Abstract
Introduction
Consensus definitions for clinical remission and super-response were recently established for severe asthma. Benralizumab is an interleukin-5 (IL-5) receptor α-directed monoclonal antibody for severe, uncontrolled asthma; efficacy and safety were demonstrated in previous pivotal phase 3 trials (SIROCCO, CALIMA, ZONDA). This analysis applied a composite remission definition to characterize individual responses to benralizumab after 6 and 12 months.
Methods
In previous phase 3 studies, eligible patients were those with severe, uncontrolled asthma receiving medium- or high-dosage inhaled corticosteroids plus long-acting β2-agonists. This post hoc analysis included patients randomized to the approved benralizumab dose and not receiving oral corticosteroids (OCS) at baseline (SIROCCO/CALIMA) or OCS ≤ 12.5 mg per day (ZONDA). Individual remission components were zero exacerbations; zero OCS use; Asthma Control Questionnaire-6 (ACQ-6) score < 1.5 or ≤ 0.75; and pre-bronchodilator forced expiratory volume in 1 s (FEV1) increase ≥ 100 mL; clinical remission incorporated zero exacerbations, zero OCS use, ACQ-6 score ≤ 0.75, and pre-bronchodilator FEV1 increase ≥ 100 mL after 6 or 12 months.
Results
Overall, 609 patients (N = 301 and N = 308) and 586 patients (N = 293 and N = 293) receiving benralizumab in SIROCCO and CALIMA were included at 6 and 12 months, respectively; 40 ZONDA patients were included after 6 months. In SIROCCO/CALIMA, similar to 6-month findings, approx. 83% and approx. 49% receiving benralizumab, and 77% and 37% on placebo achieved ≥ 2 and ≥ 3 remission components after 12 months; 14.5% (85/586) on benralizumab and 7.7% (48/620) on placebo achieved clinical remission at 12 months. Among ZONDA patients, 75% and approx. 48% on benralizumab and 35% and 20% on placebo achieved ≥ 2 and ≥ 3 remission components at 6 months, respectively; 22.5% (9/40) on benralizumab and 7.5% on placebo achieved clinical remission.
Conclusions
This analysis demonstrates clinical remission is achievable by targeting the underlying drivers of inflammation. Precision medicines can help shift treatment paradigms toward treat-to-target, with clinical remission as the ultimate therapeutic goal in severe asthma.
Clinical trial registration
SIROCCO (NCT01928771); CALIMA (NCT01914757); ZONDA (NCT02075255).
Dr. Andrew Menzies-Gow Discusses a Post Hoc Analysis of Clinical Remission in Severe Asthma with Benralizumab
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02098-1.
Keywords: Benralizumab, Biologic therapies, Oral corticosteroids (OCS), Precision medicine, Remission, Severe eosinophilic asthma, Super-response, Treat-to-target
Plain Language Summary
Widely accepted definitions for disease remission are already established for the treatment of rheumatoid arthritis, ulcerative colitis, and cancer, among others. Two separate expert groups recently collaborated to discuss clinical remission/super-response to treatment in patients with severe asthma. Both groups developed separate, yet similar ways to determine whether a patient should be considered “in remission.” In this study, we used the results from three previous trials (SIROCCO, CALIMA, and ZONDA) that were conducted to assess a therapy called benralizumab in patients with severe asthma to identify patients who met some or all of the criteria for disease remission in severe asthma. These criteria included zero asthma exacerbations; zero oral steroid (OCS) use; asthma control score; and improvement in lung function. Across all three trials, about three quarters of the patients achieved two or more remission components and about half achieved three or more remission components after 6 months of treatment; furthermore, these rates were generally similar to the numbers of patients who achieved two or more components and three or more components of remission after 12 months of treatment. Overall, 15–23% of patients achieved clinical remission in 6 months, and approximately 15% achieved remission within 12 months. The results show that biologic therapies like benralizumab help improve the symptoms of severe asthma and allow patients to achieve disease remission.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02098-1.
Key Summary Points
| Expert consensus definitions for clinical remission and super-response to treatment have recently been established for patients with severe asthma. |
| A large percentage of patients with severe asthma continue to receive treatment with oral corticosteroids (OCS) despite updated treatment recommendations to avoid OCS whenever possible and despite the substantial morbidity and mortality associated with OCS; this continued overreliance on OCS may be due to the current clinical approach to asthma management, which has been likened to treat-to-failure, whereby therapy is sequentially increased to the maximum recommended dose when patients fail to show measurable improvements or efficacy of combination therapy. |
| Subgroups of patients randomized to the approved 30 mg benralizumab Q8W dose in previous randomized, phase 3 trials (SIROCCO, CALIMA, and ZONDA) were assessed to identify patients who achieved one or more composite criteria of clinical remission at 6 or 12 months: zero exacerbations; zero OCS use; Asthma Control Questionnaire-6 (ACQ-6) score < 1.5 or ≤ 0.75; and pre-bronchodilator forced expiratory volume in 1 s (FEV1) increase ≥ 100 mL. |
| Among those in SIROCCO and CALIMA, 14.8% (90/609) and 14.5% (85/586) met the criteria for clinical remission at 6 and 12 months, respectively, whereas 22.5% (9/40) of patients in ZONDA met the criteria for clinical remission at 6 months; across all three trials, > 86% achieved ≥ 2 components and > 54% achieved ≥ 3 remission components, demonstrating that clinically meaningful improvements were often achieved regardless of whether patients ultimately achieved the stringent, full definition of clinical remission. |
| Clinical remission in severe asthma can be achieved by using targeted treatments, such as benralizumab, to address eosinophils as the underlying driver of inflammation; these precision approaches represent a promising way to end the age of treat-to-failure and usher in a new era of treat-to-target, with expert consensus definitions as a benchmark for treatment success. |
Digital Features
This article is published with digital features, including a video abstract, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.19771474.
Introduction
Asthma is a heterogeneous respiratory disease usually associated with chronic airway inflammation and airway smooth muscle hyper-responsiveness, with symptoms that vary in intensity and over time [1]. It is the most common noncommunicable respiratory disease globally, with an estimated 339 million people affected worldwide [1, 2]. Among all patients with asthma, approximately 3–10% have severe asthma, which is defined by the European Respiratory Society/American Thoracic Society as asthma that requires a high-dosage inhaled corticosteroid (ICS) plus a second controller (and/or systemic corticosteroids) to remain controlled or asthma that remains uncontrolled despite this level of treatment [3]. Greater than 50% of patients with severe asthma also have poor disease control, which carries an increased risk for asthma exacerbations [4, 5].
Recent estimates indicate that eosinophilic asthma is the most common asthma phenotype (approx. 84% in a cohort from the International Severe Asthma Registry); primary biomarkers of eosinophilic asthma include elevated sputum eosinophils and higher blood eosinophil (bEOS) levels, which may be supplemented by measuring fractional exhaled nitric oxide (FeNO) levels or atopic status/IgE [1, 4, 6–8]. As a result of the heterogeneous nature of the disease, severe asthma can have many different clinical presentations and can be associated with a substantial burden of disease [8, 9]. Common sources of morbidity, which vary with individual clinical presentation, include exacerbations, reduced lung function and fixed airway obstruction (e.g. forced expiratory volume in 1 s [FEV1]), daily symptoms (e.g. as assessed by patient-reported outcomes [PROs], like the Asthma Control Questionnaire-6 [ACQ-6]), and adverse effects of treatment, most notably those from oral corticosteroids (OCS) [5, 10–13].
Severe asthma and asthma exacerbations are frequently treated with OCS, despite their associated acute and long-term morbidity and mortality [14–18]. The 2021 recommendations from the Global Initiative for Asthma (GINA) note that maintenance (i.e. long-term) OCS should be avoided whenever possible, and instead, biologics and other add-on therapies should be the preferred options [1]. Despite these recommendations, results from two recent patient cohort studies show that approximately 50% of patients with severe asthma were receiving regular treatment with either intermittent or long-term OCS [4, 5]. This degree of OCS use may be reflective of the current clinical approach to managing asthma: inhaler-based medications and add-on treatments are sequentially increased to the maximum doses when patients fail to show improvements (treat-to-failure), despite a lacking dose–response or efficacy of combination therapy (e.g. as seen in a TENOR cohort); indeed, only then are the underlying drivers of inflammation addressed with targeted therapy [19].
