Abstract
Aims
Bone turnover markers (BTMs) follow distinct trends after fractures and limited evidence suggests differential levels in BTMs in patients with delayed healing. The effect of vitamin D, and other factors that influence BTMs and fracture healing, is important to elucidate the use of BTMs as surrogates of fracture healing. We sought to determine whether BTMs can be used as early markers of delayed fracture healing, and the effect of vitamin D on BTM response after fracture.
Methods
A total of 102 participants aged 18 to 50 years (median 28 years (interquartile range 23 to 35)), receiving an intramedullary nail for a tibial or femoral shaft fracture, were enrolled in a randomized controlled trial comparing vitamin D3 supplementation to placebo. Serum C-terminal telopeptide of type I collagen (CTX; bone resorption marker) and N-terminal propeptide of type I procollagen (P1NP; bone formation marker) were measured at baseline, six weeks, and 12 weeks post-injury. Clinical and radiological fracture healing was assessed at three months.
Results
CTX and P1NP concentrations peaked at six weeks in all groups. Elevated six-week CTX and P1NP were associated with radiological healing at 12 weeks post-injury (odds ratio (OR) 10.5; 95% confidence interval 2.71 to 53.5, p = 0.002). We found no association between CTX or P1NP and functional healing. Baseline serum 25(OH)D showed a weak inverse relationship with P1NP (p = 0.036) and CTX (p = 0.221) at 12 weeks, but we observed no association between vitamin D supplementation and either BTM.
Conclusion
Given the association between six-week BTM concentrations and three-month radiological fracture healing, CTX and P1NP appear to be potential surrogate markers of fracture healing.
Cite this article: Bone Joint Res 2022;11(4):239–250.
Keywords: Fracture, Bone turnover marker, Vitamin D, CTX, P1NP, fracture healing, femur fractures, tibia, Bone turnover markers, Serum, femoral shaft fractures, type I collagen, randomized controlled trial
Article focus
To assess the association between C-terminal telopeptide of type I collagen (CTX) and N-terminal propeptide of type I procollagen (P1NP) and early radiological fracture healing.
To assess the association between CTX and P1NP and early functional fracture healing.
To assess the association between CTX and P1NP and vitamin D.
Key messages
Elevated six-week CTX and P1NP were associated with radiological healing at 12 weeks post-injury.
No association was observed between CTX or P1NP and functional fracture healing.
A limited inverse relationship was observed between CTX and P1NP and baseline serum 25(OH)D.
Strengths and limitations
Prospective randomized trial of tibial and femoral shaft fractures, reducing bias from pre-analytical variation of CTX and P1NP.
Limited follow-up to three months.
Introduction
Nonunion after fracture results in high burdens to patients and healthcare systems, and early identification continues to be a challenge. 1 Bone turnover markers (BTMs) have been demonstrated to follow distinct trends following several fracture types, 2-7 and limited evidence suggests that differential levels of BTMs are seen in patients with delayed healing. 1,8-11 While BTMs are an appealing potential early marker of delayed healing, inadequate evidence exists to support using BTMs as surrogate markers of fracture healing. 10
Vitamin D is involved in several stages of the fracture healing process. 12 High rates of hypovitaminosis D have been observed in fracture populations; 13 however, inconclusive evidence supports a relationship between hypovitaminosis D, 12,14-16 or vitamin D supplementation, 15,17 and fracture healing. Despite the lack of evidence, many surgeons believe that vitamin D supplementation is justified for fracture healing, and some surgeons have suggested that vitamin D supplementation should be given to all fracture patients regardless of vitamin D status. 18
Further, the effect of vitamin D and other factors that may influence both BTMs and clinical healing is important to elucidate, in order to use BTMs as surrogates of fracture healing. 19,20 The BTMs C-terminal telopeptide of type I collagen (CTX) and N-terminal propeptide of type I procollagen (P1NP) have been recommended as reference BTMs for predicting fracture risk and monitoring of osteoporosis, 21,22 and have been characterized to acutely rise following fracture. 22,23 An inverse relationship with vitamin D supplementation and these BTMs has been observed in some studies, 24-27 while others found no difference. 28,29 Similarly, serum 25(OH)D levels have been observed to be inversely correlated with CTX and P1NP in several studies, albeit with some heterogeneity of results. 7,30-33 However, few studies investigating the relationship between vitamin D and BTMs were from fracture patients, and these studies consisted of low-energy fractures without fracture healing outcomes. 7,34-37
Given this unclear relationship between CTX and P1NP, fracture healing, and vitamin D, we aimed to explore five questions: 1) do BTM values change in the 12 weeks after a tibial or femoral shaft fracture?; 2) does an association exist between baseline serum 25(OH)D levels and BTM response?; 3) does an association exist between vitamin D supplementation and BTM response?; 4) does an association exist between BTM and early functional healing?; and 5) does an association exist between BTM and early radiological healing?
Methods
Trial design
This analysis is part of a larger study, the Vita-Shock trial: a four-arm, double-blinded randomized controlled trial (RCT) comparing varying doses of vitamin D3 supplementation to placebo in patients with tibial or femoral shaft fractures, with no assumption of efficacy of vitamin D3 supplementation improving fracture healing. The trial objective methods have been described in detail previously. 38 The trial was registered with ClinicalTrials.gov, number NCT02786498, and was approved by the Hamilton Integrated Research Ethics Board (2017-1952) and the University of Maryland Institutional Review Board (HP-00069705).
Participant inclusion and exclusion
We included adult patients, aged 18 to 50 years, with a closed or low-grade open (Gustilo-Anderson class I or II) tibial or femoral shaft fracture treated by reamed intramedullary nailing at the R Adams Cowley Shock Trauma Center at the University of Maryland. All participants were enrolled within seven days of injury. We excluded patients with osteoporosis, stress fractures, serum calcium > 10.5 mg/dl, atypical femur fractures, pathological fractures secondary to bone lesion, underlying disorders of bone metabolism, hyperhomocysteinemia, or vitamin D allergies. Participants were included regardless of baseline vitamin D levels. The full exclusion criteria are listed in the protocol paper. 38 Written informed consent was obtained from all participants.
Intervention and randomization
Treatment allocation was double-blinded and included four treatment arms: 1) 150,000 IU (high) loading dose plus placebo daily dose; 2) 4,000 IU (high) daily plus placebo loading dose; 3) 600 IU (low) daily plus placebo loading dose; or 4) placebo loading and daily doses (Figure 1). Participants received active or placebo loading doses within one week of injury and six weeks (± two weeks) post-injury, and doses daily for three months beginning within one week of injury. The randomization was stratified by open versus closed fractures, and by tibia versus femur fractures.
Fig. 1.
