Abstract
Introduction
Prostate cancer (PC) is the second most common cancer, and the fifth most common cause of cancer-related mortality among male patients, worldwide. In Europe and Japan, the incidence of PC in men in 2020 exceeded that of lung cancer. Although national and regional clinical guidelines for the treatment of metastatic castration-resistant prostate cancer (mCRPC) are available in Europe and Japan, a literature review did not identify a published comparison of differing guidelines, but identified a lack of studies reporting treatment patterns of approved mCRPC treatments in Europe and Japan in normal clinical practice. The objective of this real-world study was to compare national treatment guidelines and real-world treatment for mCRPC in Europe and Japan.
Methods
Physician-reported demographics, clinical characteristics, and treatment data of patients with mCRPC were drawn from the Adelphi Prostate Cancer Disease Specific Programme™, conducted in five European countries and Japan (2020) and analysed descriptively.
Results
All current treatment guidelines recommended the use of novel hormonal agents (NHA—abiraterone/enzalutamide) and chemotherapy (mainly docetaxel), with some intercountry differences, with NHA rechallenge accepted in Germany, Italy and Japan, but not in France, Spain or the United Kingdom. Overall, 271 physicians provided data for 1753 patients. At 1st-line (1L), the most common treatment was NHAs followed by (→) chemotherapy, in all countries. Chemotherapy was the most common 2nd-line (2L) treatment, except in Japan, where 2L NHA use was preferred, and Spain, where both were used equally. NHA → chemotherapy and chemotherapy → NHA were the first and second usual 1L → 2L sequence in most countries, except for France, where the second most common sequence was NHA → NHA, and Japan, with androgen deprivation therapy alone → NHA.
Conclusion
Real-world mCRPC treatment patterns largely reflected national guidelines. It is expected that guidelines and treatment patterns will change with the development of new treatment options.
Keywords: Chemotherapy, Docetaxel, Metastatic castration-resistant prostate cancer, Novel hormonal agents, Treatment guidelines, Treatment patterns
Key Summary Points
| Prostate cancer is the second most common cancer in men, with a high mortality rate |
| While androgen deprivation therapy has improved the survival of patients with prostate cancer, treatment resistance inevitably leads to a castration-resistant stage, with subsequent metastasis |
| For patients with metastatic disease, treatment options are limited, and there is a lack of consensus in national treatment guidelines |
| This study examined the differing guidelines alongside data from a point-in-time survey of men with metastatic castration-resistant prostate cancer in five European countries and Japan |
| We found a general adherence to national treatment guidelines, but, with the introduction of new, more effective treatments, there is need for constant updates of international guidelines on the optimal treatment of advanced prostate cancer. |
Introduction
Globally, prostate cancer (PC) was the second most common cancer and the fifth most common cause of cancer-related mortality among male patients in 2020, with an estimated 1.4 million new cases and 375,000 deaths worldwide [1]. In Europe and Japan, the incidence of PC in men in 2020 exceeded that of lung cancer [1]. While little has been published on the epidemiology of metastatic castration-resistant prostate cancer (mCRPC), the prevalence and incidence of mCRPC in France in 2014 were estimated as 62/100,000 and 21/100,000 men, respectively [2], and a retrospective study calculated an annual incidence of 820 mCRPC cases from 2003 to 2007 in the United Kingdom (UK) [3].
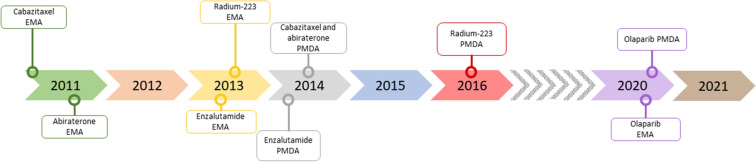
In terms of treatment, prior to 2010, chemotherapy with docetaxel was the only option shown to prolong survival in mCRPC [4]; however, the last decade has seen the introduction of several novel agents, including novel hormonal agents (NHA), such as abiraterone and enzalutamide, which provided an alternative to chemotherapy (Fig. 1), initially in second-line (2L), but more recently for more early use when conventional androgen deprivation therapy (ADT) is seen to be failing. Abiraterone and enzalutamide were approved for mCRPC by the European Medicines Agency (EMA) in September 2011 and June 2013, respectively [5, 6] and by the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan in July 2014 and March 2014, respectively [7, 8]. The EMA has also approved these drugs for metastatic hormone-sensitive prostate cancer (mHSPC).
Fig. 1.
Timeline for approval of treatments for mCRPC in Europe and Japan
In addition, cabazitaxel was approved by the EMA in January 2011 [9] for patients with mCRPC previously treated with a docetaxel-containing regimen, and by the PMDA for use in CRPC in July 2014 [10]. Radium-223, an α-emitting radioisotope, was approved for treatment of CRPC with bone metastases by the EMA in September 2013 [11] and by the PMDA in March 2016 [12] (Fig. 1).
Poly adenosine diphosphate–ribose polymerase inhibitors (PARPi) have also been shown to be efficacious in treating patients with Breast Cancer Gene (BRCA) 1/2-mutation positive mCRPC [12, 13], with olaparib monotherapy receiving EMA and PMDA approvals in September and December 2020 for the treatment of adult patients with mCRPC and BRCA1/2-mutations (germline and/or somatic) who have progressed following prior therapy that included an NHA [14], and patients with BRCA-mutation positive mCRPC [15], respectively (Fig. 1).
While national and regional clinical guidelines for the treatment of mCRPC are available in Europe and Japan, in view of the burgeoning treatment options, the recommendations vary, and a literature review did not identify a published comparison of differing guidelines. There is also a significant gap in the literature assessing treatment patterns of approved mCRPC treatments in Europe and Japan [16]. Accordingly, this study compared the guidelines for treating mCRPC in France, Germany, Italy, Spain, the UK (Europe) and Japan, and evaluated real-world treatment patterns in these countries. To the best of the authors’ knowledge, this is the first point-in-time study to assess treatment patterns of all approved treatment options in Europe and Japan.
Methods
National guidelines in Europe and Japan were reviewed to extract key recommendations on the treatment of mCRPC, and these were compared descriptively to identify areas of consistency and discrepancy. Real-world data were drawn from the Adelphi Prostate Cancer Disease Specific Programme (DSP™) conducted in Europe and Japan from January to August 2020. DSPs are point-in-time surveys of physicians and their patients presenting in a real-world clinical setting. The DSP methodology has been previously published and validated [17–19].
Using physician panels and publicly available lists, local fieldwork partners identified physicians and invited them to participate in the survey if they had a speciality in medical oncology, urology or were specialist surgeons, had personal responsibility for prescribing decisions for patients with mCRPC, were seeing ≥ four patients (≥ two patients in Japan) with metastatic PC per month, two of whom had to be diagnosed with mCRPC (one patient in Japan), after their agreement to adhere to all survey rules and regulations.
Physicians completed an online questionnaire for eligible patients whom they consecutively consulted, with the numbers of patients varying across countries. Physicians from Europe completed forms for four patients with mCRPC, plus two mCRPC patients who were currently receiving 2L or later treatment in the mCRPC setting. Once data regarding these patients had been captured, those physicians from Europe who tested for homologous recombination repair mutation (HRRm) were also requested to provide data for up to three additional consecutively consulting mCRPC patients who had tested positive for HRRm. Physicians from Japan completed forms for three patients with mCRPC. Once these patient data had been captured, physicians from Japan who tested for HRRm were also asked to provide data for one additional HRRm-positive mCRPC patient (if the physician did not see a patient meeting this criterion, they were asked to provide data for one additional mCRPC patient).
