Abstract
Introduction
Systemic sclerosis (SSc) is a complex autoimmune disease with increased mortality, and interstitial lung disease (ILD) is a major cause of death. There are no recent epidemiological data on SSc and SSc-associated ILD (SSc-ILD) in Japan and little is known about how patients with these diseases are treated.
Methods
The incidence rate and prevalence of SSc and SSc-ILD in Japan were estimated using the Japanese Medical Data Centre (JMDC) database. The demographic and clinical characteristics of patients and the immunomodulatory medications they received were also assessed using JMDC and the Medical Data Vision (MDV) databases. All analyses were descriptive.
Results
The overall incidence rates of SSc and SSc-ILD per 100,000 person-years were 6.6 (95% confidence interval [CI] 6.2–7.1) and 1.9 (95% CI 1.6–2.1), respectively, and the overall prevalence per 100,000 persons was 37.0 (95% CI 35.6–38.5) and 13.9 (95% CI 13.0–14.8), respectively. ILD was the most common comorbidity in patients with SSc present in approximately 30% of patients (JMDC, 29.3%; MDV, 30.1%). The immunomodulatory medications prescribed were similar in patients with SSc and SSc-ILD, and each of the medications in this analysis was prescribed in less than 15% of patients.
Conclusion
We have demonstrated that estimates of prevalence and incidence rates of SSc and SSc-ILD in Japan are comparable to similar database studies conducted in the USA, using a medical claims database. Only a small proportion of patients were receiving immunomodulatory treatments, suggesting undertreatment in Japan.
Incidence Rate and Prevalence of Systemic Sclerosis and Systemic Sclerosis-associated Interstitial Lung Disease in Japan: Analysis Using Japanese Claims Databases—A Video Abstract. (MP4 68892 KB)
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02078-5.
Keywords: Scleroderma, Interstitial lung disease, Systemic sclerosis, Epidemiology, Immunosuppressant
Key Summary Points
| Why carry out this study? |
| There are no recent epidemiological data on systemic sclerosis (SSc) and SSc-associated interstitial lung disease (SSc-ILD) in Japan and how patients with these diseases are treated. |
| This study aimed to estimate the incidence rate and prevalence of systemic sclerosis (SSc) and SSc-associated interstitial lung disease (SSc-ILD) in Japan, as well as to assess the clinical characteristics of and pharmacotherapy for patients with SSc and SSc-ILD in Japan, using two Japanese medical claims databases. |
| What was learned from the study? |
| The prevalence and incidence rates of SSc and SSc-ILD in Japan were comparable to published global data. |
| There is an unmet need for treatment options for patients with SSc and SSc-ILD in Japan as only a small proportion of patients were receiving immunomodulatory treatments. |
Digital Features
This article is published with digital features, including a video abstract, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.19346348.
Introduction
Systemic sclerosis (SSc) is a complex autoimmune disease that causes extensive fibrosis of the skin and multiple organs that can lead to severe organ dysfunction and/or failure [1, 2]. A major cause of death in SSc is SSc-associated interstitial lung disease (SSc-ILD), which can result in respiratory insufficiency [3]. SSc is most commonly diagnosed in women aged 40–60 years, with reported female-to-male ratios between 3:1 and 14:1 [3, 4].
Epidemiological studies are crucial to determine the risk and causal factors of SSc. However, conducting reliable epidemiological studies has been difficult because of the rarity and heterogenous clinical presentation of SSc [5]. A programme sponsored by the Ministry of Health, Labour and Welfare in Japan called ‘Intractable disease’ estimated that approximately 27,000 patients (or 21 per 100,000 people) have been diagnosed with SSc, though only patients with moderate-to-severe organ damage are eligible for this programme [6, 7]. Reported incidence rates and prevalence of SSc have increased in the last few decades, which could be due to increased physician awareness and better diagnosis [5]. There are, however, no recent epidemiological data on the entire population of patients with SSc and SSc-ILD in Japan, and little is known about how these patients are treated.
The aims of this study were to estimate the incidence rate and prevalence of SSc and SSc-ILD in Japan, as well as to assess the patient characteristics and the medications they received, using two claims databases. The databases used in the study were the Japanese Medical Data Centre (JMDC), a health insurance societies-based claims database, and the Medical Data Vision (MDV), a hospital claims database.
Methods
Databases
The JMDC database is a commercially available, health insurance societies-based claims database in Japan [8, 9]. The database includes employed persons and their families. Data are collected from over 7.3 million people across Japan, and patient tracking can be done if the person stays with the same health insurance society. This database contains information on all medical treatment received by patients at hospitals and clinics. However, this database lacks data on elderly people. Given that 2.2% of the individuals in the database are aged 65 years or more, there are insufficient data for this population aged 65 years or more, and data for those aged over 75 years are also lacking.
The MDV database is a hospital claims database that holds data on up to 30 million patients from over 400 major referral hospitals accepting acute illness, geographically spread out throughout Japan. Up to 34% of patients are aged 65 years or more [10]. Demographic characteristics, including the age and sex distributions of patients, are very similar to those of national statistics in Japan [11].
Primary Outcomes
Since only the JMDC database includes healthy individuals, only the JMDC database could be used to estimate incidence rate and prevalence.
Crude incidence rates for SSc and SSc-ILD, respectively, are presented per 100,000 person-years (PY) by sex and age group. Incidence rate was calculated as the number of new cases divided by the number of PYs at risk in the JMDC database.
Crude prevalence rates for SSc and SSc-ILD, respectively, are presented per 100,000 population by sex and age group. Prevalence was calculated as the number of existing cases (prevalent or incident) divided by the number of patients in the JMDC database.
Secondary Outcomes
We analysed the following for patients aged 20 years or more with SSc and SSc-ILD in the JMDC and MDV databases: (1) the prevalence of selected comorbidities potentially related to SSc during the baseline and follow-up periods (12 months); (2) the incidence rate of selected disease outcomes after SSc and SSc-ILD diagnosis, respectively; and (3) the frequency of prescribed medications of interest (immunomodulators) during the baseline and follow-up periods (12 months). Further details of secondary outcomes, including lists of medications and ICD-10 codes to identify comorbidities and disease outcomes, can be found in the Supplementary Methods.
