Abstract
Introduction
The history of insulin-induced skin lipohypertrophy (LH) runs parallel to that of insulin's 100 years, and an average of 47% of insulin-treated patients still suffer from it today. The metabolic and economic effects of LH are significant, with hypoglycemia being the most striking. The objective of the study was to perform a 52-week follow-up of 713 insulin-treated patients with type 2 diabetes (T2DM) and LH to detect any differences in the occurrence of hypoglycemic events (HYPOs) and related healthcare costs as well as in LH rates and injection habits between an intensive education intervention group (IG) and control group (CG) provided with a single educational session at the starting point.
Methods
All participants were trained in accurately self-monitoring blood glucose and recording all HYPOs for 6 months, which allowed baseline recordings before they were randomized into the IG, comprising 395 insulin-treated subjects undergoing repeated, structured multimodal education on correct injection techniques as a longstanding behavioral rehabilitation strategy, and the CG, comprising 318 subjects receiving the same structured, multimodal educational session, but only initially.
Results
Changes in LH rate and size and in performance were large in the IG and only slight and transient in the CG. A striking difference in the rate of decrease of HYPOs was also apparent between groups. Indeed, estimated costs of health interventions for severe and symptomatic HYPOs, which were on the order of €70,000 and €9300, respectively, in the two groups at baseline decreased by 5.9 times and 13.7 times, respectively, at the end of follow-up in the IG and by only approximately half in the CG. Full details of the changes occurring as a result of intensive education are provided in the text.
Conclusions
The effect of only initial education in the CG was not significant, thus providing evidence of the virtual worthlessness of a single training session on injection techniques, typical of worldwide daily clinical practice, and easily explaining the extremely high prevalence of LH in insulin-treated patients. Conversely, highly positive effects on LH prevalence and size as well as costs expected from decreased HYPO rate were obtained in the IG. To our knowledge, ours is the first 18-month randomized trial in the field. If our experimental model were to be used as an effective, longstanding behavioral rehabilitation strategy and therefore adapted to real-world settings universally, LH prevalence and costs related to their clinical consequences would be drastically reduced. However, only with a strong, relentless commitment of universities, scientific societies, and patient associations can we achieve this ambitious goal, which would provide great institutional savings and improved quality of life for people with diabetes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02105-5.
Keywords: Diabetes, Education, Economic burden, Hypoglycemia, Lipohypertrophy, Rehabilitation
Key Summary Points
| Lipohypertrophy (LH) is a complication affecting almost 50% of insulin-treated patients and is responsible for poor metabolic control and hazardous, unpredictable hypoglycemic events (HYPOs). |
| In our study, a single education session on correct injection techniques, which reflects everyday clinical practice worldwide, failed to improve unhealthy habits or decrease the LH rate efficiently. |
| To our knowledge, our study was the first 18-month educational trial to show that a relentless behavioral rehabilitation strategy can sustainedly and dramatically decrease LH-related prevalence of HYPOs. |
| If adapted to individual contexts, our experimental model can dramatically reduce the high costs of LH-related HYPOs and improve the quality of life of insulin-treated patients worldwide. |
Introduction
Skin lipohypertrophy (LH) is a common complication of insulin therapy. LH is characterized by the enlargement and proliferation of adipocytes in the subcutaneous tissue arising from the anabolic effect of exogenous insulin exposure and enhanced fibrin production [1].
Repeated needle utilization, missed injection site rotation, and ice-cold insulin administration are recognized LH risk factors [2, 3].
The prevalence of LH in persons with diabetes has been variably reported according to the different methods used for case definitions and study populations [4]. A recent meta-analysis of 45 studies reported a pooled prevalence estimate of 41.8% (95% CI: 35.9–47.6%) in insulin users [5], which, despite the considerable identification method-related variability, showed the wide scale of the LH issue.
Injections into lipohypertrophic tissue can change insulin absorption and action [6–9] with consequent sudden blood glucose elevations [6], variability, and hazardous, unpredictable drops [7–10], presenting at least suboptimal metabolic control conditions [10, 11]. All these consequences have an economic impact on the health system, as preliminary data indicate that optimal insulin injection habits reduce the daily doses—and therefore the costs—of insulin by about 20% [7]. However, as shown by our preliminary short report in a small cohort of patients with Type 1 diabetes mellitus (T1DM), decreasing the rate of only one LH complication, i.e., hypoglycemia, could have a great impact on health and social costs [12].
Recently, our group published two papers on the effects of education on subjects used to injecting insulin into lipodystrophic areas: after being trained again in correct injection techniques in two repeated 6-month follow-up periods, the patients experienced a significantly lower number and severity of hypoglycemic episodes, consequently with less frequent National Health System service utilization and patient/caregiver absences from work [2, 3].
The aim of our study was to evaluate the real economic burden of hypoglycemia before and after education compared to the cost of educational training on correct injection techniques in a large cohort of patients with LH for an entire 12-month observation period, which to our knowledge has never been done before. Another endpoint was to assess the durability of a single albeit structured and multimodal education session on correct injection techniques reflecting typical real-life conditions.
Methods
The study was designed as a two-arm, open-label, multicenter, randomized, 18-month case-control study. The study protocol was approved by the Ethics and Science Committee of the reference center, University Luigi Vanvitelli, Naples, Italy (trial registration no. 120-16.02.2019), which serves as the central reference ethics committee for all participating diabetes centers contributing to the Nefrocenter Research Network private consortium associated with the above-mentioned university and supported by the National Health System (SSN). Healthcare professionals (HCPs) were well trained to accurately follow the study procedures. Considering the age of enrolled subjects, the exclusion of CKD was based on serum creatinine levels within the normal range with eGFR > 75 ml/min/1.73 m2 and the absence of macroalbuminuria.
The study was carried out following the original Declaration of Helsinki and its subsequent amendments. T2DM patients were enrolled in the study when regularly attending any diabetes centers (DCs) involved in the study, meeting the inclusion criteria, and giving their informed consent to participate and have their data anonymously published.
The inclusion criteria were follows: (1) diagnosis of T2DM and age 18–75 years, (2) absence of severe disabling heart, renal, neoplastic, or cognitive diseases assessed by the Mini Mental State Examination, (3) at least 1 year of insulin treatment, (4) no add-on oral hypoglycemic therapy to exclude the bias a priori due to the lower number of insulin injections per day most often associated with it, (5) signed informed consent, and (6) full hypoglycemia awareness.
Study Protocol
O 4719 consecutive insulin-treated subjects with type 2 diabetes (T2DM) extracted from the general database of our DCUs, 1238 met all inclusion criteria. We selected 713 of them (58%) having LH lesions of varying size, shape, palpability, and texture for the study. In this entire cohort, we calculated the economic value of the health and social interventions required by hypoglycemia in the 6 months of the pre-randomization and 12 months of the post-randomization period.
At enrollment, in line with Nefrocenter’s practice, participants received thorough and structured educational training on how to perform self-monitoring of blood glucose (SMBG) and systematic recording of light symptomatic and severe hypoglycemic events (SyHs and SeHs, respectively) in the 6 months prior to randomization (T-6/T0). Hypoglycemic events (HYPOs) were defined as symptomatic or severe depending on whether they caused typical symptoms or required help from another person/physician or even hospitalization as previously described [12–15]. Data were recorded through a validated self-administered questionnaire developed as part of the original Worldwide Injection Technique Questionnaire Study 2016 [17, 18] according to a salient, nonintrusive, recent-past-oriented, well-established procedure [12, 13].
