Abstract
Background
Despite its broad range of biological activities, use of curcumin is limited because of poor bioavailability. Here we report a novel curcumin formulation, Curcuwin Ultra+ (CU+), with superior bioavailability as compared to 95% turmeric extract (TUR 1800).
Methods
A randomized, double-blind, three-treatment, crossover oral bioavailability study was conducted in 24 healthy volunteers under fasting conditions. Subjects received a single dose of CU+ 250 mg, 500 mg and 1900 mg of TUR1800 as per randomization schedule and blood samples were collected at 4 h and 0 h before dosing, and 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12, 24 h post dose. Total curcuminoids were measured as curcumin, demethoxycurcumin, bisdemethoxycurcumin, and tetrahydrocurcumin using a validated LC–MS/MS method.
Results
CU+ achieved a significantly higher (p < 0.05) maximum plasma concentration (Cmax) and total systemic exposure (AUC0–6 and AUC0–12) for total curcuminoids as compared to TUR 1800. We observed 101 and 100 times higher Cmax respectively for 250 and 500 mg doses of CU+ as compared to 1900 mg of TUR1800. Similarly, AUC0–6 was 144 and 149 times higher whereas AUC0–12 was 99 and 113 times higher respectively for 250 and 500 mg doses of CU+ as compared to 1900 mg dose of TUR1800. Further, CU+ showed 40% faster absorption (p < 0.05). No safety issues were observed.
Conclusion
CU+, which is designed for increased absorption and protection of curcuminoids from intestinal degradation, demonstrated superior bioavailability as compared to TUR1800 at considerably smaller doses. Additional clinical studies will help to demonstrate the impact of its increased bioavailability on efficacy.
Clinical Trial Registration
CTRI/2020/10/028508 (Clinical Trials Registry—India).
Keywords: Curcumin, Curcuminoids, Bioavailability, Pharmacokinetics, Absorption, Turmeric
Key Summary Points
| Curcuwin Ultra+, a unique formulation of curcumin, significantly increases plasma concentration of curcuminoids in human subjects. |
| Curcuwin Ultra+ 250 mg shows 101 times higher Cmax, 144 times higher AUC0–6, and 99 times higher AUC0–12 as compared to 1900 mg of 95% turmeric extract. |
| Curcuwin Ultra+ 500 mg shows 100 times higher Cmax, 149 times higher AUC0–6, and 113 times higher AUC0–12 as compared to 1900 mg of 95% turmeric extract. |
| Curcuwin Ultra+ shows 40% faster absorption (p < 0.05) as compared to 95% turmeric extract. |
Introduction
Turmeric (Curcuma longa L.) is a common spice extensively used in traditional medicine for management of disorders of the skin, upper respiratory tract, joints, and digestive system. Curcuminoids, the yellow pigment abundantly present in the rhizomes of turmeric, have diverse biological properties including anti-inflammatory, antioxidant, neuroprotective, antimicrobial, and anticancer activities [1–3]. Major components of curcuminoids include curcumin (CUR), demethoxycurcumin (DMC), and bisdemethoxycurcumin (BDMC), with CUR being up to 77% of the total curcuminoid content [4–6]. Tetrahydrocurcumin (THC) is a primary metabolite of curcumin that has better aqueous solubility, chemical stability, bioavailability, and antioxidative activity [7, 8].
The biological activities of curcuminoids have been extensively studied as they modulate several molecular targets and cell-signaling pathways, including anti-inflammatory, antioxidant, and pro-apoptotic pathways [4, 6]. Beneficial therapeutic effects of curcumin against cancer, diabetes, and other inflammatory disease such as osteoarthritis have been extensively reported and curcumin is being used as a dietary supplement in many countries worldwide [9]. However, low absorption, rapid metabolism, and elimination of curcuminoids in the body limits the efficacy when orally ingested as a supplement [10]. Curcumin is highly hydrophobic molecule making it practically insoluble in water [11] and it has a relatively short half-life due to alkaline instability at physiological pH [12]. Poor aqueous solubility and alkaline instability result in very low plasma levels of curcumin even after taking gram doses and this severely limits the therapeutic potential of curcumin.
Several formulations have been developed to increase the bioavailability of curcuminoids by improving lipophilicity, enhancing adsorption and dispersion of curcuminoids using micellar and nanoparticles, and chemical modification such as curcumin conjugates [13–19]. Similarly, Curcuwin®, which uses turmeric extract formulated with a hydrophilic carrier, cellulosic derivatives, and natural antioxidants, was shown to improve the curcuminoids bioavailability by 46 times as compared to 95% curcumin powder [20], leading to an improvement in endothelial function [21] and reduction in muscle damage [22].
