Abstract
Hip injection (HI) for osteoarthritis (OA) are in vogue nowadays. Corticosteroids (CSs) and hyaluronic acid (HA) gel are the two most common agents injected into the hip. Off late, platelet-rich plasma (PRP), mesenchymal stem cell (MSC), bone marrow aspirate concentrate (BMAC), local anesthetic (LA) agents, non-steroidal anti-inflammatory drugs (NSAIDs) and their different combinations have also been injected in hips to provide desired pain relief. However, there is a group of clinicians who vary of these injections. A search of the literature was performed on PubMed, Cochrane Library, and DOAJ using the keywords “hip osteoarthritis injection”. Data were analyzed and compiled. Intraarticular CSs are effective in providing the desired pain relief in OA hip, but repeated injections should be avoided and the interval between HI and hip arthroplasty must be kept for more than three months. Methylprednisolone or triamcinolone are combined with 1% lidocaine or 0.5% bupivacaine. Chondrotoxic effects of LA is a concern. Although national guidelines do not favor the use of HA for hip OA, numerous publications have favored its usage for a moderate grade of OA. The PRP, MSC, and BMAC are treatment options with great potential; however, currently, the evidence is conflicting on their role in hip OA. There is always a risk of septic arthritis, particularly when aseptic precautions are not followed, and clinicians must vary of this complication.
Keywords: Bone marrow aspirate concentrate, corticosteroids, hip injection, hip osteoarthritis, hyaluronic acid, mesenchymal stem cell, platelet-rich plasma, steroid.
The use of hip injection (HI) in the treatment of osteoarthritis (OA) has gained wide popularity. The relatively low cost, fast and simple method of pain relief are amongst its many advantages. Over time, the content of the injection has also evolved from local anesthetic (LA) agents to corticosteroids (CSs), hyaluronic acid (HA) and platelet-rich plasma (PRP).[1] The scope of use of injections in the hip region has grown from traditional aspiration to therapeutic injections. The two main substances used in recent times for pain relief are CSs and HA gel. For decades, low doses of CS were given to surgically unfit patients and to those who are not keen on joint replacement surgery.[2]
The recent surge in the use of high-molecularweight HA for knee OA has been expanded as a treatment option for hip OA. The popularity of the administration of HA has been mounting with very little outcome data to support its use. Administration of HA injections has shown some promise in a selected subset of patients suffering from early OA of the hip.[3,4] Most papers report insufficient sample size and had a varied follow-up period which results in difficulty formulating and implementing national guidelines and clinical recommendations. Current literature advocates the safe use of CS injections for early hip OA.[5] Although there is no concrete evidence supporting HA injections, this has not dissuaded researchers from injecting PRP, mesenchymal stem cells (MSCs), LA agents, NSAIDS and many different combinations into the hip. The true extent of their benefits is still being debated.[6] In this review, we outline recent trends, discuss the role of HIs, and summarize complications of the technique.
SEARCH STRATEGY AND SELECTION CRITERIA
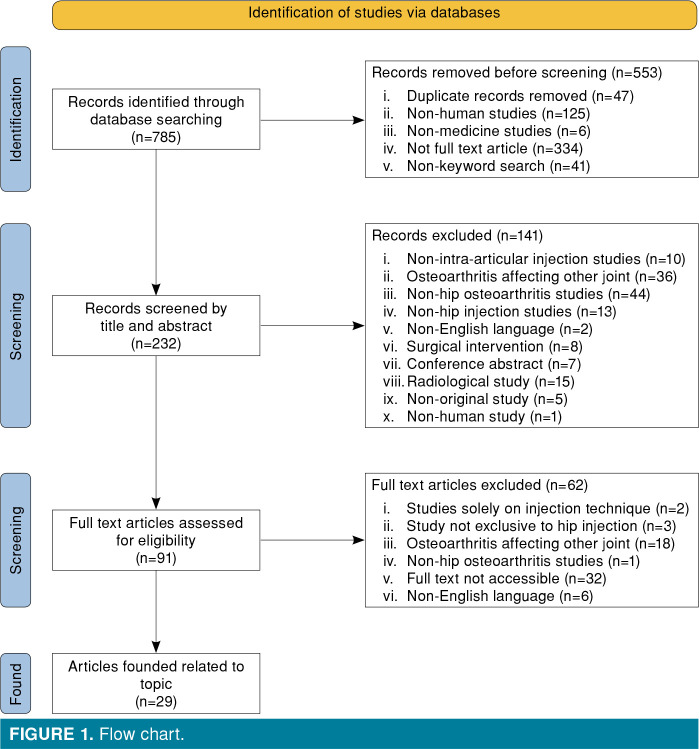
We conducted a review of literature on intraarticular injections for OA of the hip. The PubMed, Cochrane Libra and DOAJ were accessed, and articles written in the English language with keywords “hip osteoarthritis injection”, and those that published relevant literature on humans were included in our search. We focused on publications from the past 11 years (2010 to 2021). Meta-analyses, systematic reviews, randomized and non-randomized clinical trials on hip OA that were published in the English language were included. All other types of articles were excluded from this review. On typing the keywords “hip osteoarthritis and hip injection”, a total of 785 articles were identified in the search, out of which only 232 articles were found to be relevant and hence selected. Out of these 232, 141 articles were excluded due to the unsuitable nature of the article, and lack of well-defined inclusion and exclusion criteria. Ninety-one full-text articles were assessed for eligibility and finally, directly related 29 articles were found below (Figure 1).
