Abstract
A total of 105 isolates of Mycoplasma pneumoniae were evaluated for susceptibility to moxifloxacin, sparfloxacin, levofloxacin, and ciprofloxacin. Moxifloxacin, a newly synthesized compound, showed the greatest activity. The MICs and MBCs at which 50 and 90% of isolates were affected were 0.15 (MIC50 and MBC50) and 0.3 μg/ml (MIC90 and MBC90) respectively. The results indicate that moxifloxacin might be promising an antimycoplasmal agent.
Mycoplasma pneumoniae is the causative agent of upper respiratory tract infections and pneumonia in humans (6). Recently the efficacy of new quinolones has been demonstrated in vitro against various respiratory pathogens, including M. pneumoniae (2, 4, 11). In this report, we compared the in vitro activity of moxifloxacin, a new member of the fluoroquinolone group which has potent antimicrobial activities against gram-positive and gram-negative bacteria (3, 9), with those of other fluoroquinolone as references against M. pneumoniae.
A total of 105 strains of M. pneumoniae isolated from throat swabs from patients with pneumonia from 1986 (and earlier) to 2000 were used. These strains were subcultured fewer than five times in liquid medium and stored at −80°C until use. The antimicrobial agents used were moxifloxacin (Bay12-8039; Bayer Yakuhin Ltd.), sparfloxacin (SPFX; Dainippon Pharmaceutical Co., Ltd.) levofloxacin (LVFX; Daiichi Pharmaceutical Co., Ltd.), and ciprofloxacin (CPFX; Bayer Yakuhin Ltd.). The MICs and MBCs against each drug were determined by serial dilutions of the drugs in broth medium as described previously (5, 8). Briefly, each strain of M. pneumoniae cultured for 5 days was diluted to 105 CFU/ml and 200 μl of each sample was cultured in a sealed microtiter plate (Nunc Co., Ltd., Roskilde, Denmark) at 37°C. After 2 days of cultivation, twofold dilutions of the antimicrobial agents were added to samples of each strain. After an additional 2 days of cultivation, two and three 10-fold dilutions of each sample were used for plating on agar plates to provide a range of 500 to 1,000 CFU/10 μl of the sample. The number of mycoplasmas in broth without drugs reached approximately 107 CFU/ml. MIC is defined as the lowest concentration of an antimcrobial agent inhibiting more than 99% of the mycoplasmal colonies with the control. The definition of MBC most often used in clinical microbiology is the lowest drug concentration that kills 99.9% of the bacterial population in a liquid medium.
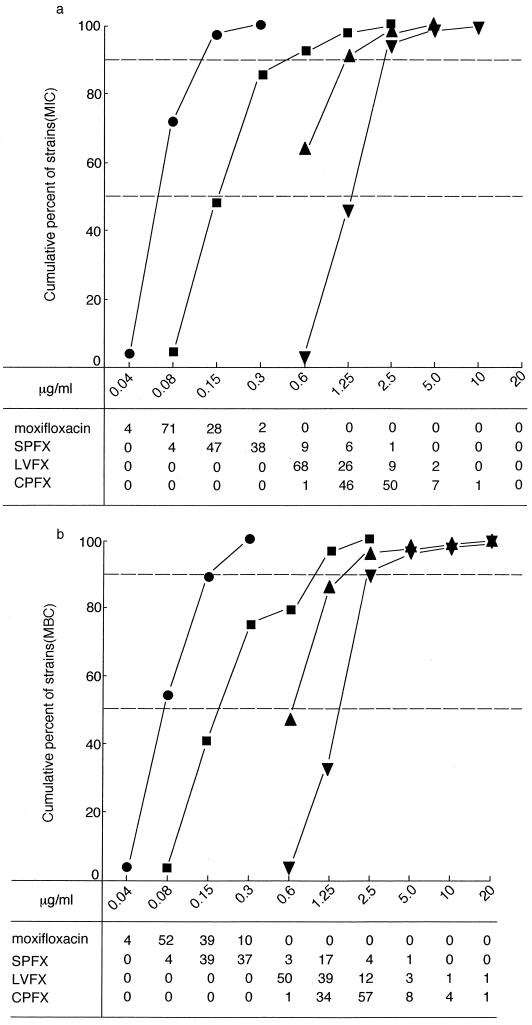
As shown in Fig. 1a, moxifloxacin (MIC for 50% of isolates [MIC50], 0.15 μg/ml; MIC for 90% of isolates [MIC90], 0.3 μg/ml) was more active than SPFX (MIC50, 0.3 μg/ml; MIC90, 1.25 μg/ml), LVFX (MIC50, 1.25 μg/ml; MIC90, 2.5 μg/ml), and CPFX (MIC50, 5.0 μg/ml, MIC90, 5.0 μg/ml). The MBCs and their distributions among the strains for these agents are shown in Fig. 1b; the MBCs of moxifloxacin (MBC50, 0.15 μg/ml; MBC90, 0.3 μg/ml) were markedly lower than those of SPFX (MBC50, 0.6 μg/ml; MBC90, 2.5 μg/ml), LVFX (MBC50, 2.5 μg/ml; MBC90, 5.0 μg/ml), and CPFX (MBC50, 5.0 μg/ml; MBC90, 5.0 μg/ml).
