Introduction
Pain is a common and debilitating symptom of inflammatory bowel disease (IBD) [4]. Unfortunately, IBD remains a significant health problem because of its high prevalence about 1.3% of US adults (3 million) are diagnosed with IBD [9; 34] and its debilitating impact on the quality of life. These two factors are exacerbated by the lack of effective therapies, which also contributes to the tremendous economic burden of IBD in terms of health care costs and lost productivity [50].
The pathophysiology of pain associated with IBD is complex but appears to be due to a heightened response to physiological stimuli within the intestine, termed visceral hypersensitivity [4; 12; 27]. Visceral hypersensitivity, in turn, appears to be due, at least in part, to the sensitization of colonic nociceptors, which is typically reflected as a reduction in the mechanical threshold for activation and an increase in the response to both innocuous and noxious stimulation of the colon [4; 47].
While the sensitization of colonic nociceptors appears to be due to intrinsic changes in colonic afferents [14], this sensitization may also be due to the loss of GABAA receptor (GAR) mediated inhibition in their peripheral terminals.
We have recently demonstrated that the excitability of colonic afferents in situ is established, at least in part, by GAR activity [28]. Importantly, GAR antagonists increased the colonic afferent excitability, suggesting that in the absence of tissue injury, there is sufficient extracellular GABA for GAR activation. There is evidence of a decrease in extracellular GABA in the colon in the presence of inflammation [1]. And there is evidence that GAR controlling the excitability of nociceptive afferents are present in the periphery [6]. Importantly, a loss of GAR inhibition of the central terminals of nociceptive afferents contributes to inflammatory hypersensitivity [2]. Interestingly, at the central terminals, there is not simply a loss of inhibition, but the emergence of a GAR-mediated excitation [7; 49]. However, this change in GAR signaling appeared to be GAR subtype-dependent because GAR-mediated excitation in the presence of inflammation appeared to be dependent on high-affinity GAR subtypes while low-affinity GAR-positive allosteric modulators (PAMs) retained their analgesic efficacy [2]. These observations raise the possibility that depending on the nature of the inflammation-induced change in GAR signaling in the colon, GAR activation may contribute to the hypersensitivity despite the apparent decrease in the extracellular GABA levels in the colon. Nevertheless, we hypothesize that visceral hypersensitivity observed in the presence of persistent inflammation is due a decrease in GAR-mediated suppression of nociceptive signaling from the colon.
To begin to test this hypothesis, we performed a series of behavioral pharmacological experiments in mice in the presence or absence of persistent inflammation of the gastrointestinal tract. Persistent inflammation was induced with dextran sodium sulfate (DSS) added to the drinking water. GAR agonists, antagonists, and PAMs were directly applied to the colon. Visceral sensitivity was assessed with the visceromotor response (VMR) evoked with balloon distention of the colon. Our results, raise the interesting possibility that activation of low-affinity GARs and/or blocking high-affinity GARs may be effective therapeutic strategies for the management of pain associated with IBD.
Methods
All procedures were approved by and performed in accordance with the guidelines of the University of Pittsburgh Institutional Animal Care and Use Committee. They were also performed in accordance with the guidelines established by the United States National Institutes of Health for the use of animals in research. All attempts were made to minimize the number of mice used and any pain or distress associated with the procedures performed.
Animals and Treatment
172 adult (8–10 weeks old) male and female C57BL/6J mice (Taconic Biosciences, Germantown, NJ, USA) were used in all experiments. Mice were housed by sex two to four per cage, in a temperature and humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) approved facility, with food and water available ad libitum, in rooms on a 12:12 h light: dark schedule with lights on at 07.00 h.
Mice were divided randomly by cage into two groups defined by the presence or absence of visceral inflammation. Those in the inflammation group received 3% (wt/vol) of dextran sodium sulfate (DSS) at a molecular weight of 36,000–50,000 Da (MP Biomedicals, Santa Ana, CA, USA) in their drinking water for 5 days. The non-inflamed, or control group received water bottles handled and filled in the same way as group receiving DSS, but DSS was omitted. Mice received DSS- or vehicle-filled water bottles for five days, and on the sixth day of treatment, mice were used for behavioral pharmacological experiments. At the end of the behavioral pharmacological experiments, mice were euthanized with an overdose of isoflurane 5%, and tissue removed for subsequent analysis.
Visceromotor response (VMR)
The VMR was evoked and recorded as previously described [28]. Mice were anesthetized using an intraperitoneal injection of urethane (1.2g/Kg). We chose urethane as an anesthetic because produces a long-lasting stable level of anesthesia, has minimal effects on autonomic and cardiovascular systems, and has minimal effect on GAR signaling [18; 29; 45]. In contrast, inhaled anesthetics such as isoflurane act to enhance responses of GAR [46]. Then the mice were place on a heating pad. The abdominal area was shaved, and a ~1 cm incision was done in the skin to expose the abdominal musculature. Positive and negative clip electrodes (Pomona Electronics) were attached to the abdominal muscle to record the VMR.
A polyethylene balloon (length,1.5 cm; diameter, 0.9 cm) affixed to the end of PE-60 tubing, was used to distend the colon. The balloon was inserted trans-anally until the proximal end of the balloon was 0.5 cm from the anal verge (total balloon insertion = 2 cm) and secured with tape to the mouse tail. The balloon was designed to enable the infusion of the test agents rostral to the balloon. The volume of each intra-colonic infusion was 0.1 ml and was given immediately after the baseline distensions. A ground electrode was attached to the tail of the mouse. The recordings and ground electrodes were attached to a differential amplifier (A-M Systems) connected to an A-D converter (Cambridge Electronic Design (CED) Micro 1401).
Colorectal distension (CRD) was performed by balloon inflation using a compressed N2 tank equipped with a pressure regulator connected to a computer-controlled air valve. CRD to 20, 30, 45, 60, and 70 mmHg were applied for 10 s every 4 min. The VMR was amplified (1000×), filtered (0.3–10 kHz bandpass), sampled at 20 kHz with 1401 interface (Cambridge Electronic Design), and stored in a PC for analysis offline. The VMR to CRD was collected before (baseline) and after instillation of test agents using Spike2 (Cambridge Electronic Design).
Analysis of VMR
The VMR was quantified as the area under the curve (AUC) of the rectified EMG signal recorded during distension minus the AUC of the rectified EMG signal recorded over the 10 s prior to distension. Prior to the intracolonic instillation of test agents, the VMR was evoked three times at each pressure (20, 30, 45, 60, and 70 mmHg). The mean VMR at each pressure was considered the baseline, and the variance around that mean was used to determine whether a mouse “responded” to a test agent. That is, a mouse was considered a responder, of the VMR evoked after the test agent was > two standard deviations from the baseline response. The response to test agents was determined in the same manner as baseline, with a VMR evoked three times at each pressure. A four min inter-stimulus interval was maintained throughout.
To be able to compare the impact of test agents on VMR from control and DSS-treated mice, data were analyzed as a % of change from baseline using the following equation: ((VMRtest agent – VMRbaseline)/VMRbaseline)*100, where VMRbaseline and VMRtest agent are the VMR determined before and after application of test agents, respectively. Thus, if a test agent increases the VMR (i.e., increases nociception), the % change from baseline will be positive, while if a test agent decreases the VMR (i.e., decreases nociception), the % change from baseline will be negative.
Intestinal permeability
Changes in intestinal permeability was determined by quantifying Evans Blue leakage from the colon lumen. The proximal colon (2.5 cm) was incised at the junction with the cecum, and the fecal contests were gently flushed with 3 ml of PBS. The ends of the colon were then ligated with a 4.0 silk suture and 0.2 ml of 1.5% (w/v) Evans Blue (Sigma-Aldrich) in 0.1 M phosphate buffered saline (PBS) was injected into the lumen of the colon with a 1 ml syringe and gavage needle. The colon sac was suspended in 50 ml of oxygenated Krebs solution (in mM: 117.9 NaCl, 4.7 KCl, 25 NaH2CO3, 1.3 NaH2PO4, 1.2 MgSO4*7H2O, 2.5 CaCl2, 11.1 D-glucose) maintained at 30–32 °C for 120 min. The colon was opened and rinsed 4 times with 3 ml PBS at room temperature. The colon was dried on filter paper at 37°C for 24 hr, weighed and incubated with 1 ml of formamide (Sigma-Aldrich) at room temperature for 24 hr. The amount of Evans Blue recovered from the tissue was estimated from a standard curve with a microplate reader at a wavelength of 655 nm (BioTek Instruments, Epoch/2 microplate reader). Evans Blue recovered (in μg) was then normalized to the dry weight of the colon (in mg).