Figure 1. Flow chart.
CONTEMPORARY TRENDS AND CLINICAL RECOMMENDATIONS
Intraarticular HIs have been administered for decades.[7] As early as 1947, Crowe[8] reported satisfactory results with intraarticular acid phosphate injections for the treatment of hip OA. He also recommended the anterior approach for administration of the injection as the easiest, least painful, and most accurate. In 1956, Leveaux and Quin[9] published their results on “Local injection of hydrocortisone and procaine in osteoarthritis of the hip joint” and concluded that the combination of these two substances was of valuable palliative management for the painful osteoarthritic hip joint. On the other hand, the American Academy of Orthopaedic Surgeons (AAOS) guidelines on the management of OA of the hip published in 2017 considered only intraarticular CSs and HA worthy of any recommendation. AAOS supported the use of intraarticular CSs to improve short-term function and pain for patients with symptomatic OA of the hip.[10] However, they did not support the use of intraarticular HA, citing equal efficacy to placebo for function, stiffness, and pain in patients with symptomatic OA of the hip.[3] In a meta-analysis conducted by Gazendam et al.,[6] only minimal clinically important differences were observed from the baseline to six months after all HIs, and the results were similar in the intervention and placebo groups.
ROLE OF INTRAARTICULAR CORTICOSTEROIDS FOR HIP OSTEOARTHRITIS
Synovitis is a major cause of pain in hip OA. Local anti-inflammatory treatment such as intraarticular CS is effective in ameliorating pain in OA of the hip.[11] Downregulating genetic expression of several proinflammatory proteins and limiting the interaction between white blood cells involved in immune response appears to be the mechanism of action of the therapy.[12] These injections are frequently given in combination with an LA agent. Methylprednisolone or triamcinolone is combined with 1% lidocaine or 0.5% bupivacaine. The dose administered depends on patientspecific factors and surgeon experience. The dose of methylprednisolone ranges from 40 to 120 mg, while the dose of triamcinolone ranges from 20 to 80 mg.[12,14] These agents have been selected by the virtue of them being less soluble in water (particulate CSs).[15] The more the solubility of a particular steroid injection, the less the duration of effect of the injection. Only preservative-free anesthetics must be used as a solvent for these steroids to prevent particulate precipitation. Mixing steroids with an LA agent has distinct advantages of reducing infiltration discomfort, increasing the volume of the injection and better distribution of the solution throughout the joint.[16]
McCabe et al.[5] published their systematic review on the efficacy of intraarticular steroids in hip OA in 2016. They recommended methodologically rigorous trials to verify whether intraarticular CSs were beneficial, and the duration of efficacy. They included randomized-controlled trials (RCTs) assessing the efficacy of hip intraarticular steroid injection on pain. A total of five RCTs were included though all were marred by a limited sample size. They determined that these injections were well tolerated and were effective in reducing some amount of pain for up to four weeks.
In 2018, Lai et al.[17] published a retrospective analysis of all intraarticular hip steroid injections performed for hip OA between January 2010 and December 2012. Around 20% showed no response, less than 50% showed an immediate response (≤2 weeks of pain relief), and the remaining showed a continued response (>2 weeks of pain relief). Age, obesity, duration of symptoms and radiological grading of hip OA were not found to have a significant correlation with intraarticular CS injection. Total hip replacement within two years was required in almost 50% of the patients, which ultimately led to the authors’ recommendation of considering hip arthroplasty early in the disease.