FIG. 1.
Susceptibilies of 105 strains of M. pneumoniae to moxifloxacin and other fluoroquinolones. (a) MICs. (b) MBCs. Symbols: ●, moxifloxacin; ■, SPFX; ▴, LVFX; ▾, CPFX. The numbers of strains are indicated at the bottom.
Next, we compared the susceptibility of strains by the year of isolation. We collected the 105 strains into groups containing 39 strains isolated prior to 1986, 24 strains isolated between 1986 and 1990, 37 strains isolated between 1991 and 1995, and 5 strains isolated between 1996 and 2000. The MBC/MIC ratio for moxifloxacin was 4 in one strain isolated between 1986 and 1990 and in one strain isolated between 1991 and 1995, with MIC of 0.08 μg/ml and MBC of 0.3 μg/ml. The MBC/MIC ratio for SPFX was 4 or more in two strains isolated prior to 1986, one strain isolated between 1986 and 1990, five strains isolated between 1990 and 1995, and five strains isolated between 1996 and 2000, but the MBCs for these 13 strains were also comparatively low (between 0.6 and 2.5 μg/ml). Similarly, for two strains isolated between 1986 and 1990 and one strain isolated between 1991 and 1995, the MBC/MIC ratio was more than 4 for LVFX; the LVFX MBCs of one strain isolated prior to 1986, one isolated between 1986 and 1990, one isolated between 1991 and 1995, and one isolated between 1996 and 2000 were high (in the range between 5 and 20 μg/ml). The MBC/MIC ratio for CPFX was more than 4 for one strain isolated prior to 1986 and one strain isolated between 1996 and 2000. The MICs and MBCs for four strains isolated between 1991 and 1995 and three strains isolated between 1996 and 2000 were in the range between 5 and 20 μg/ml.
Moxifloxacin is a fluoroquinolone designed to have a broad spectrum of in vitro activities against gram-positive and gram-negative organisms. Addition of SS-2,8-diazabicyclo[4,3,0]non-8-yl at position 7 and a methoxyl substituent at position 8 of the quinolone nucleus differentiates moxifloxacin from other fluoroquinolone compounds (1). The purpose of the present study was to evaluate the in vitro activity of moxifloxacin against M. pneumoniae. The MIC50s and MBC50s of fluoroquinolones for the isolates were similar to those for isolates obtained during prior to 2000. We reported that the MBC50/MIC50 ratios for macrolides and tetracyclines were markedly higher, with a range of 37 to 2,000, as described previously (2, 5). In the present study, we showed that the MBC50/MIC50 ratio for moxifloxacin was lower in 105 strains of M. pneumoniae isolated from 1985 (and earlier) to 2000 than were those of other fluoroquinolones. These results suggest that development of fluoroquinolone resistance of M. pneumoniae has not been recognized, although the LVFX and CPFX MICs and MBCs for several strains isolated between 1991 and 2000 were relatively high. Niitu et al. reported that the development of erythromycin resistance in M. pneumoniae was accompanied by cross-resistance to other macrolide antibiotics in vitro and in vivo (7). Toumanen et al. reported that the extension of bactericidal activity to slowly growing bacteria like mycobacteria may be a major advance in efforts to improve the chemotherapy of infectious diseases (10).