Test agents
Test agents included the non-selective GAR agonist muscimol (1–100μM), δ-subunit preferring GAR agonist 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridine-3-ol (THIP, 100 μM), the non-selective GAR antagonist bicuculline (100μM), the GAR antagonist, gabazine, which is non selective at low concentrations (1 μM), but high-affinity GAR preferring at high concentrations (100μM), and the GAR positive allosteric modulator (PAM) diazepam (1–100 nM). All test agents were obtained from Sigma-Aldrich. Muscimol and bicuculline were dissolved in distilled water at 100 mM while THIP and gabazine were dissolved in distilled water at 25mM. Stock solutions were stored at −20̊ C. On the day of the experiment, the stock solution was diluted to the final concentration in sterile saline (NaCl 0.9%) solution. Diazepam was dissolved in DMSO (100%) at 1 mM. For diazepam, the final concentration of DMSO was 0.01%.
Statistics
Data are presented as mean ± SEM. Data were analyzed using Prism 9 (GraphPad, San Diego, CA, USA), using student’s t-tests for two group comparisons, or more commonly two- or three-way analysis of variance (ANOVA) with Sidak’s, Tukey’s or Bonferroni’s post hoc analyses dependent on data distribution. Non-parametric data were analyzed using the Friedman rank test. Data were tested for normality and equal distribution prior to the application of parametric tests.
Results
Induction of colitis by DSS administration
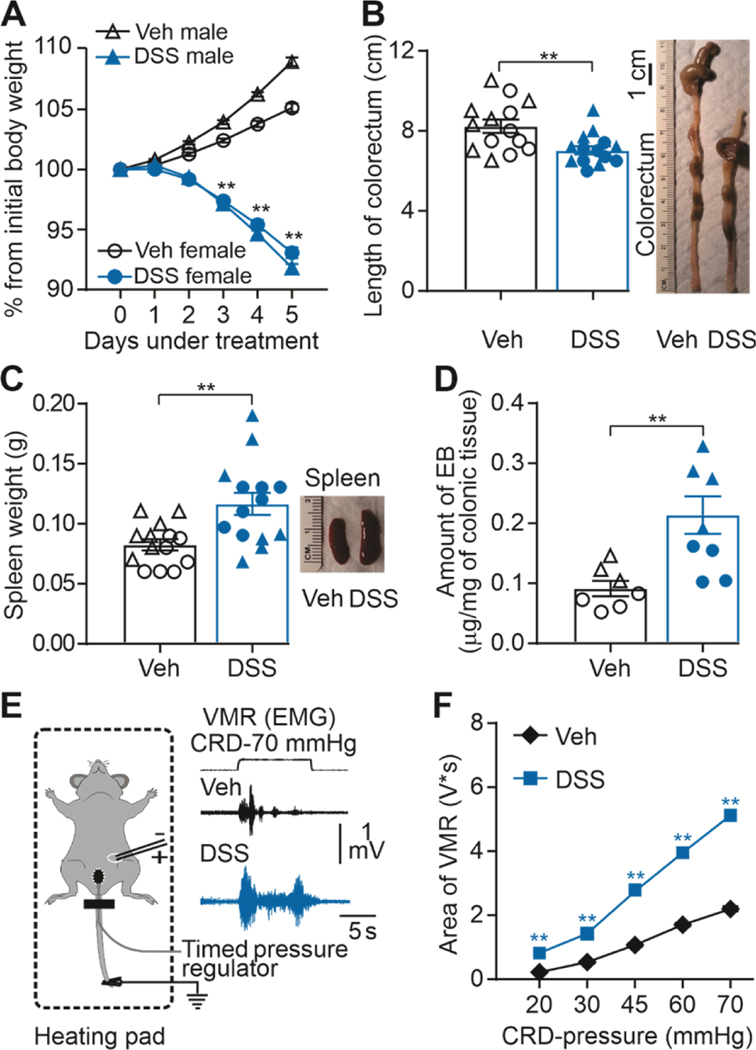
Consistent with previous results [8; 11; 33; 35; 38], 3% DSS in the drinking water was associated with changes consistent with the presence of visceral inflammation. DSS-treated mice developed diarrhea, with evidence of blood in their stool. There was a decrease in bodyweight in DSS-treated mice across the five days of treatment which was significantly lower by day three in DSS-treated mice (49 male and 37 female) compared to those with water alone (i.e., control mice, 46 male and 40 female) (Figure 1A, significant interaction between time, treatment, and sex (F (5, 800) = 71.13, p < 0.01). As colon inflammation is also associated with a decrease in colon length and an increase in spleen weight [11] we compared both in 14 mice each (7 male and 7 female) from control and DSS groups. Both endpoints were analyzed with a two-way ANOVA, which revealed a significant influence of treatment (DSS) for colon length (Figure 1B, F (1, 12) = 5.698, p < 0.01), but no significant influence of sex (F (1, 12) = 4.037, F (1, 24) = 1.257, p > 0.05), nor a significant interaction between treatment and sex (F (1, 12) = 0.1968, F (1, 24) = 0.7394, p > 0.05). Likewise, there was a significant influence of treatment (DSS) for spleen weight (Figure 1C, F (1, 24) = 10.77, p < 0.01), but no significant influence of sex (F (1, 24) = 1.257, p > 0.05), nor a significant interaction between treatment and sex (F (1, 24) = 0.7394, p > 0.05).
Figure 1. Dextran sodium sulfate (DSS) administration produces colon inflammation.
A) Percentage change from initial body weight during DSS or sterile water (Veh) treatment. Significant differences in body weight emerge at day three of treatment in male and female mice (**P < 0.01, three-way ANOVA, n = 49 and n = 37 male and female respectively). B) Pooled colorectum length data from fourteen DSS-treated and vehicle-treated mice (**P < 0.01, two-way ANOVA). At the right, representative colorectum imagen from DSS-treated and vehicle-treated mice. C) Pooled spleen weight data from fourteen DSS-treated and vehicle-treated mice (respectively.**P < 0.01; two-way ANOVA). D) Pooled EB in DSS-treated and vehicle-treated mice (**P < 0.01, n = 7 per group; two-way ANOVA). E) Experimental setting for the recording of the visceromotor response (VMR) to colorectum distention (CRD). F) Visceromotor responses- area presented as stimulus-response functions reveal significant colon hypersensitivity after DSS treatment compared with sterile- water treatment. **p < 0.01; two-way ANOVA; n=86 mice/group. Of note, in this and subsequent figures, when data from individual mice are plotted, males are represented by triangles and females by circles, with open symbols used for vehicle treated mice and closed symbols used for DSS-treated mice.
Finally, given that colon inflammation is associated with an increase in colon permeability, we evaluated the colon-permeability in separate groups of control (4 male and 3 female) and DSS-treated (4 male and 3 female) mice. Consistent with the presence of colon inflammation, there was a significant main effect of treatment (F (1, 10) = 10.47, p < 0.01) due elevated Evans Blue leakage into the colon wall in DSS-treated compared to control mice (Figure 1D). There was no significant effect of sex (F (1, 10) = 0.03842, p > 0.05), nor interaction (F (1, 10) = 0.3840, p > 0.05) between sex and treatment with respect to the amount of Evans Blue recovered from the colon wall.
DSS-treatment produces mechanical visceral hypersensitivity
Consistent with previous results [22; 24; 33; 42], DSS treatment was associated with the emergence of visceral hypersensitivity. Changes in visceral sensitivity were assessed with the VMR in response to CRD (Figure 1E). VMR data from control (46 male and 40 female) and DSS- (49 male and 37 female) treated mice were analyzed with a three-way ANOVA (treatment x sex x distension pressure). Results of this analysis revealed a significant interaction between treatment and distension pressure (F (4, 672) = 154, p < 0.01), but no significant influence of sex (F (1,168) = 0.3256, p > 0.05), nor a significant interaction between sex and treatment (F (1,168) = 0.2966, p > 0.05) or distension pressure (F (4, 672) = 1.356, p > 0.05; Figure 1F). Of note, post-hoc testing confirmed the DSS-induced increase in VMR was significant (p < 0.01), in response to both non-noxious (e.g., 20 mmHg) and noxious (e.g., 70 mmHg) distension pressures.