Multiple authors have reported that the effect of intraarticular steroid administration on pain and function disappears rapidly, but Deshmukh et al.[18] reported that steroids could provide long-term relief, unlike others. Additionally, it was reported that there was a relationship between the reduction of pain and the severity of the disease, contrary to Lai et al.[17]
Moreover, since the coronavirus pandemic has drastically reduced the occurrence of elective arthroplasty surgery, intraarticular steroids are an effective intervention for patients awaiting joint replacement surgery. However, these injections are not without risks such as the risk of infection and progression of cartilage damage. Therefore, we should remember to use these injections judiciously.[19]
ROLE OF INTRAARTICULAR VISCOSUPPLEMENTATION FOR HIP OSTEOARTHRITIS
Hyaluronic acid has been said to improve the rheological properties of synovial fluid with some chondroprotective and anti-inflammatory effects. Although the positive clinical effects of HA have been demonstrated in the knee joint, guidelines and the latest literature recommend otherwise for its use in the hip.[3,20] In 2017, Eymard et al.,[21] on behalf of the Osteoarthritis Group of the French Society of Rheumatology and the French Research Group in Interventional Rheumatology, published their results from a multi-center, open-label, prospective, trial. They elaborated on the subset of hip OA patients that benefited the most by a single intraarticular injection of a cross-linked HA combined with mannitol on Day 90. Patients with moderate pain, moderate disability, moderate joint space narrowing, superomedial and axial femoral head migration, with femoral-acetabular impingement or coxa profunda displayed better results. This drug combination was able to decrease pain by 50%, in half of their patients at day 90.
Benefits in an almost similar subset of patients have been reported by other authors, as well. Pogliacomi et al.[4] in their original study published in 2018 reported the efficacy of intraarticular HI of a single dose of high-weight HA in patients under a follow-up of 12 months. They concluded that patients with a moderate grade of OA are the ones that benefit the most from the said injection.
Extravasation of HA has been shown to cause inflammation of periarticular tissues and must be avoided, as it can sometimes mimic a septic reaction. Acute local reactions have been associated with multiple injections, leading to the conclusion that a single injection is more beneficial.[16] In a metaanalysis focused on adverse reactions after HA HIs; Wu et al.[22] could not find an increased adverse reaction rate with HA compared to controls.
ROLE OF INTRAARTICULAR PLATELET-RICH PLASMA FOR HIP OSTEOARTHRITIS
We did not find high-quality studies comparing PRP with placebo up to April 15th, 2016.[3] In 2018, the Royal Australian College of General Practitioners released their guideline for the management of knee and hip OA, and due to a lack of high-quality evidence, they were unable to make any recommendation for PRP injections.[23]
Platelet-rich plasma is a biological treatment with great perspective, but standardization of treatment is lacking, and this has led to conflicting evidence on the effect of intraarticular PRP injections for the management of hip OA. Dong et al.[24] published a meta-analysis of high-powered RCTs conducted on the effect of intraarticular PRP on OA, up to June 2019. They included three RCTs on hip OA and concluded that large scale double-blinded RCTs are required to evaluate the effect of PRP injection in hip OA. An RCT comparing the efficacy of ultrasound-guided intraarticular injections of PRP versus HA for hip OA, published by Battaglia et al.[25] recognized the efficacy of PRP in terms of functional enhancement and decrease in pain, but concluded that PRP was not superior to HA up to 12-month follow-up.
Di Sante et al.[26] published an RCT comparing the efficacy of PRP vs HA injection in hip OA and established that intraarticular PRP had a pain-relieving effect on hip OA that lasted up to four weeks. The PRP is being marketed as a promising new product of regenerative medicine that is superior to other current therapies. However, unfortunately, it still lacks robust evidence to support its use in clinical practice.[27,28] Orthopedic surgeons should be aware of the ongoing uncertainty about the evidence behind PRP therapies and inform patients about this fact.[28] Overall, there is insufficient evidence to support the use of PRP for hip OA in the current literature.
