Abstract
Background:
Evidence from longitudinal patient studies regarding gut microbial changes after bariatric surgery is limited.
Objective:
To examine intraindividual changes in fecal microbiome and metabolites among patients undergoing Roux-en-Y gastric bypass or vertical sleeve gastrectomy.
Setting:
Observational study.
Methods:
Twenty patients were enrolled and provided stool samples before and 1 week, 1 month, and/or 3 months after surgery. Shallow shotgun metagenomics and untargeted fecal metabolomics were performed. Zero-inflated generalized additive models and linear mixed models were applied to identify fecal microbiome and metabolites changes, with adjustment for potential confounders and correction for multiple testing.
Results:
We enrolled 16 women and 4 men, including 16 white and 4 black participants (median age = 45 years; presurgery body mass index = 47.7 kg/m2). Ten patients had Roux-en-Y gastric bypass, 10 had vertical sleeve gastrectomy, and 14 patients provided postsurgery stool samples. Of 47 samples, median sequencing depth was 6.3 million reads and 1073 metabolites were identified. Microbiome alpha-diversity increased after surgery, especially at 3 months. Significant genus-level changes included increases in Odoribacter, Streptococcus, Anaerotruncus, Alistipes, Klebsiella, and Bifidobacterium, while decreases in Bacteroides, Coprocosccus, Dorea, and Faecalibacterium. Large increases in Streptococcus, Akkermansia, and Prevotella were observed at 3 months. Beta-diversity and fecal metabolites were also changed, including reduced caffeine metabolites, indoles, and butyrate.
Conclusions:
Despite small sample size and missing repeated samples in some participants, our pilot study showed significant postsurgery changes in fecal microbiome and metabolites among bariatric surgery patients. Future large-scale, longitudinal studies are warranted to investigate gut microbial changes and their associations with metabolic outcomes after bariatric surgery.
Keywords: Gut microbiota, Metagenomics, Fecal metabolomics, Bariatric surgery, Longitudinal patient cohort
The obesity epidemic continues to worsen in the United States. In 2017 to 2018, 42% of U.S. adults were obese (body mass index; BMI ≥30 kg/m2) and 9% had severe obesity (BMI ≥40 kg/m2) [1]. Bariatric surgery is currently most effective treatment for severe obesity, with common procedures being Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (SG). On average, patients lose 30% of total weight and 60% of excess weight, and most patients also experience resolution of obesity-related metabolic diseases after surgery [2-5].
The gut microbiota is known to play an important role in obesity and metabolism [6]. Bariatric surgery can profoundly alter the gut microbiota, usually toward a “leaner and healthier” community [7]. Animal studies have shown that microbiota transfer from RYGB patients to germ-free mice resulted in less fat deposition and better fat utilization than mice receiving microbiota from BMI-matched controls, supporting a potential causal role of gut microbiota in weight loss and metabolic improvements after bariatric surgery [8].
However, relevant evidence from human studies is limited. To our knowledge, 11 studies have reported intraindividual gut microbiome changes after RYGB or SG [9-19], with sample sizes of 5 to 30. These studies have yielded mixed results. At community level, most studies, but not all, found increased alpha-diversity [9,13-15,17]. At phylum level, many studies found reduced Firmicutes while increased Bacteroidetes and Proteobacteria [9-12,15,16,18], but the opposite were also reported [14]. At genus/species level, promising results reported in ≥2 studies include Akkermansia muciniphila, an antidiabetic, antiinflammatory species, increased in studies of different populations (United States, Denmark, and China) after different procedures (RYGB or SG) [11,13,15,18,19]. Faecalibacterium prausnitzii, another antiinflammatory and butyrate-producing species, increased in 4 studies but decreased in another 2 studies [9,10,12,13,16,18]. Roseburia, a butyrate-producing genus, increased in 2 studies and was related to remission of diabetes [14,17]; whereas, other butyrate-producing genera (e.g., Dorea and Coprococcus), reduced in several studies and were linked to improved glycosylated hemoglobin [9,12,15,18]. Among those, 4 studies were conducted in the United States with sample sizes of 5 to 14 [11,16,18,19]. Meanwhile, few studies have evaluated fecal metabolites in addition to microbiome sequencing [12,18]. Microbial metabolites are considered functional readouts of the microbial activities and signaling molecules mediating the diet-microbiota-host interactions, providing information not captured by microbiome sequencing [20].
To provide additional data on gut microbial changes after bariatric surgery, we conducted a longitudinal study among patients undergoing RYGB or SG, collected stool samples before and after surgery, and applied metagenomics and fecal metabolomics. Leveraging the diverse population served by our hospital, this may be the first study including black patients, who have not been included in previous research.
Methods
Study population
We enrolled 20 patients during November 2018 and April 2019, who were approved for RYGB or SG, aged 20 to 70 years, and were able and willing to provide information and biospecimens needed for the study. Exclusion criteria included prior gastric operations, prevalent gastrointestinal disease (e.g., inflammatory bowel disease or celiac disease), history of cancer within 5 years (except nonmelanoma skin cancer), vomiting, constipation, or diarrhea within 7 days, or antibiotics use within 2 months before enrollment. Working with the clinical team, we approached patients who indicated interests in our study at the end of their preoperative visits and discussed study details. Written informed consent was obtained from all participants. Our study was approved and monitored by the institutional review board of our medical center.
Stool sample collection
At enrollment, each participant received a stool sample collection kit and in-person instructions on how to collect sample and fill out the collection form. The kit included disposable collection tools (e.g., plastic collection bowl, powder-free nitrile gloves, and collection spatula), tubes/cards with DNA preservatives, biohazard bags, a sample collection form, and a step-by-step, picture-illustrated instruction sheet. We used 3 collection methods in this study as follows: an OMNIgene-GUT tube, a tube with 5-mL 95% ethanol and glass beads, and 2 fecal occult blood test cards. Based on methodology studies comparing different collection methods with the “gold standard” that freezes samples immediately with no additive [21,22], OMNIgene and fecal occult blood test showed the highest reproducibility for metagenomics, and 95% ethanol method showed the best results for fecal metabolomics. Also, these methods have been shown to yield valid samples after leaving at room temperature for days to weeks, making them convenient for participants to collect samples at home and return to the clinic. Also returned collection form recorded the date and time of sample collection, Bristol stool type, use of antibiotics and other medications in last 2 months, and dietary intakes in last 7 days. We collected stool samples at the following 4 times: before, and 1 week, 1 month, and 3 months after surgery. All samples were transported from the clinic to our research lab within 3 days after receiving and aliquoted and stored in −80°C freezers until assays.
Metagenomics and metabolomics
Stool samples collected using OMNIgene-GUT were sent to CoreBiome, Inc. (St. Paul, MN, USA) for DNA extraction and shallow shotgun sequencing, which provides species-level resolution for microbiome analysis [23]. DNA was extracted using QIAGEN’s DNeasy PowerSoil Pro Kit, automated for high-throughput DNA isolation using QIAcube Connect. DNA libraries were prepared using Illumina Nextera kits. Sequencing was performed on Illumina Nova-Seq at 1 × 100 single-end reads. DNA sequences were filtered for low quality (Q-score < 30) and length (<50), and adapter sequences were trimmed using cutadapt. Samples with <10,000 sequence reads were excluded from analysis. Reads were aligned at 97% identity against a curated database containing all representative genomes in RefSeq for bacteria at NCBI with additional manually curated strains, then clustered into operational taxonomic units (OTUs). The number of counts for each OTU was normalized to OUT’s genome length. OTUs accounting for <.01% of their unique genome and <1% of the whole genome were discarded. Based on OTU table rarefied by the minimum sequencing depth of all samples (.87 million reads), diversity metrics were calculated, including Shannon index for alpha-diversity and Bray-Curtis distance for beta-diversity. For taxonomy assignment, each input sequence was assigned the lowest common ancestor consistent across ≥80% of all reference sequences tied for best hit. Taxonomy from phylum to species were assigned; relative abundance of each taxon within a sample was calculated.
