Abstract
Introduction
Ethnoracial differences in cerebrospinal fluid (CSF; amyloid beta 42 [Aβ42], total tau [t‐tau], phosphorylated tau 181 [p‐tau181], and plasma (p‐tau181, neurofilament light [NfL]) biomarkers of Alzheimer's disease (AD) are incompletely understood.
Methods
We performed cross‐sectional analyses with and without adjustment for covariates comparing baseline CSF (Aβ42, t‐tau, p‐tau181) and plasma (p‐tau181, NfL) values in 47 African Americans (AAs) matched to 141 non‐Hispanic Whites (NHWs) and 43 Latinos (LAs) matched to 129 NHWs from the Alzheimer's Disease Neuroimaging Initiative (ADNI).
Results
Unadjusted comparisons revealed no significant differences in plasma or CSF biomarkers between AAs and NHWs. A trend toward a lower CSF t‐tau and p‐tau181 in LAs compared to NHWs was observed, without significant differences in plasma biomarkers. After adjusting for covariates, there were no significant differences in CSF or plasma biomarkers between AAs and NHWs or between LAs and NHWs.
Discussion
Plasma and CSF AD biomarkers may perform similarly across diverse populations but future studies in large, diverse cohorts are needed.
Keywords: ADNI, Alzheimer's, amyloid, biomarkers, Black, CSF, ethnicity, Latino, NfL, plasma, race, tau
1. INTRODUCTION
Fluid biomarkers of Alzheimer's disease (AD) (ie, amyloid beta [Aβ], tau) and neurodegeneration (ie, neurofilament light [NfL]) are understudied in diverse populations yet represent important diagnostic tools due to their accessibility. Ethnoracial differences in biomarker concentrations across the disease spectrum must be better understood, as African American (AA) and Latino (LA) individuals will be disproportionately affected by AD and related disorders in the coming decades. 1 Validating biomarkers in diverse populations is also essential when considering implementation of the 2018 NIA‐AA (National Institute on Aging‐Alzheimer's Association) Research Framework for AD 2 and selecting appropriate candidates for emerging molecular therapies. 3
Direct comparisons of fluid biomarkers between ethnoracial groups have been limited and have yielded mixed results, although lower concentrations of cerebrospinal fluid (CSF) total tau (t‐tau) and phosphorylated tau 181 (p‐tau181) among AAs compared to non‐Hispanic Whites (NHWs) have been reported. 4 , 5 , 6 , 7 Novel AD plasma biomarkers are less studied among diverse groups. Mixed results, including an effect of race and ethnicity on plasma t‐tau, as well as no impact of race or ethnicity on biomarker concentrations have been reported. 8 , 9 , 10 , 11 , 12 Prior studies have largely included cognitively normal participants from single sites and have not fully characterized social determinants of health (eg, educational background, socioeconomic background) among participants.
We leveraged data available through the Alzheimer's Disease Neuroimaging Initiative (ADNI) to study ethnoracial differences in plasma (p‐tau181, NfL) and CSF (Aβ42, t‐tau, p‐tau181) biomarkers among AA, NHW, and LA individuals, adjusting for sociodemographic factors and comorbidities. Based on the previous literature, we hypothesized significantly lower CSF t‐tau and plasma and CSF p‐tau181 concentrations among AA and LA individuals compared to NHWs, but similar NfL levels among AAs and LAs compared to NHWs.
2. METHODS
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (or ADNI) database (adni.loni.usc.edu). (Please see supplementary materials for additional information about the ADNI.)
Data were obtained from the ADNI website accessed on October 14, 2020 (plasma data) and December 3, 2020 (non‐plasma data). We obtained demographic and additional data from all AA, LA, and NHW participants with available baseline plasma p‐tau181 and NfL data from ADNI 1, ADNI Grand Opportunity (GO), ADNI 2, and ADNI 3. Race and ethnicity in ADNI is self‐reported by participants. Availability of baseline plasma biomarker data was the primary criteria used for selection of AA and LA participants, with NHWs selected to be matched to each group (see below). All levels of impairment were included (cognitively unimpaired, mild cognitive impairment, dementia). For subjects with multiple plasma measurements, the baseline data were used. All AAs and matched NHWs used in the current study self‐identified as non‐Hispanic ethnicity. All LA individuals self‐identified as Hispanic ethnicity. Written informed consent was obtained for all participants at each site and each site operated according to institutional review board (IRB)–approved protocol.
