Abstract
Smokers are at a higher risk of delayed union or nonunion after fracture repair. Few specific interventions are available for prevention because the molecular mechanisms that result in these negative sequelae are poorly understood. Murine models that mimic fracture healing in smokers are crucial in further understanding the local cellular and molecular alterations during fracture healing caused by smoking. We exposed three murine strains, C57BL/6J, 129X1/SvJ, and BALB/cJ, to cigarette smoke for 3 months before the induction of a midshaft transverse femoral osteotomy. We evaluated fracture healing 4 weeks after the osteotomy using radiography, microcomputed tomography (μCT), and biomechanical testing. Radiographic analysis demonstrated a significant decrease in the fracture healing capacity of smoking 129X1/SvJ mice. μCT results showed delayed remodeling of fracture calluses in all three strains after cigarette smoke exposure. Biomechanical testing indicated the most significant impairment in the functional properties of 129X1/SvJ in comparison with C57BL/6J and BALB/cJ mice after cigarette smoke exposure. Thus, the 129X1/SvJ strain is most suitable in simulating smoking-induced impaired fracture healing. Furthermore, in smoking 129X1/SvJ murine models, we investigated the molecular and cellular alterations in fracture healing caused by cigarette smoking using histology, flow cytometry, and multiplex cytokine/chemokine analysis. Histological analysis showed impaired chondrogenesis in cigarette smoking. In addition, the important reparative cell populations, including skeletal stem cells and their downstream progenitors, demonstrated decreased expansion after injury as a result of cigarette smoking. Moreover, significantly increased pro-inflammatory mediators and the recruitment of immune cells in fracture hematomas were demonstrated in smoking mice. Collectively, our findings demonstrate the significant cellular and molecular alterations during fracture healing impaired by smoking, including disrupted chondrogenesis, aberrant skeletal stem and progenitor cell activity, and a pronounced initial inflammatory response. © 2020 American Society for Bone and Mineral Research (ASBMR).
Keywords: CIGARETTE SMOKING, FRACTURE HEALING, INFLAMMATORY RESPONSE, MURINE MODELS, SKELETAL STEM/PROGENITOR CELLS
Introduction
As of 2018, there are about 34.2 million adult smokers in the United States, although the prevalence of smoking is declining.(1) Smoking causes harm to the entire musculoskeletal system,(2–4) including decreased bone mineral density, increased risk of perioperative complications, and slower healing. Based on these findings, smoking cessation programs have been recommended in conjunction with musculoskeletal treatments to improve surgical prognosis for smokers.(2–4) However, implementing these programs for smokers with an acute fracture is not practical. Therefore, smokers have longer average healing time and risk of non-union after fracture repair.(5–7)
Various methods have been developed and used to investigate the mechanisms of smoking-related impaired fracture healing, including in vitro cell contact(8,9) and in vivo animals treated with individual compounds such as cigarette smoke extract (CSE)(8) or nicotine.(9) However, given the fact that cigarette smoking involves the complicated interaction of numerous compounds and causes a wide range of physiological changes in the body, the generalizability of conclusions from these studies is limited.(10) Murine models exposed to cigarette smoke have been widely used to investigate smoking-related lung diseases(11) and demonstrate efficacy and reliability because they recapitulate the physiological absorption that occurs in humans with minimal alterations of the chemicals in the whole cigarette smoke aerosol.(12) Nonetheless, studies of fracture healing with such models are limited. The determination of a suitable murine model that recapitulates the compromised fracture healing in smokers is crucial to further understand the related pathogenesis and potential interventions.
Smoking harms nearly every organ of the body and affects a person’s overall health.(10,13) It is challenging to characterize the detailed mechanisms responsible for the impaired fracture healing capacity noted in smokers under such complicated systemic changes. Nevertheless, the fracture healing process has been widely investigated and characterized with three partially overlapping phases—inflammation, repair, and remodeling—regulated by a precise sequence of growth factors, cytokines, and cells.(14) Insight into the local changes of the natural fracture healing process in cigarette smoking may help in finding critical local pathways and further enable the development of therapies to restore the reduced fracture healing capacity found in smokers. Therefore, the purpose of the present study is to (i) determine a suitable murine model that recapitulates the depressed fracture healing capacity in smokers and to (ii) investigate the significant molecular and cellular changes to the natural fracture healing process caused by cigarette smoking.
The present study evaluated the effects of chronic exposure to cigarette smoke on fracture healing in the following murine strains: C57BL/6J, 129X1/SvJ, and BALB/cJ. The findings suggest that 129X1/SvJ mice are the most suitable model for simulating the impaired fracture healing in human smokers. Also, when 129X1/SvJ mice were chronically exposed to cigarette smoke, an impairment of chondrogenesis during fracture healing was noted. Additionally, the important reparative cell populations, including skeletal stem cells (SSCs) and their downstream progenitors—the bone, cartilage, and stromal progenitor (BCSPs)—demonstrated aberrant regenerative activity after fracture as a result of cigarette smoking. Lastly, the present study suggests that the initial inflammatory response during fracture healing was significantly enhanced due to cigarette smoking. These findings may provide the potential cellular and molecular mechanisms underlying the altered bone phenotype present in cigarette smokers, thereby providing new targets for prevention.
Materials and Methods
Animals and cigarette smoke exposure
After approval from Yale University School of Medicine’s Institutional Animal Care and Use Committee, a total of 40 male 10-week-old C57BL/6, 129X1/SvJ, and BALB/cJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were maintained in sterile, ventilated cages on a 12-hour light/ dark cycle with standard food and water ad libitum in Yale University School of Medicine’s Animal Facility.
At 12 weeks of age, 20 mice from each of the three strains (C57BL/6, 129X1/SvJ, and BALB/cJ) were randomly assigned to the air-exposed control (nonsmoking cohort [NS]) or cigarette smoke-exposed groups (cigarette smoking cohort [CS]). The mice in the CS cohort were exposed to two standard research nonfiltered cigarettes (3R4F; University of Kentucky, Lexington, KY, USA) per day via a modified cigarette smoke exposure system(15) (Fig. 1A) for 6 days a week for a total of 3 months before a midshaft transverse femoral osteotomy. After surgery was performed, the mice continued the smoking regimen until euthanization. The body weights of all mice were measured every month during the first 3 months.
Fig 1.
Modified whole-body cigarette smoke exposure system (A) and procedures for femoral osteotomy (B).
Femoral osteotomy
The procedures for femoral osteotomy are shown in Fig. 1B. Briefly, the femoral shaft was exposed, and a Gigli saw wire of 0.22 mm diameter was then used to create a transverse midshaft femoral osteotomy. A 1-inch, 23-gauge needle was used for intramedullary fixation of the bone fragments. The musculature was repaired using 5–0 suture and the skin was closed with surgical staples.