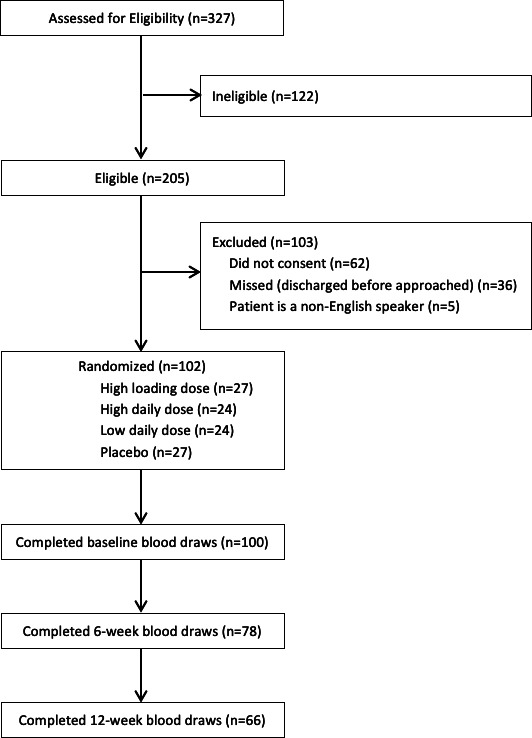
Participant flow diagram.
Participant characteristics
We enrolled 102 participants into the trial, with 27 allocated to the high loading dose group, 24 to the high daily dose group, 24 to the low daily dose group, and 27 to the placebo group. A total of 100 participants (98%) completed bloodwork at baseline, 78 (76%) at six weeks, and 66 (65%) at 12 weeks (Figure 1). The median age of study participants was 28 years (IQR 23 to 35) (Table I). The majority of the patients were male (n = 70, 69%) and were African-American (n = 47, 46%) or White (n = 48, 47%). A total of 41 (40%) patients had tibial shaft fractures and 61 (60%) patients had femoral shaft fractures. Overall 27 (26%) patients had an open fracture. A total of 47 (46%) had additional fractures, with 16 (16%) upper limb, 32 (31%) lower limb, and 9 (9%) spine. The most common other fractures were femur (n = 8, 8%), fibula (n = 8, 8%), hand (n = 7, 7%), tibia (n = 6, 6%), and radius (n = 6, 6%).
Table I.
Patient characteristics.
| Characteristic | Data (n = 102) |
|---|---|
| Median age, yrs (IQR) | 28 (23 to 35) |
| Female, n (%) | 32 (31.4) |
| Race/ethnicity, n (%) | |
| White/Caucasian | 48 (47.1) |
| Black/African-American | 47 (46.1) |
| Hispanic/Latino | 5 (4.9) |
| South Asian | 1 (1.0) |
| East Asian | 1 (1.0) |
| Fracture location, n (%) | |
| Tibia | 41 (40.2) |
| Femur | 61 (59.8) |
| Open fracture, n (%) | 27 (26.5) |
| Soft-tissue injury, n (%) | |
| Gustilo Type I | 14 (13.7) |
| Gustilo Type II | 13 (12.7) |
| Tscherne Class 0 | 18 (17.6) |
| Tscherne Class 1 | 42 (41.2) |
| Tscherne Class 2 | 12 (11.8) |
| Tscherne Class 3 | 3 (2.9) |
| Additional fractures, n (%) | 47 (46.1) |
| Upper limb | 16 (15.7) |
| Lower limb | 32 (31.4) |
| Spine | 9 (8.8) |
| Median serum albumin, g/dl (IQR) | 4.30 (4.00 to 4.60) |
| Median serum 25(OH)D, μg/l (IQR) | 18.4 (13.4 to 25.6) |
| Vitamin D deficient, 25(OH)D < 20 μg/l, n (%) | 57 (55.9) |
| Median CTX, μg/l (IQR) | 0.34 (0.27 to 0.46) |
| Median P1NP, μg/l (IQR) | 41.1 (30.0 to 55.0) |
CTX, C-terminal telopeptide of type I collagen; IQR, interquartile range; P1NP, N-terminal propeptide of type I procollagen.
Outcomes and measures
The BTMs CTX and P1NP were the variables of primary interest. CTX is a marker of bone resorption and P1NP is a marker of bone formation. 3 CTX and P1NP have been recommended as the reference BTMs for predicting fracture risk and monitoring of osteoporosis, 21,22 and have been characterized to acutely rise following fracture. 22,23 CTX and P1NP were measured at baseline (within seven days of the fracture), six weeks, and 12 weeks post-injury. Several other pre-analytic factors have been shown to influence CTX and P1NP, including age, sex, smoking status, and alcohol consumption, 22 which were abstracted from the patient charts by study personnel. Patients with underlying bone metabolic disease were excluded as described above. Blood for serum 25(OH)D levels was drawn concurrently with BTM blood draws.
Clinical fracture healing was assessed at three months by the treating surgeon using the Function IndeX for Trauma (FIX-IT). 39 FIX-IT assesses weightbearing and pain in patients with tibia and femur fractures on a scale of 4 (worst) to 12 (best). We considered a score of 12 to indicate functional healing. Radiological healing was assessed at three months using the modified Radiographic Union Score for Tibial fractures (mRUST) 40,41 by an independent orthopaedic surgeon who was blinded to the treatment allocation. The mRUST assesses radiological healing based on callus formation on four bone cortices on a scale from 4 (no callus) to 16 (remodelled). We considered a score of 12 or higher at three months to signal early radiological healing.
Blood samples
All blood samples were collected at enrolment and at clinic follow-up in gold top serum separator tubes, and were immediately refrigerated. Laboratory personnel at the University of Maryland’s Muscle Research Laboratory processed the samples on the same day and stored them in a -80°C freezer. Upon completion of all blood work for the study, the serum samples were transferred to the Institute for Clinical and Translational Research Clinical Research Unit Core Laboratory to be analyzed as a single batch to eliminate inter-batch assay variability. Immunoassays for bone metabolism biomarkers were obtained from ImmunoDiagnostic Systems (UK). Serum 25(OH)D levels were measured using a radioimmunoassay that has a sensitivity of 0.3 nmol/l, an intra-assay coefficient of variance of 5.19%, and an inter-assay coefficient of variance of 7.90%. Serum levels of CTX were determined using an enzyme-linked immunosorbent assay that has a sensitivity of 20 pg/ml, an intra-assay coefficient of variance of 8.15%, and an inter-assay coefficient of variance of 10.31%. P1NP was measured by a radioimmunoassay that had a sensitivity of 2.0 ng/ml, an intra-assay coefficient of variance of 4.14%, and an inter-assay coefficient of variance of 2.74%. The results of the analyses were sent to the Center for Evidence-Based Orthopaedics to be added to the study database, and included within the final data analysis.
Statistical analysis
The planned sample size for this phase II pilot RCT was 96 patients (24 patients per treatment group). With an a priori increased acceptable type I error threshold of 20% (α), this sample size would provide 80% power to detect a mean difference of 20% in the BTMs. Expanded details of the sample size justification have been published previously in a protocol manuscript. 38
Participant demographics and characteristics were described as medians with interquartile ranges for continuous variables and counts with proportions for categorical variables. Given the right-skewed distribution of CTX and P1NP values, we used log-transformed values for all analyses. We used random intercepts in mixed-effects models that allow each patient to have a unique baseline value, which removes this variance from our estimate and provides more precision in our sample mean estimates (the model slopes) but does not change the interpretation of the estimates. We constructed the following models to answer our five research questions.