Patient Population and Study Design
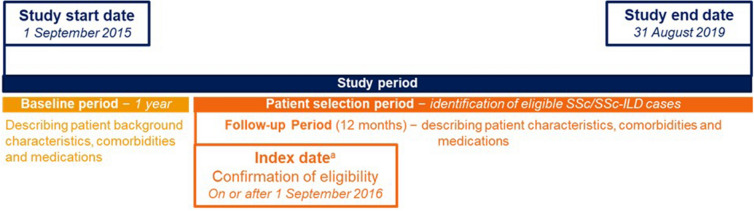
This non-interventional cohort study was carried out using data from 1 September 2015 to 31 August 2019 (Fig. 1). For the JMDC, the study population was all individuals enrolled in the JMDC database between 1 September 2015 and 31 August 2019. The study population for MDV was all patients with proxy enrolment (defined as from first encounter until end of the last encounter in the database) during the time period from 1 September 2015 to 31 August 2019 and with at least one encounter during the baseline period.
Fig. 1.
Schematic of the study design. aIndex date varied depending on the date participants fulfilled eligibility criteria. SSc systemic sclerosis, SSc-ILD SSc-associated interstitial lung disease
SSc was identified as patients having at least one administrative claims diagnosis of SSc (ICD10 M34.0, M34.1, M34.2, M34.8, or M34.9) between 1 September 2016 and 31 August 2019. The date of the first SSc diagnosis claim was defined as the SSc index date. Patients with less than 12 months of continuous enrolment prior to the index date were excluded. In addition to the criteria for SSc, the SSc-ILD population required a diagnosis code for ILD (J84.1, J84.8, J84.9, J99.0, J99.1 or J98.4) on or after the index date. Among the SSc cohort, the date of the first ILD diagnosis claim was defined as SSc-ILD index date. Only records with a confirmed diagnosis were considered for evaluating diagnosis-related inclusion or exclusion criteria.
An incident case was a new case identified with no diagnosis code for SSc or SSc-ILD in the 12 months prior to the index date. The incident cohort included all patients with newly diagnosed SSc or SSc-ILD, respectively, identified from 1 September 2016 to 31 August 2019. Prevalent cases were patients with an SSc or SSc-ILD claim from 1 September 2016 to 31 August 2019, and an SSc/SSc-ILD claim during the 1-year baseline period. The prevalent cohort included all patients with SSc or SSc-ILD (incident and prevalent cases) identified from 1 September 2016 to 31 August 2019.
Secondary outcomes were assessed in patients meeting the criteria for SSc and SSc-ILD, aged 20 years or more, and enrolled in the JMDC or MDV databases.
The follow-up time for each patient was calculated from the index date. Patients were censored on the date of the first occurrence of any of the following: discontinuation of insurance medical coverage (last date of enrolment) among patients who were not continuously enrolled through the end of the study period (JMDC only); end of the study period (for patients who were continuously enrolled through the end of the study period); or the date of diagnosis of the selected disease outcome being evaluated.
Statistical Analysis
The results of this study are reported using descriptive analyses. Continuous variables were presented as mean values, medians, minimum and maximum values, interquartile ranges (IQR), and standard deviations; categorical variables were presented as absolute and relative frequencies.
Ethics Approval
This study was approved by the ethics committee of the Kyosokai NISHI-UMEDA Clinic for Asian Medical Collaboration (approval number AMC-BI-20-004). The study was also conducted in accordance with the Declaration of Helsinki. All patient data were anonymised and included no identifying information; therefore, informed consent was not necessary.
Results
Baseline Patient Demographics
According to data from the JMDC database, the at-risk incident population for patients aged over 20 years with data for the previous 12 months and registered in the database between 1 September 2016 and 31 August 2019 was 6,881,421; of these 933 patients with SSc were identified in the incident cohort. The population at risk for SSc-ILD was 6,876,092; of these, 261 patients had SSc-ILD. The at-risk prevalent population was 6,882,535; within this, 2548 patients had SSc, and of these, 957 patients had ILD. The MDV database was at least five times larger, with 4805 patients with SSc; of these, 1810 patients with ILD were in the incident cohort, and 12,652 patients with SSc and 5505 patients with ILD were in the prevalent cohort (Table 1).
Table 1.