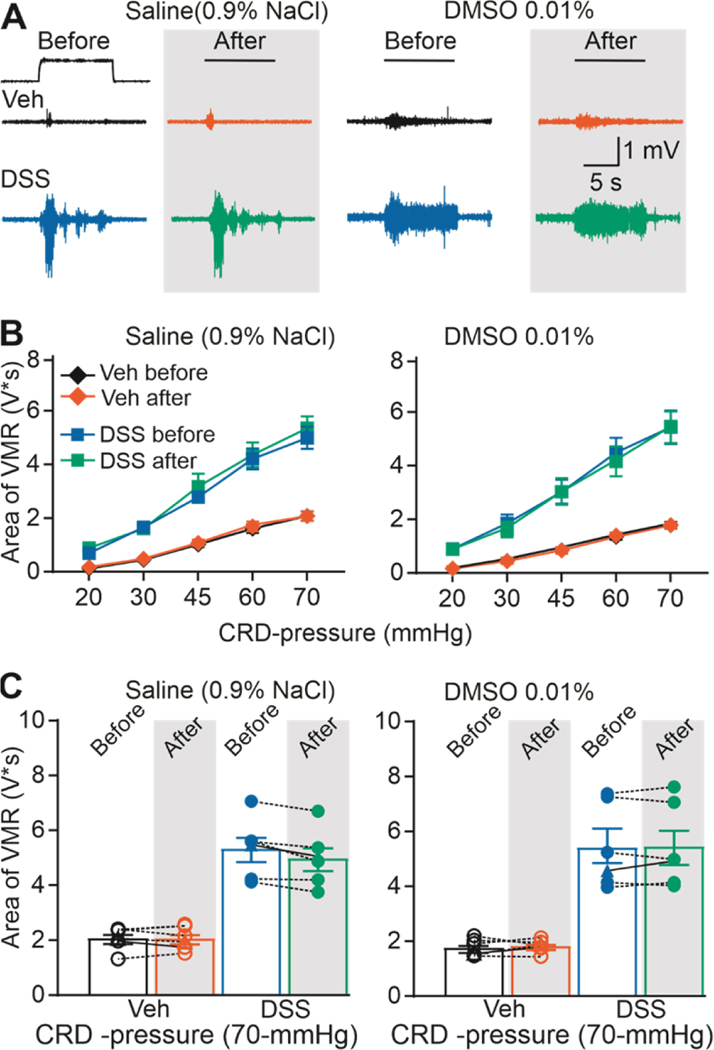
Because the response to test agents required repeated CRD, we next sough to confirm the stability of the VMR over time and following intracolonic instillation of control solutions (e.g., vehicle used for test agents: saline or DMSO). Six mice (3 male and 3 female) each were tested with saline and with DMSO in the DSS-treated and control groups (24 total). VMR data were analyzed with a three-way ANOVA (sex x treatment (+/− DSS) x intracolonic infusion (Before/After)), which revealed a significant influence of treatment (F (1, 8) = 26.92, p < 0.01), but no significant influence of sex (F (1,8) = 0.0553, p > 0.05), infusion of saline (F (1,8) = 0.0062, p > 0.05), or a significant interaction between sex, treatment and/or infusion of saline (Figure 2, F (1,8) = 0.2866, p > 0.05). Likewise, three-way ANOVA results also revealed a significant influence of treatment (F (1, 8) = 26.90, p < 0.01), but no significant influence of sex (F (1,8) = 0.0036, p > 0.05), infusion of DMSO (F (1,8) = 1.063, p > 0.05), or a significant interaction between sex, treatment and/or infusion of DMSO (Figure 2, F (1,8) = 0.1484, p > 0.05).
Figure 2. Intracolonic installation of the vehicle does not induce changes in VMR.
A) Typical original recordings of VMR from vehicle- (Veh) and DSS-treated mice (DSS) in response to CRD-pressure of 70 mmHg before and after administration of either saline or DMSO. B) Pooled stimulus-response function data before and after of either saline or DMSO relative to baseline (before saline/DMSO installation). C) VMR data for individual mice are plotted before and after either saline or DMSO.
The impact of persistent inflammation on the actions of the non-selective GABAA agonist, muscimol
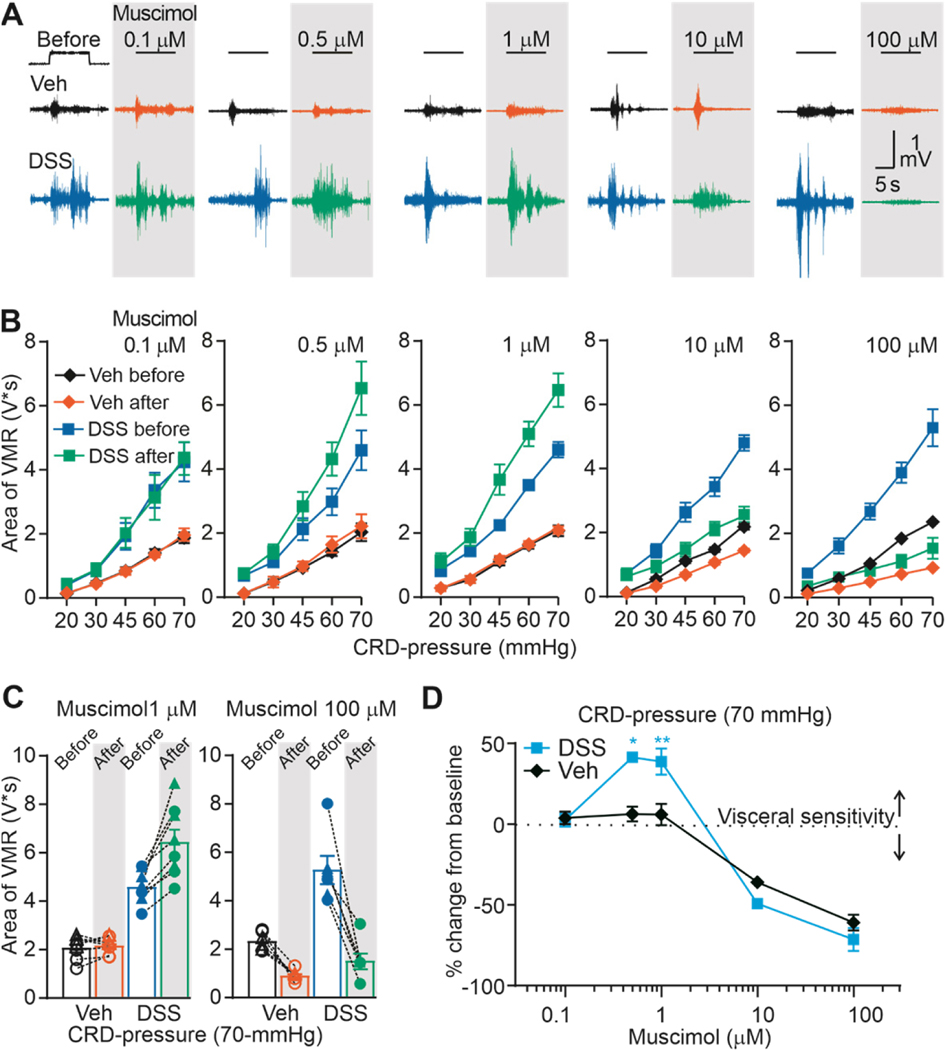
We recently reported that activation of GAR in the colon in uninflamed mice with the non-selective GAR agonist muscimol, attenuates colonic nociceptor excitability and consequently visceral sensitivity [28]. If a loss of GAR inhibition of colonic afferents contributes to inflammatory hypersensitivity, as we hypothesized, and this loss of inhibition is due to a decrease in functional GAR, we predicted we would observe a rightward shift in the muscimol concentration response curve such that more muscimol would be needed to produce the same decrease in visceral nociception. To test this prediction, we assessed the impact of the intracolonic application of muscimol (0.1 to 100 μM) on CRD-induced VMR in control and DSS-treated mice. Male and female mice were randomly assigned to DSS and control groups, and then to groups defined by the concentration of muscimol administered. As expected, based on our previous results, in mice receiving normal drinking water, muscimol produced a concentration-dependent decrease in the VMR (Figure 3). Typical examples of the effect of muscimol on VMR are shown in Figure 3A. Pooled stimulus-response function data before and after muscimol administration are shown in Figure 3B. In contrast to our prediction, the muscimol-induced suppression of the VMR in DSS-treated animals, as a percent of baseline, at least at the highest doses tested (10 and 100 μM) was comparable to that in vehicle-treated mice. Even more striking, however, was that in contrast to control mice in which there was no detectable change in VMR in response to 0.5 and 1 μM muscimol, these concentrations of muscimol increased the VMR in DSS-treated mice. Thus, the effect of muscimol in DSS-treated mice was bi-directional, increasing VMR at low concentrations and decreasing VMR at high concentrations. The variability in responses to 1 and 100 μM muscimol in vehicle- and DSS-treated mice are plotted in Figure 3C. Pooled concentration-response data plotted as percent change from baseline (Figure 3D) were analyzed with a three-way ANOVA (sex x treatment x dose). The results revealed a significant treatment x dose interaction (F (4, 38) = 7.69, p < 0.01), but no main effect or significant interactions associated with sex.