The advent of biologic therapies in severe asthma has shed light on the utility of phenotyping as a precision approach to treatment targeting key drivers of the disease pathophysiology. Benralizumab is an interleukin-5 receptor α-directed monoclonal antibody that leads to rapid, nearly complete depletion of eosinophils [20–23]. Given that eosinophilic asthma is the most common asthma phenotype, biologic therapies such as benralizumab, which target the underlying cause of inflammation, provide an opportunity to develop clinical remission as a treatment goal in severe asthma [6, 22, 24, 25]. Disease remission is an important concept in medicine, and noteworthy definitions can be found in diseases like rheumatoid arthritis (RA) [26–28]. For some conditions, the potential to achieve remission has been a more recent development, as therapies have improved over time (e.g. RA) [29], while in other diseases, remission has long been the stated goal for treatment (e.g. oncology).
Among diseases in which remission is defined by consensus as an achievable treatment outcome, the indices are often defined by one or more of a range of different disease-specific parameters, including clinical-, histological-/endoscopic-, PRO-, serological, and medication-based measures [27, 28, 30, 31]. Historically, the concept of remission in asthma has focused largely on spontaneous disease remission, which is probably more akin to disease resolution [32]. Recent work by Menzies-Gow et al. used a modified Delphi survey approach to achieve expert consensus on the key components for a definition of clinical remission in asthma [24]. This novel framework comprised at least 12 months with the absence of significant symptoms by a validated instrument, no OCS use for asthma, optimisation/stabilisation of lung function, and health care professional (HCP)/patient agreement about disease remission [24]. Upham et al. also used a Delphi process to develop a consensus definition for what they dubbed super-response to therapy in severe asthma [33]. The super-response definition comprised improvements in three domains assessed over 12 months, including improvement in at least two of the following major criteria: exacerbation elimination, ≥ 2 × minimal clinically important difference (MCID) improvements in asthma control, or cessation of long-term OCS; and improvement in one of the following minor criteria: ≥ 75% exacerbation reduction, ≥ 500 mL improvement FEV1, or achievement of well-controlled asthma [33]. Indeed, the criteria for clinical remission are broadly consistent with those for super-response; however, patients with less active disease could be considered super-responders and experience meaningful responses to therapy, while not meeting the stricter definition of clinical remission [24, 33].
Although asthma remission/super-response definitions may be revised, and or newly developed over time, the aforementioned definitions demonstrate that composite measures are necessary and achievable in the era of precision biologics. They also make it possible to address important questions regarding treatment, such as how many patients on biologics partially or completely meet the remission framework and which specific criteria are being met [34]. The efficacy and safety of benralizumab were establish through three previous phase 3 pivotal trials: SIROCCO, CALIMA, and ZONDA [35–37]. A recent post hoc analysis from SIROCCO and CALIMA evaluated the percentages of patients with improvements in six outcomes (exacerbations, ACQ-6 score, FEV1, total asthma symptom score [composite of daytime and nighttime symptoms], Asthma Quality of Life Questionnaire [AQLQ], and nights with awakenings that required asthma rescue medication). Of 504 patients with severe asthma treated with benralizumab every 8 weeks (Q8W, first three doses 4 weeks apart), 89.7% (454/504) evidenced a response in one or more outcome measures; however, the analysis did not formally apply either consensus definition [24, 33, 38].
The objective of this pooled post hoc analysis was to apply a composite remission definition—described below, and derived from two previously published frameworks (i.e. framework for asthma remission and severe asthma super-responders)—to a pooled population of patients treated with benralizumab in three prior phase 3 pivotal studies to (1) understand individual responses to benralizumab at 6 and 12 months from baseline according to composite remission criteria and (2) determine how many patients may have achieved clinical remission in severe asthma [24, 33]. This analysis focused on patients treated with the approved benralizumab regimen to emphasize the importance of considering clinical remission among individuals rather than as a comparison between treatment groups in a clinical trial.
Methods
Study Design, Patient Population, and Outcomes
This post hoc analysis included patients pooled from three predecessor, phase 3 randomized, double-blind, placebo-controlled trials of benralizumab 30 mg: SIROCCO (NCT01928771), CALIMA (NCT01914757), or ZONDA (NCT02075255). Trial designs, eligibility criteria, and primary results for all three studies have previously been described in detail elsewhere [35–37]. Briefly, eligible patients included those with severe, uncontrolled asthma receiving treatment with medium- or high-dosage inhaled corticosteroids (ICS) plus long-acting β2-agonists (ICS/LABA) at baseline. Patients in ZONDA were also required to be on stable long-term OCS therapy (equivalent to a prednisone dose of 7.5–40 mg/day) for 6 months prior to enrolment [37]. Following enrolment in their initial phase 3 trials, patients were randomized to placebo or benralizumab 30 mg by subcutaneous injection every 4 weeks or Q8W (first three doses 4 weeks apart) [35–37].
In the SIROCCO and CALIMA clinical trials, benralizumab efficacy, assessed by exacerbation rate reduction, and safety were evaluated in patients 12–75 years of age for 48 and 56 weeks, respectively [35, 36]. In the ZONDA trial, benralizumab safety and efficacy, assessed by OCS dose and exacerbation reductions, were evaluated over 28 weeks in patients 18–75 years of age [37]. Across all three trials, patients were required to remain on the same stable dose of background medication, with the exception of OCS doses in ZONDA. Background medication changes were only allowed if they were judged to be necessary by the site investigator [35–37].
This post hoc analysis only included patients from SIROCCO and CALIMA not receiving OCS at the baseline visit; patients who were receiving OCS at baseline were excluded because the trial protocols did not explicitly allow for OCS dose reductions, and therefore would not have allowed these patients to meet the composite criteria in this analysis. This analysis also included patients from ZONDA receiving OCS dosages ≤ 12.5 mg prednisone/prednisolone equivalents per day at the baseline visit; patients receiving OCS dosages > 12.5 mg prednisone/prednisolone equivalents per day at baseline in ZONDA were excluded from this analysis because the trial design did not allow them to achieve 100% OCS reductions by the end of the 28-week treatment period. For this analysis, patients from all three trials were included in this analysis for the entire duration of their study participation (i.e. from enrolment through study discontinuation) regardless of their treatment status or final trial disposition (i.e. regardless of whether they were on treatment or ultimately completed SIROCCO, CALIMA, or ZONDA).
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Remission Definitions
In keeping with criteria from the consensus asthma clinical remission framework, this post hoc analysis evaluated four components of asthma remission among patients treated with benralizumab Q8W in SIROCCO, CALIMA, or ZONDA: exacerbations, OCS use, patient-reported asthma control (ACQ-6), and lung function (FEV1) [24]. Responses to each of these criteria were defined as follows: zero exacerbations, zero OCS use, ACQ-6 score < 1.5 or ≤ 0.75 (more stringent), and pre-bronchodilator (BD) FEV1 increase ≥ 100 mL from baseline (Fig. 1). Furthermore, these components were measured after both 6 months and 12 months of treatment in patients from SIROCCO and CALIMA but were only measured after 6 months of treatment in patients from ZONDA because of the length of the trial. Consistent with previous definitions from the phase 3 trials, an exacerbation was defined as a worsening of asthma that resulted in one of the following: use of systemic corticosteroids, a temporary increase in OCS dosage (by any amount) for at least 3 days, or a dose of injectable corticosteroids; a visit to the emergency department or an urgent care centre; or an inpatient hospital stay. The ≥ 100 mL threshold for improvement in pre-BD FEV1 was selected as a clinically relevant change, although it is noteworthy that a consensus definition of FEV1 improvement has not been established, nor has the optimal way to assess lung function changes over time [39]. Patient-reported asthma control was measured using the ACQ-6, and thresholds of < 1.5 and ≤ 0.75, previously validated in patients with asthma, were used here [40, 41]. Finally, responses were also assessed for eligible patient subgroups with blood eosinophil counts of ≥ 150 eosinophils/µL or ≥ 300 eosinophils/µL at baseline. Given that meaningful responses to treatment may comprise many different combinations of composite criteria, we evaluated the percentages of patients who achieved at least one (≥ 1), at least two (≥ 2), at least three (≥ 3), or all four of the following components: zero exacerbations, zero OCS use, ACQ-6 score < 1.5 or ≤ 0.75 (more stringent), and pre-BD FEV1 increase ≥ 100 mL. Clinical remission in severe asthma was defined as achieving zero exacerbations, zero OCS use, ACQ-6 ≤ 0.75, and pre-BD FEV1 increase ≥ 100 mL (Fig. 1).