The number and severity of HYPOs, as recorded through a validated patient recall-based method, were confirmed by glucose meter recordings regularly downloaded to the dedicated digital CRF platform throughout the study [16] as part of the entire daily SMBG profile (both immediately before and 2 h after breakfast, lunch, and dinner), required at least once a week together with additional checks in case of suspected HYPOs. Adherence to the above protocol was rated as adequate with ≥ 80% successful data recording.
Only after that (at T0) were subjects divided into the control group (CG) and intensive training group (IG) through a random code generator. The CG received only the initial structured educational training on correct injection techniques reported in extenso in Supplementary Materials. The IG, instead, received the same educational training at randomization (T0) and after 6 months (T+6) together with a monthly refresher telephone call concerning salient information on correct injection techniques for the entire duration of the study.
To reduce any possible bias related to different insulin preparations and variable costs depending on manufacturers, we had all participants use KwikPens with the least expensive insulin analogues at the national level, i.e., insulin lispro U-100 at mealtime and Abasaglar (glargine biosimilar) at bedtime.
All patients performed a full visit at T–6, T0, T+6, and T+12 months. The latter, including a careful injection site examination and an ultrasound scan, allowed experimenters to fill in the electronic case report form (eCRF) with detailed injection habits and HYPOs.
Insulin Titration
As injecting into healthy skin areas leads to more predictable insulin absorption than in LH nodules [8], participants avoided HYPOs by reducing their original DID by 20% as previously recommended according to their SMBG profile [2, 3].
Education
All patients underwent structured therapeutic education sessions on correct injection techniques, including regular injection site rotation, avoidance of needle reuse, utilization of room-temperature insulin, and accurate choice of LH-free injection sites according to a structured protocol already described [2] and reported in extenso in Supplementary Materials.
Costs
Healthcare resource utilization, loss of productivity, and other indirect cost items were investigated through specific questions, as previously described [19]. We calculated costs based on official NHS average reimbursement price lists [19], including the following items: physician home visit (PHV) = €25.82 [20]; emergency room (ER) visit and treatment = €241.00 [21]; emergency medical services (EMS) utilization = €128.50 per hour [22]; family member or caregiver (FMWD and CWD, respectively) working day = €78.60 [23, 24]. Hospitalization cost (HC), obtained by multiplying the average length of stay (6.7 days) by the average daily hospitalization cost DHC (€750), was calculated as €5.025 [25]. We also calculated cost changes (rather than actual costs) of excess insulin administration required by the presence of LH nodules by applying a 0.02426 €/unit conversion factor, i.e., the only relevant one published so far to the best of our knowledge [7]. Table 1S, Supplementary Materials, provides detailed costs related to all health and social interventions, including those due to patient/caregiver's lost working days.
Statistical Analysis
Based on a previous interventional educational study on LHs, HbA1c levels decreased by 0.58% from baseline to the end of follow-up, with a standard deviation of 1.35% [26]. So, after setting the two-sided significance level at p = 0.05 and the power at 90%, the minimum sample size for each group was calculated to be 90 patients per group. However, we decided to enroll and randomize all 723 T2DM patients meeting the inclusion criteria to maximize data availability.
The analysis of HYPO rates was purposely conducted by comparing data from each of the three 6-month study periods. We compared the pre-randomization with the first and second follow-up periods separately. This choice was based on our previous studies (ISTERP1 [2], and ISTERP-2 [3], respectively), showing that behavioral changes followed a somewhat exponential trend so that overall performance, i.e., injection habits and metabolic parameters, improved by > 80% already after the first 6-month period, thus leaving participants with a < 20% chance to improve further after the full structured educational refresher offered at T+6.
Patient characteristics were reported as means ± standard deviations (SD) for continuous variables or number/percentage for categorical variables. The incidence rates (IRs) within 95% confidence intervals (95% CI) of SyHs and SeHs, expressed as the number of subjects involved and the number of episodes occurring per single subject, were calculated according to the Poisson regression model. Treatment and time-related differences were tested for significance by the repeated measures analysis of variance (rANOVA) supplemented by the two-tailed paired Student’s t-test with 95% confidence intervals for parametric variables and Mann-Whitney’s U test for nonparametric variables. The χ2 test with Yates’s correction or Fisher’s exact test was implemented to achieve categorical variable differentiation. p < 0.05 was accepted for statistical significance. All evaluations were performed using the SAS program (release 9.4, SAS Institute, Cary, NC, USA).
Results
All 713 participants completed the study. Median adherence to overall SMBG data recording was as high as 93% (range 87–100. As shown in Table 1, no statistically significant differences in general, clinical, and laboratory data or injection habits were detected between the two groups. In the pre-randomization period (T−6/T0), in agreement with our previous findings [2, 3], the habit of injecting insulin into LH nodules and overall (severe or symptomatic) HYPOs were virtually the same, both averaging 85% in each group. The daily insulin dose (DID) was also similar between groups (56 ± 12 and 58 ± 13 in the CG and IG, respectively).
Table 1.
Clinical characteristics and injection habits of the CG and IG at enrollment
| Control group n = 319 |
Intervention group n = 395 |
|
|---|---|---|
| Male gender n (%) | 167 (52.35) | 211 (53.42) |
| Age (years) | 62 ± 14 | 61 ± 19 |
| BMI (kg/m2) | 30.4 ± 7.21 | 30.72 ± 5.60 |
| HbA1c (%) | 8.32 ± 1.31 | 8.52 ± 1.10 |
| DM duration (years) | 12.51 ± 5.21 | 11.96 ± 7.5 |
| Injections/day (n) | 4 | 4 |
| Insulin treatment duration (years) | 6.91 ± 4.22 | 6.53 ± 4.41 |
| Daily insulin dose (IU/day) | 56 ± 12 | 58 ± 13 |
| HYPOs (% of patients affected) | 85.85 | 85.32 |
| Injection habits n (%) | ||
| Needle reuse (%) | 92.20 | 91.27 |
| Failure to rotate injection sites (%) | 94.31 | 93.61 |
| Cold insulin injection (%) | 72.58 | 72.70 |
| Waiting at end injection (%) | 14.13 | 13.18 |
| Leaking drop after injection (%) | 85.37 | 87.22 |
| Painful injection (%) | 10.32 | 9.98 |
| Injection into LH nodules (%) | 96.85 | 98.66 |
Data are presented as mean ± standard deviation (M ± SD) or frequencies (%). Daily insulin dose and % of subjects suffering from hypoglycemic episodes (HYPOs) were evaluated in the 6-month period before randomization (T−6/T0). No significant differences were found between groups
Painful injections are defined as those causing symptoms ranging from slight local discomfort to real pain
Injection Habits
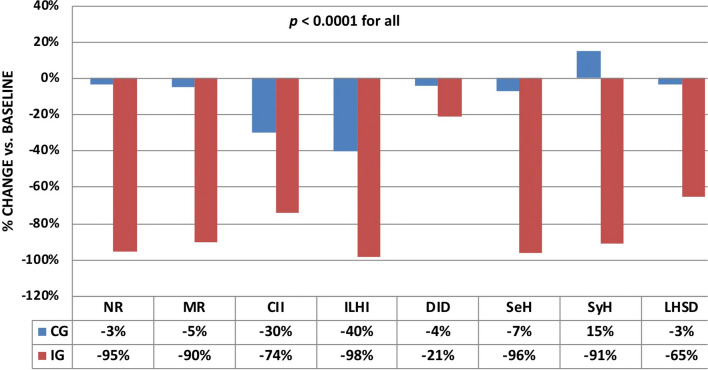
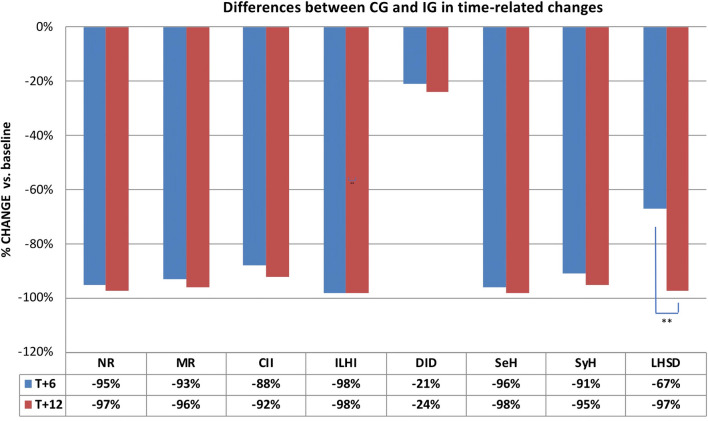
Figure 1 shows significant percentage changes occurring in the IG compared to CG between the pre-randomization and the first post-randomization period (T0/T+6). In particular, needle reuse decreased by 95% vs. 34%, as did all the following parameters: missing rotation by 90% vs. 5%, ice-cold insulin injection by 75% vs. 30%, intra-LH injection by 98% vs. 40%, LH size by 65 > % vs. 3%, DID by 21% vs. 4%, SeH rate by 96% vs. 7%, and SyH rate by 91% vs. 15%, respectively (p < 0.0001). Figure 2 summarizes percent changes of the above parameters at T+6 (blue bars) and T+12 (red bars) vs. baseline (pre-randomization period) in both groups, thus clearly showing that 55% of LHs observed at baseline was no longer present in the IG at T+12 while the astonishing improvement attained at T+12 vs. T+6 stayed virtually the same and even improved for both LH size (97% reduction at T+12 vs. 67% at T+6, p < 0.001) and DID (24% reduction at T+12 vs. 21% at T+6, p < 0.05). Contrarily, in the CG, all parameters worsened by 2–4% at T+12 compared to T+6 on average.