Do BTM values change in the 12 weeks after a tibial or femoral shaft fracture?
We fit linear mixed-effects models to determine the mean change in CTX and P1NP values at six weeks and 12 weeks post-injury compared to baseline values. We coded each patient as a unique random intercept in this model to account for baseline variation.
Does an association exist between baseline serum 25(OH)D levels and BTM response?
We fit linear mixed-effects models with the log-transformed BTM as the dependent variable. Given the non-linear association between time and BTMs, we included time as a non-linear fixed effect using natural splines. Patients were uniquely coded as a random intercept. Baseline serum 25(OH)D was modelled as a continuous variable, as well as a binary indicator, which stratified patients above and below the vitamin D deficiency threshold of 20 μg/l.
Does an association exist between vitamin D supplementation and BTM response?
These models were similar to our serum 25(OH)D level models, except we used a four-level indicator for the assigned vitamin D supplementation regimen as the primary independent variable.
Does an association exist between BTM and early functional healing?
To determine the association between BTMs and early functional healing, we fit multilevel generalized linear models with binomial distributions. The binary dependent variable was a FIX-IT score of 12 at 12 weeks post-injury. The independent variables were the log-transformed BTM of interest and the four-level indicator for vitamin D3 supplementation group. We included time as a random effect and a unique patient identifier as the random intercept.
Does an association exist between BTM and early radiological healing?
This model was very similar to model four, except the dependent variable was a modified RUST score of 12 or more at 12 weeks post-injury to indicate early radiological healing. Given the observed difference in BTMs among patients with early radiological healing at six weeks post-injury, we also fit a generalized linear model with a binomial distribution that includes only BTM data observed at six weeks post-injury.
Our analyses included all enrolled patients. Missing FIX-IT and mRUST data were imputed using multiple imputations. Missing BTM data were assumed to be missing at random in our mixed-effects models. We did not adjust our significance level to account for multiple testing, and all results should be considered exploratory. All p-values were calculated using t-tests with Satterthwaite’s method or Wald z-test as appropriate, and statistical significance was set at p < 0.05. Analysis was performed using R (version 4.0.0, R Foundation for Statistical Computing, Austria).
Results
Changes in CTX and P1NP over time
The median baseline serum CTX level was 0.34 μg/l and ranged from 0.09 μg/l to 2.8 μg/l. At six weeks after injury, serum CTX levels were 64% higher (95% confidence interval (CI) 47% to 82%, p < 0.001, t-tests with Satterthwaite’s method) than baseline (Supplementary Figure a). The 12-week serum CTX levels were 32% higher (95% CI 18% to 48%, p < 0.001, t-tests with Satterthwaite’s method) than baseline (Table II). The median baseline serum P1NP level was 41.13 μg/l and ranged from 9.51 μg/l to 208.29 μg/l. Six weeks after injury, serum P1NP levels were 240% higher (95% CI 206% to 278%, p < 0.001, t-tests with Satterthwaite’s method) than baseline levels. Serum P1NP levels were 217% higher (95% CI 183% to 255%, p < 0.001, t-tests with Satterthwaite’s method) at 12 weeks compared to baseline levels.
Table II.
Mean percentage change in C-terminal telopeptide of type I collagen and N-terminal propeptide of type I procollagen value compared to baseline.
| Variable | Mean, μg/l (SD) | Mean percentage change from baseline (95% CI) | p-value* |
|---|---|---|---|
| CTX | |||
| Baseline | 0.42 (0.32) | N/A | N/A |
| 6 wks | 0.71 (0.44) | 64 (47 to 82) | < 0.001 |
| 12 wks | 0.55 (0.25) | 32 (18 to 48) | < 0.001 |
| P1NP | |||
| Baseline | 45.5 (26.1) | N/A | N/A |
| 6 wks | 154.0 (76.1) | 240 (206 to 278) | < 0.001 |
| 12 wks | 147.0 (64.9) | 217 (183 to 255) | < 0.001 |
p < 0.05 indicates statistical significance.
t-tests with Satterthwaite’s method.
CI, confidence interval; CTX, C-terminal telopeptide of type I collagen; N/A, not applicable; P1NP, N-terminal propeptide of type I procollagen; SD, standard deviation.
Association of baseline serum 25(OH)D levels with CTX and P1NP
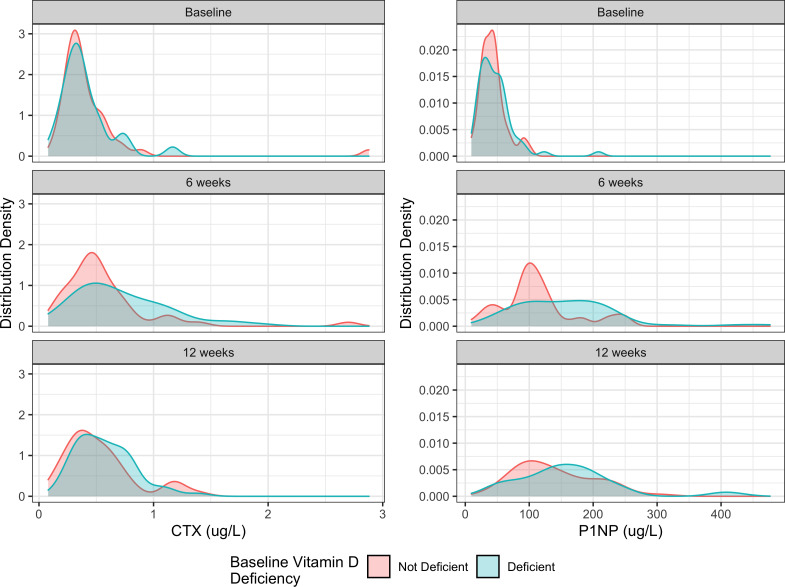
The data suggest that baseline serum 25(OH)D levels were not associated with CTX markers. Specifically, we observed that each 1 ng/ml increase in baseline serum 25(OH)D corresponded with a 0.7% decrease in mean CTX levels over 12 weeks from injury (95% CI -1.9% to 0.0%, p = 0.221, t-tests with Satterthwaite’s method). There was no association between baseline vitamin D deficiency and mean CTX levels (difference, 0%, 95% CI -17% to 22%, p = 0.976, t-tests with Satterthwaite’s method) (Figure 2). Each 1 ng/ml increase in baseline serum 25(OH)D was associated with a 1.1% decrease in the patient’s mean P1NP levels from baseline to 12 weeks post-injury (95% CI -2.1% to -0.1%, p = 0.036, t-tests with Satterthwaite’s method). Patients with baseline vitamin D deficiency had a 12% increase in their mean P1NP levels, yet this estimate had considerable uncertainty (95% CI -5.4% to 33%, p = 0.193, t-tests with Satterthwaite’s method).
Fig. 2.