Baseline patient demographics
| Incident cohort | Prevalent cohorta | |||||||
|---|---|---|---|---|---|---|---|---|
| SSc | SSc-ILD | SSc | SSc-ILD | |||||
| JMDC | MDV | JMDC | MDV | JMDC | MDV | JMDC | MDV | |
| N | 933 | 4805 | 261 | 1810 | 2548 | 12,652 | 957 | 5505 |
| Sex, % | ||||||||
| Male | 21.9 | 18.7 | 20.7 | 17.2 | 17.5 | 16.0 | 17.8 | 18.1 |
| Female | 78.1 | 81.3 | 79.3 | 82.8 | 82.5 | 84.0 | 82.2 | 81.9 |
| Age, years | ||||||||
| Mean (SD) | 49.0 (13.5) | 67.2 (13.7) | 50.8 (12.9) | 66.9 (13.1) | 51.5 (12.6) | 66.6 (13.1) | 53.2 (11.4) | 67.0 (12.3) |
| Median (IQR) | 51 (42–58) | 69 (60–77) | 52 (45–60) | 69 (60–76) | 53 (45–60) | 68 (59–76) | 55 (47–61) | 68 (60–76) |
| Min, max | 3, 74 | 2, 99 | 9, 74 | 3, 96 | 2, 74 | 2, 102 | 3, 74 | 3, 96 |
| Age groups, % | ||||||||
| < 20 | 4.9 | 0.6 | 3.5 | 0.4 | 3.5 | 0.5 | 1.6 | 0.3 |
| 20–29 | 3.9 | 1.0 | 4.2 | 1.1 | 3.1 | 1.0 | 2.9 | 0.7 |
| 30–39 | 11.5 | 2.3 | 9.2 | 1.7 | 9.0 | 3.5 | 7.6 | 2.7 |
| 40–49 | 24.2 | 7.5 | 22.6 | 7.2 | 25.0 | 8.5 | 22.6 | 7.9 |
| 50–59 | 34.0 | 13.2 | 34.9 | 13.8 | 33.7 | 15.9 | 35.2 | 16.3 |
| 60–69 | 19.2 | 25.7 | 23.4 | 28.0 | 21.9 | 31.8 | 26.0 | 32.1 |
| 70–79 | 2.4 | 32.4 | 2.3 | 32.7 | 3.8 | 28.8 | 4.1 | 30.2 |
| ≥ 80 | 0.0 | 17.3 | 0.0 | 15.2 | 0.0 | 10.2 | 0.0 | 9.8 |
| < 65 | 90.4 | 34.2 | 88.1 | 35.4 | 88.6 | 42.9 | 86.8 | 41.7 |
| ≥ 65 | 9.7 | 65.8 | 11.9 | 64.6 | 11.4 | 57.2 | 13.2 | 58.3 |
| < 75 | 100.0 | 66.2 | 100.0 | 68.6 | 100.0 | 76.5 | 100.0 | 76.4 |
| ≥ 75b | 0.0 | 33.8 | 0.0 | 31.4 | 0.0 | 23.5 | 0.0 | 23.7 |
IQR interquartile range, JMDC Japanese Medical Data Centre, MDV Medical Data Vision, SD standard deviation, SSc systemic sclerosis, SSc-ILD SSc-associated interstitial lung disease
aPrevalent cohort includes prevalent and incident cases of SSc and SSc-ILD
bAs JMDC is an employment-related claims database, no data available for elderly people (≥ 75 years)
In both databases, approximately 80% of patients with SSc and SSc-ILD in both the incident and prevalent cohorts were female. The majority of patients with SSc and SSc-ILD in the incident cohort were aged 40–69 years in the JMDC database, whereas it was 60–79 years in the MDV database. It is important to note that the age distribution of the two databases was different since the JMDC database represents a younger population and the MDV database represents an older population who have visited the hospital.
Incidence Rate
Table 2 shows the crude incidence rates of SSc and SSc-ILD from the JMDC database. The overall incidence rates of SSc and SSc-ILD per 100,000 PY were 6.6 (95% confidence interval [CI] 6.2–7.1) and 1.9 (95% CI 1.6–2.1), respectively. Incidence rates were numerically higher in female patients, with overall female and male incidence rates per 100,000 PY being 11.8 (95% CI 10.9–12.7) and 2.6 (95% CI 2.3–3.0), respectively, for SSc and 3.3 (95% CI 2.9–3.8) and 0.7 (95% CI 0.6–0.9), respectively, for SSc-ILD.
Table 2.
Crude incidence rate per 100,000 person-years and prevalencea per 100,000 persons of SSc and SSc-ILD (95% CI) by age groups and sex (JMDC)
| Sex | SSc | SSc-ILD | |||||
|---|---|---|---|---|---|---|---|
| Overall | < 65 years | ≥ 65 years | Overall | < 65 years | ≥ 65 years | ||
| Incidence rates per 100,000 person-years (95% CI) | |||||||
| Population at risk, n | M | 3,749,068 | 3,669,882 | 79,186 | 3,746,204 | 3,667,536 | 78,668 |
| F | 3,132,353 | 3,058,146 | 74,207 | 3,129,888 | 3,055,999 | 73,889 | |
| All | 6,881,421 | 6,728,028 | 153,393 | 6,876,092 | 6,723,535 | 152,557 | |
| Incident events, nb | M | 204 | 175 | 29 | 54 | 45 | 9 |
| F | 729 | 668 | 61 | 207 | 185 | 22 | |
| All | 933 | 843 | 90 | 261 | 230 | 31 | |
| Person-years at risk, total | M | 7,896,552 | 7,762,000 | 134,553 | 7,890,285 | 7,756,632 | 133,653 |
| F | 6,197,567 | 6,074,213 | 123,354 | 6,193,081 | 6,070,250 | 122,832 | |
| All | 14,094,119 | 13,836,212 | 257,907 | 14,083,366 | 13,826,882 | 256,484 | |
| Crude incidence rate per 100,000 person-years (95% CI) | M | 2.6 | 2.3 | 21.6 | 0.7 | 0.6 | 6.7 |
| (2.3–3.0) | (1.9–2.6) | (15.0–31.0) | (0.6–0.9) | (0.4–0.8) | (3.6–12.8) | ||
| F | 11.8 | 11.0 | 49.5 | 3.3 | 3.1 | 17.9 | |
| (10.9–12.7) | (10.2–11.9) | (38.5–63.5) | (2.9–3.8) | (2.6–3.5) | (11.9–27.1) | ||
| All | 6.6 | 6.1 | 34.9 | 1.9 | 1.7 | 12.1 | |
| (6.2–7.1) | (5.7–6.5) | (28.4–42.9) | (1.6–2.1) | (1.5–1.9) | (8.5–17.2) | ||
| Prevalence of SSc and SSc-ILD per 100,000 persons (95% CI) | |||||||
| Population at risk, n | M | 3,749,261 | 3,670,063 | 79,198 | 3,749,261 | 3,670,069 | 79,192 |
| F | 3,133,274 | 3,058,935 | 74,339 | 3,133,274 | 3,058,956 | 74,318 | |
| All | 6,882,535 | 6,728,998 | 153,537 | 6,882,535 | 6,729,025 | 153,510 | |
| Prevalent events, nc | M | 446 | 399 | 47 | 170 | 152 | 18 |
| F | 2102 | 1858 | 244 | 787 | 679 | 108 | |
| All | 2548 | 2257 | 291 | 957 | 831 | 126 | |
| Crude prevalence rate per 100,000 persons (95% CI) | M | 11.9 | 10.9 | 59.3 | 4.5 | 4.1 | 22.7 |
| (10.8–13.1) | (9.8–12.0) | (43.6–78.9) | (3.9–5.3) | (3.5–4.9) | (13.5–35.9) | ||
| F | 67.1 | 60.7 | 328.2 | 25.1 | 22.2 | 145.3 | |
| (64.3–70.0) | (58.0–63.6) | (288.4–372.0) | (23.4–26.9) | (20.6–23.9) | (119.2–175.4) | ||
| All | 37.0 | 33.5 | 189.5 | 13.9 | 12.4 | 82.1 | |
| (35.6–38.5) | (32.2–35.0) | (168.4–212.6) | (13.0–14.8) | (11.5–13.2) | (68.4–97.7) | ||
CI confidence interval, F female, JMDC Japanese Medical Data Centre, M male, SSc systemic sclerosis, SSc-ILD SSc-associated interstitial lung disease
aPrevalence was calculated by including prevalent and incident cases of SSc and SSc-ILD
bThe total number of patients with a new diagnosis of SSc or SSc-ILD identified during the entire patient selection period for the overall incidence rate (1 September 2016 to 31 August 2019)
cThe total number of patients with a diagnosis of SSc or SSc-ILD identified anytime during the patient selection period, for the overall prevalence (1 September 2016 to 31 August 2019)
Incidence rates of SSc and SSc-ILD were higher in people aged over 65 years (Table 2), although only 2.2% (153,393/6,881,421) of patients over 65 years of age were from the JMDC database.
