Abstract
In vitro susceptibility tests were performed to document the inhibitory activities of three nonfluorinated quinolone (NFQ) compounds (PGE 9262932, PGE 9509924, and PGE 4175997) compared to those of ciprofloxacin, levofloxacin, and trovafloxacin against 3,030 bacterial isolates. The spectra of the NFQ agents included most gram-positive species as well as quinolone-susceptible Enterobacteriaceae. Ciprofloxacin-resistant, methicillin-resistant Staphylococcus aureus strains were inhibited by the NFQ series at ≤1.0 μg/ml. The NFQ compounds were not very active against Pseudomonas aeruginosa and most other nonfermentative gram-negative bacilli. Against other species, the potency of the NFQ agents was similar to that of trovafloxacin. Continued investigation of the NFQ compounds seems to be warranted.
Nalidixic acid was the first of a series of compounds belonging to the naphthyridine series; its use was limited to treatment of urinary tract infections. By replacing the nitrogen at position 8 with a carbon atom oxolinic acid was developed, and that was the first quinolone to be developed for clinical use. Against the Enterobacteriaceae, oxolinic acid was much more potent than its predecessors, but its clinical utility was still limited. The status of the quinolones was dramatically changed after the development of a series of quinolones with one or more fluorine atoms. The fluoroquinolones (FQs) could be administered parenterally or orally and they were at least 100-fold more active than their naphthyridine predecessors (1). More recently, a series of extended-spectrum FQs have been developed to obtain better activity against gram-positive species without substantial loss of activity against gram-negative species. Unfortunately, use of many FQs is limited because of the potential for adverse side effects (2, 3). Some FQs have been removed from the market because of more serious adverse events (3). Other FQ antimicrobial agents are now widely used for a variety of infections.
Scientists at Procter & Gamble Pharmaceuticals (Cincinnati, Ohio) have recently synthesized a series of nonfluorinated quinolones (NFQs) (B. Ledoussal, J. K. Almstead, S. M. Flaim, C. P. Gallagher, J. L. Gray, X. E. Hu, N. K. Kim, H. D. McKeever, C. J. Miley, T. L. Twinem, and S. X. Zheng, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F544, p. 303, 1999). These compounds maintain an extended spectrum of antibacterial activity even though they contain no fluorine at the 6 position of the quinolone nucleus. As part of the early in vitro screening tests, we evaluated the in vitro activities of three such NFQ compounds, compared to that of three FQs, against recent clinical isolates and appropriate stock cultures. This report summarizes the results of antibiotic dilution tests of 3,030 bacterial isolates.
(Abstracts of the data included in this report were presented at the 39th and 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., 1999, and Toronto, Canada, 2000.)
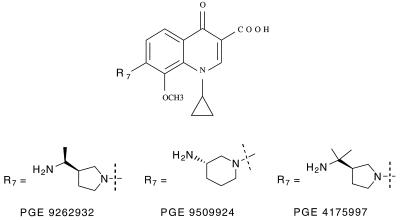
Antimicrobial susceptibility tests were performed exactly as described in the National Committee for Clinical Laboratory Standards M7-A5 and M11-A4 documents (4, 5). Agar dilution methods were used for testing Haemophilus influenzae, Neisseria gonorrhoeae, and anaerobic bacteria. All other species were tested by the broth microdilution procedure using cation-adjusted Mueller-Hinton broth. Lysed horse blood (3 to 5%) was added to the broth when testing streptococci. The NFQ compounds that were provided by Procter & Gamble Pharmaceuticals were designated as PGE 9262932, PGE 9509924, and PGE 4175997. The chemical structures of these compounds are shown in Fig. 1. The three FQ comparator drugs that we tested were obtained from their respective U.S. manufacturers. Standard quality control strains were included with each batch of tests; the control strains were those appropriate for the species being studied on each test day. The microorganisms that were selected for this study were all clinical isolates originally obtained over the past 10 years from a variety of medical centers throughout North America. Isolates were selected to represent a wide variety of bacterial species and to include antibiotic-resistant strains, when available. The species identification reported by the contributing laboratory was confirmed by appropriate conventional tests when there was a reason to question the initial identification.
FIG. 1.
Chemical structure of three NFQ compounds included in this study.