Figure 3. Direct comparation of the effect of muscimol on colon sensitivity (VMR) from vehicle-treated and DSS-treated mice.
A) Typical original recordings of VMR from vehicle-treated (Vehicle) and DSS-treated (DSS) mice in response to CRD-pressure of 70 mmHg before and after admistration of muscimol (0.1, 0.5, 1, 10,100 μM). Time of CRD-pressure 10 s (indicated by black line on top on the recordings). B) VMR data are shown as stimulus-response functions before and after muscimol (0.1, 0.5, 1, 10,100 μM). C) VMR data for individual mice are plotted before and after muscimol 1 and 100 μM. D) Pooled concentration-response data as percent from baseline. *P <0.5, **P < 0.01, n= 7 per group; three-way ANOVA.
THIP increases visceral hypersensitivity in DSS-treated mice
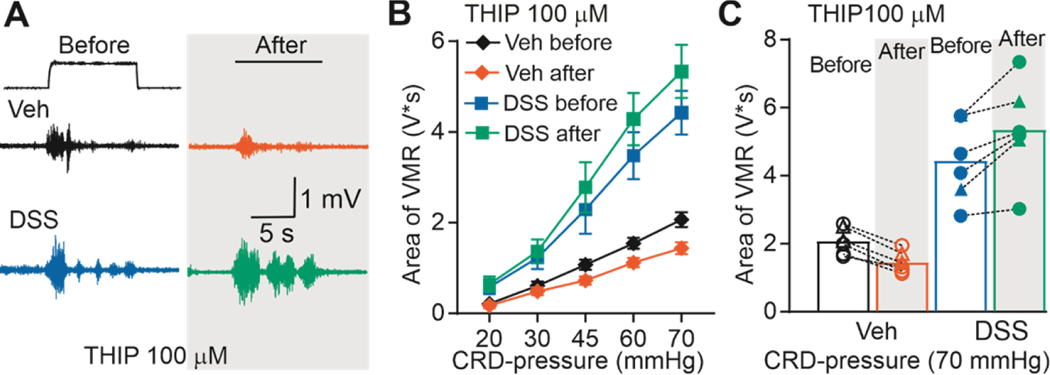
The bi-directional influence of muscimol on the VMR in DSS-treated mice suggested the emergence of a high-affinity GAR underlying the pro-nociceptive effects of the low concentrations of muscimol, in addition to a low-affinity GAR underlying the anti-nociceptive effects of the high concentrations of muscimol. To further test this possibility, we assessed the impact of the high-affinity δ-subunit preferring GAR agonist THIP (100 μM) on the VMR in DSS-treated (4 male and 2 female) and control (4 male and 2 female) mice. Given that THIP has some activity at low-affinity receptors, it was not surprising that THIP suppressed the VMR in control mice (Figure 4). However, consistent with the emergence of a pro-nociceptive high-affinity GAR, THIP increased the VMR in DSS-treated mice. VMR data calculated as a percent change from baseline were analyzed with a two-way ANOVA (sex x inflammation). Results of this analysis confirmed the presence of a significant (F (1, 8) = 51.94, p < 0.01) effect of treatment, but no effect of sex (F (1, 8) = 0.04596, p > 0.05), nor a significant interaction between sex and treatment (F (1, 8) = 0.2556, p > 0.05).
Figure 4. Effect of THIP on VMR from vehicle-treated and DSS-treated mice.
A) Typical original recordings of VMR from vehicle-treated (Vehicle) and DSS-treated mice (DSS) in response to CRD-pressure of 70 mmHg before and after administration of THIP (100 μM). Time of CRD-pressure 10 s (indicated by the black line on top of the recordings). B) VMR data are shown as stimulus-response functions before and after THIP (100 μM). C) VMR data for individual mice are plotted before and after THIP (100 μM).
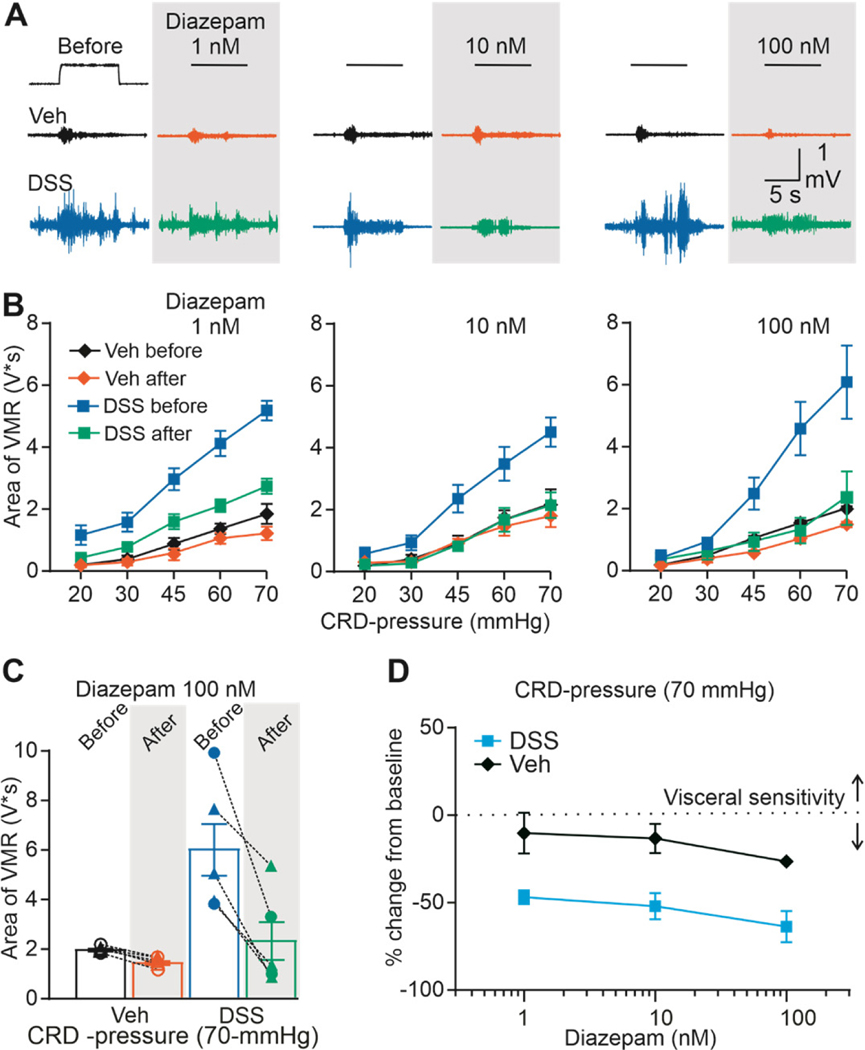
Diazepam inhibits visceral hypersensitivity in DSS-treated mice
With evidence of endogenous GAR signaling influencing the response to CRD in both control and DSS-treated mice, it was possible to further test the involvement of a low-affinity GAR in mediating the antinociceptive effects of GAR activation of the colon. We used a PAM for this purpose, because they potentiate the actions of endogenous GABA, and chose diazepam because it has preferential activity for low-affinity γ-subunit containing receptors [44].