Fig. 1.
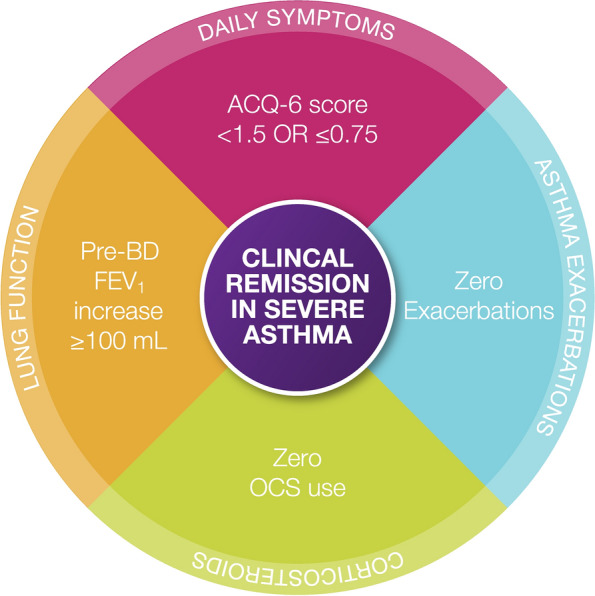
Composite definition of severe asthma clinical remission used in this post hoc analysis. This four-item composite definition was derived using criteria from two recently published expert consensus statements, which were both developed through separate, modified Delphi studies [24, 33]. ACQ-6 Asthma Control Questionnaire, 6-item, BD bronchodilator, FEV1 forced expiratory volume in 1 s, OCS oral corticosteroids
Statistical Analysis
The numbers and percentages of patients who achieved each composite component were calculated from baseline through 6 and 12 months. For visit-based components (OCS use, ACQ-6 score, and improvements in FEV1), the status was determined for individual patients at the observation period closest to the given 6- or 12-month timepoint: week 24 in SIROCCO and CALIMA and week 28 in ZONDA were used for the 6-month timepoint while week 48 in SIROCCO and week 56 in CALIMA were used for the 12-month timepoint. Exacerbations were counted from the start of treatment through each 6- or 12- month timepoint. For permutations of response to composite components, the number and percentages of patients who achieved cumulative levels of remission (e.g. ≥ 1, ≥ 2, ≥ 3, or 4 components) were calculated at each timepoint. For patients with missing values for ACQ-6 score and/or FEV1 at the aforementioned 6- and 12-month timepoints, ACQ-6 and FEV1 measurements from within ± 4 weeks of that visit were used, if available. Otherwise, if a patient had a missing measurement, the patient’s data were not included in univariate descriptions for that component; however, that patient was included in the full response score analysis, wherein missing values (except FEV1 and ACQ-6 measured from within ± 4 weeks) were counted as not achieving that remission component. This was a retrospective analysis and no hypothesis tests were planned. Unless otherwise indicated, “baseline” was defined as the baseline visit in SIROCCO, CALIMA, or ZONDA [35–37].
Ethics
For the SIROCCO, CALIMA, and ZONDA trials, ethics and compliance details have been reported elsewhere previously [35–37]. Prior to patient enrolment, institutional review boards or independent ethics committees approved the clinical study protocols, and all patients provided written informed consent at enrolment. Each study was conducted in accordance with the ethical principles set forth in the Declaration of Helsinki and consistent with the International Council for Harmonisation/Good Clinical Practice guidelines, applicable regulatory requirements, and the AstraZeneca policy on bioethics.
Results
Patient Disposition and Baseline Characteristics
Overall, 609 patients (SIROCCO N = 301; CALIMA N = 308) treated with benralizumab Q8W were included in this analysis for the 6-month timepoint, and 586 (SIROCCO N = 293; CALIMA N = 293) were included for the 12-month timepoint (Supplementary Fig. 1). A total of 40 patients from ZONDA were included in this analysis for the 6-month timepoint. Baseline demographics and clinical characteristics for patients in SIROCCO, CALIMA, and ZONDA are presented in Table 1.
Table 1.
Demographic and clinical characteristics of patients in the post hoc analysis at baselinea
| Parameter | SIROCCO | CALIMA | ZONDA | |||
|---|---|---|---|---|---|---|
| Benra Q8W (N = 327) | Placebo (N = 339) | Benra Q8W (N = 325) | Placebo (N = 329) | Benra Q8W (N = 42) | Placebo (N = 42) | |
| Demographics | ||||||
| Age, mean (SD), years | 47.2 (14.8) | 48.0 (15.3) | 50.1 (13.6) | 49.5 (14.6) | 55.0 (9.6) | 50.9 (11.5) |
| Female, n (%) | 210 (64.2) | 229 (67.6) | 199 (61.2) | 194 (59.0) | 25 (59.5) | 28 (66.7) |
| BMI, mean (SD), kg/m2 | 28.3 (6.3) | 28.9 (7.) | 28.9 (6.4) | 29.3 (6.5) | 29.2 (5.4) | 27.6 (4.5) |
| Time since asthma diagnosis, median (range), years | 14.5 (1.2, 63.1) | 13.2 (1.1, 72.4) | 16.1 (1.1, 64.6) | 16.4 (1.2, 69.9) | 14.5 (1.6, 50.5) | 8.9 (1.2, 37.6) |
| Age at asthma onset, mean (SD), years | 29.1 (19.2) | 30.2 (19.2) | 30.8 (18.9) | 29.8 (19.7) | 38.8 (17.9) | 38.1 (14.8) |
| Clinical characteristics | ||||||
| ACQ-6 score, mean (SD) | 2.8 (0.8) | 2.9 (0.9) | 2.8 (1.0) | 2.7 (0.9) | 2.3 (1.3) | 2.5 (0.9) |
| Not well controlled (≥ 1.5), n (%) | 312 (95.4) | 323 (95.3) | 302 (92.9) | 307 (93.3) | 31 (73.8) | 37 (88.1) |
| Partly/well controlled (< 1.5), n (%) | 15 (4.6) | 16 (4.7) | 23 (7.1) | 22 (6.7) | 11 (26.2) | 5 (11.9) |
| bEOS, median (range), cells/μL | 350 (0, 3100) | 380 (0, 2690) | 400 (0, 2600) | 370 (0, 3640) | 455 (170, 1630) | 510 (200, 1800) |