Table 1 shows the results of tests with gram-positive species. Against the staphylococci, the NFQ compounds were similar to trovafloxacin; ciprofloxacin was the least active quinolone tested against staphylococci. Most methicillin-resistant staphylococci are now resistant to ciprofloxacin and they show cross-resistance to other FQ compounds. The NFQs showed similar cross-resistance, as MICs at which 50% of the isolates were inhibited (MIC50s) and MIC90s were 8- to 64-fold greater than those for ciprofloxacin-susceptible strains. However, MICs of the NFQ compounds against ciprofloxacin-resistant staphylococci were substantially lower than those of the FQ drugs. The NFQ compounds have been shown to be capable of rapidly killing methicillin-resistant Staphylococcus aureus strains (S. Roychoudhury, K. M. Makin, E. J. McIntosh, H. D. McKeever, T. L. Twinem, P. M. Koenigs, and C. E. Catrenich, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F547, p. 304, 1999).
TABLE 1.
In vitro activities of six quinolone compounds against 1,481 gram-positive isolates
| Microorganism (no. of isolates tested) and antimicrobial agent | MIC (μg/ml)
|
Microorganism (no. of isolates tested) and antimicrobial agent | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Range | 50% | 90% | Range | 50% | 90% | |||
| Staphylococcus aureus | ||||||||
| Cips (231)ab | ||||||||
| PGE 9262932 | ≤0.008–0.06 | ≤0.008 | 0.03 | |||||
| PGE 9509924 | ≤0.008–0.12 | 0.016 | 0.03 | |||||
| PGE 4175997 | ≤0.008–0.06 | 0.016 | 0.03 | |||||
| Trovafloxacin | ≤0.008–0.12 | 0.016 | 0.03 | |||||
| Levofloxacin | 0.03–0.5 | 0.12 | 0.25 | |||||
| Ciprofloxacin | 0.03–1.0 | 0.25 | 0.5 | |||||
| Cipr (125)bc | ||||||||
| PGE 9262932 | 0.06–4.0 | 0.25 | 2.0 | |||||
| PGE 9509924 | 0.12–4.0 | 0.5 | 1.0 | |||||
| PGE 4175997 | 0.06–8.0 | 0.5 | 2.0 | |||||
| Trovafloxacin | 0.25–16 | 2.0 | 16 | |||||
| Levofloxacin | 2.0–>16 | 8.0 | >16 | |||||
| Ciprofloxacin | 4.0–>16 | >16 | >16 | |||||
| Coagulase-negative Staphylococcus spp. | ||||||||
| Cips (209)de | ||||||||
| PGE 9262932 | ≤0.008–0.06 | ≤0.016 | 0.03 | |||||
| PGE 9509924 | ≤0.008–0.06 | 0.03 | 0.06 | |||||
| PGE 4175997 | ≤0.008–0.06 | 0.03 | 0.03 | |||||
| Trovafloxacin | 0.016–0.12 | 0.03 | 0.06 | |||||
| Levofloxacin | 0.06–0.5 | 0.25 | 0.25 | |||||
| Ciprofloxacin | 0.03–0.5 | 0.12 | 0.25 | |||||
| Cipr (125)ef | ||||||||
| PGE 9262932 | 0.06–4.0 | 0.25 | 1.0 | |||||
| PGE 9509924 | 0.12–4.0 | 0.25 | 1.0 | |||||
| PGE 4175997 | 0.12–8.0 | 0.25 | 1.0 | |||||
| Trovafloxacin | 0.25–>16 | 4.0 | 16 | |||||
| Levofloxacin | 1.0–>16 | 8.0 | >16 | |||||
| Ciprofloxacin | 4.0–>16 | >16 | >16 | |||||
| Streptococcus pneumoniae (366)g | ||||||||
| PGE 9262932 | ≤0.008–0.03 | ≤0.008 | 0.016 | |||||
| PGE 9509924 | 0.016–0.25 | 0.03 | 0.06 | |||||
| PGE 4175997 | ≤0.008–0.12 | 0.016 | 0.03 | |||||
| Trovafloxacin | 0.03–1.0 | 0.12 | 0.12 | |||||
| Levofloxacin | 0.25–8.0 | 1.0 | 1.0 | |||||
| Ciprofloxacin | 0.5–16 | 1.0 | 2.0 | |||||
| Streptococcus viridans group (60) | ||||||||
| PGE 9262932 | ≤0.008–0.12 | 0.016 | 0.03 | |||||
| PGE 9509924 | 0.016–0.5 | 0.06 | 0.12 | |||||