ROLE OF INTRAARTICULAR MESENCHYMAL STEM CELLS FOR HIP OSTEOARTHRITIS
A study from Iran by Emadedin et al.[29] published their results on injections of autologous bone marrow-derived MSCs for patients with OA of the hip, knee, and ankle. A total of 17 patients were evaluated clinically, as well as with magnetic resonance imaging (MRI) scans for a period of up to 30 months post-injection. They did not observe any serious adverse effects (systemic effects, tumors, pulmonary embolism, and death) and found the injection to be safe. Additionally, the study group had decreased pain, improved walking ability and functional scores. Furthermore, an increase in cartilage thickness and a decrease in subchondral oedema was observed on MRI. A major drawback of this study was the small sample size, particularly while evaluating effects on hip OA.
Researchers from Chile published their welldesigned study of 10 patients who were more than 60 years old and were suffering from hip OA (up to moderate grade). These patients were given an intraarticular injection of ex vivo expanded autologous bone marrow-derived MSCs. Patients without pain or mild pain were not included in the study. Pain, stiffness, functionality, and range of motion were evaluated. Patients were followed up to a maximum of 40 weeks. Improvement in all the clinical parameters was noted in all, but one patient. The radiographic progression of OA was also arrested and these occurred without any major side effects.[30]
A systematic review published in 2018 included 28 studies for critical review. Although HIs were a minuscule part of the whole study group; the general trend was favorable to MSC injections without major complications.[31] Considering the potential of stem cell therapy, there is still a need for high-quality research on this topic.
NOVEL INTRAARTICULAR INJECTIONS FOR HIP OSTEOARTHRITIS
A combination of MSCs and constituents of PRP is known as bone marrow aspirate concentrate (BMAC). The BMAC is one of the few United States Food and Drug Administration (FDA) approved methods for providing stem cells.[32]
A study by Rodriguez-Fontan et al.[33] included 25 joints (10 knees, 15 hips), that were injected with intraarticular BMAC. Only patients with KellgrenLawrence Grade I-II/ Tönnis Grade I-II were included in the study. Maximum follow-up was up to 24 months. A total of 63.2% of patients were satisfied with the procedure and this injection was found to be safe. Darrow et al.[34] published their study of repeated BMAC injections in the hip for OA. All patients reported decreased pain and improved function. They hypothesized that multiple injections in a short period were responsible for this significant improvement from baseline. The BMAC has great potential, and large-scale, placebo-controlled RCTs can pave the way for a futuristic regenerative method of treatment for early to moderate hip OA.
Another treatment modality that is worth mentioning due to its low cost is prolotherapy; a technique that has seen more waxing and waning than any other therapy in orthopedic medicine. It has recently gained popularity for hip OA. However, placebo-controlled trials are still lacking.
A treatment method that is gaining popularity is the intraarticular injection of NSAIDs. They are less potent anti-inflammatory agents (as compared to CSs). Park et al.[35] published the results of their retrospective comparative study where 50 patients received intraarticular CS injection and 48 received intraarticular ketorolac injection. Ketorolac HI was found to be as effective as CS HI.
COMPLICATIONS OF INTRAARTICULAR HIP INJECTIONS
Procedural complications such as pain and bleeding at the injection site are easily manageable. The fear of septic arthritis although real is exceedingly rare, if proper aseptic precautions have been followed.[36] In studies on a substance other than steroids and LAs, complications related to injection have been reported with local adverse effects.[27,29]
Patients planned for intraarticular steroid injection must be warned of the side effects such as mild headaches, slight exaggeration in pain (steroid flare), lack of sleep and facial flushing (particularly common in females).[16] Diabetics often enquire regarding the influence of these injections on their blood glucose levels. Clinicians must put forward the available evidence that a transient spike in blood glucose levels lasting less than a week can occur in selected individuals after CS injections.[37] There is also an increased risk of infection in diabetic patients undergoing intraarticular steroid injections.
The list of complications of intraarticular steroids would be incomplete without mentioning the so-called “corticosteroid arthropathy”.[36] Having said that, a study with human subjects did not display radiological evidence of cartilage damage, despite repeated injections of intraarticular CSs in their knees for over two years.[38] Inversely, an annual MRI-based study comparing intraarticular triamcinolone and intraarticular saline every 12 weeks for two years found that triamcinolone resulted in significantly greater cartilage volume loss.[39]
In the practice of orthopedics, there is one common fear in the usage of intraarticular injections, which is the risk of postoperative infection in the hip that has been previously injected with a CS. Steroids are well known to dampen the intraarticular immune response. Extensive studies have been done on this topic. Werner et al.[40] proved beyond doubt that the interval between HI and hip arthroplasty must be maintained for more than three months to mitigate the risk of prosthetic joint infection. These studies would probably be quoted more frequently in the coming years as more patients are being treated with intraarticular HIs in recent times due to the coronavirus pandemic putting a halt to elective arthroplasty surgeries.