RESEARCH IN CONTEXT
Systematic Review: We conducted a literature review using traditional search engines (PubMed, Google Scholar). Ethnoracial differences in cerebrospinal fluid (CSF) and plasma biomarkers of Alzheimer's disease (AD) have been reported, yet etiology of differences is unclear. Many previously performed studies have not considered the social determinants of health and comorbidity differences between populations that could drive biomarker differences.
Interpretation: Our data demonstrated no significant differences in CSF (amyloid beta 42 [Aβ42], total tau [t‐tau], phosphorylated tau 181 [p‐tau181]) or plasma (p‐Tau181, neurofibrillary light [NfL]) biomarkers in matched comparisons between African American and Latino individuals with non‐Hispanic White individuals in Alzheimer's Disease Neuroimaging Initiative (ADNI) after adjustment for covariates. Our findings suggest that previously described ethnoracial differences in these biomarkers may be the result of differences in sociodemographics and comorbidities.
Future Directions: Large scale studies inclusive of diverse populations to further examine ethnoracial differences in plasma and CSF AD biomarkers are needed.
Information on plasma p‐tau181 and NfL (Simoa; Quanterix 13 , 14 ) and CSF Aβ42, p‐tau181, and t‐tau (Elecsys; Roche Diagnostics) measurement in ADNI is available in the supplementary materials. White matter hyperintensity volume was calculated as total volume of white matter hyperintensity divided by the total brain volume, as described in detail on the ADNI website (http://adni.loni.usc.edu).
Baseline participant and disease characteristics are summarized by median value and interquartile range (continuous variables) or counts and percentages (categorical variables). Data for 47 AAs and 43 LAs were matched to separate, mutually exclusive groups of NHWs with no overlap using a 1:3 matching strategy resulting in 141 and 129 NHWs, respectively. Variables for matching included age at blood draw, sex, years of educational attainment, level of impairment (Mini‐Mental State Examination [MMSE], Clinical Dementia Rating Sum of Boxes [CDR‐SB]), and family history of dementia. CSF measurements were available for 32 of 47 AAs and 112 of 141 matched NHWs, and for 30 of 43 LAs and 102 of 129 matched NHWs, respectively. White matter hyperintensity volume data were available for 38 of 47 AAs and 115 of 141 matched NHWs and 37 of 43 LAs and 102 of 129 matched NHWs, respectively.
Cross‐sectional analyses compared biomarker levels between the matched cohorts with (multiple regression approach) and without (non‐parametric Mann‐Whitney U test) adjustment for age, sex, years of educational attainment, MMSE, CDR‐SD, CSF Aβ42 (for t–tau, p‐tau181, and NfL comparisons), medical history of cardiovascular disease, and apolipoprotein E (APOE) ε4 carrier status. The threshold for statistical significance was set to P < .05, with a False Discovery Rate (FDR) corrected for 18 comparisons for the unadjusted tests.
3. RESULTS
Baseline demographic and clinical data of matched groups are noted in Table 1.
TABLE 1.