Radiography and micro-computed tomography (μCT) imaging
Four weeks after femoral osteotomy, femora were harvested. Anterior–posterior (AP) and lateral radiographs of the femur were obtained using in vivo Multispectral FX Pro (Bruker, Madison, WI, USA). Fracture healing was assessed using the Radiographic Union Score for Tibial fractures (RUST)(16) by two individual experienced researchers under blinded conditions. After radiography, the femur specimens were subjected to μCT (Scanco Medical, Brüttisellen, Switzerland) to determine fracture callus structure and composition. The following parameters were utilized in the scans: 10 μm voxel size, 55 KVp, 0.36° rotation step (180 angular range), and a 1000 ms exposure per view. The region of interest (ROI) was defined by manually drawing the contour directly adjacent to the callus with subtraction of the original cortex and of the callus cortex-like outer shell (Supplemental Fig. S1). Total callus volume (TV), bone volume (BV), bone volume fraction (BV/TV), bone mineral density (BMD), trabecular number (Tb.N), trabecular thickness (Tb.Th), and trabecular separation (Tb.Sp) in the fracture calluses were determined with density thresholds set at 400 mg/cm3 HA.
Biomechanical testing
The femora acquired 4 weeks after femoral osteotomy were used in biomechanical testing. In a nondestructive three-point bending test,(17) the condyles of each sample rested on the bending support, and the distance between both supports was 7 mm (l). The bending load (F) was applied on top of the callus tissue up to a maximum force of 1.5 N, and flexural rigidity (EI) was calculated from the slope (k) of the linear region of the load-deflection curve. The distances between the load vector and the proximal (a) and distal (b) supports were considered for calculating EI utilizing the following formula: . In the following destructive torsional test, the proximal and distal ends of the bone were first potted in brass cylinders and then subjected to a torsional force of 1° per second. Torque and displacement data were recorded at a rate of 20 Hz. Maximum torque and torsional rigidity were calculated using a custom routine in LABVIEW (National Instruments, Austin, TX, USA).
Blood analysis
Four weeks after femoral osteotomy, blood from each mouse was collected via cardiac puncture and then centrifuged to isolate plasma. Plasma cotinine and circulating inflammatory markers were measured by using a Cotinine ELISA kit (Calbiotech, Spring Valley, CA, USA), a Mouse Cytokine/Chemokine Kit (Millipore Corp., Billerica, MA, USA), and a C-Reactive Protein (CRP) ELISA Kit (Abcam, Cambridge, MA, USA) following the manufacturers’ instructions.
Histological and histomorphometric analyses
One week after femoral osteotomy, femora were harvested, fixed in 4% paraformaldehyde, decalcified in 10% EDTA, and embedded in paraffin. Longitudinal 5-μm sections were stained with hematoxylin and eosin (H&E) as well as safranin O. Gen5 (BioTek Instruments, Winooski, VT, USA) was used to obtain histology images. ImageJ (NIH, Bethesda, MD, USA) was used for quantification of callus and cartilage areas.
Multi-immunofluorescence staining
The paraffin sections of femora acquired 1 week after femoral osteotomy underwent multi-immunofluorescence staining. Briefly, sections were deparaffinized and the antigen was retrieved. Then, slides were incubated with primary Ki67 antibody (1:300, ab16667, Abcam) and localized with TSA-Plus Cyanine 5 System. Antigen retrieval and staining procedures with CD105 antibody (1:200, ab221675, Abcam) were repeated with TSA-Plus Fluorescein System. Slides were washed and mounted with VectaShield containing DAPI. All images were captured with Gen5 and quantified with MIPAR (MIPAR, Worthington, OH, USA).
Identification and analysis of mouse SSCs and BCSPs
The identification and analysis of mouse SSCs (CD45 − Tie2 − AlphaV+Thy − 6C3−.CD105-) and BCSPs (CD45 − Tie2 − AlphaV+Thy − 6C3 − CD105+) were performed by flow cytometry based on previous reports.(18–20) Briefly, uninjured femora and dissected fracture calluses were collected, crushed, and serially digested in 0.25% type-1 collagenase (STEMCELL, Vancouver, Canada) at 37°C for 30 minutes under gentle agitation. Dissociated cells were filtered with a 40-mm nylon mesh, pelleted, and resuspended with staining buffer (2% fetal bovine serum in PBS). The cells were then treated with red blood cell lysis buffer, centrifuged, and resuspended with buffer. Next, 106 cells per sample were seeded into 1 mL staining buffer in a 12 × 75 mm tube and stained with LIVE/DEAD stain (Invitrogen, Waltham, MA, USA) and fluorochrome-conjugated antibodies against CD45, Tie2, αV integrin, Ly51, Thy1.2, and CD105 (all BioLegend, San Diego, CA, USA). An LSR II flow cytometer (BD, Franklin Lakes, NJ, USA) and FlowJo software (FlowJo, Ashland, OR, USA) were used for analysis.
Analysis of inflammatory mediators in fracture hematoma
The fracture hematoma at 6 and 24 hours after femoral osteotomy was carefully harvested, shredded using micro-pestles, and digested with 1 mL 0.25% type-1 collagenase at room temperature for 10 minutes. The mixture was centrifuged at 3000g for 15 minutes, and the supernatant was collected and stored at −80°C. The concentrations of inflammatory mediators were determined with a MILLIPLEX MAP Mouse Cytokine/Chemokine Kit following the manufacturer’s instructions.
Analysis of immune cell populations in bone marrow and fracture hematoma
Inflammatory cells including neutrophils (Ly-6G+, F4–80-), inflammatory monocytes (Ly-6G+, F4–80+), macrophages (Ly-6G-, F4–80+), B cells (CD3-, CD19+), and T cells (CD3+, CD19+) in the fracture hematoma at 6 hours after femoral osteotomy and bone marrow from unfractured femora were analyzed by flow cytometry as previously described.(21,22) The proximal and distal ends of the intact femur were removed, and bone marrow was forced out of the intramedullary space. The fracture hematoma was carefully collected and digested with 1 mL 0.25% type-1 collagenase at room temperature for 10 minutes. Bone marrow samples and hematoma samples were filtered with a 70-mm nylon mesh, centrifuged, and lysed to remove erythrocytes. Then, 106 cells per sample were stained with fluorochrome-conjugated antibodies against CD3, CD19, F4/80, and Ly-6G (all BioLegend). An LSR II flow cytometer and FlowJo software were used for analysis.