Patients were eligible for initial inclusion if they were aged ≥ 18 years, had a physician-confirmed diagnosis of mCRPC, and had never been involved in a clinical trial. The physicians provided data on patient demographics, clinical characteristics, PC history and treatment history. Data were drawn from existing patient clinical records, and were based on the judgment and diagnostic skills of the respondent physician. No additional tests, treatments or investigations were conducted.
All descriptive analyses were conducted using UNICOM® Intelligence Reporter, Version 7.5.
Ethics
Using a checkbox, physicians provided informed consent to take part in the survey. Data were collected in such a way that both patients and physicians could be anonymised.
The questionnaires used in the PC DSP were reviewed and given exemption by the Western Institutional Review Board (reference number: 1-1261035-1), and data collection was undertaken in accordance with European Pharmaceutical Marketing Research Association guidelines [20]. As data were collected according to market research guidelines, no source data validation was possible or required. The survey was performed in full accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act 1996 [21] and the Health Information Technology for Economic and Clinical Health Act legislation [22].
Results
Key points from national clinical practice guidelines for the treatment of mCRPC in Europe and Japan are summarised in Table 1. In total, 271 physicians (France n = 50, Germany n = 50, Italy n = 45, Spain n = 45, UK n = 35, Japan n = 46) took part in the survey. Of these, 28 (56%), 10 (20%), 17 (38%), 38 (84%), 28 (80%), and 19 (41%), respectively, were based in an academic/cancer centre, with the remainder being community-based, while 41 (82%), 32 (64%), 45 (100%), 38 (84%), 33 (94%), and 4 (9%), respectively, were oncologists. The balance of physician specialities was made up by urologists, except for Spain, where 2 (4%) physicians were prostate/specialist cancer surgeons.
Table 1.
National treatment guidelines for mCRPC
| Guideline |
|---|
| European [35] |
| Abiraterone or enzalutamide is recommended for asymptomatic/mildly symptomatic men with chemotherapy-naive mCRPC |
| Docetaxel is recommended in 1L for men with mCRPC |
| In patients with mCRPC in the post-docetaxel setting, abiraterone, enzalutamide, and cabazitaxel are recommended 2L options |
| Olaparib can be considered after NHAs for patients with mCRPC with alteration in BRCA1 or BRCA2 |
| In patients with bone metastases from CRPC at risk for clinically significant skeletal-related events, a bisphosphonate or denosumab is recommended |
| 223Ra is recommended for men with bone-predominant, symptomatic mCRPC without visceral metastases, but not recommended in combination with abiraterone and prednisolone |
| The use of a second NHA (abiraterone after enzalutamide or vice versa) is not recommended |
| France [36] |
| Abiraterone, enzalutamide or docetaxel are 1L options if ADT alone is given prior to progression to mCRPC |
| Docetaxel is a 1L option if ADT + NHA given prior to progression to mCRPC |
| NHAs are 1L options if ADT + docetaxel given prior to progression to mCRPC |
| Chemotherapy is the 2L option if 1L treatment was NHA |
| Cabazitaxel and NHAs are 2L options if 1L treatment was docetaxel |
| If rapid progression on NHAs pre/post docetaxel, 2L chemotherapy is cabazitaxel |
| Rechallenge with NHAs is not a recommended option |
| PARPi use is an option at 2L for patients with BRCA2 or ATM mutations, but only within the framework of therapeutic trials |
| Germany [37] |
| Abiraterone (+ prednisone/prednisolone), enzalutamide or docetaxel are 1L options: abiraterone (+ prednisone/prednisolone) or enzalutamide if no/mild symptoms, docetaxel if symptomatic/progressive disease |
| Patients progressing on NHAs should be offered changed therapy strategy and tested for BRCA1/2 mutations |
| If BRCA1/2 mutation detected, olaparib is recommended |
| Abiraterone/prednisone, enzalutamide or cabazitaxel are 2L options if 1L treatment was docetaxel |
| Rechallenge with NHAs is an option |
| Italy [38] |
| Abiraterone, enzalutamide or docetaxel are 1L options: abiraterone or enzalutamide if asymptomatic, docetaxel if symptomatic |
| Docetaxel is the recommended 2L option following 1L treatment with abiraterone or enzalutamide |
| Enzalutamide is a 2L option if 1L treatment was abiraterone |
| Abiraterone is a 2L option if 1L treatment was enzalutamide |
| Cabazitaxel is the recommended 3L option, with abiraterone or enzalutamide also 3L options |
| Potential use of olaparib is currently under review |
| Spain [39] |
| Docetaxel/prednisone is a 1L option if symptomatic and ADT alone given prior to progression to mCRPC |
| Docetaxel, abiraterone/prednisone or enzalutamide are 1L options if asymptomatic |
| Abiraterone/prednisone, enzalutamide or cabazitaxel are 2L options if 1L treatment was docetaxel |
| Docetaxel/prednisone is a 2L option following 1L treatment with NHAs |
| Cabazitaxel is the recommended 3L option after docetaxel and NHAs |
| Olaparib is recommended in BRCA1/BRCA2 mutated CRPC patients after progression on at least one new hormonal therapy |
| United Kingdom [40] |
| Abiraterone (+ prednisone/prednisolone) or enzalutamide is 1L option after ADT has failed, if no/mild symptoms, and before chemotherapy is indicated |
| Docetaxel is a 1L option if symptomatic |
| Docetaxel is the 2L option after abiraterone/enzalutamide |
| Cabazitaxel (+ prednisone/prednisolone), abiraterone and enzalutamide are options if disease has progressed during or after docetaxel |
| Rechallenge with NHAs is not a recommended option |
| Guidance on the use of PARPi (only olaparib) in mCRPC is in development |
| Japan [41] |
| Docetaxel + prednisolone is the recommended treatment option |
| Abiraterone + prednisolone and enzalutamide are 1L options, prior to chemotherapy |
| Abiraterone + prednisolone and enzalutamide are 2L options, following chemotherapy |
| Cabazitaxel is a 2L option, following docetaxel |
| No recommendations on the order of successive treatments |
| Rechallenge with NHAs is an option |
1L 1st-line treatment, 2L 2nd-line treatment, 223Ra radium-223, 3L 3rd-line treatment, ADT androgen deprivation therapy, ATM ataxia telangiectasia, BRCA breast cancer susceptibility gene, CRPC castration-resistant prostate cancer, mCRPC metastatic castration-resistant prostate cancer, NHA novel hormonal agents, PARPi poly adenosine diphosphate-ribose polymerase inhibitor
Physicians provided data for 1753 patients (France n = 356, Germany n = 350, Italy n = 315, Spain n = 274, UK n = 216, Japan n = 242). Patient demographics and clinical characteristics are presented in Table 2. Overall, patient mean age was just over 70 years, and most were retired. Demographics were similar across countries, but, while mean age was highest in Japan (75 years), only 48% of Japanese patients were retired. At PC diagnosis, ≥ 87% of patients in all countries had an Eastern Cooperative Oncology Group (ECOG) performance status of 0/1, indicating that they were fully active or restricted only in physically strenuous activity [23]. While at data collection < 75% of patients overall had an ECOG performance status of 0/1, the results showed significant country differences: in Spain, the UK and Japan, ≥ 80% of men still had an ECOG performance status of 0/1, but it was only 51% in Germany (Table 2).
Table 2.
