Abstract
The antimalarial trioxaquine derivative DU-1102, synthesized by covalent linkage between aminoquinoline and trioxane moieties, was highly active against Cameroonian isolates (mean 50% inhibitory concentration of 43 nmol/liter) of Plasmodium falciparum. There was no correlation between the responses to DU-1102 and chloroquine and only a low correlation between the responses to DU-1102 and pyrimethamine, suggesting an independent mode of action of the trioxaquine against the parasites.
Because of the extensive spread of drug resistance involving primarily 4-aminoquinolines and antifolate drugs in most of the geographic areas where Plasmodium falciparum is endemic, there is a need for a sustained search for promising compounds with new chemical structures and mechanisms of action. In the past 2 decades, only a few compounds belonging to a new class of antimalarial drugs, including aminoalcohols (mefloquine, halofantrine, lumefantrine), sesquiterpene trioxanes (artemisinin derivatives), and naphthoquinones (atovaquone), were developed for commercialization.
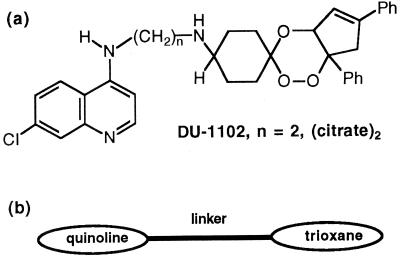
DU-1102 (Fig. 1a) is a new candidate compound that belongs to a novel chemical class named trioxaquine (7, 11) (Fig. 1b). Trioxaquines are new modular molecules obtained by covalently attaching a trioxane motif, present in artemisinin, to a 4-aminoquinoline entity, contained in chloroquine. As the trioxane moiety is a potential alkylating agent after reductive activation by heme (6, 10, 15–18) and the 4-aminoquinoline entity is known to easily penetrate within infected erythrocytes (9) and then interact with heme, such modular molecules are expected to combine the properties of both fragments. Although it remains to be experimentally confirmed, we hypothesized that trioxaquine is able to penetrate infected red blood cells and then interact with the free heme liberated during the hemoglobin digestion.
FIG. 1.
(a) Chemical structure of DU-1102; (b) general structure of trioxaquines.
The in vitro activity of DU-1102 was assessed against clinical isolates of P. falciparum obtained in Yaoundé, Cameroon, where previous in vitro and in vivo studies have shown a high proportion (>40%) of chloroquine and/or pyrimethamine resistance (3, 14). We also evaluated the potential for in vitro cross-resistance between DU-1102 and chloroquine and pyrimethamine, which are first- and second-line (pyrimethamine-sulfadoxine combination) drugs in Cameroon, respectively.
Fresh clinical isolates of P. falciparum were obtained before treatment from adult Cameroonian patients attending the Nlongkak Catholic missionary dispensary in Yaoundé in 2000. The patients presented with signs and symptoms of acute uncomplicated malaria and had at least 0.1% asexual parasitemia. The Saker-Solomons colorimetric test for 4-aminoquinolines, quinine, and antifolate drugs in urine was negative for each patient (12). The patients were treated with oral amodiaquine, according to the national antimalarial guidelines set by the Cameroonian Ministry of Public Health. The study was approved by the Cameroonian National Ethics Committee and the Cameroonian Ministry of Public Health.
Chloroquine sulfate was obtained from Rhône-Poulenc-Rorer (Antony, France). Pyrimethamine base was obtained from Sigma Chemical Company (St. Louis, Mo.). The procedures involved in the chemical synthesis of DU-1102 (molecular weight = 952.4) were recently reported (7) (it should be noted that DU-1102 is a new serial number of the trioxaquine derivative named ODC218 in reference 7). Stock solutions of chloroquine, pyrimethamine, and DU-1102 were prepared in sterile distilled water, absolute ethanol, and dimethyl sulfoxide, respectively. Twofold serial dilutions of chloroquine (final concentration, 25 to 1,600 nmol/liter) and DU-1102 (final concentration, 3.8 to 484 nmol/liter) and fourfold dilutions of pyrimethamine (final concentration, 0.05 to 51,200 nmol/liter) were prepared in sterile distilled water. The final concentration of dimethyl sulfoxide was <0.1%, which is nontoxic for parasite growth. Each concentration was distributed in triplicate in 96-well tissue culture plates. The isotopic microtest developed by Desjardins et al. was used in this study (2, 8). The suspension of infected erythrocytes (200 μl) was distributed in each well of the 96-well tissue culture plates. The parasites were incubated at 37°C in 5% CO2 for 18 h. To assess parasite growth, [3H]hypoxanthine (specific activity, 16.3 Ci/mmol; 1 μCi/well; Amersham, Little Chalfont, Buckinghamshire, United Kingdom) was added. After an additional 24 h of incubation (48 h for pyrimethamine and DU-1102), the plates were frozen to terminate the in vitro assay. The incorporation of [3H]hypoxanthine was quantitated using a liquid scintillation counter, and the 50% inhibitory concentrations (IC50s) were determined by nonlinear regression analysis.