Prevalence
Table 2 shows the crude prevalence of SSc and SSc-ILD from the JMDC database. The overall prevalence of SSc and SSc-ILD per 100,000 persons was 37.0 (95% CI 35.6–38.5) and 13.9 (95% CI 13.0–14.8), respectively. Prevalence of SSc and SSc-ILD was numerically higher in female compared with male patients (Table 2).
Comorbidities
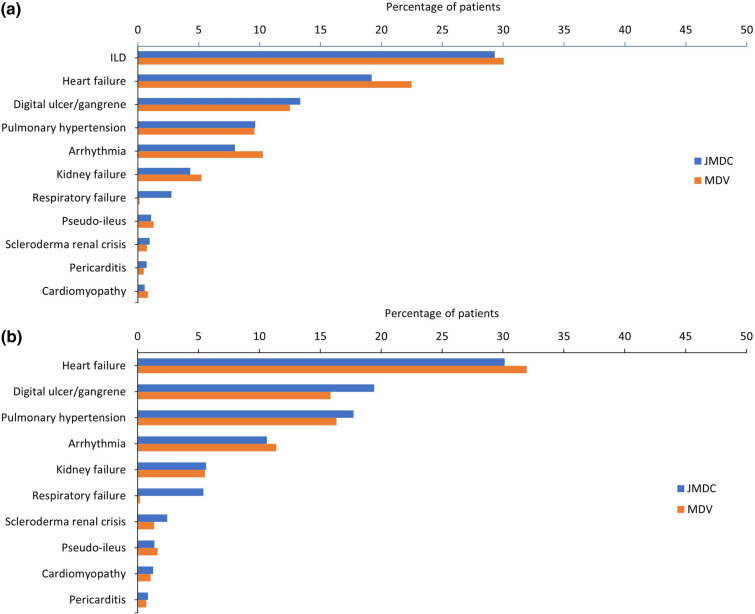
In the JMDC and the MDV databases, patient comorbidities were analysed at baseline and at 12-month follow-up after SSc/SSc-ILD diagnosis (Fig. 2). Prevalence and ranking of SSc-related comorbidities were similar in JMDC and MDV databases.
Fig. 2.
Comorbidities at baseline in a patients with SSc and b patients with SSc-ILD (JMDC and MDV). Comorbidities were defined by ICD10 MHLW2013. ILD: J84.x; Heart failure: I50.x; Digital ulcer/gangrene: L98.x; Pulmonary hypertension: I27.x; Arrhythmia: I44.x, I45.x, I47.x, I48.x, I49.x; Kidney failure: N17.x, N18.x, N19.x; Respiratory failure: J96.x; Pseudo-ileus: K56.x; Scleroderma renal crisis: M34.8; Pericarditis: I30.x, I31.x, I32.x; Cardiomyopathy: I42.x, I43.x. ICD International Classification of Disease, ILD interstitial lung disease, JMDC Japanese Medical Data Centre, MDV Medical Data Vision, SSc systemic sclerosis, SSc-ILD SSc-associated interstitial lung disease
In the JMDC database, the most common SSc-related comorbidity at baseline was ILD, which was present in 29.3% of patients with SSc. The most common SSc-related comorbidity at baseline in the MDV database was also ILD, which was present in 30.1% of patients with SSc (Fig. 2). For patients with SSc-ILD, the most common comorbidity at baseline was heart failure in the JMDC and the MDV databases. In terms of SSc-related comorbidity, digital ulcer/gangrene was most prevalent in the JMDC (present in 19.4% of patients) and pulmonary hypertension was most prevalent in the MDV database (present in 16.3% of patients).
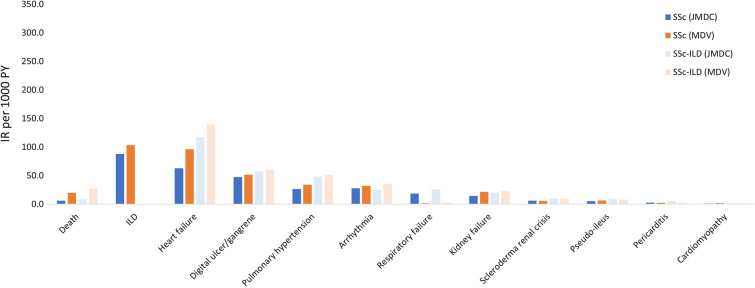
Incidence Rates of Disease Outcomes
There were 6.3 deaths per 1000 PY in patients with SSc and 8.6 per 1000 PY for patients with SSc-ILD in the JMDC. In the MDV, there were 20.2 and 27.5 deaths per 1000 PY in patients with SSc and SSc-ILD, respectively (Fig. 3). The incidence of mortality was higher in the MDV database than in the JMDC, likely reflecting differences in the patient populations, since the MDV includes older patients who attended hospital.
