Abstract
For centuries, species of the genus Consolida (Ranunculaceae) have been extensively utilized for their extremely high ornamental and medicinal values. Phytochemical investigations of Consolida species have revealed the presence of multiple active ingredients, including diterpenoid alkaloids, flavonoids, phenolic acids, phytosterols, fatty acids, and volatile constituents. These chemical constituents are of great research significance due to their novel structures and broad biological activities. This review addresses, for the first time, the chemical constituents of Consolida plants and the biological activities of these compounds to facilitate future research.
Multiple constituents from Consolida species showed great research significance for their novel structures and broad bioactivities.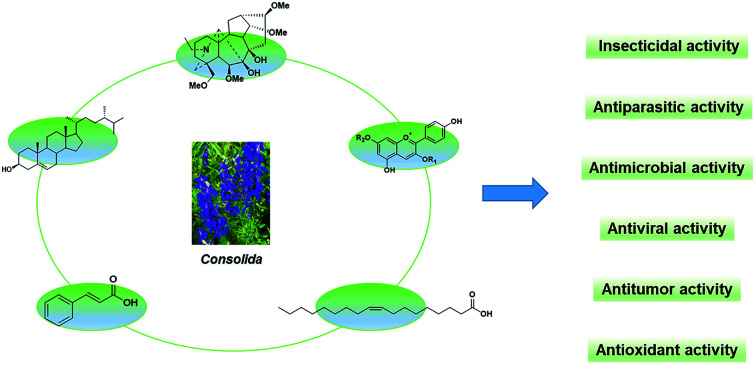
1. Introduction
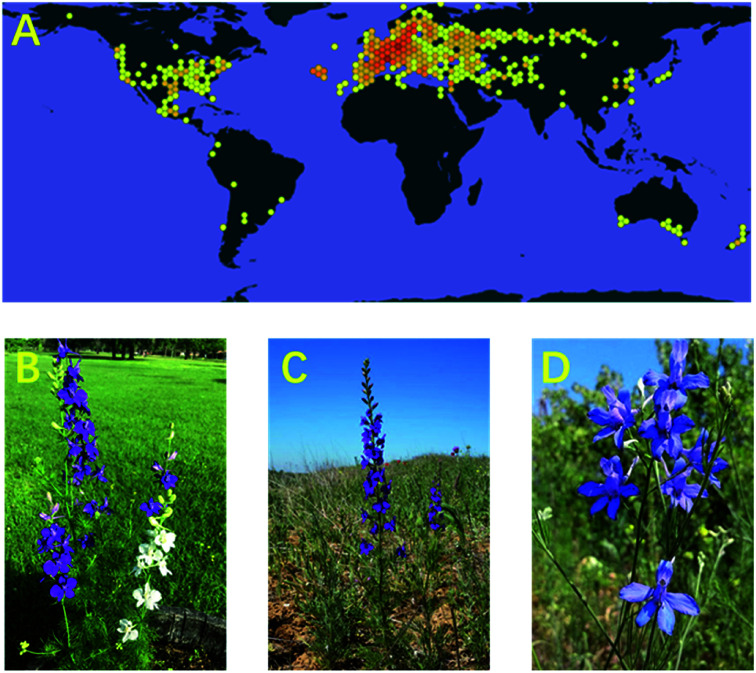
The genus Consolida, a highly specialized genus of Ranunculaceae, is composed of approximately 50 species. Consolida plants are mainly distributed in drought regions in southern Europe, northern Africa, and western Asia, with a centre of diversity and speciation in Anatolia, as at least 29 Consolida species have been found in this region (Fig. 1A).1,2Consolida plants have adapted to the seasonal drought climate and often grow on dry stony slopes in steppes, semideserts, and even deserts. In addition, some of its representatives, such as C. ambigua (formerly known as D. ajacis) (Fig. 1B), have been widely cultivated in bonsai pots, gardens, and greenbelts around the world. Plants of the Consolida genus are morphologically very similar to those of Delphinium and are frequently mistaken. In fact, the Consolida genus has been treated as a phytogroup in the genus Delphinium for many years and was even given the same trivial name larkspur. However, in 1821, Gray raised Consolida to the species level, and now in most cases, Consolida is regarded as a different genus from the genus Delphinium.3 Generally, Consolida plants are annual herbals approximately 10–60 cm in height, possessing single petals and single follicles that distinguish them from Delphinium plants.4,5
Fig. 1. (A) The global distributions of plants from the genus Consolida (https://www.gbif.org/species/3033827); (B) C. ambigua, created by latormentanegra (https://www.inaturalist.org/observations/40694233); (C) C. regalis, created by Anastasiya Ishkova (https://www.inaturalist.org/observations/45139852); (D) C. orientalis, created by Sergei (https://www.inaturalist.org/observations/46362639).
Plants from the genus Consolida have received considerable interest due to their extremely high ornamental and medicinal values. Consolida plants feature showy purple petals, which have been widely cultivated for centuries not only as fresh and dried flowers but also as seasonal outdoor flowers. Some species of Consolida, such as C. ambigua, C. regalis (D. consolida) (Fig. 1C), and C. orientalis (Fig. 1D), have become some of the most famous and popular horticultural plants around the world, especially in Europe and America. In addition to ornamental plants, Consolida plants are also of great medicinal value. In Turkey, China, and some other countries and regions, especially the Mediterranean and western Asia, various Consolida species have been extensively employed as herbal medicines for hundreds of years to treat multiple kinds of diseases, such as traumatic injury, rheumatism, sciatica, enteritis, stomach ache, ringworm, scabies and other skin diseases.6,7 In addition, Consolida plants can also be used externally against body lice.8 Generally, the medicinal uses of Consolida plants are similar to plants from its highly related genus Delphinium, as they are similar in chemical composition.
The chemical constituents of Consolida plants have been investigated since the beginning of the 20th century. These earlier studies attempted to isolate and identify the alkaloidal and pigmental compositions of several widespread Consolida species, such as C. ambigua and C. regalis. In 1914, Keller and Voelker first reported the isolation of two diterpenoid alkaloids (DAs), ajacine and ajaconine, from the seeds of C. ambigua.9 The first anthocyanin, delphinin, was identified from the petals of C. regalis by Mieg in 1915.10 The DAs and flavonoids of Consolida plants have attracted considerable attention for a long period of time, and many phytochemical investigations have been devoted to them. In addition, a series of studies performed by using high-performance liquid chromatography (HPLC), gas chromatography (GC) or their combination with mass spectrometry (MS) techniques revealed that a large number of other chemical components, such as phenolic acids, phytosterols, fatty acids (FAs) and other volatile constituents, exist in Consolida plants. The constituents of Consolida plants have exhibited a high diversity of chemical structures and biological activities, and these constituents can serve as a potential medicinal resource for drug discovery.
Several already published review articles and monographs have involved the DAs from Consolida.11–13 However, to date, there has been no individual and systematic review of the chemical constituents in the genus Consolida in addition to their biological activities. Hence, this review has been prepared to summarize the structural features and biological activities of the chemical constituents in the genus Consolida for the first time. The aim of this review is to provide a complete overview on the existing knowledge of the chemical constituents and biological properties of plant species from Consolida, which will facilitate further research and exploitation of this genus.
2. Chemical constituents
To date, investigations on the chemical constituents of Consolida plants have led to the isolation and identification of approximately 143 distinct compounds, including 126 alkaloids and 17 flavonoids. In addition, phenolic acids, phytosterols, and FAs of several Consolida species have been investigated by using HPLC, GC, and MS methods. Herein, the studied chemical constituents of Consolida plants are summarized by category.
2.1. Alkaloids
In addition to the Aconitum and Delphinium genera, Consolida is another genus in the Ranunculaceae family that is well known for its characteristic DA components.11,14 DAs are unambiguously the most predominant and representative constituents of Consolida plants and have attracted much research interest since the beginning of the 20th century.9 However, studies on the DA composition of Consolida plants increased only in the 1980s due to the difficulty associated with the structural identification of DAs, which possess a fused polycyclic skeleton substituted with multiple oxygenated groups. To date, a total of 121 DAs (1–121) along with five other alkaloids (122–126) have been isolated from Consolida species. Table 1 lists the names, types, corresponding plant sources and references of alkaloids isolated and identified from Consolida species.
Alkaloids from Consolida plants.