Fig. 1.
Comparison between the CG (blue bars) and IG (red bars) in terms of percent changes in injection habits, daily insulin doses, and hypoglycemic episodes (severe and symptomatic) occurring after the first 6-month follow-up with respect to baseline (pre-randomization period). All differences between the IG and the CG are statistically significant (p < 0.0001). NR, needle reuse; MR, missing rotation; CII, cold insulin injection; ILHI, intra LH injection; DID, daily insulin dose; SeH, severe hypoglycemia; SyH, symptomatic hypoglycemia; LHSD, LH size decrease
Fig. 2.
Comparison between the first (T+6; blue bars) and second (T+12; red bars) follow-up period in terms of percent changes vs. baseline in injection habits, daily insulin doses, and hypoglycemic episodes (severe and symptomatic) within the only IG, showing that, despite analyzed parameters keeping the same decreasing trend, only LHSD decrease attained statistical significance (**p < 0.01). NR, needle reuse; MR, missing rotation; CII, cold insulin injection; ILHI, intra LH injection; DID, daily insulin dose; SeH, severe hypoglycemia; SyH, symptomatic hypoglycemia; LHSD, LH size decrease
Hypoglycemia
During follow-up, a noticeably and significantly reduced number of patients from the IG had severe or symptomatic HYPOs (Table 2) compared to the pre-randomization period. However, this showed only a non-significant trend in the CG. Therefore, differences between the CG and the IG were highly significant in both the first and second 6-month follow-up period (p < 0.0001).
Table 2.
Comparison of subjects suffering from at least a single episode of HYPO (severe and symptomatic) in the various 6-month periods between groups, and at different time points, defined as: A = T−6/T0 (pre-randomization period); B = T0/T+6 (first follow-up period); C = T+6/T+12 (second follow-up period)
| Severe HYPOs | Symptomatic HYPOs | |||||
|---|---|---|---|---|---|---|
| Control group n. 318 |
Intervention group n. 395 |
p | Control group n. 318 |
Intervention group n. 395 |
p | |
|
T−6/T0 (A baseline) n. (%) |
55 (17.31) |
58 (14.72) |
n.s. |
218 (68.52) |
279 (70.91) |
n.s. |
|
T0/T+6 (B period) n (%) |
49 (15.41) |
25* (6.32) |
< 0.001 |
207 (65.93) |
34** (8.61) |
< 0.0001 |
|
T+6/T+12 (C period) n (%) |
48 (15.11) |
5**& (3.81) |
< 0.0001 |
215 (67.62) |
11**& (2.73) |
< 0.00001 |
| *p vs. T−6/T0 | n.s. | < 0.0001 | n.s. | < 0.0001 | ||
| &p vs. T0/T+6 | n.s. | < 0.0001 | n.s. | < 0.0001 | ||
Severe nocturnal HYPO rates were as follows: (1) 34.83% in the CG vs. 36.46% in the IG (p n.s.) during the T−6/T0 period; (2) 41.66% in the CG vs. 31.57% in the IG (p < 0.05) during the T0/T+6 period; (3) 33.15% in the CG vs. 16.6% in the IG (p < 0.001) in the T+6/T+12 period. Symptomatic HYPO rates were as follows: (1) 12.72% in the CG vs. 14.23% in the IG during the T−6/T0 period; (2) 16.24% in the CG vs. 10.86% in the IG (p < 0.05) during the T0/T+6 period; (3) 12.51% in the CG vs. 0% in the IG during the T+6/T+12 period.
Table 3 shows the absolute number of hypoglycemic episodes recorded in the two groups during follow-up compared to baseline. This analysis confirms the results in Table 2, and shows a significant and progressive reduction of total hypoglycemic episodes in the IG vs. CG in the first (97 vs. 349, respectively, p < 0.0001) and second follow-up period (37 vs. 391, respectively; p < 0.0001) with an overall rate averaging 1.16 and 2.41 episodes per capita.
Table 3.
