Abstract
The fiber obtained by electrospinning technology is a kind of biomaterial with excellent properties, which not only has a unique micro–nanostructure that gives it a large specific surface area and porosity, but also has satisfactory biocompatibility and degradability (if the spinning material used is a degradable polymer). These biomaterials provide a suitable place for cell attachment and proliferation, and can also achieve immobilization. On the other hand, its large porosity and three-dimensional spatial structure show unique blocking properties in drug delivery applications in order to achieve the purpose of slow release or even controlled release. The immobilization effect or blocking effect of these materials is mainly reflected in the hollow or core–shell structure. The purpose of this paper is to understand the application of the electrospun fiber based on biodegradable polymers (aliphatic polyesters) in the biomedical field, especially the immobilization or blocking effect of the electrospun fiber membrane on cells, drugs or enzymes. This paper focuses on the performance of these materials in tissue engineering, wound dressing, drug delivery system, and enzyme immobilization technology. Finally, based on the existing research basis of the electrospun fiber in the biomedical field, a potential research direction in the future is put forward, and few suggestions are also given for the technical problems that urgently need to be solved.
The unique blocking and immobilization of electrospinning nanofibers play an important role in tissue engineering, wound dressings, drug delivery systems and other fields.
Introduction
When the diameter of a material reaches the micron or nanometer level, some unusual properties occur, such as a very large specific surface area, excellent mechanical properties, and a three-dimensional structure.1,2 These special properties make this material the first choice in many fields. At present, there are many technologies that can be used to prepare nano-materials, such as mechanical grinding,3 template synthesis,4 self-assembly,5 and electrospinning.6,7 The time span for forming ultrafine nanofibers using electrostatic force is not long. This technology is called electrospinning, and it is a common method for the preparation of micro- to nano-sized fibers. In the process of electrospinning, the spinning device is filled with a charged polymer solution or melt liquid. Under the action of the thruster, the droplets at the nozzle are affected by the surface tension and electrostatic field force. In the process of strengthening the external electric field, the droplets are stretched from a sphere to a cone to form a “Taylor cone”, and then randomly form nano-level fibers at the receiving end.8 As the prepared fibers have high porosity, a large specific surface area, excellent mechanical properties, flexibility and good uniformity, these fibers have a variety of different uses and functions. Coupled with the simple preparation process and the possibility of large-scale production, the technology is very attractive to many fields,9–12 such as the biomedical field, filter materials, nanosensors, and nanofiber-reinforced composites.13–17 Among them, the biomedical field is one of the most important application fields, which includes tissue engineering, wound dressing, and drug delivery systems.18–20 A number of specific articles and patents have been reported on this subject. Pokorski et al.21 reviewed the past patents of polymer nanofiber scaffolds prepared by melt extrusion and chemically modified them to add some surface functions to the fibers for applications in tissue engineering, drug delivery systems, wound healing, fabrics and filter materials. Hu et al.22 reviewed the research progress of nanofibers formed by different electrospun materials, loaded with antibiotics and various antimicrobial agents, in drug delivery and cancer treatment.
Biodegradable polymer materials are much widely used in the field of biomedicine (e.g., tissue engineering and drug delivery) including variety of natural materials (e.g., collagen, gelatin, hyaluronic acid, alginate, silk fibroin) and synthetic materials (e.g., polyolefin, polyamides, polyester, aramide, acrylic).23–25 The solution or melting solution of one or more polymer materials mentioned above can obtain ultra-fine nanofibers by electrospinning, which plays an important role in all branches of the biomedical field, especially the blocking effect and immobilization effect caused by the particularity of the structure. First of all, it can load the drug as a carrier for drug delivery26 and become a potential drug repository. This can alleviate the initial sudden release and provide continuous release. This can be attributed to the blocking effect of nanofibers. Nanofibers are composed of many filaments, which have the potential to overcome the sudden release caused by other types of drug delivery systems. In addition, compared with other drug loading systems, nanofibers can withstand the larger drug loading. On the other hand, synthetic or natural biodegradable materials can be used to prepare electrospun nanofibers, and their molecular chains are often composed of main chains and many branched chains. In the spinning process, the material is dispersed in the solvent. The originally curled chain segment is stretched out due to the pull of the electrostatic field force, and then solidified into fiber filaments. When it is used as a carrier to load the drug, the change of the original structure of the material in the medium or physiological state promotes the change of the carrier volume, which hinders the drug release and slows down the release rate to achieve the purpose of sustained release.7,27 Furthermore, ultrafine nanofibers can be processed into cell scaffolds to realize cell attachment, proliferation and differentiation.28 The phenomenon of cell growth on scaffolds is very similar to the immobilization function often mentioned in the field of microbiology. When new cells are formed, the tissue forms a reticular structure supported by the scaffold and provides immobilization for the cells that continue to proliferate. The nanofiber scaffold has a very small fiber diameter and high porosity, so that the scaffold has a loosely connected 3D structure, on which cells rely on to spread just as lianas rely on stem whiskers to climb on the support. In this way, cells not only get more space for growth, but also stack more tightly. This helps the nanofiber scaffolds integrate into the surrounding tissue, and provides a reliable platform for the formation of new tissues. Suitable cell scaffolds must meet the characteristics of tissue compatibility, so that some substances, such as collagen and peptides, can be immobilized on the modified (aminated) nanofibers to improve tissue affinity. It can also guide the growth and differentiation of functional cells or tissues.29 In addition, nanofibers can achieve the immobilization of enzymes, yeast and other biological macromolecules. This helps them achieve better catalytic efficiency and enzyme activity in the biocatalytic reaction, maintain cell vitality and correctly express the target protein.30–32 So, what is the state of drug molecules or enzymes that are loaded or immobilized on nanofibers or scaffolds?
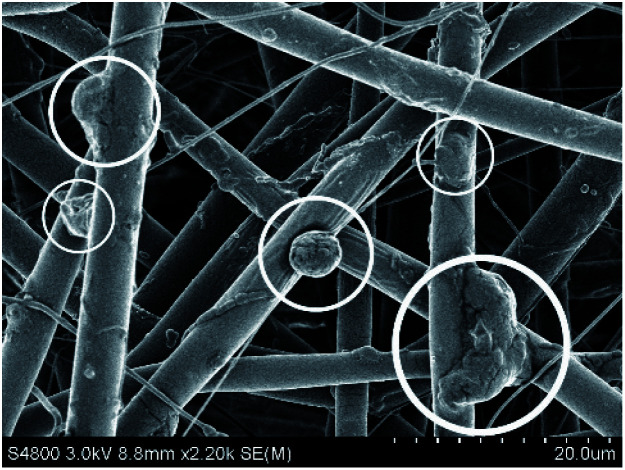
As we all know, nanofiber scaffolds provide a good space for cell growth because of their special porous structure.33 This porous structure is divided into two cases, one is the pores that are formed by the stacking of different fibers, which usually have a large space and can immobilize enzymes or load macromolecular drugs. The other is the voids that are formed by the small holes connected to each other on the outer surface of each fiber, which are supported by staggered pillars or planes to form the structural characteristics of high porosity of nanofibers. Compared with traditional scaffolds or wound coverings, this porous structure allows water and oxygen to permeate more effectively. It can better exchange nutrients and metabolize with the extracellular environment, thus transporting nutrients to specific areas to play a role. Because of the large specific surface area and the characteristics of the fiber voids, the extracellular microenvironment is simulated. This makes the scaffolds and cells achieve overall biological functionalization, which leads to the occurrence of the cell adhesion process. This also increases the biocompatibility of the nanofibers to a certain extent. Fig. 1 shows the growth of Acetobacter xylem cells on electrospun nanofibers. Acetobacter is a single cell bacterium with a size of about 2–5 μm, and can proliferate on tissue scaffolds. In the process of proliferation, the cell forms a cocoon by attaching itself to the surface of the nanofiber, and the metabolite bacterial cellulose is formed. In addition, there may be many short connections between each fiber filament. These short connections may be the same nanometer size as the fiber filament, or they may randomly form a larger cavity or bead structure. Then, through superposition and accumulation, they form a macroscopically consistent fiber membrane, which can be further assembled into a scaffold. When the fiber scaffolds are used in tissue engineering or wound management, this kind of cavity or bead structure can provide a very suitable place for cells in the implant or wound to proliferate.11,34,35 Of course, the cavity structure and core–shell structure are two different types of structures, and their formation processes are random. This makes it more suitable for immobilizing enzymes with larger molecules. More interestingly, some of the fibers obtained by electrospinning can be arranged regularly in a specific direction, which enables cells to grow in a directional manner and speed up wound healing. These kinds of fibers arranged regularly along the fiber axis are called ‘aligned fibers’.36 Aligned fibers appear in electrospinning with a polymer solution or melt solution. This is because the crystal structure of the macromolecular chain, chain segment or crystalline polymer is regularly arranged along the direction of an electrostatic field force under the action of the electrostatic field force.37,38
Fig. 1. Growth of Acetobacter xylem cells on nanofibers.
Recently, aliphatic polyesters as a class of well-known materials with low toxicity, biodegradability and good biocompatibility have been widely used in bone and cartilage tissue, drug delivery and wound healing.39,40 Polyester and its series of modified substances play an effective role in the field of biomedicine. For example, the active group was grafted onto polycaprolactone (PCL) by special ultraviolet irradiation to improve the surface function and hydrophilicity of the scaffold without affecting the inherent properties of PCL.41 Because these kinds of materials are made of renewable resources, they have good biocompatibility, can be degraded naturally, and the resulting products are non-toxic. Compared with other traditional polymers used in the medical field, these products are cheap and easy to obtain, and have excellent properties.42–44 Thus, polyester materials are commonly used in electrospinning because of these characteristics. Therefore, the purpose of this paper is to discuss and summarize the unique blocking effect and immobilization of various biomaterials prepared by polyester materials, and their series of copolymers as the raw materials of electrospinning technology, including tissue engineering, wound dressings, drug delivery carriers, enzyme immobilization and biosensors.
Tissue engineering
The concept of tissue engineering was first put forward in 1987. It is an interdisciplinary discipline involving many fields, such as medicine, biology, engineering and material science.45,46 Tissue engineering uses scaffolds to support cells and mimic the natural extracellular matrix (ECM) to regenerate new ECM. The high porosity and high surface area of the electrospun nanofiber scaffolds promote cell adhesion, and can repair and regenerate damaged tissues and organs.47 Tissue engineering is through the adsorption of non-diseased tissue cells on the scaffold which is non-toxic and self-degradable, and the degradation products are also non-toxic to the body, and then the scaffold is implanted into the lesion or injured site for treatment. When the normal cells of the body proliferate, they will be adsorbed on this scaffold to form a new scaffold with a certain morphological structure and physiological function, and achieve the purpose of working in cooperation with the original tissue and organs.18,40 The proliferation of many cells depends on a combination of growth factors, which can be released from the reticular space structure of polymer nanofibers and may stimulate the growth of damaged tissue.48 Therefore, mature fiber scaffolds can be cross-linked in different types of growth factor solutions to promote better cell growth.
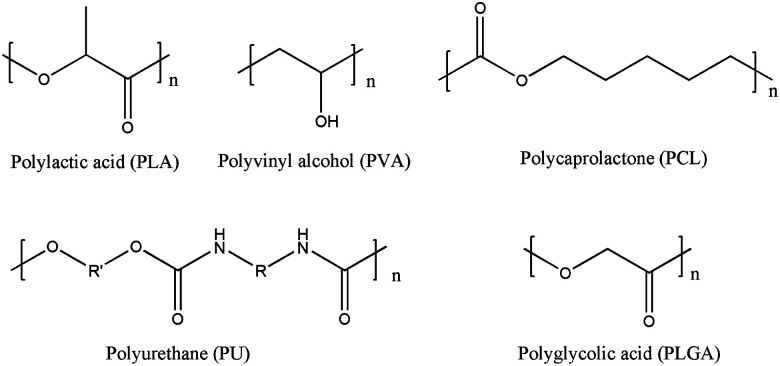
Polyester materials and their copolymers are the most commonly used raw materials in tissue engineering, such as polylactic acid (PLA), polyvinyl alcohol (PVA), PCL and their copolymers, polyurethane (PU) and polyglycolic acid (PLGA) (Fig. 2). Their extensive research and application stems from the easy degradability and excellent biocompatibility. Through the hydrolysis of ester groups, non-toxic degradation products are produced, and the degradation products can be eliminated from the body through metabolic pathways.48 These materials are designed to induce the growth and differentiation of cells in the process of forming functional tissue.
Fig. 2. The chemical structure of some polyester materials.