| Class and name (no.) | Type | Species | Ref. |
|---|---|---|---|
| C 18 -DAs | |||
| Hohenackeridine (1*) | I | C. hohenackeri | 20 |
| 14-O-Demethyldelboxine (2*) | I | C. orientalis | 21 |
| 14-Demethyltuguaconitine (3) | I | C. orientalis | 26 |
| Tuguaconitine (4) | I | C. orientalis | 21 |
| Lapaconidine (5) | II | C. scleroclada | 27 |
| C 19 -DAs | |||
| Pubescenine (6*) | III | C. pubescens, C. oliveriana, C. orientalis | 8, 21 and 28 |
| Hoheconsoline (7*) | III | C. hohenackeri | 29 |
| Consolinine (8*) | III | C. hohenackeri | 29 |
| Raveyine (8-O-methylcolumbianine, 9*) | III | C. raveyi, C. oliveriana | 23 and 30 |
| Regaline (10*) | III | C. regalis | 31 |
| Bicolorine (11) | III | C. regalis, C. hohenackeri | 31 and 32 |
| Senbusine B (12) | III | C. anthoroidea | 33 |
| Neoline (13) | III | C. thirkeana | 34 |
| 14-O-Benzoylneoline (14) | III | C. thirkeana | 34 |
| Leucanthumsine C (15) | III | C. thirkeana | 34 |
| Neolinine (16) | III | C. sulphurea | 34 |
| Aconitine (17) | III | C. scleroclada | 27 |
| Delphisine (18) | III | C. ambigua | 35 |
| Ajadelphinine (19*) | III | C. ambigua, C. orientalis, C. armeniaca, C. stenocarpa | 21, 35–37 |
| Corepanine (20*) | IV | C. regalis | 31 |
| Hohenackerine (21*) | IV | C. hohenackeri | 32 |
| Tortumine (22*) | IV | C. hohenackeri | 32 |
| Delcorine (23) | IV | C. regalis, C. hohenackeri | 31 and 32 |
| Deoxydelcorine (24) | IV | C. regalis | 31 |
| Dehyrodelcorine (25) | IV | C. regalis, C. hohenackeri | 31 and 32 |
| Delcoridine (26) | IV | C. regalis | 31 |
| Didehydrodelsoline (27) | IV | C. orientalis | 21 |
| Deltaline (28) | IV | C. ambigua | 38 |
| Delpheline (29) | IV | C. ambigua | 22 |
| Ajacusine (30*) | IV | C. ambigua | 39 |
| Ajadine (31*) | IV | C. ambigua, C. orientalis | 21 and 39 |
| 14-Deacetylajadine (32*) | IV | C. ambigua, C. orientalis | 21 and 40 |
| Ajadinine (33*) | IV | C. ambigua | 24 |
| 19-Oxoanthranoyllycotonine (34*) | IV | C. ambigua | 22 |
| Ajacisine A (35*) | IV | C. ambigua | 41 |
| Ajacisine B (36*) | IV | C. ambigua | 41 |
| Ajacisine C (37*) | IV | C. ambigua | 41 |
| Ajacisine D (38*) | IV | C. ambigua | 41 |
| Ajacisine E (39*) | IV | C. ambigua | 41 |
| Delajacine (conambine, 40*) | IV | C. ambigua | 38 and 42 |
| Delajacirine (41*) | IV | C. ambigua | 38 |
| Delajadine (42*) | IV | C. ambigua | 38 |
| Ajanine (43*) | IV | C. ambigua | 38 |
| Ajacine (44) | IV | C. ambigua, C. orientalis | 21 and 39 |
| Anthranoyllycoctonine (45) | IV | C. ambigua, C. oliveriana | 23 and 39 |
| Delectine (46) | IV | C. ambigua | 22 |
| Isodelectine (47) | IV | C. ambigua | 41 |
| Methyllycaconitine (48) | IV | C. thirkeana, C. axilliflora, C. ambigua | 34, 39 and 43 |
| 18-Hydroxy-14-O-methylgadesine (49*) | IV | C. orientalis, C. oliveriana | 23 and 44 |
| 14-O-Acetyl-8-O-methylconsolarine (50) | IV | C. orientalis | 21 |
| 18-Demethylpubescenine (51*) | IV | C. orientalis | 26 |
| Dehydrodeltatsine (52*) | IV | C. orientalis | 45 |
| 14-O-Acetyltakaosamine (53*) | IV | C. orientalis | 45 |
| 1-O-Demethyltricornine (54*) | IV | C. orientalis | 21 |
| 14-O-Benzoyltakaosamine (55*) | IV | C. orientalis | 21 |
| 1-O,19-Didehydrotakaosamine (56*) | IV | C. orientalis | 21 |
| 8-O-Methylconsolarine (14-deacetyl-18-demethylpubescenine, 57*) | IV | C. orientalis | 21 and 46 |
| 14-O-Deacetylpubescenine (58*) | IV | C. orientalis, C. oliveriana | 21 and 23 |
| 18-O-Benzoyl-14-O-deacetyl-18-O-demethylpubescenine (59*) | IV | C. orientalis | 21 |
| 18-Methoxygadesine (60*) | IV | C. orientalis, C. ambigua | 35 and 47 |
| Consolidine (61*) | IV | C. oliveriana | 8 |
| Olivimine (62*) | IV | C. oliveriana | 23 |
| Olividine (63*) | IV | C. oliveriana | 23 |
| 1-Demethylwinkleridine (64*) | IV | C. hohenackeri, C. anthoroidea | 20 and 33 |
| 18-Demethyl-14-deacetylpubescenine (65*) | IV | C. hohenackeri | 20 |
| 14,18-Di-benzoyldelcosine (66*) | IV | C. rugulosa | 48 |
| 14-Acetyl-18-benzoyldelcosine (67*) | IV | C. rugulosa | 48 |
| Ambiguine (68*) | IV | C. ambigua | 49 |
| 14-Acetylbrowniine (69*) | IV | C. ambigua | 39 |
| Ajadelphine (70*) | IV | C. ambigua | 35 |
| 19-Oxodelphatine (71*) | IV | C. ambigua, C. oliveriana | 22 and 23 |
| Paniculatine (72*) | IV | C. regalis | 31 |
| Paniculine (73*) | IV | C. regalis | 31 |
| Consolarine (74*) | IV | C. armeniaca | 36 |
| Gigactonine (75) | IV | C. orientalis, C. ambigua, C. sulphurea, C. regalis, C. oliveriana | 8, 26, 34, 35 and 50 |
| Delcosine (76) | IV | C. orientalis, C. scleroclada, C. oliveriana, C. regalis, C. ambigua | 23, 26, 27, 39 and 50 |
| Delbonine (77) | IV | C. orientalis | 45 |
| Deltatsine (78) | IV | C. orientalis, C. ambigua, | 22 and 45 |
| Delsoline (79) | IV | C. oliveriana, C. orientalis, C. regalis, C. ambigua | 8, 39, 47, 48 and 50 |
| Lycoctonine (80) | IV | C. oliveriana, C. axilliflora, C. armeniaca, C. orientalis, C. ambigua, C. hohenackeri, C. regalis | 21, 23, 32, 36, 39, 43 and 50 |
| Takaosamine (81) | IV | C. orientalis, C. oliveriana, C. regalis, C. ambigua, C. axilliflora | 22, 23, 26, 43 and 50 |
| Delphatine (82) | IV | C. olopetala, C. oliveriana, C. ambigua | 6, 23 and 39 |
| Delcaroline (83) | IV | C. olopetala | 6 |
| Browniine (84) | IV | C. olopetala, C. oliveriana, C. sulphurea, C. ambigua, C. orientalis | 6, 21, 23, 34 and 39 |
| 14-Deacetylnudicaulidine (85) | IV | C. sulphurea | 34 |
| 14-Benzoyldelcosine (86) | IV | C. rugulosa | 48 |
| 14-Acetyldelcosine (87) | IV | C. rugulosa, C. ambigua, C. orientalis | 21, 39 and 48 |
| Potanine (88) | IV | C. orientalis | 21 |
| 14-Deacetylambiguine (89) | IV | C. ambigua | 22 |
| Delectinine (90) | IV | C. hohenackeri, C. axilliflora | 32 and 43 |
| 14-O-Acetyldelectinine (91) | IV | C. orientalis | 21 |
| C 20 -DAs | |||
| Azitine (92*) | V | C. hellespontica, C. raveyi | 25 |
| Chellespontine (93*) | V | C. hellespontica, C. raveyi | 25 and 30 |
| Consorientaline (94*) | V | C. orientalis | 51 |
| Dihydroajaconine (95*) | V | C. ambigua, C. orientalis, C. oliveriana | 23, 46 and 49 |
| Spiratine A (96) | V | C. anthoroidea | 33 |
| Atisine (97) | V | C. regalis, C. anthoroidea | 33 and 50 |
| Isoatisine (98) | V | C. raveyi | 30 |
| Ajaconine (99) | V | C. anthoroidea, C. oliveriana, C. hohenackeri, C. ambigua, C. raveyi, C. axilliflora | 8, 30, 32, 33, 39 and 43 |
| 11,13-O-Diacetyl-9-deoxyglanduline (100*) | VI | C. glandulosa | 52 |
| 13-O-Acetyl-9-deoxyglanduline (101*) | VI | C. glandulosa | 52 |
| 14-O-Acetyl-9-deoxyglanduline (102*) | VI | C. glandulosa | 52 |
| 13-O-Acetyl-glanduline (103*) | VI | C. glandulosa | 52 |
| Glanduline (104*) | VI | C. glandulosa | 52 |
| 9-Deoxyglanduline (105*) | VI | C. glandulosa | 53 |
| Glandulosine (106*) | VI | C. glandulosa | 53 |
| 11,13-O-Diacetylglanduline (107*) | VI | C. glandulosa | 53 |
| 9-O-Acetylglanduline (108*) | VI | C. glandulosa | 53 |
| 7α-Hydroxycossonidine (109*) | VI | C. oliveriana | 23 |
| 13-O-Acetylvakhmatine (110*) | VI | C. ambigua | 54 |
| Vakhmatine (111) | VI | C. ambigua | 54 |
| Hetisine (112) | VI | C. olopetala, C. anthoroidea, C. stenocarpa, C. axilliflora | 6, 33, 37 and 43 |
| 13-O-Acetylhetisine (113) | VI | C. anthoroidea | 33 |
| Septentriosine (114) | VI | C. anthoroidea | 33 |
| Hetisinone (115) | VI | C. regalis, C. stenocarpa | 37 and 50 |
| Leptanine (116*) | VI | C. leptocarpum | 19 |
| Stenocarpine (117*) | VII | C. stenocarpa | 55 |
| Willipelletierine (118*) | VII | C. scleroclada | 27 |
| Ajabicine (119*) | IX | C. ambigua | 18 |
| Dehydronapelline (120) | X | C. olopetala | 6 |
| 12-Epidehydronapelline (121) | X | C. olopetala | 6 |
| Other alkaloids | |||
| β-Carboline (122) | C. ambigua | 41 | |
| Methyl-N-(3-carboxy-3-methylpropanoyl)anthranilate (123) | C. ambigua | 41 | |
| 2,4-Dihydroxy-1,4-benzoxazine-3-one 2-O-glucoside (124) | C. ambigua | 56 | |
| 2,4-Dihydroxy-1,4-benzoxazine-3-one (125) | C. ambigua | 56 | |
| Benzoxazolinone (126) | C. ambigua | 56 | |
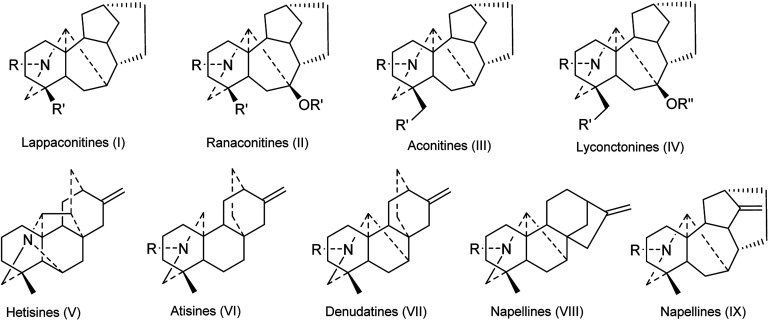
DAs are usually classified as C18-, C19-, C20- or bis-types, which can be further divided into several to dozens of subtypes.15,16 The DAs found in Consolida plants include 5 C18-DAs (1–5), 87 C19-DAs (5–91), and 29 C20-DAs (92–121). These alkaloids cover 9 subtypes of DAs, including the ranaconitine (I) and lappaconitine subtypes (II) of C18-DAs, the aconitine (III) and lycaconitine subtypes (IV) of C19-DAs, and the hetisine (V), atisine (VI), denudatine (VII), napelline (VIII), and other subtypes (IX) of C20-DAs (Fig. 2). In view of the chemical diversity, the lycaconitine-type C19-DAs contains the largest number of DAs in Consolida plants with 73 members, and they account for the largest proportion of isolated alkaloids (58%). The next largest subtypes are the hetisine-type C20-DAs with 17 members (13%) and the aconitine-type C19-DAs with 12 members (9%). Clearly, the lycaconitine-type C19-DAs are the most characteristic DA components of the genus Consolida, which is similar to its highly related genus Delphinium. In contrast, the large number of aconitine-type C19-DAs distinguishes Consolida from the genus Aconitum.17
Fig. 2. The subtypes of DAs from Consolida plants.