Demographics of matched cohorts
| Matched participants | Matched participants | |||
|---|---|---|---|---|
| AA | NHW | LA | NHW | |
| Sample size | 47 | 141 | 43 | 129 |
| Age | 74.5 (67.6‐78.1) | 73.0 (69.4‐77.9) | 72.9 (67.1‐77.6) | 72.9 (66.0‐77.3) |
| Sex (male/female) | 15/32 | 31.9%/68.1% | 48/93 | 34.0%/66.0% | 16/27 | 37.2%/62.8% | 44/85 | 34.1%/65.9% |
| Education (years) | 16 (14‐18) | 16 (13‐18) | 16 (14‐17) | 16 (14‐18) |
| MMSE | 28 (26‐29) | 28 (26‐29) | 28 (25.5‐29) | 28 (26‐29) |
| CDR‐SB | 0.5 (0‐1.3) | 1 (0‐2) | 1 (0‐2.5) | 1.5 (0.5‐3) |
| Level of impairment (cognitively normal/MCI/dementia) | 24/15/7 | 46/74/21 | 15/18/10 | 34/63/32 |
| Family history of dementia (no/yes) | 32/15 | 68.1%/31.9% | 101/40 | 71.6%/28.4% | 18/25 | 41.9%/58.1% | 52/77 | 40.3%/59.7% |
| Past medical history | ||||
| % with cardiovascular disease | 85.1% | 63.8% | 65.1% | 55.8% |
| % with respiratory disease | 17.0% | 25.5% | 11.6% | 20.2% |
| APOEε4 | (carrier/non‐carrier) | 20/27 | 42.6%/57.4% | 53/88 | 37.6%/62.4% | 18/25 | 41.9%/58.1% | 60/69 | 46.5%/53.5% |
Abbreviations: AA, African American; NHW, non‐Hispanic White, LA, Latino; MMSE, Mini‐Mental State Examination; CDR‐SB, Clinical Dementia Rating Sum of Boxes; MCI, mild cognitive impairment; APOE, apolipoprotein E gene
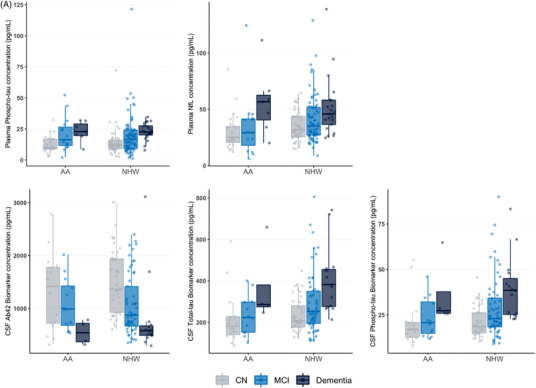
Unadjusted comparisons between AAs and NHWs revealed no significant differences in plasma NfL (pFDR = 0.21) and p‐tau181 (pFDR = 0.95) or CSF Aβ42 (pFDR = 0.95), t‐tau (pFDR = .21), or p‐tau181 (pFDR = 0.42) measures (Figure 1A, Table S1). In addition, there was no significant difference in total white matter hyperintensity volume between groups (pFDR = 0.95) (Figure 1C; Table S1). In the multiple regression model adjusting for covariates, no significant differences were identified in any of the biomarkers.
FIGURE 1.
Plasma and CSF biomarker concentration and white matter hyperintensity comparisons between ethnoracial groups. (A) Comparison of plasma (top row) and CSF (bottom row) biomarker concentrations between African American and non‐Hispanic White individuals, with stratification of individuals by level of impairment. (B) Comparison of plasma (top row) and CSF (bottom row) biomarker concentrations between Latino and non‐Hispanic White individuals, with stratification of individuals by level of impairment. (C) Comparison of ratio of white matter hyperintensity volume to intracranial volume between African American and non‐Hispanic White individuals as well as Latino and non‐Hispanic White individuals, respectively, with stratification of individuals by level of impairment. AA, African American; NHW, non‐Hispanic White; LA, Latino; NfL, neurofilament light; CSF, cerebrospinal fluid; CN, cognitively normal; MCI, mild cognitive impairment; WMH, white matter hyperintensity; ICV, intracranial volume
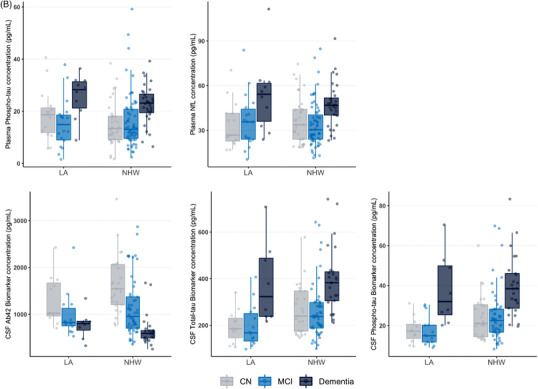
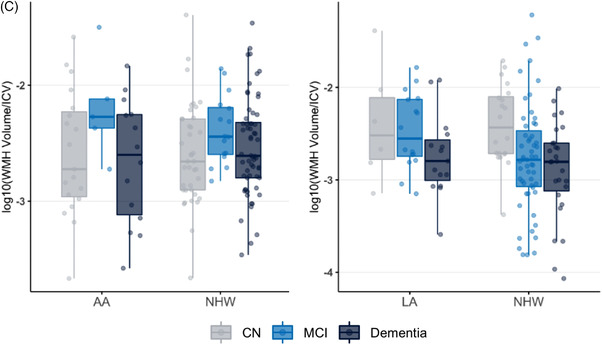
Unadjusted comparisons between LAs and NHWs revealed no significant differences in plasma NfL (pFDR = 1) and p‐tau181 (pFDR = .9) measures or CSF Aβ42 (pFDR = 1) (Figure 1B, Table S1). A trend toward lower CSF t‐tau (216.5 [147.1–270.5] vs 257.2 [197.2–360.9], pFDR = .08) and CSF p‐tau181 (19.4 [13.6–.9] vs 24.7 [17.5–33.4], pFDR = .08) in LAs compared to NHWs was observed (Figure 1B, Table S1). There were no significant differences in total white matter hyperintensity volume (pFDR = 0.73) (Figure 1C, Table S1). In the multiple regression model adjusting for covariates, LAs no longer had significantly lower levels of CSF t‐tau (standardized β = 0.134, pFDR = .09) and CSF p‐tau181 (β = 0.147, pFDR = .06). No significant differences in plasma markers were observed in the multiple regression model.