The scaffolds prepared by electrospinning technology can guide cell differentiation and strengthen cell function. Different types of fiber scaffolds have great potential in the field of tissue engineering (Table 1). In order to study the effect of fiber arrangement of scaffolds on the differentiation of phenotype of cardiomyocytes, Parrag et al.49 used an electrospinning technique to fabricate the biodegradable polyurethane (PU) into nanofiber scaffolds. Cardiomyocytes derived from mouse embryonic stem cells (mESCs) were first inoculated into mouse embryonic fibroblasts (MEFs), and then inoculated on PU scaffolds. A series of tests have shown that neatly arranged nanofiber scaffolds can improve sarcomere tissue compared with cells on aligned scaffolds. The aligned scaffolds led to the anisotropic organization of rod-shaped cells, and improved the sarcomere organization when compared to cells on the unaligned scaffolds. These results show that the arrangement of fibers can induce the differentiation of mESCs. Nanofibers can not only induce cardiomyocytes to differentiate more phenotypes, but also prepare corneal tissue stroma to replace damaged tissue.15 Poly-l-lactic acid (PLLA) nanofibers perform well in this respect. Through the preparation of acellular PLLA nanofiber networks, it was observed from the microscopic point of view that the randomly arranged fiber scaffolds are beneficial to the adhesion of corneal cells. They are very close to the natural state of cell morphology in the process of cell culture, so it shows the high bionic property of the PLLA nanofiber corneal architecture. Moreover, the fibrous stent can load eumelanin in the treatment of neurodegenerative disorders.50 A single material may not be able to fulfill the needs of tissue engineering scaffolds, so PLA and its copolymers can be compounded with natural materials (e.g., chitosan (CS), collagen) to optimize their properties (Table 1). For example, the multistage electrospun membrane was prepared by combining water-soluble collagen and PLA through pattern electrospinning.51 Compared with the single polymer, the addition of natural collagen and modified stereocomplex polylactide (sc-PLA) can improve the mechanical properties of the fiber membrane (Table 2), and the mechanical properties increase gradually with increasing addition amount. Because the modified PLA has more of a three-dimensional structure, the prepared electrospinning membrane can guide cell penetration and attachment more effectively, thus accelerating cell growth. Adding lecithin to PLA for electrospinning can also increase the flexibility and hydrophilicity of the fiber. The addition of the thermoplastic material PU improves the brittleness of PLA, and further promotes the growth and proliferation of hepatocytes.52 In addition, materials for bone tissue, cartilage tissue and vascular transplantation can also be prepared. da Silva et al.53 used PLA and PVA as materials to prepare the nanofiber scaffold with a core–shell structure by electrospinning as the carrier of the BMP-2 protein, and applied to the recovery of alveolar bone tissue. Su et al.54 compounded CS and calcium silicate into an electrospun PLA mat to observe the adhesion and proliferation of human bone marrow mesenchymal stem cells (hMSCs) on CS/CH–PLA. The results indicated that the cells could clearly proliferate, and the addition of CS and calcium silicate could promote the proliferation and osteogenesis of hMSCs. Li et al.55 prepared a PLA–silk fibroin (SF) nanofiber scaffold and studied the interaction between cell adhesion and proliferation. The results demonstrated that the scaffold had good hydrophilicity, and could effectively support the proliferation of chondrocytes. The scaffold was also beneficial to the formation of new cartilage tissue in vitro. If electrospun nanofibers are to be used as vascular grafts, they need to have the same tubular structure as human blood vessels (Fig. 3). On this basis, an appropriate pore size and cell penetration ability are needed. Of course, in order to give more function to the vascular graft material, different active ingredients can be added in the preparation process: vascular endothelial growth factor (VEGF), antiplatelet drugs (like aspirin), dipyridamole and anticoagulant drugs (like heparin).56 The addition of these ingredients makes the biological characteristics of the graft more similar to that of natural blood vessels. Li et al.57 designed a graft based on the fact that endothelial progenitor cells (EPCs) can induce vascularized bone regeneration. The graft immobilized the bioactive peptides on the PCL/PGC nanofiber scaffolds by polyglycolic acid, achieved the effective immobilization and continuous release of bioactive peptides, and guided the vascularized bone regeneration under the induction of EPCs. These dual-peptide functionalized nano-fibrous scaffolds show amazing advantages in early angiogenesis and osteogenesis. Moreover, Fiqrianti et al.58 blended PLLA, collagen and CS to prepare a vascular graft, and studied the tensile strength, burst pressure and blood compatibility of the graft. It turned out that the graft conforms to the standards of high compatibility and low cytotoxicity, and had the potential for further development. Yin et al.59 also prepared vascular grafts with polyester, collagen and CS. However, they adopted a coaxial electrospinning method, and the grafts showed satisfactory properties.
Synthetic materials commonly used in electrospinning, and the expected uses of the prepared biological materials in tissue engineering.
| Synthetic materials and additives | Spinning methods | Scaffold diameter | Cells for research | Intended use | Reference |
|---|---|---|---|---|---|
| PLLA | Single polymer solution spinning, uniaxial | 0.65 ± 0.03 μm | Decellularized corneal matrix | Corneal tissue engineering | 15 |
| PU | Single polymer solution spinning, uniaxial | 0.6–7 μm | mESCDCs | Cardiomyocytes for cardiac tissue engineering | 49 |
| PLA/EU | Single polymer solution spinning, uniaxial | — | Neuronal-like cell line (SH-SY5Y cells) | Neurodegenerative applications | 50 |
| PU/lecithin | Mixed solution spinning, uniaxial | 146.9 ± 33.7 nm | Hepatocyte | In vitro hepatocyte culture | 52 |
| PLA/CS/calcium silicate | Mixed solution spinning, uniaxial | — | hMSCs | Bone tissue engineering | 54 |
| PLLA/SFA/SF | Mixed solution spinning, uniaxial | 0.77 ± 0.16 μm | Chondrocytes | Cartilage tissue engineering | 55 |
| PLLA/collagen/CS | Mixed solution spinning, stainless-steel rod collector | 89.33–246.7 nm | Lymphocyte T cell | Vascular graft tube | 58 |
| PLCL/collagen/CS | Mixed solution spinning, coaxial | 517 ± 112 nm | Porcine iliac artery endothelial cells (PIECs) | Vascular graft tube | 59 |
| PVA/PLA | Mixed solution spinning, coaxial | 149 ± 16.9 nm | MC3T3-E1 subclone 4 strain cells | Recovery of alveolar bone tissue | 53 |
| PLA/hydrosoluble collagen | Pattern template, Electrospinning | Around 200 nm | L929 cells | Scaffold, biosensor, or biofilter in tissue engineering | 51 |
| PLA/tussah silk fibroin (TSF) | Mixed solution spinning, uniaxial | 500 nm | Mouse mesenchymal stem cells (MSCs) | Repair structural bone | 60 |
| PLGA/poly(d,l-lactic acid) (PDLLA) | Mixed solution spinning, uniaxial | 2.5 ± 0.2 μm | Human embryonic kidney (HEK) 293T cells | Guided tissue regeneration | 61 |
| PLA/GO-g-PEG | Mixed solution spinning, uniaxial | 593 ± 98 nm | Swiss mouse NIH 3T3 ECACC cells | Tissue engineering | 62 |
| PCL/PGC | Mixed solution spinning, uniaxial | — | Endothelial progenitor cells | Vascularization; bone regeneration | 57 |
Mechanical properties of electrospun films with different morphologies.51 Copyright 2018, Elsevier.
| Groups | Breaking stress (MPa) | Breaking strain (%) | Young's modulus (MPa) |
|---|---|---|---|
| PDLA random | 3.9 ± 0.4 | 11.8 ± 3.0 | 302 ± 27 |
| PLLA random | 4.1 ± 0.3 | 13.2 ± 1.1 | 314 ± 30 |
| sc-PLA random | 4.8 ± 0.8 | 23.7 ± 2.8 | 331 ± 51 |
| sc-PLA/5% collagen random | 5.4 ± 0.4 | 18.0 ± 2.2 | 397 ± 29 |
| sc-PLA/10% collagen random | 5.7 ± 0.6 | 19.5 ± 3.7 | 446 ± 43 |
| sc-PLA/15% collagen random | 8.1 ± 0.7 | 15.1 ± 2.9 | 475 ± 35 |
Fig. 3. Tubular materials similar to the shape of blood vessels.

From the published research literature, it can be seen that electrospinning technology plays a very wide range of applications in tissue engineering, mainly by guiding cell differentiation and tissue regeneration to achieve the application of scaffolds in different types of tissue engineering. Of course, it is also an excellent candidate for bone tissue, cartilage tissue and vascular grafts because the microstructure of the nanoscaffolds has significant advantages. From Table 1, we can clearly understand that the materials for preparing stents are synthetic or natural biodegradable polymer materials. These special properties make this kind of material become a research hotspot in the field of biomedicine. In addition, the diameter of the scaffold is at the nanometer or micron level, which provides an ideal platform for the application of scaffolds in different fields of tissue engineering.
In the process of searching the literature, we found that neatly arranged ordered fibers have surprising performance in some aspects.49 We all know that the nanofiber scaffolds prepared by electrospinning have the characteristics of high porosity. In the spinning process, the tip of the “Taylor cone” is randomly scattered on the receiving end under the action of an electrostatic force. The microcosmic arrangement of the nanofibers is disordered, which leads to the formation of high porosity of the scaffolds. However, the physical state of the scaffold synthesized by each fiber set is orderly as a whole, and this orderly arrangement of fibers can meet the needs of the directional growth of muscle cells, vascular endothelial cells and neuronal cells.51,58
Yang et al.63 prepared the aligned PLLA nano/micro fibrous scaffolds, and observed that neural stem cells (NSCs) can grow along the electrospinning direction on the nanofibers. Xu et al.64 used poly(l-lactide-co-ε-caprolactone) [P(LLA-CL)] as a polymer material to obtain scaffolds with a fiber diameter of 500 nm, which mimics the circumferential orientation of the cells and fibrils found in the medial layer of a native artery. Using the smooth muscle cell (SMCs) as a model cell, it was observed that SMCs could grow along the fiber direction.
Wound dressing
There are four wound healing stages: hemostasis, inflammation, proliferation and remodeling. When acute wounds (cuts, trauma) are formed, these four processes are triggered over time. In the early stages of wound healing, it is often infected. It shows persistent abnormal inflammation, which increases the permeability of the capillaries, causing tissue fluid in the body to extravasate from capillaries.65 At this time, a dressing is needed to absorb the exudate from the wound, block the contact between the wound and the bacteria and microorganisms in the external environment, and avoid the occurrence of an inflammatory reaction.
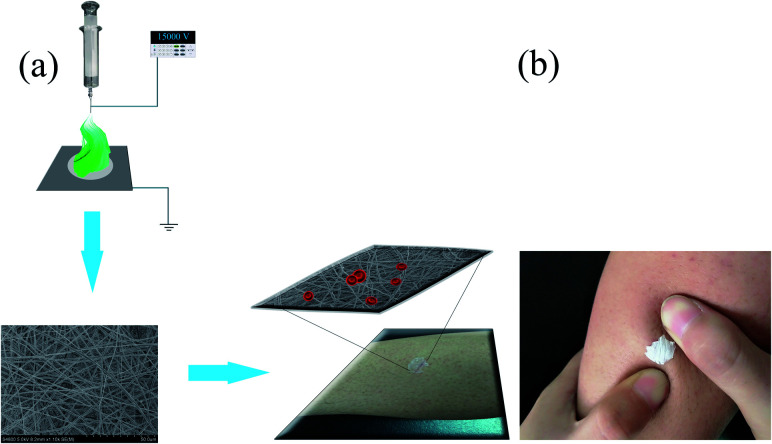
Medical wound dressing is a material used to cover the wound, and its main function is to absorb the exudate from the wound and protect the wound from the pollution of bacteria and dust in the air.66 The ideal wound dressing should have the following characteristics: (a) good hemostatic ability and absorptive capacity; (b) suitable voids in exchange for gases; (c) good biocompatibility, easy to remove, and low cost.67 Suitable wound dressings, such as materials made of biopolymer polymers, can reduce the incidence of infection and accelerate healing. This is especially true for biopolymer nanofiber dressings, which can increase the specific surface area. Then, antibiotics or antimicrobials are delivered to the wound environment to control infection.65,68 The application of a nanofiber membrane prepared by electrospinning technology for wound dressings fully satisfy the above characteristics, and it has many advantages. First of all, the materials for preparing the dressings are natural or synthetic biodegradable polymer materials (such as PLA, PVA). This satisfies the requirements of biocompatibility, and avoids the secondary injury of traditional dressings. Secondly, the nanofiber membrane can effectively hinder the contact between the external dust, bacteria and the wound, which is conducive to wound recovery. Finally, its high porosity is conducive to cell adhesion and proliferation, and the loose porous structure can exchange gases for wound healing.7,69Fig. 4(a) shows a schematic diagram of the process of preparing nanofiber membranes by electrospinning, and applying it to the wound dressing. Through observation, it can be found that the diameter of the fiber membrane is very small and uniformly distributed, showing a loose porous structure. The fibrous membrane is attached to the wound as a wound dressing (Fig. 4(b)). Its surface can adhere well to the skin. From a microscopic point of view, it was found that the reticular structure of the fibrous membrane can block the escape of blood cells, thus achieving the effect of hemostasis. In addition, the characteristic structure of the nanofiber membrane can slow down the rate of drug release, so as to avoid the toxicity and side effects of sudden drug release into the human body.70
Fig. 4. (a) Schematic diagram of the process of preparing a nanofiber membrane by electrospinning, and applying it to a wound dressing. (b) Nanofiber membrane is applied to the wound.
Loading drugs on nanofiber membranes is a common means to achieve antibacterial purposes. Under this background, many compounds with antibacterial activity have been widely studied, such as CS, a natural substance with antibacterial activity,71,72 the antibacterial drug ketoprofen,73 and antibiotics.74,75 Ranjith et al.72 reviewed the latest research progress of CS nanofiber wound dressings, and systematically introduced the latest research on CS-based nanofiber membrane-loading therapeutic agents (e.g., curcumin, asiaticoside, ferulic acid, tannic acid). It involved a variety of compounds with antibacterial activity from natural plants. Quercetin (from natural plants) is a kind of flavonoid that not only has anti-inflammatory, antibacterial and antiviral properties, but also has the effect of scavenging free radicals and can improve the process of wound healing.76 Based on this, Kost et al.77 prepared quercetin-loaded polylactic acid fibers. The star-shaped chiral polymers were synthesized by ring-opening polymerization, and then PDLA was prepared by β-cyclodextrin as the initiator. Next, quercetin and the above polymers were dissolved in CH3Cl to obtain inclusion complexes. Electrospinning technology was then used to prepare nanofibers with good antibacterial activity, which revealed their great potential in anti-microbial infections from bacteria such as Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli). Essential oils of clary sage and black pepper also have antibacterial activity, and are from natural plants. The nanofiber membrane obtained by combining it with PLA demonstrated antibacterial activity against E. coli and Staphylococcus epidermidis (S. epidermidis), and also had the potential to regulate cell behavior.78 In the aspect of an antibacterial property, lactoferrin also has good performance. Machado et al.79 prepared the PLA–bovine lactoferrin composite film as an antibacterial wound dressing, which manifested antifungal activity against Aspergillus nidulans by inhibiting spore germination and mycelial growth. Sponge spicule is an interesting substance. It comes from the animal kingdom and has good antibacterial properties. Wu et al.80 committed to the research of new biomaterials with antibacterial properties. He and his team compounded siliceous sponge spicules (SSS) and PLA to obtain nano-scale PLA/SSS composite fibers, which showed good cytocompatibility and antibacterial properties.
The drugs loaded on the fiber mat are not limited to antimicrobial agents. Other substances with antibacterial properties have also been widely studied (Table 3). Including antibacterial nanoparticles (such as zinc oxide, titanium dioxide, silver and gold), these nanoparticles exhibit some advantages because of their higher thermal stability. This will endow wound dressings with better antibacterial activity and other physical properties.69 Silver ions are a well-known metal ion with antibacterial properties, which can kill a variety of pathogenic microorganisms. Silver nanocomposite fibers can be obtained from synthetic nanoparticles or some silver salt solutions. Zhang et al.81 prepared a silver(i) metal–organic frameworks–polylactic acid composite fibrous mat with high antibacterial activity. The fiber mat has unique broad-spectrum antibacterial activity against E. coli, Pseudomonas aeruginosa (P. aeruginosa), S. aureus and Mycobacterium smegmatis (M. smegmatis). The antibacterial mechanism may be related to the dissipation of proton power, the destruction of the energy metabolism pathway and the loss of a transport system. Alippilakkotte et al.82 reported the green synthesis of colloidal silver nanoparticles, in which plant extracts with medicinal value were introduced as reducing agents. In order to further enhance the antibacterial activity of the system, they used balsam pear extract to reduce silver and synthesized colloidal Ag-NPs in an organic medium to obtain highly uniform nanoparticles. So as to stabilize the silver nanoparticles, PLA was added to the organic solvent to reduce the size of the colloidal Ag-NPs and narrow the particle size distribution. Finally, PLA/Ag nanofibers were prepared by electrospinning. The upshot showed that the fibers could promote the proliferation of epidermal cells and fibroblasts, and to repair wounds. Similarly, Alipour et al.83 also studied the wound healing properties of electrospun nanofibers with silver nanoparticles. The dermis regeneration of the dressings was studied by animal model, and it was found that the silver nanoparticles could significantly promote the wound healing of New Zealand white rabbits.