Fig. 3.
Incidence rates of selected disease outcomes among patients with SSc or SSc-ILD (prevalence cohort): JMDC and MDV databases. Incidence rates for ILD in patients with SSc-ILD are not shown as all the patients in this group were diagnosed with ILD. Death: disenrolment from the insurance system with a cause categorized as “death” in JMDC and I46.1, I46.9, R96, R98, R99 or discharge summary (FF1 data) in MDV. Other selected disease outcomes were defined by ICD10 MHLW2013. ILD: J84.x; Heart failure: I50.x; Digital ulcer/gangrene: L98.x; Pulmonary hypertension: I27.x; Arrhythmia: I44.x, I45.x, I47.x, I48.x, I49.x; Respiratory failure: J96.x; Kidney failure: N17.x, N18.x, N19.x; Scleroderma renal crisis: M34.8; Pseudo-ileus: K56.x; Pericarditis: I30.x, I31.x, I32.x; Cardiomyopathy: I42.x, I43.x. ICD International Classification of Disease, ILD interstitial lung disease, IR incidence rate, PY patient years, SSc systemic sclerosis, SSc-ILD SSc-associated ILD
The incidence rate of ILD in patients with SSc was similar in both databases (JMDC vs MDV, 88.1 vs 103.6 per 1000 PY). In both databases, the incidence rate of heart failure per 1000 PY was numerically higher in patients with SSc-ILD (JMDC, 117.3; MDV, 139.7) than in patients with SSc (JMDC, 63.0; MDV, 96.3). The incidence rate of pulmonary hypertension per 1000 PY was also numerically higher in patients with SSc-ILD (JMDC, 48.5; MDV, 51.2) than in patients with SSc (JMDC, 26.8; MDV, 34.3).
Immunomodulatory Medications
The JMDC and MDV databases were used to assess the immunomodulators prescribed to patients with SSc and SSc-ILD. As a result of the nature of claims databases, there were no data describing the disease or condition for which these were prescribed.
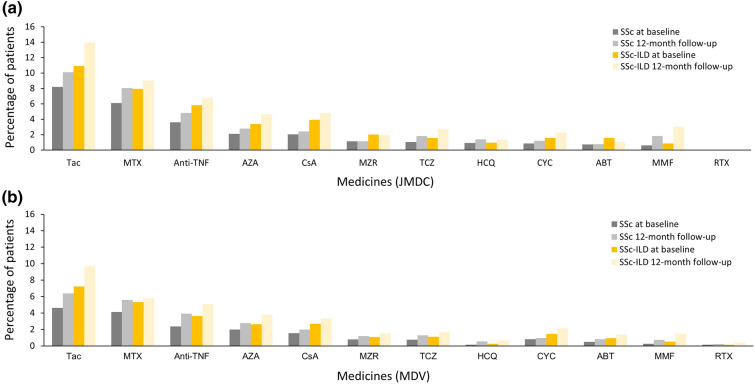
In both databases, cyclophosphamide hydrate (CYC) and mycophenolate mofetil (MMF) were not commonly prescribed to patients with SSc and SSc-ILD at follow-up (Fig. 4). In the JMDC database, CYC was prescribed in 1.2% and 2.3% of patients with SSc and SSc-ILD, respectively, whereas MMF was prescribed in 1.8% and 3.0% of patients with SSc and SSc-ILD, respectively. In the MDV database, CYC was prescribed in 1.0% and 2.1% of patients respectively, whereas MMF was prescribed in 0.7% and 1.5% of patients with SSc and SSc-ILD, respectively. In addition, methotrexate (MTX) was prescribed at the 12-month follow-up in 8.0% and 9.0% of patients with SSc and SSc-ILD, respectively, in the JMDC database, and in 5.6% and 5.8% of patients with SSc and SSc-ILD, respectively, in the MDV database.
Fig. 4.
Medications to treat patients with SSc or SSc-ILD aged 20 years or more (prevalence cohort): a JMDC and b MDV databases. ABT abatacept, AZA azathioprine, CsA cyclosporine, CYC cyclophosphamide hydrate, HCQ hydroxychloroquine sulfate, MMF mycophenolate mofetil, MTX methotrexate, MZR mizoribine, RTX rituximab, SSc systemic sclerosis, SSc-ILD SSc-associated interstitial lung disease, Tac tacrolimus hydrate, TCZ tocilizumab, TNF tumour necrosis factor
In both databases, the most commonly prescribed immunomodulator was tacrolimus hydrate, which is not approved for SSc or SSc-ILD in Japan, but is approved for the treatment of rheumatoid arthritis, lupus nephritis and polymyositis/dermatomyositis-associated ILD. Tacrolimus hydrate was prescribed in 10.1% of patients with SSc and 14.0% of patients with SSc-ILD during follow-up in the JMDC database, and in 6.4% of patients with SSc and 9.7% of patients with SSc-ILD during follow-up in the MDV database (Fig. 4). Azathioprine (AZA) and anti-tumour necrosis factor (TNF) biologics and non-anti-TNF biologics were prescribed in a minority of patients with SSc and SSc-ILD.
Each of the medications of interest in this analysis was prescribed in less than 15% of the patients in both databases, and the medications prescribed were similar in patients with SSc and SSc-ILD.
Discussion
In this study, we have successfully investigated the epidemiology and clinical characteristics of SSc and SSc-ILD in Japan, using the JMDC and MDV medical claims databases, although only the JMDC database could be used for estimating incidence rate and prevalence.