Unadjusted comparisons between AAs and LAs revealed no significant differences in plasma or CSF biomarkers (data not shown). No significant differences between these groups were identified in any of the biomarkers after adjustment for relevant covariates.
4. DISCUSSION
Advancement in therapies for AD coupled with the new NIA‐AA Research Framework have brought characterization of fluid biomarkers for AD and non‐AD pathology within diverse populations into the spotlight. The majority of biomarker studies to date have lacked inclusion of diverse populations that are disproportionately impacted by disease. In our study, after adjustment for covariates, we found no significant difference in biomarker levels of Aβ (CSF only), tau, or neurodegeneration (CSF and plasma) in matched cohorts of AAs, Las, and NHWs enrolled in the ADNI.
Our study is unique in that it includes data from the ADNI, a large study inclusive of over 60 sites in the United States and Canada. Previous studies have largely been limited to a single site or a small number of sites. We included a wide range of impairment levels and did not limit to the cognitively unimpaired. We had data from both sexes. We employed a matching strategy to match for multiple sociodemographic and clinical variables that may have differed between ethnoracial groups. We also included measurements of white matter pathology in addition to fluid biomarker data. There is no biological basis for racial and ethnic categories, and our findings suggest that ethnoracial differences in plasma and CSF biomarkers may be the result of sociodemographic and comorbidity differences as well as differences in other unmeasured variables (social determinants of health).
Current literature on the direct comparison of plasma biomarkers between ethnic and racial groups is limited. Grewal 8 found racial differences in plasma t‐tau but not p‐tau181 among 10 AA and 10 NHW females, all with mild cognitive impairment (MCI). A multi‐site study of 1193 LAs and 650 NHWs with varying levels of impairment found no interaction between ethnicity and plasma p‐tau or NfL. 9 Barker 11 found no impact of ethnicity on plasma NfL among 309 individuals with varying impairment from a single Alzheimer's Disease Research Center (ADRC) with significant Latino representation. This is consistent with findings from a study comparing 890 Mexican Americans and 813 NHWs, although there were significant differences in plasma t‐tau between groups. 12 Finally, Brickman 10 found no ethnoracial differences in plasma p‐tau181, t‐tau, or NfL among 297 combined AAs, LAs, and NHWs with varying levels of impairment from a community‐dwelling group of individuals in New York. These studies all varied in scale and characterization of sociodemographic profiles and comorbidities of participants.
Lower CSF t‐tau and p‐tau181 among cognitively normal and impaired AAs compared to NHWs has been reported. 4 , 5 , 6 , 7 This finding comes from single‐site studies with relatively small numbers of AA participants. In our multi‐linear regression analysis, we found a trend toward lower CSF t‐tau and p‐tau181 among LAs compared to NHWs; however, this different was not statistically significant after adjustment for covariates. We found no statistically significant differences in CSF t‐tau and p‐tau181 between AAs and NHWs.