Electrospun fibers loaded with drugs or active components, and their antibacterial properties.
| Polymer | Antimicrobial agents or other antibacterial substances | Nozzle type | Antibacterial properties | Cell models and cytotoxicity | Reference |
|---|---|---|---|---|---|
| PCL/CA/PEO | CS | Double nozzle | — | L929 mouse fibroblast cells; no cytotoxicity | 71 |
| Eudragit® S100 (ES100) | Nitrofurazone | Single or coaxial nozzles | E. coli | — | 74 |
| PU–Mt | Tetracycline hydrochloride | Single nozzle | S. aureus and E. coli | Human fibroblast cell; apoptosis occurs when the drug concentration is high | 74 |
| PLA/MβCD | Quercetin | Single nozzle | S. aureus, E. coli and Klebsiella pneumoniae | — | 77 |
| PLA | Essential oils (EOs) of clary sage and black pepper | Single nozzle | E. coli and S. epidermidis | — | 78 |
| PLLA | Bovine lactoferrin (bLF) | Single nozzle | S. aureus, and P. aeruginosa | Human skin fibroblasts (BJ-5ta cell line); no obvious cytotoxicity | 79 |
| PLA | Siliceous sponge spicules (SSS) | Single nozzle | S. aureus and E. coli | CCD966SK cell; good cell compatibility | 80 |
| PLA/HBTC/im | Ag ions | Single nozzle | E. coli, P. aeruginosa, S. aureus and M. smegmatis | — | 81 |
| PLA | Colloidal nanosilver | Single nozzle | S. aureus and E. coli | L929 cells; it shows a toxic effect with increased silver concentration | 82 |
| PVA/PVP/PEC/MF | Silver nanoparticles (AgNPs) | Single nozzle | E. coli, P. aeruginosa, and S. aureus | HSF-PI 18 fibroblast cells; no obvious cytotoxicity | 83 |
| PCL/CS | Silver nanoparticles (AgNPs) | Coaxial nozzles | S. aureus and E. coli | — | 84 |
In order to make the composite polymer fiber membrane perform better, a more sophisticated technology can be used to create nanofibers with a core–sheath structure, which is coaxial electrospinning technology. Kalwar et al.84 designed an electrospun fiber based on PCL/CS (core/sheath). The addition of CS improved the hydrophilicity and biocompatibility of the electrospun fiber. On the other hand, CS interacted with silver nanoparticles in the form of hydroxyl and amide, so that the silver nanoparticles were uniformly dispersed in CS. Additionally, the obtained fiber materials had a good inhibitory effect on the growth of S. aureus and E. coli in vitro.
The nanofibers obtained by electrospinning must have appropriate in vivo properties, e.g., good biocompatibility and low cytotoxicity, if they are to become potential application materials in the field of wound dressings to ensure that cells in the body can survive effectively when in contact with the dressing. Therefore, it is necessary not to use or minimize the use of hazardous solvents during the preparation process.71 In addition, wound dressings are generally applied to the affected area to have an effect. Thus, the nanofibers as an alternative material should have appropriate mechanical properties. On the other hand, compared with mechanical properties, good biocompatibility and low cytotoxicity are the most important conditions to judge nanofiber materials.
It should be emphasized that in the research literature listed above, it can be found that some have studied the biocompatibility and cytotoxicity of composite fiber pads, while only a few have studied the wound healing ability of dressings. They used a rat full-layer skin infection model to test the anti-infective healing effect of the composite fiber dressings. The results indicated that the wound healing speed was accelerated, and the healing rate was as high as 99.9%. Furthermore, the antibacterial mechanism was also discussed. From the point of view of the structure, the antibacterial activity was closely related to the skeleton knot of the composite fiber dressing because the three-dimensional structure of the fiber membrane could realize the controlled release of antibiotics to a certain extent. It has realized the strong development situation of the new strategy of antibiotic-free antibacterial dressings in the field of trauma treatment.81 Furthermore, Kost et al.77 proposed that nanofibers as antibacterial dressings may interact with antibacterial substances on the cell wall, and affect cell membrane production. This view is helpful to further clarify the mechanism of wound dressings, and lay a foundation for the follow-up study of biocompatibility and cytotoxicity.
Drug delivery system
Because of its structural characteristics, the electrospun fiber membrane can be used in drug delivery systems because it can directly wrap drugs. Hydrophilic or hydrophobic drugs and metal nanoparticles can be loaded onto the electrospun membrane to exert better function (Table 4). Compared with other forms of drug carriers, such as liposomes and nanoparticles, electrospinning technology provides great flexibility in the selection of drug delivery materials and drugs. The electrospun fiber membrane has the characteristics of large drug loading, high entrapment efficiency, simultaneous delivery of a variety of therapeutic drugs, and simple operation. The drug release behavior is decided by the drug diffusion and degradation in the carrier polymer, while the electrospinning technology can control the drug distribution in the fiber. The three-dimensional network structure of the fiber can change the drug release strategy and reduce the frequency of sudden drug release. Consequently, it is easier to achieve controlled drug release.85–87 For example, Zhu et al.88 prepared biodegradable PLLA nanofibers using lovastatin as a model drug to reduce the risk of cholesterol, stroke and heart attack. The characterization results proved that adding a certain amount of lovastatin can improve the orientation and surface smoothness of the fibers. The study of drug release in vitro shows that the release behavior is divided into two stages. The release rate of the first stage is fast, while the second stage becomes very slow to achieve the purpose of sustained release.
Structure and release behavior of electrospun fibers loaded with some drugs.
| Polymer | Fiber structure | Spinning type | Loaded drug | Drug release study | Bacteriostatic activity/cytotoxicity | Reference |
|---|---|---|---|---|---|---|
| PCL | Single and binary ketoprofen-loaded mats of ultrathin fibers | Ordinary solution spinning and emulsion spinning | Ketoprofen | Single mats exhibited burst release/binary mats exhibited sustained release | No sign of cytotoxicity | 73 |
| PLA | Core/shell structure | Emulsion spinning | Vancomycin hydrochloride | Sudden release in the initial stage (about 10% in 5 hours) and then slow release | S. aureus | 89 |
| PLA/collagen | Core/shell structure | Coaxial and uniaxial electrospinning | Gentamicin | The early release rate is fast, and there is a certain degree of retention behavior before reaching equilibrium | E. coli, P. aeruginosa, S. epidermidis; without any cytotoxicity | 91 |
| PMMA/PCL | Core/shell structure | Coaxial electrospinning | Nimesulide | The release of the drug in the shell is faster and more complete (almost 50% over 20 h), and the release of the drug in the core is slow (cumulative release 20% over 20 h) | — | 93 |
| GMS/EC | Core/shell structure | Modified coaxial electrospinning | Berberine hydrochloride | Showed good slow release performance (about 90% of 20 h release) | — | 94 |
| PLA/cellulose acetate | Core/shell structure | Coaxial wet-electrospinning | Citalopram | — | Have a positive effect on cell viability | 96 |
| PCL | Core/shell structure | Coaxial wet-electrospinning | BSA | Sudden release at the initial stage, followed by slow release, and the release rate decreased with the increase of PCL content | No cytotoxicity and good biocompatibility | 97 |
| Gliadin/cellulose acetate | Core/shell structure | Tri-axial electrospinning | Ibuprofen | Extended release duration and eliminated initial burst release | — | 101 |
| PLGA/PVP | Core/shell structure | Coaxial electrospinning | Metronidazole/naringin | The sudden release of MNA was more than 60% in the first hour, and the initial release of NAR was slow | Fusobacterium nucleatum; no cytotoxicity | 102 |
| PLLA | Core/shell structure | Emulsion spinning | Domiphen® | The sudden release of the drug was less, and entered the stage of drug release on the platform within 48 h | — | 105 |
| PCL | Hollow magnetic fibers | One-step coaxial electrospinning | Ketoconazole/Fe3O4 NPs | Rapid release within the first 0.5 h, followed by sustained release within 360 h | Has potential cytotoxicity | 107 |
Most of the studies focused on the effect of the morphology of electrospun nanofibers on drug delivery, such as core–sheath structures and hollow structures. The core–sheath structure nanofiber is a novel type of nanofiber, which is composed of two independent parts: an internal “core” and an external “sheath” layer. Since these two parts are spatially independent, they each perform their own duties.89 Many research studies have shown that nanofibers with core–sheath structure have many advantages in drug delivery:47,84 (a) drugs of different properties can be loaded and released at the same time to achieve biphasic drug release; (b) some unstable drugs can be protected in core materials; (c) the drug release behavior is controlled by the core and sheath, and this special structure can inhibit the initial sudden release of the drug, thus providing an appropriate release rate for the purpose of treatment (Table 4). In general, nanofibers with a core–sheath structure are prepared by coaxial electrospinning and emulsion electrospinning.90 Coaxial electrospinning is a method to prepare nanofibers with a complex structure through two or more concentric aligned nozzles. For example, Torres-Giner et al.91 utilized coaxial electrospinning technology to prepare PLA/collagen composite nanofibers containing gentamicin. It was found that the composite fibers could be used as an advanced drug delivery system with good controllable antibacterial properties. Furthermore, Pant et al.92 found that a large number of studies have reported that core–sheath nanofibers were prepared by coaxial electrospinning, and used for drug delivery systems. Therefore, they reviewed the physical phenomena generated during coaxial electrospinning, the influence of various parameters on the spinning process, and the application and challenges of nanofibers with this structure in drug delivery. For instance, the solution parameters include the solvent type, polymer molecular weight, solution concentration, viscosity, and electrical conductivity. The process parameters include the applied electric field voltage, distance from tip to trap, and flow rate. The environmental parameters include the temperature and relative humidity. They proposed that the spinning equipment could be further studied and improved to make this technology more attractive for large-scale production. Besides, hydrogels could be introduced into the composite system to change the properties of the drug delivery system. Simões et al.93 successfully prepared the drug loading system of the anti-inflammatory drug nimesulide using polymethyl methacrylate (PMMA) and PCL as raw materials by coaxial electrospinning. It turned out that the diameter of these fibers was larger than that of the control fibers (prepared by PMMA and PCL), and the existence of nimesulide improved the thermal stability of the core–sheath fibers. Moreover, drug release studies showed that nimesulide was released faster when it was located in the shell, which proved that the PMMA–PCL core–sheath structure fibers have great application potential in the loading of hydrophobic drugs. In nanofibers with a core–sheath structure, sheath polymers can not only contribute to the long-term release of drugs, but also protect the internal core components from direct exposure to the biological environment,22 enabling the drug to be released safely and controllably from the nanofiber membrane. As mentioned above, the preparation of electrospun fibers with a core–sheath structure was based on PCL/CS.84 Hai et al.94 continued to explore the application of nanotechnology in drug carriers. He and his group improved coaxial electrospinning to prepare novel nanofibers with a hybrid structure. Glycerol monostearate was used as the shell material, and berberine hydrochloride and ethyl cellulose were loaded internally. In vitro dissolution experiments showed that this structure had a good sustained release effect, and is better than monolithic fibers in the initial drug release.
Wet electrospinning is an improved electrospinning method that can be used to prepare fibers with a core–sheath structure without adding special chemical additives to the fibers. In this method, the traditional metal-receiving terminal is replaced by the liquid-receiving terminal. It is a simple and effective method to prepare nanofibers with three-dimensional structure.95 Thus, Mahdi et al.96 used PLA as the core material and cellulose acetate as the fiber layer to prepare three-dimensional fibrillated biodegradable scaffolds by coaxial wet electrospinning. Gelatin nano-carriers loaded with antidepressant citalopram were also coated on the scaffolds to promote the attachment and proliferation of nerve cells, showing the potential of nerve regeneration. Similarly, Rafiei et al.97 also used a new method of coaxial electrospinning and wet electrospinning to develop a core–sheath three-dimensional sheet scaffold. The scaffold uses PCL as shell material, and is loaded with bovine serum album internally. It has sufficient porosity and suitable pore size to meet the needs of cell growth, and the in vitro release test also reflects sustained release performance. We can then come to the conclusion that coaxial electrospinning is a promising method to maintain the bioactivity of the drugs loaded on the fibers. Triaxial electrospinning (using three concentric nested needles) is also an improved electrospinning method. Although there are few studies, it has shown potential in adjusting the mechanical properties, biological properties and drug release of the materials.98–100 Yang et al.101 applied triaxial electrospinning to prepare a new structure of core–sheath nanofibers, the internal structure of which is coated with drugs, and the outermost layer is thin cellulose acetate. They make core–sheath nanofibers by simultaneously treating external solvents, non-electrospun intermediate fluids and electrospun cores. Its microstructure has linear and cylindrical morphology, and the core–shell structure is clear. In addition, the outermost coating of cellulose acetate avoids the initial sudden release of ibuprofen.
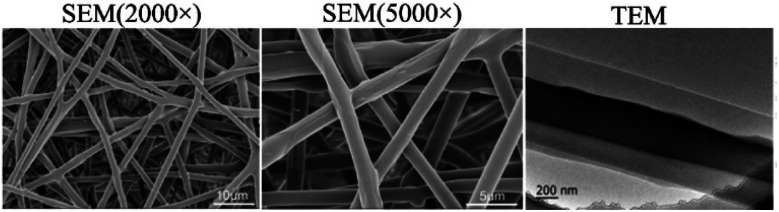
As mentioned above, core–sheath nanofibers are excellent carriers for loading two different drugs. He et al.102 prepared double drug-loaded nanofibers with core–sheath structure by coaxial electrospinning. Naringin and PVP were used as core materials to promote tissue regeneration, and metronidazole and PLGA were used as the shell fibers. Transmission electron microscopy analysis showed that the fiber had obvious core–sheath structure (Fig. 5), and the addition of double drugs reduced the tensile strength and elongation of the coaxial fiber. The in vitro release test indicated that metronidazole was released in the fiber for a short time. Conversely, naringin showed long-term release behavior, which effectively inhibited the proliferation of anaerobes. This dual drug-loading model is also used to treat corneal abrasions, a behavior that can cause corneal infection and scarring. If there is a drug delivery system that can prevent both infection and scar formation, it will greatly reduce the risk of infection and scar formation.103 Therefore, in one study,104 antibiotics and antiscar agents were loaded into different chambers by coaxial electrospinning to achieve two therapeutic purposes. They used PLGA as the shell material, which was loaded with pirfenidone and moxifloxacin to make a smooth surface fiber. The characterization outcome showed a clear core–sheath structure, and the existence of two drugs were observed in the fiber. It also demonstrated a sustained release effect, which can be used to treat corneal abrasions.