The current study provides epidemiological data about SSc and SSc-ILD in Japan, including recent incidence and prevalence rates, clinical characteristics and patient outcomes. Previous studies have successfully looked at prevalence and incidence rates of SSc and SSc-ILD using insurance claims databases in the USA [12, 13]. A study using the US IBM MarketScan claims database and a definition requiring two or more diagnostic claims estimated the crude prevalence of SSc and SSc-ILD to be 41.5 and 13.3, respectively, per 100,000 people [12]; this was similar to our crude estimates in Japan of 37.0 and 13.9 per 100,000 people. Another study in the USA, based on the Optum Clinformatics Data Mart claims database, estimated the crude prevalence at 24.4 (SSc) and 6.9 (SSc-ILD) per 100,000 people [13]. The IBM MarketScan study estimated age-adjusted incidence rates to be 8.8 and 1.6 per 100,000 PY in SSc and SSc-ILD, respectively, similar to the crude incidence rates we found in Japan (6.6 and 1.9 per 100,000 PY) [12]. The Optum Clinformatics study estimated the incidence rates to be 16.4 (SSc) and 1.2 (SSc-ILD) per 100,000 PY [13].
The MarketScan claims database included patient, inpatient and pharmaceutical claims for 40–50 million privately insured employed and retired individuals from employer-sponsored health insurance plans across the USA, whereas the Optum Clinformatics database included administrative claims (medical/pharmacy claims and linked demographic information) for over 180 million commercially insured individuals in the USA. Similarly, the JMDC is based on health insurance societies claims and contains data on medical treatments received in hospitals or clinics; however, as the database includes employed persons and their families, data is lacking on patients aged over 65 years. Patients can be tracked if they stay with the same insurance society; similarly, in the MarketScan database, member identification codes allow individuals to be followed longitudinally. The MDV hospital claims includes 30 million patients, with demographic characteristics that are very similar to those of national statistics in Japan. It complements the JMDC by including older patients; up to 34% of patients are aged 65 years or more.
Studies previously conducted in Taiwan and in Korea found lower prevalence and incidence of SSc than in our study, though this may be due to differences in methodology and in the characteristics of the different databases [14, 15]. In Taiwan, a nationwide database study by Kuo et al. estimated a mean annual prevalence of SSc of 5.6 cases per 100,000 persons and an overall annual incidence of 1.1 per 100,000 PY [14]. In this study, patients who had a catastrophic illness certificate for SSc were included, so patients with milder disease may have been excluded. A study based on the Korean Rare Intractable Disease Registry that covers the entire Korean population (n = 50 million) estimated the annual prevalence of SSc as 7.8 per 100,000 persons and the incidence of SSc was 0.8 per 100,000 PY [15]. To be included in the registry used in this study, patients had to be diagnosed by a physician, with the diagnosis reviewed by a second physician, so it is likely that milder cases may have been under-represented. In contrast, health insurance claims have a potential limitation of uncertainty of diagnosis but are more likely to include mild cases. In Europe, estimates of the prevalence of SSc have ranged between 7.2 and 33.9 per 100,000 individuals; in North America, estimates range between 13.5 and 44.3 per 100,000 individuals [16]. Estimates of the annual incidence have been 0.6–2.3 and 1.4–5.6 per 100,000 PY in Europe and North America, respectively [16].
Globally, estimates of ILD prevalence in patients with SSc vary depending on the methodology and definition of ILD used (between 19% and 52%) [16–19]. ILD has been estimated to occur in 32.3–47.0% of patients with SSc in Europe and around 52% of patients with SSc in North America [16]. The percentage of patients with SSc who had ILD in Japan in our study (29–30%) falls within the range observed globally. Patient demographics from this study were comparable to previously published data in Japan [20–22], though the JMDC database lacks adequate data on elderly people.
Regarding disease outcomes, our findings on the prevalence of heart failure, digital ulcer/gangrene, and pulmonary hypertension were generally consistent with previous studies in Japanese patients with SSc [21, 23, 24]. In our study, the prevalence of heart failure and pulmonary hypertension was increased in patients with SSc-ILD, compared with the entire SSc population. This might be explained by higher frequencies of pulmonary arterial hypertension and diastolic dysfunction in patients with ILD, compared with those without ILD [25, 26].
Immunomodulatory therapy with CYC, MMF, or MTX has been the mainstay of treatment for SSc and/or SSc-ILD. These drugs have been shown to promote improvement of skin thickness and/or prevent progression of skin thickness and/or ILD in randomised controlled trials and have been proposed as suitable treatments in SSc [27, 28]. In this study, we found that only small percentages of patients with SSc or SSc-ILD were treated with CYC, MMF, or MTX, suggesting that patients are not appropriately treated in Japan. In a US cohort study, 12.7% of the SSc-ILD cohort initiated immunomodulators after diagnosis versus 8.2% for SSc; MMF and MTX were the most commonly prescribed immunomodulators. During the whole study period, 42.5% of the SSc cohort and 45.0% of the SSc-ILD cohorts started a stable immunomodulatory regimen [12]. In another US healthcare claims database-based study, 30.8% of patients with SSc received immunomodulatory therapy during the first year after diagnosis [29]. Comparatively in Japan, immunomodulatory therapy was used infrequently. Although CYC and AZA are approved for treatment of SSc in Japan, MMF and MTX—commonly used globally—are not approved for treatment of SSc or SSc-ILD in Japan.
Our study was conducted before the approval of nintedanib for SSc-ILD in Japan in December 2019. After approval of nintedanib in the USA, Japan, and the European Union, the recommendation for SSc or SSc-ILD treatment algorithms globally included the use of nintedanib for SSc-ILD [30, 31]. Notably, in the treatment algorithm for SSc-ILD proposed by the Japan Respiratory Society and Japan College of Rheumatology in 2020, in addition to immunomodulators, nintedanib is recommended for the treatment of SSc-ILD [32]. The treatment landscape of SSc-ILD in Japan may have changed after this survey period (September 2015–August 2019) and nintedanib could be a common treatment option in future real-world studies and in clinical practice.