The threshold IC50s for in vitro resistance to chloroquine and pyrimethamine were estimated to be >100 nmol/liter (3, 14). Data were expressed as geometric mean IC50s. The mean IC50s of DU-1102 for the chloroquine-sensitive and the chloroquine-resistant isolates were compared using the unpaired t test. Correlation of the IC50s of different drugs was calculated by Spearman rank correlation. The significance level was fixed at 0.05.
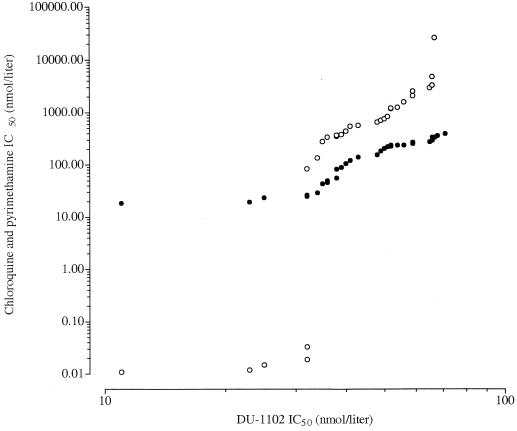
The in vitro activity of DU-1102 against 32 P. falciparum isolates was assessed. Thirteen isolates (41%) were chloroquine sensitive (mean IC50 = 36.0 nmol/liter; range, 18.6 to 90.0 nmol/liter), and 19 (59%) were chloroquine resistant (mean IC50 = 227 nmol/liter; range, 106 to 390 nmol/liter) (Fig. 2). Thirty of 32 isolates developed adequately in p-aminobenzoic acid- and folic acid-free RPMI 1640 culture medium used to determine the in vitro activity of pyrimethamine. Six isolates (20%) were pyrimethamine sensitive (geometric mean IC50 = 0.068 nmol/liter; range, 0.011 to 84.3 nmol/liter), and 24 (80%) were pyrimethamine resistant (geometric mean IC50 = 922 nmol/liter; range, 136 to 26,000 nmol/liter). The geometric mean IC50 of DU-1102 was 43.1 nmol/liter (range, 11.2 to 71.0 nmol/liter). There was no significant difference (P > 0.05) between the mean IC50s of DU-1102 for chloroquine-sensitive isolates (48 nmol/liter) and chloroquine-resistant isolates (40 nmol/liter). Moreover, the IC50s of DU-1102 were not correlated with those of chloroquine (coefficient of correlation [r] = −0.140; n = 32; P > 0.05). The correlation coefficient between the IC50s of DU-1102 and those of pyrimethamine was low (−0.439 for pyrimethamine-resistant isolates; P < 0.05). This result seems to suggest a low but existing probability of crossresistance between pyrimethamine and DU-1102. In fact, because 80% of the isolates used in this study were pyrimethamine resistant, the low correlation between the responses to pyrimethamine and DU-1102 is most likely due to the skewed distribution of resistant parasites.
FIG. 2.
Scatterplots showing the in vitro activities of DU-1102 versus chloroquine (black circles) and pyrimethamine (open circles) against Cameroonian clinical isolates of P. falciparum.
A previous study on other trioxaquine derivatives has shown that these compounds are highly active against chloroquine-sensitive and chloroquine-resistant reference clones of P. falciparum, with IC50s of <90 nmol/liter and in most cases of <40 nmol/liter (7). The trioxaquine derivative DU-1102 used in the present study is in fact the molecule which has been considered, up to now, the most active when using reference clones of P. falciparum. This trioxaquine has citrate as a counterion, and the linker between the trioxane and the aminoquinoline structure contains two methylene groups (Fig. 1a).
Combination in antimalarial therapy is advocated by many malaria researchers as one of the means to fight against the spread of resistance to classic and new drugs (13, 19). By analogy with this therapeutic approach, trioxaquines have been prepared by linking two active pharmacophores. The quinoline nucleus has been a chemical reference of highly active antimalarial drugs for many decades, and several effective drugs containing this entity (chloroquine, amodiaquine, primaquine, quinine, and mefloquine) have been developed. Trioxane contains a peroxide bridge which has been shown to be essential for the high activity of artemisisnin (1). The reductive activation of artemisinin and other endoperoxide-containing drugs by FeII-heme generates alkylating C-centered radicals (4, 5, 10, 15). The derivatives containing trioxane moiety, including artemether, arteether, dihydroartemisinin, and artesunate, are currently used as efficient and rapidly acting antimalarial drugs (18). Although the exact mechanism of action of trioxaquines is still under investigation, the available in vitro data indicate promising results for further development of this new class of antimalarial drugs based on a modular molecule strategy.
Acknowledgments
We are grateful to Sr. Marie Solange Oko and the nursing and laboratory staff at the Nlongkak Catholic missionary dispensary (Yaoundé, Cameroon) for invaluable help in recruiting patients.