Fig. 5. SEM and TEM images of double drug-loaded nanofibers with core–sheath structure.102 Copyright 2018, Elsevier.
On the other hand, compared with coaxial electrospinning, only one nozzle is needed for the preparation of core–sheath nanofibers by emulsion electrospinning. This shows that emulsion electrospinning can be used in the development of core–sheath nanofibers because of its convenience. A stable emulsion is the key to obtaining core–sheath structure nanofibers, so it is very important for emulsifiers to prepare a static spinning solution. Cai et al.89 adopted emulsifiers that were different from traditional surfactants, and they used Pickering emulsion electrospinning to prepare functional nanofibers for the first time. In this way, magnetic iron oxide nanoparticles coated with oleic acid were used to prepare stable oil-in-water Pickering emulsions. The addition of these magnetic nanoparticles made the system stable because it could self-assemble, and thus inhibit the aggregation of droplets. Then, the nanofibers with good antibacterial and mechanical properties were prepared by electrospinning via the addition of vancomycin hydrochloride and PLA to the emulsion. In general, when using an emulsion for electrospinning, it is necessary to add surfactants to the system to make the spinning solution into a water-in-oil or oil-in-water emulsion. As we all know, there are many types of surfactants, among which anionic surfactants are irritating to the mucous membrane. Nonionic surfactants are easily soluble in water, and are easily affected by the spinning environment during spinning. So, Wang et al.105 found dodecyldimethyl(2-phenoxyethyl)ammonium bromide (commercially named Domiphen®) to be a suitable cationic surfactant. Although it is also toxic, it can effectively reduce the surface tension, and possesses strong emulsifying ability. They dissolved PLLA to separately form the homogeneous solution and water-in-oil emulsion with Domiphen®, and then prepared nanofibers by electrospinning. The interaction and release behavior between Domiphen® and PLLA in the structure of the PLLA-electrospun fiber was revealed in depth. It was revealed that Domiphen® was easily complexed in the fiber, but existed in different ways. Domiphen® was mostly accumulated at the edge of the fiber cross section. Part of it was accumulated in the inner position of the cross section, and the release behavior showed that the cavity formed by the evaporation of water in the inner phase accumulated more Domiphen®. Through this study, it was found that these fibers can load and control the release of therapeutic drugs.
Similar to core–sheath nanofibers, hollow nanofibers also have many advantages.106,107 The loading and release of drugs in hollow nanofibers will reduce the side effects caused by the long-term transport of matrix materials. In addition, it can increase the interaction surface area to facilitate the double-sided release of thinner tubular structures. Currently, the main preparation methods of the hollow fiber are template synthesis and coaxial electrospinning.108,109 In the preparation of hollow nanofibers, coaxial electrospinning is of more concern for researchers. Wang et al.106 synthesized the triggered drug release hollow fiber by coaxial electrospinning. In the process of electrospinning, antifungal ketoconazole and Fe3O4 nanoparticles were encapsulated in polymer materials in the form of composites. They used dimethyl silicone oil as a flow solution in the fiber, which flowed out of the microstructure of the fiber to form a hollow fiber. The outcome showed that the addition of antifungal drugs had a great effect on the fiber morphology, drug loading rate and release rate. The in vitro release test showed that the release rate was very fast at the initial stage, and slowed down at the later stage. The analysis of this phenomenon is as follows: the early rapid release may be due to the distribution of the drug on the outer surface and inner surface of the composite fiber, and the contact release medium will directly enter the solution. In the later stage, because part of the drug is encapsulated in the polymer, the drug needs to cross the matrix material. Thus, the release rate slows down, and hundreds of hours of release can be observed. Furthermore, the transdermal drug delivery system is also a branch of drug delivery system. Studies have illustrated that CS nanoparticles can deliver drugs to the skin through their unique physical and chemical properties. PLA is a safe biodegradable polyester, so graft copolymers composed of CS and PLA can be developed and applied in transdermal drug delivery systems.110 In order to ensure the low toxicity of the synthesis process and obtain the target product with high grafting rate, Engkagul et al.111 prepared a green grafting system for the first time. In the heterogeneous system, nano-sized CS whiskers can effectively cross-link with lactic acid and undergo polycondensation to form low-polylactic acid. The content of lactic acid can regulate the lipophilicity of the CS whisker and oligolactic acid-grafted polymer. The size of the nanoparticles can then be adjusted, as well as the entrapment efficiency of lidocaine loaded with hydrophobic drug.
The study of the drug release behavior is a project that any kind of drug carrier must study. In clinical drug use, it is the expectation of many drug research and development institutions, doctors and patients to achieve the sustained and controlled release of drugs as much as possible. If the slow and controlled release of the drug can be achieved, then the bioavailability and therapeutic effect of the drug will be significantly improved, and the effective blood concentration will be stabilized. As the carrier of the drug delivery system, the nanofiber has achieved a great leap from the traditional sudden release to slow release, which is the fundamental reason why it has been widely studied as a drug delivery carrier. Of course, the researchers will not stop here; the ultimate goal is to achieve the controlled release of the drug.
Coaxial electrospinning is widely used in drug delivery systems because it can produce very exquisite fiber structures (core–shell or hollow fibers) (Table 4), and it can give nanofibers two different properties (protection of internal core drugs and loading of biphasic drugs). This exquisite fiber structure has good performance in controlling drug release, and the drug release behavior of the drug loaded in this structure has undergone ingenious changes. Whether it is a core–sheath fiber or hollow fiber, the drug release is controlled by two different parts arranged in space, which slows down the initial sudden release of the drug and prolongs the release time. However, in some conclusions, it can be observed that there is a sudden release of a small number of drugs loaded on nanofibers at the initial stage (mentioned above is only a slow release). In the special structure of the nanofiber, the drug is not only wrapped in the inner core or the internal cavity of the hollow fiber, but also adsorbed on the outer surface of the nanofiber structure, resulting in a sudden release at the initial stage.93,97 To some extent, this is not a “satisfactory” phenomenon, although it is inevitable.
In addition to focusing on the drug release behavior, the biocompatibility, cytotoxicity and in vivo research play an extremely important role in the field of medicine. These properties are closely related to the selection of spinning solution solvents (Table 4). As we all know, organic solvents have varying degrees of toxicity, and their residues on nanofibers are also studied by many people. However, the raw materials of electrospinning are mostly high molecular weight polymers, most of which can only be dissolved in organic solvents. This forms a tangled contradiction. Therefore, the selection of solvents is a key step. On the basis of ensuring the dissolution of polymers, it is a solution to avoid this contradiction by selecting the commonly used dissolving organic agents in the pharmaceutical field as far as possible. In addition, polymers can be combined with natural polymer compounds. This will not only improve the performance of the fiber to increase drug loading, but also avoid the problem of a large amount of organic solvents caused by the use of pure polymers.
Immobilization of enzymes
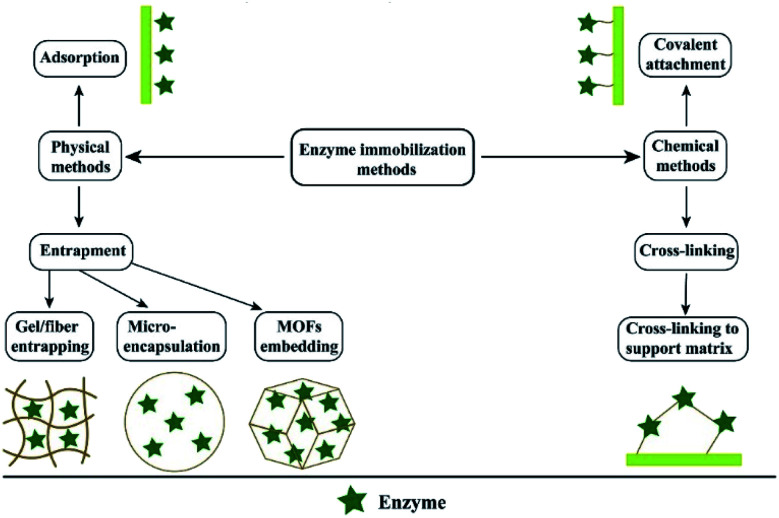
Immobilized enzyme technology is widely used in bio-pharmaceutical, chemical, cosmetic and food industries. For example, penicillin G-amylase is used in the production of β-lactam antibiotics, continuous production of high fructose corn syrup and cocoa butter analogues in the food industry, and the synthesis of acrylates and silicone esters or amides in the chemical industry. Myristic acid lipase is used in the cosmetic industry to produce myristic acid. In addition, oxygenase CSO2 is used to produce vanillin.112,113 The purpose of enzyme immobilization is to improve the rate and stability of biological reaction.114 In the actual industrial production process, almost all large-scale industrial operations are more inclined to immobilize the enzyme. Because this method can achieve continuous operation, the product purity is higher and easier to recover. The commonly used methods are physical immobilization (adsorption or embedding) and chemical immobilization (covalent binding or cross-linking)113 (Fig. 6). Usually, the relevant synthase is immobilized on the insoluble material to improve the durability and activity of the enzyme. Similar to the drug carrier, the activity of the enzyme is closely related to the properties of the carrier material.115 The reduction of the geometric size of the carrier material can greatly optimize the catalytic efficiency of the enzyme. This is because it can significantly reduce the diffusion resistance. Nanomaterials are ideal carriers for immobilized enzymes because of their large specific surface area, excellent chemical and mechanical properties and cost-effectiveness, and make enzyme immobilization more stable.114 Poorakbar et al.116 prepared magnetic gold mesoporous silica nanoparticle core–shells (mAu-PSNs) as the carrier, and the cellulase was immobilized on the nanoparticles by the covalent method of chemical immobilization. Fourier transform infrared (FTIR) spectroscopy confirmed the successful combination of the enzyme and nano-carrier. The characterization upshot explained that this method improved the thermal stability of cellulase. It provides the possibility for the long-term preservation of cellulase. Jankowska et al.117 prepared a novel electrospun polymethyl methacrylate/polyaniline fiber as the carrier material of laccase immobilization by two methods of adsorption and covalent combination, which demonstrated high activity and significantly improved stability. It still maintains more than 80% relative activity after repeated catalytic cycles for 10 times, and the decolorization test of adsorption laccase in model aqueous solution indicate a good decolorization rate. Another study118 adopted the polyacrylonitrile/gold salt solution as a spinning solution, and then the composite membrane was prepared by electroless gold plating. Laccase was immobilized on the surface of the composite film by the combination of the terminal amino self-assembly monolayer and glutaraldehyde cross-linking treatment, and the characterization outcome showed good recyclability. As everyone knows, the enzyme reaction is mild, and the temperature of the reaction is usually low. However, for those thermophilic enzymes, the practical application is usually limited due to the lack of enzyme stability. Thus, Tang et al. reported a method of immobilization of α-galactosidase from Thermotoga maritima using cross-linked PVA nanofibers at high temperature (105 °C). They found that the high specific surface area provided by the nanofibers could intercept the enzyme in the pores of the fiber, achieving very efficient immobilization, and it was important to maintain the activity and thermal stability of the enzyme.119
Fig. 6. The enzyme immobilization methods.120 Copyright 2020, Elsevier.
It is a difficult problem to separate the enzyme from the enzyme immobilization solution, and the appropriate carrier must have this characteristic.121 The nanofiber membrane performs well in this respect. Liu et al.120 reviewed various carriers conducive to lipase immobilization. It was mentioned that nanofibers with an ultra-high specific surface area, volume ratio and uniform diameter are suitable to be used as carriers for lipase immobilization, and can be easily separated from the reaction medium, which is exactly what we need. Electrospinning technology is an effective method for the preparation of nano-thin films. It also has a variety of characteristics mentioned above, so it is an ideal candidate material for the immobilized enzyme. Therefore, electrospinning technology has great application potential in the preparation of nano-carriers of immobilized enzyme (Table 5). With regard to the application of electrospinning technology in enzyme immobilization, Jia et al.122 used electrospinning technology to prepare enzyme-loaded polymer nanofibers as early as 2002, which is a unique biocatalyst to improve the catalytic efficiency of the immobilized enzymes. R-Chymotrypsin was loaded onto polystyrene nanofibers, and the nanofibers had high enzyme loading (1.4%) (wt/wt), equaling 27.4% coverage of the outer surface of the nanofibers. The apparent hydrolytic activity in aqueous solution is more than 65% of that of the natural enzyme, which shows high catalytic efficiency. Similarly, it also shows good activity in a non-aqueous solvent.
Enzyme immobilized on a nanofiber mat and enzyme activity.
| Nanofibers | Immobilized enzymes | Type of immobilization method | Enzyme activity | Reference |
|---|---|---|---|---|
| PMMA/PANI | Laccase | Adsorption and covalent binding | Retained over 80% relative activity after 30 days of storage and after 10 consecutive catalytic cycles | 117 |
| PAN/gold salt | Laccase | Adsorption | The enzyme activity after 7 days was 62.7% of that on the first day | 118 |
| PS | α-Chymotrypsin | Covalent binding | The activity of the immobilized enzyme was 65% of that of the free enzyme, and showed high activity in both aqueous and organic media | 122 |
| PVA | Cellulase | Covalent binding | The activity of the immobilized enzyme was more than 65% of that of the free enzyme, and the initial activity remained 36% after being reused 6 times | 124 |
| PVA | Cyclodextrin glucanotransferase | Covalent binding | Compared with the ordinary film, the enzyme activity immobilized on the nanofiber increased by 31% | 127 |
| P(GMA-co-MA)-g-PEO | Lipase | Covalent binding | The maximum activity of 0.673 U mg−1 | 130 |
| P(GMA-co-MA)/FP | Lipase | Covalent binding | The residual relative activity of the immobilized lipase was 62% after 7 reuses | 131 |
| PVA/BSA | Horseradish peroxidase | Covalent binding | The residual activity of the immobilized HRP were 73% after 11 reuse cycles | 132 |
| PVA/CS | β-Galactosidase | Add the enzyme directly to the solution | The catalytic activity of enzyme-NF was 57.03% of free enzyme activity at pH 6.8 and 4 °C | 133 |
| CNTs-COOH/GO | Lysozyme | Covalent binding | The enzyme was considerably active | 136 |
| PANCMA | Lipase | Covalent binding | The enzyme loading and the activity retention of the immobilized lipase on the nanofibrous membrane increase from 2.36 ± 0.06 to 21.2 ± 0.7 mg g−1 and from 33.9 to 37.6%, respectively | 137 |
| PS/PSMA | Bovine carbonic anhydrase | Covalent binding, precipitation, and cross-linking | The enzyme maintained 65.3% of its initial activity after being incubated in aqueous solution at room temperature under shaking at 200 rpm for 868 days | 138 |
As an insoluble polymer, cellulose is a low-cost substrate for the production of a large number of cellulose derivatives in the pharmaceutical field. Cellulase hydrolysis in a variety of production methods has attracted much attention because of its mild conditions.123 However, there are still some limitations in enzymatic hydrolysis, such as difficult separation and easy inactivation of enzymes, so the immobilized enzyme technology with the advantages of easy separation and easy purification of products can solve these limitations. We mentioned above that Poorakbar and his team116 used nanoparticles as carriers of cellulase to improve its thermal stability. Different from the research content of their team, Wu et al.124 used electrospinning technology to study the immobilization of cellulase on the PVA nanofiber membranes. They dissolved PVA and cellulase in acidic acetic acid buffer under the action of an external electric field. The fiber membrane with a diameter of about 200 nm was formed by electrospinning, and then glutaraldehyde water vapor was used as a cross-linking agent to investigate the biocatalysis efficiency of the fiber membrane. The upshots showed that the activity of the immobilized enzyme was more than 65% of that of the free enzyme, and still maintained a certain activity after being reused 6 times.