Another popular opinion is the chondrotoxic effect of LA agents used either alone or along with particulate CSs for intraarticular injection. Jayaram et al.[41] recognized that there was variability in the chondrotoxic effect of different LA agents and there was no consensus on an ideal LA for intraarticular injection. They carried out a systematic review on the effect of LA agents on knee cartilage. Literature from 1990 to 2018 was utilized with 16 studies included using chondrocyte viability, morphology, and histology as the outcome measures. Data related to commonly used LA agents such as lidocaine, bupivacaine, ropivacaine, levobupivacaine, and mepivacaine were extracted. Each drug had different spectrums of chondrocyte damage culminating in increased apoptosis, extracellular matrix damage and mitochondrial dysfunction. These effects were dose- and duration-dependent and concomitant CS administration was found to exacerbate these effects. The toxicity spectrum was found to be maximum for bupivacaine and the least for ropivacaine (in concentrations less than 0.75%). However, we should keep in mind that these observations were largely based on in vitro studies.
IMAGING-GUIDED INTRAARTICULAR INJECTION
The intraarticular injection procedure can be technically challenging due to the deep placement of the hip joint. Unsuccessful injection potentially exposes the patient to complications from the procedure (pain, infection, bleeding) and may contribute to diagnosis and treatment delays. Intraarticular HI with the help of anatomic landmarks is frequently performed over the signs defined for hip arthroscopy by Wettstein and Dienst[42] for accessing the peripheral compartment of the hip joint. However, the success rate of HIs without imaging guidance is not satisfactory. A meta-analysis about success rates of injection techniques reported that operators using ultrasound had an injection success of 100%, while operators using anatomical landmark-guided injections were 72% accurate.[43]
The most used imaging method for intraarticular injections of the hip is the fluoroscopic technique. Radiopaque contrast material can be used for confirming that the needle is in the joint. However, access to the fluoroscopy device can sometimes be not possible. Due to the difficulty, ultrasound guidance, which can be easily applied in office conditions, is recommended for HIs by several authors.[44,45] In an animal experiment evaluating the success rate, the accuracy was 90% for the ultrasound-guided procedure and 75% for the fluoroscopy-guided intraarticular injection. There was a statistically significant difference between the two groups.[46] Computed tomography (CT) can theoretically provide joint injection with high success, but it is not preferred due to the high radiation exposure of the patient and operator. There is a rare need to CT guidance for magnetic resonance arthrography, when qualified personnel are not available, with reduced radiation dose can be used for contrast agent injection (Table 1).[47]
Table 1. Comparisons of guidance techniques for intra-articular hip injections.
| Guidance | Success rate (%) | Radiation exposure | Cost | Time to success | Experience |
| Anatomic landmarks | 32-89 | N/A | Low-priced | High | Intermediate |
| Fluoroscopy | 76 | Low dose | Low-priced | High | Intermediate |
| Ultrasonography | 90-100 | N/A | Medium-priced | Low | Advanced |
| Computed tomography | 100 | High dose | High-priced | Moderate | Advanced |
| Magnetic resonance imaging | 100 | N/A | High-priced | Moderate | Advanced |
| N/A: Not applicable. | |||||
In conclusion, intraarticular CSs have established themselves as a pain-alleviating agent for hip OA; however, they must not be administered frequently, as a gap of three to six months must be maintained after the injection and before hip arthroplasty surgery. Additionally, the choice of LA that is combined with CS must be evidence-based and the clinician must be aware of the chondrotoxic effects of LA agents. The HA has not been able to replicate the results obtained in knee OA. At best, it has shown a promising effect in moderate grade hip OA. Biological agents including PRP/ MSC/ BMAC have great potential, but unrestricted usage cannot be recommended, as high-quality evidence is still lacking in modern literature.[48]