Meanwhile, stool samples collected using 95% ethanol method were sent to Metabolon, Inc. (Morrisville, NC, USA) for untargeted metabolomics profiling [24]. To account for water content, fecal samples were homogenized, freeze-dried, and added solvent to be extracted for analysis. Ultrahigh-performance liquid chromatography with tandem mass spectrometry was used with 4 complimentary columns. Mass spectra features were searched in an in-house reference library with >4000 authenticated standard compounds. For metagenomic and metabolomic assays, all pre- and postsurgery samples from the same patient were placed adjacently in one batch in random orders; laboratory personnel were blinded to the surgical status of the samples.
Statistical analysis
Generalized linear mixed models were used to evaluate the effect of surgery and different postsurgery time periods on Shannon index (log-transformed), adjusting for age (years), sex (male or female), race (black or non-Hispanic white), procedure type (RYGB or SG), Bristol stool type (5 categories; extreme types were combined because of small numbers), and recent use of antibiotics (no use in last 2 mo, used in last 2–8 wk, or used in last 2 wk). Generalized additive models for location, scale, and shape with zero-inflated beta distribution [25] were used to evaluate the effects of surgery and postsurgery time on the relative abundances of microbiome taxa, adjusting for above-listed covariates and considering repeated measures from the same patients. Taxa with relative abundance <.005% and prevalence <5% were excluded from analysis. BMI was further added to the model to evaluate if microbiome changes are independent of or related to BMI changes. An interaction term of surgical status × procedure type was added to the model to evaluate potential procedure-specific microbiome changes. Permutation multivariate analysis of variance was used to evaluate the effect of surgery on Bray-Curtis distance between samples. Missing values of fecal metabolites were replaced by half of the minimum of nonmissing values. Levels of metabolites were batch-normalized, log-transformed, and standardized by its mean and standard deviation. Generalized linear mixed models were used to evaluate the effect of surgery on metabolites levels with the same covariate adjustment. For diversity metrics, P <.05 was considered statistically significant. For individual microbial taxa and fecal metabolites, false discovery rates (FDR) were presented in addition to P values. Software SAS (version 9.4; procedures “mixed” and “multtest”; Cary, NC, USA) and R (version 3.6.3; package ‘metamicrobimeR’ [25]) and a web-based tool ‘MicrobiomeAnalyst’ (https://www.microbiomeanalyst.ca/) were used for data analysis and visualization.
Results
We enrolled 16 women and 4 men, including 16 non-Hispanic white and 4 black participants (Table 1). The median age was 45 years. The median presurgery BMI was 47.7 kg/m2. The median weight loss was 4.5% of total weight at 1 week, 9.1% at 1 month, and 14.8% at 3 months post surgery and 8.5% of excess weight at 1 week, 17.5% at 1 month, and 27.0% at 3 months post surgery. Ten participants had RYGB and 10 had SG; the patient characteristics were similar between these 2 procedure groups in present study (all P > .05), although RYGB patients seemed to be older and have a higher median presurgery BMI than SG patients. All 20 participants provided a presurgery stool sample, and 14 patients provided at least one postsurgery stool sample (n = 12, 9, and 6 at 1 wk, 1 mo, and 3 mo, respectively). Of total 47 stool samples, majority (81%) were collected with no use of antibiotics in last 2 months (15% reported use in last 2–8 wk; 4% used in last 2 wk).
Table 1.
Characteristics of study participants
| Characteristics | Total (n = 20) | RYGB (n = 10) | VSG (n = 10) |
|---|---|---|---|
| Age, yr, median (range) | 45 (23, 68) | 49 (28, 68) | 40 (23, 50) |
| Male sex, n (%) | 4 (20) | 2 (20) | 2 (20) |
| Black, n (%) | 4 (20) | 1 (10) | 3 (30) |
| Body mass index before surgery, kg/m2, median (range) | 47.7 (39.9, 69.3) | 49.5 (40.2, 69.3) | 47.2 (39.9, 49.6) |
| Weight loss 1 wk after surgery, % of total weight, median (range) | 4.5 (.6, 7.3) | 3.0 (.6, 5.1) | 5.8 (3.8, 7.3) |
| Weight loss 1 mo after surgery, % of total weight, median (range) | 9.1 (1.5, 22.5) | 8.8 (1.5, 22.5) | 10.0 (9.0, 11.1) |
| Weight loss 3 mo after surgery, % of total weight, median (range) | 14.8 (13.4, 16.3) | 14.8 (13.4, 16.3) | -* |
| Weight loss 1 wk after surgery, % of excess weight, median (range) | 8.5 (1.3, 15.5) | 5.8 (1.3, 13.1) | 12.7 (8.0, 15.5) |
| Weight loss 1 mo after surgery, % of excess weight, median (range) | 17.5 (3.0, 35.2) | 15.5 (3.0, 35.2) | 21.3 (19.0, 23.7) |
| Weight loss 3 mo after surgery, % of excess weight, median (range) | 27.0 (22.0, 32.0) | 27.0 (22.0, 32.0) | -* |
| Shannon index before surgery, median (range) | 3.55 (2.19, 4.44) | 3.54 (2.95, 3.95) | 3.59 (2.19, 4.44) |
| Shannon index 1 wk after surgery, median (range) | 3.72 (3.33, 4.29) | 3.67 (3.49, 4.09) | 3.78 (3.33, 4.29) |
| Shannon index 1 mo after surgery, median (range) | 3.38 (2.82, 3.86) | 3.37 (2.82, 3.86) | 3.38 (2.82, 3.86) |
| Shannon index 3 mo after surgery, median (range) | 3.76 (3.35, 3.91) | 3.78 (3.35, 3.91) | 3.75 (3.64, 3.86) |
RYGB = Roux-en-Y gastric bypass; VSG = vertical sleeve gastrectomy.
Sample size was too small to make an estimate.
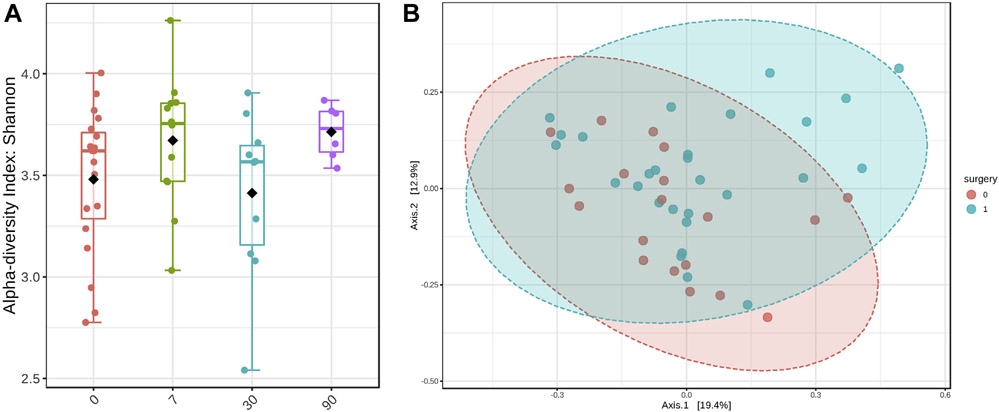
The median sequence depth was 6.3 million reads (range, .87–16.1 million). The median of Shannon index was 3.55 (range, 2.19–4.44) before surgery, 3.72 (3.33–4.29) at 1 week, 3.38 (2.82–3.86) at 1 month, and 3.76 (3.35–3.91) at 3 month after surgery (Fig. 1a; P value = .004 for 1 wk, .54 for 1 mo, and .04 for 3 mo versus before surgery). A significant effect of surgery on Bray-Curtis distance was also found (Fig. 1b; P value = .015). We did not find significant associations of age, sex, race, or procedure type with Shannon index or Bray-Curtis distance (data not shown).