The effect of diazepam (1, 10, and 100 nM) on the VMR was assessed in groups of DSS-treated (4–5 male and 1–2 female for each concentration) and control (3–5 male and 2–3 female for each concentration) mice. Diazepam 100 nM decreased the VMR in both control and DSS-treated mice. Typical examples of the effect of diazepam on VMR are shown in Figure 5A. Results of a three-way ANOVA revealed a significant influence of treatment (F (1, 80) = 35.13, p < 0.01), CRD-pressure (F (4, 80) = 27.61, p < 0.01), diazepam (F (1, 80) = 30.42, p < 0.01), and a significant interaction between treatment, CRD-pressure, and diazepam (100nM, F (4, 80) = 2.884, p = 0.028). Post hoc tests indicated that significant interactions were due to a preferential influence of diazepam on the VMR response to noxious distension pressures in DSS-treated mice (Fig. 5B). Variability in the response to diazepam (100 nM) in the inhibition of the VMR to 70 mmHg of distension is shown in Figure 5C. Despite the fact that the baseline response to CRD was smaller in the absence of inflammation, the relative impact of diazepam was significantly greater in DSS-treated mice (F (1, 32) = 29.89, p < 0.01), main effect of treatment, but no effect of diazepam concentration nor a significant interaction between treatment and concentration). This is most easily seen in data plotted as a percent change from baseline (Figure 5D), where the diazepam-induced decrease in VMR was larger in DSS-treated mice at every dose of diazepam tested.
Figure 5. Effect of diazepam on VMR from vehicle-treated and DSS-treated mice.
A) Typical original recordings of VMR from vehicle-treated (Vehicle) and DSS-treated mice (DSS) in response to CRD-pressure of 70 mmHg before and after administration of diazepam (1, 10,100 nM). Time of CRD-pressure 10 s (indicated by the black line on top of the recordings). B) VMR data are shown as stimulus-response functions before and after diazepam (1, 10,100 μM). C) VMR data for individual mice are plotted before and after diazepam 100 μM. D) Pool concentration-response data as percent from baseline.
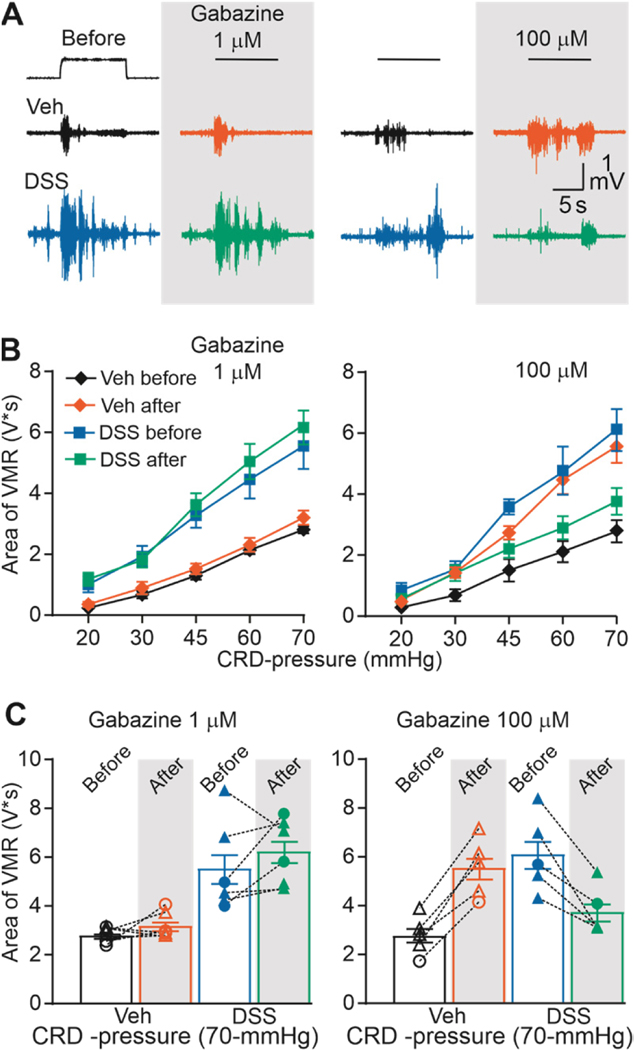
GABAA receptor antagonists reveal a pro-nociceptive role for endogenous GABA in DSS-treated mice
Our data with GABA agonists in DSS-treated mice combined with our previous data indicating that endogenous GAR activation contributes to the establishment of visceral nociceptive threshold [28], suggest that DSS-induced visceral hypersensitivity may be due, at least in part to the constitutive activation of a high-affinity GAR. If true, it should be possible to attenuate DSS-induced hypersensitivity with a high-affinity GAR antagonist. To test this possibility, we assessed the impact of intracolonic gabazine on the VMR in inflamed and non-inflamed mice.
Gabazine is considered a competitive antagonist of GAR subtypes [19]. However, gabazine is more potent against low-affinity than high-affinity GAR [31] and concentrations of less than 10 μM, has no effect on tonic high-affinity GAR currents [51]. Thus, to assess the relative contribution of high- and low-affinity GAR in DSS-induced hypersensitivity, 1 μM and 100 μM gabazine were used in different groups of DSS-treated (3–4 male and 1–3 female for each concentration) and control (4 male and 1–2 female for each concentration) mice.
There were marked differences in the response to gabazine that were dependent on concentration and DSS-treatment (Figure 6A and B). Indeed, analyzing VMR data with a three-way ANOVA (treatment x gabazine dose x CRD-pressure), revealed significant main effects of inflammation (F (1, 30) = 12.08, p < 0.01) and CRD-pressure (F (1, 30) = 6.92, p <0.01), and a significant interaction between all three parameters (F (4, 72) = 7.972, p < 0.01). Post-hoc analysis suggested that this was due to the fact that gabazine had no detectable influence on nociceptive threshold at a concentration of 1 μM, but at a concentration of 100 μM, drove changes in nociceptive threshold in opposite directions in control and DSS-treated mice. That is, gabazine increased visceral sensitivity in the absence of inflammation, consistent with the anti-nociceptive role of endogenous GAR signaling previously described in naïve animals. However, in DSS-treated mice, 100 μM gabazine was antinociceptive, consistent with the predicted emergence of pro-nociceptive GAR signaling in the colon (Figure 6B). Taken together, these data add additional support to the suggestion that inflammation of the colon results in a shift in the actions of a high-affinity GAR from inhibition to excitation such that this receptor contributes to inflammation-induced visceral hypersensitivity.
Figure 6. Effect of gabazine on VMR from vehicle-treated and DSS-treated mice.
A) Typical original recordings of VMR from vehicle-treated (Vehicle) and DSS-treated mice (DSS) in response to CRD-pressure of 70 mmHg before and after administration of gabazine (1 and 100 μM). Time of CRD-pressure 10 s (indicated by the black line on top of the recordings). B) VMR data are shown as stimulus-response functions before and after gabazine (1 and 100 μM C) VMR data for individual mice are plotted before and after gabazine 1 and 100 μM.