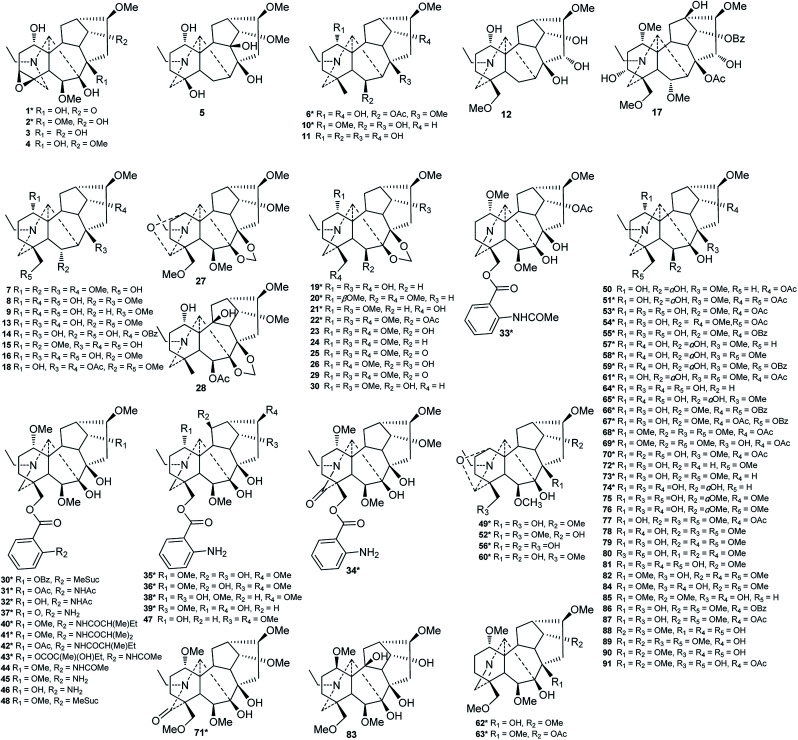
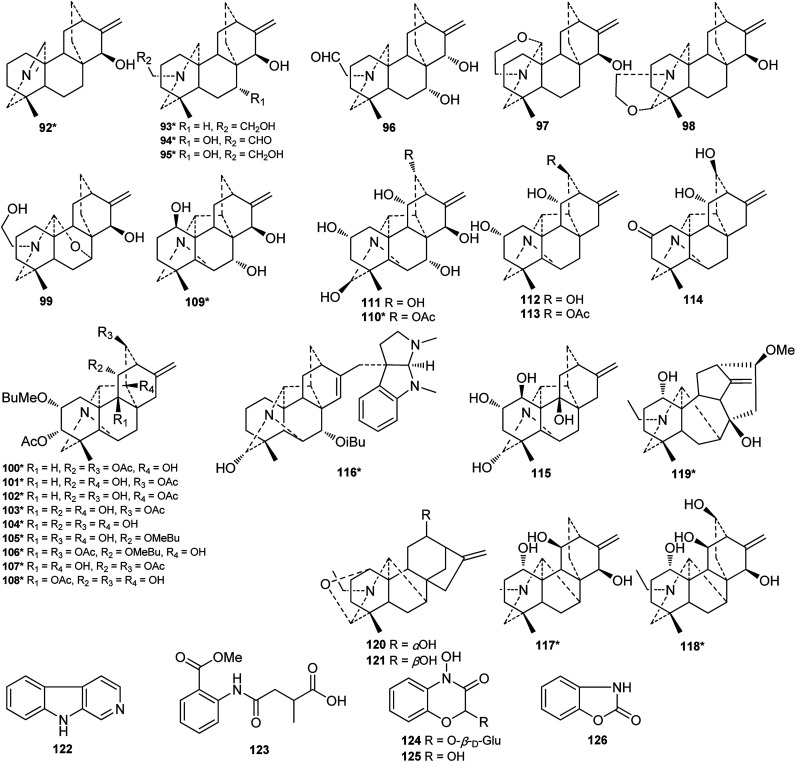
Of the 122 DAs presented in Consolida plants (Fig. 3 and 4), 69 were isolated as new compounds (labeled with *). Among them, several of the new alkaloids possess novel DA skeletons. Ajabicine (119) from C. ambigua belongs to the infrequent actaline-type C20-DAs bearing a rare C-14 exocyclic olefin methylene group, which may be produced biogenetically by a Wagner–Meerwein rearrangement of a denudatine-type DA.12,18 Leptanine (116) from C. leptocarpum (D. leptocarpum) is a dimeric alkaloid consisting of a hetisine-type C20-DA part and an indolinopyrrole fragment. The indolinopyrrole fragment is bound to the hetisine-type C20-DA part through an α-directed (relative to the indoline core) C-17–C-3 bond according to an X-ray crystal structure analysis.19 In addition, several of the new alkaloids possess at least one uncommon substituent. For example, new C18-DAs 1 and 2 possess an uncommon 3,4-epoxide unit,20,21 and new alkaloids 35 and 71 have an N–C(19) O lactam group.22,23 New alkaloids 33, 62 and 63 possess an imine group at C-19,23,24 while alkaloid 92 has an imine group at C-17, a rare substituent position.25 The other new alkaloids mainly vary in the variety, quantity, position, and orientation of oxygenated substituents. The common oxygenated substituents found in DAs from Consolida plants include hydroxyl (OH), carbonyl ( O), methoxyl (OMe), methylenedioxy (OCH2O) groups and various ester groups, such as acetyl (Ac), 2-methylbutyryl (MeBu), benzoyl (Bz), and anthranoyl groups.
Fig. 3. Alkaloids 1–91 from Consolida plants.
Fig. 4. Alkaloids 92–126 from Consolida plants.
2.2. Flavonoids
Flavonoids, which are composed of C6–C3–C6 structural units biosynthesized from phenylalanine, are one of the most widespread types of natural products in the plant kingdom.61,62 The reported studies have revealed that a certain amount of flavonoids, including anthocyanin and flavanol glycosides, exist in Consolida plants, especially in their aerial parts.63
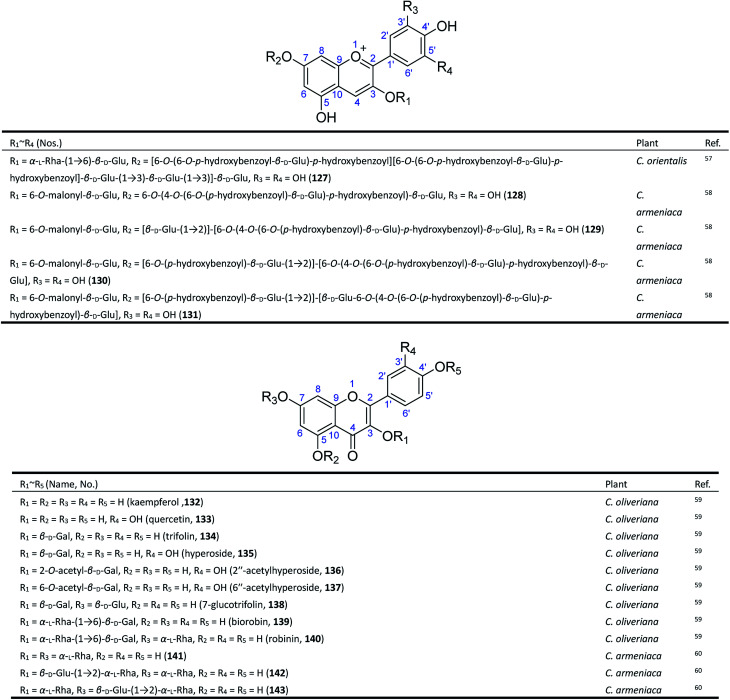
Anthocyanins are the major pigments of Consolida flowers, which are of interest to the food industry because of their antioxidant power, attractive colour, and stability in highly acidic foods.64,65 As early as 1915, Mieg isolated the first anthocyanin delphinin from the purple petals of C. regalis (D. consolida) and proposed its structure to be di-(p-hydroxybenzoyl)delphin,10 but the existence of a p-hydroxybenzoyl group was doubted by Harborne in 1964.66 Finally, in 1975, Asen revised its structure as delphinidin 3-di-(p-hydroxybenzoyl)-glucosylglucoside.67 The reported discrepancies of the major anthocyanins found in C. regalis flowers may be attributed to the use of different plant materials, since there are a number of C. regalis varieties that have been cultivated all over the world. It should be noted that these early studies did not establish the location of substitutes and the linkage of glucoses in the molecules of anthocyanins until 1985. Sulyok and Balint yielded an anthocyanin from C. orientalis and identified its structure as delphinidin-3-rutinoside-5-glucoside (127) (Fig. 5).57 More recently, in 1995, four new acylated delphinidin 3,7-glycosides (128–131) were isolated from the blue-violet flowers of C. armeniaca as major anthocyanin pigments.58
Fig. 5. Flavonoids from Consolida plants.
The flavanol glycosides in Consolida have also drawn attention from scientists. Twelve known flavonol glycosides (132–143) have been isolated from two Consolida species, C. oliveriana and C. armeniaca.59,60,68 These flavonols only possess common structures but have attracted considerable interest because of their extensive pharmacological activities, including antitumor, antitrypanosomatid, and antioxidant activities.
2.3. Phenolic acids
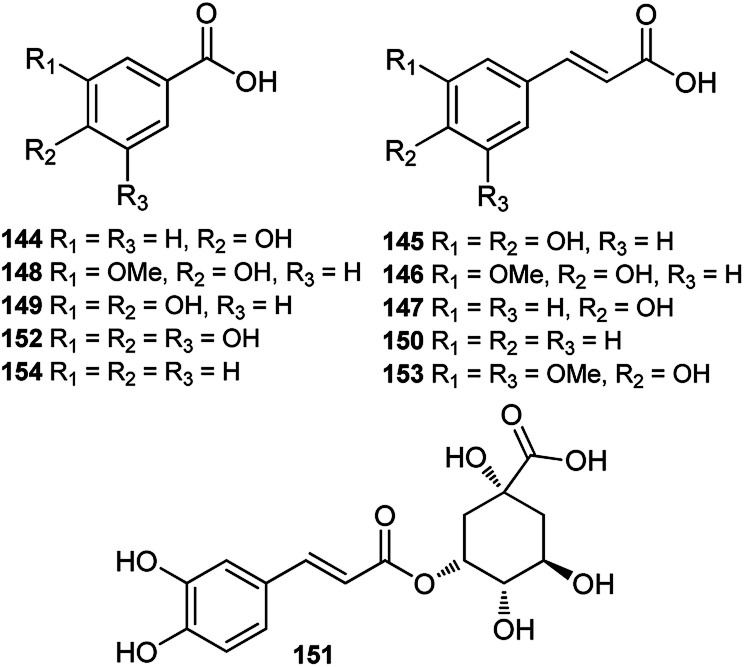
Until now, only a few studies on the phenolic acids of Consolida plants have been reported, and these studies were performed using HPLC or HPLC-MS techniques. A series of phenolic acids, mainly common phenylpropionic and benzoic acids, have been detected in the flowers of Consolida species (Fig. 6). For example, p-hydroxybenzoic (144), caffeic (145), ferulic (146) and p-coumaric (147) acids have been detected as the main phenolic compounds in C. armeniaca flowers,69 and protocatechuic (149), vanillic (148), cinnamic (150), chlorogenic (151), gallic (152), sinapic (153), and benzoic acids(154), in addition to acids 144–147, were identified in C. orientalis flowers.70,71
Fig. 6. Phenolic acids from Consolida plants.
2.4. Phytosterols
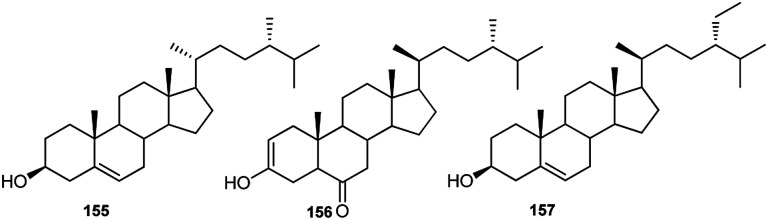
Although phytosterols are widely distributed in higher plants, little attention has been paid to Consolida phytosterols. To the best of our knowledge, the only investigation on Consolida phytosterols was performed by Waller et al. in 1981.72 In this study, 16 phytosterols were identified and quantified from the whole C. ambigua plant using GC-MS, and the study revealed that the major sterols in the C. ambigua plants were β-sitosterol (155), campesterol (156) and stigmasterol (157) (Fig. 7).
Fig. 7. Main phytosterols in Consolida species.