Fig. 1.
Fecal microbiome alpha- and beta-diversity indices before and after bariatric surgery. (a) Shannon index (0 = presurgery; 7 = 1 wk; 30 = 1 mo; 90 = 3 mo after surgery; linear mixed model, P = .004 for pre versus 1 wk; .54 for pre versus 1 mo; .04 for pre versus 3 mo). (b) Bray-Curtis distance (principle co-ordinates analysis plot; red = presurgery; blue = postsurgery; permutation multivariate analysis of variance P = .015)
Significant changes in microbiome relative abundance were observed at each taxonomic level, including 1 phylum, 5 classes, 6 orders, 8 families, 10 genera, and 11 species with FDR < .1 (Table 2). Genus (species)-level changes included increases in Odoribacter (splanchnicus), Streptococcus (parasanguinis), Anaerotruncus (hadrus), Alistipes (finegoldii and putredinis), Actinomyces, Klebsiella, Bifidobacterium, Lachnoclostridium, and Akkermansia (muciniphila), while decreases in Bacteroides (finegoldii, stercoris, and ovatus), Coprocosccus (comes), Dorea (longicatena and formicigenerans), Fusicatenibacter (saccharivorans), Faecalibacterium (prausnitzii), Roseburia, and Eubacterium (rectale and hallii). These significant results were similar between our main model and a minimally adjusted model (Supplemental Table 1).
Table 2.
Gut microbiome taxa changes after bariatric surgery*
| Taxonomic level (total number of tests) | Median relative abundance |
Effect of surgery (SE) |
P value | FDR q-value |
|---|---|---|---|---|
| Phylum (n = 7) | ||||
| Proteobacteria | 2.91% | .71 (.19) | .0006 | .02 |
| Actinobacteria | 1.45% | .32 (.12) | .02 | .17 |
| Verrucomicrobia | .007% | .63 (.27) | .03 | .25 |
| Class (n = 15) | ||||
| Proteobacteria/Gammaproteobacterial | .62% | 1.42 (.26) | 5.3 × 10−6 | .0008 |
| Firmicutes/Bacilli | 1.07% | 1.11 (.23) | 1.9 × 10−5 | .002 |
| Actinobacteria/Actinobacteria | .46% | .58 (.12) | 7.6 × 10−5 | .004 |
| Proteobacteria/Betaproteobacteria | .76% | −.35 (.10) | .002 | .05 |
| Proteobacteria/Deltaproteobacteria | .60% | .34 (.10) | .003 | .05 |
| Verrucomicrobia/Verrucomicrobiae | .007% | .63 (.27) | .03 | .25 |
| Order (n = 22) | ||||
| Firmicutes/Bacilli/Lactobacillales | 1.06% | 1.11 (.23) | 1.9 × 10−5 | .002 |
| Proteobacteria/Gammaproteobacterial/Enterobacterales | .45% | 1.29 (.26) | 3.1 × 10−5 | .002 |
| Proteobacteria/Betaproteobacteria/Burkholderiales | .76% | −.36 (.10) | .002 | .04 |
| Actinobacteria/Actinobacteria/Actinomycetales | .05% | .91 (.29) | .002 | .04 |
| Proteobacteria/Deltaproteobacteria/Desulfovibrionales | .60% | .34 (.10) | .003 | .05 |
| Actinobacteria/Actinobacteri/Bifidobacteriales | .24% | .41 (.14) | .007 | .09 |
| Verrucomicrobia/Verrucomicrobiae/Verrucomicrobiales | .007% | .63 (.27) | .03 | .25 |
| Family (n = 39) | ||||
| Bacteroidetes/Bacteroidia/Bacteroidales/Odoribacteraceae | .50% | .45 (.08) | 1.9 × 10−5 | .002 |
| Firmicutes/Bacilli/Lactobacillales/Streptococcaceae | .89% | 1.15 (.24) | 3.0 × 10−5 | .002 |
| Proteobacteria/Gammaproteobacterial/Enterobacterale/Enterobacteriaceae | .45% | 1.21 (.27) | 8.5 × 10−5 | .004 |
| Bacteroidetes/Bacteroidia/Bacteroidales/Bacteroidaceae | 35.6% | −.48 (.11) | .0003 | .009 |
| Bacteroidetes/Bacteroidia/Bacteroidales/Rikenellaceae | 6.88% | .58 (.15) | .0006 | .02 |
| Actinobacteria/Actinobacteria/Actinomycetales/Actinomycetaceae | .05% | .91 (.27) | .002 | .04 |
| Proteobacteria/Deltaproteobacteria/Desulfovibrionales/Desulfovibrionaceae | .60% | .34 (.10) | .003 | .05 |
| Actinobacteria/Actinobacteria/Bifidobacteriales/Bifidobacteriaceae | .24% | .41 (.14) | .006 | .09 |
| Actinobacteria/Coriobacteriia/Eggerthellales/Eggerthellaceae | .19% | .56 (.22) | .02 | .17 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae | 11.4% | −.28 (.11) | .02 | .18 |
| Proteobacteria/Betaproteobacteria/Burkholderiales/Sutterellaceae | .40% | −.26 (.10) | .02 | .19 |
| Firmicutes/Clostridia/Clostridiales/Clostridiales family xiii. incertae sedis | .02% | .66 (.28) | .03 | .25 |
| Verrucomicrobia/Verrucomicrobiae/Verrucomicrobiales/Akkermansiaceae | .007% | .63 (.27) | .03 | .25 |
| Genus (n = 120) | ||||
| Bacteroidetes/Bacteroidia/Bacteroidales/Odoribacteraceae/Odoribacter | .40% | .52 (.08) | 2.3 × 10−6 | .0005 |
| Firmicutes/Bacilli/Lactobacillales/Streptococcaceae/Streptococcus | .89% | 1.17 (.25) | 2.6 × 10−5 | .002 |
| Firmicutes/Clostridia/Clostridiales/Ruminococcaceae/Anaerotruncus | .11% | .91 (.21) | .0002 | .007 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Coprococcus | .11% | −.67 (.16) | .0002 | .009 |
| Bacteroidetes/Bacteroidia/Bacteroidales/Bacteroidaceae/Bacteroides | 35.6% | −.48 (.11) | .0003 | .009 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Dorea | .24% | −.54 (.13) | .0003 | .01 |
| Bacteroidetes/Bacteroidia/Bacteroidales/Rikenellaceae/Alistipes | 6.87% | .58 (.15) | .0006 | .02 |
| Actinobacteria/Actinobacteria/Actinomycetales/Actinomycetaceae/Actinomyces | .05% | .85 (.27) | .004 | .07 |
| Proteobacteria/Gammaproteobacterial/Enterobacterales/Enterobacteriaceae/Klebsiella | .01% | .88 (.30) | .006 | .09 |
| Actinobacteria/Actinobacteria/Bifidobacteriales/Bifidobacteriaceae/Bifidobacterium | .22% | .42 (.14) | .007 | .09 |
| Actinobacteria/Coriobacteriia/Eggerthellales/Eggerthellaceae/Eggerthella | .11% | .71 (.25) | .007 | .10 |
| Firmicutes/Clostridia/Clostridiales//Intestinimonas | .07% | .74 (.27) | .009 | .12 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Fusicatenibacter | .29% | −.61 (.24) | .01 | .16 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Lachnoclostridium | .69% | .55 (.24) | .03 | .25 |
| Verrucomicrobia/Verrucomicrobiae/Verrucomicrobiales/Akkermansiaceae/Akkermansia | .007% | .63 (.27) | .03 | .25 |
| Firmicutes/Clostridia/Clostridiales/Ruminococcaceae/Faecalibacterium | .84% | −.49 (.22) | .03 | .26 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Roseburia | .24% | −.55 (.26) | .03 | .26 |
| Species (n = 384) | ||||
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Eubacterium rectale | .07% | −1.41 (.20) | 2.2 × 10−7 | .0001 |
| Bacteroidetes/Bacteroidia/Bacteroidales/Odoribacteraceae/Odoribacter splanchnicus | .39% | .56 (.09) | 2.8 × 10−7 | .0005 |
| Bacteroidetes/Bacteroidia/Bacteroidales/Bacteroidaceae/Bacteroides finegoldii | .06% | −.72 (.16) | .0001 | .004 |
| Firmicutes/Bacilli/Lactobacillales/Streptococcaceae/Streptococcus unclassified | .32% | 1.04 (.26) | .0003 | .01 |
| Bacteroidetes/Bacteroidia/Bacteroidales/Rikenellaceae/Alistipes unclassified | .46% | .63 (.16) | .0006 | .02 |
| Bacteroidetes/Bacteroidia/Bacteroidales/Bacteroidaceae/Bacteroides stercoris | .45% | −.68 (.18) | .001 | .03 |
| Bacteroidetes/Bacteroidia/Bacteroidales/Bacteroidaceae/Bacteroides ovatus | .31% | −.58 (.18) | .002 | .04 |
| Bacteroidetes/Bacteroidia/Bacteroidales/Rikenellaceae/Alistipes finegoldii | 1.90% | .52 (.16) | .004 | .07 |
| Bacteroidetes/Bacteroidia/Bacteroidales/Rikenellaceae/Alistipes putredinis | 2.86% | .28 (.09) | .005 | .09 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Blautia unclassified | 1.35% | ‒.62 (.21) | .006 | .09 |
| Proteobacteria/Gammaproteobacterial/Enterobacterales/Enterobacteriaceae/Klebsiella unclassified | .01% | .87 (.30) | .006 | .09 |
| Firmicutes/Clostridia/Clostridiales/Eubacteriaceae/Eubacterium hallii | .30% | −.58 (.21) | .009 | .12 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Blautia/Ruminococcus torques | .18% | −.55 (.20) | .01 | .13 |
| Bacteroidetes/Bacteroidia/Bacteroidales/Rikenellaceae/Alistipes onderdonkii | .06% | .46 (.17) | .01 | .14 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Fusicatenibacter saccharivorans | .29% | −.62 (.24) | .01 | .15 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Blautia wexlerae | .21% | −.47 (.18) | .01 | .16 |
| Firmicutes/Bacilli/Lactobacillales/Streptococcaceae/Streptococcus parasanguinis | .04% | .68 (.29) | .02 | .24 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Anaerostipes hadrus | .04% | −.62 (.26) | .02 | .24 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Dorea longicatena | .06% | −.37 (.15) | .02 | .24 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Lachnoclostridium unclassified | .15% | .61 (.26) | .02 | .24 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Dorea formicigenerans | .03% | −.53 (.23) | .03 | .25 |
| Firmicutes/Clostridia/Clostridiales/Lachnospiraceae/Coprococcus comes | .04% | −.51 (.22) | .03 | .25 |
| Firmicutes/Clostridia/Clostridiales/Ruminococcaceae/Faecalibacterium prausnitzii | .84% | −.49 (.22) | .03 | .25 |
| Verrucomicrobia/Verrucomicrobiae/Verrucomicrobiales/Akkermansiaceae/Akkermansia muciniphila | .007% | .61 (.27) | .03 | .26 |
| Bacteroidetes/Bacteroidia/Bacteroidales/Bacteroidaceae/Bacteroides caccae | .42% | −.26 (.12) | .04 | .31 |
Generalized additive model for location, scale, and shape with zero-inflated beta distribution was used, considering repeated measures from the same patients and adjusting for age, sex, race, procedure type, stool type, and recent use of antibiotics. Results with P <.05 were shown. Within each taxonomic level, results were sorted in ascending order of P values.
Some taxa showed potential time-varying changes. Table 3 lists significantly changed genera and species at 3 months after surgery, as at this time most patients have returned to a normal diet, and their microbiome may have been stabilized and have longer-lasting effect on host health; changes of these genera/species at 1 week and 1 month post surgery are also shown. Throughout postsurgery period, Streptococcus, Odoribacter, and Anaerotruncus were higher than before surgery; while Streptococcus showed a continuous increase, and probiotic species S. thermophilus showed a significant increase only at 3 months. Other genera/species showed significant increases only at 3 months were Prevotella, Alistipe shahii, Akkermansia muciniphila, and Eubacterium siraeum. In contrast, Bacteroides (stercoris) showed the largest decrease at 1 week.
Table 3.
Gut microbiome genera and species changes at 1 week, 1 month, and 3 months after bariatric surgery*
| Significantly changed genera and species at 3 mo | Postsurgery time points | 1 wk |
1 mo |
3 mo |
|||
|---|---|---|---|---|---|---|---|
| β (SE) | P value | β (SE) | P value | β (SE) | P value | ||
| Firmicutes/Bacilli/Lactobacillales/Streptococcaceae/Streptococcus | .80 (.29) | .008 | 1.49 (.27) | 3.1 × 10−6 | 2.10 (.28) | 7.1 × 10−9 | |
| Firmicutes/Bacilli/Lactobacillales/Streptococcaceae/Streptococcus unclassified | .69 (.31) | .04 | 1.34 (.31) | .0001 | 1.86 (.33) | 1.8 × 10−6 | |
| Bacteroidetes/Bacteroidia/Bacteroidales/Odoribacteraceae/Odoribacter | .69 (.08) | 4.2 × 10−8 | .32 (.09) | .002 | .59 (.10) | 5.9 × 10−6 | |
| Bacteroidetes/Bacteroidia/Bacteroidales/Odoribacteraceae/Odoribacter splanchnicus | .74 (.08) | 4.5 × 10−8 | .35 (.10) | .002 | .61 (.10) | 8.2 × 10−6 | |
| Firmicutes/Clostridia/Clostridiales/Ruminococcaceae/Anaerotruncus | 1.59 (.19) | 4.2 × 10−8 | .68 (.22) | .006 | .94 (.23) | .004 | |
| Bacteroidetes/Bacteroidia/Bacteroidales/Tannerellaceae/Parabacteroides merdae | −.02 (.14) | .89 | −.36 (.15) | .02 | −.87 (.22) | .001 | |
| Firmicutes/Bacilli/Lactobacillales/Streptococcaceae/Streptococcus thermophilus | .15 (.34) | .68 | .59 (.37) | .12 | 1.39 (.39) | .001 | |
| Bacteroidetes/Bacteroidia/Bacteroidales/Prevotellaceae/Prevotella | .15 (.30) | .62 | .61 (.30) | .05 | 1.22 (.34) | .002 | |
| Bacteroidetes/Bacteroidia/Bacteroidales/Bacteroidaceae/Bacteroides massiliensis | −.27 (.13) | .05 | .07 (.16) | .65 | −1.08 (.31) | .002 | |
| Bacteroidetes/Bacteroidia/Bacteroidales/Rikenellaceae/Alistipes shahii | .18 (.19) | .34 | .15 (.23) | .52 | .62 (.23) | .01 | |
| Verrucomicrobia/Verrucomicrobiae/Verrucomicrobiales/Akkermansiaceae/Akkermansia | .52 (.32) | .12 | .54 (.36) | .15 | 1.06 (.40) | .01 | |
| Firmicutes/Clostridia/Clostridiales/Eubacteriaceae/Eubacterium siraeum | .04 (.35) | .91 | .19 (.38) | .62 | 1.05 (.42) | .02 | |
| Firmicutes/Clostridia/Clostridiales/Unclassified/Intestinimonas | .82 (.32) | .02 | .57 (.35) | .12 | .94 (.38) | .02 | |
| Verrucomicrobia/Verrucomicrobiae/Verrucomicrobiales/Akkermansiaceae/Akkermansia municiphila | .51 (.32) | .12 | .56 (.36) | .13 | .97 (.40) | .02 | |
| Firmicutes/Bacilli/Lactobacillales/Streptococcaceae/Streptococcus parasanguinis | .50 (.35) | .16 | .79 (.37) | .04 | 1.00 (.44) | .03 | |
| Bacteroidetes/Bacteroidia/Bacteroidales/Tannerellaceae/Parabacteroides | −.10 (.13) | .42 | −.16 (.13) | .24 | −.40 (.18) | .03 | |
| Firmicutes/Clostridia/Clostridiales/Eubacteriaceae/Eubacterium eligens | −.23 (.35) | .52 | −.04 (.38) | .92 | .90 (.41) | .04 | |
| Proteobacteria/Gammaproteobacterial/Enterobacterales/Enterobacteriaceae/Klebsiella | .67 (.36) | .07 | 1.54 (.36) | .0002 | 1.00 (.46) | .04 | |
| Bacteroidetes/Bacteroidia/Bacteroidales/Bacteroidaceae/Bacteroides | −.73 (.13) | 6.5 × 10−6 | −.23 (.14) | .11 | −.38 (.17) | .04 | |
| Bacteroidetes/Bacteroidia/Bacteroidales/Bacteroidaceae/Bacteroides stercoris | −1.34 (.22) | 5.8 × 10−6 | −.25 (.17) | .16 | −.59 (.27) | .04 | |
SE = standard error.
Generalized additive model for location, scale, and shape with zero-inflated beta distribution was used, considering repeated measures from the same patients and adjusting for age, sex, race, procedure type, stool type, and recent use of antibiotics. Results for genus/species with P <.05 at 3 months postsurgery were shown. Results were sorted in ascending order of the P values at 3 months.
Most of the results were not affected or strengthened by further adjusting for BMI, except that the associations for genus Klebsiella and Bacteroides species (ovatus and caccae) were changed after BMI adjustment (Supplemental Table 1). Among surgery-altered genera/species, Bacteroides ovatus was positively associated with BMI and reduced at 1 week (β-BMI = .07, P = .003, FDR = .08; β-1 wk = −.63, P = .01), and Prevotella was inversely associated with BMI and increased at 3 months (β-BMI = −.14, P = .0003, FDR = .01; β-3 mo = .74, P = .04). Meanwhile, most of the taxa changes appeared similar between RYGB and SG patients, except that some Bacteroides species (caccae and fragilis) showed significant decreases only among SG patients (both P interaction with procedure type < .0001, FDR < .05).
Significant changes in fecal metabolites were shown in Table 4. The untargeted metabolomics yielded 1073 named and 230 unnamed metabolites. Among named metabolites, 25 reached FDR < .1, including reduced caffeine and metabolites (paraxanthine and theophylline, especially within 1 mo), fructose, nicotinate ribonucleotide, pyrraline (a major dietary advanced glycation end-product), indole derivatives, butyrate/isobutyrte, lysophospholipids (e.g., lysophosphatidylethanolamine 18:2 and lysophosphatidylinositol 16:0, especially at 1 wk), and nucleotides and their metabolites (e.g., 2-deoxyadenosine and 8-hydroxyguanine, oxidative stress markers), whereas increased drug metabolites (e.g., rocuronium, only at 1 wk) and fatty acid hydroxyl fatty acid (e.g., oleic acid-hydroxystearic acid).
Table 4.
Fecal metabolite changes after bariatric surgery*
| All postsurgery |
1 wk |
1 mo |
3 mo |
|||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | P value | β (SE) | P value | β (SE) | P value | β (SE) | P value | |
| Caffeine | −1.22 (.23) | 4.7 × 10−5 | −1.27 (.28) | .0003 | −1.17 (.32) | .002 | −1.12 (.44) | .02 |
| Fructose | −1.14 (.27) | .0004 | −1.43 (.32) | .0003 | −.87 (.35) | .03 | −.77 (.49) | .13 |
| Nicotinate ribonucleotide | −1.01 (.24) | .0005 | −.78 (.28) | .01 | −1.09 (.31) | .004 | −1.66 (.43) | .001 |
| Pyrraline | −1.05 (.25) | .0005 | −1.01 (.31) | .004 | −1.08 (.34) | .006 | −1.14 (.47) | .03 |
| 3-Indoleglyoxylic acid | −1.16 (.28) | .0005 | −1.16 (.34) | .003 | −.99 (.38) | .02 | −1.54 (.52) | .009 |
| Butyrate/isobutyrate | −1.05 (.27) | .001 | −.60 (.30) | .06 | −1.51 (.33) | .0003 | −1.59 (.46) | .003 |
| Theophylline | −1.04 (.27) | .001 | −1.04 (.33) | .005 | −1.16 (.37) | .006 | −.75 (.51) | .16 |
| 3-Sulfo-L-alanine | −.92 (.24) | .001 | −1.14 (.28) | .0008 | −.56 (.32) | .09 | −.95 (.44) | .04 |
| Lysophosphatidylethanolamine (18:2) | −1.03 (.28) | .002 | −1.32 (.31) | .0006 | −.52 (.35) | .16 | −1.12 (.49) | .03 |
| Paraxanthine | −1.07 (.29) | .002 | −1.08 (.35) | .007 | −1.16 (.40) | .01 | −.84 (.55) | .14 |
| N-carboxymethylalanine | −1.09 (.29) | .002 | −1.09 (.36) | .008 | −1.14 (.40) | .01 | −.96 (.56) | .11 |
| Lysophosphatidylinositol (16:0) | −1.02 (.28) | .002 | −1.39 (.31) | .0003 | −.41 (.35) | .25 | −1.13 (.48) | .03 |
| Rocuronium | 1.10 (.30) | .002 | 1.87 (.25) | 9.3 × 10−7 | .50 (.28) | .09 | −.25 (.39) | .53 |
| 12,13-DiHOME | −1.10 (.30) | .002 | −1.20 (.36) | .004 | −.86 (.41) | .05 | −1.26 (.56) | .04 |
| Lysine | −.91 (.25) | .002 | −.95 (.30) | .006 | −.68 (.34) | .06 | −1.26 (.47) | .02 |
| Gentisate | −1.06 (.29) | .002 | −.95 (.35) | .02 | −1.02 (.40) | .02 | −1.55 (.55) | .01 |
| 1,2-Dilinolenoyl-galactosylglycerol (18:3/18:3) | −1.04 (.29) | .002 | −1.00 (.36) | .01 | −.96 (.40) | .03 | −.88 (.51) | .11 |
| 2’-Deoxyadenosine | −1.05 (.30) | .002 | −.68 (.34) | .06 | −1.42 (.39) | .002 | −1.50 (.53) | .01 |
| Oleic acid-hydroxystearic acid (18:1/OH-18:0) | .91 (.26) | .003 | .65 (.31) | .05 | 1.27 (.34) | .002 | .97 (.47) | .06 |
| 8-Hydroxyguanine | −.98 (.29) | .003 | −.76 (.34) | .04 | −1.14 (.38) | .009 | −1.38 (.50) | .02 |
| 4-Guanidinobutanoate | −.96 (.28) | .003 | −.81 (.34) | .03 | −1.01 (.38) | .02 | −1.40 (.52) | .02 |
| Nicotinamide riboside | −.92 (.27) | .003 | −.67 (.31) | .05 | −.95 (.35) | .01 | −1.64 (.48) | .003 |
| Stachydrine | −.98 (.29) | .003 | −1.00 (.35) | .01 | −.91 (.40) | .03 | −1.04 (.55) | .07 |
| Adenosine | −1.03 (.30) | .003 | −.70 (.35) | .07 | −1.44 (.40) | .002 | −1.24 (.55) | .04 |
| Branched-chain dicarboxylic acid (14:0) | −.87 (.26) | .003 | −.65 (.31) | .05 | −1.21 (.34) | .003 | −.89 (.47) | .08 |
| Guanosine | −.98 (.29) | .003 | −.70 (.30) | .05 | −.98 (.37) | .02 | −2.00 (.51) | .001 |
| Ferulate | −.90 (.28) | .004 | −1.24 (.32) | .001 | −.43 (.25) | .25 | −.77 (.49) | .13 |
| Indolepropionate | −.92 (.29) | .006 | −.41 (.31) | .21 | −1.30 (.35) | .002 | −1.82 (.49) | .002 |
| Thymidine | −.94 (.30) | .006 | −.47 (.32) | .16 | −1.08 (.36) | .007 | −2.22 (.49) | .0003 |
| Valerate (5:0) | −.80 (.28) | .009 | −.32 (.30) | .30 | −1.22 (.33) | .002 | −1.56 (.46) | .003 |
| Caproate (6:0) | −.78 (.27) | .009 | −.45 (.30) | .16 | −1.23 (.34) | .002 | −.89 (.47) | .08 |
| Ondansetron | .85 (.31) | .01 | 1.60 (.28) | 2.6 × 10−5 | .08 (.31) | .81 | −.01 (.43) | .98 |
| Gabapentin | .83 (.31) | .01 | 1.58 (.28) | 2.9 × 10−5 | .21 (.31) | .52 | −.35 (.43) | .43 |
| Inosine | −.77 (.31) | .02 | −.52 (.33) | .13 | −.59 (.37) | .13 | −2.08 (.51) | .0008 |
| Uridine | −.68 (.30) | .04 | −.44 (.30) | .19 | −.48 (.37) | .20 | −1.95 (.51) | .001 |
| Urolithin A | .60 (.34) | .09 | .31 (.36) | .41 | .45 (.41) | .28 | 1.97 (.57) | .003 |
| Retinol | .10 (.32) | .75 | .50 (.30) | .11 | .29 (.33) | .40 | −1.70 (.46) | .002 |
SE = standard error.
Generalized linear mixed model was used, adjusting for age, sex, race, procedure type, stool type, and recent use of antibiotics. Results with false discovery rate <.1 (approximately P value <.003) in any pre- versus postsurgery time points were shown. Results were sorted in ascending order of P values in pre- versus all postsurgery comparison.
Discussion
In a longitudinal study of patients undergoing RYGB or SG, we applied metagenomics and fecal metabolomics and observed significant pre- to postsurgery gut microbial changes, suggesting potentially important microbial taxa and metabolites that may play a role in weight loss and/or metabolic improvements after bariatric surgery. Nevertheless, given the small sample size, multiple comparisons, and short follow-up, the present study should be considered exploratory, and all results need to be validated in future studies.
Overall, we found an increase in microbiome alpha-diversity after bariatric surgery, in line with most previous studies [9,13-15,17]. At phylum level, we found an increase in Proteobacteria as many reported [8-10,13,18,19], but we did not find significant changes in other reported phyla (e.g., Firmicutes or Bacteroidetes). We observed changes in 17 genera and 25 species at P < .05 and 10 genera and 11 species at FDR < .1, including increases in oral/aero-tolerant bacteria (Odoribacter and Streptococcus), lactic acid/probiotic bacteria (S. thermophilus, Klebsiella, and Bifidobacterium), and A. muciniphila (especially at 3 mo post surgery), while decreases in Bacteroides (e.g., B. stercoris and B. ovatus, especially at 1 wk postsurgery) and several butyrate-producing bacteria (Coprocosccus, Dorea, Faecalibacterium, and Roseburia). Yet, some butyrate-producing bacteria (e.g., Prevotella) showed time-varying changes that they only increased at 3 months. We also observed changes in fecal metabolites, including reduced caffeine metabolites, advanced glycation end-product, oxidative stress markers, nucleotides, indoles, and butyrate/isobutyrate.
Increases in oral-originated or aero-tolerant bacteria have been reported in several studies [9,10,13], which may be resulted from altered gastrointestinal anatomy after bariatric surgery, leading to increased levels of oxygen and pH in the colon [26]. Yet, whether their increases are mainly a consequence or may affect weight loss or other metabolic outcomes after surgery is unclear. On the other hand, increased A. muciniphila, as previously found [11,13,15,18,19], may confer health effects among bariatric surgery patients. Mechanistic studies have shown that A. muciniphila has antiinflammatory and antidiabetic properties [27], and a recent randomized trial further showed that supplementation of A. muciniphila for 3 months can improve insulin resistance, blood lipids, and inflammation among obese adults, suggesting its therapeutic potential on metabolic disorders [28]. In this study, we found a significant increase in A. muciniphila at 3 months after surgery, independent of concurrently changed BMI. Unfortunately, we could not evaluate its associations with metabolic biomarkers (e.g., glucose and blood lipids, as we did not collect postsurgery blood samples) nor its long-term effects on BMI or metabolic health in the present study.
In present study, 2 taxa were significantly related to BMI and altered by surgery, that is, increase in Prevotella (especially at 3 mo) and decrease in Bacteroides (B. ovatus, especially at 1 wk). Increase in Prevotella has been reported in a recent U.S. study of 9 RYGB patients at 6 and 12 months after surgery [18]; otherwise, the relevant evidence from bariatric patient studies is limited. However, in population-based studies, Prevotella and Bacteroides have long been suggested as microbial features of plant/carbohydrate-rich diets and animal protein/fat-rich diets, respectively [29]. Studies have shown higher abundance of Prevotella in agrarian than Western populations and gradually decreased Prevotella among populations undergoing urbanization or immigration to Western countries [29,30]. Within Western populations (e.g., Germany and Italy), vegetarians/vegans have been found to have higher Prevotella and lower Bacteroides than omnivores [31-33], although a U.S. study found no differences [34]. In terms of potential health effects of Prevotella and Bacteroides, evidence from observational human studies (mostly cross-sectional) has been mixed [29]. Of note, some recent intervention studies have found that individuals with a high Prevotella-Bacteroides ratio lost more body fat and had a greater glucose improvement after high-fiber/whole-grain dietary interventions [35,36]. It would be of clinical impact to examine whether presurgery Prevotella and Bacteroides abundance may predict weight loss and/or metabolic improvements after bariatric surgery and whether bariatric surgery can lead to sustained changes in these bacteria and their long-term health consequences.
The seemingly temporal changes in butyrate-producing bacteria need to be interpreted with caution as the sample size at each study time point was small. We observed significant decreases in several genera of family Lachnospiraceae, including Coprocosccus, Dorea, Faecalibacterium, and Roseburia, at 1 week and 1 month but not at 3 months, while some genera (e.g., Prevotella) did not change at 1 week or 1 month but increased significantly at 3 months. Usually, patients have liquid diet for 1 to 2 weeks, then soft diet for another 2 to 4 weeks, and gradually return to normal diet approximately 2 months after surgery. These fiber-degrading, butyrate-producing bacteria may change during this postsurgery period, thus it is important to consider study time points when interpreting their changes. Previous studies have yielded mixed findings, several reported decreased abundance of butyrate-producing bacteria, including Coprocosccus, Dorea, Faecalibacterium, and Roseburia [9,10,12,13,15,18], consistent with our current findings; whereas, others reported increased abundance of Faecalibacterium and Roseburia [12,14,17]. Our present study only collected stool samples within 3 months after surgery, thus we could not evaluate changes in butyrate-producing bacteria in a longer term. However, studies with multiple follow-up time points until 1 year after surgery have suggested that most of microbiome changes occurred within 3 months, and then microbiome maintained stable throughout the first year [9,13,17]. Overall, more longitudinal studies are needed to examine microbiome changes from before surgery to short and long terms after bariatric surgery and their health effects.
Furthermore, our findings of changed fecal metabolites should be considered exploratory, as sample size of our present study is much smaller than the number of metabolites tested. Still, some of our findings showed the validity of our method for a fecal metabolomics study. For example, we found substantially increased levels of drug metabolites at 1 week (i.e., rocuronium, ondansetron, and gabapentin), which diminished at 1 to 3 months, and the decreased butyrate seemed to agree with decreased butyrate-producing bacteria. To date, only a few studies have examined changes in fecal metabolites after RYGB or SG as follows: in terms of fecal butyrate, 1 reported a tendency of decreased level [8], 1 reported no alteration [12], and another reported an increased level [18]. On the other hand, many studies have examined changes in circulating metabolites after bariatric surgery, and the most commonly reported are reduced levels of branched-chain and aromatic amino acids and other amino acids, like glutamate [37]. We did not find significant changes in fecal excretion of these amino acids in present study. Changes in circulating microbial metabolites were also reported, including increased short-chain fatty acids, indoles, secondary bile acids, hippurate, and trimethylamine n-oxide [19,38-44]. It is unclear whether the decreased fecal butyrate and indoles observed in this study is because of decreased microbial metabolism or increased absorption of these metabolites into circulation. Microbial metabolites have been increasingly recognized as important signaling molecules that mediate the microbe-host communication and in turn affect human health [20,37,45]. Future studies with both blood and fecal metabolomics, in addition to functional metagenomics, will help advance our understanding of the role of microbial metabolites in bariatric surgery.
As discussed above, major limitations of this study include a small sample size, a short follow-up, and a lack of metabolic phenotype data. However, we consider this study the first step to establish methods and generate pilot data for future large-scale, multiomic investigations on the role of gut microbiota in bariatric surgery. To our knowledge, studies to date that reported intraindividual microbiome changes after RYGB or SG had ≤30 patients [9-19], which may be prone to false negatives due to low statistical power and false positives due to unstable estimates. Well-powered studies with internal/external validations are needed in this research area, especially given the multiple testing nature of microbiome and other -omics research. Studies with longitudinal sample collection and medium/long-term follow-up are also needed to evaluate long-term health effects of altered gut microbiota after surgery. Still, some microbial species that have been repeatedly reported and with known biological effects (e.g., A. muciniphila) may inform the development of novel therapeutics for obesity and related metabolic diseases. Another limitation of our present study is the very small number of male or nonwhite patients, although this is probably the first study of its kind to include black patients, and only a handful of prior studies have included male patients (n = 3–8) [10,13-15,18,19]. Our present study is also underpowered to evaluate potential procedure-specific or temporal tends of microbial changes, although we observed potential SG-specific decreases in some Bacteroides species and time-varying changes in some butyrate-producing bacteria. The major reasons for dropouts during follow-up were reluctance to collect stool samples, lack of compensation, and lack of time, issues need to be addressed in future studies. Meanwhile, whether gut microbial changes after bariatric surgery may differ by sex, race/ethnicity, presence of co-morbidities, procedure type, or time period remain to be evaluated in well-powered, longitudinal, multiracial/ethnic studies.
Conclusions
In a longitudinal study of bariatric surgery patients, we observed significant pre- to postsurgery changes in the fecal microbiome diversity, composition, and metabolites, which might play a role in weight loss and/or metabolic improvements after surgery. Our study provided novel yet preliminary data for future large-scale, multiethnic, and multiomic investigations on the role of gut microbiota in bariatric surgery.
Supplementary Material
Acknowledgments
We thank all patients who participated in this study and the clinical team at the Vanderbilt Weight Loss Center for supporting our research. We particularly thank Ms. Tina Higginbotham, Director of the GI Clinical Research Enterprise at the Vanderbilt University Medical Center, Ms. Shea Scudder, Research Nurse Specialist, and Ms. Brittany Littleton, Study Coordinator, who helped with the institutional review board application, patient enrollment and follow-up, and collection of data and biospecimen.
W.J.E. and C.R.F. contributed equally to this work. D.Y., X.O.S., W.J.E., and C.R.F. conceived and designed the study. D.Y., E.F.H., and C.R.F. enrolled participants and collected data. E.F.H. and W.J.E. provided clinical support. D.Y. analyzed data and drafted the manuscript. All authors contributed to interpretation of the data and critical revision of the manuscript and had final approval of the submitted manuscript.
The study was funded by pilot and feasibility grants from the Vanderbilt Digestive Disease Research Center and Vanderbilt Epidemiology Center and National Institutes of Health R01 DK105847 to C.R.F. D.Y. is supported by the Vanderbilt University Medical Center Faculty Research Scholar program.
Footnotes
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.1016/j.soard.2020.06.032.
References
- [1].Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief 2020;(360):1–8. [PubMed] [Google Scholar]
- [2].Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309(21):2240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Isaman DJ, Rothberg AE, Herman WH. Reconciliation of type 2 diabetes remission rates in studies of Roux-en-Y gastric bypass. Diabetes Care 2016;39(12):2247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Carswell KA, Belgaumkar AP, Amiel SA, Patel AG. A systematic review and meta-analysis of the effect of gastric bypass surgery on plasma lipid levels. Obes Surg 2016;26(4):843–55. [DOI] [PubMed] [Google Scholar]
- [5].Schiavon CA, Bersch-Ferreira AC, Santucci EV, et al. Effects of bariatric surgery in obese patients with hypertension: the GATEWAY Randomized Trial (Gastric Bypass to Treat Obese Patients With Steady Hypertension). Circulation 2018;137(11):1132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aron-Wisnewsky J, Clement K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol 2016;12(3):169–81. [DOI] [PubMed] [Google Scholar]
- [7].Sweeney TE, Morton JM. The human gut microbiome: a review of the effect of obesity and surgically induced weight loss. JAMA Surg 2013;148(6):563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tremaroli V, Karlsson F, Werling M, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab 2015;22(2):228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kong LC, Tap J, Aron-Wisnewsky J, et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr 2013;98(1):16–24. [DOI] [PubMed] [Google Scholar]
- [10].Graessler J, Qin Y, Zhong H, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J 2013;13(6):514–22. [DOI] [PubMed] [Google Scholar]
- [11].Ward EK, Schuster DP, Stowers KH, et al. The effect of PPI use on human gut microbiota and weight loss in patients undergoing laparoscopic Roux-en-Y gastric bypass. Obes Surg 2014;24(9):1567–71. [DOI] [PubMed] [Google Scholar]
- [12].Damms-Machado A, Mitra S, Schollenberger AE, et al. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed Res Int 2015;2015:806248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Palleja A, Kashani A, Allin KH, et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med 2016;8(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Murphy R, Tsai P, Jullig M, Liu A, Plank L, Booth M. Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. Obes Surg 2017;27(4):917–25. [DOI] [PubMed] [Google Scholar]
- [15].Liu R, Hong J, Xu X, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med 2017;23(7):859–68. [DOI] [PubMed] [Google Scholar]
- [16].Sanmiguel CP, Jacobs J, Gupta A, et al. Surgically induced changes in gut microbiome and hedonic eating as related to weight loss: preliminary findings in obese women undergoing bariatric surgery. Psychosom Med 2017;79(8):880–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aron-Wisnewsky J, Prifti E, Belda E, et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut 2019;68(1):70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ilhan ZE, DiBaise JK, Dautel SE, et al. Temporospatial shifts in the human gut microbiome and metabolome after gastric bypass surgery. NPJ Biofilms Microbiomes 2020;6(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shen N, Caixàs A, Ahlers M, et al. Longitudinal changes of microbiome composition and microbial metabolomics after surgical weight loss in individuals with obesity. Surg Obes Relat Dis 2019;15(8):1367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science 2012;336(6086):1262–7. [DOI] [PubMed] [Google Scholar]
- [21].Wang Z, Zolnik CP, Qiu Y, et al. Comparison of fecal collection methods for microbiome and metabolomics studies. Front Cell Infect Microbiol 2018;8:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Loftfield E, Vogtmann E, Sampson JN, et al. Comparison of collection methods for fecal samples for discovery metabolomics in epidemiologic studies. Cancer Epidemiol Biomarkers Prev 2016;25(11):1483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hillmann B, Al-Ghalith GA, Shields-Cutler RR, et al. Evaluating the information content of shallow shotgun metagenomics. MSystems 2018;3(6):e000069–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 2009;81(16):6656–67. [DOI] [PubMed] [Google Scholar]
- [25].Ho NT, Li F, Wang S, Kuhn L. metamicrobiomeR: an R package for analysis of microbiome relative abundance data using zero-inflated beta GAMLSS and meta-analysis across studies using random effects models. BMC Bioinformatics 2019;20(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hartman AL, Lough DM, Barupal DK, et al. Human gut microbiome adopts an alternative state following small bowel transplantation. Proc Natl Acad Sci U S A 2009;106(40):17187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cani PD, de Vos WM. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front Microbiol 2017;8:1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Depommier C, Everard A, Druart C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 2019;25(7):1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Precup G, Vodnar DC. Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: a comprehensive literature review. Br J Nutr 2019;122(2):131–40. [DOI] [PubMed] [Google Scholar]
- [30].Kaplan RC, Wang Z, Usyk M, et al. Gut microbiome composition in the Hispanic Community Health Study/Study of Latinos is shaped by geographic relocation, environmental factors, and obesity. Genome Biol 2020;21(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zimmer J, Lange B, Frick JS, et al. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur J Clin Nutr 2012;66(1):53–60. [DOI] [PubMed] [Google Scholar]
- [32].Ferrocino I, Di Cagno R, De Angelis M, et al. Fecal microbiota in healthy subjects following omnivore, vegetarian and vegan diets: culturable populations and rRNA DGGE profiling. PLoS One 2015;10(6):e0128669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].De Filippis F, Pellegrini N, Vannini L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016;65(11):1812–21. [DOI] [PubMed] [Google Scholar]
- [34].Wu GD, Compher C, Chen EZ, et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016;65(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kovatcheva-Datchary P, Nilsson A, Akrami R, et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab 2015;22(6):971–82. [DOI] [PubMed] [Google Scholar]
- [36].Hjorth MF, Roager HM, Larsen TM, et al. Pre-treatment microbial Prevotella-to-Bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int J Obes (Lond) 2018;42(2):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Samczuk P, Ciborowski M, Kretowski A. Application of metabolomics to study effects of bariatric surgery. J Diabetes Res 2018;2018:6270875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mutch DM, Fuhrmann JC, Rein D, et al. Metabolite profiling identifies candidate markers reflecting the clinical adaptations associated with Roux-en-Y gastric bypass surgery. PLoS One 2009;4(11):e7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yu H, Ni Y, Bao Y, et al. Chenodeoxycholic acid as a potential prognostic marker for Roux-en-Y gastric bypass in chinese obese patients. J Clin Endocrinol Metab 2015;100(11):4222–30. [DOI] [PubMed] [Google Scholar]
- [40].Zhao L, Ni Y, Yu H, et al. Serum stearic acid/palmitic acid ratio as a potential predictor of diabetes remission after Roux-en-Y gastric bypass in obesity. FASEB J 2017;31(4):1449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Modesitt SC, Hallowell PT, Slack-Davis JK, et al. Women at extreme risk for obesity-related carcinogenesis: baseline endometrial pathology and impact of bariatric surgery on weight, metabolic profiles and quality of life. Gynecol Oncol 2015;138(2):238–45. [DOI] [PubMed] [Google Scholar]
- [42].Albaugh VL, Flynn CR, Cai S, Xiao Y, Tamboli RA, Abumrad NN. Early increases in bile acids post Roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J Clin Endocrinol Metab 2015;100(9):E1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sarosiek K, Pappan KL, Gandhi AV, et al. Conserved metabolic changes in nondiabetic and type 2 diabetic bariatric surgery patients: global metabolomic pilot study. J Diabetes Res 2016;2016:3467403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Luo P, Yu H, Zhao X, et al. Metabolomics study of Roux-en-Y gastric bypass surgery (RYGB) to treat type 2 diabetes patients based on ultra-performance liquid chromatography-mass spectrometry. J Proteome Res 2016;15(4):1288–99. [DOI] [PubMed] [Google Scholar]
- [45].Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med 2016;22(10):1079–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.