Distribution of serum C-terminal telopeptide of type I collagen (CTX) and N-terminal propeptide of type I procollagen (P1NP) at baseline, six weeks post-injury, and 12 weeks post-injury stratified by the patient’s baseline vitamin D deficiency status.
Association of vitamin D3 supplementation with CTX and P1NP
We found no association between vitamin D3 supplementation and mean CTX levels from injury to 12 weeks post-injury (Figure 3). Specifically, the high loading dose corresponded with a 2.6% reduction (-24.5% to 25%, p = 0.836, t-tests with Satterthwaite’s method) in CTX levels compared to the placebo group. A high daily dose corresponded with a 4.2% reduction (95% CI -26.2% to 24%, p = 0.746, t-tests with Satterthwaite’s method) in CTX levels compared to the placebo group, and a low daily regimen corresponded with a 24% increase (95% CI -4.6% to 61%, p = 0.112, t-tests with Satterthwaite’s method) in CTX levels relative to placebo. Similarly, we found no association between vitamin D3 supplementation and mean P1NP levels in the 12 weeks after injury. The P1NP levels of patients allocated to the high loading dose were 5.9% lower (95% CI -25.4% to 19%, p = 0.607, t-tests with Satterthwaite’s method) than the placebo group. The P1NP levels of patients assigned to high daily doses were 9% higher (95% CI -14.4% to 38%, p = 0.497, t-tests with Satterthwaite’s method) than placebo, and patients assigned to low daily doses were 15% higher (95% CI -9.4% to 47%, p = 0.249, t-tests with Satterthwaite’s method) than placebo.
Fig. 3.
Distribution of serum C-terminal telopeptide of type I collagen (CTX) and N-terminal propeptide of type I procollagen (P1NP) at baseline, six weeks post-injury, and 12 weeks post-injury stratified by vitamin D3 supplementation treatment allocation.
Association of CTX or P1NP and vitamin D3 supplementation with early functional healing
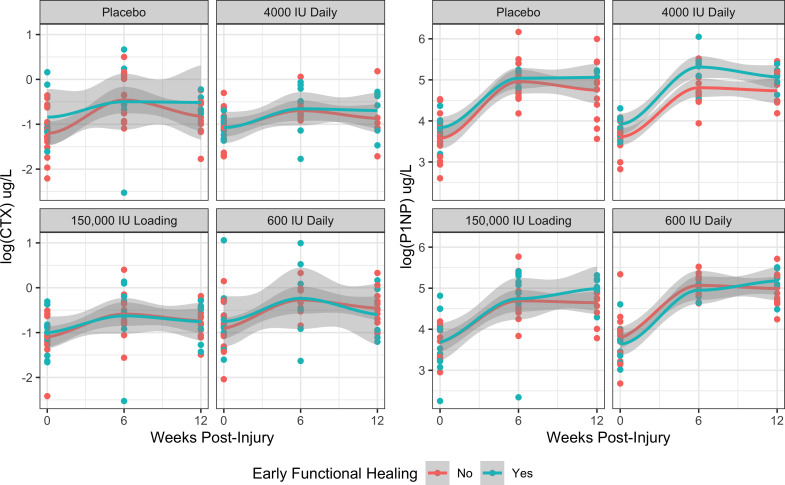
We found no evidence that CTX concentrations in the 12 weeks after injury and the allocated vitamin D3 supplementation increased the odds of early functional healing (FIX-IT = 12) (Figure 4). A 1 log μg/l increase in mean CTX corresponded with an 82% increase in the odds of early functional healing (odds ratio (OR) 1.82; 95% CI 0.76 to 4.40, p = 0.181, Wald z-test). In that same model, neither a high loading dose (OR 1.98, 95% CI 0.58 to 6.78, p = 0.276, Wald z-test), a high daily dose (OR 1.34; 95% CI 0.38 to 4.71, p = 0.651, Wald z-test), or a low daily dose (OR 1.41; 95% CI 0.39 to 5.09, p = 0.595, Wald z-test) was associated with increased odds of early functional healing. The results of our P1NP model were similar. We found no association between P1NP and early functional healing (OR 1.49 per 1 log μg/l increase; 95% CI 0.62 to 3.56, p = 0.377, Wald z-test). There was also no evidence that vitamin D3 supplementation significantly increased early functional healing in the P1NP model (high loading – OR 2.00; 95% CI 0.59 to 6.81, p = 0.271, Wald z-test; high daily – OR 1.32; 95% CI 0.38 to 4.61, p = 0.657, Wald z-test; low daily – OR 1.59; 95% CI 0.46 to 5.58, p = 0.470, Wald z-test; all compared to placebo).
Fig. 4.
Mean C-terminal telopeptide of type I collagen (CTX) and N-terminal propeptide of type I procollagen (P1NP) levels within 12 weeks of injury stratified by vitamin D3 supplementation treatment allocation and early functional healing (FIX-IT = 12).
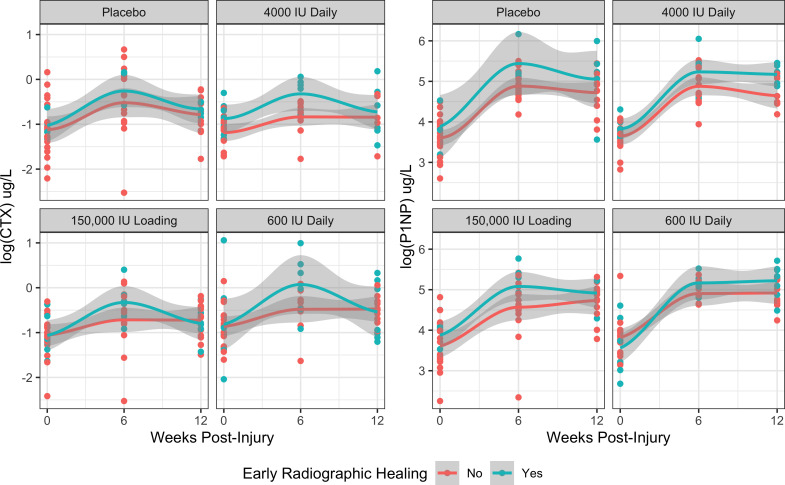
Association of CTX or P1NP and vitamin D3 supplementation with early radiological healing
We found no evidence that the mean CTX concentrations in the 12 weeks after were associated with early radiological healing (mRUST ≥ 12) (OR 1.61 per 1 log μg/l increase; 95% CI 0.64 to 4.03, p = 0.309, Wald z-test) (Figure 5). In that same model, we did not observe significant associations between the patients’ vitamin D3 supplementation group and early radiological healing (high loading – OR 1.94; 95% CI 0.48 to 7.83, p = 0.353, Wald z-test; high daily – OR 3.31; 95% CI 0.81 to 13.5, p = 0.095, Wald z-test; low daily – OR 2.44; 95% CI 0.58 to 10.2, p = 0.221, Wald z-test; all compared to placebo). However, we did observe a strong association between CTX levels at six weeks with early radiological healing (OR 5.39; 95% CI 1.75 to 21.0, p = 0.008, Wald z-test). Mean P1NP levels within 12 weeks of injury were not associated with an increased odds of early radiological healing (OR 1.50 per 1 log μg/l increase; 95% CI 0.59 to 3.83, p = 0.396, Wald z-test). In the same model, we did not observe an association between vitamin D3 supplementation and early radiological healing (high loading – OR 1.94; 95% CI 0.48 to 7.90, p = 0.352, Wald z-test; high daily – OR 3.28; 95% CI 0.80 to 13.5, p = 0.100, Wald z-test; low daily – OR 2.68; 95% CI 0.65 to 11.0, p = 0.172, Wald z-test; all compared to placebo). However, each 1 log μg/l increase in P1NP concentrations measured at six weeks post-injury was associated with a ten-fold increase in the odds of early radiological healing (OR 10.5; 95% CI 2.71 to 53.5, p = 0.002, Wald z-test).