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Urits I, Orhurhu V, Powell J, Murthy A, Kiely B, Shipon S, et al. Minimally invasive therapies for osteoarthritic hip pain: A comprehensive review. Curr Pain Headache Rep. 2020;24:37–37. doi: 10.1007/s11916-020-00874-8. [DOI] [PubMed] [Google Scholar]
- 2.Pereira LC, Kerr J, Jolles BM. Intra-articular steroid injection for osteoarthritis of the hip prior to total hip arthroplasty: Is it safe? A systematic review. Bone Joint J. 2016;98-B:1027–1035. doi: 10.1302/0301-620X.98B8.37420. [DOI] [PubMed] [Google Scholar]
- 3.Quinn RH, Murray J, Pezold R, Hall Q. Management of osteoarthritis of the hip. e434-e436J Am Acad Orthop Surg. 2018;26 doi: 10.5435/JAAOS-D-18-00351. [DOI] [PubMed] [Google Scholar]
- 4.Pogliacomi F, Schiavi P, Paraskevopoulos A, Leigheb M, Pedrazzini A, Ceccarelli F, et al. When is indicated viscosupplementation in hip osteoarthritis. Acta Biomed. 2018;90:67–74. doi: 10.23750/abm.v90i1-S.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCabe PS, Maricar N, Parkes MJ, Felson DT, O'Neill TW. The efficacy of intra-articular steroids in hip osteoarthritis: A systematic review. Osteoarthritis Cartilage. 2016;24:1509–1517. doi: 10.1016/j.joca.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Gazendam A, Ekhtiari S, Bozzo A, Phillips M, Bhandari M. Intra-articular saline injection is as effective as corticosteroids, platelet-rich plasma and hyaluronic acid for hip osteoarthritis pain: A systematic review and network meta-analysis of randomised controlled trials. Br J Sports Med. 2021;55:256–261. doi: 10.1136/bjsports-2020-102179. [DOI] [PubMed] [Google Scholar]
- 7.Dodré E, Lefebvre G, Cockenpot E, Chastanet P, Cotten A. Interventional MSK procedures: The hip. Br J Radiol. 2016;89:20150408–20150408. doi: 10.1259/bjr.20150408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowe HW. Osteo-arthritis of the hip-joint treated by intraarticular injections. Proc R Soc Med. 1947;40:486–487. [PMC free article] [PubMed] [Google Scholar]
- 9.Leveaux VM, Quin CE. Local injection of hydrocortisone and procaine in osteo-arthritis of the hip joint. Ann Rheum Dis. 1956;15:330–337. doi: 10.1136/ard.15.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Academy of Orthopaedic Surgeons, Management of Osteoarthritis of the Hip Evidence-Based Clinical Practice Guideline. 2017. Available from: https://www.aaos.org/globalassets/quality-and-practice-resources/osteoarthritisof-the-hip/oa-hip-cpg_6-11-19.pdf/ [Accessed: March 13, 2020]
- 11.Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res Ther. 2017;19:18–18. doi: 10.1186/s13075-017-1229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caldwell JR. Intra-articular corticosteroids. Guide to selection and indications for use. Drugs. 1996;52:507–514. doi: 10.2165/00003495-199652040-00004. [DOI] [PubMed] [Google Scholar]
- 13.Kullenberg B, Runesson R, Tuvhag R, Olsson C, Resch S. Intraarticular corticosteroid injection: Pain relief in osteoarthritis of the hip. J Rheumatol. 2004;31:2265–2268. [PubMed] [Google Scholar]
- 14.Qvistgaard E, Christensen R, Torp-Pedersen S, Bliddal H. Intra-articular treatment of hip osteoarthritis: A randomized trial of hyaluronic acid, corticosteroid, and isotonic saline. Osteoarthritis Cartilage. 2006;14:163–170. doi: 10.1016/j.joca.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Dietrich TJ, Sutter R, Froehlich JM, Pfirrmann CW. Particulate versus non-particulate steroids for lumbar transforaminal or interlaminar epidural steroid injections: An update. Skeletal Radiol. 2015;44:149–155. doi: 10.1007/s00256-014-2048-6. [DOI] [PubMed] [Google Scholar]
- 16.Rastogi AK, Davis KW, Ross A, Rosas HG. Fundamentals of joint injection. AJR Am J Roentgenol. 2016;207:484–494. doi: 10.2214/AJR.16.16243. [DOI] [PubMed] [Google Scholar]