Patient demographics and clinical characteristics (at the time of data collection, unless otherwise specified), overall and by country
| Total (n = 1753) | France (n = 356) | Germany (n = 350) | Italy (n = 315) | Spain (n = 274) | UK (n = 216) | Japan (n = 242) | |
|---|---|---|---|---|---|---|---|
| Age, years | |||||||
| Mean (SD) | 71.9 (8.1) | 72.3 (7.6) | 70.6 (6.76) | 71.8 (8.9) | 70.6 (8.7) | 71.2 (7.5) | 75.0 (8.8) |
| Range | 45, 90 | 45, 90 | 52, 87 | 47, 90 | 45, 90 | 45, 90 | 49, 90 |
| Employment status, n (%) | |||||||
| Working full-time | 96 (6) | 10 (3) | 9 (3) | 28 (9) | 7 (3) | 4 (2) | 38 (16) |
| Working part-time | 70 (4) | 1 (< 1) | 11 (3) | 22 (7) | 5 (2) | 17 (8) | 14 (6) |
| Long-term sick leave | 100 (6) | 18 (5) | 18 (5) | 13 (4) | 32 (12) | 12 (6) | 7 (3) |
| Homemaker | 4 (0) | 0 (0) | 0 (0) | 1 (< 1) | 2 (1) | 1 (1) | 0 (0) |
| Student | 2 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1) | 0 (0) | 0 (0) |
| Retired | 1358 (78) | 319 (90) | 301 (86) | 230 (73) | 218 (80) | 173 (80) | 117 (48) |
| Unemployed | 63 (4) | 5 (1) | 8 (2) | 2 (1) | 4 (2) | 3 (1) | 41 (17) |
| Unknown | 60 (3) | 3 (1) | 3 (1) | 19 (6) | 4 (2) | 6 (3) | 25 (10) |
| ECOG performance status at initial prostate cancer diagnosis, n (%) | |||||||
| 0–1 | 1589 (91) | 314 (88) | 325 (93) | 275 (87) | 251 (92) | 197 (91) | 227 (94) |
| 2 + | 139 (8) | 41 (12) | 24 (7) | 20 (6) | 23 (8) | 19 (9) | 12 (5) |
| Unknown | 25 (1) | 1 (< 1) | 1 (0) | 20 (6) | 0 (0) | 0 (0) | 3 (1) |
| ECOG performance status, n (%) | |||||||
| 0–1 | 1284 (73) | 250 (70) | 179 (51) | 250 (79) | 220 (80) | 189 (88) | 196 (81) |
| 2 + | 453 (26) | 101 (28) | 171 (49) | 56 (18) | 54 (20) | 27 (13) | 44 (18) |
| Unknown | 16 (1) | 5 (1) | 0 (0) | 9 (3) | 0 (0) | 0 (0) | 2 (1) |
| Has a caregiver, n (%) | |||||||
| Yes | 523 (30) | 84 (24) | 181 (52) | 96 (31) | 91 (33) | 39 (18) | 32 (13) |
| No | 1006 (57) | 243 (68) | 141 (40) | 145 (46) | 137 (50) | 168 (78) | 172 (71) |
| Unknown | 224 (13) | 29 (8) | 28 (8) | 74 (23) | 46 (17) | 9 (4) | 38 (16) |
| Family history of prostate cancer, n (%) | |||||||
| Yes | 225 (13) | 46 (13) | 55 (16) | 35 (11) | 65 (24) | 16 (7) | 8 (3) |
| No | 1378 (79) | 295 (83) | 280 (80) | 249 (79) | 187 (68) | 180 (83) | 187 (77) |
| Unknown | 150 (9) | 15 (4) | 15 (4) | 31 (10) | 22 (8) | 20 (9) | 47 (19) |
| Location of metastasesa, n (%) | |||||||
| Bone | 1529 (87) | 327 (92) | 268 (77) | 269 (85) | 253 (92) | 198 (92) | 214 (88) |
| Non-regional/distant lymph nodes | 637 (36) | 123 (35) | 127 (36) | 150 (48) | 110 (40) | 64 (30) | 63 (26) |
| Lung | 246 (14) | 50 (14) | 44 (13) | 67 (21) | 42 (15) | 28 (13) | 15 (6) |
| Brain | 22 (1) | 3 (1) | 8 (2) | 3 (1) | 3 (1) | 1 (1) | 4 (2) |
| Otherb | 284 (16) | 58 (16) | 78 (22) | 40 (13) | 65 (24) | 32 (15) | 11 (5) |
| Number of bone metastatic sites | |||||||
| N | 1235 | 275 | 207 | 232 | 209 | 139 | 173 |
| Mean (SD) | 5.2 (4.4) | 5.4 (4.1) | 4.4 (3.8) | 5.8 (4.6) | 5.2 (4.2) | 5.8 (5.2) | 4.8 (4.5) |
| Range | 1, 40 | 1, 20 | 1, 25 | 1, 30 | 1, 30 | 1, 40 | 1, 25 |
| Risk statusc, n (%) | |||||||
| Low | 766 (44) | 168 (47) | 177 (51) | 129 (41) | 110 (40) | 89 (41) | 93 (38) |
| High | 784 (45) | 148 (42) | 130 (37) | 150 (48) | 149 (54) | 86 (40) | 121 (50) |
| Unknown | 203 (12) | 40 (11) | 43 (12) | 36 (11) | 15 (6) | 41 (19) | 28 (12) |
| Most recent PSA level, ng/ml | |||||||
| N | 1540 | 310 | 293 | 286 | 235 | 188 | 228 |
| Mean (SD) | 43.6 (210.4) | 49.9 (269.4) | 15.2 (18.0) | 34.8 (115.5) | 47.9 (177.1) | 47.6 (76.7) | 74.7 (380.3) |
| Range | 0, 4807 | 0, 3650 | 0, 147 | 0, 1500 | 0, 1750 | 0, 452 | 0, 4807 |
| Most recent alkaline phosphatase level, U/L | |||||||
| N | 778 | 120 | 60 | 148 | 176 | 104 | 170 |
| Mean (SD) | 236 (205) | 201 (145) | 164 (96) | 202 (121) | 231 (194) | 182 (103) | 355 (310) |
| Range | 20, 3133 | 32, 958 | 38, 392 | 20, 710 | 27, 1844 | 46, 534 | 52, 3133 |
ECOG Eastern Cooperative Oncology Group, PSA prostate-specific antigen, SD standard deviation, UK United Kingdom
aPatients might have more than one metastatic site, so total might be > 100%
bOther includes pancreas, liver, adrenal glands, peritoneal, non-regional/distant lymph nodes, and other sites of metastases specified by physicians
cPatients were considered high risk if they had two of the following: Gleason score of ≥ 8, presence of visceral metastases or ≥ 3 bone lesions
Only 13% of patients had a known family history of PC, ranging from 3% in Japan to 24% in Spain. At data collection, metastases were reported for a wide range of sites, with the vast majority of patients having bone metastases, and a mean of just over five bone metastatic sites/patient (Table 2). The lowest proportion of patients with bone metastases was reported in Germany (77%) and the highest in Spain and the UK (both 92%). Patients were split fairly evenly between those with low and those with high risk status, with high risk defined as having at least two of the following: Gleason score of ≥ 8, presence of visceral metastases or ≥ 3 bone lesions.