Statistical analysis
Analyses were performed with SPSS 17.0 (SPSS, Chicago, IL, USA) using Mann–Whitney U or independent two-tailed Student’s t test. Results are represented as box plots showing individual data points with median and interquartile ranges in the figures and mean values and standard deviation in the tables. Differences were considered as statistically significant for p < 0.05. All sample sizes are n ≥ 3. All experiments were repeated in triplicate or more to confirm the results unless otherwise stated.
Results
Smoking causes decreased weight and increased plasma cotinine levels
Over the first 3-month investigation, the body weight of C57BL/6J and 129X1/SvJ mice in CS groups was significantly lower when compared with NS mice of the same strain. BALB/ cJ mice showed a similar trend, but this trend did not reach statistical difference during the last 2 months (Fig. 2A). Plasma cotinine levels in CS groups were significantly higher than that in the corresponding NS group (Fig. 2B).
Fig 2.
Systemic phenotype in chronic exposure to cigarette smoke. (A) Changes to body weight during the first 3 months (n = 18–20). (B) CS groups showed increases in the plasma cotinine levels (n = 5–7). (C) Plasma CRP concentrations were not significantly changed in all three strains (n = 6). (D) Smoking altered the profile of systemic inflammatory mediators (n = 4–5).
Smoking induces systemic inflammation
When evaluating the inflammatory marker CRP, there was no significant difference between CS and NS mice in all three strains (Fig. 2C). However, multiplex analysis of systemic inflammatory cytokines and chemokines demonstrated that the overall inflammatory profile was altered in CS mice (Fig. 2D; Supplemental Table S1). In all CS groups, the circulating plasma levels of MCP-1, MIP-1β, and TNF-α were significantly increased. Additionally, CS C57BL/6J mice demonstrated increased plasma levels of RANTES, IL-2, IL-5, Eotaxin, and GM-CSF. CS 129X1/SvJ demonstrated an increase in KC, IFN-γ, IL-2, IL-6, IL-10, and G-CSF. CS BALB/cJ mice showed an increase in RANTES, KC, IL-3, IL-12p70, and Eotaxin. Moreover, most inflammatory cytokines and chemokines tested were increased in CS mice when compared with their corresponding NS controls; however, the differences were not statistically significant.
Radiographic evaluation reveals compromised fracture healing in CS 129X1/SvJ mice
On inspection of radiographs of both CS and NS mice, callus formation and bridging of the proximal and distal portions of the femoral osteotomy were found in all groups except CS 129X1/ SvJ mice at 4 weeks post-osteotomy (Fig. 3A). On RUST score analysis, there was no significant difference for C57BL/6J and BALB/cJ strains. RUST score analysis of 129X1/SvJ mice demonstrated a statistically significant lower fracture healing capacity in the CS compared with NS group (Fig. 3B).
Fig 3.
Radiologic assessment of fracture repair. (A) Representative radiographs of repaired femora at 4 weeks postoperatively. (B) RUST scoring based on radiography (n = 7–9).
μCT analysis reveals delayed callus remodeling in CS mice
On μCT analysis of fractured femora, robust fracture healing with an invisible fracture line and continuous cortical bone was observed in all NS mice. Conversely, impaired fracture healing with a clear fracture gap and extensive trabecular bone formation was noted in all murine models of CS groups (Fig. 4A). Inspection of fracture calluses on μCT showed larger calluses and disorganized trabecular microarchitecture—signs of deficient fracture healing—in all strains subjected to cigarette smoking compared with nonsmoking controls (Fig. 4B).
Fig 4.
μCT analysis of healing fracture calluses. (A) Representative three-dimensional reconstructions of fracture calluses. (B) Representative cross-sectional μCT images of the fracture site. (C) Quantitative analysis of TV, BV, BV/TV, and BMD (n = 7–8). (D) Quantitative analysis of Tb.N, Tb.Th, and Tb. Sp (n = 7–8).
Quantitatively, both CS C57BL/6J and BALB/cJ mice demonstrate increased TV and BV 4 weeks after femoral osteotomy compared with their corresponding NS mice. There was no statistically significant difference in the BV/TV ratio, however, between the CS and the corresponding NS group (Fig. 4C). BMD was significantly reduced in all strains of CS groups. Quantitative analysis of trabecular microarchitecture demonstrated elevated Tb.N, reduced Tb.Th, and reduced Tb.Sp in all CS groups when compared with corresponding NS groups (Fig. 4C, D).
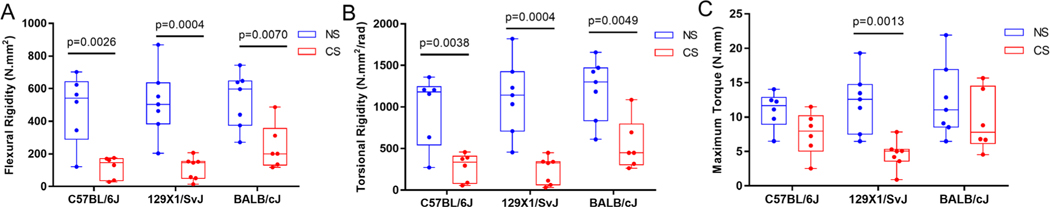
Biomechanical stress testing demonstrates poor functional fracture healing outcomes in CS mice
The flexural rigidity of fractured femora from all CS mice was significantly lower compared with the corresponding NS mice 4 weeks after femoral osteotomy (Fig. 5A). Decreased torsional rigidity of bones was observed in all CS groups (Fig. 5B). The maximal torque required to induce a second fracture in CS 129X1/SvJ mice was significantly reduced compared with NS 129X1/SvJ mice; however, no significant difference was noted for C57BL/6J and BALB/cJ strains (Fig. 5C).
Fig 5.
Biomechanical properties of fractured femora. (A) Flexural rigidity of femora (n = 6–7). (B) Torsional rigidity of femora (n = 6–7). (C) Maximal torque of femora (n = 6–7).
Based on radiography, μCT, and biomechanical data, 129X1/ SvJ mice are the most suitable model for simulating smoking-induced impaired fracture healing. Thus, we elected to use this strain for all subsequent experiments aimed at understanding the potential molecular and cellular alterations during fracture healing caused by cigarette smoking.
Smoking impairs chondrogenesis in fracture healing
129X1/SvJ CS mice demonstrated an average callus area that was smaller compared with NS mice, but this did not reach statistical significance (Fig. 6A, B). 129X1/SvJ CS mice demonstrated a significantly lower area of cartilage and percentage of cartilage in the fracture calluses compared with control mice (Fig. 6B, C).
Fig 6.