| Pre-bronchodilator FEV1, mean (SD), % predicted | 57.0 (14.4) | 57.7 (15.1) | 57.1 (14.3) | 58.4 (14.6) | 58.8 (17.1) | 66.0 (15.5) |
| Reversibility, mean (SD), % | 27.6 (24.6) | 24.7 (22.3) | 25.374 (22.6) | 27.7 (49.3) | 23.5 (18.6) | 19.3 (14.5) |
| Exacerbations in past 12 months, mean (SD) | 2.6 (1.3) | 2.8 (1.5) | 2.6 (1.2) | 2.6 (1.1) | 3.0 (2.9) | 2.8 (1.8) |
| 1, n (%) | 0 | 0 | 1 (0.3) | 0 | 11 (26.2) | 10 (23.8) |
| 2, n (%) | 220 (67.3) | 215 (63.4) | 215 (66.2) | 225 (68.4) | 15 (35.7) | 12 (28.6) |
| ≥ 3, n (%) | 107 (32.7) | 124 (36.6) | 109 (33.5) | 104 (31.6) | 16 (38.1) | 20 (47.6) |
| Medication use | ||||||
| ICS, n (%)b | 327 (100) | 339 (100) | 324 (99.7) | 329 (100) | 42 (100) | 42 (100) |
| LABA, n (%) | 327 (100) | 339 (100) | 321 (98.8) | 329 (100) | 42 (100) | 42 (100) |
| ICS/LABA, n (%) | 0 | 0 | 0 | 0 | 0 | 0 |
| LAMA, n (%) | 17 (5.2) | 25 (7.4) | 29 (8.9) | 23 (7.0) | 15 (35.7) | 10 (23.8) |
| LTRA, n (%) | 111 (33.9) | 123 (36.3) | 89 (27.4) | 80 (24.3) | 13 (31.0) | 13 (31.0) |
ACQ-6 Asthma Control Questionnaire, 6-item, benra benralizumab, bEOS blood eosinophils, BMI body mass index, FEV1 forced expiratory volume in 1 s, ICS inhaled corticosteroids, LABA long-acting β2-agonist, LAMA long-acting muscarinic antagonist, LTRA leukotriene receptor antagonist, OCS oral corticosteroids, Q8W every 8 weeks, SD standard deviation
aBaseline N values include all patients who qualified for this analysis; some of these baseline patients left the trials before 6 or 12 months and thus were not included at those timepoints
bICS may have been taken in a separate inhaler or as part of a fixed-dose ICS/LABA combination device
Response to Separate Remission Criteria
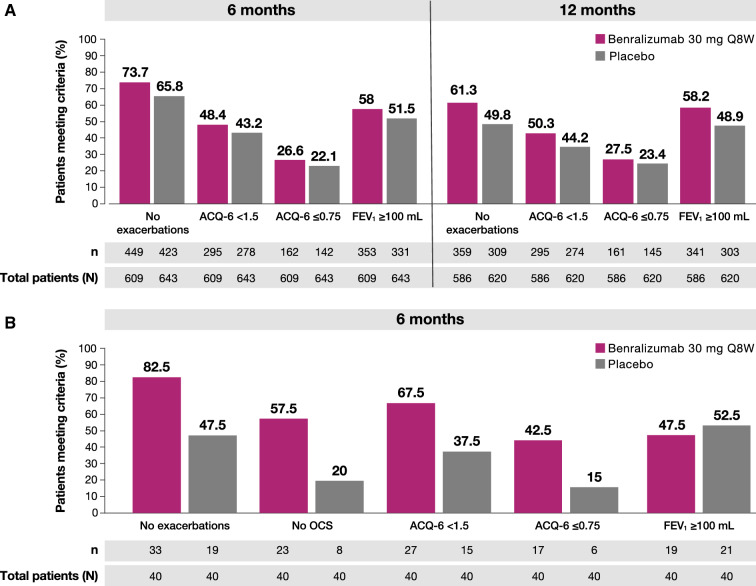
For SIROCCO/CALIMA patients treated with benralizumab, 73.7% (449/609) had no exacerbations, 48.4% (295/609) had an ACQ-6 score of < 1.5, 26.6% (159/609) had an ACQ-6 score ≤ 0.75, and 58% (353/609) had a pre-BD FEV1 increase ≥ 100 mL at the 6-month timepoint (Fig. 2a). At the 12-month timepoint, 61.3% (359/586) had no exacerbations, 50.3% (295/586) had an ACQ-6 score of < 1.5, 27.5% (161/586) had an ACQ-6 score ≤ 0.75, and 58.2% (341/586) had a pre-BD FEV1 increase ≥ 100 mL. At both the 6-month (65.8% [423/643], 43.2% [278/643], 22.1% [142/643], and 51.5% [331/643], respectively) and 12-month (49.8% [309/620], 44.2% [274/620], 23.4% [145/620], and 48.9% [303/620], respectively) timepoints, fewer SIROCCO/CALIMA patients receiving placebo met these criteria. In ZONDA patients treated with benralizumab, 82.5% (33/40) had no exacerbations, 57.5% (23/40) had no OCS, 67.5% (27/40) had an ACQ-6 score of < 1.5, 42.5% (17/40) had an ACQ-6 score ≤ 0.75, and 47.5% (19/40) had a pre-BD FEV1 increase ≥ 100 mL after 6 months (Fig. 2b); smaller percentages of patients treated with placebo met these criteria (47.5% [19/40], 20% [8/40], 37.5% [15/40], 15% [6/40], and 52.5% [21/40], respectively).
Fig. 2.
Patients achieving no exacerbations, no OCS, ACQ-6, or FEV1 ≥ 100 mL improvements a in SIROCCO/CALIMA after 6 months and 12 months or b in ZONDA after 6 monthsa. ACQ-6 Asthma Control Questionnaire, 6-item, FEV1 forced expiratory volume in 1 s, OCS oral corticosteroids, Q8W every 8 weeks. aN includes all patients still in the study at visit closest to timepoint. 6 months = week 24 (SIROCCO, CALIMA) or week 28 (ZONDA); 12 months = week 48 (SIROCCO) or week 56 (CALIMA)
Response to Multiple Components of Remission
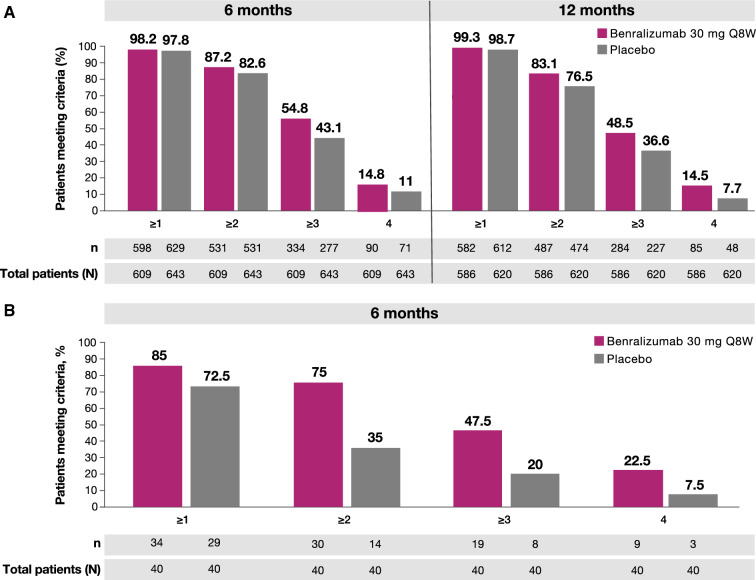
In SIROCCO/CALIMA patients treated with benralizumab, 87.2% (531/609) achieved ≥ 2 components of clinical remission, while 54.8% (334/609) and 14.8% (90/609) achieved ≥ 3 and 4 components at the 6-month timepoint, respectively (Fig. 3a). At the 12-month timepoint, 83.1% (487/586) of patients achieved ≥ 2 components of clinical remission, while 48.5% (284/586) and 14.5% (85/586) achieved ≥ 3 and 4 components, respectively. Fewer SIROCCO/CALIMA patients treated with placebo achieved ≥ 2, ≥ 3, or 4 components of remission by the 6-month (82.6% [531/643], 43.1% [277/643], and 11% [71/643]) and 12-month (76.5% [474/643], 36.6% [227/620], and 7.7% [48/620]) timepoints. In ZONDA patients treated with benralizumab, 85.0% (34/40) achieved ≥ 1 component of clinical remission, while 75.0% (30/40), 47.5% (19/40), and 22.5% (9/40) achieved ≥ 2, ≥ 3, and 4 components, respectively, at the 6-month timepoint (Fig. 3b). Fewer ZONDA patients treated with placebo achieved, ≥ 2, ≥ 3, or 4 components of remission by the 6-month timepoint (35.0% [14/40], 20% [8/40], and 7.5% [3/40]).
Fig. 3.