Current Treatment
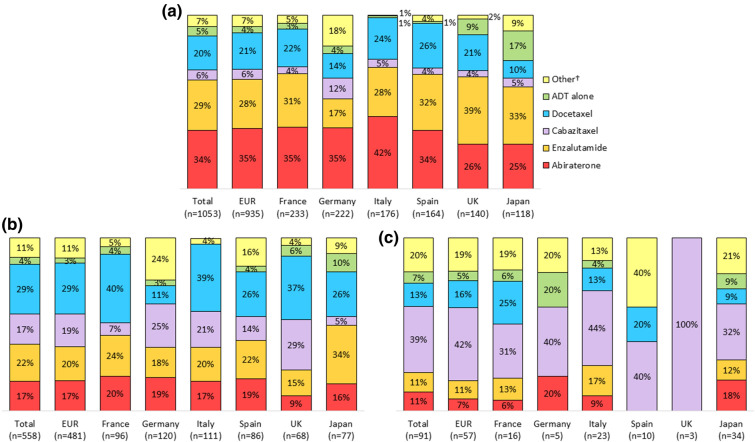
All treatments reported were ± ADT, unless otherwise stated. Treatments received at data collection are presented in Fig. 2 for the 1702 patients for whom data were available and line of therapy could be derived. Treatment data at 1L, 2L and third-line or later (3L+) were available for 1053, 558 and 91 patients, respectively. In all countries, the most common 1L treatment was NHA use. Abiraterone was the most commonly used, except in the UK and Japan, where enzalutamide was preferred, and Spain, where abiraterone and enzalutamide had similar usage (Fig. 2).
Fig. 2.
Current treatment, NHA and chemotherapy breakout: a at 1L of therapy, b at 2L of therapy, c at 3L+ of therapy, overall and by country
Chemotherapy was the second most common current 1L treatment in all countries, with docetaxel being used in 22% of patients overall. Chemotherapy was the preferred current 2L therapy in all countries except Japan, where chemotherapy was received by 34% and NHAs by 49% of patients on 2L, and Spain, where equal proportions of patients (41%) received chemotherapy and NHAs. Higher levels of chemotherapy use were associated with later treatment lines overall and in each of the countries except Italy, where 60% of patients at 2L and 57% at 3L+ were receiving chemotherapy. Overall, 26% of patients were receiving chemotherapy in 1L, but 52% of patients received chemotherapy at 3L+ . Cabazitaxel preference increased with later treatment lines in all countries, and it was received by more patients than docetaxel at 2L in Germany, and at 3L+ in all other countries.
1L to 2L Treatment Sequence Following mCRPC Diagnosis
Treatment sequences from 1L to 2L for 649 patients who were diagnosed with mCRPC are shown in Table 3. The most common 1L to 2L treatment sequence overall and in each country was NHA followed by ( →) chemotherapy, which was received by 35% of patients. Abiraterone → docetaxel was the most frequently reported sequence in 16% of patients overall, and was the most common NHA → chemotherapy sequence in most countries, while enzalutamide → docetaxel was more frequent in the UK and Japan, and abiraterone → cabazitaxel slightly more common in Germany (Table 3).
Table 3.
1L to 2L mCRPC treatment sequence, overall, in EUR, and by country
| 1L → 2L sequence | mCRPC patients treated with 1L and 2L therapy | |||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 649) | EUR (n = 538) | France (n = 112) | Germany (n = 125) | Italy (n = 134) | Spain (n = 96) | UK (n = 71) | Japan (n = 111) | |
| NHA → chemotherapy, n (%) | ||||||||
| Total | 225 (35) | 196 (37) | 48 (43) | 28 (22) | 55 (41) | 29 (30) | 36 (51) | 29 (26) |
| Abiraterone → docetaxel | 104 (16) | 95 (18) | 28 (25) | 11 (9) | 33 (25) | 16 (17) | 7 (10) | 9 (8) |
| Enzalutamide → docetaxel | 71 (11) | 56 (10) | 16 (14) | 2 (2) | 15 (11) | 6 (6) | 17 (24) | 15 (14) |
| Abiraterone → cabazitaxel | 30 (5) | 29 (5) | 3 (3) | 12 (10) | 6 (4) | 5 (5) | 3 (4) | 1 (1) |
| Enzalutamide → cabazitaxel | 15 (2) | 13 (2) | 0 (0) | 1 (1) | 1 (1) | 2 (2) | 9 (13) | 2 (2) |
| Chemotherapy → NHAa, n (%) | ||||||||
| Total | 107 (16) | 102 (19) | 20 (18) | 23 (18) | 33 (25) | 16 (17) | 10 (14) | 5 (5) |
| Docetaxel → abiraterone | 58 (9) | 55 (10) | 9 (8) | 13 (10) | 17 (13) | 12 (13) | 4 (6) | 3 (3) |
| Docetaxel → enzalutamide | 45 (7) | 44 (8) | 11 (10) | 8 (6) | 15 (11) | 4 (4) | 6 (8) | 1 (1) |
| NHA → NHAb, n (%) | ||||||||
| Total | 84 (13) | 65 (12) | 24 (21) | 17 (14) | 8 (6) | 15 (16) | 1 (1) | 19 (17) |
| Abiraterone → enzalutamide | 58 (9) | 44 (8) | 15 (13) | 12 (10) | 4 (3) | 13 (14) | 0 (0) | 14 (13) |
| Enzalutamide → abiraterone | 17 (3) | 15 (3) | 9 (8) | 1 (1) | 3 (2) | 2 (2) | 0 (0) | 2 (2) |
| Chemotherapy → chemotherapy, n (%) | ||||||||
| Total | 54 (8) | 50 (9) | 5 (4) | 12 (10) | 20 (15) | 5 (5) | 8 (11) | 4 (4) |
| Docetaxel → cabazitaxel | 53 (8) | 49 (9) | 5 (4) | 12 (10) | 19 (14) | 5 (5) | 8 (11) | 4 (4) |
| Other 1L → 2L sequences, n (%) | ||||||||
| ADT alone → NHA | 55 (8) | 32 (6) | 3 (3) | 5 (4) | 8 (6) | 9 (9) | 7 (10) | 23 (21) |
| ADT alone → chemotherapy | 17 (3) | 10 (2) | 3 (3) | 4 (3) | 1 (1) | 1 (1) | 1 (1) | 7 (6) |
| NHA → ADT alone | 16 (2) | 12 (2) | 4 (4) | 2 (2) | 1 (1) | 3 (3) | 2 (3) | 4 (4) |
| ADT alone → ADT alone | 9 (1) | 3 (1) | 1 (1) | 0 (0) | 1 (1) | 0 (0) | 1 (1) | 6 (5) |
| Chemotherapy → ADT alone | 7 (1) | 3 (1) | 0 (0) | 2 (2) | 1 (1) | 0 (0) | 0 (0) | 4 (4) |
| Otherc | 75 (11) | 65 (12) | 4 (4) | 32 (26) | 6 (4) | 18 (19) | 5 (7) | 10 (9) |
Treatments are ± ADT, unless otherwise stated
→ , followed by; 1L 1st-line treatment, 2L 2nd-line treatment, ADT androgen deprivation therapy, EUR Europe (France, Germany, Italy, Spain, the UK), mCRPC metastatic castration-resistant prostate cancer, NHA novel hormonal agent, UK United Kingdom
aOverall, the treatment sequences cabazitaxel → enzalutamide and cabazitaxel → abiraterone were received by 2 patients (0.3%) and 1 patient (0.2%), respectively
bOverall, the treatment sequences apalutamide → abiraterone and apalutamide → enzalutamide were received by 3 patients each (0.5%), enzalutamide → enzalutamide was received by 2 patients (0.3%), and abiraterone → abiraterone was received by 1 patient (0.2%)
cOther 1L to 2L sequences observed overall (n = 75) included: NHA → other combination including NHA (n = 10), NHA → chemotherapy combination (n = 1), NHA → any other combination (n = 22), chemotherapy → other combination including NHA (n = 4), chemotherapy → any other combination (n = 7), ADT alone → other combination including NHA (n = 3), ADT alone → any other combination (n = 2), other combination including NHA → NHA (n = 2), other combination including NHA → chemotherapy (n = 3), other combination including NHA → other combination including NHA (n = 4), other combination including NHA → any other combination (n = 6), chemotherapy combination → chemotherapy (n = 3), any other combination → NHA (n = 5), any other combination → chemotherapy (n = 2), any other combination → ADT alone (n = 1)
The second most common 1L to 2L treatment sequence in all countries except France and Japan was chemotherapy → NHA, received by 16% of patients overall. In France, NHA rechallenge was the second most common sequence and was reported for 21% of patients, while chemotherapy → NHA was chosen in 18% of cases. In contrast, in Japan, ADT alone → NHA was reported for 21% of patients, and chemotherapy → NHA in only 5%. Docetaxel → abiraterone was the most frequently reported drug sequence overall for patients receiving chemotherapy → NHA, with 9% of patients receiving this treatment sequence; however, France and the UK reported a higher proportion of patients who were receiving docetaxel → enzalutamide (Table 3).