Compared HYPO (severe and symptomatic) number per person between groups and at different time points, defined as: A = T−6/T0 (pre-randomization period); B = T0/T+6 (first follow-up period); C = T+6/T+12 (second follow-up period)
| Groups | HYPOs | T−6/T0 (A = baseline) Episode n |
T0/T+6 (B period) Episode n |
T+6/T+12 (C period) Episode n |
|---|---|---|---|---|
|
Control group (n. 359) |
Severe n Mean/person |
89 1.61 |
72* 1.46 |
80 1.66 |
|
Symptomatic n Mean/person |
316 1.12 |
277* 1.22 |
311 1.08 |
|
| Total | 405 | 349 | 391 | |
|
Intervention group (n. 359) |
Severe n Mean/person |
91 1.56 |
38** 1.52 |
12***&& 2.10 |
|
Symptomatic n Mean/person |
308 1.10 |
59** 1.73 |
25***&& 2.27 |
|
| Total | 399 | 97 | 37 |
B vs. A: *p < 0.05; **p < 0.01: ***p < 0.001; C vs. A: && p < 0.01
Costs
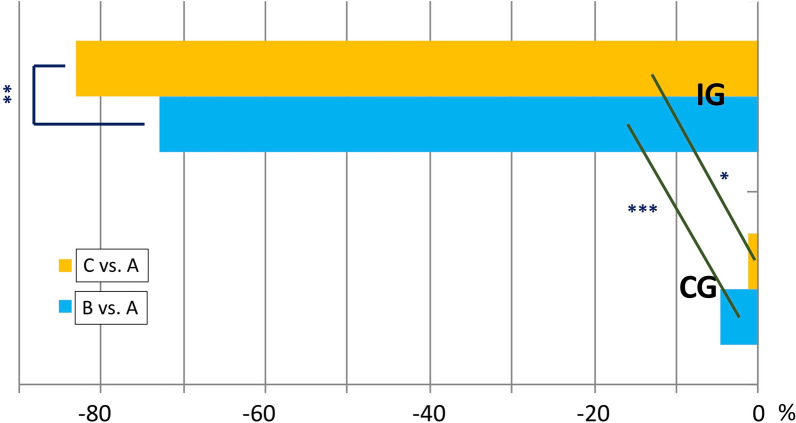
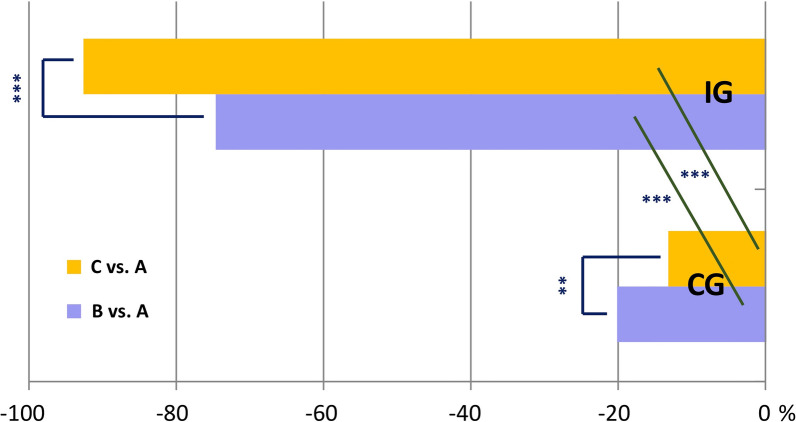
Table 4 describes the average cost of health services, working days, and single insulin units as calculated from official sources. It is immediately evident that utilization of emergency services was the most frequent health intervention required from both the CG and IG in the pre-randomization period. All interventions, including the latter, showed different results during the follow-up in the two groups, entailing significantly different costs at T+12. Indeed. while a dramatic drop in costs was apparent for SeHs (from €77,107.50 to €13,045.50; Δ − 83.08%, p < 0.00001) in the IG, costs remained substantially unchanged in the CG (i.e., from €69,245.00 to €68,349.40 (Δ − 1.3%, p ns) (Fig. 3). Similar results were observed for SyHs, with costs dropping from €9327.0 to €678 (Δ − 92.7%, p < 0.00001) in the IG and staying unchanged (from €9246 to €8013.4, Δ − 13.3, p ns) in the CG (Fig. 4). Until T + 12, costs further decreased in the IG while only slightly decreasing (− 4.6% and − 20.1% for SeHs and SyHs, respectively) during the first semester with reversal of the trend (− 1.2% and 13.3% for SeHs and SyHs, respectively) after that in the CG.
Table 4.
Average cost of health services, working day, and insulin UNITS, calculated on the basis of official sources
| Acronym | Description | Cost (€) | References N |
|---|---|---|---|
| PHV | Physician home visit | 25.82 | [20] |
| ER | Emergency room visit and treatment | 241.00 | [21] |
| EMS | Emergency medical services utilization per hour | 128.50 | [22] |
| FMWD/CWD | Working day of family member or caregiver (mean) | 78.60 | [23, 24] |
| DHC | Average daily hospitalization cost | 750 | [25] |
| HC | Mean duration (6.6 days) of hospitalization | 5.025 | [25] |
| IUC | Cost/single unit | 0.02426 | [7] |
In particular, it should be noted that the costs of health services are calculated at the minimum expenditure; as for the regionalization of the National Health System, there are also considerable cost differences between the various regions
Fig. 3.
Percent decrease of severe hypoglycemic events between/within groups in the three study periods. In the IG, it was markedly greater than in the CG (p < 0.0001) during the first follow-up period (B) after randomization (A) and only slightly greater (p < 0.042) in the second one (C). For within-group results, the decrease observed in the IG was already impressive (p < 0.0001) in the first follow-up period (B) after randomization (A) and continued (p < 0.0001) during the second one (C) while the decrease observed in the CG, besides being much smaller than in the IG, followed an opposite trend, being much more prominent (p < 0.01) in the first follow-up period (B) after randomization (A) than in the second one (C) [p < 0.031 vs. A and p < 0.05 vs. B). A = T-6/T0 (pre-randomization period); B = T0/T + 6 (first 6-month follow-up period); C = T + 6/T + 12 (second 6-month follow-up period). *p < 0.05; **p < 0.01; ***p < 0.0001
Fig. 4.
Percent decrease of symptomatic hypoglycemic events between/within groups in the three study periods. It was significantly higher in the IG than in the CG (p < 0.0001) during the first follow-up period, despite the further decrease experienced by the IG in the following follow-up period. For within-group results, the decrease observed in the IG was already impressive (p < 0.0001) in the first follow-up period (B) after randomization (A) and continued (p < 0.0001) during the second one (C) while the decrease observed in the CG, besides being much smaller than in the IG, followed an opposite trend, being greater in the first follow-up period (B) after randomization (A) (p < 0.01) and dropping dramatically in the second one (C) (p < 0.01 vs. A and p < 0.01 vs. B, yet). A = T-6/T0 (pre-randomization period); B = T0/T + 6 (first six-month follow-up period); C = T + 6/T + 12 (second six-month follow-up period). **p < 0.01; ***p < 0.0001
Based on the 24% reduction observed in the IG and 4% reduction observed in the CG, an overall 13.9 IU decrease in DID was calculated for the IG versus a 2.2 IU decrease in the CG, leading to annual savings of €123.26 per single IG member and €2.2 per single CG member. Although intrinsically modest, when reported in huge numbers, i.e., 100 or 1000 subjects, such figures represent a conspicuous saving in insulin-related healthcare costs.
Factors Associated with Hypoglycemia
Table 5 shows the RRs resulting from a multivariate analysis involving factors significantly associated HYPOs in subjects with LH, the most relevant being large, mainly abdominal LH lesions (for SeHs), ice-cold insulin intranodular injections, not rotating the site, and needle reuse. Injected insulin doses and the post-injection drop-leaking phenomenon are less relevant.
Table 5.
Multivariate analysis of factors significantly associated with severe and symptomatic HYPOs
| (95% CI) | RR | |
|---|---|---|
| Factors associated with severe hypoglycemia (SeH) | ||
| Diameter ≥ 4 cm | 2.24–5.37 | 3.38 |
| Abdominal site | 1.55–2.88 | 2.04 |
| LH at the abdominal site | 1.86–2.91 | 2.38 |
| LHs at multiple sites | 2.68–4.79 | 3.95 |
| Needle reuse | 2.22–3.87 | 3.46 |
| Failure to rotate injection sites | 2.89–4.90 | 3.67 |
| Insulin dose > 40 units/day | 1.15–2.19 | 1.68 |
| Ice-cold insulin | 2.75–4.88 | 3.39 |
| Factors associated with symptomatic hypoglycemia (SyH) | ||
| LH at the abdominal site | 1.22–2.63 | 2.17 |
| LHs at multiple sites | 1.85–2.78 | 2.39 |
| Injection into LH nodules | 2.59–5.98 | 4.21 |
| Needle reuse | 2.25–6.65 | 4.74 |
| Failure to rotate injection sites | 2.48–4.56 | 3.49 |
| Insulin dose > 40 units/day | 1.93–2–21 | 1.48 |
| Post-injection drop-leaking | 0.98–1.88 | 1.21 |
| Ice-cold insulin | 2.06–3.98 | 2.58 |
Discussion
To our knowledge, ours is the first 52-week, two-arm, open-label, multicenter, randomized, case-control trial aiming to compare the number and costs of LH-related HYPOs caused by incorrect injection techniques in two large cohorts of subjects with insulin-treated T2DM undergoing either (1) a long-duration, intensive educational training or (2) a single, fully structured, multimodal training session at enrollment. During the 6-month pre-randomization period, both groups proved to have virtually the same clinical features and to be similar in terms of injection habits as well as HYPO number and rates: this allowed reliable results to be analyzed when comparing data from subsequent periods and indeed made our study the first 18-month trial in the field to our knowledge.