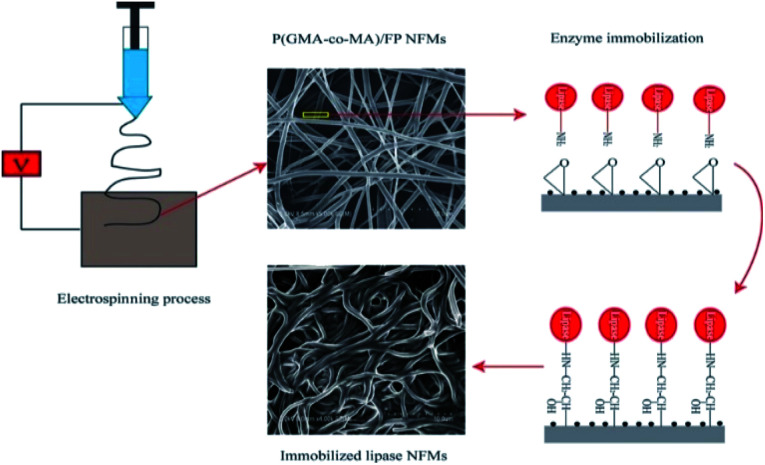
Compared with cellulose, cyclodextrin is also an important pharmaceutical excipient in the pharmaceutical field. It has a unique hydrophilic outer surface and the hydrophobic cavity can form inclusion complexes with guest molecules. In general, free cyclodextrin glucan transferase can catalyze starch to prepare cyclodextrin. However, the industrial price of free enzyme is relatively high and its stability is low. Therefore, the theme of this section in enzyme immobilization technology can be used to make this process more economical.125,126 Saallah et al.127 immobilized cyclodextrin glucan transferase on PVA nanofibers by electrospinning. It was then cross-linked with glutaraldehyde steam, and the ordinary film was set by loading with cyclodextrin glucan transferase and PVP as control. The results showed that the nanofibers showed better properties, and improved the loading and activity of the enzyme. Enzyme immobilization technology is not only suitable for the pharmaceutical industry to produce medicinal excipients, but can also be used to screen active components for use in clinical research and application. For instance, lipase inhibitors are a popular drug in the clinical treatment of obesity. With the prevalence of obesity, this treatment has received more attention. Thus, there is an urgent need to develop more lipase inhibitors. At present, the use of immobilized lipase to screen inhibitors is a promising research method by many scholars.128,129 Liu et al.130 synthesized a new terpolymer containing a reactive epoxy group and hydrophilic polyoxyethylene branched chain. The terpolymer was then prepared into a nanofiber by electrospinning technology, and then the lipase molecules were immobilized by epoxy covalent bonding, which has high enzyme loading and enzyme activity under the optimum immobilization conditions. In addition, the thermal stability, reusability and organic solvent stability of the immobilized lipase were inspected, and the results were satisfactory. In the same year, their team also prepared a new type of nanofiber membrane containing a reactive epoxy group and biocompatible feather peptide (FP) for lipase immobilization.131 The detailed preparation process is shown in Fig. 7. This study revealed that the nanofibrous membrane stabilized the enzyme conformation and improved the activity of the immobilized enzyme. Compared with the results of the previous study, this study also showed higher thermal stability and good reusability, and it is worth paying attention to the good durability of the immobilized enzyme in the organic solvent methanol. Furthermore, the pH tolerance was also examined in this study, and the results showed that the lipase immobilized on this carrier had a wide pH tolerance.
Fig. 7. Schematic diagram of the preparation process of lipase immobilized on electrospun nanofibers.131 Copyright 2018, Elsevier.
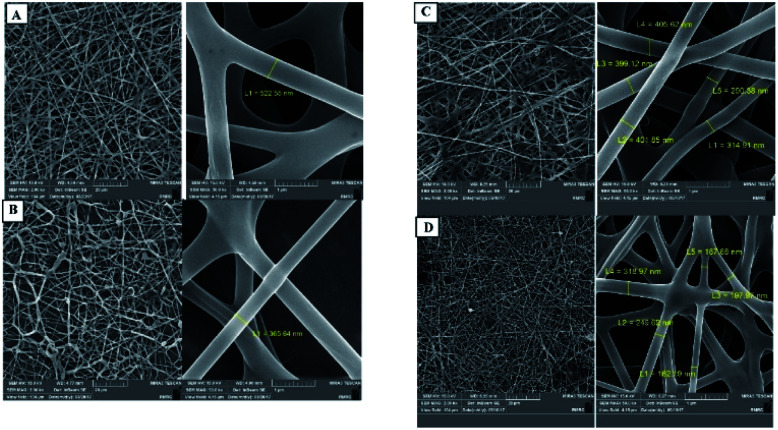
There are many reports about the application of electrospinning technology in the field of enzyme immobilization. Some research groups120 proposed that the preparation of a nanofiber carrier by electrospinning technology is the most general, simple and efficient method. There are usually two main ways to assemble the enzyme and the electrospun polymer.132 The first is to attach the enzyme directly to the outer surface of the nanofiber. Second, the enzyme is mixed with the polymer solution embedded in the nanofiber by the spinning process. Haghju et al.133 immobilized β-galactosidase on the CS/PVA nanofibers by the second method, and immobilized a specified number of β-galactosidase on the nanofibers. The effects of different polymer solution parameters (such as the CS/PVA mass ratio, electrical conductivity, spinning parameters) on the enzyme activity were also studied. The results showed that these parameters had no significant effect on the catalytic activity of the enzyme. It is well known that the spinning parameters affect the morphology and diameter of the nanofibers. With the change of the CS/PVA mass ratio and the increase of voltage, the fiber diameter decreases greatly. With the increase of the flow rate, the fiber diameter increased greatly, but the distribution range decreased (Fig. 8). Fazel et al.132 used the above two methods to prepare PVA and BAS biocomposite nanofibers. Horseradish peroxidase (HRP) was used as a model enzyme in order to obtain composites with ultra-fine diameter and high enzyme activity at the same time. Compared with HRP immobilized in an ordinary nanofiber membrane, the enzyme activity immobilized in PVA/BAS composite nanofiber was significantly higher, and had higher tolerance and reusability than the free enzyme. Their results show that HRP can be efficiently immobilized in PVA/BAS biocomposite nanofibers, which is a promising strategy. In addition, an electrospun nanofiber membrane combined with immobilized enzyme technology can be used to prepare materials with healing and anti-inflammatory properties to promote different types of wound healing and recovery. Melo-Brites et al.134 synthesized cellulose triacetate (CTAB) using cellulose acetate extracted from bagasse as the raw material. Bromelain was immobilized on the nanofiber membrane by glutaraldehyde cross-linking direct electrospinning method, which showed good enzyme activity. The in vitro release test showed that bromelain was completely released within 3 days. It has been reported that lysozyme is widely used in biomedical field.135 As its name implies, lysozyme can decompose the cell wall of bacteria by catalyzing the hydrolysis of glycosidic bonds. We have mentioned many times in the previous article that nanofibers have many advantages, and are widely used in tissue scaffolds and wound healing materials. So, Liu et al.136 used recycled cotton-based regenerated cellulose as basic materials in combination with nanotubes and graphene oxide (GO), integrated these materials into composite nanofiber pads by electrospinning technology, and fixed lysozyme on the fiber pads. The test results showed that the solidification of lysozyme did not affect the morphology of these composite fiber pads, and still maintained a three-dimensional structure. Cytotoxicity tests verified that the fiber pads had no cytotoxicity. Compared with the free enzyme, lysozyme had higher activity after solidification, and the antibacterial property test also proved that these fiber mats had higher antibacterial activity after the immobilization of lysozyme.
Fig. 8. Effects of different parameters (voltage, flow velocity) on the fiber diameter.133 Copyright 2018, Elsevier. (A) 20 kV, 0.5 ml h−1; (B) 20 kV, 0.1 ml h−1; (C) 30 kV, 0.5 ml h−1; (D) 30 kV, 0.1 ml h−1.
As a new type of technology with great application prospect, enzyme immobilization technology is valued by the majority of scholars. Of course, it is not limited to the biomedical field (preparation of bioactive materials, production of medicinal excipients, efficient catalysis of biological reaction). It also has good performance in filtration and sewage treatment117 (Table 5). It is a very important and necessary development trend to be able to connect technologies in different fields, and integrate the advantages of different technologies to achieve unexpected results. Electrospinning technology is well known because of its many advantages, so it is combined with enzyme immobilization technology. The nanofiber prepared by electrospinning technology as the enzyme carrier can provide the functional groups needed for enzyme immobilization. In addition, its uniform diameter and easy separation greatly improve the immobilization efficiency and catalytic efficiency.120
However, there is a phenomenon that has to be mentioned. In fact, the use of the nanofiber membrane for enzyme immobilization is still in a very primary stage of research. After obtaining the enzyme immobilized nanofiber membrane, most of the studies carry on the necessary characterization, and observe the activity and stability of the enzyme. Among them, only a few reports point out its potential application fields. For example, laccase immobilized on nanofibers can be used for the removal of pollutants from aqueous solutions and wastewaters, and as a raw material for flow-through reactors.117,118 Lysozyme nanofibers can be used as bioactive biomaterials. Furthermore, the application of this technique in actual production has not been reported, which is the same as the lack of in vivo studies on the wound dressings and drug delivery systems mentioned above. Despite the current situation, in practical work, it is more necessary to explore the potential application value of using nanofibers as the carrier of enzyme immobilization in the biomedical field (and even other fields) in order to promote the development of related fields faster and better.
Biosensor
Biosensor is a kind of analytical equipment that combines biologically derived molecular recognition signals with appropriate physical and chemical converters, usually by generating electronic or optical signals that interact with target recognition molecules to detect the change of the signal. It has the advantages of fast response, high sensitivity, and simple operation. It usually loads active enzymes with different functions to achieve the purpose of detection. It is widely used in food industry, agriculture, environmental monitoring, clinical testing and other fields.139,140
The combination of enzyme immobilization technology and biosensor has become a research hotspot. Its purpose is to further improve the stability of the enzyme used in the biological detection system, and the modification of the sensor surface plays a key role in this process. Various research groups have studied the role of different types of modification methods (Table 6) in sensor detection, and whether they have achieved good detection results. Unal et al.141 modified organoclay-montmorillonite (Mt) with PAMAM generation-2 (PAMAMG2) dendrimers, and PVA/PAMAM–Mt nanofibers were prepared by electrospinning with PVA as the polymer matrix. Glucopyranose oxidase was immobilized on the surface of the nanofiber-modified electrode to obtain the biosensor. It has been applied to the quantitative analysis of glucose in beverages, and the results show that the detection of glucose in samples can be realized without any interference. In another study,142 polyaniline and polystyrene nanofibers were formed by electrospinning. Cholesterol oxidase (COX) was immobilized on nanofibers by electrostatic layer-by-layer adsorption technique to make cholesterol biosensors. The electrostatic layer-by-layer adsorption technique is assembled into a film by alternately adsorbing charged proteins and materials with opposite charges from the solution by electrostatic interaction. The measurement results show that a precise response to the cholesterol concentration was obtained when 5 cycles of COX had been deposited on the nanofiber mat. Moreover, Chen et al.143 used electrospinning technology and the one-step reduction method to prepare ultrafine composite fibers based on gold nanoparticles and cross-linked zein. Studies have shown that a new type of biosensor based on the natural protein can be used for the detection of catechol. The high sensitivity of the biosensor is due to the electron transfer ability of gold nanoparticles and the catalytic ability to catechol. Another study144 proposed that GO and PVA were blended into a mixed solution, and the above solution was coated on the surface of the gold sheet by electrospinning to form nanofibers. The gold nanoparticles were then coated on the nanofibers. Finally, horseradish peroxidase (HRP)–Cu-nanoflowers were deposited on the nanofibers. The results showed that the nanofibers showed significant electrochemical response to the concentration of glucose. Coincidentally, Wu and Yin145 also carried out biosensors in glucose detection, which is quantitatively measured based on the sensitivity of hydrogen peroxide, an intermediate product catalyzed by glucose oxidase. The PVA–CS composite nanofiber membrane was selected as the protective film of Prussian blue (reducing hydrogen peroxide) to maintain its stability and efficient enzyme immobilization. In the end, the detection limit of the sensor made by this team is slightly higher than that of the previous team, but the linear range is wider. For this phenomenon, it may be that the modification method used by the previous group is relatively special. This improves the selectivity for the conversion of glucose to gluconic acid and the electrochemical catalytic performance in the presence of Gox–HRP–Cu nano-flowers.
Effect of modification methods of electrospun polymer nanofibers on the detection effect of biosensors.
| Electrospun polymer fibers | Modification method | Detection object | Test effect | Reference |
|---|---|---|---|---|
| PVA/PAMAM–Mt | Modification of Mt by PAMAMG2 | Glucose in beverages | The recovery rate is 100.45% | 141 |
| Polyaniline and polystyrene | Electrostatic layer-by-layer adsorption technique | Cholesterol | Accurate data can be obtained by depositing five cycles of COX on nanofibers | 142 |
| Gold nanoparticles (Au NPs)-crosslinked zein ultrafine fibers (CZUF) | Adding gold nanoparticles; combining electrospinning and one-step reduction method | Catechol | The interference is very small, and the sensitivity is very high | 143 |
| Cu-nanoflower@AuNPs–GO | Blending of GO and PVA | Glucose in biological fluids | The detection limit is 0.018 mM. The linear range is 1 × 10−6 to 1 × 10−4 M | 144 |
| CS–PVA | Add natural biopolymers | Glucose | The detection limit is 0.361 mM, and the linear range is 3.30 × 10−6 M to 5.56 × 10−2 M | 145 |
With the biosensor as an efficient detection equipment, high sensitivity and high selectivity are very important criteria. We know that the combination of enzyme immobilization technology and biosensor can meet different detection objectives. In this process, the electrospun nanofiber membrane plays an outstanding role because of its special structure. Because the biosensor is a kind of high precision instrument, small changes in the fiber membrane structure and electrode can cause great changes in the detection ability. Then, the modification of the biosensor can characterize its detection performance to a certain extent. Of course, the modification method is not limited to the modification of the nanofiber membrane using the fine structure of the nanofiber membrane and the electrode of the biosensor. It can efficiently immobilize the enzyme, increasing the stability of the enzyme and the sensitivity and selectivity of the biosensor.