2.5. Fatty acids and essential oils
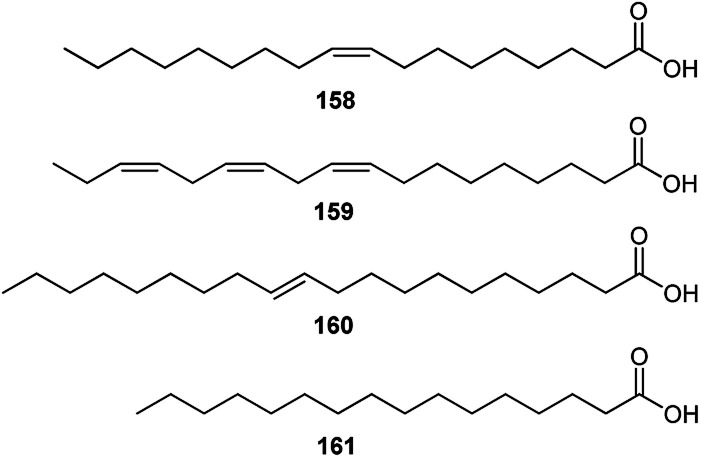
Several studies have revealed that the seeds of Consolida species are a rich source of FAs. FAs are the major constituents of the oils from Consolida plants; for example, FA components are 87.16% of the seed oils of C. regalis.73 It has also been found that oleic acid (158), with a carbon chain length (CCL) of 18 : 1, is the most dominant FA in all studied Consolida plants (more than 50% of the total FAs), namely, C. regalis, C. orientalis, C. armeniaca, C. glandulosa and C. hohenackeri (Fig. 8).73–76Consolida plants also contain certain amounts of linoleic (159), eicosenoic (160), and palmitic (161) acids, whereas other FAs are almost negligible.
Fig. 8. Main FAs in Consolida species.
To date, only one species of Consolida, namely, C. regalis, has been investigated for its volatile constituents by using GC-MS,73 and a total of 66 compounds have been identified, representing 99.86% of the total content (Table 2). The analyses showed that the major constituents of the oils from C. regalis seeds were FAs (87.16%). In addition to the FAs, the carbonyl compounds (total content 8.57%), heptadecenal (3.58%), heptadecadienal (3.24%), and esters (total content 2.37%), particularly methyl octadecenoate (1.06%), were the main volatile constituents.
Chemical constituents of oils from seeds of C. regalis.
| Compounds and class | CAS no. | Molecular formula | Relative content |
|---|---|---|---|
| Hydrocarbons | |||
| 2,6-Dimethyldecane | 13150-81-7 | C12H26 | 0.04 |
| Undecane | 1120-21-4 | C11H24 | 0.03 |
| Dodecane | 112-40-3 | C12H26 | 0.05 |
| Tridecane | 629-50-5 | C13H28 | 0.07 |
| Tetradecane | 629-59-4 | C14H30 | 0.04 |
| Pentadecane | 629-62-9 | C15H32 | 0.02 |
| Hexadecatriene | 25167-60-6 | C16H28 | 0.02 |
| Hexadecane | 544-76-3 | C16H34 | 0.03 |
| Heptadecadiene, isomer I | 58045-14-0 | C17H32 | 0.06 |
| Heptadecadiene, isomer II | 81265-03-4 | C17H32 | 0.02 |
| Heptadecane | 629-78-7 | C17H36 | 0.02 |
| Octadecane | 593-45-3 | C18H38 | 0.03 |
| Nonadecane | 629-92-5 | C19H40 | 0.02 |
| Carbonylic compounds | |||
| Nonan-2-one | 30642-09-2 | C9H18O | 0.02 |
| Nonanal | 124-19-6 | C9H18O | 0.07 |
| Non-2-enal | 2463-53-8 | C9H16O | 0.02 |
| Decan-2-one | 693-54-9 | C10H20O | 0.02 |
| Decanal | 112-31-2 | C10H20O | 0.02 |
| Dec-2-enal | 3913-71-1 | C10H18O | 0.04 |
| Deca-2,4-dienal | 5910-88-3 | C10H16O | 0.04 |
| Undec-2-enal | 53 448-07-0 | C11H20O | 0.07 |
| Tetradecanal | 124-25-4 | C14H28O | 0.05 |
| Pentadecanal | 2765-11-9 | C15H30O | 1.02 |
| Hexadecanal | 629-80-1 | C16H32O | 0.14 |
| Hexadecenal | 76261-03-5 | C16H30O | 0.03 |
| 6,10,14-Trimethylpentadecan-2-one | 16825-16-4 | C18H36O | 0.21 |
| Heptadecadienal | 56797-42-3 | C17H30O | 3.24 |
| Heptadecenal | 98028-42-3 | C17H32O | 3.58 |
| Aliphatic alcohols | |||
| Octan-1-ol | 111-87-5 | C8H18O | 0.02 |
| Nonan-2-ol | 628-99-9 | C9H20O | 0.14 |
| Nonan-1-ol | 143-08-8 | C9H20O | 0.04 |
| Undecan-2-ol | 1653-30-1 | C11H24O | 0.01 |
| Tridecan-1-ol | 61725-89-1 | C18H38O3 | 0.01 |
| Aromatic compounds | |||
| 2-(tert-Butyl)-1,4-dimethoxybenzene | 21112-37-8 | C12H18O2 | 0.02 |
| Fatty acids | |||
| Dodecanoic acid | 143-07-7 | C12H24O2 | 0.07 |
| Tetradecanoic acid | 62217-70-3 | C14H28O2 | 0.22 |
| Pentadecanoic acid | 1002-84-2 | C15H30O2 | 0.03 |
| Hexadecenoic acid | 629-56-1 | C16H30O2 | 0.06 |
| Hexadecanoic acid | 57-10-3 | C16H32O2 | 8.34 |
| Octadecenoic acid | 2825-79-8 | C18H34O2 | 77.79 |
| Octadecanoic acid | 85541-42-0 | C18H36O2 | 0.16 |
| Icosenoic acid | 7050-07-9 | C20H38O2 | 0.49 |
| Esters | |||
| Methyl tetradecanoate | 124-10-7 | C15H30O2 | 0.02 |
| Methyl hexadecanoate | 112-39-0 | C17H34O2 | 0.20 |
| Ethyl hexadecanoate | 628-97-7 | C18H36O2 | 0.07 |
| Isopropyl hexadecanoate | 142-91-6 | C19H38O2 | 0.03 |
| Methyl octadecadienoate | 112-63-0 | C19H34O2 | 0.40 |
| Methyl octadecenoate | 14620-36-1 | C19H36O2 | 1.06 |
| Ethyl octadecenoate | 1260505-83-6 | C20H38O3 | 0.40 |
| Monoterpenoids | |||
| Methyl icosenoate | 2390-09-2 | C21H40O2 | 0.19 |
| Estragole | 140-67-0 | C10H12O | 0.06 |
| β-Ionone | 79-77-6 | C13H20O | 0.02 |
| Sesquiterpenoids | |||
| Copaene | 138874-68-7 | C15H24 | 0.01 |
| β-Caryophyllene | 87-44-5 | C15H24 | 0.04 |
| α-Bergamotene | 17699-05-7 | C15H24 | 0.02 |
| β-Farnesene | 3899-18-1 | C15H26 | 0.04 |
| Germacrene D | 37839-63-7 | C15H24 | 0.09 |
| β-Selinene | 17066-67-0 | C15H24 | 0.01 |
| α-Muurolene | 10208-80-7 | C15H24 | 0.02 |
| Himachalene | 1461-03-6 | C15H24 | 0.17 |
| Cadinene | 523-47-7 | C15H24 | 0.18 |
| Carotol | 465-28-1 | C15H26O | 0.08 |
| Cedrol | 77-53-2 | C15H26O | 0.09 |
| Dihydrofarnesol | 51411-24-6 | C15H28O | 0.04 |
| Higher isoprenoids | |||
| Squalene | 111-02-4 | C30H50 | 0.17 |
| Others | |||
| 2-Isopropyl-3-methoxypyrazine | 25773-40-4 | C8H12N2O | 0.03 |
3. Biological activities
The crude extracts and isolated compounds (mainly DAs and flavanols) of Consolida plants have been widely screened for their bioactivity. Preliminary screening tests revealed that Consolida-derived constituents possessed broad and impressive biological activities, including insecticide, antileishmanial, antimicrobial, antiviral, antitumor, and antioxidant activities. Herein, the biological activities of the crude extracts and isolated compounds of Consolida plants are summarized and discussed.
3.1. Insecticidal activity
Similar to its related genera Aconitum and Delphinium, the extracts or powders from plants in the Consolida genus have also been used widely as natural insecticides against various kinds of agricultural pests, which indicates that the constituents of Consolida plants possess insecticidal activities. Early in the mid-1980s, it was reported that the C19-DA methyllycaconitine, which can also be found in Consolida plants, displayed high affinity to insect nicotinic receptors and had evolved to protect plants against pests in their early growth stages.77,78 Thus, the DAs in Consolida may play a vital role in the insecticidal activities of Consolida plants, and the results from several studies seem to support this viewpoint. Ulubelen et al. tested the insect repellent activities of 29 natural DA components, six of which (79, 80, 84, 99, 112 and 115) were isolated from Turkish Consolida species, against a common household pest, the red flour beetle (Tribolium casteneum Herbst.).79 C20-DA hetisine (112) (repellency of 59.12% at 3 mg mL−1) was found to have the highest activity among all tested alkaloids, suggesting that it is a promising candidate for insecticide development. In addition, the C19-DAs lycoctonine (80) and browniine (84) and the C20-DA ajaconine (100) also showed a repellency class III effect (40.1–60%) with repellency values of 46.87%, 46.87%, and 53.12% at 3 mg mL−1, respectively, while delsoline (79) and hetisinone (115) showed only a low class II repellent effect (both with a repellency value of 37.50% at 3 mg mL−1).
A series of C19- and C20-DAs isolated from Consolida species were evaluated for their insect antifeedant activities on polyphagous Spodoptera littoralis and the Colorado potato beetle Leptinotarsa decemlineata, as well as their toxicity to insect-derived Sf9 cells (derived from S. frugiperda pupal ovarian tissue) and mammalian Chinese hamster ovary (CHO) cells (Table 3).80,81 Most of the tested DAs showed notable antifeedant effects on these two pests (EC50 < 50 μg cm−2), and the antifeedant effects of DAs were found to be species- and structure-dependent (Table 4). Overall, DAs were more effective on L. decemlineata than on S. littoralis. Among these Consolida-derived DAs, the most active antifeedant to L. decemlineata was lycaconitine-type C19-DA 8-O-methylconsolarine (57, EC50 = 0.13 μg cm−2), followed by lycaconitine-type C19-DAs 91, 78, 51, 81, 31, and aconitine-type DA 9 (EC50 < 1 μg cm−2). Ajadine (31, EC50 = 0.1 μg cm−2) exerted the strongest antifeedant effect on S. littoralis, followed by alkaloids 78 (EC50 = 0.84 μg cm−2) and 87 (EC50 = 1.51 μg cm−2). Only a few tested DAs showed toxicity to insect-derived Sf9 cells (LD50 < 100 μg mL−1), and the most toxic compound was 14-O-deacetylpubescenine (58, LD50 = 0.38 μg mL−1), followed by tuguaconitine (4, LD50 = 1.83 μg mL−1) and 14-O-demethyldelboxine (2, LD50 = 6.27 μg mL−1). In addition, none of the tested DAs showed cytotoxicity to CHO cells (LD50 > 100 μg mL−1). In general, C19-DAs demonstrated better antifeedant activities than C20-DAs, especially lycaconitine-type C19-DAs. From the viewpoint of chemical structure, it seemed that lycaconitine-type C19-DAs with ester substituents were more effective, but more research is needed for confirmation. The data described above, combined with the fact that more C19-DAs are present in Consolida plants, indicate that C19-DAs play a key role in the insecticidal activity of Consolida plants. These results also encourage further in-depth research on the antifeedant activities of Consolida-derived C19-DAs.