This subject has already been investigated by other authors and our group in a much smaller series and for no longer than 6 months [2, 3, 26–28]. These initial experiences provided some information on the effectiveness of education in the short term and, in our case, on reducing HYPO-related costs. However, no one has collected data for a more extended period so far, and, above all, no one has investigated time-related behavioral changes in subjects with bad injection habits undergoing only one educational session. The latter, indeed, reflects common real-life conditions due to the significant resource- and time-consuming efforts required by continuous contacts with patients for any intensive education program.
Our results allowed us to trace the typical phenotype of the subject exposed to a significant risk of severe and symptomatic HYPOs, i.e., a subject with large LH lesions located mainly in the abdominal area used to repeatedly inject ice-cold insulin by the same needle directly into LH nodules without paying attention to injection site rotation.
However, they also showed very clearly that an intensive long-term educational approach induced a significant behavioral change with a consequent decrease in LH size and in SeHs (− 72% at 6 and − 83% at 12 months), in contrast to the very modest and transitory effect of a single educational intervention (− 4.0% at 6 and − 1.3% at 12 months); similar results were also observed for SyHs, despite decidedly less prominent economic advantages due to lower SyH requirements in terms of emergency services and hospitalization. This suggests that, in the absence of continuous educational refreshers, subjects tend to turn back to the same mistakes they made before and that a longstanding behavioral rehabilitation strategy is needed to obtain actual results.
The relevant economic benefits of an intensive educational program have to be considered as well. For instance, health interventions required by SeHs, which were on the order of €70,000 at baseline in both groups, declined by 5.9 times in the IG while only halving in the CG. For SyHs, the initial cost of about €9300 decreased 13.7 times at the end of the follow-up in the IG while only halving or so in the CG. Also, the observed DID reduction by 24 IU/day/patient would account for an annual saving of €123.26 per IG member, i.e., about 56 times that estimated for single CG members (€2.2/person). Calculating these apparently trivial annual differences to a larger scale, e.g., 10, 100, or 1000 patients, savings could reach impressive figures in a general context.
Many studies have already focused attention on factors associated with hypoglycemia in subjects with LH [2, 3, 7, 14, 26], such as rotation failure, needle reuse, ice-cold insulin injection, injection into LH nodules. Our paper fully confirms these observations and, for the first time to our knowledge, while showing the effectiveness of a 12-month intensive educational program, provides evidence that a single educational session provides only marginal and hardly any behavioral effects within 6 and 12 months, respectively. Unfortunately, our data cannot identify the reasons behind “lost adherence” to best injection practice recommendations. Therefore, we can only hypothesize laziness, depression, poor personal health care, or inadvertent repetition of acquired movements as factors behind longstanding hard-to-break unhealthy habits. Indeed, apart from long-term behavioral gains being quite rare per se, many patients admitted injections into LH lesions to be highly preferred because of their painlessness because of partial repeated trauma-dependent skin denervation [14].
Limitations
We are well aware that, especially for the IG, our study protocol, despite being well suited for a trial, is not easily adapted to real life. However, we are equally aware that a strategy must be identified to disseminate therapeutic paths aimed at improving the current critical situation. Indeed, according to most recent literature reports, LH frequency averages 41.8% (95% CI: 35.9% to 47.6%) with 10 to over 60% range of insulin-treated TDMs affected [5].
Conclusions
Our results provide evidence that insulin-treated subjects with T2DM need intensive education through regular refresher courses. So, based on such lessons learned, clinicians should pay much more attention to structured therapeutic education on best injection practices than they do now. In this way, they could provide people suffering from this disabling chronic disease with a valuable long-standing rehabilitation tool oriented to the prevention of LH and related complications [14, 28].
Therefore, in our view, the reduced economic burden on the NHS because of patients being educated about correct injection habits should encourage the international scientific community to:
work to obtain adequate reimbursement from as many national regulators as possible for diabetes teams engaging in sustained education programs;
have universities and scientific societies update all healthcare providers on correct injection techniques;
have physician and nurse groups disseminate adequate extracurricular knowledge about correct injection techniques in all care settings;
encourage manufacturers to add detailed instructions on correct injection practice to insulin leaflets and packages;
encourage Scientific Journal Editorial Boards to perform a more careful analysis of submitted papers dealing with lipodystrophies to avoid acceptance of methodologically poor manuscripts, thus preventing inexpert readers from being misled by lacking, incomplete, or even incorrect information (as already outlined by our group many times) [4, 6, 14, 29, 29].
From the results we obtained, the goal of decreasing the LH rate by an intensive, long-standing educational approach appears feasible. To our knowledge, this is the first study to evaluate the 52-week course despite massive literature on LH lesions. The most accessed bibliographic sources (mainly PubMed and Embase) have many papers on lipodystrophies, but research on LH is rare and is widespread in at least half of insulin-treated patients. In addition to frustrating care teams, this dire situation shows the lack of interest or inability of health professionals to systematically address and solve the problem of LH and its metabolic and economic consequences. In 100 years of insulin history, and of lipodystrophies [31], the solution to the problem is still missing. So, patients continue to inject insulin into LH nodules, preventing insulin from effectively playing its role. As a consequence, we are still at the starting point of the path that should lead to the eradication of this frustrating, dangerous, and expensive local complication of injection therapy without being able to identify a winning strategy yet.
We are aware that this is a strong statement and, for some, probably also irritating. However, in addition to the evidence derived from our study, a recent survey conducted on a significant number of healthcare workers operating in primary, secondary, and tertiary Chinese order hospitals confirmed our findings [33]. The result of this survey is that > 50% of the doctors and nurses interviewed know about LH, but, in most cases, they neither know about nor diagnose them, nor do they know how to implement the necessary procedures to prevent them or to reduce their size and metabolic consequences!
Our study shows that this war can be won, but efforts in multiple sectors and a general sharing of this goal are needed.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are indebted to all experimenters and members of the AMD-OSDI study group and of the Nefrocenter working group, all of whom are listed below. All DCs were part of the Nefrocenter Research Network in Southern Italy, a private consortium supported by the National Health System in association with Naples University Luigi Vanvitelli in several clinical aspects, including the Ethics Committee’s authorizations (Trial Registration no. 120-16.02.2019). Special thanks are due to Carolina La Rocca, national president of OSDI, Marcello Grussu, National President of ANIAD, and Paola Murano, General Manager of the Nefrocenter Research Network for the effective and continuous, spontaneously offered support toward carrying out the study. We are also indebted to all patients who generously agreed to participate in the study.
Funding
No funding or sponsorship was received for this study or the publication of this article. None of the authors or coworkers received funding or another type of payment for this paper.
Authorship
All named authors (Sandro Gentile, Giuseppina Guarino, Teresa Della Corte, Giampiero Marino, Ersilia Satta, Carmine Romano, Carmelo Alfarone, Maria Pasquarella, Laura Giordano, Fabrizio Loiacono, Maurizio Capece, Rossella Lamberti and Felice Strollo) meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article taking responsibility for the integrity of the work as a whole and gave their approval for this version to be published.