Other aspects
Electrospun nanofibers are widely used not only in tissue engineering, wound dressings, drug delivery systems, enzyme immobilization and biosensors, but also in protective textiles, cosmetics, facial masks and antibacterial materials.92,115,146,147
Sinha et al.148 described the characteristics and specific applications of electrospun nanofibers as biomedical textiles, with special emphasis on its application in strategic defense. The nonwovens prepared have sufficient bending resistance and wear resistance. Compared with the traditional needle and syringe methods, the production method combined with nanospider technology has the possibility of large-scale production. The purpose of this study is to combine the filtration and antibacterial properties of nanofiber membranes to provide defensive biological protective clothing with lightweight and good antibacterial properties. Zaitoon and Lim149 also paid attention to the large-scale production of electrospinning technology. They chose free surface electrospinning technology to prepare an ethyl cellulose composite fiber membrane. This method has the characteristics of a simple setting, high yield, high fiber uniformity and large coverage area, so it is very suitable for large-scale production. Another study150 also noted that the combination of antimicrobial agents and fabrics can have an unexpected effect. Their team deposited antibacterial electrospun nanofibers into cotton fiber networks, and then used the composites to make textiles with lasting antibacterial activity. The inhibition rate against E. coli and Staphylococcus aureus was as high as 99.99%. On this basis, the antibacterial fabric sportswear was prepared. After many times of washing, the antibacterial rate remained above 95%. It has been reported that the fiber fabric prepared by mixing nano-oxidized metal particles in spinning solution can play the role of flame retardant and antibacterial, and is expected to play a role in the field of soldier protective clothing.151 In the study of protective fabrics, fabrics with deworming activity have been developed because they can be loaded with different insect repellents to control pathogen vectors. Muñoz et al.152 loaded the natural biological protective agent lemonol on a nanofiber mat, and then made it into gloves, covering the hand and forearm. The arm was then stretched into the test room to evaluate the effect of mosquito avoidance, showing 100% avoidance. The preparation of this biopolymer nanofiber pad provides an excellent strategy for the development of disposable outdoor products. In addition to the electrospun nanofiber fabric as a new material, the removal of harmful pollutants in the air also has great application potential.153
Electrospun nanofiber membranes are also used as facial masks for skin care or other therapeutic purposes. Skin activating factors (such as l-ascorbic acid) are loaded on electrospun nanofibers, and applied on the skin to gradually dissolve and release active components. The high specific surface area ensures maximum contact with the skin surface of the nanofiber mask to improve skin quality.154 Furthermore, electrospun nanofiber membranes are also often used as antibacterial materials. In addition to being widely used in wound dressings, it is also used as an active packaging in food packaging. This can inhibit or delay the growth of microorganisms, so as to prolong the shelf life of food.155 Altan et al.156 used electrospinning technology to prepare composite fiber membranes with zein and PLA matrix materials, and added carvanol. The characterization outcome showed that the content of carvanol had an effect on the fiber morphology and size, and showed good antioxidant activity when the addition was 20%. Whole wheat bread samples were used to study the preservation properties of the composite fiber membranes, and the preliminary results showed that these fiber membranes could better preserve bread. Another study157 used β-cyclodextrin and cinnamon essential oil to form an inclusion complex. A new type of antibacterial packaging material was prepared by PLA electrospinning, which showed good antibacterial activity. Besides, curcumin,158 solid triclosan,159 soybean protein,160 and wheat gluten proteins161 can be loaded to achieve the purpose of antibacterial, preventing photolysis or barrier properties.
Conclusions
Electrospinning is an excellent technology for the preparation of nanofibers, and its operation is simple. It solidifies the fluid (including polymer solution and molten polymer), and forms a random arrangement or nanofibers under the action of an external electrostatic field force. At present, the materials obtained by electrospinning technology have the characteristics of high porosity, large specific surface area, good biocompatibility, biodegradable, and a three-dimensional structure. In recent years, its extensive research has set off a new wave of applications in various fields. More scholars tend to use electrospinning technology to contribute to different fields, such as filter materials and biomedical fields. Nanofibers or three-dimensional scaffolds produced by electrospinning technology are outstanding in tissue engineering, wound dressings and drug delivery systems.
On the other hand, although there are more studies in the literature about the application of electrospinning technology in the biomedical field, these applications are still in a relatively primary stage. Electrospinning is not a single technology, it also includes coaxial electrospinning, emulsion electrospinning, triaxial electrospinning, and melt electrospinning. These methods have different advantages. Various parameters involved in the spinning process have great influence on the morphology and function of nanofibers. For example, coaxial electrospinning, which is widely studied in the drug delivery system, can encapsulate hydrophilic or lipophilic drugs in the internal fiber nucleus to achieve continuous drug release. Sheath fibers can play a role of protection or support. In that case, we must pay attention to the optimization of electrospinning parameters in order to obtain ideal nanofibers. This process often becomes complex. In addition, the selection of an internal fiber core and sheath spinning solution is also very important. As we all know, materials in the biomedical field must have the characteristics of non-toxicity, but organic solvents are often added in the spinning process. There will inevitably be some solvent residues in the material, and the control of the process parameters in the spinning process will also affect the volatilization of the solvent. Therefore, it is necessary to select the most suitable process parameters, solvent system and spinning materials. Furthermore, the improvement of spinning equipment also plays an important role. The nanofibers prepared by traditional uniaxial or ordinary solution electrospinning may not meet the required properties. Consequently, triaxial electrospinning and emulsion electrospinning can be used to meet different research purposes. So far, most of the reports on electrospun nanofibers are studied in vitro, so it is necessary to improve the contents of in vivo research and preclinical research in order to speed up the practical application and scale of electrospinning technology in the field of biomedicine. Finally, as electrospinning materials, polyester materials have the characteristics of easy degradation and a wide range of sources. After all, it is not an endogenous substance of the human body, and it cannot reach the required safety level (emphasized non-toxicity and degradability), and the physical properties to a certain extent. Hence, future research work should be devoted to the structural modification of this kind of materials, or combined with suitable natural polymer materials to improve their biological and mechanical properties. On the basis of constantly tamping the application of electrospinning technology in the biomedical field, it further promotes the development of the biomedical field.
Generally speaking, as an emerging technology, electrospinning technology has excellent application potential. It is expected that more reports will be published and play a more extensive role in the biomedical field.
Abbreviations
- ECM
Extracellular matrix
- PLA
Polylactic acid
- sc-PLA
Stereocomplex polylactide
- PLLA
Poly-l-lactic acid
- PDLLA
Poly(d,l-lactic acid)
- PVA
Polyvinyl alcohol
- PCL
Polycaprolactone
- PU
Polyurethane
- PLGA
Polyglycolic acid
- CS
Chitosan
- hMSCs
Human bone marrow mesenchymal stem cell
- SF
Silk fibroin
- VEGF
Vascular endothelial growth factor
- EPCs
Endothelial progenitor cells
- TSF
Tussah silk fibroin
- mESCs
Mouse embryonic stem cells
- MEFs
Mouse embryonic fibroblasts
- NSCs
Neural stem cells
- P(LLA-CL)
Poly(l-lactide-co-ε-caprolactone)
- PGC
Poliglecaprone
- SMCs
Smooth muscle cells
- SSS
Siliceous sponge spicules
- CA
Cellulose acetate
- PEO
Poly(ethylene oxide)
- HBTC
1,3,5-Benzenetricarboxylate
- im
Imidazole ligand
- PVP
Polyvinylpyrrolidone
- TEM
Transmission electron microscope
- SEM
Scanning electron microscope
- PEC
Pectin
- MF
Mafenide acetate
- DMF
N,N-Dimethyl formamide
- PMMA
Poly(methyl methacrylate)
- THF
Tetrahydrofuran
- DCM
Dichloromethane
- GMS
Glycerol monostearate
- EC
Ethylcellulose
- DMAc
Dimethylacetamide
- HFP
1,1,1,3,3,3-Hexafluoro-2-propanol
- BSA
Bull serum album
- FTIR
Fourier transform infrared
- HRP
Horseradish peroxidase
- GO
Graphene oxide
- PAN
Polyacrylonitrile
- PMMA/PANI
Poly(methyl methacrylate)/polyaniline
- PS
Polystyrene
- P(GMA-co-MA)-g-PEO
Poly(glycidyl methacrylate-co-methylacrylate)-g-polyethylene oxide
- P(GMA-co-MA)/FP
Poly(glycidyl methacrylate-co-methylacrylate)/feather polypeptide
- PANCMA
Poly-(acrylonitrile-co-maleic acid)
- PSMA
Poly(styrene-co-maleic anhydride)
- COX
Cholesterol oxidase
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was financially supported by the Key Research and Development Program of Shaanxi, China (No. 2020SF-423; No. 2020FP-029).
References
- Sun B. Long Y. Z. Zhang H. D. Li M. M. Duvail J. L. Jiang X. Y. Yin H. L. Prog. Polym. Sci. 2014;39:862–890. doi: 10.1016/j.progpolymsci.2013.06.002. [DOI] [Google Scholar]
- Huang Z. M. Zhang Y. Z. Kotaki M. Ramakrishna S. Compos. Sci. Technol. 2003;63:2223–2253. doi: 10.1016/S0266-3538(03)00178-7. [DOI] [Google Scholar]
- Koch C. C. Nanostruct. Mater. 1993;2:109–129. doi: 10.1016/0965-9773(93)90016-5. [DOI] [Google Scholar]
- Nicolas A. Jean-Pierre B. Patrick S. J. Controlled Release. 2008;128:185–199. doi: 10.1016/j.jconrel.2008.02.007. [DOI] [Google Scholar]
- Cheng W. L. Campolongo M. J. Tan S. J. Luo D. Nano Today. 2009;4:482–493. doi: 10.1016/j.nantod.2009.10.005. [DOI] [Google Scholar]
- Lee J. Chen N. Peng S. Li L. Tian L. Thakor N. v. Ramakrishna S. Prog. Polym. Sci. 2018;86:40–84. doi: 10.1016/j.progpolymsci.2018.07.002. [DOI] [Google Scholar]
- Agarwal S. Wendorff J. H. Greiner A. Polymer. 2008;49:5603–5621. doi: 10.1016/j.polymer.2008.09.014. [DOI] [Google Scholar]
- Thillaipandian H. Venkateshwarapuram Rengaswami G. D. Int. J. Biol. Macromol. 2018;106:712–718. doi: 10.1016/j.ijbiomac.2017.08.079. [DOI] [PubMed] [Google Scholar]
- Chen M. J. Zhang Y. C. Li H. Y. Li X. N. Ding Y. M. Bubakir M. M. Yang W. M. Advanced Industrial and Engineering Polymer Research. 2019;2:110–115. doi: 10.1016/j.aiepr.2019.09.001. [DOI] [Google Scholar]