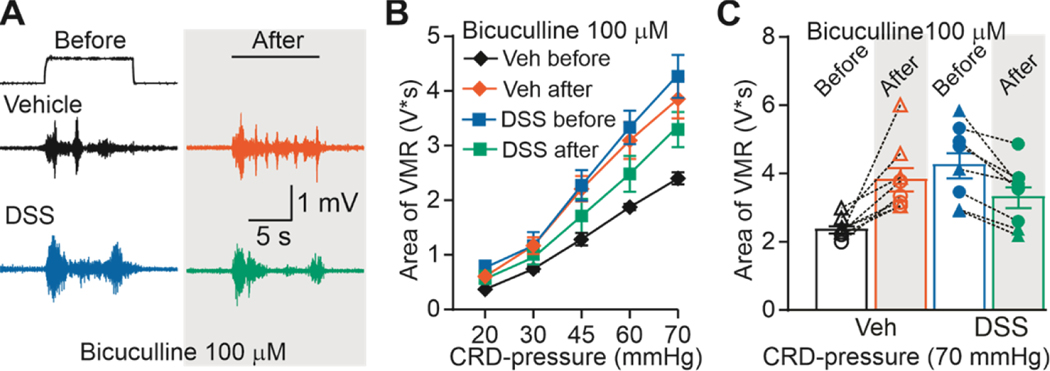
Bicuculline, another competitive GAR antagonist was used to further assess the contribution of pronociceptive GAR signaling to inflammation-induced visceral hypersensitivity. CRD-induced VMR was measured in additional groups of DSS-treated (4 male and 4 female) and control (4 male and 4 female) mice before and after intracolonic infusion of bicuculline (100 μM). The effects of bicuculline were similar to the effect of 100 μM gabazine (Figure 7A): VMR was increased in control animals but decreased in DSS-treated animals (Figure 7B). Thus, there were again significant main effects of treatment (F (1, 140) = 9.608, p < 0.01) and distension pressure (F (4, 140) = 100.5, p < 0.01), and a significant interaction between the two (F (1, 140) = 43.86, p < 0.01). Variability in the response to bicuculline can be seen in Figure 7C. These data are consistent with an inflammation-induced shift in the valence of endogenous high-affinity GAR signaling in the colon.
Figure 7. Effect of bicuculline on VMR from vehicle-treated and DSS-treated mice.
A) Typical original recordings of VMR from vehicle-treated (Vehicle) and DSS-treated mice (DSS) in response to CRD-pressure of 70 mmHg before and after administration of bicuculline (100 μM). Time of CRD-pressure 10 s (indicated by the black line on top of the recordings). B) VMR data are shown as stimulus-response functions before and after bicuculline (100 μM). C) VMR-data for individual mice are plotted before and after bicuculline (100 μM).
Discussion
The purpose of this study was to test the hypothesis that visceral hypersensitivity observed in the presence of persistent inflammation is due a decrease in GAR-mediated suppression of nociceptive signaling from the colon. The model of persistent inflammation used was DSS-induced colitis and consistent with previoius results with this model, DSS treatment was associated with weight loss, a decrease in colon length, an increase in colon permeability, an increase in spleen weight, and an increase in the response of CRD. Persistent inflammation was associated with a qualitative shift in the response to intracolonic muscimol administration, with the emergence of a muscimol-induced increase in the VMR in response to low concentrations (100 and 500 nM) of muscimol, that was not present in control mice. The response to THIP was also shifted in inflamed mice from supression to potentiation of the VMR. In contrast, persistent inflammation was associated with an increase in the antinocicetive efficacy of diazepam. There was no detectable response to gabazine at concentrations selective for low-affinity GARs (1 μM). But at a gabazine concentration that blocks both low- and high-affinity GAR (100 μM), the changes in VMR in inflammed and control mice were in opposite directions: the VMR was increased in control mice but decreased in inflamed mice. Comparable results were obtained with the non-selective GAR antagonist bicuculline. Taken together, these results suggest persistent inflammation-induced visceral hypersensitivity is not simply due to a loss of GAR inhibition, but the emergence of a high-affinity GAR-induced increase in nociception.
Our results with GAR agonists and diazepam suggest that there are at least two distinct GAR subtypes in the colon that influence visceral nociception. This includes at least one high-affinity GAR subtype, and at least one low-affinity GAR subtype. That is, while muscimol is generally considered a non-subtype selective GAR agonist [10; 26], it has an exceptionally high-affinity for δ subunit-containing GARs [39], a subunit commonly associated with high-affinity GARs. Thus, we suggest that the effects of low concentrations of muscimol were mediated by high-affinity receptors, while those associated with high concentrations of muscimol were mediated by low-affinity receptors. The hyperalgesia associated with THIP in DSS-treated mice is consistent with the emergence of a pro-nociceptive high-affinity GAR in the presence of persistent inflammation. Similarly, the observation that the PAM diazepam, thought to facilitate activity of γ subunit-containing low-affinity receptors [44], attenuated the VMR in both the presence and absence of inflammation, is consistent with the presence of a low-affinity GAR that retains antinociceptive efficacy. Taken together, these observations suggest that different GAR subtypes mediate the pro- and anti-nociceptive effects of GAR signaling in the colon. This has potentially important therapeutic implications as it suggests two different approaches to the treatment of visceral hypersensitivity, with a high-affinity GAR antagonist and a low-affinity GAR agonist. Although, most of the drugs used in this research are not currently used for treatment of IBD in humans, diazepam has been used clinically for the treatment of high-tone pelvic floor dysfunction [32; 41], a condition that is frequently comorbid with painful bladder syndrome [36].
Our GAR antagonist data not only add additional support the suggestion that there are at least two GAR subtypes in the colon involved in the regulation of nociceptive threshold, but that inflammatory hypersensitivity involves a shift in the valence of the high-affinity GAR from inhibition to excitation. That is, the absence of a detectable influence of 1 μM gabazine on the VMR regardless of the presence of inflammation, suggests that the low-affinity GAR, while present and functional in the colon as suggested by the agonist data, is not sufficiently engaged by endogenous GABA to play a significant role in regulating the response to CRD. Thus, the change in VMR associated with 100 μM gabazine appears to be due to the inhibition of a high-affinity GAR that suppresses nociception in the absence of inflammation, but contributes to inflammatory hypersensitivity. It is certainly possible that this shift reflects the emergence of a receptor on another cell type in the colon that dominates the inhibitory signaling detected in the absence of inflammation. However, based on inflammation-induced changes previously described in the spinal cord dorsal horn on the central terminals of nociceptive afferents [17], we suggest a more likely possibility is a change in the functional consequences of GAR activation of the same cell.
Two models have been proposed to account for the inflammation-induced change in GAR signaling. In one, there is a depolarizing shift in the Cl- equilibrium potential, secondary to an increase in activity of the Na+-K+-Cl-Co transporter, NKCC1 [2]. The second model involves an inflammation-induced decrease in a low threshold voltage-gated K+ current in the face of an already depolarized Cl- equilibrium potential [52; 53] coupled to an increase in GAR channel density [52]. The result of both models is the same, and that is an increase in the depolarizing drive associated with GAR activation, and the emergence of GAR mediated excitation of nociceptive afferents. Additional studies will be needed to identify the mechanism(s) responsible for the shift in GAR signaling in the colon. Nevertheless, the emergence of a pro-nociceptive role for GABA is consistent with the observation that GAR agonists potentiate nociceptive behavior evoked with formalin [5].