Histological analysis of fracture calluses. (A) Representative H&E staining images (scale bars = 1000 μm). (B) Quantitative assessment of fracture callus area, cartilage area, and the percentage of cartilage in the fracture calluses (n = 5–6). (C) Representative safranin O staining images. Outlined areas are magnified (scale bars = 1000 μm [left] and 200 μm [right]).
Smoking induces impaired SSC and BCSP reparative activity after fracture
The identification and analysis of mSSCs and BCSPs in uninjured femora and fracture calluses utilizing flow cytometry are briefly illustrated in Fig. 7A. The results suggested that fracture induced a significant increase in the absolute cell number of BCSPs but did not stimulate the expansion of mSSCs (Fig. 7B). Analysis of the cells in the uninjured femora demonstrated no statistically significant difference in the mSSC number nor the BCSP number between CS and NS groups. However, when analyzing the number of mSSCs and BCSPs in fracture calluses 1 week after femoral osteotomy, there was a markedly lower number of mSSCs and BCSPs in the CS group compared with the NS group (Fig. 7B). Furthermore, when utilizing multi-immunofluorescence with Ki67- and CD105-specific antibodies to evaluate actively proliferating BCSPs in fracture calluses 1 week after femoral osteotomy (Fig. 7C), the number of proliferating BCSPs (CD105+/Ki67+ cells) were significantly reduced in CS mice compared with NS mice (Fig. 7D).
Fig 7.

Analysis of mSSC and BCSP reparative ability after fracture. (A) Gating flow chart for the identification of mSSCs and BCSPs in uninjured femora and fracture calluses using flow cytometry. (B) Analysis of mSSCs and BCSPs in uninjured femora and fracture calluses (n = 5). (C) Representative images of fracture calluses that are double stained for Ki67 and CD105 (scale bars = 300 μm). BM = bone marrow; Ct = cortex; FG = fracture gap; C = callus). (D) Quantitative analysis of CD105+/Ki67+ cells in fracture calluses (n = 7).
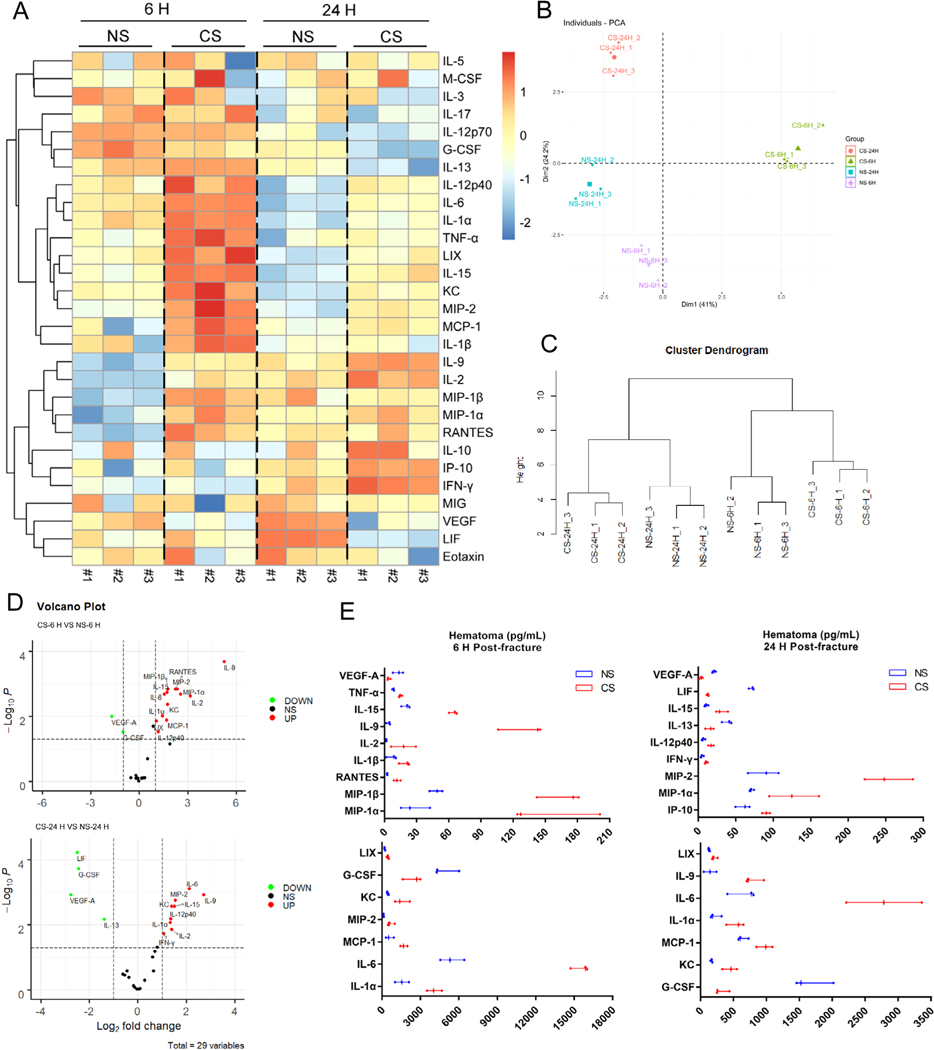
Smoking causes an enhanced initial inflammatory response in fracture healing
A heat map was generated using the data of 29 inflammatory mediators tested in fracture hematomas that demonstrated a significant difference between CS and NS groups at both the 6-hour and 24-hour time marks after femoral osteotomy (Fig. 8A). This difference was also confirmed on principal component analysis (PCA), which showed separate distributions of each group (Fig. 8B). Although individual differences in the same group were observed in a few markers’ expressions (Fig. 8A), the cluster dendrogram results suggested that the profiles of tested mediators among individuals in the same group were close, demonstrating reliable results of the entire analysis (Fig. 8C). Further analysis of the markers revealed that within the fracture hematoma 6 hours after femoral osteotomy, the CS group showed higher concentrations of the pro-inflammatory cytokines IL-1α, IL-1β, IL-2, IL-6, IL-9, IL-15, and TNF-α, pro-inflammatory chemokines KC, LIX, macrophage inflammatory proteins (MIP-1α, MIP-1β, MIP-2), MCP-1, and RANTES (Fig. 8D, E; Supplemental Table S2). Analysis of the markers within the hematoma 24 hours after femoral osteotomy demonstrated that IL-1α, IL-6, IL-9, IL-15, KC, LIX, MIP-1α, MIP-2, and MCP-1 remained at higher concentrations in the CS group compared with the NS group. In addition, other pro-inflammatory cytokines, including IFN-γ and IL-12p40, and the pro-inflammatory chemokine IP-10 also showed increased levels in the CS group (Fig. 8D, E; Supplemental Table S2).
Fig 8.
Smoking alters the profile of local inflammatory mediators at the early phase of fracture healing. (A) A heat map was generated using the data of 29 inflammatory mediators tested in reclaimed fracture hematomas. (B) PCA shows separate distributions of each group. (C) The cluster dendrogram results suggest that the profiles of tested mediators among individuals in the same group were close. (D) Volcano plots identify changes of 29 inflammatory mediators tested in the hematoma between NS and CS groups. (E) Statistically different mediators in the hematoma between NS and CS groups (n = 3).
The cellular composition of the bone marrow and fracture hematoma was assessed pre-femoral osteotomy and 6 hours after femoral osteotomy. In the bone marrow, CS mice displayed significantly increased numbers of neutrophils, macrophages, B cells, and T cells compared with NS mice (Fig. 9A). Not only were the numbers elevated, but the proportion of neutrophils, macrophages, and B cells were also markedly increased (Fig. 9A). Flow cytometry analysis of the fracture hematoma 6 hours after femoral osteotomy demonstrated that the number of infiltrated neutrophils, macrophages, B cells, and T cells were significantly increased in CS mice compared with NS mice. Additionally, the proportions of neutrophils, B cells, and T cells were also elevated, whereas the proportion of monocytes was reduced (Fig. 9B).
Fig 9.
Smoking alters the cellular composition of immune cells in the bone marrow and fracture hematoma. (A) Immune cell populations of the bone marrow were analyzed (n = 4). (B) Immune cell populations in fracture hematoma 6 hours after femoral osteotomy were analyzed (n = 4).
Discussion
Cigarette smoking is associated with delayed fracture union and nonunion. Indeed, Schmitz and colleagues(5) reported a significant increase (98%) of the overall median clinical time to union from a closed or grade I open tibial shaft fracture (269 days versus 136 days; p = 0.0001) in smokers compared with nonsmokers. Additionally, Castillo and colleagues(6) conducted a prospective clinical study comprising 268 open tibia fracture patients and reported that, after adjusting for potential confounders, current and previous smokers were 37% and 32% less likely to achieve union than nonsmokers over the course of the 2-year follow-up period. Moreover, Scolaro and colleagues(7) reviewed published studies regarding long-bone fractures and cigarette smoking. Results of their study showed a longer mean healing time for smokers (30.2 weeks; 95% confidence interval [CI] 22.7–37.7 weeks) than nonsmokers (24.1 weeks; 95% CI 17.3–30.9 weeks; p = 0.18) and an increased risk of fracture nonunion among smokers compared with nonsmokers, with an odds ratio of 2.32 (95% CI 1.76–3.06). There are no targeted therapies for preventing the potential risk of inhibited fracture healing in smokers. Therefore, suitable animal models for cigarette smoke–induced impaired fracture healing are crucial in helping explore the potential mechanisms and develop new modalities to improve clinical outcomes.
Systemic phenotype in chronic exposure to cigarette smoke
Cotinine is a standard indicator that distinguishes smokers from nonsmokers in epidemiologic studies and smoking-cessation clinical trials.(23,24) The serum cotinine cut-off value for distinguishing smokers from nonsmokers has been established as 3 ng/mL in human subjects.(24) In our study, the cotinine levels in CS mice met the criteria used in humans who are current smokers and thus verify our chronic cigarette smoke exposure models.
Long-term smoking is associated with increased systemic inflammation characterized by elevated levels of CRP, IL-6, and increased white blood cell counts in humans.(25,26) Similarly, murine studies have reported increased plasma levels of pro-inflammatory cytokines and chemokines.(8) The CS mice in our study demonstrated increased systemic inflammation mediators, including MCP-1, MIP-1β, and TNF-α compared with NS mice in all strains. CS C57BL/6J mice also showed increased RANTES, IL-2, IL-5, Eotaxin, and GM-CSF; CS 129X1/SvJ mice showed increased KC, IFN-γ, IL-2, IL-6, IL-10, and G-CSF; CS BALB/cJ mice showed an increase in RANTES, KC, IL-3, IL-12p70, and Eotaxin. It is evident that mice exposed to cigarette smoke showed significant systemic inflammation, which is consistent with studies of direct smoke exposure in humans.(25,26)
An inverse relation between smoking and body weight has been demonstrated in both clinical and murine animal studies.(27–29) In our study, C57BL/6J and 129X1/SvJ mice showed significant failure to gain weight in cigarette smoke exposure. CS BALB/cJ mice also showed similar trend, but this trend did not reach statistical difference during the last 2 months.
Collectively, the murine models chronically exposed to cigarette smoke in our study showed a systemic phenotype like long-term human smokers, including significantly increased plasma cotinine levels, systemic inflammation, and decreased weight gain, verifying the efficacy and reliability of the models.
129X1/SvJ murine strain is the most suitable for modeling cigarette smoke–induced compromised fracture healing
Although smoking is an important risk factor for impaired fracture healing, there have been limited animal studies on smoke exposure and fracture healing. Cigarette smoke–induced delayed union models can be complex because bone healing is a continuous process in which specific stages can be delayed but compensated during later stages; for example, deficient soft callus formation can be compensated by increased hard callus formation or bone remodeling.(30) Defining a model of delayed fracture healing in cigarette smoking that recapitulates the phenotype in human smokers is critical but challenging.
Clinically, smokers show a prolonged average time to heal after fractures, which is based on radiological as well as clinical indicators of bone union.(5,6) In the present study, we evaluated healing 4 weeks after femoral osteotomy since the remodeling process in mice is initiated around 3 to 4 weeks post-fracture.(31) The formation of calluses and the visibility of fracture lines on radiographs of femora were observed. Afterwards, the RUST score,(16) which has been proved to be a reliable and valid strategy to assess fracture healing in both clinical and animal studies,(32,33) was used to provide a way for quantification and statistical analysis. Our results showed that CS 129X1/SvJ mice exhibited less callus formation and visible fracture lines, while other groups showed robust healing. A significantly lower RUST score in CS 129X1/SvJ mice compared with NS 129X1/SvJ mice was also observed. Additionally, CT is a commonly used method with clear and multidimensional images able to evaluate fracture callus structure and composition and further determine the progression of fracture repair.(34,35) In the present study, μCT data showed delayed remodeling of the fracture callus in all CS groups, mainly characterized by lower BMD, more trabecular bone, and more unorganized trabecular microarchitecture. Furthermore, the recovery of biological and physical function is a critical indicator in defining skeletal healing.(14,34) Functional properties, assessed by biomechanical tests,(36) were significantly impaired in all CS groups, which were more evident in the 129X1/SvJ strain.