- Liu Y. B. Hao M. Chen Z. J. Liu L. L. Liu Y. Yang W. X. Ramakrishna S. Curr. Opin. Biomed. Eng. 2020;13:174–189. doi: 10.1016/j.cobme.2020.02.001. [DOI] [Google Scholar]
- Jain R. Shetty S. Yadav K. S. J. Drug Delivery Sci. Technol. 2020;57:101604. doi: 10.1016/j.jddst.2020.101604. [DOI] [Google Scholar]
- Solomin E. Sirotkin E. Sirotkin A. A. Procedia Eng. 2017;206:1371–1375. doi: 10.1016/j.proeng.2017.10.647. [DOI] [Google Scholar]
- Zhang H. Jia X. L. Han F. X. Zhao J. Zhao Y. H. Fan Y. B. Yuan X. Y. Biomaterials. 2013;34:2202–2212. doi: 10.1016/j.biomaterials.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Shao W. L. He J. X. Sang F. Wang Q. Chen L. Cui S. Z. Ding B. Mater. Sci. Eng., C. 2016;62:823–834. doi: 10.1016/j.msec.2016.01.078. [DOI] [PubMed] [Google Scholar]
- Aslan B. Guler S. Tevlek A. Aydin H. M. J. Biomed. Mater. Res., Part B. 2018;106:2157–2168. doi: 10.1002/jbm.b.34022. [DOI] [PubMed] [Google Scholar]
- Wang X. X. Xiang H. F. Song C. Zhu D. Y. Sui J. X. Liu Q. Long Y. Z. J. Hazard. Mater. 2020;385:121535. doi: 10.1016/j.jhazmat.2019.121535. [DOI] [PubMed] [Google Scholar]
- Kacmaz S. Ertekin K. Mercan D. Oter O. Cetinkaya E. Celik E. Spectrochim. Acta, Part A. 2015;135:551–559. doi: 10.1016/j.saa.2014.07.056. [DOI] [PubMed] [Google Scholar]
- Zhao X. X. Lui Y. S. Choo C. K. C. Sow W. T. Huang C. L. Ng K. W. Tan L. P. Loo J. S. C. Mater. Sci. Eng., C. 2015;49:746–753. doi: 10.1016/j.msec.2015.01.084. [DOI] [PubMed] [Google Scholar]
- Morgane S. L. Anne-Claude C. Séverine V. Guy S. Anne H. Carbohydr. Polym. 2019;207:276–287. doi: 10.1016/j.carbpol.2018.11.085. [DOI] [PubMed] [Google Scholar]
- Kenawy E. R. Abdel-Hay F. I. El-Newehy M. H. Wnek G. E. Mater. Chem. Phys. 2009;113:296–302. doi: 10.1016/j.matchemphys.2008.07.081. [DOI] [Google Scholar]
- Pokorski J. K., Baer E., Sieun K. and Wang J., US Pat., US2015/015243, 2015
- Hu X. L. Liu S. Zhou G. Y. Huang Y. B. Xie Z. G. Jing X. B. J. Controlled Release. 2014;185:12–21. doi: 10.1016/j.jconrel.2014.04.018. [DOI] [PubMed] [Google Scholar]
- Juncos Bombin A. D. Dunne N. J. McCarthy H. O. Mater. Sci. Eng., C. 2020;114:110994. doi: 10.1016/j.msec.2020.110994. [DOI] [PubMed] [Google Scholar]
- Frenot A. Chronakis I. S. Curr. Opin. Colloid Interface Sci. 2003;8:64–75. doi: 10.1016/S1359-0294(03)00004-9. [DOI] [Google Scholar]
- Cestari M. Muller V. Rodrigues J. H. d. S. Nakamura C. V. Rubira A. F. Muniz E. C. Biomacromolecules. 2014;15:1762–1767. doi: 10.1021/bm500132g. [DOI] [PubMed] [Google Scholar]
- Prajapati S. K. Jain A. Jain A. Jain S. Eur. Polym. J. 2019;120:109191. doi: 10.1016/j.eurpolymj.2019.08.018. [DOI] [Google Scholar]
- Sill T. J. von Recum H. A. Biomaterials. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Kim J. Y. Kim J. I. Park C. H. Kim C. s. Mater. Lett. 2018;236:521–525. doi: 10.1016/j.matlet.2018.10.087. [DOI] [Google Scholar]
- Mattanavee W. Suwantong O. Puthong S. Bunaprasert T. Hoven V. P. Supaphol P. ACS Appl. Mater. Interfaces. 2009;1:1076–1085. doi: 10.1021/am900048t. [DOI] [PubMed] [Google Scholar]
- Dai Y. R. Niu J. F. Liu J. Yin L. F. Xu J. J. Bioresour. Technol. 2010;101:8942–8947. doi: 10.1016/j.biortech.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Yao Q. K. Hu Y. Yu F. Zhang W. J. Fu Y. RSC Adv. 2018;8:18372–18380. doi: 10.1039/C7RA13551C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canbolat M. F. Gera N. Tang C. Monian B. Rao B. M. Pourdeyhimi B. Khan S. A. ACS Appl. Mater. Interfaces. 2013;5:9349–9354. doi: 10.1021/am4022768. [DOI] [PubMed] [Google Scholar]
- Huang C. Thomas N. L. Eur. Polym. J. 2018;99:464–476. doi: 10.1016/j.eurpolymj.2017.12.025. [DOI] [Google Scholar]
- Cheng H. L. Yang X. Y. Che X. Yang M. S. Zhai G. X. Mater. Sci. Eng., C. 2018;90:750–763. doi: 10.1016/j.msec.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Cheng J. Jun Y. Qin J. Lee S.-H. Biomaterials. 2017;114:121–143. doi: 10.1016/j.biomaterials.2016.10.040. [DOI] [PubMed] [Google Scholar]
- Grey C. P. Newton S. T. Bowlin G. L. Haas T. W. Simpson D. G. Biomaterials. 2013;34:4993–5006. doi: 10.1016/j.biomaterials.2013.03.033. [DOI] [PubMed] [Google Scholar]
- Li X. Y. Wang X. F. Yao D. S. Jiang J. Guo X. Gao Y. H. Li Q. Shen C. Y. Colloids Surf., B. 2018;171:461–467. doi: 10.1016/j.colsurfb.2018.07.045. [DOI] [PubMed] [Google Scholar]
- Ge Y. Chen R. P. Li H. Guo Z. Z. Mater. Lett. 2018;228:301–304. doi: 10.1016/j.matlet.2018.06.034. [DOI] [Google Scholar]
- Dhandayuthapani B. Yoshida Y. Maekawa T. Kumar S. Int. J. Polym. Sci. 2011;2011:609–618. [Google Scholar]
- Sartore L. Pandini S. Dey K. Bignotti F. Chiellini F. Materialia. 2020;9:100615. doi: 10.1016/j.mtla.2020.100615. [DOI] [Google Scholar]
- Amokrane G. Humblot V. Jubeli E. Yagoubi N. Ramtani S. Migonney V. Falentin-Daudré C. ACS Omega. 2019;4:17194–17208. doi: 10.1021/acsomega.9b01647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlotta D. J. Polym. Environ. 2001;9:63–84. doi: 10.1023/A:1020200822435. [DOI] [Google Scholar]
- Li L. Y. Chen Y. Yu T. X. Wang N. Wang C. S. Wang H. P. Compos. Commun. 2019;16:162–167. doi: 10.1016/j.coco.2019.10.004. [DOI] [Google Scholar]
- Hamad K. Kaseem M. Yang H. W. Deri F. Ko Y. G. eXPRESS Polym. Lett. 2015;9:435–455. doi: 10.3144/expresspolymlett.2015.42. [DOI] [Google Scholar]
- Tamayol A. Akbari M. Annabi N. Paul A. Khademhosseini A. Juncker D. Biotechnol. Adv. 2013;31:669–687. doi: 10.1016/j.biotechadv.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain J. R. Transplant. Proc. 1998;30:2762–2767. doi: 10.1016/S0041-1345(98)00804-5. [DOI] [PubMed] [Google Scholar]
- Li H. Y. Xu Y. C. Xu H. Chang J. J. Mater. Chem. B. 2014;2:5492–5510. doi: 10.1039/C4TB00913D. [DOI] [PubMed] [Google Scholar]
- dos Santos Jr A. R. Bioresorbable Polymers for Tissue Engineering. InTech. 2010:226–246. [Google Scholar]
- Parrag I. C. Zandstra P. W. Woodhouse K. A. Biotechnol. Bioeng. 2012;109:813–822. doi: 10.1002/bit.23353. [DOI] [PubMed] [Google Scholar]
- Fasolino I. Bonadies I. Ambrosio L. Raucci M. G. Carfagna C. Caso F. M. Cimino F. Pezzella A. ACS Appl. Mater. Interfaces. 2017;9:40070–40076. doi: 10.1021/acsami.7b13257. [DOI] [PubMed] [Google Scholar]
- Kang Y. Chen P. Shi X. T. Zhang G. C. Wang C. L. Polymer. 2018;156:250–260. doi: 10.1016/j.polymer.2018.10.009. [DOI] [Google Scholar]
- Liu X. Zhou L. T. Heng P. Xiao J. C. Lv J. P. Zhang Q. M. Hickey M. E. Tu Q. Wang J. Y. Colloids Surf., B. 2019;175:264–271. doi: 10.1016/j.colsurfb.2018.09.069. [DOI] [PubMed] [Google Scholar]
- da Silva T. N. Gonçalves R. P. Rocha C. L. Archanjo B. S. Barboza C. A. G. Pierre M. B. R. Reynaud F. de Souza Picciani P. H. Mater. Sci. Eng., C. 2019;97:602–612. doi: 10.1016/j.msec.2018.12.020. [DOI] [PubMed] [Google Scholar]
- Su C. J. Tu M. G. Wei L. J. Hsu T. T. Kao C. T. Chen T. H. Huang T. H. Materials. 2017;10:501. doi: 10.3390/ma10050501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. Q. Liu P. Yang T. Sun Y. You Q. Li J. L. Wang Z. L. Han B. J. Biomater. Appl. 2016;30:1552–1565. doi: 10.1177/0885328216638587. [DOI] [PubMed] [Google Scholar]
- Norouzi S. K. Shamloo A. Mater. Sci. Eng., C. 2019;94:1067–1076. doi: 10.1016/j.msec.2018.10.016. [DOI] [PubMed] [Google Scholar]
- Li L. Liu W. Q. Zhao Y. L. Ma P. P. Zha S. F. Chen P. X. Lu H. W. Jiang X. R. Wan S. Luo J. M. Dai Q. J. Hu J. X. Utomo Y. K. S. Han X. Y. Yang Z. W. Yang L. He Q. Y. ACS Appl. Mater. Interfaces. 2020;12:3474–3493. doi: 10.1021/acsami.9b21434. [DOI] [PubMed] [Google Scholar]
- Fiqrianti I. A. Widiyanti P. Manaf M. A. Savira C. Y. Cahyani N. R. Bella F. R. J. Funct. Biomater. 2018;9:32. doi: 10.3390/jfb9020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin A. L. Luo R. F. Li J. K. Mo X. M. Wang Y. B. Zhang X. D. Colloids Surf., B. 2017;152:432–439. doi: 10.1016/j.colsurfb.2017.01.045. [DOI] [PubMed] [Google Scholar]
- Shao W. L. He J. X. Han Q. M. Sang F. Wang Q. Chen L. Cui S. Z. Ding B. Mater. Sci. Eng., C. 2016;67:599–610. doi: 10.1016/j.msec.2016.05.081. [DOI] [PubMed] [Google Scholar]
- Zhang E. Zhu C. S. Yang J. Sun H. Zhang X. M. Li S. H. Wang Y. L. Sun L. Yao F. L. Mater. Sci. Eng., C. 2016;58:278–285. doi: 10.1016/j.msec.2015.08.032. [DOI] [PubMed] [Google Scholar]
- Zhang C. M. Wang L. W. Zhai T. L. Wang X. C. Dan Y. Turng L. S. J. Mech. Behav. Biomed. Mater. 2016;53:403–413. doi: 10.1016/j.jmbbm.2015.08.043. [DOI] [PubMed] [Google Scholar]
- Yang F. Murugan R. Wang S. Ramakrishna S. Biomaterials. 2005;26:2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Xu C. Y. Inai R. Kotaki M. Ramakrishna S. Biomaterials. 2004;25:877–886. doi: 10.1016/S0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- Ambekar R. S. Kandasubramanian B. Eur. Polym. J. 2019;117:304–336. doi: 10.1016/j.eurpolymj.2019.05.020. [DOI] [Google Scholar]
- Zhao W. Liu W. L. Li J. J. Lin X. Wang Y. J. Biomed. Mater. Res., Part A. 2015;103:807–818. doi: 10.1002/jbm.a.35187. [DOI] [PubMed] [Google Scholar]
- Lin L. Chen J. M. Wang H. Li J. S. Chen J. Y. Zeng X. Q. Materials Reports. 2019;33:65–72. [Google Scholar]
- Homaeigohar S. Boccaccini A. R. Acta Biomater. 2020;107:25–49. doi: 10.1016/j.actbio.2020.02.022. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Tobías H. Morales G. Grandel D. Mater. Sci. Eng., C. 2019;101:306–322. doi: 10.1016/j.msec.2019.03.099. [DOI] [PubMed] [Google Scholar]
- Freeman A. I. Mayhew E. Cancer. 1986;58:573–583. doi: 10.1002/1097-0142(19860715)58:2+<573::AID-CNCR2820581328>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Trinca R. B. Westin C. B. da Silva J. A. F. Moraes Â. M. Eur. Polym. J. 2017;88:161–170. doi: 10.1016/j.eurpolymj.2017.01.021. [DOI] [Google Scholar]
- Ranjith R. Balraj S. Ganesh J. Milton J. Mater. Today Chem. 2019;12:386–395. doi: 10.1016/j.mtchem.2019.03.008. [DOI] [Google Scholar]
- Basar A. O. Castro S. Torres-Giner S. Lagaron J. M. Turkoglu Sasmazel H. Mater. Sci. Eng., C. 2017;81:459–468. doi: 10.1016/j.msec.2017.08.025. [DOI] [PubMed] [Google Scholar]
- Saha K. Dutta K. Basu A. Adhikari A. Chattopadhyay D. Sarkar P. Nano-Struct. Nano-Objects. 2020;21:100418. doi: 10.1016/j.nanoso.2019.100418. [DOI] [Google Scholar]
- Rivero G. Meuter M. Pepe A. Guevara M. G. Boccaccini A. R. Abraham G. A. Colloids Surf., A. 2020;587:124313. doi: 10.1016/j.colsurfa.2019.124313. [DOI] [Google Scholar]
- Yang Y. Liu X. K. Wu T. Zhang W. P. Shu J. H. He Y. L. Tang S. J. Sci. Rep. 2018;8:6194. doi: 10.1038/s41598-018-24618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B. Svyntkivska M. Brzeziński M. Makowski T. Piorkowska E. Rajkowska K. Kunicka-Styczyńska A. Biela T. Colloids Surf., B. 2020;190:110949. doi: 10.1016/j.colsurfb.2020.110949. [DOI] [PubMed] [Google Scholar]
- Wang P. W. Mele E. Materials. 2018;11:923. doi: 10.3390/ma11060923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado R. da Costa A. Silva D. M. Gomes A. C. Casal M. Sencadas V. Macromol. Biosci. 2018;18:1700324. doi: 10.1002/mabi.201700324. [DOI] [PubMed] [Google Scholar]
- Wu C. S. Wu D. Y. Wang S. S. Mater. Sci. Eng., C. 2020;108:110506. doi: 10.1016/j.msec.2019.110506. [DOI] [PubMed] [Google Scholar]
- Zhang S. Q. Ye J. W. Sun Y. Kang J. Liu J. H. Wang Y. Li Y. C. Zhang L. H. Ning G. L. Chem. Eng. J. 2020;390:124523. doi: 10.1016/j.cej.2020.124523. [DOI] [Google Scholar]
- Alippilakkotte S. Kumar S. Sreejith L. Colloids Surf., A. 2017;529:771–782. doi: 10.1016/j.colsurfa.2017.06.066. [DOI] [Google Scholar]
- Alipour R. Khorshidi A. Shojaei A. F. Mashayekhi F. Moghaddam M. J. M. Polym. Test. 2019;79:106022. doi: 10.1016/j.polymertesting.2019.106022. [DOI] [Google Scholar]