We report a novel curcumin formulation, Curcuwin Ultra+ (CU+), with improved oral bioavailability at a considerably smaller dose when compared against standard 95% turmeric extract. CU+ showed 64.7 times higher systemic exposure in the body (AUC0–t) as compared to 95% turmeric powder in an animal pharmacokinetic study which was further associated with amelioration of experimentally induced osteoarthritis in a rat model [23]. In the current study we evaluated the oral bioavailability of CU+ at two doses—250 mg providing 50 mg of total curcuminoids (CU+ 50) and 500 mg providing 100 mg of total curcuminoids (CU+ 100)—as compared to 95% turmeric extract at a dose of 1900 mg providing 1800 mg of total curcuminoids (TUR 1800), in healthy human subjects. Safety and tolerability of CU+ was also examined. We report pharmacokinetics parameters for total curcuminoids for CU+ as determined by measuring plasma levels of CUR, DMC, BDMC, and THC.
Methods
The study was conducted in accordance with the protocol, guidelines of the Declaration of Helsinki, and International Council for Harmonization—Good Clinical Practices (ICH-GCP) after obtaining approval from Maarg Independent Ethics Committee, Secunderabad, India. The study was registered on Clinical Trials Registry—India (registration number CTRI/2020/10/028508). All subjects voluntarily signed the consent before commencing study activities.
Study Design
This was a randomized, double-blind, single-dose, three-treatment, three-period, crossover oral bioavailability study in healthy adult human subjects under fasting conditions. Twenty-four subjects were enrolled in the study (Fig. 1). Randomization scheme was generated using SAS® software 9.4 for Windows (SAS Inst. Inc., Cary, NC, USA). TUR 1800 (lot number J190389) was acquired from Sabinsa Corporation, East Windsor, NJ, USA, and CU+ 50 (lot number CU20DNS3-096(04)/069) and CU+ 100 (lot number CU20DNS3-096(04)/073) were provided by OmniActive Health Technologies, India. An inert filler (microcrystalline cellulose) was used to match the total weight of each of the study materials. The study blinding was achieved by delivering the yellowish powder of study products in opaque scarlet red-colored capsules. Additionally, the number of capsules dosed per group were kept uniform across groups, i.e., six capsules to keep the study double blinded. The principal investigator, subjects, and the study personnel involved in the study were kept blinded from the randomization schedule during the entire study. After an overnight fasting of at least 10 h, six capsules (equally distributed doses) of CU+ 50, CU+ 100, and TUR 1800 were administered orally to the subjects in sitting posture with at least 500 ± 2 mL water, at ambient temperature in each period (period I, II, and III) as per the randomization scheme.
Fig. 1.
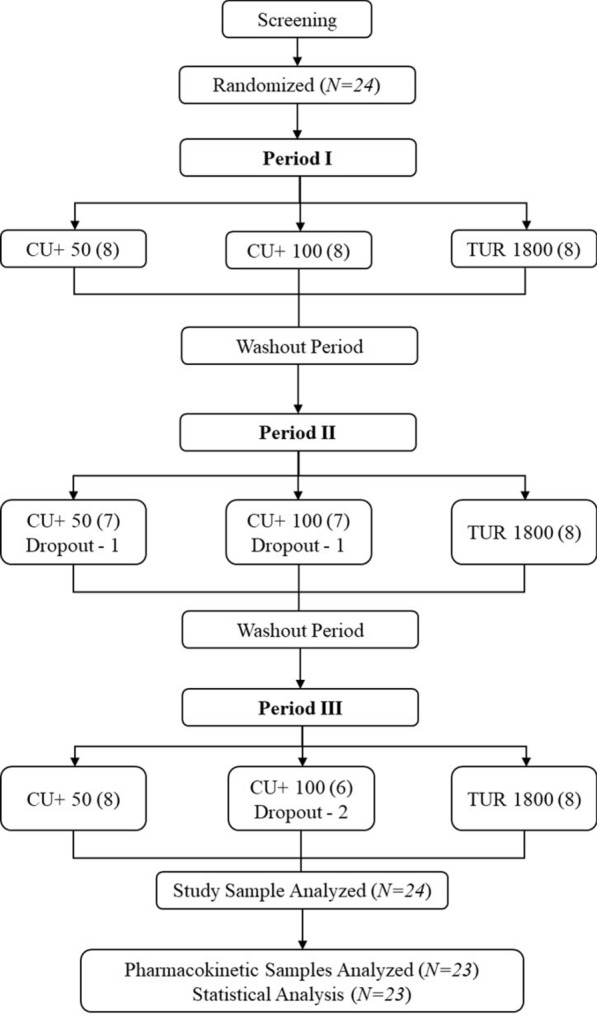
Study flow diagram
The subjects were housed for at least 60 h prior to investigational product administration and up to 24 h post dose in each period with at least 7 days washout between each consecutive treatment period. This was done so that the subjects were provided with curcumin and black pepper-free diet to control for any plasma curcuminoid levels from the diet. Dosing was done under yellow monochromatic light. All the screening procedures were performed within 28 days before the start of the study and no volunteer screening activities were done on the day of the subject’s check-in into the study.
Inclusion/Exclusion Criteria
Subjects of both genders were included in the study if they were healthy and aged between 18 and 55 years (both limits inclusive); with a BMI between 20 and 27 kg/m2; willing to provide voluntary consent; avoided food containing curcumin and black pepper for at least 48 h before dosing with absence of significant disease or clinically significant abnormal laboratory values, medical history, physical examination, and systemic examination during the screening; a normal 12-lead ECG; a normal chest X-ray done within past 6 months; stable weight (weight change less than 3 kg during the last 6 months); willing to comply with the requirement of the entire protocol; able to communicate effectively; non-smoker and non-alcoholic.
Subjects with following conditions were excluded from the study: history or evidence of hypersensitivity to curcumin or its metabolites; history or presence of any medical condition or disease; history or evidence of chronic diseases; participating in aerobic exercise more than three times per week; high caffeine intake (more than five cups of coffee or tea/day); participation in a clinical research study within the past 90 days prior to check-in; use of supplements containing curcumin or its metabolites 30 days prior to period I dosing and during the study duration; consumption of food and beverages containing xanthine (chocolates, tea, coffee, or cola drinks) for at least 48 h prior to check-in of each period; consumption of grapefruit and grapefruit-like citrus fruit (mosumbi/sweet lime) or juice within the 7 days prior to check-in of each period; positive results for drugs of abuse (benzodiazepines, opioids, amphetamines, cannabinoids, cocaine, and barbiturates) in urine during the check-in of each period; positive results for urine alcohol analysis using a kit during the check-in of each period; difficulty in swallowing solid dosage forms like tablets or capsules; a positive pregnancy test; pregnant or breast-feeding or likely to become pregnant during the study; use of implanted or injected hormonal contraceptives anytime during the 6 months prior to study or hormonal contraceptives within 30 days before dosing.
Pharmacokinetic Sampling and Analysis
A total of 13 blood samples (6 mL each) were collected from each subject in each period, pre-dose samples at 4 h and 0 h before dosing, and post-dose blood samples at 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12, and 24 h, with ± 2 min of scheduled sampling time. All blood samples were collected in dipotassium ethylenediaminetetraacetic acid (EDTA)-containing tubes at the bedside and under yellow monochromatic light. The samples were centrifuged at 4000 rpm for 5 min at 4 °C to separate plasma and stored immediately in a deep freezer at − 70 ± 10 °C until analysis.
The plasma samples were analyzed for CUR, DMC, BDMC, and THC using a validated LC–MS/MS method. The analytes were extracted from human plasma by liquid–liquid extraction method. The 20 μL of internal standard working solution (curcumin D6 and warfarin) was added to all tubes containing 200 μL of plasma sample with the help of micropipette and vortexed. In addition, 20 μL of diluent solution was added for the blank sample. These samples were treated with 100 μL enzyme solution containing 1000 U of β-glucuronidase isolated from Helix pomatia (Sigma, St. Louis, MO) in 0.1 M phosphate buffer (pH 6.86), and 50 μL of 50 U of sulfatase obtained from Helix pomatia (Sigma, St. Louis, MO) in 0.1 M sodium acetate buffer (pH 5.0). The resulting mixture was then vortexed for 10 s and incubated at 37 °C for 1 h to hydrolyze conjugates of curcuminoids. Then, 0.2 mL of 100 mM potassium dihydrogen phosphate and 0.1 mL of 100% methanol was further added, vortexed for 30 s followed by addition of 2.4 mL of t-butyl methyl ether to all tubes, vortexed for 10 min, and centrifuged for 10 min at 4200 rpm at 4 °C. The 2.4 mL of organic layer was transferred into another pre-labeled RIA vial and evaporated to dryness at 40 °C until drying. Then, 200 μL of mobile phase was added to all the dried samples and vortexed for 30 s, transferred into pre-labeled autosampler vials, and submitted for analysis. All the sample processing was done under monochromatic light.
Curcumin D6 (Clearsynth Labs Ltd.) was used as an internal standard for the analysis. The separation was achieved by phenyl-hexyl column (4.6 mm × 100 mm, 5 μm) using acetonitrile/methanol/ultra-pure water (60:5:35 v/v/v) with 0.2% acetic acid as mobile phase. Analyte concentrations were quantified using Analyst® 1.6.1, software with API 3200 Q Trap [API 3200 Q Trap from AB Sciex, Framingham, USA]. The measured concentrations for each subject for all the time points were calculated against the calibration curve prepared with known standards. All pharmacokinetic (PK) parameters were calculated using WinNonlin Software version 8.2.
Statistical Analysis
Statistical analysis was performed using SAS® system for Windows version 9.4 (SAS Inst. Inc., Cary, NC, USA). Descriptive statistics of all the PK parameters were computed and reported for CUR, DMC, BDMC, THC, and TC. Mean, standard deviation, minimum, and maximum were calculated for plasma concentrations at each individual time point as well as for the pharmacokinetic parameters (AUC0–6, AUC0–12, Cmax, Tmax, and t1/2) for CUR, DMC, BDMC, THC, and TC. In addition, geometric least squares means were calculated for AUC0–6, AUC0–12, and Cmax. Relative absorption was calculated using Cmax and AUC values for CU+ 50 and CU+ 100 as compared to TUR 1800. The relative absorption for each analyte was calculated using the following formula:
Sample Size Determinations
A sample size of 18–21 subjects was calculated as sufficient to prove superiority of test versus reference considering 85% power and a significance level of 5% to detect minimum mean difference between Cmax of two groups as 200 ng/mL and SD 120 ng/mL. Assuming a dropout rate of 15–20% in this three-way crossover design, 24 subjects were considered for the study.
