad S., et al. Inhibition of UVB-induced pro-inflammatory cytokines and MMP expression by Zanthoxylum rhetsa bark extract and its active constituent hesperidin. Phytotherapy Research . 2018;32(8):1608–1616. doi: 10.1002/ptr.6092. [DOI] [PubMed] [Google Scholar]
- 7.Kammeyer A., Luiten R. M. Oxidation events and skin aging. Ageing Research Reviews . 2015;21:16–29. doi: 10.1016/j.arr.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Mota M. D., da Boa Morte A. N., Silva L. C. R. C. e., Chinalia F. A. Sunscreen protection factor enhancement through supplementation with Rambutan (Nephelium lappaceum L) ethanolic extract. Journal of Photochemistry and Photobiology B: Biology . 2020;205 doi: 10.1016/j.jphotobiol.2020.111837.111837 [DOI] [Google Scholar]
- 9.Saewan N., Jimtaisong A. Natural products as photoprotection. Journal of Cosmetic Dermatology . 2015;14(1):47–63. doi: 10.1111/jocd.12123. [DOI] [PubMed] [Google Scholar]
- 10.Lin D., Xiao M., Zhao J., et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules . 2016;21(10) doi: 10.3390/molecules21101374.1374 [DOI] [Google Scholar]
- 11.Boo Y. C. Can plant phenolic compounds protect the skin from airborne particulate matter? Antioxidants . 2019;8(9):p. 379. doi: 10.3390/antiox8090379. [DOI] [Google Scholar]
- 12.Ravichandran V., Arunachalam G., Subramanian N., Suresh B. Pharmacognostical and phytochemical investigations of Moringa concanensis (Moringaceae) an ethnomedicine of Nilgiris. Journal of Pharmacognosy and Phytotherapy . 2009;1:76–81. [Google Scholar]
- 13.Manzoor M., Anwar F., Iqbal T., Bhanger M. I. Physico-chemical characterization of Moringa concanensis seeds and seed oil. Journal of the American Oil Chemists’ Society . 2007;84(5):413–419. doi: 10.1007/s11746-007-1055-3. [DOI] [Google Scholar]
- 14.Anbazhakan S., Dhandapani R., Anandhakumar P., Balu S. Traditional medicinal knowledge on Moringa concanensis Nimmo of perambalur district, tamilnadu. Ancient Science of Life . 2007;26:42–45. [PMC free article] [PubMed] [Google Scholar]
- 15.Balakrishnan B. B., Krishnasamy K., Choi K. C. Moringa concanensis Nimmo ameliorates hyperglycemia in 3T3-L1 adipocytes by upregulating PPAR-gamma, C/EBP alpha via Akt signaling pathway and STZ- induced diabetic rats. Biomedicine & Pharmacotherapy . 2018;103:719–728. doi: 10.1016/j.biopha.2018.04.047. [DOI] [PubMed] [Google Scholar]
- 16.Santhanam R., Ahmad S., Abas F., Ismail I. S., Rukayadi Y., Shaari K. Photoprotective properties of Zanthoxylum rhetsa: an in vitro analysis. Journal of Chemical and Pharmaceutical Research . 2013;5:1512–1520. [Google Scholar]
- 17.Wang C., Gao X., Santhanam R. K., et al. Effects of polysaccharides from Inonotus obliquus and its chromium (III) complex on advanced glycation end-products formation, α-amylase, α-glucosidase activity and H2O2-induced oxidative damage in hepatic L02 cells. Food and Chemical Toxicology . 2018;116:335–345. doi: 10.1016/j.fct.2018.04.047. [DOI] [PubMed] [Google Scholar]
- 18.Raseetha S., Zulkifli M. F., Nurul-Nabilah M., Ismail W. I. W., Jayasinghe L. Inhibition of lipid accumulation in 3T3-L1 adipocytes by chlorogenic acid derived from green coffee (Robusta sp.) beans and pulps. Malaysian Applied Biology . 2017;46:163–170. [Google Scholar]
- 19.Chen W., Liu Y., Xue G., Zhang L., Shao S. Diazoxide protects L6 skeletal myoblasts from H2O2-induced apoptosis via the phosphatidylinositol-3 kinase/Akt pathway. Inflammation Research . 2016;65:53–60. doi: 10.1007/s00011-015-0890-1. [DOI] [PubMed] [Google Scholar]
- 20.Lin H., Zhu H., Tan J., et al. Comparative analysis of chemical constituents of Moringa oleifera leaves from China and India by ultra-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. Molecules . 2019;24(5):p. 942. doi: 10.3390/molecules24050942. [DOI] [Google Scholar]