| PGE 4175997 | 0.016–0.25 | 0.03 | 0.06 | |||||
| Trovafloxacin | 0.06–8.0 | 0.12 | 0.25 | |||||
| Levofloxacin | 0.25–8.0 | 1.0 | 2.0 | |||||
| Ciprofloxacin | 0.25–16 | 1.0 | 1.0 | |||||
| Streptococcus agalactiae (77) | ||||||||
| PGE 9262932 | ≤0.008–0.03 | 0.016 | 0.016 | |||||
| PGE 9509924 | 0.03–0.12 | 0.06 | 0.06 | |||||
| PGE 4175997 | 0.016–0.06 | 0.03 | 0.06 | |||||
| Trovafloxacin | 0.12–0.5 | 0.25 | 0.25 | |||||
| Levofloxacin | 0.5–2.0 | 0.5 | 1.0 | |||||
| Ciprofloxacin | 0.25–2.0 | 0.5 | 1.0 | |||||
| Streptococcus pyogenes (90) | ||||||||
| PGE 9262932 | ≤0.008–0.016 | ≤0.008 | ≤0.008 | |||||
| PGE 9509924 | 0.03–0.06 | 0.03 | 0.06 | |||||
| PGE 4175997 | 0.016–0.06 | 0.016 | 0.03 | |||||
| Trovafloxacin | 0.06–0.5 | 0.06 | 0.12 | |||||
| Levofloxacin | 0.25–2.0 | 0.5 | 0.5 | |||||
| Ciprofloxacin | 0.25–2.0 | 0.5 | 0.5 | |||||
| Enterococcus faecalis | ||||||||
| Vans (63) | ||||||||
| PGE 9262932 | ≤0.008–1.0 | 0.06 | 0.5 | |||||
| PGE 9509924 | 0.06–8.0 | 0.12 | 2.0 | |||||
| PGE 4175997 | 0.03–4.0 | 0.12 | 2.0 | |||||
| Trovafloxacin | 0.06–>16 | 0.25 | 16 | |||||
| Levofloxacin | 0.5–>16 | 1.0 | >16 | |||||
| Ciprofloxacin | 0.25–>16 | 1.0 | >16 | |||||
| Vanr (17) | ||||||||
| PGE 9262932 | 0.06–1.0 | 0.5 | 1.0 | |||||
| PGE 9509924 | 0.12–4.0 | 4.0 | 4.0 | |||||
| PGE 4175997 | 0.12–4.0 | 2.0 | 4.0 | |||||
| Trovafloxacin | 0.06–16 | 4.0 | 8.0 | |||||
| Levofloxacin | 0.5–>16 | >16 | >16 | |||||
| Ciprofloxacin | 0.5–>16 | >16 | >16 | |||||
| Enterococcus faecium | ||||||||
| Vans (36) | ||||||||
| PGE 9262932 | 0.016–16 | 1.0 | 8.0 | |||||
| PGE 9509924 | 0.06–>16 | 1.0 | >16 | |||||
| PGE 4175997 | 0.03–>16 | 1.0 | 16 | |||||
| Trovafloxacin | 0.12–>16 | 16 | >16 | |||||
| Levofloxacin | 0.5–>16 | >16 | >16 | |||||
| Ciprofloxacin | 0.25–>16 | >16 | >16 | |||||
| Vanr (43) | ||||||||
| PGE 9262932 | 0.12–16 | 4.0 | 8.0 | |||||
| PGE 9509924 | 2.0–>16 | 16 | >16 | |||||
| PGE 4175997 | 0.5–>16 | 4.0 | 16 | |||||
| Trovafloxacin | 1.0–>16 | 16 | >16 | |||||
| Levofloxacin | 2.0–>16 | >16 | >16 | |||||
| Ciprofloxacin | 4.0–>16 | >16 | >16 | |||||
| Clostridium difficile (9) | ||||||||
| PGE 9262932 | 0.5–2.0 | 0.5 | —h | |||||
| PGE 9509924 | 1.0–1.0 | 1.0 | — | |||||
| PGE 4175997 | 0.5–1.0 | 0.5 | — | |||||
| Trovafloxacin | 0.5–1.0 | 1.0 | — | |||||
| Levofloxacin | 4.0–4.0 | 4.0 | — | |||||
| Ciprofloxacin | 8.0–8.0 | 8.0 | — | |||||
| Other Clostridium species (21)i | ||||||||
| PGE 9262932 | 0.06–0.5 | 0.25 | 0.5 | |||||
| PGE 9509924 | 0.12–0.5 | 0.25 | 0.5 | |||||
| PGE 4175997 | 0.12–1.0 | 0.5 | 1.0 | |||||
| Trovafloxacin | 0.06–1.0 | 0.25 | 0.5 | |||||
| Levofloxacin | 0.12–2.0 | 0.5 | 1.0 | |||||
| Ciprofloxacin | 0.06–2.0 | 1.0 | 1.0 | |||||
| Peptostreptococcus spp. (9) | ||||||||
| PGE 9262932 | 0.016–0.5 | 0.12 | —h | |||||
| PGE 9509924 | 0.03–4.0 | 0.5 | — | |||||
| PGE 4175997 | 0.03–1.0 | 0.12 | — | |||||
| Trovafloxacin | 0.016–8.0 | 0.5 | — | |||||
| Levofloxacin | 0.03–>16 | 4.0 | — | |||||
| Ciprofloxacin | 0.03–>16 | 4.0 | — | |||||
Includes 215 methicillin-susceptible and 16 methicillin-resistant strains.