Although, overall, the third most preferred 1L to 2L sequence was NHA rechallenge, the third most commonly reported 1L to 2L sequence in Italy and the UK was chemotherapy rechallenge, NHA rechallenge in Japan, and chemotherapy → NHA in France. Chemotherapy rechallenge was reported in 10%-15% of patients in Germany, Italy, and the UK.
Treatment in mHSPC Setting Prior to mCRPC Diagnosis
Most patients in all countries (81% overall) had mHSPC prior to progression to mCRPC. Treatments received by patients with mHSPC immediately prior to being diagnosed with mCRPC are shown in Table 4 for the 1131 patients for whom data were available. In all countries, the most common treatment received prior to the patient progressing to mCRPC was ADT alone, with frequency of use ranging from 64% in Spain to 84% in France and Japan. Overall, chemotherapy and NHAs were used in the same proportions of patients with mHSPC prior to mCRPC (both 13%). Chemotherapy use was lowest in Japan (2%) and highest in the UK (24%), predominantly docetaxel, which was received by 12% of patients in the mHSPC state overall, ranging from 0% in Japan to 24% in the UK. NHA uptake was lowest in the UK (10%) and highest in Spain (18%), predominantly abiraterone (9%), ranging from 5% in the UK to 14% in Spain, whilst enzalutamide, which was more recently approved, was only received by 3% of patients overall.
Table 4.
Treatment received by mHSPC patients immediately prior to being diagnosed with mCRPCa, overall, in EUR, and by country
| Total (n = 1131) | EUR (n = 1005) | France (n = 267) | Germany (n = 223) | Italy (n = 217) | Spain (n = 126) | UK (n = 172) | Japan (n = 126) | |
|---|---|---|---|---|---|---|---|---|
| ADT alone, n (%) | ||||||||
| Total | 839 (74) | 733 (73) | 225 (84) | 148 (66) | 166 (76) | 81 (64) | 113 (66) | 106 (84) |
| Chemotherapyb, n (%) | ||||||||
| Total | 147 (13) | 145 (14) | 14 (5) | 41 (18) | 25 (12) | 23 (18) | 42 (24) | 2 (2) |
| Docetaxel | 137 (12) | 137 (14) | 12 (4) | 40 (18) | 23 (11) | 21 (17) | 41 (24) | 0 (0) |
| Cabazitaxel | 6 (1) | 6 (1) | 0 (0) | 1 (< 1) | 2 (1) | 2 (2) | 1 (1) | 0 (0) |
| NHA, n (%) | ||||||||
| Total | 142 (13) | 125 (12) | 28 (10) | 32 (14) | 26 (12) | 22 (17) | 17 (10) | 17 (13) |
| Abiraterone | 99 (9) | 86 (9) | 19 (7) | 22 (10) | 18 (8) | 18 (14) | 9 (5) | 13 (10) |
| Enzalutamide | 34 (3) | 31 (3) | 9 (3) | 2 (1) | 8 (4) | 4 (3) | 8 (5) | 3 (2) |
| Apalutamide | 9 (1) | 8 (1) | 0 (0) | 8 (4) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
Treatments are ± ADT, unless otherwise stated
ADT androgen deprivation therapy, mCRPC metastatic castration-resistant prostate cancer, mHSPC metastatic hormone sensitive prostate cancer, NHA novel hormonal agent, UK United Kingdom
aOther treatment combinations including NHA and any other treatment combinations were both being used in 0.1% of patients overall
bChemotherapy other than docetaxel or cabazitaxel was being used in 0.3% of patients overall
Treatment Sequence from mHSPC to 1L mCRPC Following mCRPC Diagnosis
The most common mHSPC to 1L mCRPC treatment sequence overall in 1131 patients for whom data were available was ADT alone → NHA, received by 52% of patients, ranging from 43% in Germany to 65% in France (Table 5). Overall, ADT → abiraterone was reported in 28% of patients and enzalutamide in 23%. The preference for abiraterone over enzalutamide was reported for all countries except the UK and Japan.
Table 5.