Fig. 5.
Mean C-terminal telopeptide of type I collagen (CTX) and N-terminal propeptide of type I procollagen (P1NP) levels within 12 weeks of injury stratified by vitamin D3 supplementation treatment allocation and early radiological healing (mRUST ≥ 12).
Discussion
The results of this study add confirmatory evidence that CTX and P1NP serum levels are elevated between six and 12 weeks after tibial and femoral shaft fractures relative to baseline measures. We demonstrated that baseline serum 25(OH)D trended towards an inverse relationship to CTX and P1NP in these fracture patients but found no evidence of an association between vitamin D deficiency and BTMs within 12 weeks of injury. We also found no evidence that vitamin D3 supplementation affects BTMs during the 12 weeks post-injury. The mean CTX and P1NP concentrations over the 12-week period were not associated with early functional or radiological healing. However, elevated CTX and P1NP levels at six weeks after injury had a strong association with radiological healing at 12 weeks post-injury.
CTX and P1NP after fracture and relationship with fracture healing
Trends in CTX and P1NP following fracture have been consistently demonstrated. Studies in tibial and femoral shaft fractures have shown a peak in CTX from two to four weeks post-fracture and remain elevated through 12 to 24 weeks, 5,9 and P1NP peaks from four to 12 weeks with elevated levels persisting a year after fracture. 5,8,9 These trends are similar to the larger body of evidence from fractures in older patients with several other fracture types. 2,4,7,23 Our results are in agreement with these reports demonstrating maximally elevated CTX and P1NP at six weeks.
Given the high burden of nonunion, 42 and current practice of diagnosing nonunion through clinical exam and radiological findings several months after fracture, a sensitive early marker has significant potential benefit. As CTX and P1NP follow distinct trends during normal fracture healing, it has been hypothesized that these markers may follow distinct trends in patients with delayed healing or those who progress to nonunions. Several studies have investigated BTMs as early indicators of fracture healing, although strong evidence supporting this use has not been accumulated. 10,11 Moghaddam et al, 9 in a cohort consisting primarily of tibia fractures, observed lower levels of CTX in the first week following fractures in those with delayed fracture healing compared to those with normal fracture healing. No differences were observed in P1NP between groups. In contrast, Kumar et al 8 recently presented evidence that suggests P1NP was lower at eight, 12, and 24 weeks in those who experienced delayed union after tibia fractures. They did not measure CTX, but found no difference in N-terminal telopeptide of type I collagen (NTX), another marker of bone resorption, between groups. Our study observed significantly lower values of both CTX and P1NP at six weeks in those with delayed radiological healing at 12 weeks. Lower CTX in patients with delayed healing corresponds with Moghaddam et al’s findings, 9 although they observed a difference in CTX at one week but not at four or eight weeks, whereas we observed a difference at six weeks. We did not measure CTX at one week, so a difference may have existed that was not captured in the present study. Moghaddam et al’s sample of 15 delayed healing and 15 controls may have been too small to detect a difference at four or eight weeks. Kumar et al 8 observed lower P1NP in their delayed healing group at eight through 24 weeks, while we only noted this association at six weeks. Overall, our observed changes in CTX and P1NP at six-week follow-up suggests that these markers may provide earlier indication of delayed fracture healing than three-month radiographs.
CTX and P1NP relationship with vitamin D
Vitamin D deficiency has been associated with higher levels of BTMs, presumably due to secondary hyperparathyroidism and the resulting elevated bone turnover, although the literature specific to CTX and P1NP is limited. 22 The role of vitamin D supplementation in clinical outcomes after fracture has been a challenge to elucidate. 14 Similarly, the relationship between vitamin D supplementation and CTX and P1NP has shown varying results. Studies in patients without fractures have demonstrated an inverse relationship between vitamin D supplementation and CTX or P1NP, 24-27 or no difference. 28,29 In hip fracture populations, Hitz et al 37 found an inverse relationship with supplementation and P1NP, and Torbergsen et al 36 found no difference between supplementation and P1NP or CTX. Significant heterogeneity in vitamin D dosing existed among these studies. Our data are from the first prospective randomized trial to combine both clinical and biological measures of fracture healing with multiple commonly prescribed vitamin D doses in patients after fracture. We found no evidence of an association between vitamin D supplementation and CTX or P1NP in this fracture population. While Hitz et al 37 found an inverse relationship between vitamin D supplementation and P1NP, many differences exist between our study cohorts that influence BTMs including vitamin D dose, baseline vitamin D levels, 24 age, 22 and fracture type. 4,5 Results in non-fracture patients have shown an inverse relationship, 24-27 or noted no difference, 28,29 highlighting the modest association between vitamin D supplementation and CTX and P1NP.
Similar results have been observed in the relationship between serum 25(OH)D and CTX and P1NP. An inverse relationship was seen between CTX and 25(OH)D in two studies on older hip fractures, 7,43 with no association between 25(OH)D and P1NP in hip or distal radius fractures. 7,34 Literature from individuals without fractures similarly showed an inverse relationship, 30,31 or did not observe a difference. 32,33 We add to the limited evidence in younger fracture populations, showing a trend towards higher CTX and P1NP in patients with lower levels of serum 25(OH)D.