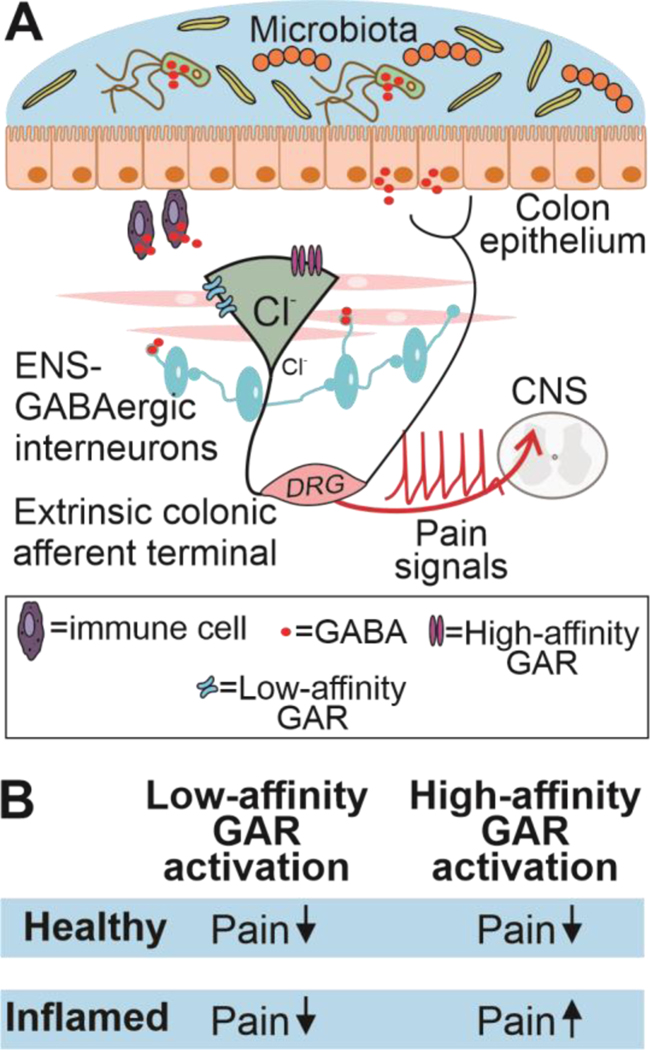
We originally hypothesized that visceral hypersensitivity would be due to a loss of GABAergic inhibition of colonic afferents. This could have been due to a decrease in GABA and/or a decrease in receptors. While our antagonist data argue against a decrease in GABA, an increase in GABA could contribute to the shift in the functional impact of high-affinity GAR activity. That is, if, as appears to be the case at the central terminals of nociceptive afferents [7; 17], and has been demonstrated at the sensory neuron cell body [2; 52], the intracellular Cl- concentration in the peripheral terminals of nociceptive afferents is also elevated, GAR activation of peripheral terminals of colonic afferents may normally drive membrane depolarization. An increase in GABA may result in sufficient GAR activation to increase the excitability of nociceptive afferents. In the GI tract, there are several sources of GABA, including enteric GABAergic neurons [15; 16; 25], epithelial cells [48], immune cells [3] and components of the gut microbiome [37], (see Figure 8A). With evidence of GABA synthetic machinery in the colon including GAD65 and GAD67 [30; 48], GABA transporters (GAT1–3, [13]) and GABA release [23] it is also possible that GABA is made locally from glutamate released from primary afferents as has been suggested in the CNS [40]. Interestingly, in the fecal retention model of visceral hypersensitivity, there is evidence that GABA expressing bacteria can be used to attenuate visceral hypersensitivity, at least as measured by changes in colonic DRG neuron excitability [37]. While the suggestion that it is possible to treat visceral pain with pro-biotics is intriguing, it will be important to determine the behavioral consequences of the increased colonic GABA concentration associated with the increase in GABA producing bacteria in the colon, as the decrease in colonic afferent excitability observed in vitro in this previous study may reflect a compensatory mechanism associated with an increase in GABA-induced activation of these neurons.
Figure 8. Schematic diagram of the proposed GAR mechanism during healthy conditions.
A) In the healthy colon endogenous GABA released from GABAergic enteric neurons, epithelial, immune cells or components of the gut microbita attenuate nociceptive pain via activation of both low- and high-GAR. B) Summary of our findings on visceral sensitivity during health and inflamed conditions.
As implied above, the most straight forward explanation for the impact of GAR activation on visceral sensitivity is that GAR is present on colonic afferents, enabling a direct modulation of their excitability [28] and the propagation of nociceptive input to central nervous system. This is consistent with the widespread distribution of GAR subunits among colonic afferents [20]. However, an indirect mechanism(s) cannot be ruled out either as these receptors are also present on a number of additional cell types in the colon [43], including immune cells [21], which may be a source of mediators ultimately responsible for the DSS-induced hypersensitivity.
In summary, our results suggest that there are at least two distinct GAR subtypes in the periphery that are contribute to the modulation of nociception. Importantly, while we and others have previously demonstrated that GARs are present and functional in the peripheral terminals of nociceptive afferents, this is the first study to demonstrate that peripheral GAR activation contributes to inflammatory hypersensitivity. Potentially more importantly, however, our results suggest that it may not only be possible to treat visceral pain with peripherally restricted low-affinity GAR agonists or PAMs, but with high-affinity GAR antagonists (Figure 8B). In this regard, it will be important to not only determine the GAR subunit(s) responsible for the divergent effects GAR subtypes, but their location.
Supplementary Material
Acknowledgements:
The authors acknowledge and thank Drs Gerald F. Gebhart and Bin Feng for their help in getting this project started, Dr. Jane Hartung and the rest of the Gold Lab for helpful comments in the preparation of the manuscript and Dr. Martha Canto-Bustos for help with figure design. Work was supported by a grant from the National Institutes of Health (R01 DK107966 (MSG)).
Abbreviations:
- (AUC)
Area under the curve
- (CRD)
Colorectal distension
- (DSS)
Dextran sodium sulfate
- (DMSO)
Dimethylsulfoxide
- (EB)
Evan Blue
- (GAR)
GABAA receptor
- (IBD)
Inflammatory bowel disease
- (PBS)
Phosphate buffered saline
- (PAM)
Positive allosteric modulator
- (THIP)
4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridine-3-ol
- (VMR)
Visceromotor response
Footnotes
Authors have no conflict of interest.
References
- [1].Aggarwal S, Ahuja V, Paul J. Attenuated GABAergic Signaling in Intestinal Epithelium Contributes to Pathogenesis of Ulcerative Colitis. Dig Dis Sci 2017;62(10):2768–2779. [DOI] [PubMed] [Google Scholar]
- [2].Anseloni VC, Gold MS. Inflammation-induced shift in the valence of spinal GABA-A receptor-mediated modulation of nociception in the adult rat. J Pain 2008;9(8):732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW, Steinman L. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci U S A 2010;107(6):2580–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bielefeldt K, Davis B, Binion DG. Pain and inflammatory bowel disease. Inflammatory bowel diseases 2009;15(5):778–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bravo-Hernandez M, Feria-Morales LA, Torres-Lopez JE, Cervantes-Duran C, Delgado-Lezama R, Granados-Soto V, Rocha-Gonzalez HI. Evidence for the participation of peripheral alpha5 subunit-containing GABAA receptors in GABAA agonists-induced nociception in rats. Eur J Pharmacol 2014;734:91–97. [DOI] [PubMed] [Google Scholar]
- [6].Carlton SM, Zhou S, Coggeshall RE. Peripheral GABA(A) receptors: evidence for peripheral primary afferent depolarization. Neuroscience 1999;93(2):713–722. [DOI] [PubMed] [Google Scholar]
- [7].Chen JT, Guo D, Campanelli D, Frattini F, Mayer F, Zhou L, Kuner R, Heppenstall PA, Knipper M, Hu J. Presynaptic GABAergic inhibition regulated by BDNF contributes to neuropathic pain induction. Nature communications 2014;5:5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen L, Zhou Z, Yang Y, Chen N, Xiang H. Therapeutic effect of imiquimod on dextran sulfate sodium-induced ulcerative colitis in mice. PLoS One 2017;12(10):e0186138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of Inflammatory Bowel Disease Among Adults Aged >/=18 Years - United States, 2015. MMWR Morb Mortal Wkly Rep 2016;65(42):1166–1169. [DOI] [PubMed] [Google Scholar]
- [10].DeFeudis FV. Physiological and behavioral studies with muscimol. Neurochem Res 1980;5(10):1047–1068. [DOI] [PubMed] [Google Scholar]
- [11].Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Büchler MW. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion 2000;62(4):240–248. [DOI] [PubMed] [Google Scholar]
- [12].Farthing MJ, Lennard-jones JE. Sensibility of the rectum to distension and the anorectal distension reflex in ulcerative colitis. Gut 1978;19(1):64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fletcher EL, Clark MJ, Furness JB. Neuronal and glial localization of GABA transporter immunoreactivity in the myenteric plexus. Cell Tissue Res 2002;308(3):339–346. [DOI] [PubMed] [Google Scholar]
- [14].Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med 2010;16(11):1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Grider JR. Regulation of excitatory neural input to longitudinal intestinal muscle by myenteric interneurons. Am J Physiol 1998;275(5 Pt 1):G973–978. [DOI] [PubMed] [Google Scholar]
- [16].Grider JR, Makhlouf GM. Enteric GABA: mode of action and role in the regulation of the peristaltic reflex. Am J Physiol 1992;262(4 Pt 1):G690–694. [DOI] [PubMed] [Google Scholar]