- 17.Lai WC, Arshi A, Wang D, Seeger LL, Motamedi K, Levine BD, et al. Efficacy of intraarticular corticosteroid hip injections for osteoarthritis and subsequent surgery. Skeletal Radiol. 2018;47:1635–1640. doi: 10.1007/s00256-018-3052-z. [DOI] [PubMed] [Google Scholar]
- 18.Deshmukh AJ, Panagopoulos G, Alizadeh A, Rodriguez JA, Klein DA. Intra-articular hip injection: Does pain relief correlate with radiographic severity of osteoarthritis. Skeletal Radiol. 2011;40:1449–1454. doi: 10.1007/s00256-011-1120-8. [DOI] [PubMed] [Google Scholar]
- 19.Wall C, Johnson T, de Steiger R. Symptom management for patients awaiting joint replacement surgery. Aust J Gen Pract. 2020;49:444–446. doi: 10.31128/AJGP-03-20-5286. [DOI] [PubMed] [Google Scholar]
- 20.Hunter DJ. Viscosupplementation for osteoarthritis of the knee. N Engl J Med. 2015;372:1040–1047. doi: 10.1056/NEJMct1215534. [DOI] [PubMed] [Google Scholar]
- 21.Eymard F, Maillet B, Lellouche H, Mellac-Ducamp S, Brocq O, Loeuille D, et al. Predictors of response to viscosupplementation in patients with hip osteoarthritis: Results of a prospective, observational, multicentre, openlabel, pilot study. BMC Musculoskelet Disord. 2017;18:3–3. doi: 10.1186/s12891-016-1359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu B, Li YM, Liu YC. Efficacy of intra-articular hyaluronic acid injections in hip osteoarthritis: A meta-analysis of randomized controlled trials. Oncotarget. 2017;8:86865–86876. doi: 10.18632/oncotarget.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basedow M, Williams H, Shanahan EM, Runciman WB, Esterman A. Australian GP management of osteoarthritis following the release of the RACGP guideline for the nonsurgical management of hip and knee osteoarthritis. BMC Res Notes. 2015;8:536–536. doi: 10.1186/s13104-015-1531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong Y, Zhang B, Yang Q, Zhu J, Sun X. The effects of platelet-rich plasma injection in knee and hip osteoarthritis: A meta-analysis of randomized controlled trials. Clin Rheumatol. 2021;40:263–277. doi: 10.1007/s10067-020-05185-2. [DOI] [PubMed] [Google Scholar]
- 25.Battaglia M, Guaraldi F, Vannini F, Rossi G, Timoncini A, Buda R, et al. Efficacy of ultrasound-guided intra-articular injections of platelet-rich plasma versus hyaluronic acid for hip osteoarthritis. e1501-8Orthopedics. 2013;36 doi: 10.3928/01477447-20131120-13. [DOI] [PubMed] [Google Scholar]
- 26.Di Sante L, Villani C, Santilli V, Valeo M, Bologna E, Imparato L, et al. Intra-articular hyaluronic acid vs plateletrich plasma in the treatment of hip osteoarthritis. Med Ultrason. 2016;18:463–468. doi: 10.11152/mu-874. [DOI] [PubMed] [Google Scholar]
- 27.Sezgin EA, Atik OŞ. Are orthobiologics the next chapter in clinical orthopedics. A literature review. Eklem Hastalik Cerrahisi. 2018;29:110–116. doi: 10.5606/ehc.2018.005. [DOI] [PubMed] [Google Scholar]
- 28.Atik OŞ. Do patients benefit from platelet-rich plasma. Jt Dis Relat Surg. 2020;31:409–410. doi: 10.5606/ehc.2020.57895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emadedin M, Ghorbani Liastani M, Fazeli R, Mohseni F, Moghadasali R, Mardpour S, et al. Long-term follow-up of intra-articular injection of autologous mesenchymal stem cells in patients with knee, ankle, or hip osteoarthritis. Arch Iran Med. 2015;18:336–344. [PubMed] [Google Scholar]
- 30.Mardones R, Jofré CM, Tobar L, Minguell JJ. Mesenchymal stem cell therapy in the treatment of hip osteoarthritis. J Hip Preserv Surg. 2017;4:159–163. doi: 10.1093/jhps/hnx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIntyre JA, Jones IA, Han B, Vangsness CT Jr. Intraarticular mesenchymal stem cell therapy for the human joint: A systematic review. Am J Sports Med. 2018;46:3550–3563. doi: 10.1177/0363546517735844. [DOI] [PubMed] [Google Scholar]