Limitations and strengths
Blood draws in this study were limited to three months. As described previously, CTX and P1NP may be elevated for at least a year. However, our data suggest that peak values occur near six weeks, consistent with previous reports. Further, three months is sufficient to monitor early signs of healing. 38,44 A potential weakness of using BTMs as predictors of fracture healing is the rapid increase after fracture. Our baseline blood draws occurred within seven days, introducing the possibility that some patients had elevated baseline BTM values compared to immediately post-fracture. 5 As previously demonstrated, peak CTX and P1NP occur later than one week post-fracture, and the initial rise occurs near two weeks. 4,5,9 Further, our blood draws were unfasted, potentially introducing variability to the CTX measurements. 22 Attrition in the sample reached approximately 35% despite multiple phone calls to try to contact study participants. However, the analyses used mixed-effects repeated-measures models and data were assumed to be missing at random, limiting the bias of missing data on the estimates. Delayed healing and nonunions are influenced by many factors, 45 such as reduction and fixation quality, which our study did not assess. Similarly, BTM response is influenced by factors such as location of fracture. 4 Our sample included tibia and femur fractures, potentially introducing variability to the BTM response. Open fractures may also exhibit differential BTM responses and have higher rates of nonunion compared to closed fractures. While our study included both low-grade open fractures and closed fractures, the increase in nonunion rates is largely driven by grade III fractures, 45 which were not included in the study cohort. Further, we conducted several tests to confirm homogeneity, and found no model that included an open fracture interaction term with fracture healing or BTM outcomes. Finally, we did not record other BTMs that have shown association with delayed healing such as the osteoclast-associated tartrate-resistant acid phosphatase (TRACP) 5b enzyme. 9,11 However, strong evidence does not support use of specific BTMs as markers of fracture healing at present. Future prospective studies may consider incorporating additional BTMs in light of previous reports. 9,11
This trial has several strengths that add confidence to the results. Multiple commonly prescribed vitamin D doses were studied, including both loading dose and daily dose regimens, allowing for generalizability to many fracture patients receiving vitamin D regardless of dosing. BTMs have several sources of pre-analytical variation; the prospective nature of this study, randomization, fracture population aged 18 to 50 years, and exclusion of many diseases that confound BTMs served to limit many sources of potential bias. The patient population of tibial and femoral shaft fractures in patients aged 18 to 50 years allows for generalizability of the results to these common fractures. However, our results may not be generalizable to other populations, such as older vitamin D-deficient patients or those treated nonoperatively, which are important areas of future investigation. Finally, our finding of differential response of CTX and P1NP in delayed union is an important contribution to the current understanding of BTMs and fracture healing.
In conclusion, CTX and P1NP concentrations increase during acute fracture healing. Elevated CTX and P1NP levels at six weeks after injury may be an early marker for radiological healing at 12 weeks post-injury. Vitamin D3 supplementation appears to have a limited effect on CTX (bone resorption marker) and P1NP (bone formation marker) responses. Our data suggest a limited relationship between baseline serum 25(OH)D levels and early CTX and P1NP measures. To date, clinically relevant BTM ranges have not been robustly defined in their relationship to either normal or impaired healing. While this study adds significantly to our understanding of these BTMs across routine and delayed healing, future studies are required to determine if there is an optimal range or pattern of change of CTX and P1NP, in combination with other factors, 42 that may be used as early predictors of nonunion.
Author contributions
C. C. Stewart: Conceptualization, Writing – original draft, Writing – review & editing.
N. N. O’Hara: Conceptualization, Formal analysis, Methodology, Project administration, Writing – original draft, Writing – review & editing.
S. Bzovsky: Data curation, Writing – review & editing.
C. Bahney: Validation, Writing – review & editing.
S. Sprague: Conceptualization, Methodology, Writing – review & editing.
G. P. Slobogean: Conceptualization, Formal analysis, Methodology, Writing – review & editing.
Funding statement
The authors disclose receipt of the following financial or material support for the research, authorship, and/or publication of this article: this work was supported by research grants from the Orthopaedic Trauma Association. Research reported in this publication was also partially supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K24AR076445. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding bodies had no role in the design of the study and collection of data and will have no role in the analysis and interpretation of data and in writing the manuscript.
ICMJE COI statement
G. P. Slobogean is a consultant for Smith & Nephew, Nuvasive, and Zimmer, and receives funding from the Patient-Centered Outcomes Research Institute and the US Department of Defense, not related to this study.
S. Sprague reports personal fees from Global Research Solutions and is a paid employee of McMaster University, not related to this study.
C. S. Bahney discloses an unpaid Board of Directors or Committee position for the Tissue Engineering and Regenerative Medicine International Society (TERMIS), Orthopaedic Research Society (ORS) International Section of Fracture Repair (ISFR), the Orthopaedic Trauma Association (OTA), receives funding from the Orthopaedic Research and Education Foundation, received royalties from the following US patent 041263: Implants using ultrasonic backscatter for sensing electrical impedance of tissue, and is a paid employee of the non-profit Steadman Philippon Research Institute (SPRI), all unrelated to this study.
Acknowledgements
Vita-Shock Investigators:
Principal Investigators: Gerard P. Slobogean (University of Maryland School of Medicine) and Sheila Sprague (McMaster University).
Co-Investigators: Jonathan D. Adachi, Mohit Bhandari, Lehana Thabane (McMaster University), and Michael F. Holick (Boston University).
McMaster University Methods Centre: Sofia Bzovsky (Project Manager and Data Analyst); Nicole Simunovic (Grants Management); Kim Madden (Unblinded Project Manager, Data Management); Taryn Scott (Unblinded Project Manager, Data Management); Andrew Duong (Unblinded Project Manager, Data Management); and Diane Heels-Ansdell (Statistical Analysis).
R Adams Cowley Shock Trauma Center, University of Maryland: Zachary D. Hannan, Daniel Connelly, Joshua Rudnicki, Andrew N. Pollak, Robert V. O’Toole, Christopher LeBrun, Jason W. Nascone, Marcus F. Sciadini, Yasmin Degani, Raymond Pensy, Theodore Manson, W. Andrew Eglseder Jr., Christopher G. Langhammer, Aaron J. Johnson, Nathan N. O’Hara, Haley Demyanovich, Andrea Howe, Dimitrius Marinos, Daniel Mascarenhas, George Reahl, Katherine Ordonio, Marckenley Isaac, Ugochukwu Udogwu, Mitchell Baker, Alexandra Mulliken, Jared Atchison, Michael G. Schloss, Syed M. R. Zaidi, Phillip C. McKegg, Genaro A. DeLeon, Qasim M. Ghulam, and Megan Camara.
Adjudicator: Lucas S. Marchand.
Ethical review statement
Trial registration: Vita-Shock (A Blinded Exploratory Randomized Controlled Trial to Determine Optimal Vitamin D3 Supplementation Strategies for Acute Fracture Healing) was registered at ClinicalTrials.gov (identifier NCT02786498) prior to enrolment of participants.
Open access funding
The authors report that the open access funding for their manuscript was self-funded.
Supplementary material
Figure showing the distribution of serum C-terminal telopeptide of type I collagen and N-terminal propeptide of type I procollagen at baseline, six weeks post-injury, and 12 weeks post-injury.
© 2022 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
Contributor Information
Christopher C. Stewart, Email: stewart.ccs@gmail.com.
Nathan N. O’Hara, Email: no’hara@som.umaryland.edu.
Sofia Bzovsky, Email: bzovskys@mcmaster.ca.
Chelsea S. Bahney, Email: Chelsea.Bahney@ucsf.edu.
Sheila Sprague, Email: sprags@mcmaster.ca.
Gerard P. Slobogean, Email: gslobogean@som.umaryland.edu.