Author Contributions
Sandro Gentile and Felice Strollo designed the study and wrote the article. Ersilia Satta, Teresa Della-Corte, Giuseppina Guarino, Giampiero Marino, Carmine Romano, Carmelo Alfarone, Maria Pasquarella, Laura Giordano, Fabrizio Loiacono, Maurizio Capece, and Rossella Lamberti critically read and approved the paper. All authors contributed to data acquisition, critically assessed the results, and approved the final text. All collaborators critically read and approved the final text. Members of the AMD-OSDI Study Group: Stefano De-Riu, Nicoletta De-Rosa, Giorgio Grassi, Gabriella Garrapa, Laura Tonutti, Katija Speese, Lia Cucco, MariaTeresa Branca, Amodio Botta, Carolina La Rocca, Marcello Grussu. Members of the Nefrocenter Research and Nyx Start-Up Study Group: Diabetologists: Sandro Gentile, Giuseppina Guarino, Felice Strollo, Gerardo Corigliano, Marco Corigliano, Carmine Martino, Antonio Fasolino, Antonio Vetrano, Agostino Vecchiato, Domenica Oliva, Clelia Lamberti, Giuseppe Cozzolino, Clementina Brancario, Luca Franco, Enrico Visconti. Luisa Anna Stile, Antonella Raffaele, Massimo Amodio, Antonello Selleri. Nutritionist: Teresa Della-Corte. Nephrologists: Carmelo Alfarone, Maria Luisa Abate Giovanna, Maria Amicone, Giovanni Apuzzo, Gennaro Barbuto, Antonio Bassi, Pasquale Boccia, Francesca Borghesi, Alfonso Bosco, Francesco Buono, Tiziana Castellano, Giorgio Chianese, Michele Cicala, Alfonso Ciotta, Secondino Cipriano, Fabrizio G. Crisci, Cristiano Pina, Iris Cupic, Marco De Chiara, Alfonso De Maio, Carlo Del Piano, Chiara Del Prete, Luigi Di Leva, Monica Di Maio, Mauro Di Monte, Alfonso Donnarumma, Enzo Di Stazio, Michele Fabozzi, Maria Gallo, Laura Giordano, Monte Giovanni, Manuela Guerri, Giulia Esposito Iacobitti, Anna Maria La Manna, Gianluca Latte, Fabrizio Lo Iacono, Donato Maietta, Chiara Marano, Maria Federica Finelli, Maria Pasquarella, Mario Acquaviva, Massimo Romano, Gennaro Mattiello, Pietro Miano, Silvia Migliaccio, Alfredo Mignone, Giovanni Monte, Alfredo Fabio Murano, Simona Oliviero, Teresa Pagano, Gabriele Palmentola, Salvatore Postiglione, Pvalo Yavorskiy, Raffaela Esposito, Rosario Reggio, Bruno Riccardi, Eleonora Riccio, Giuseppe Romano, Ersilia Satta, Francesco Antonio Savino, Luisa Scarpati, Domenico Schettino, Giuseppe Spinoso, Erika Troncone, Pasquale Vendemia, Olga Yushkova. Nurses: Paolino Albertini, Lucio Ambrosino, Rosa Vitale Amoroso, Marilena Angrisani, Rosa Apuzzo, Angela Auletta, Fabrizio Barbaro, Gilda Barrella, Alfonso Bartiromo, Maria Battipaglia, Orfeo Belardo, Roberto Bernardo, Valentina Bianco, Pasquale Biondillo, Lucia Bottiglieri, Michela Brida, Orsola Brusco, Giuseppina Buonocore, Zaira Buonocore, Halina Buska, Giuseppe Calabrese, Ida Campolattano, Margherita Capasso, Cecilia Caracciolo, Teresa Carrara, Angela Casaburo, Sara Caso, Marina Assunta Cesarano, Tiziana Cesarini, Annunziata Cherillo, Enrico Cicchella, Michelina Cicchella, Angelo Cimmarosa, Simone Cimmino, Carmelina Cirillo, Pasquale Como, Tiziana Conturso, Giuseppina Cozzolino, Adele Crispino, Raffaele D’Angelo, Salvatore De Felice, Adriano De Filippis, Margarita De Lucia, Lucia De Micco, Maria Andrea De Vita, Antonio Decostanzo, Carmine D’Elia, Salvatore De Felice, Eligio Della Monica, Angela D’Errico, Veronica D’Esculapio, Marialucia Di Riso, Giovanna Di Maio, Roberta Di Maio, Assunta Di Matola, Assunta Di Nardo, Elisabetta Di Virgilio, Davide Doriano, Eliana Ebraico, Gioacchino Erbaggio, Luca Erpete, Roberta Errichiello, Santo Esca, Gennaro Esposito, Virginia Esposito, Rinaldo Fargnoli, Pasquale Ferrante, Oriana Ferraro, Marco Festinese, Carmen Figlioli, Giovanni Fiorenza, Filomena Fontanella, Michela Fusco, Carmela Gigante, Carmelina Giove, Ruslava Gladka, Anna Guerra, Achille Iannone, Lucia Imbembo, Concetta Imbimbo, Melania Imbimbo, Grazia Indaco, Felice Marco Isola, Antonietta Izzo, Beata Jeschke, Onorina Letizia, Danilo Lettieri, Anna Maria Mandato, Donatella Mannato, Erika Manzi, Lucia Manzo, Carmela Marano, Zuzanna Matusz, Emilio Menna, Sara Milano, Joanna Mlynarska Malgorzata, Carmela Montesarchio, Vincenza Morgillo, Vincenzo Morgillo, Teresa Morrone, Teresa Napolitano, Maria Teresa Natale, Aldo Occhio, Livio Orropesa, Daniela Palmeri, Angela Palmiero, Antonietta Pandolfo, Valentina Passa, Assunta Pastore, Teresa Jadwiga Pazdior, Annamaria Pellino, Elena Petrone, Valentina Pettinati, Filomena Piccolo, Catello Polichetti, Milena Puce, Angela Rainone, Emanuela Repola, Raffaele Riccio, Amelia Ricuperati, Maria Roselli, Enza Ruotolo, Eva Russo, Francesco Russo, Antonietta Salsano, Andrea Schettino, Annalisa Siani, Marilina Siani, Immacolata Silvestri, Pellegrino Spallieri, Annunziata Stasio, Claudia Tabacco, Francesca Tammaro, Maria Emanuela Toscano, Gabriele Ummarino, Federica Variselli, Francesca Vela, Mario Vitale, Paolo Vitale.
Disclosures
Sandro Gentile, Giuseppina Guarino, Teresa Della Corte, Giampiero Marino, Ersilia Satta, Carmine Romano, Carmelo Alfarone, Maria Pasquarella, Laura Giordano, Fabrizio Loiacono, Maurizio Capece, Rossella Lamberti, and Felice Strollo have no financial interests to declare in relation to the present study.
Compliance with Ethics Guidelines
This study was carried out in compliance with good clinical practice standards and in accordance to the ethical guidelines of the 1964 Declaration of Helsinki and its subsequent amendments. The study protocol was approved (2) by the Ethical and Scientific Committee of the reference center, University Luigi Vanvitelli, Naples, Italy (trial registration no. 120, 16.02.2019), which served as the central reference ethics committee for all of the participating diabetes centers, with the latter an integral part of the same private consortium, Nefrocenter Research Network, associated with the above-mentioned University. All of the subjects with T2DM participating in the study signed an informed consent form to be included in the present investigation.
Data Availability
The datasets analyzed during the present study are available from the corresponding author on reasonable request.