Percentages of patients achieving at least 1, 2, 3, or 4 composite remission componentsa a in SIROCCO/CALIMA after 6 months and 12 months or b in ZONDA after 6 monthsb. ACQ-6 Asthma Control Questionnaire, 6-item, FEV1 forced expiratory volume in 1 s, Q8W every 8 weeks. aComponents of response include no exacerbations, no oral corticosteroid use, ACQ-6 score ≤ 0.75, and pre-BD FEV1 increase ≥ 100 mL. bN includes all patients still in the study at visit closest to timepoint. 6 months = week 24 (SIROCCO, CALIMA) or week 28 (ZONDA); 12 months = week 48 (SIROCCO) or week 56 (CALIMA)
Response Outcomes Leading to Remission
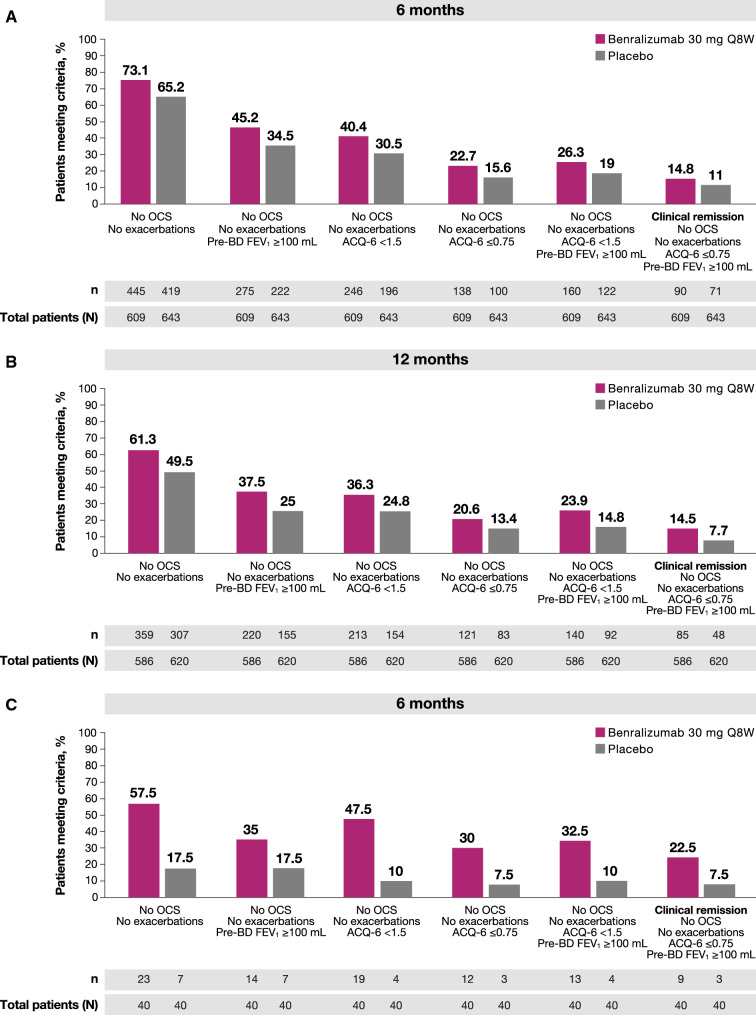
In SIROCCO/CALIMA patients treated with benralizumab, 45.2% (275/609) had a combination of no exacerbations, no OCS use, and a pre-BD FEV1 increase ≥ 100 mL after 6 months, whereas 37.5% (220/586) met those three criteria after 12 months (Fig. 4a, b). At the 6-month timepoint, 26.3% (160/609) of SIROCCO/CALIMA patients had no exacerbations, no OCS use, a pre-BD FEV1 increase ≥ 100 mL, and an ACQ-6 score of < 1.5; furthermore, 23.9% (140/586) met those four criteria after 12 months (Fig. 4a, b). Overall, among SIROCCO/CALIMA patients, 14.8% (90/609) of patients on benralizumab met all four criteria for clinical remission—no exacerbations, no OCS use, an ACQ-6 score ≤ 0.75, and a pre-BD FEV1 increase ≥ 100 mL—at the 6-month timepoint, and 14.5% (85/586) met all four criteria at the 12-month timepoint; in total, 11% [71/643] and 7.7% [48/620] of patients treated with placebo achieved remission at these same timepoints (Fig. 4a, b). Finally, responses to components of the remission definition were similar in patients from SIROCCO and CALIMA when patients were stratified according to baseline bEOS counts (≥ 150 bEOS/µL or ≥ 300 eosinophils/µL; Supplementary Tables 1 and 2).
Fig. 4.
Percentages of patients achieving permutations of composite remission componentsa a in SIROCCO/CALIMA after 6 months; b in SIROCCO/CALIMA after 12 months; or c in ZONDA after 6 monthsb. ACQ-6 Asthma Control Questionnaire, 6-item, exac exacerbation, FEV1 forced expiratory volume in 1 s, OCS oral corticosteroids, Q8W every 8 weeks. aComponents of response include no exacerbations, no OCS use, ACQ-6 score < 1.5 or ≤ 0.75, and pre-BD FEV1 increase ≥ 100 mL. bN includes all patients still in the study at visit closest to timepoint. 6 months = week 24 (SIROCCO, CALIMA) or week 28 (ZONDA); 12 months = week 48 (SIROCCO) or week 56 (CALIMA)
In ZONDA patients treated with benralizumab, 57.5% (23/40) had no exacerbations and no OCS use, while 35.0% (14/40) had no exacerbations, no OCS use, and a pre-BD FEV1 increase ≥ 100 mL at the 6-month timepoint (Fig. 4c). Furthermore, 32.5% (13/40) of patients had no exacerbations, no OCS use, a pre-BD FEV1 increase ≥ 100 mL, and an ACQ-6 score of < 1.5. Among ZONDA patients, 22.5% (9/40) of those on benralizumab met all four criteria for clinical remission—no exacerbations, no OCS use, an ACQ-6 score ≤ 0.75, and a pre-BD FEV1 increase ≥ 100 mL—after 6 months; in total, 7.5% [3/40] of ZONDA patients treated with placebo achieved remission after 6 months. Responses to components of the remission definition were similar in ZONDA patients when stratified according to baseline bEOS counts (≥ 150 bEOS/µL or ≥ 300 eosinophils/µL; Supplementary Table 3).
Discussion
This analysis was conducted to evaluate levels of response leading to clinical remission, based on a novel asthma clinical remission framework, through a post hoc analysis of patients from prior pivotal phase 3 trials with benralizumab [24]. In patients from SIROCCO and CALIMA, more than 87% achieved ≥ 2 components of clinical remission, more than half achieved ≥ 3 components within 6 months of initiating treatment with benralizumab, and these response rates were generally similar after 12 months of treatment. Indeed, more than 26% of these patients had achieved no exacerbations, no OCS, pre-BD FEV1 increase ≥ 100 mL, and ACQ-6 < 1.5 by 12 months, with almost 15% achieving clinical remission in severe asthma after 1 year of treatment with benralizumab. These findings suggest a gradient of responses to benralizumab among patients with severe asthma on high-dose ICS/LABA.
Consistent with the heterogeneous clinical presentation of severe asthma, meaningful responses to benralizumab from the patient perspective also likely vary, and it is noteworthy that significant improvement in at least one component can be meaningful to the patient [34]. For example, a patient with frequent exacerbations and poor lung function might derive greater benefits from achieving the FEV1 component and no exacerbations than from achieving the ACQ-6 or OCS components. In patients from ZONDA, 85% achieved at least one component of clinical remission, while 75% achieved ≥ 2 components, nearly half (47.5%) achieved ≥ 3 components, and nearly one quarter (23%) achieved clinical remission in severe asthma after 6 months of treatment with benralizumab. Across all three trials, zero exacerbations were the most frequently achieved of all the individual composite remission criteria. Contrary to the patient with frequent exacerbations and poor lung function in the aforementioned example, a patient with a high steroid burden and frequent exacerbations may derive substantially more benefit from achieving no OCS use and no exacerbations than they would from achieving the ACQ-6 and FEV1 components.
A high percentage of patients across all three trials (> 86%; 561/649) had enough improvement to achieve ≥ 2 components (no exacerbations, no OCS use, FEV1 improvement, and ACQ-6 ≤ 0.75) of clinical remission within 6 months of treatment. Higher percentages of patients on benralizumab achieved either clinical remission or ≥ 2 or ≥ 3 components of remission compared to placebo. Since patients in the SIROCCO, CALIMA, and ZONDA trials were on high levels of maintenance medication, the high percentages of patients achieving multiple components of response in this study reinforce the notion that the traditional “treat-to-failure” approach represents a less effective way to manage asthma. Indeed, given that after initiating a precision treatment that directly targets the inflammatory driver (i.e. treat-to-target) of their asthma, the eosinophil, these patients were more likely to achieve meaningful responses [19, 42]. This observation may suggest that some patients could begin precision treatments sooner. Indeed, the notion is also consistent with recent findings from the ANDHI In Practice substudy, which show that 53% of non-OCS-dependent patients treated with benralizumab successfully reduced at least one background medication over the course of the study, while 51% of OCS-dependent patients treated with benralizumab eliminated OCS use by the end of treatment [43]. Moreover, results from the recent open-label PONENTE trial also underscore the advantages of precision treatments, as 63% of patients treated with benralizumab successfully eliminated OCS use while 82% eliminated OCS or achieved the maximum possible OCS reduction over the course of the study [44].