Excludes one ciprofloxacin-intermediate strain of methicillin-resistant S. aureus.
Includes 11 methicillin-susceptible and 114 methicillin-resistant strains.
Includes 148 methicillin-susceptible and 61 methicillin-resistant strains.
Excludes 12 ciprofloxacin-intermediate (MIC, 2.0 μg/ml) strains.
Includes 10 methicillin-susceptible and 115 methicillin-resistant strains.
Includes 132 penicillin-susceptible, 84 penicillin-intermediate, and 150 penicillin-resistant strains.
MIC90s were not calculated for species with fewer than 10 strains.
Includes 19 C. perfringens, 1 C. tertium, and 1 C. histolyticum isolates.
The NFQ compounds and trovafloxacin were especially potent against Streptococcus pneumoniae and other streptococci. Ciprofloxacin and levofloxacin were less active. Resistance to the FQ compounds is currently uncommon among these pathogens and they all appear to be active, but the NFQ compounds are 2 to 32 times more potent.
Among the enterococci, resistance to vancomycin is now a major clinical concern in many institutions. Most of our vancomycin-resistant strains were relatively resistant to ciprofloxacin, levofloxacin, and trovafloxacin. The NFQs displayed some activity against such strains, although they were more active against vancomycin-susceptible strains. The NFQ compounds were active against most Enterococcus faecalis strains, which were rarely resistant to vancomycin. Enterococcus faecium strains were commonly resistant to vancomycin and they were often resistant to the FQ agents; the NFQ compounds were also less potent against these strains.
The nine strains of Clostridium difficile were inhibited by 1.0 to 2.0 μg of trovafloxacin or of the NFQ compounds per ml, but ciprofloxacin and levofloxacin were four to eight times less active. The 21 other Clostridium spp. and the nine Peptostreptococcus spp. were also inhibited by the NFQ compounds at concentrations of ≤1.0 μg/ml. On the other hand, all six study drugs showed less activity against members of the Bacteroides fragilis group (Table 2).
TABLE 2.
In vitro activities of six quinolone compounds against 647 gram-negative species other than the Enterobacteriaceae
| Microorganism (no. of isolates tested) and antimicrobial agent | MIC (μg/ml)
|
Microorganism (no. of isolates tested) and antimicrobial agent | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Range | 50% | 90% | Range | 50% | 90% | |||
| Pseudomonas aeruginosa | ||||||||
| Cips (241)a | ||||||||
| PGE 9262932 | 0.12–16 | 1.0 | 4.0 | |||||
| PGE 9509924 | 0.12–8.0 | 1.0 | 4.0 | |||||
| PGE 4175997 | 0.25–>16 | 4.0 | 8.0 | |||||
| Trovafloxacin | 0.06–8.0 | 0.5 | 2.0 | |||||
| Levofloxacin | 0.06–4.0 | 0.5 | 4.0 | |||||
| Ciprofloxacin | 0.03–1.0 | 0.12 | 1.0 | |||||
| Cipr (72)a | ||||||||
| PGE 9262932 | 2.0–>16 | 16 | >16 | |||||
| PGE 9509924 | 2.0–>16 | 16 | >16 | |||||
| PGE 4175997 | 4.0–>16 | >16 | >16 | |||||
| Trovafloxacin | 0.5–>16 | >16 | >16 | |||||