Based on the aforementioned results, the 129X1/SvJ murine strain is the most suitable for constructing models of cigarette smoke–induced compromised fracture healing compared with C57BL/6J and BALB/cJ strains. The identification of this suitable murine strain can help provide a viable model for future investigations of the pathogenesis and interventions for cigarette smoke–related compromised fracture healing.
Smoking delays chondrogenesis during fracture healing
In systemic diseases with multiple complications, it is challenging to characterize the intact mechanisms of tissue-specific dysfunction. Instead, greater comprehension of the cellular and molecular changes during fracture healing in cigarette smoking may be critical for combating its negative effects. El-Zawawy and colleagues(37) partially reported the alterations in a smoking mouse fracture model. They found that the smoking-induced delay was initiated at the early stages of healing due to an interruption in cellular differentiation into chondrocytes that resulted in an impaired development of the soft cartilaginous callus. However, additional knowledge of fracture healing in the context of cigarette smoking is limited. Our histological data also demonstrate the impaired early chondrogenesis caused by cigarette smoking as shown by marked decreases in both cartilage size and the percentage of cartilage to total callus.
Smoking causes aberrant stem and progenitor cell activity after fracture
MSCs are crucial regenerative cells involved in fracture repair through recruitment, proliferation, and differentiation of MSCs into bone-forming cells.(38,39) Therefore, some studies have focused on the regenerative potential of MSCs in the context of cigarette smoking. Cyprus and colleagues(8) exposed human MSCs to CSE and found that they had lower mRNA expression of osteoblast differentiation markers and reduced production of osteogenic proteins, indicating an attenuated osteogenic differentiation. Zhang and colleagues(9) discovered that in vitro and in vivo use of low-dose nicotine concentrations inhibited the migration and homing of bone marrow MSCs. Kung and colleagues(40) demonstrated inhibited chondrogenesis of mesenchymal cells treated with either benzo(α)pyrene (BaP), a prototypical polycyclic aromatic hydrocarbon found in cigarette smoke, or CSE. Moreover, aryl hydrocarbon receptor signaling was shown to be one underlying molecular mechanism in the inhibition of chondrogenesis. However, the use of individual components of cigarette smoke, like nicotine, BaP, or CSE, and treatment routes that are different from that of human smoking limit the generalizability of these conclusions.
Increasing evidence demonstrates that MSCs are a heterogeneous mixture of multiple stem cell populations with indeterminate and varied contributions to the many cell lineages, such as bone, cartilage, fat, muscle, fibroblast, endothelial, and stromal cells. Thus, MSCs do not exactly represent the properties of the uniform purified stem cells that exclusively participate in skeletal repair.(41,42) Under such circumstances, the concept of skeletal stem cells emerged, and a significant amount of effort has been devoted toward defining these cells with rigorous functional characterization.(18,43,44) Chan and colleagues identified the mouse SSC(18) and its downstream BCSP(19) and proved the critical roles of these specific stem and progenitor cells in fracture repair, including massive expansion and enhanced osteogenic capacity after injury.(18,20) In impaired fracture murine models caused by irradiation or diabetes mellitus, the expansion of mSSC and BCSP in the callus at 1 week after fracture were both significantly reduced compared with normal mice.(18,20,45) In the present study, we also found that the numbers of these two cell types, in calluses collected from CS mice at 1 week after fracture, were markedly decreased. In addition, multi-immunofluorescence staining analysis of Ki67 and CD105 in the sections of fracture calluses collected at 1 week after femoral osteotomy indicated that the proliferative activity of BCSPs was impaired in the CS group. Thus, aberrant stem and progenitor cell activity after fracture caused by smoking may be a potential mechanism that results in impaired bone healing.
Smoking causes a pronounced inflammatory response after fracture
The intimate interaction between the immune system and bone healing has been recognized in the emerging field of osteoimmunology. Clinically, patients suffering from disorders that impact their immune function, such as autoimmune diseases or malignancies, often exhibit delayed or insufficient fracture healing.(46–48) The reasons for the impaired fracture healing in these patients are not yet known in detail. However, with attention to the local immune reaction of fracture healing and the determination of its importance in the entire healing process, the imbalance of the initial inflammatory response offers a potential reason.(49,50)
Paula and colleagues(51) investigated the early immune response in the fracture hematomas and surrounding bone marrow in immunocompromised patients. Results showed higher numbers of immune cells like monocytes, macrophages, natural killer T cells, and activated T helper cells in these patients. Meanwhile, several pro-inflammatory cytokines such as IL-6 and TNF-α and chemokines such as Eotaxin and RANTES were found at higher levels. Haffner-Luntzer and colleagues(22) employed an established mouse model for posttraumatic stress disorder (CSC) and discovered that mice subjected to CSC displayed increased numbers of neutrophils in the early fracture hematoma, whereas markers for cartilage-to-bone transition and angiogenesis were reduced. They indicated that changes to the early inflammatory processes might disturb the cartilage-to-bone transition, resulting in poor fracture healing outcomes.
It has been well documented that cigarette smoking can also impact both innate and adaptive immunity and interrupt systemic immunological homeostasis.(4,52–54) However, the effects of cigarette smoking on the local immune response at the fracture site remain to be clarified. In the present study, we revealed an imbalanced initial inflammatory response after fracture caused by cigarette smoking. Within the bone fracture-induced hematoma at 6 hours post-fracture, CS groups showed high concentrations of the cytokines IL-1α, IL-1β, IL-2, IL-6, IL-9, IL-15, and TNF-α, the chemokines KC and LIX, macrophage inflammatory proteins (MIP-1α, MIP-1β, MIP-2), MCP-1, and RANTES. Moreover, most of the increased concentrations of mediators including IL-1α, IL-6, IL-9, IL-15, KC, LIX, MIP-1α, MIP-2, and MCP-1 remained elevated in the hematomas of CS mice 24 hours post-fracture. Meanwhile, the cytokines IFN-γ and IL-12p40, and the chemokine IP-10 also showed increased levels. Cellularly, the recruitment of neutrophils, macrophages, B cells, and T cells was enhanced in the hematoma of CS mice 6 hours after femoral osteotomy.
IL-1, IL-6, IL-12p40, IFN-γ, IP-10, TNF-α, KC, MCP-1, macrophage inflammatory proteins, and RANTES are known pro-inflammatory mediators that regulate the migration, infiltration, and functions of different immune cells in the acute inflammatory phase of fracture healing.(51,55,56) The effects of the cytokines IL-2, IL-9, IL-15, and LIX in fracture healing remain unclear, but they have been shown to accelerate inflammation in other diseases.(55,57) Significantly increased pro-inflammatory mediators and inflammatory cells in the fracture hematoma indicate a severe and probably prolonged inflammatory response after fracture due to cigarette smoking. The natural pro-inflammatory reaction after fracture is an essential initiator of the healing process. However, the imbalance of this reaction, either excessive or insufficient, may negatively impact the healing process. The pronounced initial inflammatory response after fracture caused by cigarette smoking provides a potential immunological mechanism as well as a possible target for immune-modulatory interventions of cigarette smoke–induced compromised fracture healing.