- 21.Vijayarajan M., Pandian R.-M. Cytotoxicity of methanol and acetone root bark extracts of Moringa concanensis against A549, hep-G2 and HT-29 cell lines. J. Acad. ind. Res, . 2016;5:45–49. [Google Scholar]
- 22.Gori A., Brunetti C., dos Santos Nascimento L. B., et al. Photoprotective role of photosynthetic and non-photosynthetic pigments in Phillyrea latifolia: is their “Antioxidant” function prominent in leaves exposed to severe summer drought? International Journal of Molecular Sciences . 2021;22(15):p. 8303. doi: 10.3390/ijms22158303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geoffrey K., Mwangi A. N., Maru S. M. Sunscreen products: rationale for use, formulation development and regulatory considerations. Saudi Pharmaceutical Journal . 2019;27(7):1009–1018. doi: 10.1016/j.jsps.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kale S., Gajbhiye G., Chaudhari N. Determination and comparison of in vitro SPF of topical formulation containing lutein ester from Formulation and in- vitro Evaluation of Moringa concanensis, Nimmo. Seed Oils Sunscreen Cream. Int. J. Pharmtech Res, . 2010;2:2060–2062. [Google Scholar]
- 25.Nunes A. R., Rodrigues A. L. M., de Queiróz D. B., et al. Photoprotective potential of medicinal plants from Cerrado biome (Brazil) in relation to phenolic content and antioxidant activity. Journal of Photochemistry and Photobiology B: Biology . 2018;189:119–123. doi: 10.1016/j.jphotobiol.2018.10.013. [DOI] [Google Scholar]
- 26.Lionetti N., Rigano L. The new sunscreens among formulation strategy, stability issues, changing norms, safety and efficacy evaluations. Cosmetics . 2017;4(2):p. 15. doi: 10.3390/cosmetics4020015. [DOI] [Google Scholar]
- 27.Maeda K. Analysis of ultraviolet radiation wavelengths causing hardening and reduced elasticity of collagen gels in vitro. Cosmetics . 2018;5(1):p. 14. doi: 10.3390/cosmetics5010014. [DOI] [Google Scholar]
- 28.Iswarya S., Radhakrishnan N., Kavitha V., Mandal A. B., Gnanamani A. Collagenase inhibition activity of Indian medicinal plants: an approach to moderate collagen turnover. International Journal of Bioassays . 2014;3:2075–2078. [Google Scholar]
- 29.Thring T. S., Hili P., Naughton D. P. Anti‐collagenase, anti‐elastase and anti‐oxidant activities of extracts from 21 plants. BMC Complementary and Alternative Medicine . 2009;9(1):p. 27. doi: 10.1186/1472-6882-9-27. [DOI] [Google Scholar]
- 30.Ambarwati N. S. S., Elya B., Desmiaty Y., Omar H. Anti-elastase of leaves and stem bark extract of Garcinia daedalanthera Pierre. International Journal of Pharma Sciences and Research . 2020;12:592–596. [Google Scholar]
- 31.Balamurugan V., Balakrishnan V., Robinson P. J., Ramakrishan M. Anti-cancer and apoptosis-inducing effects of Moringa concanensis using hepG2 cell lines. Bangladesh Journal of Pharmacology . 2014;9:604–609. doi: 10.3329/bjp.v9i4.20481. [DOI] [Google Scholar]
- 32.Sivakumar D., Manikandan R., Velu P. Antimicrobial activity of Moringa concanensis flower against human pathogens and its cytotoxic effects on HepG2 cell Line. Research Journal of Microbiology . 2020;15:51–60. [Google Scholar]
- 33.Sin B. Y., Kim H. P. Inhibition of collagenase by naturally-occurring flavonoids. Archives of Pharmacal Research . 2005;28(10):1152–1155. doi: 10.1007/bf02972978. [DOI] [PubMed] [Google Scholar]
- 34.Zofia N. Ł., Martyna Z. D., Aleksandra Z., Tomasz B. Comparison of the antiaging and protective properties of plants from the Apiaceae family. Oxidative Medicine and Cellular Longevity . 2020;2020 doi: 10.1155/2020/5307614.5307614 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used to support the findings of this study are obtained from the corresponding author.