Treatment sequence from mHSPC to 1L mCRPC following mCRPC diagnosis, overall, in EUR, and by countrya
| mCRPC patients who received a treatment in mHSPC | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 1131) | EUR (n = 1005) | France (n = 267) | Germany (n = 223) | Italy (n = 217) | Spain (n = 126) | UK (n = 172) | Japan (n = 126) | |
| ADT alone → NHAb, n (%) | ||||||||
| Total | 583 (52) | 512 (51) | 173 (65) | 97 (43) | 106 (49) | 60 (48) | 76 (44) | 71 (56) |
| ADT alone → abiraterone | 321 (28) | 290 (29) | 91 (34) | 73 (33) | 65 (30) | 36 (29) | 25 (15) | 31 (25) |
| ADT alone → enzalutamide | 259 (23) | 219 (22) | 81 (30) | 22 (10) | 41 (19) | 24 (19) | 51 (30) | 40 (32) |
| ADT alone → chemotherapy, n (%) | ||||||||
| Total | 196 (17) | 188 (19) | 49 (18) | 41 (18) | 53 (24) | 20 (16) | 25 (15) | 8 (6) |
| ADT alone → docetaxel | 169 (15) | 164 (16) | 43 (16) | 25 (11) | 52 (24) | 20 (16) | 24 (14) | 5 (4) |
| ADT alone → cabazitaxel | 24 (2) | 24 (2) | 6 (2) | 16 (7) | 1 (< 1) | 0 (0) | 1 (1) | 0 (0) |
| Chemotherapy → NHAb, n (%) | ||||||||
| Total | 93 (8 | 93 (9) | 9 (3) | 18 (8) | 17 (8) | 15 (12) | 34 (20) | 0 (0) |
| Docetaxel → abiraterone | 61 (6) | 61 (6) | 7 (3) | 14 (6) | 12 (6) | 13 (10) | 15 (9) | 0 (0) |
| Docetaxel → enzalutamide | 30 (3) | 30 (3) | 2 (1) | 3 (1) | 4 (2) | 2 (2) | 19 (11) | 0 (0) |
| NHA → chemotherapyb, n (%) | ||||||||
| Total | 76 (7) | 72 (7) | 17 (6) | 12 (5) | 18 (8) | 15 (12) | 10 (6) | 4 (3) |
| Abiraterone → docetaxel | 57 (5) | 54 (5) | 13 (5) | 8 (4) | 15 (7) | 11 (9) | 7 (4) | 3 (2) |
| Enzalutamide → docetaxel | 13 (1) | 12 (1) | 3 (1) | 0 (0) | 3 (1) | 4 (3) | 2 (1) | 1 (1) |
| NHA → NHAb, n (%) | ||||||||
| Total | 40 (4) | 28 (3) | 3 (1) | 15 (7) | 7 (3) | 3 (2) | 0 (0) | 12 (10) |
| Abiraterone → enzalutamide | 19 (2) | 10 (1) | 2 (1) | 4 (2) | 1 (< 1) | 3 (2) | 0 (0) | 9 (7) |
| Enzalutamide → abiraterone | 9 (1) | 7 (1) | 1 (< 1) | 2 (1) | 4 (2) | 0 (0) | 0 (0) | 2 (2) |
| NHA → ADT alone, n (%) | ||||||||
| Total | 15 (1) | 15 (1) | 6 (2) | 1 (< 1) | 1 (< 0) | 2 (2) | 5 (3) | 0 (0) |
| Chemotherapy → chemotherapy, n (%) | ||||||||
| Total | 31 (3) | 31 (3) | 4 (1) | 10 (4) | 7 (3) | 5 (4) | 5 (3) | 0 (0) |
| Docetaxel → cabazitaxel | 23 (2) | 23 (2) | 2 (1) | 7 (3) | 6 (3) | 4 (3) | 4 (2) | 0 (0) |
| Docetaxel → docetaxel | 3 (< 10) | 3 (< 1) | 0 (0) | 3 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Cabazitaxel → docetaxel | 3 (< 10) | 3 (< 10) | 0 (0) | 0 (0) | 1 (< 1) | 1 (1) | 1 (1) | 0 (0) |
| ADT alone → ADT alone, n (%) | ||||||||
| Total | 43 (4) | 18 (2) | 0 (0) | 3 (1) | 4 (2) | 1 (1) | 10 (6) | 25 (20) |
Treatments are ± ADT, unless otherwise stated
→ , followed by, ADT androgen deprivation therapy, mCRPC metastatic castration-resistant prostate cancer, mHSPC metastatic hormone sensitive prostate cancer, NHA novel hormonal agent, UK United Kingdom
aOther treatment sequences from prior to mCRPC diagnosis to 1L following mCRPC diagnosis observed overall (n = 47) included: ADT alone → other combination including NHA (n = 4), ADT alone → chemotherapy (n = 1), ADT alone → any other combination (n = 12), chemotherapy → other combination including NHA (n = 13), chemotherapy → any other combination (n = 3), NHA → other combination including NHA (n = 5), NHA → chemotherapy combination (n = 1), NHA → any other combination (n = 5), other combination including NHA → NHA (n = 1), other combination including NHA → other combination including NHA (n = 1), any other combination → chemotherapy (n = 1)
bOverall, ADT alone → apalutamide was received by 3 patients (0.3%), cabazitaxel → abiraterone by 2 patients (0.2%), apalutamide → docetaxel by 2 patients (0.2%), abiraterone → cabazitaxel by 2 patients (0.2%), enzalutamide → cabazitaxel by 2 patients (0.2%), abiraterone → apalutamide by 3 patients (0.3%), abiraterone → abiraterone by 2 patients (0.2%)
The second most common treatment sequence overall, received by 17% of patients, was ADT alone → chemotherapy, ranging between 6% of patients in Japan and 24% in Italy. Docetaxel was the most common chemotherapeutic agent received in this treatment sequence overall (15%) and in all countries. In Japan, the second most common treatment sequence after progression to mCRPC was repeat ADT alone, with 20% of patients in Japan receiving this treatment sequence. Of the 13% of patients receiving NHAs in the mHSPC setting (Table 4), 54% received chemotherapy at mCRPC 1L, while 28% received an alternative NHA. NHA rechallenge was uncommon, being reported in 4% of patients overall, with the highest occurrence in Japan (10%).
Discussion
In this study, we evaluated country-specific treatment guidelines and assessed the treatment patterns in mCRPC patients from France, Germany, Italy, Spain, the UK, and Japan. Overall, treatment patterns were generally in accordance with country guidelines and were consistent with the available treatment options that were reimbursed. The first and second most common treatments in the 1L mCRPC setting were NHAs and chemotherapy, respectively, in all countries. This reflected guidelines from Europe and Japan, which specify abiraterone, enzalutamide or docetaxel as 1L options. A published analysis of Prostate Cancer Registry data collected between 2013 and 2018 in 16 European countries reported that, of mCRPC patients prescribed abiraterone, enzalutamide or docetaxel at 1L, 48%, 14%, and 38%, respectively, received these treatments [16]. Equivalent analysis of Europe data from our dataset showed that the current 1L share between abiraterone, enzalutamide and docetaxel to be 34%, 29%, and 20%, respectively. The higher use of enzalutamide highlighted through our analysis compared with the cited source might reflect differences in the timing of data collection in the two studies, as well as the more recent approval of enzalutamide compared with the other two treatments. A recent analysis of medical records for 422 patients with mCRPC from a number of Japanese centres reported 32%, 36%, and 32% receiving abiraterone, enzalutamide, and docetaxel, respectively, in the 1L mCRPC setting [24], compared with 25%, 33%, and 10%, respectively, of current 1L treatment of Japanese patients in our dataset.
In Japan, docetaxel is the recommended 1L treatment, although NHAs are also recommended at 1L if chemotherapy is likely to be received at 2L. Interestingly, our data showed the use of chemotherapy at 1L to be lower in Japan than in Europe. We also observed that ADT use as a current 1L treatment option in Japan was higher compared to other countries (17% vs. 5% overall). This may be attributed to Japanese patients in this study being older than those from other countries, and the willingness to undergo active treatment may be lower in Japan. It may also reflect that not all patients in Japan have access to NHAs [25]. Current cabazitaxel use at 1L in 6% of patients overall, and 12% in Germany, is surprising, since it does not represent a recommended 1L option in any country.
In the current 2L mCRPC setting, chemotherapy and NHAs were the first and second most common treatments, respectively (48% and 39% of patients, overall), in all countries except Japan, where NHAs were preferred. Two published studies of real-world 2L mCRPC treatment that included only patients who had received docetaxel at 1L reported the percentage of patients receiving 2L abiraterone after 1L docetaxel to be 67% in Spain [26] and 55% in Italy [27], compared with 13% in both Spain and Italy in our study.
In our analysis of 1L to 2L mCRPC treatment sequences, NHA → chemotherapy was the most common treatment sequence in all countries. This is consistent with recommendations from most guidelines, although German and Japanese guidelines do not specify a 2L treatment following NHAs. Other treatment sequences observed in the total study population were chemotherapy → NHA (16%), NHA → NHA (13%), and chemotherapy → chemotherapy (8%). Country differences were observed within Europe in treatment sequences, with chemotherapy → NHA more common than NHA → NHA and chemotherapy → chemotherapy in Germany, Italy, and UK, whereas the use of chemotherapy → NHA and NHA → NHA was similar in France and Spain. In Japan, the most common 1L → 2L treatment sequence was NHA → chemotherapy; however, NHA rechallenge (specifically abiraterone → enzalutamide) was the second most common treatment sequence observed, although some reimbursement agencies (e.g. NICE) state that NHAs cannot be used in sequence, and can only be used once within the treatment pathway.