- 32.Kraeutler MJ, Chahla J, LaPrade RF, Pascual-Garrido C. Biologic options for articular cartilage wear (platelet-rich plasma, stem cells, bone marrow aspirate concentrate) Clin Sports Med. 2017;36:457–468. doi: 10.1016/j.csm.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Fontan F, Piuzzi NS, Kraeutler MJ, PascualGarrido C. Early clinical outcomes of intra-articular injections of bone marrow aspirate concentrate for the treatment of early osteoarthritis of the hip and knee: A cohort study. PM R. 2018;10:1353–1359. doi: 10.1016/j.pmrj.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Darrow M, Shaw B, Darrow B, Wisz S. Short-term outcomes of treatment of hip osteoarthritis with 4 bone marrow concentrate injections: A case series. Clin Med Insights Case Rep. 2018;11:1179547618791574–1179547618791574. doi: 10.1177/1179547618791574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park KD, Kim TK, Bae BW, Ahn J, Lee WY, Park Y. Ultrasound guided intra-articular ketorolac versus corticosteroid injection in osteoarthritis of the hip: A retrospective comparative study. Skeletal Radiol. 2015;44:1333–1340. doi: 10.1007/s00256-015-2174-9. [DOI] [PubMed] [Google Scholar]
- 36.Gray RG, Gottlieb NL. Intra-articular corticosteroids. Gray RG, Gottlieb NL. Intra-articular corticosteroids. An updated assessment. Clin Orthop Relat Res 1983;(177):235-63. [PubMed] [Google Scholar]
- 37.Habib GS. Systemic effects of intra-articular corticosteroids. Clin Rheumatol. 2009;28:749–756. doi: 10.1007/s10067-009-1135-x. [DOI] [PubMed] [Google Scholar]
- 38.Raynauld JP, Buckland-Wright C, Ward R, Choquette D, Haraoui B, Martel-Pelletier J, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: A randomized, double-blind, placebocontrolled trial. Arthritis Rheum. 2003;48:370–377. doi: 10.1002/art.10777. [DOI] [PubMed] [Google Scholar]
- 39.McAlindon TE, LaValley MP, Harvey WF, Price LL, Driban JB, Zhang M, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: A randomized clinical trial. JAMA. 2017;317:1967–1975. doi: 10.1001/jama.2017.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Werner BC, Cancienne JM, Browne JA. The timing of total hip arthroplasty after intraarticular hip injection affects postoperative infection risk. J Arthroplasty. 2016;31:820–823. doi: 10.1016/j.arth.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 41.Jayaram P, Kennedy DJ, Yeh P, Dragoo J. Chondrotoxic effects of local anesthetics on human knee articular cartilage: A systematic review. PM R. 2019;11:379–400. doi: 10.1002/pmrj.12007. [DOI] [PubMed] [Google Scholar]
- 42.Wettstein M, Dienst M. Arthroscopy of the peripheral compartment of the hip. Operative Techniques in Orthopaedics. 2005;15:225–230. [Google Scholar]
- 43.Hoeber S, Aly AR, Ashworth N, Rajasekaran S. Ultrasoundguided hip joint injections are more accurate than landmarkguided injections: A systematic review and meta-analysis. Br J Sports Med. 2016;50:392–396. doi: 10.1136/bjsports-2014-094570. [DOI] [PubMed] [Google Scholar]
- 44.Sofka CM, Saboeiro G, Adler RS. Ultrasound-guided adult hip injections. J Vasc Interv Radiol. 2005;16:1121–1123. doi: 10.1097/01.RVI.0000167855.43900.74. [DOI] [PubMed] [Google Scholar]
- 45.Smith J, Hurdle MF. Office-based ultrasound-guided intraarticular hip injection: Technique for physiatric practice. Arch Phys Med Rehabil. 2006;87:296–298. doi: 10.1016/j.apmr.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Wang S, Luan S, Yu S, Zheng Y, Ma C, et al. Accuracy and feasibility of ultrasound-guided intraarticular injection of the rat hip joint. Ultrasound Med Biol. 2021;47:2936–2940. doi: 10.1016/j.ultrasmedbio.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Goeller A, Pogarell T, May MS, Uder M, Dankerl P. Evaluation of CT-guided ultra-low-dose protocol for injection guidance in preparation of MR-arthrography of the shoulder and hip joints in comparison to conventional and low-dose protocols. Diagnostics (Basel) 2021;11:1835–1835. doi: 10.3390/diagnostics11101835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atik OŞ. What are the expectations of an editor from a scientific article. Jt Dis Relat Surg. 2020;31:597–598. doi: 10.5606/ehc.2020.57896. [DOI] [PMC free article] [PubMed] [Google Scholar]