| Levofloxacin | 1.0–>16 | >16 | >16 | |||||
| Ciprofloxacin | 4.0–>16 | 8.0 | >16 | |||||
| Pseudomonas fluorescens/putida group (31) | ||||||||
| PGE 9262932 | 0.12–8.0 | 1.0 | 8.0 | |||||
| PGE 9509924 | 0.12–8.0 | 1.0 | 8.0 | |||||
| PGE 4175997 | 0.5–16 | 2.0 | 8.0 | |||||
| Trovafloxacin | 0.06–4.0 | 0.5 | 1.0 | |||||
| Levofloxacin | 0.06–>16 | 0.5 | 2.0 | |||||
| Ciprofloxacin | 0.06–>16 | 0.12 | 4.0 | |||||
| Burkholderia cepacia (23) | ||||||||
| PGE 9262932 | 0.06–>16 | 8.0 | >16 | |||||
| PGE 9509924 | 0.06–>16 | 4.0 | >16 | |||||
| PGE 4175997 | 0.25–>16 | 16 | >16 | |||||
| Trovafloxacin | 0.016–>16 | 2.0 | >16 | |||||
| Levofloxacin | ≤0.008–>16 | 2.0 | >16 | |||||
| Ciprofloxacin | ≤0.008–>16 | 2.0 | >16 | |||||
| Stenotrophomonas maltophilia (54) | ||||||||
| PGE 9262932 | 0.25–>16 | 4.0 | 16 | |||||
| PGE 9509924 | 0.25–>16 | 2.0 | 16 | |||||
| PGE 4175997 | 0.5–>16 | 4.0 | >16 | |||||
| Trovafloxacin | 0.06–16 | 1.0 | 4.0 | |||||
| Levofloxacin | 0.25–>16 | 1.0 | 8.0 | |||||
| Ciprofloxacin | 0.5–>16 | 4.0 | 16 | |||||
| Acinetobacter baumanii (70) | ||||||||
| PGE 9262932 | 0.06–>16 | 0.5 | >16 | |||||
| PGE 9509924 | 0.06–>16 | 0.5 | >16 | |||||
| PGE 4175997 | 0.12–>16 | 1.0 | >16 | |||||
| Trovafloxacin | ≤0.008–>16 | 0.06 | 16 | |||||
| Levofloxacin | 0.06–>16 | 0.25 | 16 | |||||
| Ciprofloxacin | 0.06–>16 | 0.5 | >16 | |||||
| Haemophilus influenzae (30) | ||||||||
| PGE 9262932 | 0.016–0.25 | 0.12 | 0.12 | |||||
| PGE 9509924 | 0.016–0.25 | 0.06 | 0.12 | |||||
| PGE 4175997 | 0.03–1.0 | 0.25 | 0.5 | |||||
| Trovafloxacin | ≤0.008–0.12 | 0.016 | 0.03 | |||||
| Levofloxacin | ≤0.008–0.06 | 0.03 | 0.03 | |||||
| Ciprofloxacin | ≤0.008–0.03 | 0.016 | 0.03 | |||||
| Moraxella catarrhalis (15) | ||||||||
| PGE 9262932 | 0.016–0.06 | 0.03 | 0.06 | |||||
| PGE 9509924 | 0.03–0.12 | 0.06 | 0.12 | |||||
| PGE 4175997 | ≤0.008–0.12 | 0.03 | 0.12 | |||||
| Trovafloxacin | ≤0.008–0.03 | 0.016 | 0.03 | |||||
| Levofloxacin | 0.02–0.06 | 0.06 | 0.06 | |||||
| Ciprofloxacin | ≤0.008–0.06 | 0.03 | 0.03 | |||||
| Neisseria gonorrhoeae (25)b | ||||||||
| PGE 9262932 | ≤0.008–0.06 | 0.016 | 0.03 | |||||
| PGE 9509924 | ≤0.008–0.12 | 0.03 | 0.12 | |||||
| PGE 4175997 | ≤0.008–0.12 | 0.03 | 0.12 | |||||
| Trovafloxacin | ≤0.008–0.06 | ≤0.008 | 0.12 | |||||
| Levofloxacin | ≤0.008–0.12 | ≤0.008 | 0.12 | |||||
| Ciprofloxacin | ≤0.008–0.25 | ≤0.008 | 0.12 | |||||
| Bacteroides fragilis group (86)c | ||||||||
| PGE 9262932 | 0.25–>16 | 4.0 | >16 | |||||
| PGE 9509924 | 0.5–>16 | 4.0 | >16 | |||||
| PGE 4175997 | 0.5–>16 | 2.0 | >16 | |||||
| Trovafloxacin | 0.12–>16 | 1.0 | 8.0 | |||||
| Levofloxacin | 0.5–>16 | 8.0 | >16 | |||||
| Ciprofloxacin | 0.5–>16 | 16 | >16 | |||||
Excludes 20 ciprofloxacin-intermediate (MIC, 2.0 μg/ml) strains.
Includes seven strains with elevated quinolone MICs; they were ciprofloxacin intermediate (MIC, 0.12 to 0.25 μg/ml).