Although ESMO and EAU guidelines also explicitly discourage NHA → NHA usage, in Germany, Italy, and Japan, NHA rechallenge is a treatment option specified in local guidelines. Overall, 13% of patients received NHA rechallenge at 2L after 1L, with 21% of patients in France and 16% in Spain receiving this treatment sequence, despite not being recommended by respective local guidelines. In the UK, where NHA rechallenge is also not recommended, this treatment sequence was reported for 1% of patients. The potential for inter-country differences in treatment guidelines and treatment sequencing to have differential impact on patient outcomes would be an interesting topic for future research.
Most patients had mHSPC immediately prior to their mCRPC diagnosis, with ADT alone as the most common treatment in mHSPC in all countries. Chemotherapy use was lower overall than ADT alone and showed a greater range, from 2% in Japan to 24% in the UK. NHA use was fairly low, with between 10% (UK) and 17% (Spain) of patients across countries receiving NHAs during the mHSPC disease state. Enzalutamide received approval for an indication in mHSPC from the PMDA in 2020 [15], and the EMA in 2021 [28]; as these approvals were during and after the data collection period for this survey, the low level of use was to be expected. Apalutamide was approved for mHSPC by the EMA in 2019 [29] and the PMDA in 2020 [15]; as patients included in this analysis had to be diagnosed with mCRPC between January and August 2020, and patients progress after a median of 3 years [30, 31]), low levels of apalutamide prior to mCRPC were also not surprising. However, abiraterone was approved for use in mHSPC in Europe in 2011 [32], and in Japan in 2018 [33], so its relatively low adoption rates were unexpected.
Although regulatory approvals suggest NHAs are moving to be used in pre-mCRPC disease states, and treatment intensification is the new standard of care in mHSPC in many developed countries, in our study only 13% of patients received NHAs in the mHSPC setting, while higher uptake in the UK may be attributed to its approval by NICE. There are several potential reasons for slow adoption of NHAs in the earlier setting [34]. Clinical trials of NHAs in mHSPC have involved high number of patients newly-diagnosed with metastatic disease, while the real-world patient population is likely to have already received radical treatment (prostatectomy). Patients in clinical practice tend to be older and have more comorbidities than those in clinical trials, with competing risks complicating treatment decisions, and ADT monotherapy a reasonable option for patients with high mortality risk due to comorbidities. Only indirect comparisons of NHAs with other treatments are available in the mHSPC setting, due to the lack of head-to-head trials. Finally, cost and reimbursement might influence treatment selection.
Currently, in France and Spain, docetaxel is recommended as the mCRPC 1L option if patients received NHA treatment in the mHSPC setting, and docetaxel could potentially become the frontline treatment in the mCRPC setting in countries where NHA rechallenge is not recommended, such as the UK and Spain. German, Italian, and Japanese guidelines do not specify the treatment in mCRPC for patients treated with NHAs in the mHSPC setting, but, as NHA rechallenge is allowed, this will likely continue to be the frontline treatment in mCRPC. Health Technology Assessment guidance is evolving and may evolve further once NHA adoption in mHSPC increases.
Limitations
Our methodological approach had a number of potential limitations. The DSP was not based on a true random sample of physicians or patients. Despite minimal inclusion criteria, physician participation was influenced by their willingness to complete the survey. Physicians were asked to provide data for a consecutive series of patients to avoid selection bias, but no formal patient selection verification procedures were in place. Identification of eligible patients was based on physician judgment and not on a formalised diagnostic checklist. Recall bias might have affected the responses; however, the data for these analyses were collected at the time of each patient’s appointment and this is expected to reduce the likelihood of recall bias. Missing data were not imputed; therefore, the base of patients for analysis could vary from variable to variable and is reported separately for each analysis. Finally, the point-in-time design of this survey prevented any conclusions about causal relationships. All comparisons between treatments and countries were descriptive; no formal statistical testing was performed, and comparative terms such as ‘higher’ or ‘more’ should not be taken to imply statistical significance.
Despite such limitations, real-world studies highlight concerns that are not addressed in clinical trials. Patients included in clinical trials represent a small proportion of the consulting population, as a result of age restrictions and failure to meet stringent eligibility criteria. Data from real-world studies can complement clinical trials and provide insight into the effectiveness of interventions seen in clinical practice.
Conclusions
To our knowledge, this was the first study to assess mCRPC treatment sequencing and to demonstrate that overall real-world treatment sequencing was in line with country treatment guidelines. This real-world study of patients with mCRPC across five countries in Europe, and Japan confirmed that the treatment patterns are largely in line with treatment guidelines, although a proportion of patients received treatment that was not recommended in their country. Clear guidelines on optimal treatment sequences for patients with mCRPC are needed. As clinicians become more familiar with recently approved treatments in various PC disease states, and new treatment options such as PARPi become available, it will be interesting to investigate how these agents are introduced into treatment guidelines in different regions, and how they may impact real-world treatment patterns in the future.
Acknowledgements
The authors wish to thank the participants of the study.
Funding
This project, including the journal’s Rapid Service and Open Access Fees, was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and AstraZeneca UK Limited.
Medical Writing and Editorial Assistance
Medical writing and editing support under the guidance of the authors was provided by Carole Evans PhD, and K Ian Johnson BSc MBPS SRPharmS, on behalf of Adelphi Real World, and was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and AstraZeneca UK Limited in accordance with Good Publication Practice guidelines (Ann Intern Med 2015;163:461-464).
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Amanda Ribbands, Andrea Leith and Emily Clayton. The first draft of the manuscript was written by Carole Evans and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Andrea Leith, Amanda Ribbands, Emily Clayton declare that they have no conflict of interest; Jeri Kim and Sameer R. Ghate are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and hold stock in Merck & Co., Inc., Kenilworth, NJ, USA. Lingfeng Yang was a previous employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and holds stock in Merck & Co., Inc., Kenilworth, NJ, USA. Lingfeng Yang is an employee of Janssen Global Services, LLC, Horsham, PA, USA.
Compliance with Ethics Guidelines
Using a checkbox, physicians provided informed consent to take part in the survey. Data were collected in such a way that both patients and physicians could be anonymised. The questionnaires used in the PC DSP were reviewed and given exemption by the Western Institutional Review Board (reference number: 1-1261035-1), and data collection was undertaken in accordance with European Pharmaceutical Marketing Research Association guidelines [20]. As data were collected according to market research guidelines, no source data validation was possible or required. The survey was performed in full accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act 1996 [21] and the Health Information Technology for Economic and Clinical Health Act legislation [22].
Data Availability
The datasets generated during and/or analysed during the current study are not publicly available due to all data, i.e. methodology, materials, data and data analysis, that support the findings of this survey are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to Andrea Leith at andrea.leith@adelphigroup.com.
Footnotes
Lingfeng Yang: Employee at the time the study was conducted.