Footnotes
AMD = Associazione Medici Diabetologi (Italian Association of Diabetes Specialists)- OSDI = Operatori Sanitari di Diabetologia Italiani (Italian Diabetes Healthcare Professionals) Study Group on Injection Techniques and ANIAD = Associazione Nazionale Italiana Atleti Diabetici (Italian National Association of Athletes with Diabetes) collaborating members are listed in the Acknowledgements section.
Change history
4/28/2022
A Correction to this paper has been published: 10.1007/s12325-022-02147-9
Contributor Information
Felice Strollo, Email: felix.strollo@gmail.com.
Nefrocenter and Nyx Start-up, AMDOSDI Study Group on Injection Techniques, and ANIAD:
Stefano De-Riu, Nicoletta De-Rosa, Giorgio Grassi, Gabriella Garrapa, Laura Tonutti, Katija Speese, Lia Cucco, MariaTeresa Branca, Amodio Botta, Carolina La Rocca, Marcello Grussu, Sandro Gentile, Giuseppina Guarino, Felice Strollo, Gerardo Corigliano, Marco Corigliano, Carmine Martino, Antonio Fasolino, Antonio Vetrano, Agostino Vecchiato, Domenica Oliva, Clelia Lamberti, Giuseppe Cozzolino, Clementina Brancario, Luca Franco, Enrico Visconti. Luisa Anna Stile, Antonella Raffaele, Massimo Amodio, Antonello Selleri, Teresa Della-Corte, Carmelo Alfarone, Maria Luisa Abate Giovanna, Maria Amicone, Giovanni Apuzzo, Gennaro Barbuto, Antonio Bassi, Pasquale Boccia, Francesca Borghesi, Alfonso Bosco, Francesco Buono, Tiziana Castellano, Giorgio Chianese, Michele Cicala, Alfonso Ciotta, Secondino Cipriano, Fabrizio G. Crisci, Cristiano Pina, Iris Cupic, Marco De Chiara, Alfonso De Maio, Carlo Del Piano, Chiara Del Prete, Luigi Di Leva, Monica Di Maio, Mauro Di Monte, Alfonso Donnarumma, Enzo Di Stazio, Michele Fabozzi, Maria Gallo, Laura Giordano, Monte Giovanni, Manuela Guerri, Giulia Esposito Iacobitti, Anna Maria La Manna, Gianluca Latte, Fabrizio Lo Iacono, Donato Maietta, Chiara Marano, Maria Federica Finelli, Maria Pasquarella, Mario Acquaviva, Massimo Romano, Gennaro Mattiello, Pietro Miano, Silvia Migliaccio, Alfredo Mignone, Giovanni Monte, Alfredo Fabio Murano, Simona Oliviero, Teresa Pagano, Gabriele Palmentola, Salvatore Postiglione, Pvalo Yavorskiy, Raffaela Esposito, Rosario Reggio, Bruno Riccardi, Eleonora Riccio, Giuseppe Romano, Ersilia Satta, Francesco Antonio Savino, Luisa Scarpati, Domenico Schettino, Giuseppe Spinoso, Erika Troncone, Pasquale Vendemia, Olga Yushkova, Paolino Albertini, Lucio Ambrosino, Rosa Vitale Amoroso, Marilena Angrisani, Rosa Apuzzo, Angela Auletta, Fabrizio Barbaro, Gilda Barrella, Alfonso Bartiromo, Maria Battipaglia, Orfeo Belardo, Roberto Bernardo, Valentina Bianco, Pasquale Biondillo, Lucia Bottiglieri, Michela Brida, Orsola Brusco, Giuseppina Buonocore, Zaira Buonocore, Halina Buska, Giuseppe Calabrese, Ida Campolattano, Margherita Capasso, Cecilia Caracciolo, Teresa Carrara, Angela Casaburo, Sara Caso, Marina Assunta Cesarano, Tiziana Cesarini, Annunziata Cherillo, Enrico Cicchella, Michelina Cicchella, Angelo Cimmarosa, Simone Cimmino, Carmelina Cirillo, Pasquale Como, Tiziana Conturso, Giuseppina Cozzolino, Adele Crispino, Raffaele D’Angelo, Salvatore De Felice, Adriano De Filippis, Margarita De Lucia, Lucia De Micco, Maria Andrea De Vita, Antonio Decostanzo, Carmine D’Elia, Salvatore De Felice, Eligio Della Monica, Angela D’Errico, Veronica D’Esculapio, Marialucia Di Riso, Giovanna Di Maio, Roberta Di Maio, Assunta Di Matola, Assunta Di Nardo, Elisabetta Di Virgilio, Davide Doriano, Eliana Ebraico, Gioacchino Erbaggio, Luca Erpete, Roberta Errichiello, Santo Esca, Gennaro Esposito, Virginia Esposito, Rinaldo Fargnoli, Pasquale Ferrante, Oriana Ferraro, Marco Festinese, Carmen Figlioli, Giovanni Fiorenza, Filomena Fontanella, Michela Fusco, Carmela Gigante, Carmelina Giove, Ruslava Gladka, Anna Guerra, Achille Iannone, Lucia Imbembo, Concetta Imbimbo, Melania Imbimbo, Grazia Indaco, Felice Marco Isola, Antonietta Izzo, Beata Jeschke, Onorina Letizia, Danilo Lettieri, Anna Maria Mandato, Donatella Mannato, Erika Manzi, Lucia Manzo, Carmela Marano, Zuzanna Matusz, Emilio Menna, Sara Milano, Joanna Mlynarska Malgorzata, Carmela Montesarchio, Vincenza Morgillo, Vincenzo Morgillo, Teresa Morrone, Teresa Napolitano, Maria Teresa Natale, Aldo Occhio, Livio Orropesa, Daniela Palmeri, Angela Palmiero, Antonietta Pandolfo, Valentina Passa, Assunta Pastore, Teresa Jadwiga Pazdior, Annamaria Pellino, Elena Petrone, Valentina Pettinati, Filomena Piccolo, Catello Polichetti, Milena Puce, Angela Rainone, Emanuela Repola, Raffaele Riccio, Amelia Ricuperati, Maria Roselli, Enza Ruotolo, Eva Russo, Francesco Russo, Antonietta Salsano, Andrea Schettino, Annalisa Siani, Marilina Siani, Immacolata Silvestri, Pellegrino Spallieri, Annunziata Stasio, Claudia Tabacco, Francesca Tammaro, Maria Emanuela Toscano, Gabriele Ummarino, Federica Variselli, Francesca Vela, Mario Vitale, and Paolo Vitale
References
- 1.Hauner H, Stockamp B, Haastert B. Prevalence of lipohypertrophy in insulin-treated diabetic patients and predisposing factors. Exp Clin Endocrinol. 1996;104:106–110. doi: 10.1055/s-0029-1211431. [DOI] [PubMed] [Google Scholar]
- 2.Gentile S, Guarino G, Della Corte T, et al. Role of structured education in reducing lypodistrophy and its metabolic complications in insulin-treated people with type 2 diabetes: a randomized multicenter case-control study. Diabetes Ther. 2021;12(5):1379–1398. doi: 10.1007/s13300-021-01006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gentile S, Guarino G, Della Corte T, et al. The durability of an intensive, structured education-based rehabilitation protocol for best insulin injection practice: the ISTERP-2 study. Diabetes Ther. 2021;12(9):2557–2569. doi: 10.1007/s13300-021-01108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gentile S, Guarino G, Giancaterini A, Guida P, Strollo F. A suitable palpation technique allows to identify skin lipohypertrophic lesions in insulin-treated people with diabetes. Springerplus. 2016;5(5):563. doi: 10.1186/s40064-016-1978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K, Zhang S, Liu C, Chen Y. A meta-analysis and meta-regression on the prevalence of lipohypertrophy in diabetic patients on insulin therapy. Therapies. 2021;76:617–628. doi: 10.1016/j.therap.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Strollo F, Guarino G, Armentano V, et al. Unexplained hypoglycaemia and large glycaemic variability: skin lipohypertrophy as a predictive sign. Diabetes Res Open J. 2016;2:24–32. doi: 10.17140/DROJ-2-126. [DOI] [Google Scholar]