On behalf of Vita-Shock Investigators:
Gerard P. Slobogean, Sheila Sprague, Jonathan D. Adachi, Mohit Bhandari, Lehana Thabane, Michael F. Holick, Sofia Bzovsky, Nicole Simunovic, Kim Madden, Taryn Scott, Andrew Duong, Diane Heels-Ansdell, Zachary D. Hannan, Daniel Connelly, Joshua Rudnicki, Andrew N. Pollak, Robert V. O’Toole, Christopher LeBrun, Jason W. Nascone, Marcus F. Sciadini, Yasmin Degani, Raymond Pensy, Theodore Manson, W. Andrew Eglseder Jr., Christopher G. Langhammer, Aaron J. Johnson, Nathan N. O’Hara, Haley Demyanovich, Andrea Howe, Dimitrius Marinos, Daniel Mascarenhas, George Reahl, Katherine Ordonio, Marckenley Isaac, Ugochukwu Udogwu, Mitchell Baker, Alexandra Mulliken, Jared Atchison, Michael G. Schloss, Syed M. R. Zaidi, Phillip C. McKegg, Genaro A. DeLeon, Qasim M. Ghulam, Megan Camara, and Lucas S. Marchand
References
- 1. Cunningham BP, Brazina S, Morshed S, Miclau T. Fracture healing: A review of clinical, imaging and laboratory diagnostic options. Injury. 2017;48 Suppl 1:S69–S75. 10.1016/j.injury.2017.04.020 [DOI] [PubMed] [Google Scholar]
- 2. Yan J, Liu HJ, Li H, et al. . Circulating periostin levels increase in association with bone density loss and healing progression during the early phase of hip fracture in Chinese older women. Osteoporos Int. 2017;28(8):2335–2341. 10.1007/s00198-017-4034-z [DOI] [PubMed] [Google Scholar]
- 3. Cox G, Einhorn TA, Tzioupis C, Giannoudis PV. Bone-turnover markers in fracture healing. J Bone Joint Surg Br. 2010;92-B(3):329–334. 10.1302/0301-620X.92B3.22787 [DOI] [PubMed] [Google Scholar]
- 4. Ivaska KK, Gerdhem P, Akesson K, Garnero P, Obrant KJ. Effect of fracture on bone turnover markers: a longitudinal study comparing marker levels before and after injury in 113 elderly women. J Bone Miner Res. 2007;22(8):1155–1164. 10.1359/jbmr.070505 [DOI] [PubMed] [Google Scholar]
- 5. Veitch SW, Findlay SC, Hamer AJ, Blumsohn A, Eastell R, Ingle BM. Changes in bone mass and bone turnover following tibial shaft fracture. Osteoporos Int. 2006;17(3):364–372. 10.1007/s00198-005-2025-y [DOI] [PubMed] [Google Scholar]
- 6. Ingle BM, Hay SM, Bottjer HM, Eastell R. Changes in bone mass and bone turnover following distal forearm fracture. Osteoporos Int. 1999;10(5):399–407. 10.1007/s001980050246 [DOI] [PubMed] [Google Scholar]
- 7. Stewart CC, O’Hara NN, Orwig D, et al. . Serum 25(OH)D is associated with an altered bone turnover marker response after a hip fracture. J Orthop Res. 2019;37(3):535–540. 10.1002/jor.24200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kumar M, Shelke D, Shah S. Prognostic potential of markers of bone turnover in delayed-healing tibial diaphyseal fractures. Eur J Trauma Emerg Surg. 2019;45(1):31–38. 10.1007/s00068-017-0879-2 [DOI] [PubMed] [Google Scholar]
- 9. Moghaddam A, Müller U, Roth HJ, Wentzensen A, Grützner PA, Zimmermann G. TRACP 5b and CTX as osteological markers of delayed fracture healing. Injury. 2011;42(8):758–764. 10.1016/j.injury.2010.11.017 [DOI] [PubMed] [Google Scholar]
- 10. Pountos I, Georgouli T, Pneumaticos S, Giannoudis PV. Fracture non-union: Can biomarkers predict outcome? Injury. 2013;44(12):1725–1732. 10.1016/j.injury.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 11. Sousa CP, Dias IR, Lopez-Peña M, et al. . Bone turnover markers for early detection of fracture healing disturbances: A review of the scientific literature. An Acad Bras Cienc. 2015;87(2):1049–1061. 10.1590/0001-3765201520150008 [DOI] [PubMed] [Google Scholar]
- 12. Gorter EA, Hamdy NAT, Appelman-Dijkstra NM, Schipper IB. The role of vitamin D in human fracture healing: a systematic review of the literature. Bone. 2014;64:288–297. 10.1016/j.bone.2014.04.026 [DOI] [PubMed] [Google Scholar]
- 13. Sprague S, Petrisor B, Scott T, et al. . What is the role of vitamin D supplementation in acute fracture patients? A systematic review and meta-analysis of the prevalence of hypovitaminosis D and supplementation efficacy. J Orthop Trauma. 2016;30(2):53–63. 10.1097/BOT.0000000000000455 [DOI] [PubMed] [Google Scholar]
- 14. Bergin PF, Tarkin IS, Kempton LB, Sagi HC, Hsu J, Archdeacon MT. Optimizing the host in fracture surgery. J Orthop Trauma. 2019;33 Suppl 6:S34–S38. 10.1097/BOT.0000000000001477 [DOI] [PubMed] [Google Scholar]
- 15. Gorter EA, Krijnen P, Schipper IB. Vitamin D status and adult fracture healing. J Clin Orthop Trauma. 2017;8(1):34–37. 10.1016/j.jcot.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bodendorfer BM, Cook JL, Robertson DS, et al. . Do 25-Hydroxyvitamin D levels correlate with fracture complications? J Orthop Trauma. 2016;30(9):e312-7. 10.1097/BOT.0000000000000639 [DOI] [PubMed] [Google Scholar]
- 17. Haines N, Kempton LB, Seymour RB, et al. . The effect of a single early high-dose vitamin D supplement on fracture union in patients with hypovitaminosis D: a prospective randomised trial. Bone Joint J. 2017;99-B(11):1520–1525. 10.1302/0301-620X.99B11.BJJ-2017-0271.R1 [DOI] [PubMed] [Google Scholar]
- 18. Sprague S, Bhandari M, Devji T, et al. . Prescription of vitamin D to fracture patients: a lack of consensus and evidence. J Orthop Trauma. 2016;30(2):e64-9. 10.1097/BOT.0000000000000451 [DOI] [PubMed] [Google Scholar]
- 19. Morshed S. Current options for determining fracture union. Adv Med. 2014;2014:708574. 10.1155/2014/708574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125(7):605–613. 10.7326/0003-4819-125-7-199610010-00011 [DOI] [PubMed] [Google Scholar]
- 21. Vasikaran S, Eastell R, Bruyère O, et al. . Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391–420. 10.1007/s00198-010-1501-1 [DOI] [PubMed] [Google Scholar]
- 22. Szulc P, Naylor K, Hoyle NR, Eastell R, Leary ET, National Bone Health Alliance Bone Turnover Marker Project . Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int. 2017;28(9):2541–2556. 10.1007/s00198-017-4082-4 [DOI] [PubMed] [Google Scholar]
- 23. Højsager FD, Rand MS, Pedersen SB, Nissen N, Jørgensen NR. Fracture-induced changes in biomarkers CTX, PINP, OC, and BAP-a systematic review. Osteoporos Int. 2019;30(12):2381–2389. 10.1007/s00198-019-05132-1 [DOI] [PubMed] [Google Scholar]