References
- 1.Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Thurin NH, et al. Epidemiology of metastatic castration-resistant prostate cancer: a first estimate of incidence and prevalence using the French nationwide healthcare database. Cancer Epidemiol. 2020;69:101833. doi: 10.1016/j.canep.2020.101833. [DOI] [PubMed] [Google Scholar]
- 3.Morgan C, et al. PCN17 Castration-resistant prostate cancer (CRPC): a UK epidemiology study. Value Health. 2010;13(3):A26. doi: 10.1016/S1098-3015(10)72108-2. [DOI] [Google Scholar]
- 4.Tannock IF, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.Gravanis I, et al. The European medicines agency review of abiraterone for the treatment of metastatic castration-resistant prostate cancer in adult men after docetaxel chemotherapy and in chemotherapy-naive disease: summary of the scientific assessment of the committee for medicinal products for human use. Oncologist. 2013;18(9):1032–1042. doi: 10.1634/theoncologist.2013-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Vida A, et al. Enzalutamide for the treatment of metastatic castration-resistant prostate cancer. Drug Des Dev Ther. 2015;9:3325–3339. doi: 10.2147/dddt.s69433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda H, Saito A. Enzalutamide-a novel androgen receptor inhibitor that provides treatment options for patients with castration-resistant prostate cancer. Gan To Kagaku Ryoho. 2014;41(7):805–810. [PubMed] [Google Scholar]
- 8.Ueda Y, et al. A multicenter retrospective analysis of sequential treatment of abiraterone acetate followed by docetaxel in Japanese patients with metastatic castration-resistant prostate cancer. Jpn J Clin Oncol. 2015;45(8):774–779. doi: 10.1093/jjco/hyv070. [DOI] [PubMed] [Google Scholar]
- 9.EMA. Assessment report Jevtana. 2011. www.ema.europa.eu/en/documents/assessment-report/jevtana-epar-public-assessment-report_en.pdf. Accessed 23 June 2021.
- 10.PMDA. New drugs approved in FY 2014. 2014 19 July 2021; 000229076.pdf (pmda.go.jp). Accessed 19 July 2021.
- 11.EMA. Assessment report Xofigo. 2013. www.ema.europa.eu/en/documents/assessment-report/xofigo-epar-public-assessment-report_en.pdf. Accessed 19 July 2021.
- 12.PMDA. New drugs approved in FY 2015. 2016. 000229077.pdf (pmda.go.jp). Accessed 19 July 2021.
- 13.Hussain M, et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med. 2020;383(24):2345–2357. doi: 10.1056/NEJMoa2022485. [DOI] [PubMed] [Google Scholar]
- 14.EMA. Assessment report Lynparza. 2020. https://www.ema.europa.eu/en/documents/variation-report/lynparza-h-c-3726-ii-0036-epar-assessment-report-variation_en.pdf. Accessed 23 June 2021.
- 15.PMDA. New drugs approved in FY 2020. 2020. Available from: 000241459.pdf (pmda.go.jp). Accessed 16 Aug 2021.
- 16.Chowdhury S, et al. Real-world outcomes in first-line treatment of metastatic castration-resistant prostate cancer: the prostate cancer registry. Target Oncol. 2020;15(3):301–315. doi: 10.1007/s11523-020-00720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson P, et al. Real-world physician and patient behaviour across countries: disease-Specific Programmes—a means to understand. Curr Med Res Opin. 2008;24(11):3063–3072. doi: 10.1185/03007990802457040. [DOI] [PubMed] [Google Scholar]
- 18.Babineaux SM, et al. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the Disease Specific Programme. BMJ Open. 2016;6(8):e010352. doi: 10.1136/bmjopen-2015-010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins V, et al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes. 2016;9:371–380. doi: 10.2147/DMSO.S120101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EphMRA, 2019. European Pharmaceutical Market Research Association (EphMRA) Code of Conduct 2019. Association EPMR.
- 21.US HHS. Summary of the HIPAA Privacy Rule. 2003. www.hhs.gov/sites/default/files/privacysummary.pdf. Accessed 3 Mar 2022.
- 22.US HHS. HITECH Act Enforcement Interim Final Rule. 2017. www.healthit.gov/sites/default/files/hitech_act_excerpt_from_arra_with_index.pdf.
- 23.Oken MM, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Uchimoto T, et al. Early prostate-specific antigen (PSA) change at four weeks of the first-line treatment using abiraterone and enzalutamide could predict early/primary resistance in metastatic castration-resistant prostate cancer. Cancers (Basel) 2021;13(3):526. doi: 10.3390/cancers13030526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uemura H, et al. The treatment patterns of castration-resistant prostate cancer in Japan, including symptomatic skeletal events and associated treatment and healthcare resource use. Expert Rev Pharmacoecon Outcomes Res. 2017;17(5):511–517. doi: 10.1080/14737167.2017.1300530. [DOI] [PubMed] [Google Scholar]
- 26.Puente J, et al. Novel agents’ sequencing following first-line docetaxel in mCRPC patients: CAPRO study. J Clin Oncol. 2016;34(2_suppl):229–229. doi: 10.1200/jco.2016.34.2_suppl.229. [DOI] [Google Scholar]
- 27.Caffo O, et al. Outcomes of metastatic castration-resistant prostate cancer (mCRPC) patients (pts) treated with different new agents (NAs) sequence in post-docetaxel (DOC) setting. An updated analysis from a multicenter Italian study. Ann Oncol. 2016;27:vi252. doi: 10.1093/annonc/mdw372.27. [DOI] [Google Scholar]
- 28.EMA. Assessment report. 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/xtandi#product-information-section. Accessed 9 Sept 2021.
- 29.EMA. Assessment report Erleada. 2019. https://www.ema.europa.eu/en/medicines/human/EPAR/erleada#product-information-section. Accessed 9 Sept 2021.
- 30.Chi KN, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13–24. doi: 10.1056/NEJMoa1903307. [DOI] [PubMed] [Google Scholar]
- 31.Chi KN, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021;39(20):2294–2303. doi: 10.1200/JCO.20.03488. [DOI] [PubMed] [Google Scholar]
- 32.EMA. Assessment report Zytiga. 2011. https://www.ema.europa.eu/en/medicines/human/EPAR/zytiga. Accessed 9 Sept 2021.
- 33.PMDA. New drugs approved in FY 2017. 2018. 000232769.pdf (pmda.go.jp). Accessed 16 Aug 2021.
- 34.Ng K, Smith S, Shamash J. Metastatic hormone-sensitive prostate cancer (mHSPC): advances and treatment strategies in the first-line setting. Oncol Ther. 2020;8(2):209–230. doi: 10.1007/s40487-020-00119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker C, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(9):1119–1134. doi: 10.1016/j.annonc.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Rozet F, et al. French ccAFU guidelines—update 2020–2022: prostate cancer. Prog Urol. 2020;30(12s):S136–s251. doi: 10.1016/S1166-7087(20)30752-1. [DOI] [PubMed] [Google Scholar]
- 37.AWMF. SW-Leitlinie Prostatakarzinom. 2021. https://www.awmf.org/uploads/tx_szleitlinien/043-022OLl_S3_Prostatakarzinom_2021-08.pdf. Accessed 9 Sept 2021.
- 38.AIOM. Linee Guida: Carcinoma Della Prostata. 2020. https://www.aiom.it/wp-content/uploads/2020/12/2020_LG_AIOM_Carcinoma_Prostata.pdf. Accessed 9 Sept 2021.
- 39.González Del Alba A, et al. SEOM clinical guidelines for the treatment of advanced prostate cancer (2020) Clin Transl Oncol. 2021;23(5):969–979. doi: 10.1007/s12094-021-02561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.NICE, Treating hormone-relapsed metastatic prostate cancer. 2021, National Institute for Health and Care Excellence.
- 41.Kakehi Y, Sugimoto M, Taoka R. Evidenced-based clinical practice guideline for prostate cancer (summary: Japanese Urological Association, 2016 edition) Int J Urol. 2017;24(9):648–666. doi: 10.1111/iju.13380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available due to all data, i.e. methodology, materials, data and data analysis, that support the findings of this survey are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to Andrea Leith at andrea.leith@adelphigroup.com.