Consistent with evidence that eosinophilic asthma is the most common asthma phenotype, the high percentages of patients who achieved ≥ 2 composite remission criteria also reflects that many patients enrolled in benralizumab trials have an eosinophilic component underlying their asthma; indeed, in these patients, using benralizumab as a precision approach to directly target, and almost entirely deplete, eosinophils led to clinically meaningful improvements. Patients with eosinophilic asthma or a history of elevated bEOS levels are more likely to experience asthma exacerbations or have worse lung function [6, 9, 45]. Exacerbations represent a substantial source of morbidity and mortality for patients with severe asthma, and they are also associated with considerable economic burden as well as a progressive decline in lung function [12, 13, 46–48]. Indeed, exacerbations, and in particular severe exacerbations, represent a major risk factor for future adverse outcomes [49]. Furthermore, exacerbations are often treated with OCS, which also carry substantial risk for acute and chronic morbidity and mortality, which further underscores the importance of the treat-to-target approach and the importance of clinical remission in severe asthma [14, 16, 17, 50].
Since the Delphi-derived asthma clinical remission and super-responder frameworks are expert consensus-based definitions, the components of these frameworks will likely evolve and change with time. For example, both temporal and HCP/patient agreement components were included in the recent clinical remission consensus. Although HCP/patient agreement could not be assessed in this study because of the post hoc nature of this analysis, it is nevertheless a key aspect of identifying patients in asthma remission in the clinical setting. Indeed, the remission framework also defines asthma remission through at least 12 months [24], whereas in some diseases, such as autoimmune conditions, the state of remission is a state of being and thus can change over both short and long periods of time (e.g. relapse and remit) in a given patient [24, 26–28].
The ACQ-6 as a measure of control, and pre-BD FEV1 as a measure of lung function, are other components that may evolve with future definitions of asthma remission, because both measures have several limitations. For example, some established PROs like the ACQ-6 or the Asthma Control Test (ACT) only evaluate a subset of asthma control (i.e. impairment but not risk) and thus fail to measure important aspects of the disease (e.g. OCS use) [51]. Additionally, the ACQ was initially developed to assess clinically important differences in asthma control at the individual level (e.g. MCID) whereas cut-points for levels of control were not determined until later [10, 40, 41]. Moreover, caution should be applied when extrapolating results for subjective measures like patient-reported outcomes from double-blind trials to what a patient may experience in clinical practice or an open-label setting. The ACQ-6 thresholds used in this study align with validated cut-points for well-controlled and partially controlled asthma, despite the fact that they may not be clinically relevant in patients with severe asthma, airway limitations, or even lung damage [10, 52]. For example, the percentages of patients who achieved ACQ-6 scores ≤ 0.75 in this analysis were substantially lower than the degrees of response to other components (e.g. ACQ-6 < 1.5, exacerbations, etc.) of remission (i.e. 27% vs. 48–74%; and 28% versus 50–61% in SIROCCO/CALIMA at 6 and 12 months, respectively). These low rates of ACQ-6 response at the ≤ 0.75 score threshold relative to the other objective measures, such as exacerbations and lung function, suggest there may be other drivers of symptom perception beyond asthma.
In addition to variability in how ACQ-6 scores may be interpreted, the ACQ-6 is also limited by virtue of having been developed in a small, homogeneous population of patients with asthma [10]. ACQ-6 cut-points have not been established for patients with severe asthma and thus may be too limited for these patients given the high rates of fixed airway obstructions in severe asthma. Furthermore, it is unclear how differences in asthma phenotypes might affect the accuracy of ACQ-6 measurements in certain patients and therefore validated cut-points would be necessary to accurately deploy the instrument across a range of phenotypes/endotypes [10]. Finally, the ACQ-6 was not developed to assess composite criteria such as clinical remission. Indeed, as noted above, the higher rates of response to the other remission criteria could suggest there may be other drivers of symptom perception, including patient specific attributes other than asthma, such as deconditioning or subjective drivers that are not underpinned by airway inflammation.
Ultimately, future ideations of consensus definitions for clinical remission in severe asthma could incorporate a different or more comprehensive PRO assessment of asthma control, such as the Asthma Impairment and Risk Questionnaire (AIRQ) or the Predominant Symptom and Impairment Assessment (PSIA) [51, 53]. Furthermore, regardless of clinical improvements, patients with a long history of asthma may not score as “normal” on the ACQ because of the presence of chronic symptoms, side effects from long-term OCS use, or other comorbidities. In such cases, the ACQ does not provide insights into the reasons for such below “normal” scores; as an example, in a case where physical deconditioning, rather than the underlying inflammatory airway disease, is driving perceptions of certain symptoms, this can limit the potential to reach ACQ-6 scores ≤ 0.75 or even < 1.5 [54].
Likewise, FEV1 measurements may be refractory to increases in some patients because of the presence of airway remodelling [55, 56]. Ultimately, as a wider range of methods become available to measure different individual treatment response types, precision medicine approaches like treat-to-target will be used more widely to manage asthma. For example, the ANDHI trial employed the novel outcome measure PSIA that asked individual patients to identify their most bothersome symptoms at the outset of the study and then measured response of these symptoms over the course of therapy [53]. In fact, this approach could prove to be a more accurate way to measure clinically meaningful improvements in patients, versus a composite PRO, given the heterogeneous clinical presentation of severe asthma.
Clinical remission in severe asthma should be considered distinct from the concept of reversal of airway remodelling or the loose vernacular of disease modification, as these are long-term outcomes that currently lack a consensus assessment method in severe asthma. Nevertheless, there are suggestions that treatment with benralizumab may lead to beneficial structural changes in the lungs. In a study of patients with severe eosinophilic asthma, ventilation heterogeneity, as measured by 129Xe MRI and oscillometry, significantly improved 28 days after treatment with benralizumab [57]. A separate study of bronchial biopsies from patients with severe eosinophilic asthma showed a decrease in airway smooth muscle mass following treatment with benralizumab [58]. Those findings are also consistent with a recent report, which showed pre-BD FEV1 improved for patients on benralizumab, despite consistent bronchodilator responses over the course of treatment, suggesting that benralizumab-induced lung function changes may result from airway structural improvements rather than changes in airway hyperreactivity [59]. Whatever the case may be, results from the ongoing phase 4 CHINOOK trial, which is evaluating the effects of benralizumab on structural and lung function changes in patients with severe eosinophilic asthma, are expected to clarify the impact of benralizumab on airway remodelling [60].
Indeed, as a post hoc analysis, this study is limited by the eligibility criteria and outcomes of the original three pivotal, phase 3 trials: SIROCCO, CALIMA, and ZONDA. The study designs for SIROCCO and CALIMA prevented patients from reducing OCS dosages during the trial and therefore only SIROCCO and CALIMA patients who were not using OCS at the baseline visit could be included in this analysis. Similarly, as a result of the design of the ZONDA trial, this post hoc analysis could only include patients who had OCS dosages of ≤ 12.5 mg prednisone/prednisolone equivalents per day at the baseline visit. Likewise, as discussed earlier, although other PRO tools (e.g. AIRQ or PSIA) may ultimately provide additional, and possibly more accurate, insights into levels of symptom control among patients with severe asthma, only ACQ-6 absolute scores were available from the benralizumab pivotal trials, which is why they were used for this post hoc analysis [51, 53]. Finally, despite our efforts to identify similar patient populations between all three studies, differences between the trial designs (e.g. 48 weeks in SIROCCO vs. 56 weeks in CALIMA) and patient populations (e.g. no OCS at baseline in SIROCCO and CALIMA vs. ≤ 12.5 mg/day in ZONDA) make it difficult to extrapolate the results to longer periods of time on treatment. Future studies should apply the consensus clinical remission framework and super-responder definitions to patients in real-world evidence settings (i.e. outside the clinical trial setting) as well as in patient populations from other completed trials. Furthermore, additional studies are also necessary to understand how achieving the clinical remission and/or super-responder criteria correlates clinically to underlying disease pathology or progression. Finally, a comparison of remission rates across the available biologics could be useful for clinicians.