Includes 55 B. fragilis, 16 B. thetaiotaomicron, 9 B. uniformis, 5 B. vulgaris, and 1 B. distasonis isolates.
Against the Enterobacteriaceae, levofloxacin and trovafloxacin were 2- to 16-fold more active than the NFQ compounds (Table 3). Ciprofloxacin was the most potent agent that we evaluated, but a few ciprofloxacin-resistant strains were found among the recent clinical isolates included in this study. Ciprofloxacin resistance was observed with 15 Providencia spp., 4 Morganella morganii, 6 Proteus mirabilis, 8 Serratia marcescens, 6 Klebsiella pneumoniae, 3 Enterobacter spp., and 1 of the Escherichia coli isolates. Among those 43 isolates, 13 had high-level resistance to ciprofloxacin (MIC, ≥16 μg/ml) and the other isolates were only relatively resistant to ciprofloxacin (MIC, 4.0 or 8.0 μg/ml). The 43 ciprofloxacin-resistant strains showed cross-resistance to the other quinolones studied (Table 3). Against the 72 ciprofloxacin-resistant Pseudomonas aeruginosa isolates that were included in this series, the NFQ compounds had little activity (Table 2). Against quinolone-susceptible P. aeruginosa, Pseudomonas fluorescens, and Pseudomonas putida, ciprofloxacin and trovafloxacin were 2 to 16 times more potent than the NFQs.
TABLE 3.
In vitro activities of three NFQ compounds and three FQs against 869 isolates belonging to Enterobacteriaceae
| Microorganism (no. of isolates tested) and antimicrobial agent | MIC (μg/ml)
|
Microorganism (no. of isolates tested) and antimicrobial agent | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Range | 50% | 90% | Range | 50% | 90% | |||
| Escherichia coli (102) | ||||||||
| PGE 9262932 | 0.03–>16 | 0.06 | 0.12 | |||||
| PGE 9509924 | 0.03–>16 | 0.12 | 0.12 | |||||
| PGE 4175997 | 0.06–>16 | 0.25 | 0.5 | |||||
| Trovafloxacin | ≤0.008–>16 | 0.016 | 0.03 | |||||
| Levofloxacin | 0.016–>16 | 0.03 | 0.06 | |||||
| Ciprofloxacin | 0.016–>16 | 0.016 | 0.016 | |||||
| Citrobacter freundii (25) | ||||||||
| PGE 9262932 | 0.06–8.0 | 0.25 | 2.0 | |||||
| PGE 9509924 | 0.12–16 | 0.25 | 2.0 | |||||
| PGE 4175997 | 0.25–>16 | 0.5 | 4.0 | |||||
| Trovafloxacin | 0.016–16 | 0.06 | 1.0 | |||||
| Levofloxacin | 0.016–8.0 | 0.06 | 0.5 | |||||
| Ciprofloxacin | 0.016–8.0 | 0.03 | 0.25 | |||||
| Enterobacter aerogenes (72) | ||||||||
| PGE 9262932 | 0.06–8.0 | 0.25 | 2.0 | |||||
| PGE 9509924 | 0.12–8.0 | 0.25 | 2.0 | |||||
| PGE 4175997 | 0.25–>16 | 0.25 | 4.0 | |||||
| Trovafloxacin | 0.016–16 | 0.06 | 0.5 | |||||
| Levofloxacin | 0.03–8.0 | 0.06 | 0.5 | |||||
| Ciprofloxacin | 0.016–16 | 0.016 | 0.25 | |||||
| Enterobacter cloacae (70) | ||||||||
| PGE 9262932 | 0.06–>16 | 0.25 | 1.0 | |||||
| PGE 9509924 | 0.016–16 | 0.25 | 1.0 | |||||
| PGE 4175997 | 0.12–>16 | 0.5 | 2.0 | |||||
| Trovafloxacin | ≤0.008–16 | 0.06 | 0.25 | |||||
| Levofloxacin | 0.016–16 | 0.06 | 0.25 | |||||
| Ciprofloxacin | ≤0.008–16 | 0.016 | 0.25 | |||||
| Klebsiella pneumoniae (122) | ||||||||
| PGE 9262932 | 0.03–>16 | 0.25 | 4.0 | |||||
| PGE 9509924 | 0.06–>16 | 0.25 | 4.0 | |||||
| PGE 4175997 | 0.12–>16 | 0.5 | 8.0 | |||||
| Trovafloxacin | ≤0.008–>16 | 0.06 | 1.0 | |||||