Study Limitations
The study benefitted from analysing data from two different databases that have different characteristics. However, as JMDC is an employment-related claims database, people aged 65 years or more represented just 2.2% of the database and there were no data for people aged 75 years or more. Thus, our estimates of incidence rate and prevalence are only valid for the population aged less than 65 years. It should also be noted that the JMDC database covers only 4% of the Japanese population and may not constitute a representative population. Whilst the MDV is a much larger database, patient tracking is difficult in case of transfer, and there are no data tracking the history of patients’ hospital visits. Although the JMDC database is smaller than the MDV database, we used it to estimate crude incidence rate/prevalence because it contains healthy population data.
The follow-up period was short and the algorithms defining SSc and SSc-ILD have not been validated, thus the results should be interpreted with caution.
Conclusions
The estimated prevalence and incidence rates of SSc and SSc-ILD in Japan obtained in this study are comparable to published global data. These data suggest that in Japan during the period studied, patients with SSc and SSc-ILD may have been undertreated. Further studies on treatment and patient outcomes, both from national databases and prospective clinical cohorts, would provide valuable information for planning and designing future clinical trials in patients with SSc and SSc-ILD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge the contribution of Jenifer Gao, senior analyst from Panalgo, Boston, MA, USA, for the data analysis. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive payment related to the development of the manuscript. Nippon Boehringer Ingelheim Co., Ltd (NBI) was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. This study was funded by NBI.
Funding
This study was funded by NBI. The rapid service fee and open access fee was also funded by NBI.
Medical Writing, Editorial, and Other Assistance
Shivani Singh, PhD, and Helen Keyworth, PhD, of Nucleus Global provided writing, editorial support, and formatting assistance, which was contracted and funded by NBI.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. All authors contributed to study design, data interpretation, manuscript preparation and manuscript approval. Wataru Sakamoto and Christina Raabe analysed the data.
Prior Presentation
The contents were previously presented at the 65th Annual General Assembly and Scientific Meeting of the Japan College of Rheumatology which is held virtually and in Kobe, Japan from 26 to 28 April 2021.
Disclosures
Masataka Kuwana has received research grants and personal fees from Boehringer Ingelheim, MBL and Ono Pharmaceuticals; and personal fees from AbbVie, Asahi-Kasei, Astellas, Bayer, Chugai, Corbus, Galapagos NV, GlaxoSmithKline, MBL, Mochida, Mitsubishi-Tanabe, Nippon Shinyaku, Pfizer, Reata Pharmaceuticals and Janssen. Aiko Saito, Wataru Sakamoto, Christina Raabe and Kumiko Saito report being employed by Boehringer Ingelheim at the time of the study.
Compliance with Ethics Guidelines
This study was approved by the ethics committee of the Kyosokai NISHI-UMEDA Clinic for Asian Medical Collaboration (approval number AMC-BI-20-004). The study was also conducted in accordance with the Declaration of Helsinki. All patient data were anonymized and included no identifying information; therefore, informed consent was not necessary.
Data Availability
We conducted a retrospective study using patient data from the JMDC database and the MDV database, a commercially available databases of health claims and administrative data from health insurance associations and Japanese acute hospitals respectively. The data sets generated during and/or analysed during the current study are not publicly available because of a contract between JMDC/MDV and Boehringer Ingelheim. For inquiries on the database analysed in this study, please contact JMDC (https://www.jmdc.co.jp/en/index) or MDV (https://en.mdv.co.jp/).
References
- 1.Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–1699. doi: 10.1016/S0140-6736(17)30933-9. [DOI] [PubMed] [Google Scholar]
- 2.Moore DF, Steen VD. Overall mortality. J Scleroderma Relat Disord. 2020;6(1):3–10. doi: 10.1177/2397198320924873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR scleroderma trials and research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809–1815. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 4.Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum. 2008;37(4):223–235. doi: 10.1016/j.semarthrit.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Ranque B, Mouthon L. Geoepidemiology of systemic sclerosis. Autoimmun Rev. 2010;9(5):A311–A318. doi: 10.1016/j.autrev.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Health administration report for the first year of Reiwa. Ministry of Health Labor and Welfare Japan. 2019. https://www.e-stat.go.jp/stat-search/files?page=1&bunya_l=15&bunya_s=1504&layout=datalist&cycle=8&toukei=00450027&tstat=000001031469&tclass1=000001148807&tclass2=000001148808&tclass3=000001148810&cycle_facet=cycle&tclass4val=0&stat_infid=000032045204. Accessed Apr 2021.
- 7.Summary of population estimates (as of October 1, 2019 (first year of Reiwa)). 2019. https://www.stat.go.jp/data/jinsui/2019np/index.html. Accessed Apr 2021.
- 8.JMDC Claims Database. Japan medical data center. https://www.jmdc.co.jp/en/jmdc-claims-database. Accessed Apr 2021.
- 9.Nagai K, Tanaka T, Kodaira N, Kimura S, Takahashi Y, Nakayama T. Data resource profile: JMDC claims databases sourced from medical institutions. J Gen Fam Med. 2020;21(6):211–218. doi: 10.1002/jgf2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medical Data Vision. About MDV database. https://en.mdv.co.jp/about-mdv-database/. Accessed April 2021.
- 11.Pletcher H. Age distribution in Japan 2009–2019. https://www.statista.com/statistics/270087/age-distribution-in-japan/. Accessed April 2021.