- 24. Jorde R, Stunes AK, Kubiak J, et al. . Effects of vitamin D supplementation on bone turnover markers and other bone-related substances in subjects with vitamin D deficiency. Bone. 2019;124:7–13. 10.1016/j.bone.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 25. Yadav AK, Kumar V, Kumar V, Banerjee D, Gupta KL, Jha V. The effect of vitamin D supplementation on bone metabolic markers in chronic kidney disease. J Bone Miner Res. 2018;33(3):404–409. 10.1002/jbmr.3314 [DOI] [PubMed] [Google Scholar]
- 26. Grimnes G, Joakimsen R, Figenschau Y, Torjesen PA, Almås B, Jorde R. The effect of high-dose vitamin D on bone mineral density and bone turnover markers in postmenopausal women with low bone mass--a randomized controlled 1-year trial. Osteoporos Int. 2012;23(1):201–211. 10.1007/s00198-011-1752-5 [DOI] [PubMed] [Google Scholar]
- 27. Nahas-Neto J, Cangussu LM, Orsatti CL, et al. . Effect of isolated vitamin D supplementation on bone turnover markers in younger postmenopausal women: a randomized, double-blind, placebo-controlled trial. Osteoporos Int. 2018;29(5):1125–1133. 10.1007/s00198-018-4395-y [DOI] [PubMed] [Google Scholar]
- 28. Macdonald HM, Wood AD, Aucott LS, et al. . Hip bone loss is attenuated with 1000 IU but not 400 IU daily vitamin D3: a 1-year double-blind RCT in postmenopausal women. J Bone Miner Res. 2013;28(10):2202–2213. 10.1002/jbmr.1959 [DOI] [PubMed] [Google Scholar]
- 29. Madar AA, Knutsen KV, Stene LC, et al. . Effect of vitamin D3-supplementation on bone markers (serum P1NP and CTX): A randomized, double blinded, placebo controlled trial among healthy immigrants living in Norway. Bone Rep. 2015;2:82–88. 10.1016/j.bonr.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lu HK, Zhang Z, Ke YH, et al. . High prevalence of vitamin D insufficiency in China: relationship with the levels of parathyroid hormone and markers of bone turnover. PLoS One. 2012;7(11):e47264. 10.1371/journal.pone.0047264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao J, Xia W, Nie M, et al. . The levels of bone turnover markers in Chinese postmenopausal women: Peking Vertebral Fracture study. Menopause. 2011;18(11):1237–1243. 10.1097/gme.0b013e31821d7ff7 [DOI] [PubMed] [Google Scholar]
- 32. Bhattoa HP, Nagy E, More C, et al. . Prevalence and seasonal variation of hypovitaminosis D and its relationship to bone metabolism in healthy Hungarian men over 50 years of age: the HunMen Study. Osteoporos Int. 2013;24(1):179–186. 10.1007/s00198-012-1920-2 [DOI] [PubMed] [Google Scholar]
- 33. Szulc P, Munoz F, Marchand F, Chapuy MC, Delmas PD. Role of vitamin D and parathyroid hormone in the regulation of bone turnover and bone mass in men: the MINOS study. Calcif Tissue Int. 2003;73(6):520–530. 10.1007/s00223-002-2103-5 [DOI] [PubMed] [Google Scholar]
- 34. Rozental TD, Herder LM, Walley KC, et al. . 25-Hydroxyvitamin-D and bone turnover marker levels in patients with distal radial fracture. J Bone Joint Surg Am. 2015;97-A(20):1685–1693. 10.2106/JBJS.O.00313 [DOI] [PubMed] [Google Scholar]
- 35. Ting BL, Walley KC, Travison TG, Rozental TD. Elevated bone turnover markers are associated with distal radius fractures in premenopausal women. J Hand Surg Am. 2017;42(2):71–77. 10.1016/j.jhsa.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 36. Torbergsen AC, Watne LO, Frihagen F, Wyller TB, Mowè M. Effects of nutritional intervention upon bone turnover in elderly hip fracture patients. Randomized controlled trial. Clin Nutr ESPEN. 2019;29:52–58. 10.1016/j.clnesp.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 37. Hitz MF, Jensen JE, Eskildsen PC. Bone mineral density and bone markers in patients with a recent low-energy fracture: effect of 1 y of treatment with calcium and vitamin D. Am J Clin Nutr. 2007;86(1):251–259. 10.1093/ajcn/86.1.251 [DOI] [PubMed] [Google Scholar]
- 38. Sprague S, Bzovsky S, Connelly D, et al. . Study protocol: design and rationale for an exploratory phase II randomized controlled trial to determine optimal vitamin D3 supplementation strategies for acute fracture healing. Pilot Feasibility Stud. 2019;5:135. 10.1186/s40814-019-0524-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhandari M, Wasserman SM, Yurgin N, Petrisor B, Sprague S, Dent RE. Development and preliminary validation of a Function IndeX for Trauma (FIX-IT). Can J Surg. 2013;56(5):E114-20. 10.1503/cjs.004312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mitchell SL, Obremskey WT, Luly J, et al. . Inter-rater reliability of the modified Radiographic Union Score for diaphyseal tibial fractures with bone defects. J Orthop Trauma. 2019;33(6):301–307. 10.1097/BOT.0000000000001445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Litrenta J, Tornetta P, Mehta S, et al. . Determination of radiographic healing: an assessment of consistency using RUST and Modified RUST in metadiaphyseal fractures. J Orthop Trauma. 2015;29(11):516–520. 10.1097/BOT.0000000000000390 [DOI] [PubMed] [Google Scholar]
- 42. Ross KA, O’Halloran K, Castillo RC, et al. . Prediction of tibial nonunion at the 6-week time point. Injury. 2018;49(11):2075–2082. 10.1016/j.injury.2018.07.033 [DOI] [PubMed] [Google Scholar]
- 43. Nuti R, Martini G, Valenti R, et al. . Vitamin D status and bone turnover in women with acute hip fracture. Clin Orthop Relat Res. 2004;422:208–213. 10.1097/01.blo.0000129163.97988.06 [DOI] [PubMed] [Google Scholar]
- 44. Lack WD, Starman JS, Seymour R, et al. . Any cortical bridging predicts healing of tibial shaft fractures. J Bone Joint Surg Am. 2014;96-A(13):1066–1072. 10.2106/JBJS.M.00385 [DOI] [PubMed] [Google Scholar]
- 45. Tian R, Zheng F, Zhao W, et al. . Prevalence and influencing factors of nonunion in patients with tibial fracture: systematic review and meta-analysis. J Orthop Surg Res. 2020;15(1):377. 10.1186/s13018-020-01904-2 [DOI] [PMC free article] [PubMed] [Google Scholar]