- Kalwar K. Sun W. Li D. Zhang X. Shan D. React. Funct. Polym. 2016;107:87–92. doi: 10.1016/j.reactfunctpolym.2016.08.010. [DOI] [Google Scholar]
- Zamani M. Prabhakaran M. P. Ramakrishna S. Int. J. Nanomed. 2013;8:2997–3017. doi: 10.2147/IJN.S43575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. N. Mo H. Y. Yu D. G. Int. J. Pharm. 2012;438:232–239. doi: 10.1016/j.ijpharm.2012.08.053. [DOI] [PubMed] [Google Scholar]
- Zelko R. Lamprou D. A. Sebe I. Pharmaceutics. 2020;12:5. doi: 10.3390/pharmaceutics12010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. Z. Pyda M. Cebe P. J. Appl. Polym. Sci. 2017;134:45287. doi: 10.1002/app.45287. [DOI] [Google Scholar]
- Cai N. Han C. Luo X. G. Chen G. Dai Q. Yu F. Q. Macromol. Mater. Eng. 2017;302:1600364. doi: 10.1002/mame.201600364. [DOI] [Google Scholar]
- Wei K. Li Y. Mugishima H. Teramoto A. Abe K. Biotechnol. J. 2012;7:677–685. doi: 10.1002/biot.201000473. [DOI] [PubMed] [Google Scholar]
- Sergio T. G. Antonio M. A. Jose G. A. Maria O. Jose Maria L. Adv. Eng. Mater. 2012;14:B112–B122. doi: 10.1002/adem.201180006. [DOI] [Google Scholar]
- Pant B. Park M. Park S. J. Pharmaceutics. 2019;11:305. doi: 10.3390/pharmaceutics11070305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões M. Cragg S. Barbu E. De Sousa F. J. Mater. Sci. 2019;54:5712–5725. doi: 10.1007/s10853-018-03261-2. [DOI] [Google Scholar]
- Hai T. Wan X. Yu D. G. Wang K. Yang Y. Y. Liu Z. P. Mater. Des. 2018;162:70–79. doi: 10.1016/j.matdes.2018.11.036. [DOI] [Google Scholar]
- Naseri Nosar M. Salehi M. Ghorbani S. Beiranvand S. P. Goodarzi A. Azami M. Cellulose. 2016;23:3239–3248. doi: 10.1007/s10570-016-1026-7. [DOI] [Google Scholar]
- Naseri-Nosar M. Salehi M. Hojjati-Emami S. Int. J. Biol. Macromol. 2017;103:701–708. doi: 10.1016/j.ijbiomac.2017.05.054. [DOI] [PubMed] [Google Scholar]
- Rafiei M. Jooybar E. Abdekhodaie M. J. Alvi M. Mater. Sci. Eng., C. 2020;113:110913. doi: 10.1016/j.msec.2020.110913. [DOI] [PubMed] [Google Scholar]
- Zong H. X. Xia X. Liang Y. R. Dai S. Y. Alsaedi A. Hayat T. Kong F. T. Pan J. H. Mater. Sci. Eng., C. 2018;92:1075–1091. doi: 10.1016/j.msec.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Han D. Sherman S. Filocamo S. Steckl A. J. Acta Biomater. 2017;53:242–249. doi: 10.1016/j.actbio.2017.02.029. [DOI] [PubMed] [Google Scholar]
- Zanjani J. S. M. Saner Okan B. Yilmaz C. Menceloglu Y. Yildiz M. Composites, Part A. 2017;99:221–232. doi: 10.1016/j.compositesa.2017.04.017. [DOI] [Google Scholar]
- Yang Y. Y. Li W. B. Yu D. Wang G. H. Williams G. Zhang Z. Carbohydr. Polym. 2018;203:228–237. doi: 10.1016/j.carbpol.2018.09.061. [DOI] [PubMed] [Google Scholar]
- He P. Zhong Q. Ge Y. Guo Z. A. Tian J. H. Zhou Y. H. Ding S. Li H. Zhou C. R. Mater. Sci. Eng., C. 2018;90:549–556. doi: 10.1016/j.msec.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Wipperman J. L. Dorsch J. N. Am. Fam. Physician. 2013;87:114–120. [PubMed] [Google Scholar]
- Tawfik E. A. Craig D. Q. M. Barker S. A. Int. J. Pharm. 2020;581:119296. doi: 10.1016/j.ijpharm.2020.119296. [DOI] [PubMed] [Google Scholar]
- Wang H. Cui Y. Wen M. Yi Feng E. Bo Wen C. J. Ind. Text. 2019;48:1024–1043. doi: 10.1177/1528083718754902. [DOI] [Google Scholar]
- Wang S. F. Ren Z. H. Li J. P. Ren Y. Q. Zhao L. Yu J. RSC Adv. 2014;4:31300–31307. doi: 10.1039/C4RA04383A. [DOI] [Google Scholar]
- Wang B. L. Zheng H. X. Chang M. W. Ahmad Z. Li J. S. Colloids Surf., B. 2016;145:757–767. doi: 10.1016/j.colsurfb.2016.05.092. [DOI] [PubMed] [Google Scholar]
- Khajavi R. Abbasipour M. Sci. Iran. 2012;19:2029–2034. doi: 10.1016/j.scient.2012.10.037. [DOI] [Google Scholar]
- Yi Z. C. Wang K. Tian J. R. Shu Y. Yang J. Xiao W. Q. Li B. Liao X. L. Ceram. Int. 2016;42:19079–19085. doi: 10.1016/j.ceramint.2016.09.067. [DOI] [Google Scholar]
- Bodnar M. Hartmann J. F. Borbely J. Biomacromolecules. 2005;6:2521–2527. doi: 10.1021/bm0502258. [DOI] [PubMed] [Google Scholar]
- Engkagul V. Klaharn I. y. Sereemaspun A. Chirachanchai S. Nanomedicine. 2017;13:2523–2531. doi: 10.1016/j.nano.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Furuya T. Kuroiwa M. Kino K. J. Biotechnol. 2017;243:25–28. doi: 10.1016/j.jbiotec.2016.12.021. [DOI] [PubMed] [Google Scholar]
- Basso A. Serban S. Mol. Catal. 2019;479:110607. doi: 10.1016/j.mcat.2019.110607. [DOI] [Google Scholar]
- Liu D. M. Dong C. Process Biochem. 2020;92:464–475. doi: 10.1016/j.procbio.2020.02.005. [DOI] [Google Scholar]
- Haider A. Haider S. Kang I. K. Arabian J. Chem. 2018;11:1165–1188. doi: 10.1016/j.arabjc.2015.11.015. [DOI] [Google Scholar]
- Poorakbar E. Shafiee A. Saboury A. A. Rad B. L. Khoshnevisan K. Ma'mani L. Derakhshankhah H. Ganjali M. R. Hosseini M. Process Biochem. 2018;71:92–100. doi: 10.1016/j.procbio.2018.05.012. [DOI] [Google Scholar]
- Jankowska K. Zdarta J. Grzywaczyk A. Kijeńska-Gawrońska E. Biadasz A. Jesionowski T. Environ. Res. 2020;184:109332. doi: 10.1016/j.envres.2020.109332. [DOI] [PubMed] [Google Scholar]
- Sawada K. Sakai S. Taya M. Chem. Eng. J. 2015;280:558–563. doi: 10.1016/j.cej.2015.06.029. [DOI] [Google Scholar]
- Tang C. Saquing C. D. Morton S. W. Glatz B. N. Kelly R. M. Khan S. A. ACS Appl. Mater. Interfaces. 2014;6:11899–11906. doi: 10.1021/am5033633. [DOI] [PubMed] [Google Scholar]
- Liu J. Ma R. T. Shi Y. P. Anal. Chim. Acta. 2020;1101:9–22. doi: 10.1016/j.aca.2019.11.073. [DOI] [PubMed] [Google Scholar]
- Liu D. M. Chen J. Shi Y. P. TrAC, Trends Anal. Chem. 2018;102:332–342. doi: 10.1016/j.trac.2018.03.011. [DOI] [Google Scholar]
- Jia H. F. Zhu G. Y. Vugrinovich B. Kataphinan W. Reneker D. H. Wang P. Biotechnol. Prog. 2002;18:1027–1032. doi: 10.1021/bp020042m. [DOI] [PubMed] [Google Scholar]
- Nathan V. Rani M. Rathinasamy G. Dhiraviam K. Jayavel S. SpringerPlus. 2014;3:92. doi: 10.1186/2193-1801-3-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. L. Yuan X. Y. Sheng J. J. Membr. Sci. 2005;250:167–173. doi: 10.1016/j.memsci.2004.10.024. [DOI] [Google Scholar]
- Martin del Valle E. Process Biochem. 2004;39:1033–1046. doi: 10.1016/S0032-9592(03)00258-9. [DOI] [Google Scholar]
- Martín M. T. Plou F. J. Alcalde M. Ballesteros A. J. Mol. Catal. B: Enzym. 2003;21:299–308. doi: 10.1016/S1381-1177(02)00264-3. [DOI] [Google Scholar]
- Saallah S. Naim M. N. Lenggoro I. W. Mokhtar M. N. Abu Bakar N. F. Gen M. Biotechnol. Rep. 2016;10:44–48. doi: 10.1016/j.btre.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y. Zhang Y. F. Wang Y. Cheng Y. Y. Anal. Chim. Acta. 2013;785:75–81. doi: 10.1016/j.aca.2013.04.058. [DOI] [PubMed] [Google Scholar]
- Rajan L. Palaniswamy D. Mohankumar S. K. Pharmacol. Res. 2020;155:104681. doi: 10.1016/j.phrs.2020.104681. [DOI] [PubMed] [Google Scholar]
- Liu X. H. Fang Y. C. Yang X. Li Y. Wang C. Chem. Eng. J. 2018;336:456–464. doi: 10.1016/j.cej.2017.12.048. [DOI] [Google Scholar]
- Liu X. H. Fang Y. C. Yang X. Li Y. Wang C. Colloids Surf., B. 2018;166:277–285. doi: 10.1016/j.colsurfb.2018.03.037. [DOI] [PubMed] [Google Scholar]
- Fazel R. Torabi S. F. Naseri-Nosar P. Ghasempur S. Ranaei-Siadat S. O. Khajeh K. Enzyme Microb. Technol. 2016;93–94:1–10. doi: 10.1016/j.enzmictec.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Haghju S. Bari M. R. Khaled-Abad M. A. Carbohydr. Polym. 2018;200:137–143. doi: 10.1016/j.carbpol.2018.07.096. [DOI] [PubMed] [Google Scholar]
- de Melo Brites M. Cerón A. A. Costa S. M. Oliveira R. C. Ferraz H. G. Catalani L. H. Costa S. A. Enzyme Microb. Technol. 2020;132:109384. doi: 10.1016/j.enzmictec.2019.109384. [DOI] [PubMed] [Google Scholar]
- Datta S. Christena R. Rajaram Y. R. S. 3 Biotech. 2013;3:1–9. doi: 10.1007/s13205-012-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Vincent Edwards J. Prevost N. Huang Y. Chen J. Y. Mater. Sci. Eng., C. 2018;91:389–394. doi: 10.1016/j.msec.2018.05.061. [DOI] [PubMed] [Google Scholar]
- Ye P. Xu Z. K. Wu J. Innocent C. Seta P. Macromolecules. 2006;39:1041–1045. doi: 10.1021/ma0517998. [DOI] [Google Scholar]
- Jun S. H. Yang J. Jeon H. Kim H. S. Pack S. P. Jin E. Kim J. Environ. Sci. Technol. 2020;54:1223–1231. doi: 10.1021/acs.est.9b05284. [DOI] [PubMed] [Google Scholar]
- Bahadır E. B. Sezgintürk M. K. Anal. Biochem. 2015;478:107–120. doi: 10.1016/j.ab.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Mercante L. A. Scagion V. P. Migliorini F. L. Mattoso L. H. C. Correa D. S. TrAC, Trends Anal. Chem. 2017;91:91–103. doi: 10.1016/j.trac.2017.04.004. [DOI] [Google Scholar]
- Unal B. Yalcinkaya E. E. Demirkol D. O. Timur S. Appl. Surf. Sci. 2018;444:542–551. doi: 10.1016/j.apsusc.2018.03.109. [DOI] [Google Scholar]
- Shin Y. J. Kameoka J. J. Ind. Eng. Chem. 2012;18:193–197. doi: 10.1016/j.jiec.2011.11.009. [DOI] [Google Scholar]
- Chen X. D. Li D. W. Li G. H. Luo L. Ullah N. Wei Q. F. Huang F. L. Appl. Surf. Sci. 2015;328:444–452. doi: 10.1016/j.apsusc.2014.12.070. [DOI] [Google Scholar]
- Baek S. H. Roh J. Park C. Y. Kim M. W. Shi R. Kailasa S. K. Park T. J. Mater. Sci. Eng., C. 2020;107:110273. doi: 10.1016/j.msec.2019.110273. [DOI] [PubMed] [Google Scholar]
- Wu J. P. Yin F. J. Electroanal. Chem. 2013;694:1–5. doi: 10.1016/j.jelechem.2013.02.003. [DOI] [Google Scholar]
- Liu C. Shen J. Yeung K. W. K. Tjong S. C. ACS Biomater. Sci. Eng. 2017;3:471–486. doi: 10.1021/acsbiomaterials.6b00766. [DOI] [PubMed] [Google Scholar]
- Liu Z. L. Shang S. M. Chiu K. L. Jiang S. X. Dai F. Y. Mater. Sci. Eng., C. 2020;107:110308. doi: 10.1016/j.msec.2019.110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M. K. Das B. R. Bharathi D. Prasad N. E. Kishore B. Raj P. Kumar K. Mater. Today: Proc. 2020;21:1818–1826. [Google Scholar]
- Zaitoon A. Lim L. T. Materialia. 2020;10:100649. doi: 10.1016/j.mtla.2020.100649. [DOI] [Google Scholar]
- Qiu Q. H. Chen S. Y. Li Y. P. Yang Y. C. Zhang H. N. Quan Z. Z. Qin X. H. Wang R. W. Yu J. Y. Chem. Eng. J. 2020;384:123241. doi: 10.1016/j.cej.2019.123241. [DOI] [Google Scholar]
- Dhineshbabu N. R. Gopalu K. Rangaraj S. Manivasakan P. Venkatachalam R. Nano-Micro Lett. 2014;6:46–54. doi: 10.1007/BF03353768. [DOI] [Google Scholar]
- Muñoz V. Buffa F. Molinari F. Hermida L. G. García J. J. Abraham G. A. Mater. Lett. 2019;253:289–292. doi: 10.1016/j.matlet.2019.06.091. [DOI] [Google Scholar]
- Ge J. C. Kim J. Y. Yoon S. K. Choi N. J. Fuel. 2019;237:236–244. doi: 10.1016/j.fuel.2018.09.068. [DOI] [Google Scholar]
- Fathi Azarbayjani A. Qun L. Chan Y. Chan S. AAPS PharmSciTech. 2010;11:1164–1170. doi: 10.1208/s12249-010-9475-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares R. M. D. Siqueira N. M. Prabhakaram M. P. Ramakrishna S. Mater. Sci. Eng., C. 2018;92:969–982. doi: 10.1016/j.msec.2018.08.004. [DOI] [PubMed] [Google Scholar]
- Altan A. Aytac Z. Uyar T. Food Hydrocolloids. 2018;81:48–59. doi: 10.1016/j.foodhyd.2018.02.028. [DOI] [Google Scholar]
- Wen P. Zhu D. H. Feng K. Liu F. J. Lou W. Y. Li N. Zong M. H. Wu H. Food Chem. 2016;196:996–1004. doi: 10.1016/j.foodchem.2015.10.043. [DOI] [PubMed] [Google Scholar]
- Wang H. L. Hao L. L. Wang P. Chen M. M. Jiang S. W. Jiang S. T. Food Hydrocolloids. 2017;63:437–446. doi: 10.1016/j.foodhyd.2016.09.028. [DOI] [Google Scholar]
- Kayaci F. Umu O. C. O. Tekinay T. Uyar T. J. Agric. Food Chem. 2013;61:3901–3908. doi: 10.1021/jf400440b. [DOI] [PubMed] [Google Scholar]
- Aydogdu A. Yildiz E. Ayhan Z. Aydogdu Y. Sumnu G. Sahin S. Eur. Polym. J. 2019;112:477–486. doi: 10.1016/j.eurpolymj.2019.01.006. [DOI] [Google Scholar]
- Rocca-Smith J. R. Pasquarelli R. Lagorce-Tachon A. Rousseau J. Fontaine S. Aguié-Béghin V. Debeaufort F. Karbowiak T. ACS Sustainable Chem. Eng. 2019;7:3759–3771. doi: 10.1021/acssuschemeng.8b04064. [DOI] [Google Scholar]