Fluoroquinolones might show antimycoplasmal activities against macrolide- and tetracycline-resistant strains of M. pneumoniae, because of their different mechanisms of action from those of the other classes of drugs. Fluoroquinolone antimicrobial agents show excellent penetration into lung tissue, in particular bronchial secretions (2, 5). The proved susceptibility of fluoroquinolones, especially moxifloxacin, described above and the characteristics of fluoroquinolones in terms of organ distribution might be useful in treating respiratory tract infections caused by M. pneumoniae.
To clarify the correlation between the activities of fluoroquinolones against M. pneumoniae and the clinical effects of these antibiotics on mycoplasma infections, further work is needed.
REFERENCES
- 1.Angela B B, Kari C K, Gary V D. In vitro activity of BAY 12-8039, a novel 8-methoxyquinolone, compared to activities of six fluoroquinolones against Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob Agents Chemother. 1997;41:1594–1597. doi: 10.1128/aac.41.7.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai S, Gohara Y, Akashi A, Kuwano K, Nishimoto M, Yano T, Oizumi K, Takeda K, Yamaguchi T. Effects of new quinolones on Mycoplasma pneumoniae-infected hamasters. Antimicrob Agents Chemother. 1993;37:287–292. doi: 10.1128/aac.37.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boswell F J, Andrews J M, Wise R. Pharmacodynamic properties of BAY 12-8039 on gram-positive and gram-negative organisms as demonstrated by studies of time-kill kinetics and post antibiotic effect. Antimicrob Agents Chemother. 1997;41:1377–1379. doi: 10.1128/aac.41.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassell G H, Waites K B, Pate M S, Canupp K C, Duffy L B. Comparative susceptibility of Mycoplasma pneumoniae to erythromycin, ciprofloxacin, and lomefloxacin. Diagn Microbiol Infect Dis. 1989;12:433–435. doi: 10.1016/0732-8893(89)90115-6. [DOI] [PubMed] [Google Scholar]
- 5.Gohara Y, Arai S, Akashi A, Kuwano K, Tseng C, Matsubara S, Matumoto M, Furudera T. In vitro and in vivo activities of Q-35, a new fluoroquinolone, against Mycoplasma pneumoniae. Antimicrob Agents Chemother. 1993;37:1826–1830. doi: 10.1128/aac.37.9.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grayston J T, Foy H M, Kenny G E. The epidemiology of mycoplasma infections of the human respiratory tract. In: Hayflick L, editor. The mycoplasmatales and the L-phase of bacteria. Amsterdam, The Netherlands: Appelton-Century-Crofts; 1969. pp. 651–682. [Google Scholar]
- 7.Niitu Y, Hasegawa S, Suetake T, Kubota H, Komatsu S, Horikawa M. Registance of Mycoplasma pneumoniae to erythromycin and other antibiotics. J Pediatr. 1970;76:438–443. doi: 10.1016/s0022-3476(70)80485-1. [DOI] [PubMed] [Google Scholar]
- 8.Pearson R D, Steigbigel R T, Davis H T, Chapman S W. Method for reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980;18:699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen U, Bartel S, Bremm K-D, Himmler T, Krebs A, Schenke T. The synthesis and biological properties of 6-fluoroquinolone-carboxylic acids. Bull Soc Chim Belg. 1996;105:689–699. [Google Scholar]
- 10.Tuomanen E. Phenotypic tolerance: the search for β-lactam antibiotics that kill nongrowing bacteria. Rev Infect Dis. 1986;8:s279–s291. doi: 10.1093/clinids/8.supplement_3.s279. [DOI] [PubMed] [Google Scholar]
- 11.Waites K B, Cassell G H, Canupp K C, Fernandes P B. In vitro susceptibilities of mycoplasmas and ureaplasma macrolides and aryl-fluroquinolones. Antimicrob Agents Chemother. 1988;32:1500–1502. doi: 10.1128/aac.32.10.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]