Conclusions
Among patients in the SIROCCO/CALIMA and ZONDA trials, 87% and 75%, respectively, achieved ≥ 2 composite remission components and roughly half (54.8% and 48.5%, respectively) achieved ≥ 3 remission components within 6 months of beginning treatment with benralizumab; the response rates were generally similar after 12 months of treatment. Results from this post hoc analysis show that clinical remission in severe asthma is achievable with benralizumab, which is targeted to treat the underlying drivers of inflammation. Furthermore, precision medicine represents a promising way to end the age of treat-to-failure and usher in a new era of treat-to-target, with clinical remission as the ultimate therapeutic goal for patients with severe asthma.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study, the Rapid Service Fee, and the Open Access Fee were funded by AstraZeneca (Gaithersburg, MD, USA).
Medical Writing and Editorial Assistance
The authors wish to acknowledge Yasa Reddy, Yunhui Cao, Satya Sanikommui, Surendra Saimpu, and Liliya Landsman for support with the statistical analyses. Medical writing support was provided by Dan Jackson, Ph.D., CMPP (CiTRUS Health Group), and was funded by AstraZeneca (Gaithersburg, Maryland, USA) in accordance with Good Publication Practice (GPP3) guidelines.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Rohit Katial, Peter Barker, and David Cohen contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Peter Barker and David Cohen. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Andrew Menzies-Gow has attended advisory boards for AstraZeneca, GlaxoSmithKline, Novartis, Sanofi, and Teva; has received speaker fees from AstraZeneca, Novartis, Sanofi, and Teva; has participated in research with AstraZeneca for which his institution has been remunerated and has attended international conferences with Teva; and has had consultancy agreements with AstraZeneca and Sanofi. Flavia L. Hoyte has attended advisory boards for AstraZeneca; has received speaker fees from AstraZeneca and GlaxoSmithKline; and has participated in research sponsored by AstraZeneca, GlaxoSmithKline, Genentech, Teva, Sanofi, and the National Institute of Allergy and Infectious Diseases (NIAID), for which her institution has been remunerated. David B. Price has board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, and Thermofisher; consultancy agreements with Airway Vista Secretariat, AstraZeneca, Boehringer Ingelheim, Chiesi, EPG Communication Holdings Ltd, FIECON Ltd, Fieldwork International, GlaxoSmithKline, Mylan, Mundipharma, Novartis, OM Pharma SA, PeerVoice, Phadia AB, Spirosure Inc, Strategic North Limited, Synapse Research Management Partners S.L., Talos Health Solutions, Theravance, and WebMD Global LLC; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Theravance, and the UK National Health Service; received payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, and Sanofi Genzyme; received payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis, and Thermofisher; stock/stock options from AKL Research and Development Ltd, which produces phytopharmaceuticals; ownership of 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp, which develops adherence monitoring technology; a peer reviewer role for grant committees of the UK Efficacy and Mechanism Evaluation programme and the Health Technology Assessment; and served as an expert witness for GlaxoSmithKline. David Cohen, Peter Barker, James Kreindler, Maria Jison, Chris Brooks, Peggy Papeleu, and Rohit Katial are employees of AstraZeneca.
Compliance with Ethics Guidelines
Before patient enrolment in the initial trials, the studies were approved by an independent ethics committee or institutional review board at each study centre. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data underlying the findings described in this manuscript may be requested in accordance with AstraZeneca’s data-sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home.
References
- 1.Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention, 2021 report. 2021 https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf. Accessed 1 Mar 2022.
- 2.Global Burden of Disease and Injury Incidence and Prevalence Collaborators VT. Abajobir AA, Abbafati C, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 4.Jackson DJ, Busby J, Pfeffer PE, et al. Characterisation of patients with severe asthma in the UK severe asthma registry in the biologic era. Thorax. 2021;76(3):220–227. doi: 10.1136/thoraxjnl-2020-215168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry. Chest. 2020;157(4):790–804. doi: 10.1016/j.chest.2019.10.053. [DOI] [PubMed] [Google Scholar]
- 6.Heaney LG, Perez-de-Llano L, Al-Ahmad M, et al. Eosinophilic and noneosinophilic asthma: an expert consensus framework to characterize phenotypes in a global real-life severe asthma cohort. Chest. 2021;160:814–830. doi: 10.1016/j.chest.2021.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Pavlidis S, Takahashi K, Ng Kee-Kwong F, et al. "T2-high" in severe asthma related to blood eosinophil, exhaled nitric oxide and serum periostin. Eur Respir J. 2019;53(1):1800938. doi: 10.1183/13993003.00938-2018. [DOI] [PubMed] [Google Scholar]
- 8.Kaur R, Chupp G. Phenotypes and endotypes of adult asthma: moving toward precision medicine. J Allergy Clin Immunol. 2019;144(1):1–12. doi: 10.1016/j.jaci.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 9.McDowell PJ, Heaney LG. Different endotypes and phenotypes drive the heterogeneity in severe asthma. Allergy. 2020;75(2):302–310. doi: 10.1111/all.13966. [DOI] [PubMed] [Google Scholar]
- 10.Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 11.Bourdin A, Bjermer L, Brightling C, et al. ERS/EAACI statement on severe exacerbations in asthma in adults: facts, priorities and key research questions. Eur Respir J. 2019;54(3):1900900. doi: 10.1183/13993003.00900-2019. [DOI] [PubMed] [Google Scholar]
- 12.Zeiger RS, Schatz M, Li Q, Chen W, Khatry DB, Tran TN. Burden of chronic oral corticosteroid use by adults with persistent asthma. J Allergy Clin Immunol Pract. 2017;5(4):1050–1060.e1059. doi: 10.1016/j.jaip.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Humbert M, Bourdin A, Papadopoulos NG, et al. Reducing the hidden burden of severe asthma: recognition and referrals from primary practice. J Asthma. 2020;58:1–6. doi: 10.1080/02770903.2020.1759084. [DOI] [PubMed] [Google Scholar]
- 14.Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204. doi: 10.2147/JAA.S176026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canonica GW, Colombo GL, Bruno GM, et al. Shadow cost of oral corticosteroids-related adverse events: a pharmacoeconomic evaluation applied to real-life data from the Severe Asthma Network in Italy (SANI) registry. World Allergy Organ J. 2019;12(1):100007. doi: 10.1016/j.waojou.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bleecker ER, Menzies-Gow AN, Price DB, et al. Systematic literature review of systemic corticosteroid use for asthma management. Am J Respir Crit Care Med. 2020;201(3):276–293. doi: 10.1164/rccm.201904-0903SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suehs CM, Menzies-Gow A, Price D, et al. Expert consensus on the tapering of oral corticosteroids for the treatment of asthma: a Delphi study. Am J Respir Crit Care Med. 2020;203:871–881. doi: 10.1164/rccm.202007-2721OC. [DOI] [PubMed] [Google Scholar]
- 18.Cataldo D, Louis R, Michils A, et al. Severe asthma: oral corticosteroid alternatives and the need for optimal referral pathways. J Asthma. 2021;58(4):448–458. doi: 10.1080/02770903.2019.1705335. [DOI] [PubMed] [Google Scholar]
- 19.Calhoun WJ, Haselkorn T, Mink DR, Miller DP, Dorenbaum A, Zeiger RS. Clinical burden and predictors of asthma exacerbations in patients on guideline-based steps 4–6 asthma therapy in the TENOR cohort. J Allergy Clin Immunol Pract. 2014;2(2):193–200. doi: 10.1016/j.jaip.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mab with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125(6):1344–1353.e1342. doi: 10.1016/j.jaci.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Maselli DJ, Rogers L, Peters JI. Benralizumab, an add-on treatment for severe eosinophilic asthma: evaluation of exacerbations, emergency department visits, lung function, and oral corticosteroid use. Ther Clin Risk Manag. 2018;14:2059–2068. doi: 10.2147/TCRM.S157171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pham TH, Damera G, Newbold P, Ranade K. Reductions in eosinophil biomarkers by benralizumab in patients with asthma. Respir Med. 2016;111:21–29. doi: 10.1016/j.rmed.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Korn S, Bourdin A, Chupp G, et al. Integrated safety and efficacy among patients receiving benralizumab for up to 5 years. J Allergy Clin Immunol Pract. 2021;9:4381–4392. doi: 10.1016/j.jaip.2021.07.058. [DOI] [PubMed] [Google Scholar]