| Levofloxacin | 0.016–>16 | 0.06 | 1.0 | |||||
| Ciprofloxacin | ≤0.008–>16 | 0.03 | 0.5 | |||||
| Klebsiella oxytoca (50) | ||||||||
| PGE 9262932 | 0.12–8.0 | 0.12 | 1.0 | |||||
| PGE 9509924 | 0.12–8.0 | 0.12 | 1.0 | |||||
| PGE 4175997 | 0.25–>16 | 0.5 | 2.0 | |||||
| Trovafloxacin | 0.016–4.0 | 0.03 | 0.25 | |||||
| Levofloxacin | 0.03–2.0 | 0.03 | 0.25 | |||||
| Ciprofloxacin | 0.016–2.0 | 0.016 | 0.12 | |||||
| Serratia marcescens (120) | ||||||||
| PGE 9262932 | 0.06–>16 | 1.0 | 4.0 | |||||
| PGE 9509924 | 0.12–>16 | 0.5 | 4.0 | |||||
| PGE 4175997 | 0.25–>16 | 2.0 | 16 | |||||
| Trovafloxacin | 0.03–>16 | 0.5 | 4.0 | |||||
| Levofloxacin | 0.03–>16 | 0.12 | 1.0 | |||||
| Ciprofloxacin | 0.016–>16 | 0.06 | 1.0 | |||||
| Proteus mirabilis (120) | ||||||||
| PGE 9262932 | 0.12–>16 | 0.5 | 1.0 | |||||
| PGE 9509924 | 0.12–>16 | 0.5 | 2.0 | |||||
| PGE 4175997 | 0.25–>16 | 1.0 | 2.0 | |||||
| Trovafloxacin | 0.06–>16 | 0.25 | 0.5 | |||||
| Levofloxacin | 0.03–>16 | 0.06 | 0.12 | |||||
| Ciprofloxacin | 0.016–>16 | 0.016 | 0.06 | |||||
| Proteus vulgaris (44) | ||||||||
| PGE 9262932 | 0.06–2.0 | 0.25 | 1.0 | |||||
| PGE 9509924 | 0.06–2.0 | 0.25 | 1.0 | |||||
| PGE 4175997 | 0.12–4.0 | 0.5 | 2.0 | |||||
| Trovafloxacin | 0.016–4.0 | 0.12 | 0.25 | |||||
| Levofloxacin | 0.06–0.12 | 0.03 | 0.06 | |||||
| Ciprofloxacin | 0.016–0.06 | 0.016 | 0.03 | |||||
| Morganella morganii (40) | ||||||||
| PGE 9262932 | 0.06–>16 | 0.25 | 1.0 | |||||
| PGE 9509924 | 0.06–>16 | 0.25 | 2.0 | |||||
| PGE 4175997 | 0.12–>16 | 1.0 | 2.0 | |||||
| Trovafloxacin | 0.03–>16 | 0.25 | 0.5 | |||||
| Levofloxacin | 0.016–8.0 | 0.03 | 0.12 | |||||
| Ciprofloxacin | ≤0.008–16 | 0.016 | 0.06 | |||||
| Providencia rettgeri (28) | ||||||||
| PGE 9262932 | 0.12–16 | 0.5 | 2.0 | |||||
| PGE 9509924 | 0.12–8.0 | 0.25 | 4.0 | |||||
| PGE 4175997 | 0.25–>16 | 1.0 | 2.0 | |||||
| Trovafloxacin | 0.03–>16 | 0.25 | 4.0 | |||||
| Levofloxacin | 0.03–8.0 | 0.12 | 2.0 | |||||
| Ciprofloxacin | 0.016–4.0 | 0.016 | 0.5 | |||||
| Providencia stuartii (36) | ||||||||
| PGE 9262932 | 0.06–>16 | 0.5 | >16 | |||||
| PGE 9509924 | 0.12–>16 | 1.0 | >16 | |||||
| PGE 4175997 | 0.25–>16 | 2.0 | >16 | |||||
| Trovafloxacin | 0.03–>16 | 0.25 | >16 | |||||
| Levofloxacin | 0.06–>16 | 0.5 | >16 | |||||
| Ciprofloxacin | 0.016–>16 | 0.12 | >16 | |||||
| Shigella spp. (20)a | ||||||||
| PGE 9262932 | 0.03–0.12 | 0.06 | 0.12 | |||||
| PGE 9509924 | 0.06–0.25 | 0.25 | 0.25 | |||||
| PGE 4175997 | 0.03–0.25 | 0.25 | 0.25 | |||||
| Trovafloxacin | ≤0.008–0.06 | 0.03 | 0.03 | |||||
| Levofloxacin | 0.016–0.06 | 0.06 | 0.06 | |||||
| Ciprofloxacin | ≤0.016–0.06 | ≤0.016 | 0.03 | |||||
| Salmonella enteritidis (20) | ||||||||
| PGE 9262932 | 0.12–0.25 | 0.25 | 0.25 | |||||
| PGE 9509924 | 0.25–0.5 | 0.25 | 0.5 | |||||
| PGE 4175997 | 0.25–0.5 | 0.5 | 0.5 | |||||
| Trovafloxacin | 0.03–0.06 | 0.03 | 0.06 | |||||
| Levofloxacin | 0.03–0.06 | 0.06 | 0.06 | |||||
| Ciprofloxacin | ≤0.016–0.03 | ≤0.016 | 0.03 | |||||
| All Enterobacteriaceae | ||||||||
| Cips (816)b | ||||||||
| PGE 9262932 | 0.03–8.0 | 0.12 | 1.0 | |||||