- 7.Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39:445–453. doi: 10.1016/j.diabet.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Ibarra LS, Gallego F. Factors related to lipohypertrophy in insulin-treated diabetic patients: role of educational intervention. Pract Diabetes Int. 1998;15:9–11. doi: 10.1002/pdi.1960150108. [DOI] [Google Scholar]
- 9.Gentile S, Agrusta M, Guarino G, et al. Metabolic consequence of incorrect insulin administration techniques in aging subjects with diabetes. Acta Diabetol. 2011;48:121–125. doi: 10.1007/s00592-009-0172-x. [DOI] [PubMed] [Google Scholar]
- 10.Deeb A, Abdelrahman L, Tomy M, et al. Impact of insulin injection and infusion routines on lipohypertrophy and glycemic control in children and adults with diabetes. Diabetes Ther. 2019;10(1):259–267. doi: 10.1007/s13300-018-0561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji L, Sun Z, Li Q, et al. Lipohypertrophy in China: prevalence, risk factors, insulin consumption, and clinical impact. Diabetes Technol Ther. 2017;19(1):61–67. doi: 10.1089/dia.2016.0334. [DOI] [PubMed] [Google Scholar]
- 12.Gentile S, Strollo F. Cost saving effects of a short-term educational intervention entailing lower hypoglycaemic event rates in people with type 1 diabetes and lipo-hypertrophy. Diabetes Res Clin Pract. 2018;143:320–321. doi: 10.1016/j.diabres.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Hsu PF, Sung SH, Cheng HM, Yeh JS, Liu WL. Association of clinical symptomatic hypoglycemia with cardiovascular events and total mortality in type 2 diabetes: a nationwide population-based study. Diabetes Care. 2013;36:894–900. doi: 10.1001/jamainternmed.2013.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentile S, Guarino G, Della Corte T, et al. Insulin-induced skin lipohypertrophy in type 2 diabetes: a multicenter regional survey in Southern Italy. Diabetes Ther. 2020;11(9):2001–2017. doi: 10.1007/s13300-020-00876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentile S, Guarino G, Della-Corte T, et al. Lipohypertrophy in elderly insulin-treated patients with type 2 diabetes. Diabetes Ther. 2020 doi: 10.1007/s13300-020-00954-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentile S, Strollo F, Satta E, et al. Insulin-related lipohypertrophy in hemodialyzed diabetic people: a multicenter observational study and a methodological approach. Diabetes Ther. 2019;10(4):1423–1433. doi: 10.1007/s13300-019-0650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch LJ, Strauss KW. The injection technique factor: what you don’t know or teach can make a difference. Clin Diabetes. 2019;37(3):227–233. doi: 10.2337/cd18-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frid AH, Hirsch LJ, Menchior AR, Morel DR, Strauss KW. Worldwide injection technique questionnaire study: injecting complications and the role of the professional. Mayo Clin Proc. 2016;91(9):1224–1230. doi: 10.1016/j.mayocp.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Frid AH, Hirsch LJ, Menchior AR, Morel DR, Strauss KW. Worldwide injection technique questionnaire study: population parameters and injection practices. Mayo Clin Proc. 2016;91(9):1212–1223. doi: 10.1016/j.mayocp.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Council of Italian General Practitioners. Least accepted medical fees. 2020. http://www1.ordinemediciroma.it/elenco-completo/regolamenti/53-tariffaminima-nazionale-deglionorari.html. Accessed 7 Jan 2020.
- 21.Emergency cost evaluation. 2020. http://www.mattoni.salute.gov.it/mattoni/documenti/11_Valutazione_costi_dell_emergenza.pdf. Accessed 7 Jan 2020.
- 22.Emergency cost evaluation. 2020. http://www.sanita24.ilsole24ore.com/art/regioni-e-aziende/2015-02-12/emergenza-fiaso-ecco-costi-113448.php?uuidZAbcvOTBL&refresh_ceZ1. Accessed 7 Jan 2020.
- 23.Archives of the Italian Institute of Statistics. 2020. http://www.istat.it/it/archivio/75111. Accessed 7 Jan 2020.
- 24.Demographic balance as recorded in 2013. 2020. http://demo.istat.it/ricostruzione2013/. Accessed 7 Jan 2020.
- 25.Ministry of Health, hospitalization cost evaluation. 2020. http://www.salute.gov.it/portale/news/p3_2_1_1_1.jsp?menuZnotizie&pZdalministero&idZ1411). Accessed 7 Jul 2020.
- 26.Grassi G, Scuntero P, Trepiccioni R, Marubb F, Strauss K. Optimizing insulin injection technique and its effect on blood glucose control. J Clin Transl Endocrinol. 2014;1(4):145–150. doi: 10.1016/j.jcte.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith M, Clapham L, Strauss K. UK lipohypertrophy interventional study. Diabetes Res Clin Pract. 2017;2017(126):248–253. doi: 10.1016/j.diabres.2017.01.020(Epub. [DOI] [PubMed] [Google Scholar]
- 28.Campinos C, Le-Floch JP, Petit C, et al. An effective intervention for diabetic lipohypertrophy: results of a randomized, controlled, prospective multicenter study in France. Diabetes Technol Ther. 2017;19(11):623–632. doi: 10.1089/dia.2017.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gentile S, Strollo F, Guarino G, et al. Why are so huge differences reported in the occurrence rate of skin lipohypertrophy? Does it depend on method defects or on lack of interest? Diabetes Metab Syndr. 2019;13(1):682–686. doi: 10.1016/j.dsx.2018.11.042. [DOI] [PubMed] [Google Scholar]
- 30.Gentile S, Strollo F, Della Corte T, Marino G, Guarino G. Insulin related lipodystrophic lesions and hypoglycemia: double standards? Diabetes Metab Syndr. 2018;12(5):813–818. doi: 10.1016/j.dsx.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 31.Williams JR. Lipoatrophy following the injection of insulin. J Metabol Resh. 1922;2:729. [Google Scholar]
- 32.Famulla S, Hovelmann U, Fischer A, Coester HV, Hermanski L, Kaltheuner M. Insulin injection into lipohypertrophic tissue: blunted and more variable insulin absorption and action and impaired postprandial glucose control. Diabetes Care. 2016;39:1486–1492. doi: 10.2337/dc16-0610. [DOI] [PubMed] [Google Scholar]
- 33.Shen M, Shi Y, Zheng S, Fan H, Xu J, Yang T. A systematic survey of physicians' insights into lipohypertrophy. Front Public Health. 2021;23(9):738179. doi: 10.3389/fpubh.2021.738179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forum for injection Technique (FIT). 2021. https://www.fit4diabetes.com/about-this-site/. Accessed 23 Dec 2021 (in supplementary material).
- 35.International Forum for Injection Technique (FITTER; Rome 2015) Optimize insulin delivery to help improve your patients’ lives and reduce the burden of care. Rome. 2015. https://www.fitter4diabetes.com/pages/the-rome-congress. Accessed 23 Dec 2021. (in supplementary material).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the present study are available from the corresponding author on reasonable request.