- 12.Li Q, Wallace L, Patnaik P, Alves M, Gahlemann M, Kohlbrenner V, et al. Disease frequency, patient characteristics, comorbidity outcomes and immunosuppressive therapy in systemic sclerosis and systemic sclerosis-associated interstitial lung disease: a US cohort study. Rheumatology (Oxford) 2021;60(4):1915–1925. doi: 10.1093/rheumatology/keaa547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan Y, Bender S, Shi W, Zoz D. Incidence and prevalence of systemic sclerosis and systemic sclerosis with interstitial lung disease in the United States. J Manag Care Spec Pharm. 2020;26(12):1539–1547. doi: 10.18553/jmcp.2020.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo CF, See LC, Yu KH, Chou IJ, Tseng WY, Chang HC, et al. Epidemiology and mortality of systemic sclerosis: a nationwide population study in Taiwan. Scand J Rheumatol. 2011;40(5):373–378. doi: 10.3109/03009742.2011.553736. [DOI] [PubMed] [Google Scholar]
- 15.Kang GW, Jung KH, Lee YS, Kim HJ, Yoon DY, Lee SH, et al. Incidence, prevalence, mortality and causes of death in systemic sclerosis in Korea: a nationwide population-based study. Br J Dermatol. 2018;178(1):e37–e39. doi: 10.1111/bjd.15838. [DOI] [PubMed] [Google Scholar]
- 16.Bergamasco A, Hartmann N, Wallace L, Verpillat P. Epidemiology of systemic sclerosis and systemic sclerosis-associated interstitial lung disease. Clin Epidemiol. 2019;11:257–273. doi: 10.2147/CLEP.S191418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker UA, Tyndall A, Czirjak L, Denton C, Farge-Bancel D, Kowal-Bielecka O, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR scleroderma trials and research group database. Ann Rheum Dis. 2007;66(6):754–763. doi: 10.1136/ard.2006.062901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steele R, Hudson M, Lo E, Baron M, Canadian Scleroderma Research Group Clinical decision rule to predict the presence of interstitial lung disease in systemic sclerosis. Arthritis Care Res (Hoboken) 2012;64(4):519–24. doi: 10.1002/acr.21583. [DOI] [PubMed] [Google Scholar]
- 19.Vonk MC, Broers B, Heijdra YF, Ton E, Snijder R, van Dijk AP, et al. Systemic sclerosis and its pulmonary complications in the Netherlands: an epidemiological study. Ann Rheum Dis. 2009;68(6):961–5. doi: 10.1136/ard.2008.091710. [DOI] [PubMed] [Google Scholar]
- 20.Tamaki T, Mori S, Takehara K. Epidemiological study of patients with systemic sclerosis in Tokyo. Arch Dermatol Res. 1991;283(6):366–371. doi: 10.1007/BF00371817. [DOI] [PubMed] [Google Scholar]
- 21.Nishioka K, Katayama I, Kondo H, Shinkai H, Ueki H, Tamaki K, et al. Epidemiological analysis of prognosis of 496 Japanese patients with progressive systemic sclerosis (SSc) Scleroderma Research Committee Japan. J Dermatol. 1996;23(10):677–82. doi: 10.1111/j.1346-8138.1996.tb02680.x. [DOI] [PubMed] [Google Scholar]
- 22.Ohta A, Nagai M, Nishina M, Tomimitsu H, Kohsaka H. Age at onset and gender distribution of systemic lupus erythematosus, polymyositis/dermatomyositis, and systemic sclerosis in Japan. Mod Rheumatol. 2013;23(4):759–64. doi: 10.3109/s10165-012-0733-7. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto A, Endo H, Kondo H, Hirohata S. Clinical features of 405 Japanese patients with systemic sclerosis. Mod Rheumatol. 2012;22(2):272–9. doi: 10.3109/s10165-011-0515-7. [DOI] [PubMed] [Google Scholar]
- 24.Kuwana M, Kaburaki J, Okano Y, Tojo T, Homma M. Clinical and prognostic associations based on serum antinuclear antibodies in Japanese patients with systemic sclerosis. Arthritis Rheum. 1994;37(1):75–83. doi: 10.1002/art.1780370111. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Y, Turk MA, Pope JE. Factors associated with pulmonary arterial hypertension (PAH) in systemic sclerosis (SSc) Autoimmun Rev. 2020;19(9):102602. doi: 10.1016/j.autrev.2020.102602. [DOI] [PubMed] [Google Scholar]
- 26.Vemulapalli S, Cohen L, Hsu V. Prevalence and risk factors for left ventricular diastolic dysfunction in a scleroderma cohort. Scand J Rheumatol. 2017;46(4):281–287. doi: 10.1080/03009742.2016.1206963. [DOI] [PubMed] [Google Scholar]
- 27.Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. 2017;76(8):1327–39. doi: 10.1136/annrheumdis-2016-209909. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Codina A, Walker KM, Pope JE, Scleroderma Algorithm Group Scleroderma Algorithm Group. Treatment algorithms for systemic sclerosis according to experts. Arthritis. Rheumatol. 2018;70(11):1820–8. doi: 10.1002/art.40560. [DOI] [PubMed] [Google Scholar]
- 29.Gale SL, Trinh H, Mathew N, Jahreis A, Lin CJF, Sarsour K. Characterizing disease manifestations and treatment patterns among adults with systemic sclerosis: a retrospective analysis of a US healthcare claims population. Rheumatol Ther. 2020;7(1):89–99. doi: 10.1007/s40744-019-00181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann-Vold A-M, Maher TM, Philpot EE, Ashrafzadeh A, Barake R, Barsotti S, et al. The identification and management of interstitial lung disease in systemic sclerosis: evidence-based European consensus statements. Lancet Rheumatol. 2020;2(2):e71–83. doi: 10.1016/S2665-9913(19)30144-4. [DOI] [PubMed] [Google Scholar]
- 31.Roofeh D, Distler O, Allanore Y, Denton CP, Khanna D. Treatment of systemic sclerosis-associated interstitial lung disease: lessons from clinical trials. J Scleroderma Relat Disord. 2020;5(2):61–71. doi: 10.1177/2397198320903208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kondoh Y, Makino S, Ogura T, Suda T, Tomioka H, Amano H, et al. 2020 guide for the diagnosis and treatment of interstitial lung disease associated with connective tissue disease. Respir Invest. 2021;2:2. doi: 10.1016/j.resinv.2021.04.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We conducted a retrospective study using patient data from the JMDC database and the MDV database, a commercially available databases of health claims and administrative data from health insurance associations and Japanese acute hospitals respectively. The data sets generated during and/or analysed during the current study are not publicly available because of a contract between JMDC/MDV and Boehringer Ingelheim. For inquiries on the database analysed in this study, please contact JMDC (https://www.jmdc.co.jp/en/index) or MDV (https://en.mdv.co.jp/).