Safety Analysis
Adverse event (AE) monitoring, systolic and diastolic blood pressure, radial pulse rate, aural temperature, and laboratory parameters including hematology, biochemistry, and urine analysis were evaluated for safety assessments.
Results
Subject Disposition
A total of 24 subjects were enrolled in the study and 23 subjects completed the study. Out of 23, 15 subjects were male and 8 were female (Table 1). The mean age of the participants was 34.30 ± 5.47 years, and the mean BMI was 24.53 ± 1.65 kg/m2. The mean age for men was 34.60 ± 6.23 years and mean BMI was 23.75 ± 1.40 kg/m2, whereas the mean age for women was 33.75 ± 3.99 years and mean BMI was 26.00 ± 0.94 kg/m2. Subject #6 did not come back for periods II and III and hence did not complete the study. Subject #7 completed periods I and III but did not attend period II. Similarly, subject #9 completed periods I and II but did not come for period III (Fig. 1).
Table 1.
Summary of demographics of the study participants
| Parameter | Numbera | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| Total subjects | 23 | – | – | – | – |
| Age (years) | 34.30 | 5.47 | 26.00 | 44.00 | |
| Height (cm) | 162.80 | 9.80 | 147.00 | 180.00 | |
| Weight (kg) | 64.97 | 6.74 | 56.64 | 80.41 | |
| BMI (kg/m2) | 24.53 | 1.65 | 20.92 | 26.97 | |
| Male | 15 | – | – | – | – |
| Age (years) | 34.60 | 6.23 | 26.00 | 44.00 | |
| Height (cm) | 168.33 | 6.69 | 159.00 | 180.00 | |
| Weight (kg) | 67.44 | 7.01 | 56.64 | 80.41 | |
| BMI (kg/m2) | 23.75 | 1.40 | 20.92 | 25.78 | |
| Female | 8 | – | – | – | – |
| Age (years) | 33.75 | 3.99 | 27.00 | 38.00 | |
| Height (cm) | 152.38 | 4.75 | 147.00 | 161.00 | |
| Weight (kg) | 60.33 | 2.62 | 57.11 | 64.80 | |
| BMI (kg/m2) | 26.00 | 0.94 | 24.15 | 26.97 |
BMI body mass index, SD standard deviation
aNumber of subjects in the specified category
Pharmacokinetics
Pharmacokinetic samples for 23 subjects were analyzed for CUR, DMC, BDMC, THC, and TC for groups CU+ 50, CU+ 100, and TUR 1800. Baseline corrected data was used for analysis. Cmax, Tmax, t1/2, AUC0–6, AUC0–12, and relative absorption were calculated for total curcuminoids and all the analytes (Table 2).
Table 2.
Summary of pharmacokinetic results for CU+ 50, CU+ 100, and TUR 1800
| Formulation | Cmax (ng/ml) | 90% CI | Relative absorption based on Cmax data | Tmax (h) | t1/2 (h) | AUC0–6 (ng/ml h) | 90% CI | Relative absorption based on AUC0–6 data | AUC0–12 (ng/ml h) | 90% CI | Relative absorption based on AUC0–12 data |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Curcumin | |||||||||||
| TUR 1800 | 9.85 ± 6.82 | – | 1 | 8 | 9.45 ± 5.25 | 15.43 ± 30.77 | – | 1 | 40.21 ± 44.98 | – | 1 |