- 24.Menzies-Gow A, Bafadhel M, Busse WW, et al. An expert consensus framework for asthma remission as a treatment goal. J Allergy Clin Immunol. 2020;145(3):757–765. doi: 10.1016/j.jaci.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Menzies-Gow A, Szefler SJ, Busse WW. The relationship of asthma biologics to remission for asthma. J Allergy Clin Immunol Pract. 2021;9(3):1090–1098. doi: 10.1016/j.jaip.2020.10.035. [DOI] [PubMed] [Google Scholar]
- 26.Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011;70(3):404–413. doi: 10.1136/ard.2011.149765. [DOI] [PubMed] [Google Scholar]
- 27.Travis SP, Higgins PD, Orchard T, et al. Review article: defining remission in ulcerative colitis. Aliment Pharmacol Ther. 2011;34(2):113–124. doi: 10.1111/j.1365-2036.2011.04701.x. [DOI] [PubMed] [Google Scholar]
- 28.van Vollenhoven RF, Bertsias G, Doria A, et al. 2021 DORIS definition of remission in SLE: final recommendations from an international task force. Lupus Sci Med. 2021;8(1):181–182. doi: 10.1136/lupus-2021-000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emery P, Salmon M. Early rheumatoid arthritis: time to aim for remission? Ann Rheum Dis. 1995;54(12):944–947. doi: 10.1136/ard.54.12.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danese S, Allez M, van Bodegraven AA, et al. Unmet medical needs in ulcerative colitis: an expert group consensus. Dig Dis. 2019;37(4):266–283. doi: 10.1159/000496739. [DOI] [PubMed] [Google Scholar]
- 31.Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. Acg clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018;113(4):481–517. doi: 10.1038/ajg.2018.27. [DOI] [PubMed] [Google Scholar]
- 32.Upham JW, James AL. Remission of asthma: the next therapeutic frontier? Pharmacol Ther. 2011;130(1):38–45. doi: 10.1016/j.pharmthera.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Upham JW, Le Lievre C, Jackson DJ, et al. Defining a severe asthma super-responder: findings from a Delphi process. J Allergy Clin Immunol Pract. 2021;9(11):3997–4004. doi: 10.1016/j.jaip.2021.06.041. [DOI] [PubMed] [Google Scholar]
- 34.Rupani H, Hew M. Super-responders to severe asthma treatments: defining a new paradigm. J Allergy Clin Immunol Pract. 2021;9(11):4005–4006. doi: 10.1016/j.jaip.2021.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 36.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 37.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–2458. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- 38.Katial R, Hirsch I, Barker P, Garcia GE, Hoyte F. Eosinophil as a biomarker of severity and clinically important asthma outcomes in benralizumab phase III studies. Am. J. Respir. Crit. Care Med. 2021;203(60):A1468.
- 39.Tepper RS, Wise RS, Covar R, et al. Asthma outcomes: pulmonary physiology. J Allergy Clin Immunol. 2012;129(3 Suppl):S65–87. doi: 10.1016/j.jaci.2011.12.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the Asthma Control Questionnaire. Respir Med. 2005;99(5):553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Juniper EF, Bousquet J, Abetz L, Bateman ED, GOAL Committee Identifying 'well-controlled' and 'not well-controlled' asthma using the Asthma Control Questionnaire. Respir Med. 2006;100(4):616–621. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Zeiger RS, Schatz M, Dalal AA, et al. Utilization and costs of severe uncontrolled asthma in a managed-care setting. J Allergy Clin Immunol Pract. 2016;4(1):120–129.e123. doi: 10.1016/j.jaip.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Louis R, Chanez P, Menzella F, et al. Standard-of-care asthma controller regimen reduction with benralizumab treatment: ANDHI In Practice study. European Respiratory J. 2021;58:PA1114.10.1183/13993003.congress-2021.PA1114 .
- 44.Menzies-Gow A, Gurnell M, Heaney LG, et al. Oral corticosteroid elimination via a personalised reduction algorithm in adults with severe, eosinophilic asthma treated with benralizumab (PONENTE): a multicentre, open-label, single-arm study. Lancet Respir Med. 2021;10:47–58. doi: 10.1016/S2213-2600(21)00352-0. [DOI] [PubMed] [Google Scholar]
- 45.Tran TN, Kerkhof M, Carter V, Price DB. Persistence of eosinophilic asthma endotype and clinical outcomes: a real-world observational study. J Asthma Allergy. 2021;14:727–742. doi: 10.2147/JAA.S306416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bourdin A, Fabry-Vendrand C, Ostinelli J, et al. The burden of severe asthma in France: a case-control study using a medical claims database. J Allergy Clin Immunol Pract. 2019;7(5):1477–1487. doi: 10.1016/j.jaip.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 47.Suruki RY, Daugherty JB, Boudiaf N, Albers FC. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17(1):74. doi: 10.1186/s12890-017-0409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ortega H, Yancey SW, Keene ON, Gunsoy NB, Albers FC, Howarth PH. Asthma exacerbations associated with lung function decline in patients with severe eosinophilic asthma. J Allergy Clin Immunol Pract. 2018;6(3):980–986.e981. doi: 10.1016/j.jaip.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 49.Miller MK, Lee JH, Miller DP, Wenzel SE, Group TS. Recent asthma exacerbations: a key predictor of future exacerbations. Respir Med. 2007;101(3):481–489. doi: 10.1016/j.rmed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Bourdin A, Molinari N, Vachier I, Pahus L, Suehs C, Chanez P. Mortality: a neglected outcome in OCS-treated severe asthma. Eur Respir J. 2017;50(5):1701486. doi: 10.1183/13993003.01486-2017. [DOI] [PubMed] [Google Scholar]
- 51.Murphy KR, Chipps B, Beuther DA, et al. Development of the Asthma Impairment and Risk Questionnaire (AIRQ): a composite control measure. J Allergy Clin Immunol Pract. 2020;8(7):2263–2274.e2265. doi: 10.1016/j.jaip.2020.02.042. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan A, Szefler SJ, Halpin DMG. Impact of comorbid conditions on asthmatic adults and children. NPJ Prim Care Respir Med. 2020;30(1):36. doi: 10.1038/s41533-020-00194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harrison TW, Chanez P, Menzella F, et al. Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): a randomised, controlled, phase 3b trial. Lancet Respir Med. 2021;9(3):260–274. doi: 10.1016/S2213-2600(20)30414-8. [DOI] [PubMed] [Google Scholar]
- 54.van’t Hul AJ, Frouws S, van den Akker E, et al. Decreased physical activity in adults with bronchial asthma. Respir Med. 2016;114:72–77. doi: 10.1016/j.rmed.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 55.Tashkin DP, Chipps BE, Trudo F, Zangrilli JG. Fixed airflow obstruction in asthma: a descriptive study of patient profiles and effect on treatment responses. J Asthma. 2014;51(6):603–609. doi: 10.3109/02770903.2014.895012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kozlik P, Zuk J, Bartyzel S, et al. The relationship of airway structural changes to blood and bronchoalveolar lavage biomarkers, and lung function abnormalities in asthma. Clin Exp Allergy. 2020;50(1):15–28. doi: 10.1111/cea.13501. [DOI] [PubMed] [Google Scholar]
- 57.McIntosh M, Eddy R, Knipping D, et al. Response to benralizumab in severe asthma: oscillometry and clinical measurements. Am J Respir Crit Care Med. 2020;201:A6244. [Google Scholar]
- 58.Chachi L, Diver S, Kaul H, et al. Computational modelling prediction and clinical validation of impact of benralizumab on airway smooth muscle mass in asthma. Eur Respir J. 2019;54(5):1900930. doi: 10.1183/13993003.00930-2019. [DOI] [PubMed] [Google Scholar]
- 59.Mathur SK, Modena BD, Coumou H, Barker P, Kreindler JL, Zangrilli JG. Postbronchodilator lung function improvements with benralizumab for patients with severe asthma. Allergy. 2020;75(6):1507–1510. doi: 10.1111/all.14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.AstraZeneca. Benralizumab airway remodeling study in severe eosinophilic asthmatics ClinicalTrials.gov registration number: NCT03953300 2021. Accessed 1 Mar 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings described in this manuscript may be requested in accordance with AstraZeneca’s data-sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home.