Although we show that cigarette smoking impairs chondro-genesis, skeletal stem cell, and progenitor cell activity after fracture while simultaneously amplifying the initial inflammatory response of fracture healing, our study still possesses limitations. Our data are based solely on murine models, and a vast gap exists between men and mice in the pathophysiology of disease development in response to external noxious compounds.(58,59) Moreover, owing to the differences in smoking patterns (eg, components, amount, and length of smoking history) and individual characteristics (eg, age, sex, and medical comorbidities) between human smokers, the effects of smoking can be diverse, such as the dual influence on the immune system—pro-inflammatory and immunosuppressive.(4,52–54) Thus, elucidation of the fracture healing process in smoking should be further studied in primate and human subjects.
In the present study, we establish reliable and valid murine models to mimic long-term cigarette smoking and compare different susceptibilities to cigarette smoke–induced compromised fracture healing among murine strains. The 129X1/SvJ murine strain seems to be most suitable in constructing models of cigarette smoke–induced compromised fracture healing compared with C57BL/6J and BALB/cJ strains. Furthermore, we demonstrate the significant molecular and cellular changes to the natural fracture healing process caused by cigarette smoking, including interrupted chondrogenesis, aberrant SSC and BCSP activity, and a pronounced initial inflammatory response. Insights into these changes may be helpful for explaining the mechanisms of smoking-induced impaired fracture healing and developing interventions to prevent this problem. These alterations are potentially connected because the inflammatory response plays an important role in migration, proliferation, and differentiation of stem and progenitor cells in fracture repair. Defining the cross-talk between altered mediators and stem cells as well as progenitors is an important goal in future studies.
Supplementary Material
Acknowledgments
Funding was provided by R01AR073607 (FYL), R01AR056246 (FYL), and Musculoskeletal Transplantation Foundation (FYL).
Authors’ roles: Study design: ZH, JB, SX, MK, and FYL. Study conduct: ZH, JL, BL, KDA, SVC, JB, and IL. Data collection: ZH, JL, BL, SVC, and IL. Data analysis: ZH, H-KK, and JB. Data interpretation: ZH, KDA, AMM, H-KK, SX, MK, and FYL. Drafting manuscript: ZH and KDA. Revising manuscript content: ZH, KDA, SVC, AMM, and FYL. All authors approved the final manuscript.
Footnotes
Disclosures
All authors state that they have no conflicts of interest.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4175.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States. Morb Mortal Wkly Rep. 2019;68(45):1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JJ, Patel R, Biermann JS, Dougherty PJ. The musculoskeletal effects of cigarette smoking. J Bone Joint Surg Am. 2013;95(9):850–9. [DOI] [PubMed] [Google Scholar]
- 3.Abate M, Vanni D, Pantalone A, Salini V. Cigarette smoking and musculoskeletal disorders. Muscles Ligaments Tendons J. 2013;3(2):63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehnert S, Aspera-Werz RH, Ihle C, et al. Smoking dependent alterations in bone formation and inflammation represent major risk factors for complications following total joint arthroplasty. J Clin Med. 2019;8(3):406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitz MA, Finnegan M, Natarajan R, Champine J. Effect of smokingon tibial shaft fracture healing. Clin Orthop Relat Res. 1999;365: 184–200. [DOI] [PubMed] [Google Scholar]
- 6.Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM. Impact of smokingon fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151–7. [DOI] [PubMed] [Google Scholar]
- 7.Scolaro JA, Schenker ML, Yannascoli S, Baldwin K, Mehta S, Ahn J. Cigarette smoking increases complications following fracture: a systematic review. J Bone Joint Surg Am. 2014;96(8):674–81. [DOI] [PubMed] [Google Scholar]
- 8.Cyprus GN, Overlin JW, Hotchkiss KM, Kandalam S, Olivares-Navarrete R. Cigarette smoke increases pro-inflammatory markers and inhibits osteogenic differentiation in experimental exposure model. Acta Biomater. 2018;76:308–18. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Wan Q, Yu X, Cheng G, Ni Y, Li Z. Low-dose nicotine reduces the homing ability of murine BMSCs during fracture healing. Am J Transl Res. 2018;10(9):2796–809. [PMC free article] [PubMed] [Google Scholar]
- 10.CDC. How tobacco smoke causes disease: the biology and behavioural basis for smoking-attributable disease: a report of the surgeon general. Atlanta: Centers for Disease Control and Prevention (US); 2010. [PubMed] [Google Scholar]
- 11.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277(5334):2002–4. [DOI] [PubMed] [Google Scholar]
- 12.Camlin NJ, McLaughlin EA, Holt JE. Through the smoke: use of in vivo and in vitro cigarette smoking models to elucidate its effect on female fertility. Toxicol Appl Pharmacol. 2014;281(3):266–75. [DOI] [PubMed] [Google Scholar]
- 13.CDC. The health consequences of smoking—50 years of progress: a report of the surgeon general. Atlanta: Centers for Disease Control and Prevention (US); 2014. [PubMed] [Google Scholar]
- 14.Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11(1):45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glynos C, Bibli SI, Katsaounou P, et al. Comparison of the effects of e-cigarette vapor with cigarette smoke on lung function and inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2018;315(5): L662–72. [DOI] [PubMed] [Google Scholar]
- 16.Whelan DB, Bhandari M, Stephen D, et al. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J Trauma. 2010;68(3): 629–32. [DOI] [PubMed] [Google Scholar]
- 17.Röntgen V, Blakytny R, Matthys R, et al. Fracture healing in mice under controlled rigid and flexible conditions using an adjustable external fixator. J Orthop Res. 2010;28(11):1456–62. [DOI] [PubMed] [Google Scholar]
- 18.Chan CK, Seo EY, Chen JY, et al. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160(1–2):285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan CK, Lindau P, Jiang W, et al. Clonal precursor of bone, cartilage, and hematopoietic niche stromal cells. Proc Natl Acad Sci U S A. 2013; 110(31):12643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marecic O, Tevlin R, McArdle A, et al. Identification and characterization of an injury-induced skeletal progenitor. Proc Natl Acad Sci U S A. 2015;112(32):9920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroner J, Kovtun A, Kemmler J, et al. Mast cells are critical regulators of bone fracture-induced inflammation and osteoclast formation and activity. J Bone Miner Res. 2017;32(12):2431–44. [DOI] [PubMed] [Google Scholar]