| PGE 9509924 | 0.016–16 | 0.25 | 1.0 | |||||
| PGE 4175997 | 0.03–16 | 0.5 | 4.0 | |||||
| Trovafloxacin | ≤0.008–16 | 0.06 | 0.5 | |||||
| Levofloxacin | ≤0.016–4.0 | 0.06 | 0.25 | |||||
| Ciprofloxacin | ≤0.016–0.5 | ≤0.016 | 0.12 | |||||
| Cipr (43)b | ||||||||
| PGE 9262932 | 4.0–>16 | 16 | >16 | |||||
| PGE 9509924 | 4.0–>16 | 16 | >16 | |||||
| PGE 4175997 | 8.0–>16 | >16 | >16 | |||||
| Trovafloxacin | 1.0–>16 | 16 | >16 | |||||
| Levofloxacin | 2.0–>16 | 8.0 | >16 | |||||
| Ciprofloxacin | 4.0–>16 | 8.0 | >16 | |||||
Includes six S. boydii, five S. dysenteriae, six S. flexneri, and three S. sonnei isolates.
Excludes 10 ciprofloxacin-intermediate (MIC, 2.0 μg/ml) strains: one C. freundii, one E. aerogenes, two K. pneumoniae, two K. oxytoca, one P. mirabilis, and three S. marcescens strains.
H. influenzae, Moraxella catarrhalis, and N. gonorrhoeae are three other gram-negative species that were uniformly susceptible to the FQs; the NFQs were also active at low concentrations (Table 2).
All three nonflourinated quinolones are potentially useful antimicrobial agents. The NFQ agents demonstrated broad spectrums of antibacterial activity that are similar to that of trovafloxacin. They are more active than trovafloxacin against gram-positive pathogens, somewhat less active against the Enterobacteriaceae, and have little activity against Pseudomonas spp. The absence of fluorine atoms at position 6 of the quinolone nucleus in these molecules might reduce the frequency of toxic side effects but that remains to be demonstrated. Clearly, the antibacterial activity of the quinolone compounds is not necessarily related to the fluorine component alone. Providing that appropriate pharmacokinetic properties (N. L. Mallalieu, D. H. Ellis, P. H. Zoutendam, M. Gavin, M. K. Dirr, M. J. Martin, and B. Ledoussal, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F550, p. 305, 1999) can be achieved in humans and that adverse events are minimized (2, 3), these agents might fill a valuable role in today's ever-increasing concerns about infections due to antibiotic-resistant bacteria.
Acknowledgments
This study was supported by a grant from Procter & Gamble Pharmaceuticals, Cincinnati, Ohio.
REFERENCES
- 1.Barry A L. In vitro activity of the quinolones and related compounds. In: Siporin C, Heifetz C L, Domagala J M, editors. The new generation of quinolones. New York, N.Y: Marcel Dekker, Inc; 1990. pp. 79–105. [Google Scholar]
- 2.Hooper D C. New uses for new and old quinolones and the challenge of resistance. Clin Infect Dis. 2000;30:243–254. doi: 10.1086/313677. [DOI] [PubMed] [Google Scholar]
- 3.Lipsky B A, Baker C A. Fluoroquinolone toxicity profiles: a review focusing on newer agents. Clin Infect Dis. 1999;28:352–364. doi: 10.1086/515104. [DOI] [PubMed] [Google Scholar]
- 4.National Committee for Clinical Laboratory Standards. Methods for antimicrobial susceptibility tests of anaerobic bacteria, 4th ed. Approved standard M11–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 5.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7–A5. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]