| CU+ 50 | 28.36 ± 31.21* | 212.66–389.60 | 103.62* | 3* | 5.33 ± 3.70* | 86.53 ± 90.33* | 362.46–867.41 | 201.86* | 119.12 ± 120.81* | 199.05–440.83 | 106.64* |
| CU+ 100 | 54.11 ± 40.11*† | 392.77–767.85 | 98.85* | 3* | 3.97 ± 2.27* | 160.99 ± 112.64*† | 629.30–1726.37 | 187.62* | 251.12 ± 164.55*† | 403.54–966.41 | 112.41* |
| Demethoxycurcumin | |||||||||||
| TUR 1800 | 3.20 ± 1.40 | – | 1 | 3 | 8.37 ± 5.66 | 6.42 ± 5.91 | – | 1 | 10.37 ± 10.04 | – | 1 |
| CU+ 50 | 4.33 ± 4.91 | 104.93–174.79 | 48.75* | 3 | 6.53 ± 5.25 | 9.35 ± 13.81 | 99.29–213.67 | 52.44* | 12.19 ± 16.92 | 84.12–164.30 | 42.32* |
| CU+ 100 | 7.98 ± 5.65*† | 186.36–337.84 | 45.16* | 3 | 3.60 ± 3.68 | 20.28 ± 15.46*† | 185.78–526.61 | 56.30* | 28.42 ± 21.96*† | 172.24–428.26 | 48.89* |
| Bisdemethoxycurcumin | |||||||||||
| TUR 1800 | 2.15 ± 1.05 | – | 1 | 2 | 3.24 ± 1.39 | 4.89 ± 2.82 | – | 1 | 6.38 ± 5.04 | – | 1 |
| CU+ 50 | 1.05 ± 1.19+ | 30.86–77.04 | 17.55* | 8+ | – | 0.49 ± 1.17+ | 2.62–38.50 | 3.62 | 1.46 ± 3.28+ | 11.26–46.72 | 8.26* |
| CU+ 100 | 1.15 ± 1.04+ | 33.48–84.55 | 9.58* | 5+ | – | 0.82 ± 1.20+ | 7.10–36.47 | 2.90 | 2.09 ± 2.11+ | 18.49–56.61 | 5.82* |
| Tetrahydrocurcumin | |||||||||||
| TUR 1800 | 5.38 ± 3.63 | – | 1 | 4.5 | 11.92 ± 7.60 | 7.88 ± 12.19 | 1 | 10.28 ± 16.86 | – | 1 | |
| CU+ 50 | 13.98 ± 13.63* | 184.37–366.04 | 93.52* | 3* | 4.89 ± 3.67* | 40.81 ± 41.12* | 301.11–890.97 | 186.47* | 52.55 ± 57.19* | 327.02–799.37 | 184.06* |
| CU+ 100 | 26.24 ± 21.34*† | 348.33–669.98 | 86.96* | 3* | 5.25 ± 3.05* | 81.54 ± 57.15*† | 552.03–1879.20 | 183.33* | 115.44 ± 80.58*† | 649.28–1863.39 | 197.99* |
| Total curcuminoids | |||||||||||
| TUR 1800 | 15.65 ± 9.41 | – | 1 | 5 | 9.04 ± 7.34 | 33.21 ± 43.34 | – | 1 | 66.57 ± 64.71 | – | 1 |
| CU+ 50 | 43.83 ± 48.20* | 203.73–384.89 | 100.81* | 3* | 3.88 ± 2.04* | 133.24 ± 138.50* | 276.61–581.74 | 144.41* | 182.65 ± 182.82* | 196.42–383.29 | 98.78* |
| CU+ 100 | 86.98 ± 62.94*† | 401.02–770.05 | 100.03* | 3* | 4.29 ± 3.95* | 274.35 ± 169.57*† | 534.48–1282.65 | 149.04* | 419.14 ± 248.98*† | 434.96–911.44 | 113.33* |
Data expressed as geometric least squares mean (Cmax, AUC) or arithmetic mean (t1/2) ± standard deviation and Tmax expressed as median. N = 24 for TUR1800, N = 23 for CU+ 50, N = 21 for CU+ 100
AUC area under the curve, CU+ Curcuwin Ultra+, SD standard deviation, TUR turmeric extract
*P value < 0.05, CU+ significant over TUR 1800
+P value < 0.05, TUR 1800 significant over CU+
†P value < 0.05, CU+ 100 significant over CU+ 50
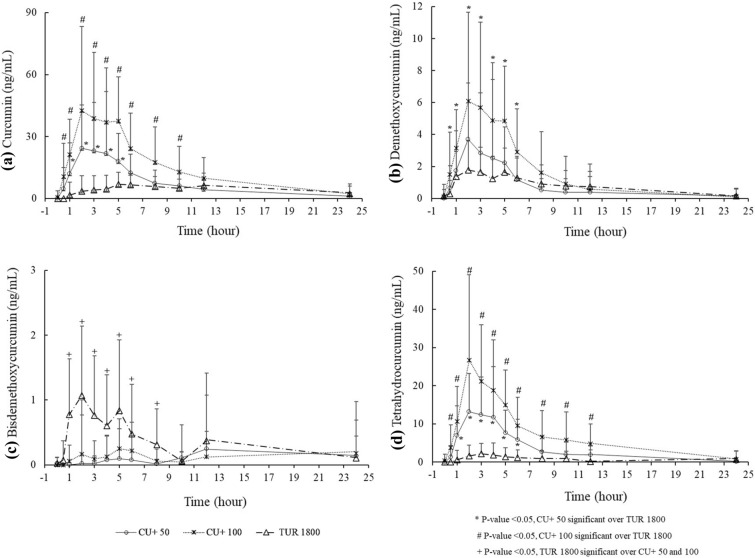
Plasma TC concentrations were significantly higher (p < 0.05) for CU+ 50 from 1 to 5 h and CU+ 100 from 0.5 to 12 h as compared to TUR 1800 (Fig. 2). Plasma concentration vs. time profiles of individual analytes CUR, DMC, BDMC, and THC are presented in Fig. 3.
Fig. 2.
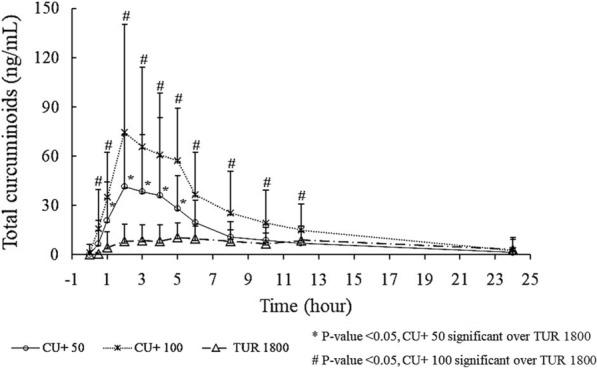
Plasma total curcuminoid concentration vs. time profiles
Fig. 3.
Plasma concentration vs. time profiles of individual curcuminoids (CUR, DMC, BDMC, and THC)
CU+ 50 showed significantly greater (p < 0.05) relative absorption for TC with 101 times higher Cmax, 144 times higher AUC0–6, and 99 times higher AUC0–12 as compared to TUR 1800 (Table 2).
Similarly, CU+ 100 showed significantly greater (p < 0.05) relative absorption for TC with 100 times higher Cmax, 149 times higher AUC0–6, and 113 times higher AUC0–12 as compared to TUR 1800 (Table 2).
Both CU+ 50 and CU+ 100 showed significantly faster absorption (40%) in blood as compared to TUR 1800.
We also observed significantly greater (p < 0.05) plasma total curcuminoid levels, Cmax, AUC0–6, and AUC0–12 for CU+ 100 as compared to CU+ 50.
Safety
There were no statistically significant differences between treatments for vital signs and laboratory variables (hematology and blood chemistry). Additionally, there were no adverse events, and the treatments were well tolerated by all the participants.
Discussion
There is an extensive published scientific evidence supporting the biological effects of curcuminoids in conditions associated with inflammation and oxidative stress. However, the poor bioavailability limits the health benefit potential of curcumin and requires large doses of the supplement. Several formulation approaches have been developed to enhance the bioavailability of curcuminoids through improved solubility, enhanced absorption, and delayed elimination [13]. CU+ , a novel curcumin formulation, was developed using widely accepted excipients that are known to improve solubility of curcumin and protect from degradation by the alkaline conditions of the small intestine. We conducted a pharmacokinetic study in healthy human volunteers and found that CU+ formulation significantly enhances bioavailability of total curcuminoids even at lower doses as compared to 95% turmeric extract.
Most of the commercial curcumin sources are available as a composition of CUR (approx. 75–80%), DMC (approx. 15–17%), BDMC (approx. 3–5%), and THC being the major metabolite of curcumin [7]. We measured the plasma levels of CUR, DMC, BDMC, and THC which are known to be responsible for the diverse bioactivity of curcuminoids [8, 24–27] and represented the data for total curcuminoids. Our study results showed that the plasma concentrations of total curcuminoids and its bioavailability were significantly higher for both doses (250 and 500 mg) of CU+ as compared to TUR 1800. The maximum plasma concentrations of total curcuminoids as measured by Cmax were 101 and 100 times higher respectively for 250 and 500 mg doses of CU+ as compared to 1900 mg of TUR 1800. Further, total systemic exposure for total curcuminoids measured between 0 and 6 h (AUC0–6) and 0–12 h (AUC0–12) were also significantly higher (p < 0.05) for both doses of CU+ as compared to TUR 1800. We observed 144 and 149 times higher AUC0–6 and 99 and 113 times higher AUC0–12 respectively for 250 and 500 mg doses of CU+ as compared to 1900 mg dose of TUR 1800. Moreover, we report a faster absorption for total curcuminoids for both doses of CU+ (250 and 500 mg) with 40% faster absorption (p < 0.05) as compared to TUR 1800. There was a significant increase in the bioavailability of total curcuminoids in the CU+ 100 group with approximately two times higher Cmax and AUC measures as compared to the CU+ 50 group demonstrating a robust dose response. Our data further validates earlier results in rats wherein CU+ showed 64.7 times higher absorption as compared to TUR 1800 that was associated with significant beneficial effects in the rat knee osteoarthritis model [23].
Curcumin has been extensively studied under experimental conditions as well as human clinical studies including treatment of osteoarthritis [23, 28–31], and bioavailability of curcuminoids plays an important role in the bioactivity such as exhibiting antioxidant and anti-inflammatory properties [32]. A recent study that used cell-based experimental models of osteoarthritis demonstrated that curcumin suppresses inflammation by blocking the NF-κB–Sox9 signaling pathway that plays a critical role in initiation of pathogenesis of osteoarthritis [29]. Curcumin enhances the antioxidant activities, inhibits oxidative stress, modulates immune functions, protects against cartilage damage, blocks inflammation pathways, and inhibits chondrocyte apoptosis [33]. Therefore, curcumin with improved bioavailability may have a significant effect on the clinical course of osteoarthritis including alleviating pain, joint stiffness, and improve functionalities of the joints.
Conclusion
The CU+ formulation shows superior bioavailability at relatively low doses of 250 and 500 mg with 144 and 149 times higher relative absorption, respectively, as compared to 1900 mg of 95% turmeric extract. Moreover, we observed a robust dose response between the two doses (250 and 500 mg) of CU+ with direct correlation to the dose administered and plasma concentration achieved. As bioavailability is the key factor in determining the biological activity of curcumin, efficacy studies of CU+ at low doses are required to demonstrate the beneficial effect of CU+ in subjects with knee osteoarthritis and other inflammatory conditions.
Acknowledgements
The authors wish to thank the participants of this study for their invaluable contribution to this work. This study was funded by OmniActive Health Technologies Ltd., which also provided the study supplements (Curcuwin Ultra+ formulations and 95% turmeric extract).
Funding
This study and journal’s Rapid Service Fee was funded by OmniActive Technologies Limited (Mumbai, India).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
KS, AS and MR were responsible for designing and conducting the study at the site, volunteer recruitment, study procedures, data collection, statistical analysis and study report. NN was responsible for volunteer recruitment, study procedures and data collection. The manuscript was drafted by KS, AS and MR. All authors read and approved the final manuscript.
Disclosures
Kothaplly Sudhakar, Alukapally Shankar, Nagula Nagaraju, and Maddela Rambabu have nothing to disclose.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the Good Clinical Practice guidelines, the ethical principles originating from the Helsinki Declaration, and in strict compliance with the “New Drugs and Clinical Trial Rules–2019,” the Ministry of Health and the Government of India, at all stages of the trial for adherence to protocol. The study activities commenced after obtaining approval from Maarg Independent Ethics Committee (EC) Secunderabad, India. The EC was duly apprised of the progress and updates of the trial at regular intervals as per prescribed guidelines. Informed consent was obtained from all individual participants included in the study.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Sudhakar Kothaplly, Email: sudhakar.k@clinsynccro.com.
Shankar Alukapally, Email: shankar.a@clinsynccro.com.
Nagaraju Nagula, Email: nagaraju@clinsynccro.com.
Rambabu Maddela, Email: rambabu.m@clinsynccro.com.
References
- 1.Siviero A, Gallo E, Maggini V, et al. Curcumin, a golden spice with a low bioavailability. J Herb Med. 2015;5:57–70. doi: 10.1016/j.hermed.2015.03.001. [DOI] [Google Scholar]
- 2.Kunnumakkara AB, Harsha C, Banik K, et al. Is curcumin bioavailability a problem in humans: lessons from clinical trials. Expert Opin Drug Metab Toxicol. 2019;15:705–733. doi: 10.1080/17425255.2019.1650914. [DOI] [PubMed] [Google Scholar]
- 3.Fan X, Zhang C, Liu D, Yan J, Liang H. The clinical applications of curcumin: current state and the future. Curr Pharm Des. 2013;19:2011–2031. [PubMed] [Google Scholar]
- 4.Salehi B, Stojanović-Radić Z, Matejić J, et al. The therapeutic potential of curcumin: a review of clinical trials. Eur J Med Chem. 2019;163:527–545. doi: 10.1016/j.ejmech.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Deb L, Prasad S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Mol Multidiscip. 2015;20:185–205. doi: 10.3390/molecules20010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purpura M, Lowery RP, Wilson JM, Mannan H, Münch G, Razmovski-Naumovski V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur J Nutr. 2018;57:929–938. doi: 10.1007/s00394-016-1376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padmanaban G, Nagaraj VA. Chapter 6—Curcumin from turmeric as an adjunct drug? Stud Nat Prod Chem. 2018;57:179–202. https://www.sciencedirect.com/science/article/pii/B9780444640574000065. Accessed 4 Sep 2021.
- 8.Panda SK, Nirvanashetty S, Missamma M, Jackson-Michel S. The enhanced bioavailability of free curcumin and bioactive-metabolite tetrahydrocurcumin from a dispersible, oleoresin-based turmeric formulation. Medicine. 2021;100:e26601. [DOI] [PMC free article] [PubMed]
- 9.Howes M-J. Phytochemicals as anti-inflammatory nutraceuticals and phytopharmaceuticals. In: Chatterjee S, Jungraithmayr W, Bagchi D, editors. Immunity and inflammation in health and disease. New York: Elsevier; 2018; p. 363–88.
- 10.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharmaceut. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 11.Wahlström B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol. 1978;43:86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 12.Tønnesen HH, Karlsen J. Studies on curcumin and curcuminoids. Z Lebensm Unters Forsch. 1985;180:402–404. doi: 10.1007/BF01027775. [DOI] [PubMed] [Google Scholar]
- 13.Stohs SJ, Chen O, Ray SD, Ji J, Bucci LR, Preuss HG. Highly bioavailable forms of curcumin and promising avenues for curcumin-based research and application: a review. Molecules. 2020;25:1397. doi: 10.3390/molecules25061397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kharat M, Du Z, Zhang G, McClements DJ. Physical and chemical stability of curcumin in aqueous solutions and emulsions: impact of pH, temperature, and molecular environment. J Agric Food Chem. 2017;65:1525–1532. doi: 10.1021/acs.jafc.6b04815. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Zou L-Q, Niu J, Liu W, Peng S-F, Liu C-M. The stability, sustained release and cellular antioxidant activity of curcumin nanoliposomes. Mol Multidiscip. 2015;20:14293–14311. doi: 10.3390/molecules200814293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S. A pilot cross-over study to evaluate human oral bioavailability of BCM-95® CG (Biocurcumax™), a novel bioenhanced preparation of curcumin. Indian J Pharmaceut Sci. 2008;70:445. [DOI] [PMC free article] [PubMed]
- 17.Cuomo J, Appendino G, Dern AS, et al. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J Nat Prod. 2011;74:664–669. doi: 10.1021/np1007262. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki H, Sunagawa Y, Takahashi K, et al. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharmaceut Bull. 2011;34:660–665. doi: 10.1248/bpb.34.660. [DOI] [PubMed] [Google Scholar]
- 19.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas P. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 20.Jäger R, Lowery RP, Calvanese AV, Joy JM, Purpura M, Wilson JM. Comparative absorption of curcumin formulations. Nutr J. 2014;13:1–8. doi: 10.1186/1475-2891-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliver JM, Stoner L, Rowlands DS, et al. Novel form of curcumin improves endothelial function in young, healthy individuals: a double-blind placebo controlled study. J Nutr Metab. 2016;2016:1089653. [DOI] [PMC free article] [PubMed]
- 22.Jäger R, Purpura M, Kerksick CM. Eight weeks of a high dose of curcumin supplementation may attenuate performance decrements following muscle-damaging exercise. Nutrients. 2019;11:1692. doi: 10.3390/nu11071692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yabas M, Orhan C, Er B, et al. A next generation formulation of curcumin ameliorates experimentally induced osteoarthritis in rats via regulation of inflammatory mediators. Front Immunol. 2021;12:157. doi: 10.3389/fimmu.2021.609629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canistro D, Chiavaroli A, Cicia D, et al. The pharmacological basis of the curcumin nutraceutical uses: an update. Pharmadvances. 2021;3:421–66. doi: 10.36118/pharmadvances.2021.06. [DOI] [Google Scholar]
- 25.Osawa T, Sugiyama Y, Inayoshi M, Kawakishi S. Antioxidative activity of tetrahydrocurcuminoids. Biosci Biotechnol Biochem. 1995;59:1609–1612. doi: 10.1271/bbb.59.1609. [DOI] [PubMed] [Google Scholar]
- 26.Mukhopadhyay A, Basu N, Ghatak N, Gujral PK. Anti-inflammatory and irritant activities of curcumin analogues in rats. Agents Actions. 1982;12:508–515. doi: 10.1007/BF01965935. [DOI] [PubMed] [Google Scholar]
- 27.Liu A, Lou H, Zhao L, Fan P. Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin. J Pharmaceut Biomed Anal. 2006;40:720–727. doi: 10.1016/j.jpba.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 28.Paultre K, Cade W, Hernandez D, Reynolds J, Greif D, Best T. Therapeutic effects of turmeric or curcumin extract on pain and function for individuals with knee osteoarthritis: a systematic review. BMJ Open Sport Exerc Med. 2021;7:e000935. doi: 10.1136/bmjsem-2020-000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shokri-Mashhadi N, Bagherniya M, Askari G, Sathyapalan T, Sahebkar A. A systematic review of the clinical use of curcumin for the treatment of osteoarthritis. Adv Exp Med Biol. 2021;2021:265–282. doi: 10.1007/978-3-030-56153-6_16. [DOI] [PubMed] [Google Scholar]
- 30.Buhrmann C, Brockmueller A, Mueller A-L, Shayan P, Shakibaei M. Curcumin attenuates environment-derived osteoarthritis by Sox9/NF-kB signaling axis. Int J Mol Sci. 2021;22:7645. doi: 10.3390/ijms22147645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng L, Yu G, Hao W, Yang K, Chen H. The efficacy and safety of Curcuma longa extract and curcumin supplements on osteoarthritis: a systematic review and meta-analysis. Biosci Rep. 2021;41:7. doi: 10.1042/BSR20210817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayaprakasha GK, Rao LJ, Sakariah KK. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006;98:720–724. doi: 10.1016/j.foodchem.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 33.Shen XY, Li Y, Zhang Z. Research progress of curcumin in the treatment of osteoarthritis. Zhonghua Wai Ke Za Zhi. 2021;59:554–557. doi: 10.3760/cma.j.cn112139-20210127-00055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.