- 22.Haffner-Luntzer M, Foertsch S, Fischer V, et al. Chronic psychosocial stress compromises the immune response and endochondral ossification during bone fracture healing via β-AR signaling. Proc Natl Acad Sci U S A. 2019;116(17):8615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattes W, Yang X, Orr MS, Richter P, Mendrick DL. Biomarkers oftobacco smoke exposure. Adv Clin Chem. 2014;67:1–45. [DOI] [PubMed] [Google Scholar]
- 24.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–48. [DOI] [PubMed] [Google Scholar]
- 25.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;131(5):1557–66. [DOI] [PubMed] [Google Scholar]
- 26.Aldaham S, Foote JA, Chow HH, Hakim IA. Smoking status effect on inflammatory markers in a randomized trial of current and former heavy smokers. Int J Inflam. 2015;2015:439396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Audrain-McGovern J, Benowitz NL. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther. 2011;90(1):164–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerassimov A, Hoshino Y, Takubo Y, et al. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am J Respir Crit Care Med. 2004;170(9):974–80. [DOI] [PubMed] [Google Scholar]
- 29.Basic VT, Tadele E, Elmabsout AA, et al. Exposure to cigarette smoke induces overexpression of von Hippel-Lindau tumor suppressor in mouse skeletal muscle. Am J Physiol Lung Cell Mol Physiol. 2012; 303(6):L519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia P, Histing T, Holstein JH, et al. Rodent animal models of delayed bone healing and non-union formation: a comprehensive review. Eur Cell Mater. 2013;26:1–12; discussion 12–4. [DOI] [PubMed] [Google Scholar]
- 31.Manigrasso MB, O’Connor JP. Characterization of a closed femur fracture model in mice. J Orthop Trauma. 2004;18(10):687–95. [DOI] [PubMed] [Google Scholar]
- 32.Fiset S, Godbout C, Crookshank MC, Zdero R, Nauth A, Schemitsch EH. Experimental validation of the Radiographic Union Score for Tibial fractures (RUST) using micro-computed tomography scanning and biomechanical testing in an in-vivo rat model. J Bone Joint Surg Am. 2018;100(21):1871–8. [DOI] [PubMed] [Google Scholar]
- 33.Cooke ME, Hussein AI, Lybrand KE, et al. Correlation between RUST assessments of fracture healing to structural and biomechanical properties. J Orthop Res. 2018;36(3):945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morshed S. Current options for determining fracture union. Adv Med.2014;2014:708574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Neill KR, Stutz CM, Mignemi NA, et al. Micro-computed tomography assessment of the progression of fracture healing in mice. Bone. 2012;50(6):1357–67. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham JL, Kenwright J, Kershaw CJ. Biomechanical measurement of fracture healing. J Med Eng Technol. 1990;14(3):92–101. [DOI] [PubMed] [Google Scholar]
- 37.El-Zawawy HB, Gill CS, Wright RW, Sandell LJ. Smoking delays chondrogenesis in a mouse model of closed tibial fracture healing. J Orthop Res. 2006;24(12):2150–8. [DOI] [PubMed] [Google Scholar]
- 38.Lemos DR, Eisner C, Hopkins CI, Rossi F. Skeletal muscle-resident MSCs and bone formation. Bone. 2015;80:19–23. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Wang Y, Gou W, Lu Q, Peng J, Lu S. Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int Orthop. 2013;37(12):2491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kung MH, Yukata K, O’Keefe RJ, Zuscik MJ. Aryl hydrocarbon receptor-mediated impairment of chondrogenesis and fracture healing by cigarette smoke and benzo(a)pyrene. J Cell Physiol. 2012;227 (3):1062–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sipp D, Robey PG, Turner L. Clear up this stem-cell mess. Nature.2018;561(7724):455–7. [DOI] [PubMed] [Google Scholar]
- 42.Galipeau J, Weiss DJ, Dominici M. Response to nature commentary “clear up this stem-cell mess”. Cytotherapy. 2019;21(1):1–2. [DOI] [PubMed] [Google Scholar]
- 43.Bianco P, Robey PG. Skeletal stem cells. Development. 2015;142(6): 1023–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrison JI, Lööf S, He P, Simon A. Salamander limb regeneration involves the activation of a multipotent skeletal muscle satellite cell population. J Cell Biol. 2006;172(3):433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tevlin R, Seo EY, Marecic O, et al. Pharmacological rescue of diabetic skeletal stem cell niches. Sci Transl Med. 2017;9(372):eaag2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woodland DL, Blackman MA. Immunity and age: living in the past. Trends Immunol. 2006;27(7):303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hadjiargyrou M, O’Keefe RJ. The convergence of fracture repair and stem cells: interplay of genes, aging, environmental factors and disease. J Bone Miner Res. 2014;29(11):2307–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bogoch ER, Moran EL. Bone abnormalities in the surgical treatment of patients with rheumatoid arthritis. Clin Orthop Relat Res. 1999; 366:8–21. [DOI] [PubMed] [Google Scholar]
- 49.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–7. [DOI] [PubMed] [Google Scholar]
- 50.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7 (4):292–304. [DOI] [PubMed] [Google Scholar]
- 51.Hoff P, Gaber T, Strehl C, et al. A pronounced inflammatory activity characterizes the early fracture healing phase in immunologically restricted patients. Int J Mol Sci. 2017;18(3):583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9(5):377–84. [DOI] [PubMed] [Google Scholar]
- 53.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2(5):372–7. [DOI] [PubMed] [Google Scholar]
- 54.Strzelak A, Ratajczak A, Adamiec A, Feleszko W. Tobacco smoke induces and alters immune responses in the lung, triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health. 2018;15(5):1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edderkaoui B. Potential role of chemokines in fracture repair. Front Endocrinol (Lausanne). 2017;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu J, Wang Y, Li J, et al. IL-12p40 impairs mesenchymal stem cell mediated bone regeneration via CD4+ T cells. Cell Death Differ. 2016;23(12):1941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nouailles G, Dorhoi A, Koch M, et al. CXCL5-secreting pulmonary epithelial cells drive destructive neutrophilic inflammation in tuberculosis. J Clin Invest. 2014;124(3):1268–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burkhardt AM, Zlotnik A. Translating translational research: mouse models of human disease. Cell Mol Immunol. 2013;10(5):373–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perlman RL. Mouse models of human disease: an evolutionary perspective. Evol Med Public Health. 2016;2016(1):170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.