- [17].Guo D, Hu J. Spinal presynaptic inhibition in pain control. Neuroscience 2014;283:95–106. [DOI] [PubMed] [Google Scholar]
- [18].Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg 2002;94(2):313–318, table of contents. [DOI] [PubMed] [Google Scholar]
- [19].Heaulme M, Chambon JP, Leyris R, Molimard JC, Wermuth CG, Biziere K. Biochemical characterization of the interaction of three pyridazinyl-GABA derivatives with the GABAA receptor site. Brain Res 1986;384(2):224–231. [DOI] [PubMed] [Google Scholar]
- [20].Hockley JRF, Taylor TS, Callejo G, Wilbrey AL, Gutteridge A, Bach K, Winchester WJ, Bulmer DC, McMurray G, Smith ESJ. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 2019;68(4):633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jin Z, Mendu SK, Birnir B. GABA is an effective immunomodulatory molecule. Amino acids 2013;45(1):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kalra J, Lingaraju MC, Mathesh K, Kumar D, Parida S, Singh TU, Sharma AK, Kumar D, Tandan SK. Betulinic acid alleviates dextran sulfate sodium-induced colitis and visceral pain in mice. Naunyn Schmiedebergs Arch Pharmacol 2018;391(3):285–297. [DOI] [PubMed] [Google Scholar]
- [23].Kerr DI, Krantis A. Uptake and stimulus-evoked release of [3H]-gamma-aminobutyric acid by myenteric nerves of guinea-pig intestine. Br J Pharmacol 1983;78(2):271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kogure Y, Kanda H, Wang S, Hao Y, Li J, Yamamoto S, Noguchi K, Dai Y. Daikenchuto attenuates visceral pain and suppresses eosinophil infiltration in inflammatory bowel disease in murine models. JGH open : an open access journal of gastroenterology and hepatology 2020;4(6):1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Krantis A, Tufts K, Nichols K, Morris GP. [3H]GABA uptake and GABA localization in mucosal endocrine cells of the rat stomach and colon. Journal of the autonomic nervous system 1994;47(3):225–232. [DOI] [PubMed] [Google Scholar]
- [26].Krogsgaard-Larsen P, Hjeds H, Curtis DR, Lodge D, Johnston GA. Dihydromuscimol, thiomuscimol and related heterocyclic compounds as GABA analogues. J Neurochem 1979;32(6):1717–1724. [DOI] [PubMed] [Google Scholar]
- [27].Lamb K, Zhong F, Gebhart GF, Bielefeldt K. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am J Physiol Gastrointest Liver Physiol 2006;290(3):G451–457. [DOI] [PubMed] [Google Scholar]
- [28].Loeza-Alcocer E, McPherson TP, Gold MS. Peripheral GABA receptors regulate colonic afferent excitability and visceral nociception. J Physiol 2019;597(13):3425–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: General considerations. Experientia 1986;42(2):109–114. [DOI] [PubMed] [Google Scholar]
- [30].Miki Y, Taniyama K, Tanaka C, Tobe T. GABA, glutamic acid decarboxylase, and GABA transaminase levels in the myenteric plexus in the intestine of humans and other mammals. J Neurochem 1983;40(3):861–865. [DOI] [PubMed] [Google Scholar]
- [31].Mortensen M, Iqbal F, Pandurangan AP, Hannan S, Huckvale R, Topf M, Baker JR, Smart TG. Photo-antagonism of the GABAA receptor. Nature communications 2014;5:4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Murina F, Felice R, Di Francesco S, Oneda S. Vaginal diazepam plus transcutaneous electrical nerve stimulation to treat vestibulodynia: A randomized controlled trial. Eur J Obstet Gynecol Reprod Biol 2018;228:148–153. [DOI] [PubMed] [Google Scholar]
- [33].Najjar SA, Ejoh LL, Loeza-Alcocer E, Edwards BS, Smith-Edwards KM, Epouhe AY, Gold MS, Davis BM, Albers KM. Optogenetic inhibition of the colon epithelium reduces hypersensitivity in a mouse model of inflammatory bowel disease. Pain 2021;162(4):1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017;390(10114):2769–2778. [DOI] [PubMed] [Google Scholar]
- [35].Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990;98(3):694–702. [DOI] [PubMed] [Google Scholar]
- [36].Peters KM, Carrico DJ, Kalinowski SE, Ibrahim IA, Diokno AC. Prevalence of pelvic floor dysfunction in patients with interstitial cystitis. Urology 2007;70(1):16–18. [DOI] [PubMed] [Google Scholar]
- [37].Pokusaeva K, Johnson C, Luk B, Uribe G, Fu Y, Oezguen N, Matsunami RK, Lugo M, Major A, Mori-Akiyama Y, Hollister EB, Dann SM, Shi XZ, Engler DA, Savidge T, Versalovic J. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol Motil 2017;29(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res 2007;140(1):12–19. [DOI] [PubMed] [Google Scholar]
- [39].Quirk K, Whiting PJ, Ragan CI, McKernan RM. Characterisation of delta-subunit containing GABAA receptors from rat brain. Eur J Pharmacol 1995;290(3):175–181. [DOI] [PubMed] [Google Scholar]
- [40].Roberts E, Frankel S. gamma-Aminobutyric acid in brain: its formation from glutamic acid. J Biol Chem 1950;187(1):55–63. [PubMed] [Google Scholar]
- [41].Rogalski MJ, Kellogg-Spadt S, Hoffmann AR, Fariello JY, Whitmore KE. Retrospective chart review of vaginal diazepam suppository use in high-tone pelvic floor dysfunction. Int Urogynecol J 2010;21(7):895–899. [DOI] [PubMed] [Google Scholar]
- [42].Scanzi J, Accarie A, Muller E, Pereira B, Aissouni Y, Goutte M, Joubert-Zakeyh J, Picard E, Boudieu L, Mallet C, Gelot A, Ardid D, Carvalho FA, Dapoigny M. Colonic overexpression of the T-type calcium channel Ca(v) 3.2 in a mouse model of visceral hypersensitivity and in irritable bowel syndrome patients. Neurogastroenterol Motil 2016;28(11):1632–1640. [DOI] [PubMed] [Google Scholar]
- [43].Seifi M, Brown JF, Mills J, Bhandari P, Belelli D, Lambert JJ, Rudolph U, Swinny JD. Molecular and functional diversity of GABA-A receptors in the enteric nervous system of the mouse colon. J Neurosci 2014;34(31):10361–10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sieghart W, Savić MM. International Union of Basic and Clinical Pharmacology. CVI: GABA(A) Receptor Subtype- and Function-selective Ligands: Key Issues in Translation to Humans. Pharmacol Rev 2018;70(4):836–878. [DOI] [PubMed] [Google Scholar]
- [45].Soma LR. Anesthetic and analgesic considerations in the experimental animal. Ann N Y Acad Sci 1983;406:32–47. [DOI] [PubMed] [Google Scholar]
- [46].Topf N, Jenkins A, Baron N, Harrison NL. Effects of isoflurane on gamma-aminobutyric acid type A receptors activated by full and partial agonists. Anesthesiology 2003;98(2):306–311. [DOI] [PubMed] [Google Scholar]
- [47].Vergnolle N. Visceral afferents: what role in post-inflammatory pain? Autonomic neuroscience : basic & clinical 2010;153(1–2):79–83. [DOI] [PubMed] [Google Scholar]
- [48].Wang FY, Zhu RM, Maemura K, Hirata I, Katsu K, Watanabe M. Expression of gamma-aminobutyric acid and glutamic acid decarboxylases in rat descending colon and their relation to epithelial differentiation. Chinese journal of digestive diseases 2006;7(2):103–108. [DOI] [PubMed] [Google Scholar]
- [49].Willis WD Jr. Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res 1999;124(4):395–421. [DOI] [PubMed] [Google Scholar]
- [50].Xu F, Liu Y, Wheaton AG, Rabarison KM, Croft JB. Trends and Factors Associated with Hospitalization Costs for Inflammatory Bowel Disease in the United States. Applied health economics and health policy 2019;17(1):77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA. Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol 2003;63(1):2–8. [DOI] [PubMed] [Google Scholar]
- [52].Zhu Y, Dua S, Gold MS. Inflammation-induced shift in spinal GABA(A) signaling is associated with a tyrosine kinase-dependent increase in GABA(A) current density in nociceptive afferents. J Neurophysiol 2012;108(9):2581–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhu Y, Lu SG, Gold MS. Persistent inflammation increases GABA-induced depolarization of rat cutaneous dorsal root ganglion neurons in vitro. Neuroscience 2012;220:330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.