Limitations of our study include small sample size; limited characterization of race and ethnicity (although in keeping with National Institutes of Health [NIH] guidelines) in the ADNI (five racial categories along with a “multiple” and “other” category as well as only two ethnic [Latino vs non‐Latino] categories), which does not capture diversity within each group; missing data for CSF biomarkers; unequal sex distribution of participants; and limited available sociodemographic data (no data regarding quality of education, income level, employment status, health insurance status, and so on). Our study emphasizes the need for larger‐scale studies to investigate possible ethnoracial differences in biomarkers of AD and non‐AD neurodegenerative disease.
CONFLICT OF INTEREST
GDR receives research support from Avid Radiopharmaceuticals, GE Healthcare, Genentech, and Life Molecular Imaging. He has received consulting fees from Eisai, Genentech, Johnson & Johnson, and Roche. He is an Associate Editor for JAMA Neurology. ALB receives research support from Biogen, Eisai, Regeneron, and Woolsey. In addition, he has participated in unfunded collaborations with Biogen, Eli Lilly, and Novartis and has received consulting fees from AGTC, Alector, Arvinas, AZ Therapeutics, GSK, Humana, Oligomerix, Oscotec, Roche, Stealth Transposon, TrueBinding, and Wave. MRM receives research support from the Genentech health equity fund and is chair of the National Institute on Aging (NIA) AGCD‐4 study section and a board member of the Alzheimer's Association New York chapter. The remaining authors have no declarations of interest.
Supporting information
SUPPORTING INFORMATION
ACKNOWLEDGEMENTS
This study was supported by Alzheimer's Association P0553569 (C.W.), National Institutes of Health/National Institute on Aging (NIH/NIA) R35‐AG072362 (GDR and CW). Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH‐12‐2‐0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol‐Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann‐La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Study design analysis, interpretation of data, and the composition of this report were performed by the stated authors.
Windon C, Iaccarino L, Mundada N, et al. Comparison of plasma and CSF biomarkers across ethnoracial groups in the ADNI. Alzheimer's Dement. 2022;14:e12315. 10.1002/dad2.12315
REFERENCES
- 1. Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer's disease and related dementias in the United States (2015‐2060) in adults aged ≥65 years. Alzheimers Dement. 2019;15(1):17‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jack CR Jr, Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cummings J, Aisen P, Apostolova LG, Atri A, Salloway S, Weiner M. Aducanumab: appropriate use recommendations. J Prev Alzheimers Dis. 2021;8(4):398‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Howell JC, Watts KD, Parker MW, et al. Race modifies the relationship between cognition and Alzheimer's disease cerebrospinal fluid biomarkers. Alzheimers Res Ther. 2017;9(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morris JC, Schindler SE, McCue LM, et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol. 2019;76(3):264‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumar VV, Huang H, Zhao L, et al. Baseline results: The association between cardiovascular risk and preclinical Alzheimer's disease pathology (ASCEND) study. J Alzheimers Dis. 2020;75(1):109‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garrett SL, McDaniel D, Obideen M, et al. Racial disparity in cerebrospinal fluid amyloid and tau biomarkers and associated cutoffs for mild cognitive impairment. JAMA Netw Open. 2019;2(12):e1917363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grewal R, Haghighi M, Huang S, et al. Identifying biomarkers of dementia prevalent among amnestic mild cognitively impaired ethnic female patients. Alzheimers Res Ther. 2016;8(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gonzales MM, Short MI, Satizabal CL, et al. Blood biomarkers for dementia in Hispanic and non‐Hispanic White adults. Alzheimers Dement (N Y). 2021;7(1):e12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brickman AM, Manly JJ, Honig LS, et al. Plasma p‐tau181, p‐tau217, and other blood‐based Alzheimer's disease biomarkers in a multi‐ethnic, community study. Alzheimers Dement. 2021. 10.1002/alz.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barker W, Quinonez C, Greig MT, et al. Utility of plasma neurofilament light in the 1Florida Alzheimer's Disease Research Center (ADRC). J Alzheimers Dis. 2021;79(1):59‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Bryant SE, Johnson LA, Barber RC, et al. The Health & Aging Brain among Latino Elders (HABLE) study methods and participant characteristics. Alzheimers Dement (Amst). 2021;13(1):e12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19(5):422‐433 [DOI] [PubMed] [Google Scholar]
- 14. Mattsson N, Andreasson U, Zetterberg H, Blennow K; Alzheimer's Disease Neuroimaging Initiative. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74(5):557‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION


