Abstract
Background:
The optimal revascularization approach for patients with multivessel coronary artery disease (MVCAD) is controversial. We sought to investigate outcomes in patients undergoing coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) for non-ST elevation myocardial infarction (NSTEMI).
Methods:
Adult patients with MVCAD and NSTEMI undergoing either CABG or PCI at a single institution between 2011 and 2018 were included. Multivariable analysis was utilized to determine independent predictors of death, major adverse cardiac and cerebrovascular events (MACCE), and readmissions. A subanalysis examined patients undergoing complete revascularization.
Results:
A total of 2001 patients were included, of whom 1480 (74.0%) underwent CABG. CABG was associated with a lower risk-adjusted hazard for death (hazard ratio, 0.59, P < .001) and with improved survival at 1 year (92.0 vs 81.8%, P < .001) and 5 years (80.7 vs 63.3%, P < .001). Additionally, freedom from MACCE (P < .001) was greater in the CABG group and cumulative readmission, rates of MI, and rates of repeat revascularization were lower with CABG (each P < .001). Among patients undergoing complete revascularization, overall survival (1 year: 92.7 vs 83.9%, P = .010; 5 years: 81.1 vs 69.4%, P < .001) and freedom from MACCE (1 year: 92.3 vs 75.2%, P < .001; 5 years: 81.7 vs 61.4%, P < .001) remained higher for the CABG group; cumulative incidence of readmission was also decreased in those undergoing CABG (P < .001).
Conclusions:
In this real-world analysis of patients with MVCAD presenting with NSTEMI, revascularization with CABG resulted in improved survival with lower rates of MACCE and readmission as compared to PCI, which persisted when accounting for complete revascularization.
Keywords: coronary, artery, disease
1 |. INTRODUCTION
Optimal revascularization approaches for patients with multivessel coronary artery disease (MVCAD) remain controversial despite multiple randomized trials and retrospective series.1–5 Indeed, many of the prior randomized clinical trials comparing coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) for MVCAD have analyzed specific subsets of patients thus making the generalizability of the results to real-world clinical practice questionable. For example, several recent trials have frequently excluded patients with MVCAD who present with non-ST elevation myocardial infarction (NSTEMI). NSTEMI is one of the most common presentations of the acute coronary syndrome and, furthermore, rates of multivessel disease among patients with NSTEMI are greater than 50%.6 There has been some investigation into the relative risks and benefits of revascularization of the culprit lesion as compared to complete revascularization in NSTEMI with MVCAD.7 Nevertheless, there is limited data regarding real-world outcomes of surgical vs percutaneous revascularization in the setting of MVCAD and NSTEMI. The aim of this study was to compare mortality, adverse events, and readmission rates among patients with MVCAD and NSTEMI undergoing either PCI or CABG.
2 |. METHODS
2.1 |. Data source
The University of Pittsburgh Medical Center is a large, multicenter health system. This study utilized institutionally-derived data from the Society of Thoracic Surgeons (STS) adult cardiac database and the National Cardiovascular Data Registry (NCDR) from five hospitals to capture patients undergoing PCI and CABG. These data were then supplemented with longitudinal data from the electronic health record. This study was approved by the Institutional Review Board at the University of Pittsburgh.
2.2 |. Study population
Adults (≥18 years old) with NSTEMI and MVCAD who underwent PCI or CABG between 2011 and 2018 were included. The criteria utilized to define MVCAD included three-vessel CAD (defined by the presence of 70% or greater stenosis in the left anterior descending, left circumflex, and right coronary arteries), left main coronary artery stenosis of at least 50%, or two-vessel stenosis of at least 70% in two of the major coronary arteries including the proximal left anterior descending. NSTEMI diagnosis consisted of both of the following: (a) cardiac biomarkers (ie, troponin, creatine kinase-myocardial band) exceeding the upper limit of normal with a clinical presentation consistent with or suggestive of cardiac ischemia and (b) absence of electrocardiogram changes diagnostic of a STEMI. Complete revascularization was defined as revascularization of all significantly stenosed (ie, >70%) major epicardial vessels. Major adverse cardiac and cerebrovascular adverse events (MACCE) included the occurrence of all-cause mortality, myocardial infarction, stroke, or the need for any repeat revascularization. Patients with a prior CABG, a nonisolated CABG (eg, combined procedures such as concomitant CABG and valve surgery), staged revascularization procedures, and a lack of follow-up were excluded.
2.3 |. Outcomes
The primary outcome was overall survival following CABG or PCI. Secondary outcomes included freedom from MACCE and readmissions. A subanalysis was conducted to examine survival, freedom from MACCE, and readmissions specifically in patients who underwent complete revascularization.
2.4 |. Statistical analysis
Demographic and clinical characteristics are presented as frequency (percentage) for categorical variables and mean ± standard deviation for Gaussian continuous variables or median (interquartile range [IQR]) for non-Gaussian continuous variables. Normality was checked with the Kolmogorov-Smirnov test. The χ2 test or the Fisher exact test was utilized for categorical variables and continuous variables were analyzed with the Student t test if normally distributed and the Mann Whitney U test if non-Gaussian. Cox proportional hazards models were constructed to determine independent predictors of survival and MACCE. A total time-restricted model was utilized to determine independent predictors of multiple readmissions. Kaplan-Meier estimates were used to compare overall survival and freedom from MACCE in the CABG and PCI groups. Cumulative rates of readmission were also modeled.
3 |. RESULTS
3.1 |. Characteristics of the study population
A total of 2001 patients (median age, 68 years; 31.7% female) with MVCAD and NSTEMI were included in the analysis (Table 1). A greater proportion underwent CABG (n = 1480, 74.0%) as compared to PCI (n = 521, 26.0%). Patients undergoing CABG were less likely to have a diagnosis of heart failure (10.4 vs 15.9%, P = .001) and were less likely to have had a prior PCI (23.6 vs 31.5%, P < .001). Additionally, patients undergoing CABG had a greater percentage of three-vessel disease (77.7 vs 59.3%, P < .001) and were more likely to undergo complete revascularization (81.0 vs 28.6%, P < .001).
TABLE 1.
Basel ine characteristics of patients with non-ST elevation myocardial infarction undergoing either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)
| Overall N = 2001 | PCI N = 521 | CABG N = 1480 | P value | |
|---|---|---|---|---|
| Age | 68 (59–76) | 70 (60–80) | 67 (59–75) | <.001 |
| Female sex, n (%) | 635 (31.7) | 190 (36.5) | 445 (30.1) | .007 |
| Race, n (%) | .090 | |||
| Caucasian | 1833 (91.6) | 474 (91.0) | 1359 (91.8) | |
| African American | 116 (5.8) | 38 (7.3) | 78 (5.3) | |
| Other | 52 (2.6) | 9 (1.7) | 43 (2.9) | |
| Body mass index, kg/m2 | 29 (26–33) | 29 (26–33) | 30 (26–33) | .533 |
| Left ventricular ejection fraction | 50 (40–56) | 50 (43–53) | 50 (40–58) | .118 |
| Body surface area, mean (SD) | 2.0 (0.2) | 2.0 (0.3) | 2.0 (0.2) | .090 |
| Chronic lung disease, n (%) | 415 (20.7) | 83 (15.9) | 332 (22.4) | .002 |
| Cerebrovascular disease, n (%) | 452 (22.6) | 110 (21.1) | 342 (23.1) | .349 |
| Heart failure, n (%) | 237 (11.8) | 83 (15.9) | 154 (10.4) | .001 |
| Dialysis, n (%) | 76 (3.8) | 26 (5.0) | 50 (3.4) | .098 |
| Current smoker, n (%) | 567 (28.3) | 115 (22.1) | 452 (30.5) | <.001 |
| Hypertension, n (%) | 1728 (86.4) | 436 (83.7) | 1292 (87.3) | .039 |
| Hyperlipidemia, n (%) | 1713 (85.6) | 384 (73.7) | 1329 (89.8) | <.001 |
| Diabetes, n (%) | 979 (48.9) | 247 (47.4) | 732 (49.5) | .421 |
| Liver Disease, n (%) | 131 (6.6) | 26 (5.0) | 105 (7.1) | .095 |
| Cancer, n (%) | 293 (14.6) | 108 (20.7) | 185 (12.5) | <.001 |
| Peripheral artery disease, n (%) | 382 (19.1) | 75 (14.4) | 307 (20.7) | .002 |
| Prior myocardial infarction, n (%) | 1618 (80.9) | 138 (26.5) | 1480 (100.0) | <.001 |
| Prior PCI, n (%) | 513 (25.6) | 164 (31.5) | 349 (23.6) | <.001 |
| Number of diseased vessels | <.001 | |||
| 1 | 34 (1.7) | 0(0) | 34 (2.3) | |
| 2 | 493 (24.6) | 198 (38.0) | 295 (19.9) | |
| 3 | 1459 (72.9) | 309 (59.3) | 1150 (77.7) | |
| Unknown | 15 (0.8) | 14 (2.7) | 1 (0.1) | |
| Completeness of revascularization, n (%) | 1348 (67.4) | 149 (28.6) | 1199 (81.0) | <.001 |
| Cardiopulmonary bypass time, min | 98 (78–120) | |||
| Cross clamp time, min | 69 (52–88) | |||
| Bilateral mammary use, n (%) | 217 (14.7) | |||
| Off-pump bypass, n (%) | 347 (23.4) | |||
| STS mortality risk percentage | 1.7 (0.9–3.9) |
3.2 |. Survival analysis
The overall median time of follow-up was 3.6 years (IQR, 2.3–5.4 years). A multivariable model for survival among patients with NSTEMI undergoing revascularization demonstrated that increasing creatinine (hazard ratio [HR], 1.17; 95% confidence interval [CI], 1.08–1.27; P < .001), older age (HR, 1.04; 95% CI, 1.03–1.05; P < .001) and comorbidities including cerebrovascular disease (HR, 1.47; 95% CI, 1.20–1.81; P < .001), end-stage renal disease (HR, 1.89; 95% CI, 1.15–3.12; P = .013), and diabetes (HR 1.45; 95% CI, 1.19–1.77; P < .001) were all associated with increased hazards for death (Table 2). Additionally, CABG was associated with a lower hazard for death (HR, 0.59; 95% CI, 0.46–0.76; P < .001) when compared to PCI. Overall survival was higher at 1 year (92.0 vs 81.8%; P < .001) and 5 years (80.7 vs 63.3%, P < .001) in patients undergoing CABG as compared to PCI (Figure 1).
TABLE 2.
Cox proportional hazards model for death in patients with non-ST elevation myocardial infarction (NSTEMI) undergoing either percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG)
| Hazard ratio | 95% Confidence interval | P value | |
|---|---|---|---|
| CABG (in reference to PCI) | 0.59 | 0.46–0.76 | <.001 |
| Age, y | 1.04 | 1.03–1.05 | <.001 |
| Left ventricular ejection fraction | 0.98 | 0.97–0.99 | <.001 |
| Creatinine | 1.17 | 1.08–1.27 | <.001 |
| Chronic lung disease | 1.64 | 1.32–2.04 | <.001 |
| Cerebrovascular disease | 1.47 | 1.20–1.81 | <.001 |
| End-stage renal disease | 1.89 | 1.15–3.12 | .013 |
| Diabetes | 1.45 | 1.19–1.77 | <.001 |
| Cancer | 1.71 | 1.36–2.15 | <.001 |
| Peripheral artery disease | 1.43 | 1.15–1.77 | .001 |
Note: Variables included in the model but not significant: prior PCI, smoking, race, hyperlipidemia, prior MI, body mass index, body surface area, completeness of revascularization, number of diseased vessels, liver disease, hypertension, heart failure.
FIGURE 1.
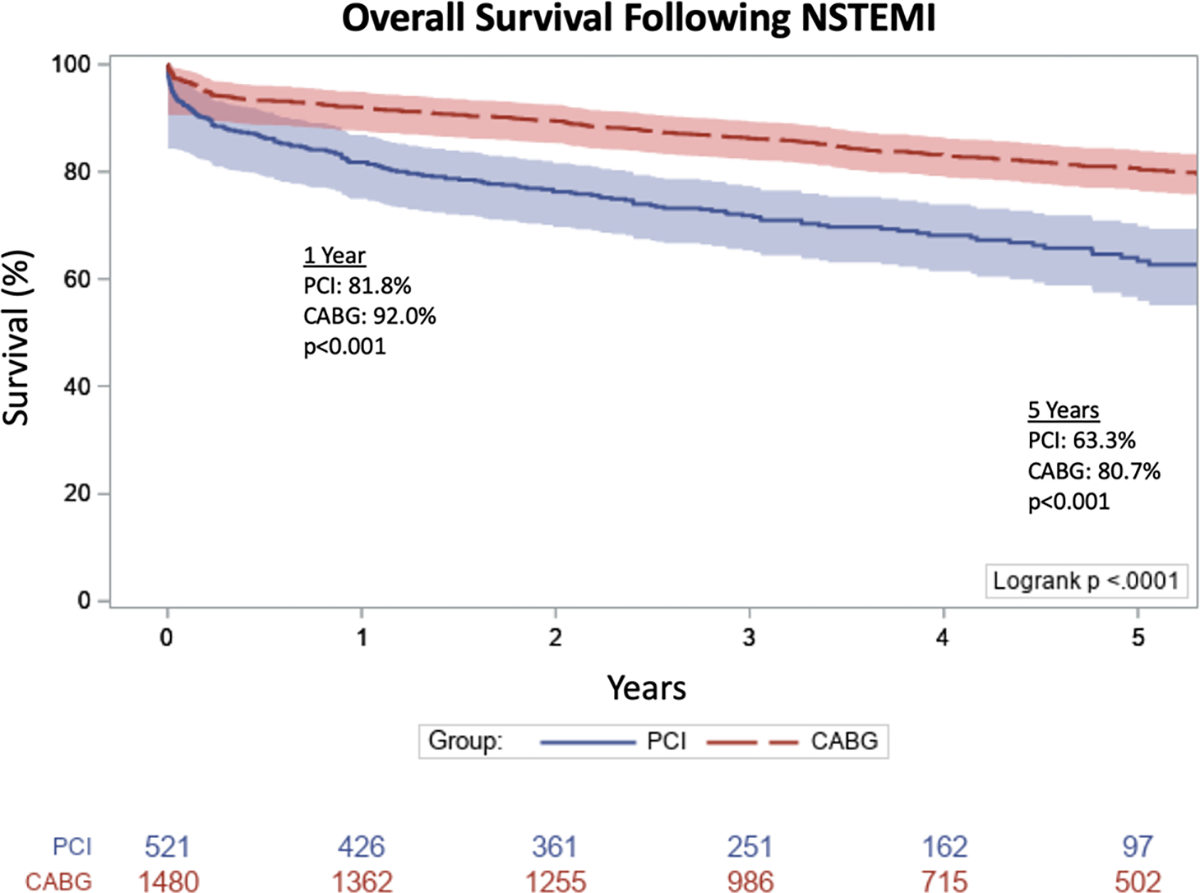
Kaplan-Meier analysis of overall survival following non-ST elevation myocardial infarction (NSTEMI) in patients undergoing percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)
3.3 |. Secondary outcomes
Analysis of adverse events revealed greater freedom from MACCE in the CABG group at 1 year (91.5 vs 75.8%, P < .001) and 5 years (81.1 vs 60.0%, P < .001) (Figure 2). Rates of MI (1.8 vs 7.5%, P < .001) and need for repeat revascularization (2.2 vs 6.5%, P < .001) were significantly lower in the CABG group; there was no significant difference in the rate of stroke (0.8% in CABG vs 1.2% in PCI, P = .479). A Cox model was constructed to explore the adjusted hazards for MACCE (Table S1). CABG was associated with a lower adjusted hazard for MACCE (HR, 0.43; 95% CI, 0.35–0.52; P < .001) when compared to PCI. Older age (HR, 1.03; 95% CI, 1.02–1.04; P < .001), increasing creatinine (HR, 1.17; 95% CI, 1.08–1.26; P < .001), and comorbidities including cerebrovascular disease (HR, 1.31; 95% CI, 1.07–1.60; P = .009), end-stage renal disease (HR, 1.86; 95% CI, 1.15–3.01; P = .012), and diabetes (HR, 1.48; 95% CI, 1.22–1.80; P < .001) were all significantly associated with MACCE.
FIGURE 2.
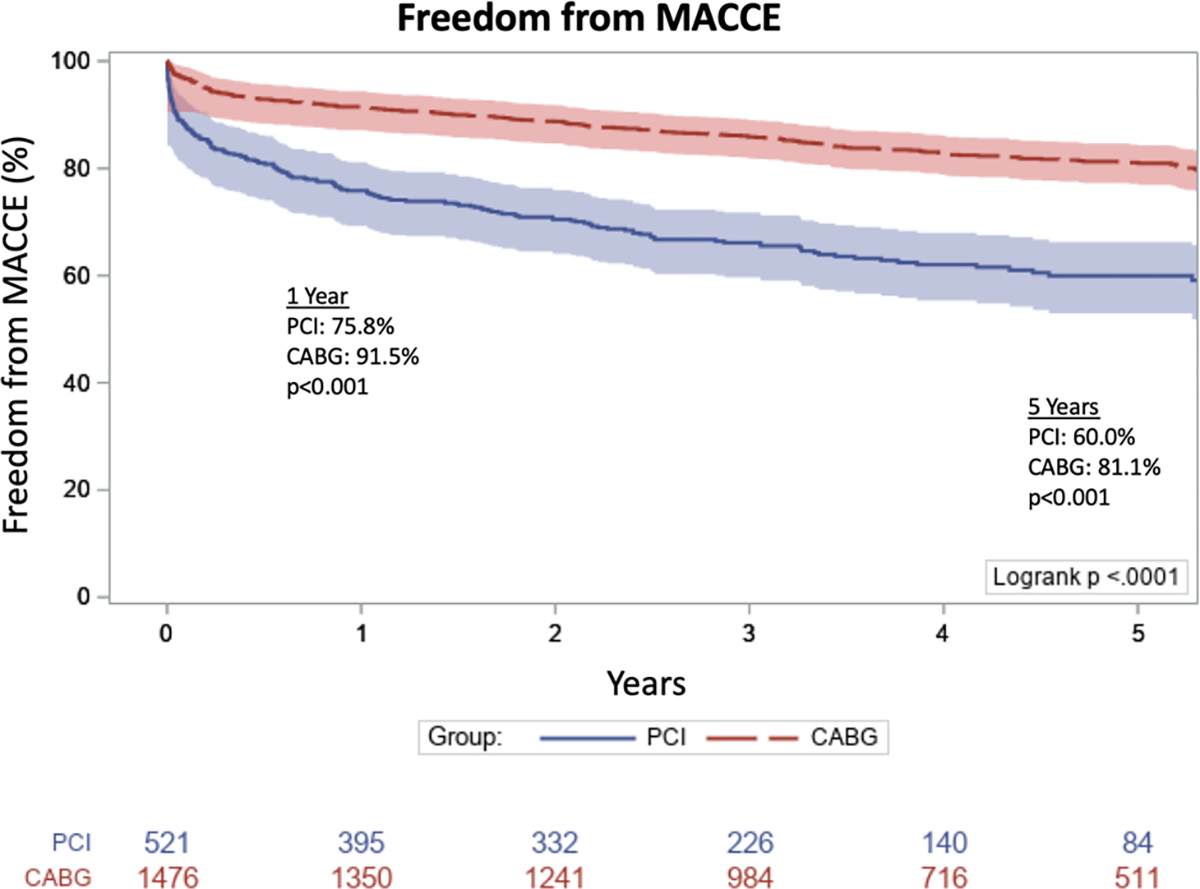
Freedom from major adverse cardiac and cerebrovascular events (MACCE) following non-ST elevation myocardial infarction (NSTEMI) in patients undergoing percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)
A total time-restricted model for multiple readmissions demonstrated a lower adjusted hazard ratio for readmission in patients undergoing CABG (HR, 0.69; 95% CI, 0.53–0.89; P = .004) as well as a lower hazard for readmission in those undergoing complete revascularization (HR, 0.74; 95% CI, 0.63–0.87; P < .001). Additionally, older age (HR, 1.01; 95% CI, 1.01–1.02; P < .001), lower ejection fraction (HR, 0.99; 95% CI, 0.99–1.00; P = .016), and comorbidities including heart failure (HR, 1.28; 95% CI, 1.08–1.52; P = .004), diabetes (HR, 1.35, 95% CI, 1.18–1.54, P < .001), and end-stage renal disease (HR, 1.61, 95% CI, 1.13–2.30, P = .008) were associated with increased hazards for multiple readmissions. The cumulative incidence of readmission was significantly lower in patients undergoing CABG (P < .001) (Figure 3).
FIGURE 3.
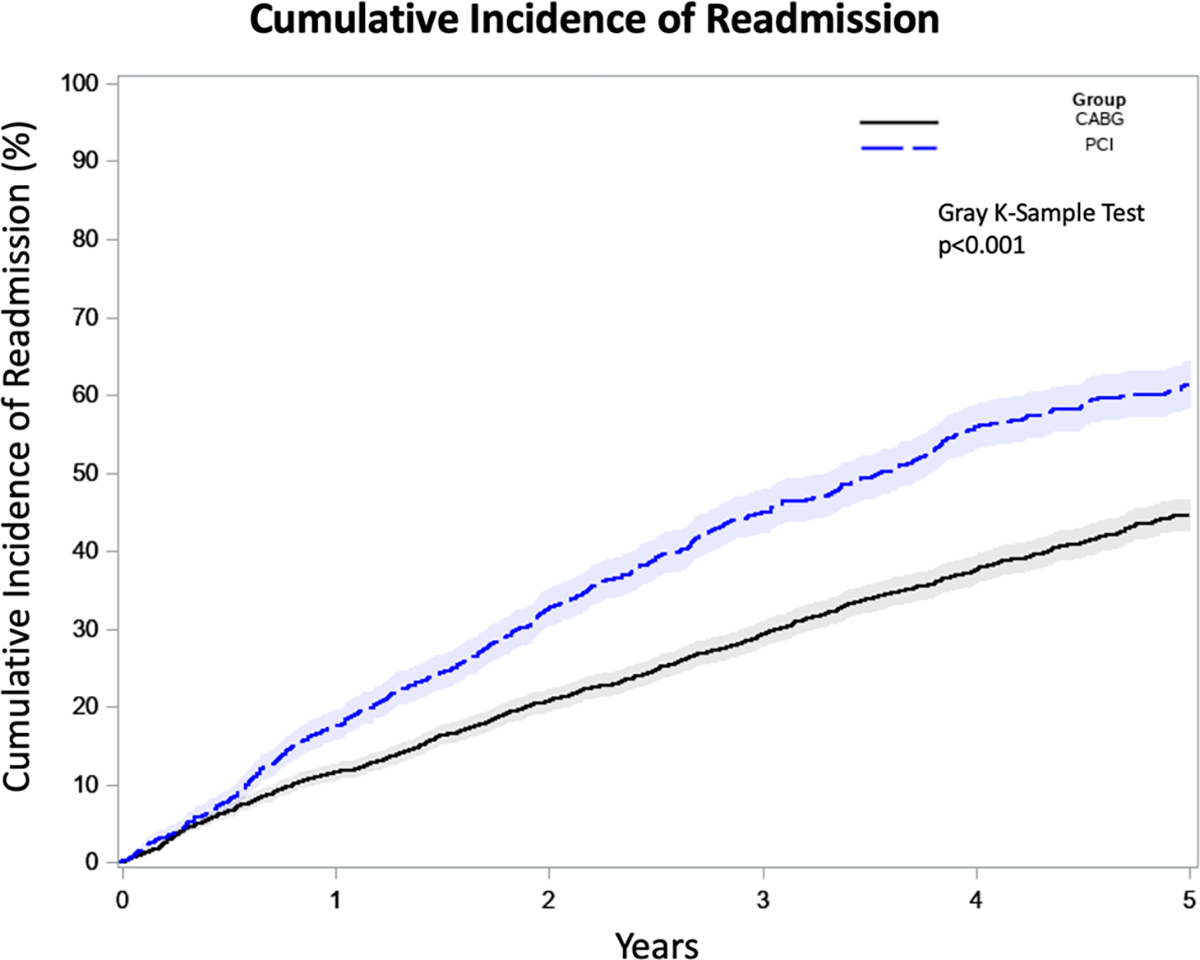
Cumulative incidence of readmission following non-ST elevation myocardial infarction (NSTEMI) in patients undergoing percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)
3.4 |. Analysis of patients undergoing complete revascularization
We performed a subanalysis of the 149 PCI patients and 1199 CABG patients who underwent complete revascularization. Overall survival was significantly higher in the CABG group at 1 year (92.7 vs 83.9%, P = .01) and 5 years (81.1 vs 69.4%, P < .001) (Figure S1). Additionally, freedom from MACCE was higher in patients undergoing CABG at 1 year (92.3 vs 75.2%, P < .001) and 5 years (81.7 vs 61.4%, P < .001) (Figure S2). By univariate analysis, rates of myocardial infarction (1.9 vs 7.4%, P < .001) and repeat revascularization (2.4 vs 8.7%, P < .001) were significantly lower in the CABG group; there was no significant difference between CABG and PCI in the rate of stroke (0.9 vs 0%, P = .240). Cumulative incidence of readmission was significantly lower in patients undergoing CABG vs PCI with complete revascularization (P < .001) (Figure S3).
4 |. CONCLUSIONS
The optimal revascularization strategy for patients with MVCAD and NSTEMI remains controversial, with most comparative trials specifically excluding this patient subset. Since the majority of NSTEMI patients have multivessel disease, treatment and timing are critical components to ensure favorable patient outcomes.6 In this study, we demonstrated improved survival and freedom from MACCE along with lower readmission rates in patients undergoing CABG as compared to PCI. In particular, rates of MI and repeat revascularization were significantly lower among patients undergoing CABG, with no significant differences in stroke rates. Additionally, given the widely discrepant rates of complete revascularization in the groups, a subanalysis of only those patients undergoing complete revascularization was conducted and demonstrated that outcomes including mortality, freedom from MACCE, and readmission rates consistently favored CABG. This real-world analysis of patients with MVCAD presenting with NSTEMI is, therefore, supportive of revascularization with CABG in this setting.
Survival following NSTEMI has improved significantly over the years and this has been attributed to more aggressive revascularization approaches.8 Although STEMI has been associated with greater mortality, NSTEMI patients are typically older and have more extensive coronary artery disease.9 Mortality rates among elderly NSTEMI patients have been quoted at around 24% at 1 year with rates of up to 45.5% among nonagenarians.10,11 We similarly found age to be an independent predictor of death. These study populations, however, included patients treated medically, which may be responsible for the elevated mortality rates compared to our 1-year mortality rates of 18.2% and 8.0% in the PCI and CABG groups, respectively. The survival benefit observed with CABG in our study may be attributable to these lower rates of MI and repeat revascularization. A recent meta-analysis corroborated these findings, demonstrating significant reductions in mortality, MI, and repeat revascularization with CABG.1 Interestingly, we also found that among NSTEMI patients, this survival benefit appears to be independent of the completeness of revascularization, as a subanalysis of our population again demonstrated improved outcomes with CABG.
Prior studies have compared strategies targeting only the culprit lesion vs complete revascularization in patients undergoing PCI. In all patients with MVCAD, complete revascularization of all significant lesions, as opposed to revascularization of the culprit lesion only, is associated with improved survival and lower rates of MI.4,12,13 This finding has also been described in patients with NSTEMI.7,14 Reflecting the superiority of addressing all significant lesions, the 2014 American Heart Association (AHA) Guidelines for patients with NSTEMI suggest multivessel PCI over culprit lesion-only PCI as a class IIb recommendation.15 Interestingly, in our analysis complete revascularization was achieved in nearly threefold more CABG patients as compared to PCI. Further, CABG was associated with a 57% reduction in MACCE after adjustment for relevant covariates and, moreover, rates of MI and repeat revascularization were significantly higher among the PCI group. Although these findings suggest a potential difference in the durability of treatment and patency rates, the occurrence of subclinical stenoses or occlusions is unable to be captured as patients in this series did not undergo routine follow-up angiography. The timing of revascularization is another important factor in treating NSTEMI. Prior studies have demonstrated no difference in mortality and outcomes noted between procedures performed within 24 hours of NSTEMI presentation compared to those performed after 3 days, although in patients with significantly elevated troponin levels there may be a benefit to waiting for troponin normalization.16,17 As it relates to CABG, this benefit stems from the belief some surgeons have that a short time interval between MI and surgical intervention results in a higher likelihood that the heart will be less apt to tolerate cardiopulmonary bypass and aortic cross-clamping, or in cases of off-pump CABG, less likely to tolerate hemodynamic fluctuations and manipulation. A recent study from our center demonstrated no difference in outcomes in those undergoing CABG within 24 hours vs after 24 hours following a MI.18
Readmission rates were also notable in this patient population with over 50% of PCI patients being readmitted within 5 years; rates in the CABG population, although lower, still approached 40%. Hospital readmissions and their associated costs are increasingly scrutinized in today’s healthcare landscape. Nonetheless, costs accumulated during readmission are only a component of overall costs with a substantial portion related to the index hospitalization. A cost analysis of PCI vs CABG for MVCAD demonstrated higher index hospitalization costs as well as cumulative costs over 5 years for CABG, despite lower rates of MACCE.19 Another study of diabetic patients with MVCAD similarly demonstrated higher costs with CABG up to 5-year follow-up, although the authors projected that during the lifetime of a patient CABG would be economically attractive relative to PCI with substantial gains in life expectancy, quality-adjusted life expectancy, and incremental cost-effectiveness ratios.20
An important implication of this real-world analysis is that a multidisciplinary heart team approach should be considered in cases of MVCAD with NSTEMI. Our group has previously demonstrated the utility of a multidisciplinary revascularization heart team as a means to support and validate clinical decisions in an individual patient-centered approach.21 In fact, joint guidelines from the American College of Cardiology and the AHA provide a class I recommendation for a heart team approach to revascularization in patients with complex MVCAD.22
This study has several limitations. Due to the retrospective nature of the study design, we were unable to account for all factors contributing to decision-making regarding revascularization approach for each individual patient. We also could not capture which patients were discussed in a multidisciplinary heart team. Potentially confounding factors that were not included in the analysis include patient preference, target vessel size and quality, and frailty. Finally, we did not capture any readmissions that occurred outside of our health system, although prior internal quality control analyses from our group have determined that more than 90% of patients that are readmitted are readmitted within our multihospital regional network.
In conclusion, we demonstrate improved outcomes including survival, MACCE, and readmissions among patients with MVCAD and NSTEMI undergoing CABG as their revascularization approach. These findings persist when evaluating only patients with complete revascularization. Continued evaluation of the best revascularization strategy for NSTEMI in MVCAD will need to be assessed in future randomized trials. In addition, the concept of individualized patient-centered therapy remains essential. Collectively taken together, these factors in conjunction with the outcomes reported in this real-world analysis are supportive of multidisciplinary discussions regarding the best revascularization therapy for patients with MVCAD and NSTEMI.
Supplementary Material
TABLE 3.
Total time-restricted model for multiple readmissions in patients with non-ST elevation myocardial infarction (NSTEMI) undergoing either percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG)
| Hazard ratio | 95% Confidence interval | P value | |
|---|---|---|---|
| CABG (in reference to PCI) | 0.69 | 0.53–0.89 | .004 |
| Complete revascularization | 0.74 | 0.63–0.87 | <.001 |
| Age, y | 1.01 | 1.01–1.02 | <.001 |
| Left ventricular ejection fraction | 0.99 | 0.99–1.00 | .016 |
| Creatinine | 1.11 | 1.05–1.17 | .001 |
| Heart failure | 1.28 | 1.08–1.52 | .004 |
| Cerebrovascular disease | 1.21 | 1.06–1.39 | .006 |
| Peripheral artery disease | 1.30 | 1.13–1.51 | .000 |
| Current smoker | 1.22 | 1.04–1.42 | .013 |
| Diabetes | 1.35 | 1.18–1.54 | <.001 |
| End-stage renal disease | 1.61 | 1.13–2.30 | .008 |
| Cancer | 1.50 | 1.27–1.76 | <.001 |
Note: Variables included in the model but not significant: sex, number of diseased vessels, liver disease, hypertension, hyperlipidemia, chronic lung disease, prior MI, body mass index, body surface area.
Footnotes
CONFLICT OF INTERESTS
Dr Arman Kilic serves on a Medical Advisory Board for Medtronic, Inc. Dr Thomas Gleason serves on a Medical Advisory Board for Abbott, Inc. The remaining authors declare that there are no conflict of interests.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Sipahi I, Akay MH, Dagdelen S, Blitz A, Alhan C. Coronary artery bypass grafting vs percutaneous coronary intervention and long-term mortality and morbidity in multivessel disease: meta-analysis of randomized clinical trials of the arterial grafting and stenting ERA. JAMA Intern Med. 2014;174(2):223–230. 10.1001/jamainternmed.2013.12844 [DOI] [PubMed] [Google Scholar]
- 2.Hannan EL, Wu C, Walford G, et al. Drug-eluting stents vs. coronary-artery bypass grafting in multivessel coronary disease. N Engl J Med. 2008;358(4):331–341. [DOI] [PubMed] [Google Scholar]
- 3.SoS Investigators. Coronary artery bypass surgery versus percutaneous coronary intervention with stent implantation in patients with multivessel coronary artery disease (the Stent or Surgery trial): a randomised controlled trial. Lancet. 2002;360(9338):965–970. 10.1016/S0140-6736(02)11078-6 [DOI] [PubMed] [Google Scholar]
- 4.Mulukutla SR, Gleason TG, Sharbaugh M, et al. Coronary bypass versus percutaneous revascularization in multivessel coronary artery disease. Ann Thorac Surg. 2019;108(2):474–480. 10.1016/j.athoracsur.2019.02.064 [DOI] [PubMed] [Google Scholar]
- 5.Javaid A, Steinberg DH, Buch AN, et al. Outcomes of coronary artery bypass grafting versus percutaneous coronary intervention with drug-eluting stents for patients with multivessel coronary artery disease. Circulation. 2007;116(11 suppl 1):200–206. 10.1161/CIRCULATIONAHA.106.681148 [DOI] [PubMed] [Google Scholar]
- 6.Hung CS, Chen YH, Huang CC, et al. Prevalence and outcome of patients with non-ST segment elevation myocardial infarction with occluded “culprit” artery—a systemic review and meta-analysis. Crit Care. 2018;22(1):1–11. 10.1186/s13054-018-1944-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rathod KS, Koganti S, Jain AK, et al. Complete versus culprit-only lesion intervention in patients with acute coronary syndromes. J Am Coll Cardiol. 2018;72(17):1989–1999. 10.1016/j.jacc.2018.07.089 [DOI] [PubMed] [Google Scholar]
- 8.Movahed MR, John J, Hashemzadeh M, Hashemzadeh M. Mortality trends for non-ST-segment elevation myocardial infarction (NSTEMI) in the United States from 1988 to 2004. Clin Cardiol. 2011;34: 689–692. 10.1002/clc.20968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbott JD, Ahmed HN, Vlachos HA, Selzer F, Williams DO. Comparison of outcome in patients with ST-elevation versus non-ST-elevation acute myocardial infarction treated with percutaneous coronary intervention. Am J Cardiol. 2007;100(2):190–195. [DOI] [PubMed] [Google Scholar]
- 10.Roe MT, Chen AY, Thomas L, et al. Predicting long-term mortality in older patients after non-ST-segment elevation myocardial infarction: The CRUSADE long-term mortality model and risk score. Am Heart J. 2011;162(5):875–883 e1. 10.1016/j.ahj.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 11.Lopes RD, Gharacholou SM, Holmes DN, et al. Cumulative incidence of death and rehospitalization among the elderly in the first year after NSTEMI. Am J Med. 2015;128(6):582–590. 10.1016/j.amjmed.2014.12.032 [DOI] [PubMed] [Google Scholar]
- 12.Lehmann R, Fichtlscherer S, SchÄchinger V, et al. Complete revascularization in patients undergoing multivessel PCI is an independent predictor of improved long-term survival. J Interv Cardiol. 2010;23(3): 256–263. 10.1111/j.1540-8183.2010.00556.x [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez AE, Baldi J, Pereira CF, et al. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II). J Am Coll Cardiol. 2005;46(4):582–588. 10.1016/j.jacc.2004.12.081 [DOI] [PubMed] [Google Scholar]
- 14.Wang T, Kaltenbach LA, Bhatt DL, et al. Long-term mortality associated with multivessel versus culprit vessel only percutaneous coronary intervention for patients with acute myocardial infarction: insights from the National Cardiovascular Cathpci Data Registry. J Am Coll Cardiol. 2011;57(14):E914. 10.1016/s0735-1097(11)60914-5 [DOI] [Google Scholar]
- 15.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394. 10.1161/CIR.0000000000000133 [DOI] [PubMed] [Google Scholar]
- 16.Davierwala PM, Verevkin A, Leontyev S, Misfeld M, Borger MA, Mohr FW. Does timing of coronary artery bypass surgery affect early and long-term outcomes in patients with non-ST-segment-elevation myocardial infarction? Circulation. 2015;132(8):731–740. 10.1161/CIRCULATIONAHA.115.015279 [DOI] [PubMed] [Google Scholar]
- 17.Dayan V, Soca G, Parma G, Mila R. Does early coronary artery bypass surgery improve survival in non-ST acute myocardial infarction? Interact Cardiovasc Thorac Surg. 2013;17(1):140–143. 10.1093/icvts/ivt128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bianco V, Kilic A, Gleason TG, et al. Timing of coronary artery bypass grafting after acute myocardial infarction may not influence mortality and readmissions. J Thorac Cardiovasc Surg. 2020. 10.1016/j.jtcvs.2019.11.061 [in press]. [DOI] [PubMed] [Google Scholar]
- 19.Krenn L, Kopp C, Glogar D, et al. Cost-effectiveness of percutaneous coronary intervention with drug-eluting stents in patients with multivessel coronary artery disease compared to coronary artery bypass surgery five-years after intervention. Catheter Cardiovasc Interv. 2014; 84(7):1029–1039. 10.1002/ccd.25397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magnuson EA, Farkouh ME, Fuster V, et al. Cost-effectiveness of percutaneous coronary intervention with drug eluting stents versus bypass surgery for patients with diabetes mellitus and multivessel coronary artery disease: Results from the FREEDOM trial. Circulation. 2013;127(7):820–831. 10.1161/CIRCULATIONAHA.112.147488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez CE, Dota A, Badhwar V, et al. Revascularization heart team recommendations as an adjunct to appropriate use criteria for coronary revascularization in patients with complex coronary artery disease. Catheter Cardiovasc Interv. 2016;88(4):E103–E112. 10.1002/ccd.26276 [DOI] [PubMed] [Google Scholar]
- 22.Fihn SD, Blankenship JC, Alexander KP, et al. ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines, a. J Am Coll Cardiol. 2014;64(18): 1929–1949. 10.1016/j.jacc.2014.07.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