This study was supported by the French Ministry of Research and Education.
REFERENCES
- 1.Avery M A, Gao F, Chong W K M, Mehrotra S, Milhous W K. Structure-activity relationships of the antimalarial artemisinin. 1. Synthesis and comparative molecular field analysis of C-9 analogs of artemisinin and 10-deoxoartemisinin. J Med Chem. 1993;36:4264–4275. doi: 10.1021/jm00078a017. [DOI] [PubMed] [Google Scholar]
- 2.Basco L K, Bickii J, Ringwald P. In vitro activity of lumefantrine (benflumetol) against clinical isolates of Plasmodium falciparum in Yaoundé, Cameroon. Antimicrob Agents Chemother. 1998;42:2347–2351. doi: 10.1128/aac.42.9.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basco L K, Ringwald P. Molecular epidemiology of malaria in Yaoundé, Cameroon. IV. Evolution of pyrimethamine resistance between 1994 and 1998. Am J Trop Med Hyg. 1999;61:802–806. doi: 10.4269/ajtmh.1999.61.802. [DOI] [PubMed] [Google Scholar]
- 4.Cazelles J, Robert A, Meunier B. Alkylation of heme by artemisinin, an antimalarial drug. C R Acad Sci Ser II C. 2001;4:85–89. [Google Scholar]
- 5.Cumming J N, Ploypradith P, Posner G H. Antimalarial activity of artemisinin (quinghaosu) and related trioxanes: mechanism(s) of action. Adv Pharmacol. 1997;37:253–297. doi: 10.1016/s1054-3589(08)60952-7. [DOI] [PubMed] [Google Scholar]
- 6.Cumming J N, Wang D, Park S B, Shapiro T A, Posner G H. Design, synthesis, derivatization, and structure-activity relationships of simplified, tricyclic, 1,2,4-trioxane-alcohol analogues of the antimalarial artemisinin. J Med Chem. 1998;41:952–964. doi: 10.1021/jm970711g. [DOI] [PubMed] [Google Scholar]
- 7.Dechy-Cabaret O, Benoit-Vical F, Robert A, Meunier B. Preparation and antimalarial activities of “trioxaquines”, new modular molecules with a trioxane skeleton linked to a 4-aminoquinoline. ChemBio Chem. 2000;1:281–283. doi: 10.1002/1439-7633(20001117)1:4<281::AID-CBIC281>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 8.Desjardins R E, Canfield C J, Haynes J D, Chulay J D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egan T J, Marques H M. The role of haem in the activity of chloroquine and related antimalarial drugs. Coord Chem Rev. 1999;190–192:493–517. [Google Scholar]
- 10.Meshnick S R, Taylor T E, Kamchonwongpaisan S. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiol Rev. 1996;60:301–315. doi: 10.1128/mr.60.2.301-315.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meunier, B., A. Robert, O. Dechy-Cabaret, and F. Benoit-Vical. April 2000. French patent 0004422. [DOI] [PubMed]
- 12.Mount D L, Nahlen B L, Patchen L C, Churchill F C. Adaptations of the Saker-Solomons test: simple, reliable colorimetric field assays for chloroquine and its metabolites in urine. Bull WHO. 1989;67:295–300. [PMC free article] [PubMed] [Google Scholar]
- 13.Peters W. The prevention of antimalarial drug resistance. Pharmacol Ther. 1990;47:499–508. doi: 10.1016/0163-7258(90)90067-c. [DOI] [PubMed] [Google Scholar]
- 14.Ringwald P, Bickii J, Basco L. Randomised trial of pyronaridine versus chloroquine for acute uncomplicated falciparum malaria in Africa. Lancet. 1996;347:24–28. doi: 10.1016/s0140-6736(96)91558-5. [DOI] [PubMed] [Google Scholar]
- 15.Robert A, Meunier B. Characterization of the first covalent adduct between artemisinin and a heme model. J Am Chem Soc. 1997;119:5968–5969. [Google Scholar]
- 16.Robert A, Meunier B. Alkylating properties of antimalarial artemisinin derivatives and synthetic trioxanes when activated by a reduced heme model. Chem Eur J. 1998;4:1287–1296. [Google Scholar]
- 17.Robert A, Meunier B. Is alkylation the main mechanism of action of the antimalarial drug artemisinin? Chem Soc Rev. 1998;27:273–279. [Google Scholar]
- 18.Vroman J A, Alvim-Gaston M, Avery M A. Current progress in the chemistry, medicinal chemistry and drug design of artemisinin based antimalarials. Curr Pharm Des. 1999;5:101–138. [PubMed] [Google Scholar]
- 19.White N J, Olliaro P L. Strategies for the prevention of antimalarial drug resistance: rationale for combination chemotherapy for malaria. Parasitol Today. 1996;12:399–401. doi: 10.1016/0169-4758(96)10055-7. [DOI] [PubMed] [Google Scholar]