Antifeedant effects of DAs on L. decemlineata and S. littoralis and cytotoxicity on Sf9 cells.
| Compounds | Type | L. decemlineata (EC50, μg cm−2) | S. littoralis (EC50, μg cm−2) | Sf9 cells (LD50, μg mL−1) |
|---|---|---|---|---|
| 14-O-Demethyldelboxine (2) | I | 1.92 | ≈50 | 6.27 |
| 14-Demethyltuguaconitine (3) | I | 2.36 | 5.38 | >100 |
| Tuguaconitine (4) | I | 3.31 | 11.79 | 1.83 |
| Pubescenine (6) | III | 12.53 | >50 | >100 |
| Raveyine (9) | III | 0.99 | >50 | >100 |
| Ajadelphinine (19) | III | 4.43 | >50 | >100 |
| Ajadine (31) | IV | 0.84 | 0.42 | >100 |
| 14-Deacetylajadine (32) | IV | nt | nt | >100 |
| 18-Hydroxy-14-O-methylgadesine (49) | IV | 0.13 | >50 | >100 |
| 18-Demethylpubescenine (51) | IV | 0.60 | >50 | 29.17 |
| 1-O,19-Didehydrotakaosamine (56) | IV | 1.49 | 14.29 | >100 |
| 8-O-Methylconsolarine (57) | IV | 0.23 | >10 | >100 |
| 14-O-Deacetylpubescenine (58) | IV | ≈50 | 17.99 | 0.38 |
| 18-O-Benzoyl-14-O-deacetyl-18-O-demethylpubescenine (59) | IV | nt | nt | >100 |
| 18-Methoxygadesine (60) | IV | 6.36 | >50 | >100 |
| Consolidine (61) | IV | ≈50 | 9.86 | >100 |
| Olivimine (62) | IV | 10.92 | >50 | >100 |
| Olividine (63) | IV | 3.62 | 3.33 | 29.45 |
| Gigactonine (75) | IV | 13.02 | 9.31 | >100 |
| Delcosine (76) | IV | 1.11 | 3.53 | 32.37 |
| Deltatsine (78) | IV | 0.54 | 0.84 | >100 |
| Delsoline (79) | IV | 2.22 | >50 | >100 |
| Lycoctonine (80) | IV | >50 | >50 | >100 |
| Takaosamine (81) | IV | 0.66 | 5.29 | >100 |
| Delphatine (82) | IV | 2.97 | 2.72 | >100 |
| Browniine (84) | IV | nt | Nt | >100 |
| 14-Acetyldelcosine (87) | IV | >50 | 1.51 | 14.88 |
| 14-O-Acetyldelectinine (91) | IV | 0.29 | 5.63 | >100 |
| Dihydroajaconine (96) | V | 5.0 | >50 | >100 |
| Isoatisine (99) | V | 3.4 | >50 | >100 |
| Ajaconine (100) | V | 5.1 | 8.2 | >100 |
| Glandulosine (107) | VI | 4.0 | >50 | >100 |
| Hetisine (113) | VI | 1.73 | ≈50 | >100 |
| Atropine | 7.38 | >50 | >100 | |
| Anabasine | >50 | ≈60 | >100 | |
| Eserine | ≈60 | >50 | >100 |
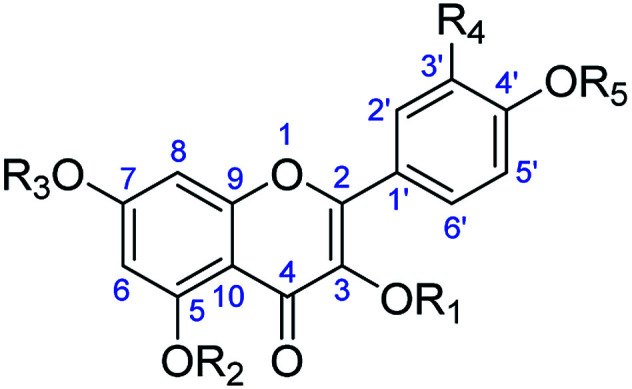
In vitro activity of flavonoids of Consolida plants on promastigotes of Leishmania species.
| |||
|---|---|---|---|
| R1–R5 (name, no.) | IC50 (μM) | ||
| L. peruviana | L. braziliensis | J774.2 cells | |
| R1 = R2 = R3 = R4 = R5 = H (kaempferol,132) | 71.29 | 53.65 | 53.67 |
| R1 = R2 = R3 = R4 = Ac, R4 = H (kaempferol tetraacetate, 132a) | 53.32 | 68.56 | 15.56 |
| R1 = R2 = R3 = R5 = H, R4 = OH (quercetin, 133) | 60.04 | 30.49 | 125.44 |
| R1 = R2 = R3 = R5 = Ac, R4 = OAc (quercetin pentaacetate, 133a) | 11.18 | 46.78 | 109.23 |
| R1 = β-d-Gal, R2 = R3 = R4 = R5 = H (trifolin, 134) | 53.34 | 52.46 | 161.32 |
| R1 = β-d-Gal Ac, R2 = R3 = R5 = H, R4 = H (trifolin heptaacetate, 134a) | 10.53 | 8.72 | 148.71 |
| R1 = 2-O-acetyl-β-d-Gal, R2 = R3 = R5 = H, R4 = OH (2′′-acetylhyperoside, 136) | 7.35 | 6.21 | 122.31 |
| R1 = 6-O-acetyl-β-d-Gal, R2 = R3 = R5 = H, R4 = OH (6′′-acetylhyperoside, 137) | 86.95 | 51.60 | 61.32 |
| Pentostam | 11.32 | 9.56 | 12.44 |
| Glucatim | 15.33 | 25.61 | 15.20 |
3.2. Antiparasitic activity
In some countries, such as Turkey and China, Consolida plants have been employed as anthelmintic herbals in traditional medicines.6,82 Several studies regarding the antiparasitic effect of the crude extracts and isolated compounds of Consolida species support the utilization of Consolida plants as anthelmintic herbals. Moreover, these results reveal the high potential of Consolida-derived compounds in the treatment of protozoal infections.
Carole et al. investigated the antileishmanial activities of 27 plants from Lebanese.83 The screened plants were extracted with water, methanol, and dichloromethane. The methanol extracts of C. rigida (white larkspur) exhibited significant antiamastigote effects on the intracellular form of Leishmania species (IC50 = 8.1 μg mL−1). Furthermore, the methanol extracts also showed no toxicity to the host cells (THP1 human monocytes, IC50 > 250 μg mL−1), exhibiting a selectivity index (SI) larger than 30. Notably, of the screened plants, the antileishmanial effects of the methanol extracts of C. rigida were next only to the aqueous extracts of Onosma aucheriana (IC50 = 5.1 μg mL−1, SI > 49) and the methanol extracts of Cytisus syriacus (IC50 = 5.8 μg mL−1, SI > 43).
From a total of 64 DAs (41 C19-DAs and 23 C20-DAs) screened by González et al., only three atisine-type C20-DAs displayed antiparasitic effects against Leishmania infantum and Trypanosoma cruzi, while none of the C19-DAs affected the parasites.80,84,85 Among these three DAs, azitine (93) has been found in Consolida species. Azitine (92) showed promising antileishmanial and antitrypanocidal properties. It was effective in vitro both against the extracellular and intracellular forms of L. infantum and could not only lower the in vitro growth rate of L. infantum but also affect the capacity to infect cells and reduce the multiplication of amastigotes. In the in vitro experiment, azitine (92) exerted an inhibitory effect against L. infantum parasites (IC50 = 10.12 μg mL−1 after 72 h of culture), which was lower than those obtained by the reference drug pentostam (IC50 = 11.32 μg mL−1 after 72 h of culture), and exhibited an inhibiting effect against T. cruzi epimastigotes (IC50 = 67.74 μg mL−1 after 72 h of culture). In the intracellular experiment, azitine (92) clearly inhibited the infection rate (approximately 53%) of L. infantum in J774A.1 macrophage cells after 48 h of culture. Moreover, this alkaloid is not toxic to host cells (IC50 > 200 μg mL−1), which highlights its potential as a lead compound in the discovery of drugs for protozoal infections.
Additionally, a set of flavonol glycosides obtained from C. oliveriana and their acetylated products have exhibited impressive antileishmaniasis activity against two Leishmania species L. peruviana and L. braziliensis (Table 4).86–88 All the compounds tested showed high inhibitory effects against their corresponding parasites, and some of them had higher effectiveness and selectivity indexes than those of their corresponding reference drugs. For example, acetylated compounds 133a, 134a, and 136 were highly active against L. peruviana, and 133a and 136 were strongly effective against L. braziliensis. Transmission electronic microscopy and nuclear magnetic resonance analysis raised the possibility that the action (or part of the action) could be at the level of the parasite membranes. Regarding structures, the acetylated compounds performed better than the phenolic analogs, and the kaempferol derivatives possessing a monosubstituted B-ring were more active than the quercetin analogs. The interesting structure–activity relationship (SAR) described above implies that the Consolida-derived flavonols can serve as a low-cost starting material for the discovery of acetylated compounds with better antileishmaniasis efficacy.
3.3. Antimicrobial activity
The crude extracts of several Consolida plants have been evaluated for their antimicrobial activities against some kinds of plant and human pathogenic bacteria and fungi. In a screening of plants with antimicrobial activity from northeastern Iran, two Consolida species, C. orientalis and C. rugulosa, were evaluated for their antimicrobial activity against several pathogenic bacteria and fungi, and C. orientalis showed significant antimicrobial activity against Morganella morganii and P. aeruginosa.89 Ucar tested the antimicrobial activity of ethanol extracts from the aerial parts (leaf, flower, and branch) of C. regalis against a series of common human pathogenic bacteria and fungi, including Staphylococcus aureus, Enterococcus faecalis, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumonia, Bacillus cereus, Candida albicans, and C. tropicalis. The extracts from the leaf, flower, and branch parts of C. regalis showed moderate antimicrobial activity against C. tropicalis with MIC values of 0.625 mg mL−1, 0.625 mg mL−1, and 0.312 mg mL−1, respectively, and the extracts from the leaf and branch parts showed moderate antimicrobial activity against S. aureus, with MIC values of 0.625 mg mL−1. The extracts showed only a weak effect on the other tested microorganisms.90
Kalpana et al. evaluated the antifungal activities of methanol extracts from the leaves, stems and flowers of C. ambigua (D. ajacis) against several phytopathogenic fungi, Alternaria solani, Rhizoctonia solani, Colletotrichum gloeosporioides and Pyricularia oryzae. All of these extracts at 10 mg mL−1 were effective at inhibiting fungal colony growth compared with that of the control. The extract of the C. ambigua leaves showed the complete inhibition of P. oryzae colony growth, followed by the almost complete inhibition of C. gloeosporioides colony growth, whereas low inhibition was observed against R. solani and A. solani. The stem extract showed the complete inhibition of the colony growth of C. gloeosporioides, P. oryzae and R. solani, followed by the inhibition of A. solani colony growth; the flower extract completely reduced the growth of the plant pathogenic fungus C. gloeosporioides, followed by P. oryzae while the least inhibition was observed against A. solani.91 In addition, Yusuf et al. tested the antifungal activity of the leaf extracts of D. consolida against Alternaria solani, an early blight disease pathogen of potato. However, the studied extracts showed no inhibitory effect on the mycelial growth of A. solani.92
The above antimicrobial activities can be attributed to their DA compositions, which have been reported to exhibit certain antibacterial and antifungal activities.15 Bilge et al. reported that five Consolida alkaloids presented a notable antibacterial effect only toward K. pneumoniae and A. baumannii with MIC values of 8 μg mL−1, while the five Consolida alkaloids exhibited considerable antifungal activity with MIC values of 4 μg mL−1 (Table 5).93
Antimicrobial activities of DAs.
| DAs | E. coli | P. aeruginosa | P. mirabilis | K. pneumoniae | A. baumannii | S. aureus | B. subtilis | C. albicans |
|---|---|---|---|---|---|---|---|---|
| Lycoctonine (80) | 32 | 64 | 32 | 8 | 8 | 64 | 128 | 4 |
| 18-O-Methyllycoctonine (61) | 32 | 64 | 32 | 8 | 8 | 64 | 128 | 4 |
| Delcosine (76) | 32 | 64 | 32 | 8 | 8 | 64 | 128 | 4 |
| 14-Acetyldelcosine (87) | 32 | 64 | 32 | 8 | 8 | 64 | 128 | 4 |
| 14-Acetylbrowniine (84) | 32 | 64 | 32 | 8 | 8 | 64 | 128 | 4 |
| Ampicilline | 2 | — | 2 | 2 | 2 | <0.12 | 0.12 | — |
| Oflaxocine | 0.12 | 1 | <0.12 | 0.12 | 0.12 | 0.5 | 0.5 | — |
| Ketocanazole | — | — | — | — | — | — | — | 2 |
3.4. Antiviral activity
The isolated DAs of Consolida plants, mainly lycaconitine-type C19-DAs, show antiviral activities toward several highly pathogenic viruses. Five known lycaconitine-type DAs from Turkish Consolida species were screened for their antiviral effects on both DNA virus herpes simplex (HSV) and RNA virus parainfluenza (PI-3) using Madin–Darby bovine kidney and Vero cell lines. The maximum non-toxic concentrations (MNTC) and cytopathogenic effects (CPE) were determined using acyclovir and oseltamivir as the references. Consequently, a selective inhibition was observed toward PI-3 virus by these alkaloids, while they were entirely unsuccessful in the inhibition of HSV (Table 6). The PI-3 inhibitory activity of the alkaloids was fairly analogous to that of the positive control oseltamivir, ranging between 8–32 μg mL−1 as the minimum and maximum inhibitory concentrations for the cytopathogenic effect (CPE).93 In addition, the new lycaconitine-type C19-DAs ajacisines C–E (37–39) and isodelectine (47) were found to exhibit moderate to weak antiviral effects against respiratory syncytial virus (RSV) with IC50 values of 75.2, 35.1, 10.1, and 50.2 μM, respectively,41 while the positive control ribavirin showed an IC50 value of 3.1 μM. The antiviral activities of DAs may be due to their high reactivity with microtubules, thus destroying their stability by polarity; this result can block cellular division and prevent the rapid growth of cancer cells.94
Antiviral effects of DAs against HSV and PI-3.
| Compounds | MDBK cells (MNTC, μg mL−1) | HSV | Vero cells (MNTC, μg mL−1) | PI-3 | ||
|---|---|---|---|---|---|---|
| Max. | Min. | Max. | Min. | |||
| Lycoctonine (80) | 64 | — | — | 32 | 32 | 8 |
| 18-O-Methyllycoctonine (61) | 64 | — | — | 64 | 32 | 1 |
| Delcosine (76) | 64 | — | — | 64 | 32 | 1 |
| 14-Acetyldelcosine (87) | 64 | — | — | 64 | 32 | 1 |
| 14-Acetylbrowniine (84) | 64 | — | — | 64 | 32 | 1 |
| Acyclovir | 16 | 16 | <0.25 | — | — | — |
| Oseltamivir | — | — | — | 32 | 32 | <0.25 |
3.5. Antitumor activity
Although no species of Consolida are traditionally used to treat cancer, several studies have revealed that the crude extracts and isolated compounds of Consolida plants possess certain antitumor effects. In a screening of anticancer plants from Iran, the ethanol extracts of C. orientalis exerted an antiproliferative effect against human cervical carcinoma HeLa cells with an IC50 value of 1.6 mg mL−1,95 which might be attributed to the high content of some DAs with cytotoxic activities in the C. orientalis extracts.17,96
De Inés et al. evaluated the cytotoxic effects of 43 DAs (40 C19-DAs and 3 C18-DAs) on CHO cells and several tumor cell lines, including CT26 (murine colon adenocarcinoma), SW480 (human colon adenocarcinoma), HeLa, SkMel25 (human melanoma) and SkMel28 (human malignant melanoma).97 As shown in Table 7, 13 of the tested alkaloids that have been found in Consolida plants produced a cytotoxic effect on the different cell lines (MICs < 100 μg mL−1). Among the various groups, the most active alkaloids were found among the lycaconitine-type C19-DAs. All the cell lines responded to 27, 56 and 60 with varying potencies. Alkaloid 27 was the most cytotoxic to CHO and SkMel28, while 56 was the most cytotoxic to CT26, SW480, HeLa and SkMel25 cells, indicating selective structure-dependent cytotoxicity for the group. Alkaloids 13 and 19 also showed relatively strong cytotoxicity toward several tumor cell lines. It is worth noting that most of the active alkaloids, including the most effective alkaloid 56, exhibited selective cytotoxicity to cancerous versus noncancerous tissues, which highlights their potential use as candidates for the treatment of cancer. In addition, the viability assays indicated that their cytotoxic effects could be related to the inhibition of ATP production.
Antitumor effects of DAs against human cancer cell lines.
| Compounds | MICs (μg mL−1) | |||||
|---|---|---|---|---|---|---|
| CHO | CT26 | SW480 | Hela | SkMel25 | Skmel28 | |
| Pubescenine (6) | >100 | 100 | 25 | 50 | 50 | >100 |
| Raveyine (9) | >100 | 50 | 50 | >100 | 50 | >100 |
| Neoline (13) | >100 | 25 | 12.5 | 6.25 | 25 | >100 |
| Ajadelphinine (19) | >100 | 50 | 25 | 12.5 | 25 | >100 |
| Didehydrodelsoline (27) | 6.25 | 12.5 | 12.5 | 12.5 | 25 | 6.25 |
| Ajadine (31) | 50 | 50 | 50 | >100 | >100 | 50 |
| 14-Deacetylajadine (32) | >100 | >100 | 100 | 50 | 100 | >100 |
| Methyllycaconitine (48) | 12.5 | 12.5 | 50 | 50 | 100 | 100 |
| 18-Demethylpubescenine (51) | >100 | >100 | >100 | >100 | 50 | >100 |
| 1-O,19-Didehydrotakaosamine (56) | >100 | 6.25 | 6.25 | 0.4 | 6.25 | 25 |
| 18-Methoxygadesine (60) | 25 | 50 | 25 | 25 | 25 | >100 |
| Lycoctonine (80) | >100 | 50 | 50 | >100 | >100 | >100 |
| Delphatine (82) | >100 | >100 | >100 | 100 | >100 | >100 |
While flavanol glycosides from Consolida themselves are slightly active against certain human cancer cell lines, increasing cytotoxic activity has been observed after the corresponding flavanols undergo acetylation. Diaz et al. prepared a series of flavanol acetates isolated from the aerial parts of C. oliveriana and tested their cytotoxicity effects against the human myeloid leukemia HL-60 and U937 cell lines and the human melanoma SK-MEL-1 cell line (Table 8).59 As shown in Table 8, some of these flavonol glycoside acetates (132a, 133a, 134a, 134b and 135a) displayed cytotoxicity against the tested cancer cell lines with IC50 values ranging from 10 to 88 μM. In particular, trifolin heptaacetate (134a) was the most effective against all assayed cell lines, with an IC50 value of approximately 10–15 μM. A subsequent pharmacological study revealed that trifolin heptaacetate could induce cancer cell apoptosis through a caspase-dependent mechanism that is associated with the release of cytochrome c.98 It has been suggested that trifolin heptaacetate has the potential to be developed as a chemopreventive agent and possibly as a therapeutic agent against cancer; however, more detailed mechanistic studies on trifolin heptaacetate are still needed.
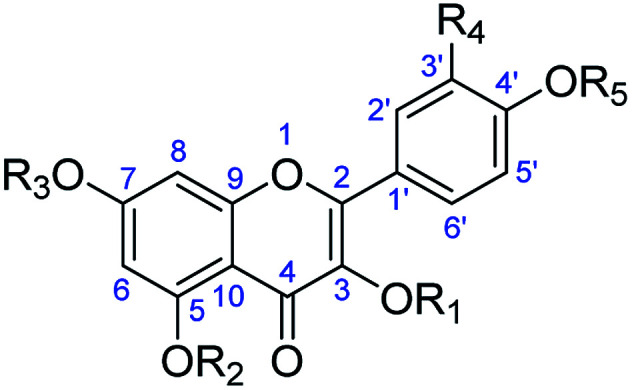
Antitumor effects of flavanol glycosides against human cancer cell lines.
| |||
|---|---|---|---|
| R1–R5 (name, no.) | IC50 (μM) | ||
| HL-60 | U937 | SK-MEL-1 | |
| R1 = R2 = R3 = R4 = Ac, R4 = H (kaempferol tetraacetate, 132a) | 45 | 48 | 37 |
| R1 = R2 = R3 = R5 = Ac, R4 = OAc (quercetin pentaacetate, 133a) | 38 | 25 | 58 |
| R1 = β-d-Gal Ac, R2 = R3 = R5 = H, R4 = H (trifolin heptaacetate, 134a) | 21 | 10 | 15 |
| R1 = β-d-Gal OMe, R2 = R3 = R5 = Me, R4 = H (heptamethyltrifolin, 134b) | 88 | >100 | >100 |
| R1 = β-d-Gal Ac, R2 = R3 = R5 = Ac, R4 = OAc (hyperoside acetate, 135a) | 15 | 19 | 23 |
| R1 = 2-O-acetyl-β-d-Gal, R2 = R3 = R5 = H, R4 = OH (2′′-acetylhyperoside, 136) | >100 | >100 | >100 |
| R1 = 6-O-acetyl-β-d-Gal, R2 = R3 = R5 = H, R4 = OH (6′′-acetylhyperoside, 137) | >100 | >100 | >100 |
| R1 = β-d-Gal Ac, R3 = β-d-Glu Ac, R2 = R5 = Ac, R4 = H (glucotrifolin acetate, 138a) | >100 | >100 | >100 |
3.6. Antioxidant activity
Several Consolida species have been evaluated for their antioxidant activities by using in vitro antioxidant assays, and considerably different effects have been observed. Zeng et al. reported that the aqueous extracts of C. ambigua (D. ajacis) flowers exhibited only a weak DPPH radical scavenging ability among 45 tested flowers, although these C. ambigua extracts could effectively scavenge hydroxyl superoxide and anion radicals.99 In contrast, investigations performed by Zengin et al. and Zengin et al. showed that C. orientalis has powerful antioxidant activities, effectively scavenging free radicals, including DPPH, ABTS, and superoxide radicals; reducing cupric and ferric ions; chelating prooxidant metal ions; and inhibiting the oxidation of linoleic acid.100,101 Another investigation also demonstrated that C. regalis possesses a powerful ability to scavenge DPPH and ABTS free radicals.90 The difference in antioxidant activities between these Consolida species may be attributed to their different phenolic contents.
4. Conclusions
To the best of our knowledge, a total of 143 distinct compounds, including 126 alkaloids (121 DAs and 5 other alkaloids) and 17 flavonoids (5 anthocyanins and 12 flavanols), have been isolated and identified from Consolida plants, which indicate that the Consolida genus is a source of abundant DAs. The DAs that have been found in Consolida plants consist of 5 C18-DAs, 87 C19-DAs and 29 C20-DAs. In terms of DA subtypes, the lycaconitine-type C19-DAs with 73 members account for the largest proportion (58%) of the isolated alkaloids; thus, lycaconitine-type C19-DAs can be regarded as the characteristic and representative components of the genus Consolida. On the other hand, of the 143 isolated compounds, 73 are novel, including 69 new DAs and 4 new anthocyanins. Among these new compounds, several possess unprecedented structures or uncommon substituents. These findings underscore the high chemical diversity among the chemical constituents of Consolida plants, which can serve as a vast resource for drug discovery.
The crude extracts and isolated compounds of Consolida plants have been reported for their various biological activities, including insecticidal, antiparasitic, antifungal, antiviral, anticancer, and antioxidant activities. Some of the reported effects are in accordance with the purported uses of Consolida plants in folk medicine, which is conducive to illuminating the pharmacodynamic material basis of Consolida-derived herbal drugs. For example, the anthelmintic effects of Consolida plants may be attributed to the anthelmintic effects of DAs. Some constituents from Consolida plants possess activities that differ from their traditional medicinal use, such as antitumor and antioxidant activities, indicating the novel potential applications for the use of Consolida plants.
Although phytochemical and biological studies on Consolida plants have attracted considerable interest, some research potential remains. First, of the 50 Consolida species around the world, only a few species have been studied for their biological constituents. The related investigations are restricted to the widespread Consolida species, such as C. ambigua, which contributes relatively more compounds than other species. Most of the less common Consolida species are still largely unstudied. Hence, an extensive investigation of the other Consolida species, especially species that are used medicinally, remains necessary.
Second, the preliminary detection performed by using LC, GC, and MS techniques reveal that there are a number of other compounds in Consolida plants, such as phenolic acids, steroids, FAs and volatile constituents, that may also possess new structures or notable biological activities, thus potentially serving as a medicinal resource for drug discovery. In addition, unlike toxic DAs, the phenolic acids, steroids, FAs and volatile constituents are generally less toxic, which is advantageous for the food and pharmaceutical industry. However, these compounds have not attracted the interest of researchers, and none have been isolated. Thus, further studies on the isolation and biological tests of these compounds are strongly encouraged.
Finally, all of the biological activities of Consolida plants have been investigated by using in vitro chemical and cellular models, and little clinical or in vivo research is currently available. These pharmacological studies are insufficient to validate the effects of Consolida plants and their derived compounds, which hinder their application and promotion. It is necessary to evaluate the biological activities of the constituents from Consolida plants using both in vitro and in vivo pharmacological models to facilitate further research and exploitation of this genus.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was financially supported by a grant from the National Natural Science Foundation of China (No. 31860095), and a grant from the Guizhou Provincial Natural Science Foundation (No. QKHJC[2018]1193).
References
- Davis P. H., Flora of Turkey and the East Aegean Islands, 1966, vol. 1, pp. 119–134 [Google Scholar]
- Kuddisi E. Emine A. Osman T. Turk. J. Biochem. 2010;35:99–104. [Google Scholar]
- Wang W. C. Guihaia. 2019;39:1425–1469. [Google Scholar]
- Jabbour F. Renner S. S. Taxon. 2011;60:1029–1040. doi: 10.1002/tax.604007. [DOI] [Google Scholar]
- Hong D. Y. Acta Bot. Sin. 1986;28:1–10. [Google Scholar]
- Bitiş L. Süzgeç S. Meriçli F. Özçelik H. Zapp J. Becker H. Meriçli A. H. Pharm. Biol. 2008;44:244–246. doi: 10.1080/13880200600713691. [DOI] [PubMed] [Google Scholar]
- Yang X. Zhen Y. Q. Hao S. J. Bian Q. W. Lin H. J. Liu G. L. Zhou X. L. Chem. Res. Appl. 2018;30:1133–1136. [Google Scholar]
- Ulubelen A. Desai H. K. Hart B. P. Joshi B. S. Pelletier S. W. Meriçli A. H. Özen H. Ç. J. Nat. Prod. 1996;59:907–910. doi: 10.1021/np960219k. [DOI] [PubMed] [Google Scholar]
- Keller O. Voelker O. Arch. Pharm. 1914;251:207–216. doi: 10.1002/ardp.19132510115. [DOI] [Google Scholar]
- Mieg W. Justus Liebigs Ann. Chem. 1915;408:61–82. doi: 10.1002/jlac.19154080105. [DOI] [Google Scholar]
- Wang F. P. Chen Q. H. Liu X. Y. Nat. Prod. Rep. 2009;27:529–570. doi: 10.1039/B916679C. [DOI] [PubMed] [Google Scholar]
- Wang F. P. and Liang X. T., C20-diterpenoid alkaloids, 2002, vol. 59, pp. 1–280 [DOI] [PubMed] [Google Scholar]
- Wang F. P. and Chen Q. H., The C19-diterpenoid alkaloids, 2010, vol. 69, pp. 1–577 [DOI] [PubMed] [Google Scholar]
- Liang X. Gao Y. Luan S. RSC Adv. 2018;8:23937–23946. doi: 10.1039/C8RA03911A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T. P. Cai L. Fang H. X. Fang Y. S. Li Z. J. Ding Z. T. Phytochemistry. 2015;116:314–319. doi: 10.1016/j.phytochem.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Yin T. P. Shu Y. Zhou H. Cai L. Ding Z. T. Fitoterapia. 2019;135:1–4. doi: 10.1016/j.fitote.2019.03.021. [DOI] [PubMed] [Google Scholar]
- Yin T. P. Cai L. Ding Z. T. RSC Adv. 2020;10:13669–13686. doi: 10.1039/D0RA00813C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi B. S. Puar M. S. Desai H. K. Ross S. A. Lu J. Pelletier S. W. Tetrahedron Lett. 1993;34:1441–1444. doi: 10.1016/S0040-4039(00)60313-1. [DOI] [Google Scholar]
- Kurbanov U. K. Tashkhodzhaev B. Turgunov K. K. Mukarramov N. I. Chem. Nat. Compd. 2019;55:197–199. doi: 10.1007/s10600-019-02653-3. [DOI] [Google Scholar]
- Almanza G. Bastida J. Codina C. De La Fuente G. Phytochemistry. 1997;45:1079–1085. doi: 10.1016/S0031-9422(97)00085-X. [DOI] [Google Scholar]
- Alva A. Grandez M. Madinaveitia A. de la Fuente G. Gavin J. A. Helv. Chim. Acta. 2004;87:2110–2119. doi: 10.1002/hlca.200490190. [DOI] [Google Scholar]
- Liang X. Ross S. A. Sohni Y. R. Sayed H. M. Desai H. K. Joshi B. S. Pelletier S. W. J. Nat. Prod. 1991;54:1283–1287. doi: 10.1021/np50077a007. [DOI] [Google Scholar]
- Grandez M. Madinaveitia A. Gavín J. A. Alva A. de la Fuente G. J. Nat. Prod. 2002;65:513–516. doi: 10.1021/np010065v. [DOI] [PubMed] [Google Scholar]
- Desai H. K. Cartwright B. T. Pelletier S. W. J. Nat. Prod. 1994;57:677–682. doi: 10.1021/np50107a022. [DOI] [PubMed] [Google Scholar]
- Desai H. K. Joshi B. S. Pelletier S. W. Sener B. Bingöl F. Heterocycles. 1993;36:1081–1089. doi: 10.3987/COM-92-6277. [DOI] [Google Scholar]
- Hohmann J. Forgo P. Hajdú Z. Varga E. Máthé I. J. Nat. Prod. 2002;65:1069–1072. doi: 10.1021/np020026z. [DOI] [PubMed] [Google Scholar]
- Mericli F. Mericli A. H. Seyhan G. V. Bahar M. Desai H. K. Ozcelik H. Ulubelen A. Pharmazie. 2002;57:761–762. [PubMed] [Google Scholar]
- De La Fuente G. Acosta R. D. Gavín J. A. Lugo R. H. Jones P. G. Tetrahedron Lett. 1988;29:2723–2726. doi: 10.1016/0040-4039(88)85270-5. [DOI] [Google Scholar]
- Ulubelen A. Meriçli A. H. Meriçli F. Özçelik H. Şener B. Becker H. Choudhary I. Phytochemistry. 1999;50:909–912. doi: 10.1016/S0031-9422(98)00593-7. [DOI] [Google Scholar]
- Meriçli A. H. Meriçli F. Seyhan V. Ulubelen A. Desai H. K. Joshi B. S. Pelletier S. W. Heterocycles. 1997;10:1955–1965. [Google Scholar]
- Sener B. Bingol F. Baykal T. Gazi Univ. Eczacilik Fak. Derg. 1988;5:79–89. [Google Scholar]
- Sener B. Bingol F. Baykal T. Gazi Univ. Eczacilik Fak. Derg. 1989;6:1–5. [Google Scholar]
- Suzgec S. Bitis L. Sozer U. Ozcelik H. Zapp J. Kiemer A. K. Mericli A. H. Chem. Nat. Compd. 2009;45:287–289. doi: 10.1007/s10600-009-9273-6. [DOI] [Google Scholar]
- Mericli A. H. Yazici S. Eroglu-Ozkan E. Sen B. Kurtoglu S. Ozcelik H. Mericli F. Chem. Nat. Compd. 2012;48:525–526. doi: 10.1007/s10600-012-0299-9. [DOI] [Google Scholar]
- Pelletier S. W. Bhandaru S. Desai H. K. Ross S. A. Sayed H. M. J. Nat. Prod. 1992;55:736–743. doi: 10.1021/np50084a005. [DOI] [Google Scholar]
- Meriçli A. H. Meriçli F. Ulubelen A. Desai H. K. Joshi B. S. Pelletier S. W. Küçükislamoglu M. Heterocycles. 1998;1:329–335. [Google Scholar]
- Mericli F. Mericli A. H. Tan N. Oezcelik H. Ulubelen A. Sci. Pharm. 1999;67:313–318. [Google Scholar]
- Lu J. Desai H. K. Ross S. A. Sayed H. M. Pelletier S. W. J. Nat. Prod. 1993;56:2098–2103. doi: 10.1021/np50102a011. [DOI] [Google Scholar]
- Pelletier S. W. Sawhney R. S. Desai H. K. Mody N. V. J. Nat. Prod. 1980;43:395–406. doi: 10.1021/np50009a011. [DOI] [Google Scholar]
- Kulanthaivel P. Desai H. K. Pelletier S. W. J. Nat. Prod. 1989;52:143–144. doi: 10.1021/np50061a018. [DOI] [Google Scholar]
- Yang L. Zhang Y. B. Zhuang L. Li T. Chen N. H. Wu Z. N. Li P. Li Y. L. Wang G. C. Planta Med. 2017;83:111–116. doi: 10.1055/s-0042-107252. [DOI] [PubMed] [Google Scholar]
- Desai H. K. Pelletier S. W. Heterocycles. 1998;7:1343–1346. [Google Scholar]
- De La Fuente G. Ruiz-Mesía L. Molero J. Blanché C. Fitoterapia. 1996;67:87–88. [Google Scholar]
- Gonzalez A. G. de la Fuente G. Munguia O. Henrick K. Tetrahedron Lett. 1981;22:4843–4844. doi: 10.1016/S0040-4039(01)92359-7. [DOI] [Google Scholar]
- Alva A. Grandez M. Madinaveitia A. de la Fuente G. Gavín J. Chem. Pharm. Bull. 2004;52:530–534. doi: 10.1248/cpb.52.530. [DOI] [PubMed] [Google Scholar]
- Hajdú Z. Forgo P. Löffler B. Hohmann J. Biochem. Syst. Ecol. 2005;33:1081–1085. doi: 10.1016/j.bse.2004.12.024. [DOI] [Google Scholar]
- Gonzalez A. G. De La Fuente G. Munguia O. Heterocycles. 1983;20:409–411. doi: 10.3987/R-1983-03-0409. [DOI] [Google Scholar]
- Lei L. Sun W. G. He L. Jiang H. F. Zhang M. He W. J. Hu Z. X. Gu Y. Song H. P. Zhang Y. H. Ecotoxicol. Environ. Saf. 2019;170:141–147. doi: 10.1016/j.ecoenv.2018.11.109. [DOI] [PubMed] [Google Scholar]
- Pelletier S. W. Sawhney R. S. Mody N. V. Heterocycles. 1978;9:1241–1247. doi: 10.3987/R-1978-09-1241. [DOI] [Google Scholar]
- Mericli F. Mericli A. H. Desai H. K. Ulubelen A. Pelletier S. W. Sci. Pharm. 2001;69:63–67. doi: 10.3797/scipharm.aut-01-07. [DOI] [PubMed] [Google Scholar]
- Meriçli F. Meriçli A. H. Ulubelen A. Desai H. K. Pelletier S. W. J. Nat. Prod. 2001;64:787–789. doi: 10.1021/np0005558. [DOI] [PubMed] [Google Scholar]
- Almanza G. Bastida J. Codina C. De La Fuente G. Phytochemistry. 1997;44:739–747. doi: 10.1016/S0031-9422(96)00567-5. [DOI] [Google Scholar]
- Ruiz-Mesía L. Madinaveitia A. Reina M. Rodriguez M. L. de la Fuente G. Ruiz-Mesía W. J. Nat. Prod. 2002;65:496–499. doi: 10.1021/np0103416. [DOI] [PubMed] [Google Scholar]
- Venkateswarlu V. Srivastava S. K. Joshi B. S. Desai H. K. Pelletier S. W. J. Nat. Prod. 1995;58:1527–1532. doi: 10.1021/np50124a008. [DOI] [PubMed] [Google Scholar]
- De La Fuente Martin G. Mesía L. R. Phytochemistry. 1997;46:1087–1090. doi: 10.1016/S0031-9422(97)00397-X. [DOI] [Google Scholar]
- Attila I. Ku M. Okatan A. J. Chromatogr. A. 1992;609:402–406. doi: 10.1016/0021-9673(92)80188-Z. [DOI] [Google Scholar]
- Sulyok G. Balint J. Stud. Org. Chem. 1985;23:261–263. [Google Scholar]
- Saito N. Toki K. Özden S. Honda T. Phytochemistry. 1996;41:1599–1605. doi: 10.1016/0031-9422(95)00808-X. [DOI] [PubMed] [Google Scholar]
- Diaz J. G. Carmona A. J. Torres F. Quintana J. Estevez F. Herz W. Planta Med. 2008;74:171–174. doi: 10.1055/s-2008-1034278. [DOI] [PubMed] [Google Scholar]
- Küçükislamoglu M. Yayli N. Şentürk H. B. Genç H. Özden S. Turk. J. Chem. 2000;24:191–198. [Google Scholar]
- Yin T. P. Zhou H. Cai L. Ding Z. T. RSC Adv. 2019;9:10184–10194. doi: 10.1039/C9RA01219B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiaka S. B. Ntie-Kang F. Ndingkokhar B. Mbah J. A. Sippl W. Yong J. N. RSC Adv. 2015;5:57704–57720. doi: 10.1039/C5RA05524E. [DOI] [Google Scholar]
- Melnichuk G. G. Ukr. Bot. Zh. 1971;28:525–527. [Google Scholar]
- Schulze-Kaysers N. Feuereisen M. M. Schieber A. RSC Adv. 2015;5:73301–73314. doi: 10.1039/C5RA11746A. [DOI] [Google Scholar]
- Fernández-López J. A. Fernández-Lledó V. Angosto J. M. RSC Adv. 2020;10:24669–24682. doi: 10.1039/D0RA03514A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne B. B. Phytochemistry. 1964;3:151–160. doi: 10.1016/S0031-9422(00)88035-8. [DOI] [Google Scholar]
- Asen S. Stewart R. N. Norris K. H. Phytochemistry. 1975;14:2677–2682. doi: 10.1016/0031-9422(75)85249-6. [DOI] [Google Scholar]
- Da Silva I. Diaz J. G. Gonzalez-platas J. J. Pharm. Sci. 2011;100:1588–1593. doi: 10.1002/jps.22379. [DOI] [PubMed] [Google Scholar]
- Ozden S. Ertepinar H. Senturk H. B. Durust N. Beyazoglu O. Pharmazie. 1990;45:803–804. [Google Scholar]
- Özden S. Küçükislamoglu M. Oezden T. Pharmazie. 1995;50:818–820. [Google Scholar]
- Attila I. Baysal A. Tufekci M. Gok Y. Ozden S. Plant. Med. Phytother. 1990;24:224–230. [Google Scholar]
- Waller G. R. Mangiafico S. Foster R. C. Lawrence R. H. Planta Med. 1981;42:344–355. doi: 10.1055/s-2007-971654. [DOI] [PubMed] [Google Scholar]
- Kokoska L. Urbanova K. Kloucek P. Nedorostova L. Polesna L. Malik J. Valterova I. Chem. Biodiversity. 2012;9:151–161. doi: 10.1002/cbdv.201100048. [DOI] [PubMed] [Google Scholar]
- Dabi E. Hethelyi E. Zambo I. Tetenyi P. Simonidesz V. Phytochemistry. 1986;25:1221–1222. doi: 10.1016/S0031-9422(00)81584-8. [DOI] [Google Scholar]
- Ayaz F. Reunanen M. Pak. J. Bot. 1996;28:155–159. [Google Scholar]
- Aitzetmüller K. Tsevegsüren N. Werner G. Plant Syst. Evol. 1999;215:37–47. doi: 10.1007/BF00984646. [DOI] [Google Scholar]
- Chen L. Shan L. Zhang J. Xu W. Wu M. Huang S. Zhou X. L. Nat. Prod. Commun. 2015;10:2063–2065. [PubMed] [Google Scholar]
- Zhang J. F. Chen L. Huang S. Shan L. H. Gao F. Zhou X. L. J. Nat. Prod. 2017;80:3136–3142. doi: 10.1021/acs.jnatprod.7b00380. [DOI] [PubMed] [Google Scholar]
- Ulubelen A. Meriçli A. H. Meriçli F. Kilinçer N. Ferizli A. G. Emekci M. Pelletier S. W. Phytother. Res. 2001;15:170–171. doi: 10.1002/ptr.688. [DOI] [PubMed] [Google Scholar]
- González-Coloma A. Reina M. Guadaño A. Martínez-Díaz R. Díaz J. G. García-Rodriguez J. Grandez M. Chem. Biodiversity. 2004;1:1327–1335. doi: 10.1002/cbdv.200490095. [DOI] [PubMed] [Google Scholar]
- González-Coloma A. Guadano A. Gutiérrez C. Cabrera R. De La Pena E. De La Fuente G. Reina M. J. Agric. Food Chem. 1998;46:286–290. doi: 10.1021/jf970585p. [DOI] [PubMed] [Google Scholar]
- Ulubelen A. and Kolak U., Innovations in Chemical Biology, 2009, pp. 39–49 [Google Scholar]
- Di Giorgio C. Delmas F. Tueni M. Cheble E. Khalil T. Balansard G. J. Altern. Complementary Med. 2008;14:157–162. doi: 10.1089/acm.2007.7041. [DOI] [PubMed] [Google Scholar]
- Gonzalez P. Marin C. Rodriguez-Gonzalez I. Hitos A. B. Rosales M. J. Reina M. Diaz J. G. Gonzalez-Coloma A. Sanchez-Moreno M. Int. J. Antimicrob. Agents. 2005;25:136–141. doi: 10.1016/j.ijantimicag.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Gonzalez P. Marin C. Rodriguez-Gonzalez I. Illana A. Mateo H. Longoni S. S. Rosales M. J. Gonzalez-Coloma A. Reina M. Sanchez-Moreno M. Pharmacology. 2006;76:123–128. doi: 10.1159/000090600. [DOI] [PubMed] [Google Scholar]
- Marín C. Díaz J. G. Irure Maiques D. Ramírez-Macías I. Rosales M. J. Guitierrez-Sánchez R. Cañas R. Sánchez-Moreno M. Phytochem. Lett. 2017;19:196–209. doi: 10.1016/j.phytol.2016.12.010. [DOI] [Google Scholar]
- Marin C. Boutaleb-Charki S. Diaz J. G. Huertas O. Rosales M. J. Pérez-Cordon G. Sánchez-Moreno M. J. Nat. Prod. 2009;72:1069–1074. doi: 10.1021/np8008122. [DOI] [PubMed] [Google Scholar]
- Boutaleb-Charki S. Sánchez-Moreno M. Diaz J. G. J Rosales M. Huertas O. Gutierrez-Sánchez R. Marín C. Open Nat. Prod. J. 2011;4:1–7. doi: 10.2174/1874848101104010001. [DOI] [PubMed] [Google Scholar]
- Bazzaz B. S. Haririzadeh G. Pharm. Biol. 2008;41:573–583. doi: 10.1080/13880200390501488. [DOI] [Google Scholar]
- Ucar E. Fresenius Environ. Bull. 2018;27:5950–5957. [Google Scholar]
- Kalpana P. Verinder K. Suman G. Anshu J. Anubhav P. Manju R. Sanjeev M. J. Chem. Pharm. Res. 2016;8:11–18. [Google Scholar]
- Yusuf Y. Ayhan G. Izzet K. Halit C. Mark W. Afr. J. Biotechnol. 2011;10:8291–8295. doi: 10.5897/AJB11.241. [DOI] [Google Scholar]
- Bilge Ş. Ilkay O. Berrin Ö. ARKIVOC. 2007;vii:265–272. [Google Scholar]
- Monajjemi M. Afr. J. Microbiol. Res. 2011;5:4344–4352. [Google Scholar]
- Nemati F. Dehpouri A. A. Eslami B. Mahdavi V. Mirzanejad S. Iran. Red Crescent Med. J. 2013;15:e8871. doi: 10.5812/ircmj.8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. X. Gao Y. Y. Luan S. X. RSC Adv. 2018;8:23937–23946. doi: 10.1039/C8RA03911A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Inés C. Reina M. Gavín J. A. González-Coloma A. Z. Naturforsch., C: J. Biosci. 2006;61:11–18. doi: 10.1515/znc-2006-1-203. [DOI] [PubMed] [Google Scholar]
- Torres F. Quintana J. Diaz J. G. Carmona A. J. Estevez F. Apoptosis. 2008;13:716–728. doi: 10.1007/s10495-008-0202-0. [DOI] [PubMed] [Google Scholar]
- Zeng Y. W. Xu L. X. Peng Y. H. Chin. J. Appl. Environ. Biol. 2004;10:699–702. [Google Scholar]
- Zengin G. Mahomoodally M. F. Picot-Allain C. M. N. Cakmak Y. S. Uysal S. Aktumsek A. S. Afr. J. Bot. 2019;120:119–123. doi: 10.1016/j.sajb.2018.01.010. [DOI] [Google Scholar]
- Seyda A. Merve B. SilaOzlem S. Nuriye K. Rezzan A. Int. J. Curr. Res. 2016;8:43735–43738. [Google Scholar]